Abstract
Context:
Intramedullary tumors are neoformations taking part on the spinal cord, and they are a rare pathology. Due to the rarity of such lesions, clinical studies take years to ensure a decent feedback with a significant number of cases.
Design:
Our study is retrospective and descriptive.
Participants:
We share a Tunisian multicentric experience of 27 years through a retrospective study of 120 cases of spinal cord tumors that have been operated in six different centers.
Outcome Measures:
The clinical, radiological, and histological findings have been analyzed along with postoperative results and tumoral progression so that we could conclude to some factors of prognosis concerning the management of these tumors.
Results:
The mean age of our patients is 33.84 years. We had 57 males and 63 females. The most frequent revealing symptom was motor trouble presented as frequent as 77.5% of the patients. Glial tumors were represented in 81 of the cases (67.5%) and nonglial by 39 cases (32.5%). Glial tumors we found were essentially 39 ependymomas and 35 astrocytomas. Surgical resection is key in the management of these lesions; the quality of tumoral resection was a significant factor of disease progression as subtotal resection is correlated to more important progression than total one.
Conclusion:
We conclude this work with some statements. In terms of functional results, age is not a significant factor. Presurgical functional state, the histological type, and the extent of surgical resection are the important factors.
Keywords: Astrocytoma, ependymoma, intramedullary tumors, prognosis, spinal cord, surgery
INTRODUCTION
Intramedullary tumors represent only 10% of the spectrum of spinal tumors as these lesions develop in 60% of the cases on the epidural aspect and are in 30% of the cases intradural and extramedullary.[1] They are mostly glial tumors. Ependymomas and low-grade astrocytomas are the leading histological types.[2] Their management is not an easy task and depends essentially on the surgical resection which can be very challenging. Adjuvant therapies have a very controversial role.
We tried by this paper to share our experience concerning intramedullary tumors, the clinical and radiological specificities, the different histological types we encountered, the management and factors of prognosis on functional results, and tumoral progression.
PATIENTS AND METHODS
We conducted an analysis of clinical cases of patients treated for intramedullary tumors in 6 centers of neurosurgery in Tunisia. We totalized 120 patients on a 27-year period between 1990 and 2017 in the National Institute of Neurology, Ben Arous Trauma Center, The Military Hospital, and Departments of Neurosurgery of Sfax, Monastir, and Sousse.
We included all the patients operated for an intramedullary tumor of all ages and all histological types. We excluded nonoperated patients and those with tumors of the terminal filum and the elongated marrow.
As it is a multicentric retrospective study, data were collected from the hospitalization files and it included epidemiologic clinical, radiological, and histological data.
The data generated were subjected to statistical analysis using IBM Corp. Released 2012. IBM SPSS Statistics for Windows, version 21.0. Armonk, NY, USA: IBM Corp, and two-tailed Fisher's t-test, Chi-square, and ANOVA tests were used to calculate significance.
RESULTS
Epidemiology
The mean age of our patients is 33.84 years with extremes between 2 and 75 years, 28.3% of which were aged between 21 and 30 years. Pediatric cases presented 17.5% of the series. We noted that there was not a sex predominance as we had 57 males (47.5%) and 63 females (52.5%).
Clinical study
The period of time separating the appearance of the first symptoms and the diagnosis was 15.76 months in our series with extremes of 15 days and 96 months, but 40% of our patients were diagnosed during the first 6 months.
These functional symptoms are variable as they can present as spinal and radicular pain, motor and sensory troubles, and vesico-sphincter disorders. Forty-seven patients (39.2%) presented with spinal pain, and it was cervical (12.5%), dorsal (16.7%), or lumbar (10%). Twenty-nine patients (24.2%) presented with radicular pain. The most frequent revealing symptom was motor trouble presented as frequent as 77.5% of the patients; this trouble interested the lower limbs in 50% of the cases and the four limbs in 17.5%. Other troubles such as spinal cord claudication, hemiparesis, or clumsiness presented only in 10% of the cases. Sensory troubles affected 35 patients (29.2%) of the series, and they varied from paresthesia (10%) to hypoesthesia (4.17%) or even total anesthesia. Vesico-sphincter disorders touched 56 of our patients (46.7%), and genital dysfunction was seen in 17.5% of the cases.
Clinical examination permitted to objectify these troubles thoroughly and thus classify these patients according to the McCormick classification [Figure 1].
Figure 1.
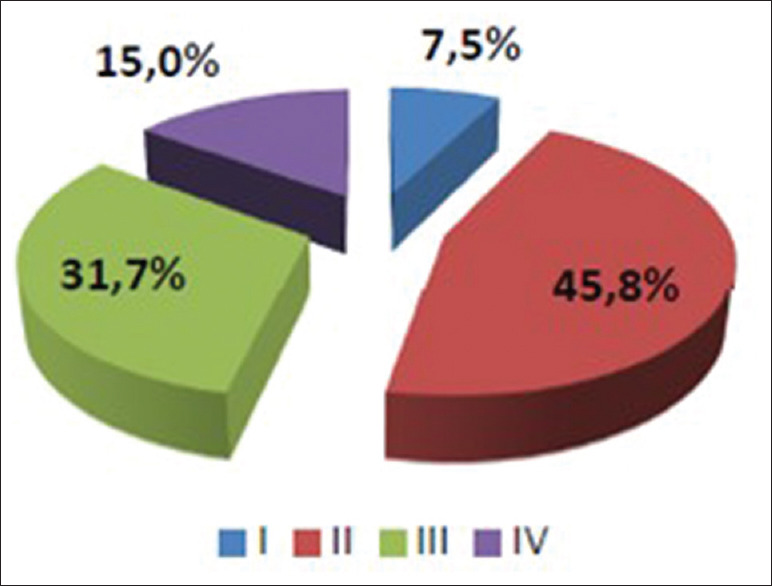
Clinical stages of McCormick Stage before surgery (I, I, III, IV) and their percentage in our series
Radiological features
Magnetic resonance imaging (MRI) is the gold standard in exploring intramedullary tumors, and it was performed on 105 of our patients; all the basic sequences were realized (T1, T2, T1 with gadolinium injection) to explore the localization, the volume, and aspects of the lesions. Other explorations were also available as computed tomography scan, myelography, Myelo scan, and arteriography for highly vascularized tumors.
Dorsal was the most frequent localization with 49 patients (40.8%). Cervical is next with 36 patients (30%). Medullary conus presented the third localization with 15.8%. Junctional zones were the least represented, with 11.7% for the cervicodorsal region and 1.6% for the cervico-occipital junction.
Treatment modalities
Medical treatment
Analgesic treatment (20% of the patients) is an important part in the management of these tumors, and it includes different types of analgesics and even injections of morphine. Nonsteroidal anti-inflammatory molecules can be beneficial in the management of radicular pain. Steroids can be used to minimize tumoral edema (85% of the patients). Prophylactic doses of heparin were used in all of the patients, and curative doses were used with 15 cases (12.5%) that presented a high risk of thromboembolic disease.
Surgery
All of our patients were operated. Posterior approach was used for all these cases; we could perform a total resection in 76 cases (63.5%), a subtotal resection with 37 patients (30.8%), and a simple biopsy for 7 of them (5.8%). Favorable outcome was seen in 84.2% of the patients, and some infectious and embolic complications were seen with 3 cases of perioperative deaths (0.025%).
Pathology
We could categorize the tumors in glial and nonglial. The first was represented in 81 of the cases (67.5%) and the latter by 39 cases (32.5%). Glial tumors we found were essentially 39 ependymomas, 35 astrocytomas, 5 glioblastomas, 1 anaplastic astrocytoma, and 1 ganglioglioma (II) [Table 1], whereas nonglial were 7 cases of neurinomas, 6 lipomas, 5 epidermoid cysts, 4 teratomas, hemangioblastomas, and nonnervous metastatic tumors, 3 cavernomas, 2 metastatic medulloblastoma, and 1 case of lymphoma, neurocytoma, dermoid cyst, and rhabdoid tumor.
Table 1.
Number and percentage of glial tumors
| n (%) of all tumors | |
|---|---|
| Ependymoma | 39 (32.5) |
| Astrocytoma | 35 (29.2) |
| Glioblastoma | 5 (4.2) |
| High-grade glioma (Grade III) | 1 (0.83) |
| Ganglioglioma Grade II | 1 (0.83) |
Radiotherapy
Eight of our patients had adjuvant radiotherapy after surgery, and they all received medullary total radiation of 45 Gy. Two of these patients did not tolerate the treatment well, and they were irradiated for a glioblastoma and a metastatic medulloblastoma [Table 2].
Table 2.
Tolerance of radiotherapy in the cases receiving adjuvant irradiation
| Pathology | Dose (Gy) | Tolerance |
|---|---|---|
| Ependymoma (Grade II) | 45 | Good |
| Rhabdoid tumor | 45 | Good |
| Glioblastoma | 45 | Good |
| Glioblastoma | 45 | Bad (nausea/vomiting) |
| Astrocytoma (Grade II) | 45 | Good |
| Metastasis | 45 | Good |
| Medulloblastoma | 45 | Bad (radio-related mucositis) |
| Astrocytoma (Grade II) | 45 | Good |
Chemotherapy
Three patients benefited from adjuvant chemotherapy, and it was done for the two cases of glioblastoma concomitant to radiotherapy and for the only case of lymphoma.
Analytical study
Radiological aspect and histological type
We analyzed different radiological features in every type of tumor, the localization, the size, and morphologic characteristics such as cystic component, necrosis, hemorrhage, edema, contrast enhancement, and limits. We dressed Table 3.
Table 3.
Radiological features by histological type
| Localization | Size (mean) in mm | Cyst, n (%) | Necrosis, n (%) | Hemorrhage, n (%) | Edema, n (%) | Contrast enhancement, n (%) | Clear limits, n (%) | |
|---|---|---|---|---|---|---|---|---|
| Astrocytoma Grade I and II | 14: Cervical 6: Cervicodorsal 14: Dorsal 1: Medullary conus |
38.4 | 23 (65.7) | 3 (8.5) | 3 (8.5) | 12 (34.3) | 10 (28.5) | 23 (65.7) |
| Astrocytoma Grade III and IV | 2: Cervical 1: Cervicodorsal 3: Dorsal 1: Medullary Conus |
37.5 | 6 (85.7) | 2 (28.6) | 3 (42.9) | 4 (57.1) | 6 (85.7) | 3 (42.9) |
| Ependymoma Grade I and II | 1: Cervico-occipital junction 10: Cervical 7: Cervicodorsal 15: Dorsal 4: Medullary conus |
32.88 | 12 (32.4) | 1 (2.7) | 2 (5.4) | 10 (27) | 15 (40.54) | 23 (62.2) |
| Ependymoma Grades III and IV | 1: Cervical 1: Dorsal |
28.4 | 0 | 1 (50) | 0 | 1 (50) | 2 (100) | 1 (50) |
| Dermoid cyst | 1: Medullary conus | 34.5 | 1 (100) | 0 | 1 (100) | 0 | 0 | 0 |
| Epidermoid cyst | 4: Medullary conus 1: Dorsal |
36.2 | 4 (66.66) | 0 | 0 | 0 | 0 | 3 (100) |
| Lipoma | 4: Medullary conus 2: Dorsal |
39.8 | 1 (16.6) | 0 | 0 | 1 (16.6) | 0 | 5 (83.3) |
| Schwannoma | 2: Cervical 3: Dorsal 2: Cervicodorsal |
22.3 | 0 | 0 | 0 | 1 (14.28) | 7 (100) | 3 (42.85) |
| Metastasis | 2: Cervical 2: Dorsal |
26.7 | 0 | 2 (50) | 1 (25) | 3 (75) | 2 (50) | 0 |
| Lymphoma | 1: Medullary conus | 34.2 | 0 | 0 | 0 | 1 | 0 | 0 |
| Hemangioblastoma | 2: Cervical 1: Dorsal 1: Medullary conus |
35.4 | 0 | 1 (25) | 2 (50) | 1 (25) | 4 (100) | 2 (50) |
| Medulloblastoma | 1: Medullary conus 1: Cervico-occipital junction |
27.9 | 0 | 1 (50) | 1 (50) | 1 (50) | 2 (100) | 0 |
| Rhabdoid tumor | 1: Dorsal | 26 | 0 | 1 | 1 | 1 | 1 | 0 |
| Extraventricular neurocytoma | 1: Cervical | 32 | 1 | 0 | 0 | 1 | 1 | 0 |
| Teratoma | 2: Medullary conus 2: Dorsal |
37.2 | 2 (66.6) | 0 | 0 | 1 (33.3) | 0 | 3 (100) |
| Cavernoma | 2: Cervical 1: Dorsal |
23.5 | 0 | 0 | 3 (100) | 0 | 1 (33.3) | 3 (100) |
Functional results
We analyzed the functional outcome of our patients according to age, preoperative McCormick score, localization of the tumor, the histological type, and the quality of resection.
When we compare results according to age, we note that both adults and children had mostly improvement after surgery, but we noticed that adults had a tendency to worsen their neurological (23.2%) status more than children who had a more stabilized neurological state (42.9%). This result is statistically not significant (P = 0.4) [Table 4].
Table 4.
Functional results after surgery depending on the age
| Age | Evolution |
|||
|---|---|---|---|---|
| Improvement, n (%) | Worsening, n (%) | Stationary, n (%) | Death, n (%) | |
| Children | 10 (47.6) | 2 (9.5) | 9 (42.9) | - |
| Adults | 42 (42.4) | 23 (23.2) | 31 (31.3) | 3 (3.0) |
Taking to note that most of our cases had a preoperative McCormick Grade II and III, we noticed that Grade III patients had the best results and mostly improved their McCormick score after surgery (57.9%). This result is also not statistically significant (P = 0.3) [Table 5].
Table 5.
Functional results depending on the McCormick score before surgery
| McCormick classification | Evolution |
|||
|---|---|---|---|---|
| Improvement, n (%) | Worsening, n (%) | Stationary, n (%) | Death, n (%) | |
| Grade I | 4 (44.4) | 1 (11.1) | 4 (44.4) | - |
| Grade II | 17 (30.9) | 16 (29.1) | 21 (38.2) | 1 (1.8) |
| Grade III | 22 (57.9) | 5 (13.2) | 9 (23.7) | 2 (5.3) |
| Grade IV | 9 (50.0) | 3 (16.7) | 6 (33.3) | - |
Patients who were treated for tumors of the cervicodorsal junction and the medullary conus had better neurological results than other localization (42.8% and 52.6%); this is statistically significant (P = 0.04) [Table 6].
Table 6.
Functional results depending on the localization
| Localization | Evolution |
|||
|---|---|---|---|---|
| Improvement, n (%) | Worsening, n (%) | Stationary, n (%) | Death, n (%) | |
| Cervico-occipital junction | - | - | 1 (50.0) | 1 (50.0) |
| Cervical | 13 (36.1) | 8 (22.2) | 14 (38.9) | 1 (2.8) |
| Cervicodorsal | 6 (42.8) | 2 (14.4) | 6 (42.8) | - |
| Dorsal | 17 (34.7) | 14 (28.6) | 17 (34.7) | 1 (2) |
| Medullary conus | 10 (52.6) | 2 (10.5) | 7 (36.8) | - |
We established a comparison of neurological postoperative status between histological groups; we compared astrocytomas, ependymomas, and nonglial tumors. The treatment of ependymomas (56.4% of postoperative improvement) and nonglial tumors (51.3%) showed better functional results than astrocytomas (23.8%), and this is statistically significant (P = 0.03) [Table 7].
Table 7.
Functional results depending on the histological type
| Histological type | Evolution |
|||
|---|---|---|---|---|
| Improvement, n (%) | Worsening, n (%) | Stationary, n (%) | Death, n (%) | |
| Astrocytoma | 10 (23.8) | 10 (23.8) | 21 (50.0) | 1 (2.4) |
| Ependymoma | 22 (56.4) | 8 (20.5) | 8 (20.5) | 1 (2.6) |
| Nonglial tumors | 20 (51.3) | 7 (17.9) | 11 (28.2) | 1 (2.6) |
We also compared low-grade astrocytomas to high-grade astrocytomas which showed better results with statistical significance (P = 0.03) [Table 8]. We dressed a table for ependymomas but could not compare the low grade and high grade due to the insufficient number of cases [Table 9].
Table 8.
Functional results depending on the grade of astrocytoma
| Astrocytoma | Evolution |
|||
|---|---|---|---|---|
| Improvement, n (%) | Worsening, n (%) | Stationary, n (%) | Death, n (%) | |
| High grade | 2 (25.0) | 5 (62.5) | 1 (12.5) | - |
| Low grade | 8 (23.5) | 5 (14.7) | 20 (58.8) | 1 (2.9) |
Table 9.
Functional results depending on the grade of the ependymoma
| Ependymoma | Evolution |
|||
|---|---|---|---|---|
| Improvement, n (%) | Worsening, n (%) | Stationary, n (%) | Death, n (%) | |
| High grade | 1 (50) | 1 (50) | - | - |
| Low grade | 21 (56.8) | 7 (18.9) | 8 (21.6) | 1 (2.7) |
The quality of resection is also correlated to better outcome with all histological types or localization, and this is also statistically significant (P = 0.04) [Table 10].
Table 10.
Functional results depending on the quality of resection
| Resection | Evolution |
|||
|---|---|---|---|---|
| Improvement, n (%) | Worsening, n (%) | Stationary, n (%) | Death, n (%) | |
| Total | 39 (51.3) | 14 (18.4) | 22 (28.9) | 1 (1.3) |
| Subtotal | 12 (32.4) | 10 (27.0) | 14 (37.8) | 1 (2.7) |
| Biopsy | 1 (14.3) | 1 (14.3) | 4 (57.1) | 1 (14.3) |
Tumoral progression
We analyzed the data concerning Tumoral Recurrence or progression according to the histological type, the quality of resection, we then did a multivariate analysis according both pathology and resection.
We first compared glial to nonglial tumors; we noticed a more frequent tumoral progression in glial lesions than nonglial ones, but this has no statistical significance. However, high-grade astrocytomas and high-grade gliomas have a statistically significant more tendency to progress or recur than low-grade astrocytomas and gliomas (P = 0.02).
The quality of tumoral resection was a significant factor of disease progression in our series as subtotal resection is correlated to more important progression than total one (P = 0.03).
DISCUSSION
Intramedullary tumors are not a frequent pathology, and few series were published. We tried to compare our series to the literature. On the epidemiologic level, the mean age of diagnosis varies between 28 and 44. C. Campello et al. published a large series of 322 cases where the mean age was 41[2] whereas it was 33.84 in ours. We had a 17.5% of pediatric cases where the other series included only adults which can explain the difference. Most of the series showed a masculine predominance contrarily to ours where there was no predominance.
Intramedullary tumors have a progressive evolution, and they usually reveal after a period of the first symptoms; this delay of diagnosis is very variable. Chandy and Babu found extremes between 3 weeks and 15 years,[3] Hausmann et al. had extremes between 3 weeks and 19 years.[4] Most of our patients were diagnosed during the first 6 months. In some cases, the symptoms can be brutal secondary to intratumoral hemorrhage, and we had one case in our series.
Spinal pain is one of the revealing symptoms, and it is secondary to osteodiscal and ligamentary suffering which explains that it is more frequent for extradural tumors and for cervical localization as it is a mobile segment. In our series, it was present in 32.9% of the cases whereas it is more frequently present in the literature such as Fisher's series where the number was 72%.[5]
Troubles of motricity and sensitivity are very frequent in patients diagnosed with intramedullary tumors as they can be revealing in most of the cases. Motor deficit varies from a simple tiredness to medullary claudication or total motor deficit. Sensitivity can be affected on different levels as you can find proprioceptive troubles or a sensitive level. Sun et al. reported that trouble of sensitivity can be revealing in 23% of the cases.[6]
Vesico-sphincter disorders appear late in the pathological progression if the tumor is not localized in the medullary conus. In our series, these troubles were discovered at the initial clinical exam in 64.2% of the cases which joins Chandy and Babu's series.[3]
Other particular clinical signs can be observed in intramedullary tumors, and spinal deformity is more specific for children. In our series, it concerned 38% of the children and 7% of the adults. Bouffet et al. reported 56% of spinal deformity in children with intramedullary tumors.[7]
Raised intracranial pressure without hydrocephalus and confirmed papillary edema is a rare manifestation which can be explained multiple causes such as the tumoral cervical localization, elevated levels of proteins in the spinal fluid, arachnoiditis, and compression of venous plexus;[8] we had one case in our series. Hydrocephalus is also a logical manifestation secondary to cervico-occipital junction compression; we reported four cases, three of which were nonsymptomatic. Syringobulbia can explain cranial nerve palsy.
Clinical and radiological particularities by histological type of tumor
We tried to review the characteristics of some of the histological types that we found on our series and compare their incidence to number reported in the literature.
Ependymoma
Ependymomas are benignly vascularized but noninfiltrating tumors. They represent 15% of intramedullary tumors and affect mostly adults (60% of intramedullary glial tumors).[9] They represent 32.5% of all intramedullary tumors in our series. When they affect children, an association with neurofibromatosis type 2 can be suspected.[10] Grade II Ependymoma progress slowly and have clear limits with the sane spinal cord while Grade III tumors show atypia and important cellularity.
On the MRI exploration, ependymomas have an intermediary signal on T1 sequences, and they are on hyper-, iso-, or heterogeneous signal on T2. Gadolinium enhancement is mostly homogeneous and can show different cysts.[11,12] Tumoral cysts are a consequence of hemorrhage and necrosis within the tumor. Polar cysts are present on the cranial and caudal aspects of the tumor and are a signal of good prognosis.[9] Syringomyelia is secondary to compression on the ependymal canal[13] [Figure 2].
Figure 2.
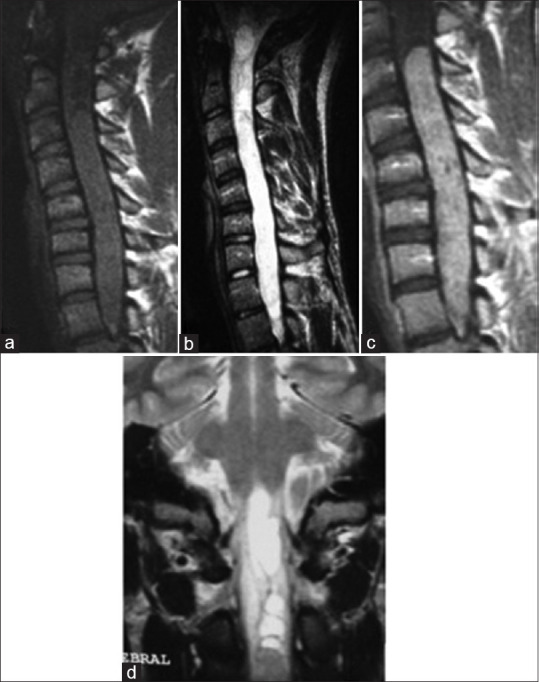
Spinal MRI: A case of Cervico Dorsal Ependymoma. Sagittal section on T1 sequence (a), T2 sequence (b), and T1 after gadolinium enhancement (c); Coronal section on T2 sequence: A tumoral process is centro medullar extended from C3 to D1 (d). This process has an isosignal on T1, it is hyperintense on t2 surrounded by a discreet T2 hypointense line. Enahancement is homogenous. There is an association with syringomyelic cavities and even syringobulbia
Surgical resection is mostly complete for Grade II tumors, and it is more difficult for infiltrative tumors which need an adjuvant therapy. The friable and nonencapsulated character makes relapse very frequent for this kind of tumor.[14]
Astrocytoma
They represent 30% of intramedullary tumors[15](29.16% in our series). They mostly affect children and usually are of low grade. Cervical and thoracic localizations are the most frequent.[16] Incidence of intramedullary astrocytoma is more important in patients with neurofibromatosis type I.[17]
Astrocytomas are usually not well limited; they are mostly infiltrative. They present a tumoral cyst in 48% of the cases[15](65.7% in our series). Hemorrhage is less frequent than ependymomas.
Clinical presentations join those of ependymoma; it is usually progressive with motor and sensitivity troubles.[18] They present on the MRI as a noncentral infiltrative tumor enlarging the spinal cord.[19]
They are hypointense on T1 and hyperintense on T2. Enhancement is heterogeneous and has no correlation to the grade of the tumor contrarily to cerebral astrocytomas. Leptomeningeal enhancement can orient for high-grade lesions[19] [Figure 3].
Figure 3.
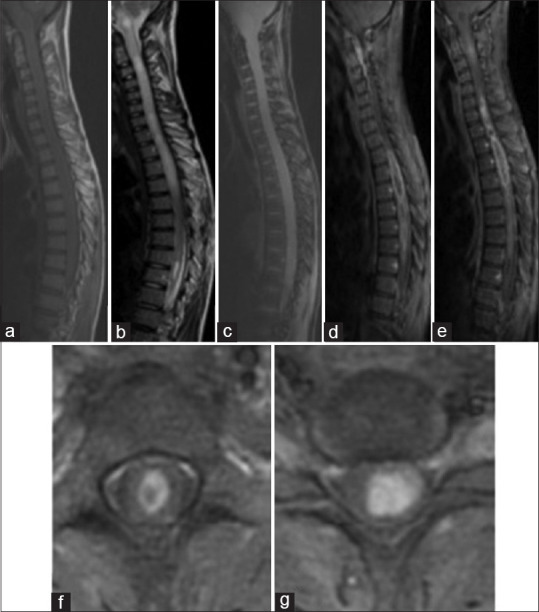
Spinal MRI: A case of Cervico Dorsal Astrocytoma, Sagittal section on T1 sequence (a), T2 sequence (b), gradient echo sequence (c) and T1 Fatsat after gadolinium enhancement (d and e); Axial sections on T1 Fatsat after Gadolinium enhancement (f,g): There is a tumoral process extended on all of the cervical and dorsal spinal cord, it has an isosignal on T1 and has a heterogenous hypersignal on T2. Enhancement is heterogenous and peripheral delimiting necrosis. It has multiple cysts with a syringomyelia extended to the bulb
Glioblastoma and high-grade gliomas
Five cases of glioblastomas were reported in our series. Contrarily to intracranial ones, they are a very rare entity when concerning intramedullary tumors. Only 200 cases were reported in the literature since 1938. Differentiating glioblastoma from other intramedullary tumors or astrocytomas is not an easy task, and for most of the cases, only biopsy can assume the diagnosis.[20]
Surgical resection is the most important therapeutic weapon facing this pathology although it rarely can be done due to the infiltrative character of the tumor.[21] In some cases of lumber and sacral localization, cordectomy can be done and it showed better results and survival going to 111 months for the few cases it was done to.[22] Radiotherapy as an adjuvant tool significantly (P = 0.007) boosts survival. There is no consensus concerning optimal doses, but medulla can tolerate 55 Gy. There is, however, not enough feedback or clinical trials concerning chemotherapy due to the low number of cases.[20]
Ganglioglioma
It a relatively rare tumor which has more predilection for temporal lobes and cerebellar hemispheres. Medullary localization is even more rare; it represents 1.1% of intramedullary tumors. They are most of the times cervical although the only case of our series is dorsal. They are composed of both astrocytes and ganglionic cells. There is no specific aspect on the MRI for these lesions; they are heterogeneous on both T1 and T2. Hemorrhage and calcifications may be seen in some cases.[23]
Hemangioblastoma
Hemangioblastomas are benign tumors that are usually localized in the posterior fossa. They may also present as an intramedullary tumor. They represent 1.6%–6.4% of the intramedullary tumors. In our series, they represent 2.5% joining what is published in the literature. They are usually unique but may be multiple which indicates a Von Hippel–Lindau syndrome. Hemangioblastomas have some specificities on the MRI exploration, they present as hypervascularized nodule associated with an extended cyst, and they are mostly on the posterior part of the spinal cord. The nodule is typically hypointense on T1 and hyperintense on T2 with a homogeneous or heterogeneous gadolinium enhancement depending on the size. The flow void sign due to the presence of vascular structures next to the nodule is also characteristic. Arteriography confirms the diagnosis specifying the nourishing arteries, the venous drainage, and the vascular blush[24,25,26] [Figure 4].
Figure 4.
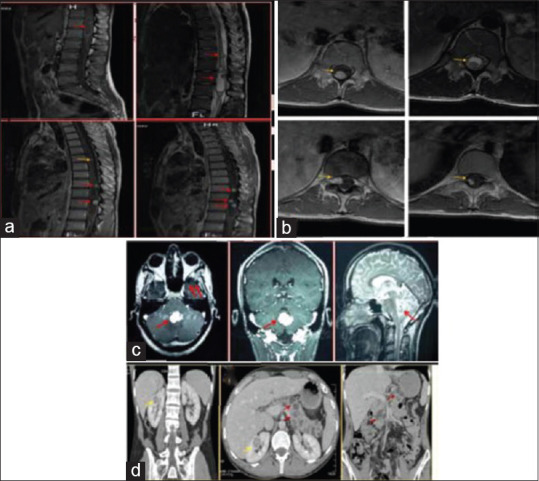
Von Hippel Lindau Disease. MRI of the Spine has showed multiple spinal tumors. The symptomatic lesions is on the medullary conus (Red Arrow). The mass shows a cystic form with a mural nodule tissular heterogenous hypointense on T1 with an intense enhancement.(a, b) The Cerebral MRI shows a cerebellar hemangioblastoma (c) Abdominal CT scan showed two renal masses associated to multiple pancreatic cysts (d)
Dermoid cyst
In our series, we report only one case of dermoid cyst (0.8%). In the literature, they present <1% of intramedullary tumors. They usually are intradural and extramedullary. These lesions are benign and may be congenital associated with a spinal dysraphism or acquired secondary to lumbar puncture, spinal surgery, or spinal trauma. They are hyperintense on T1 and T2 sequences[27] [Figure 5].
Figure 5.
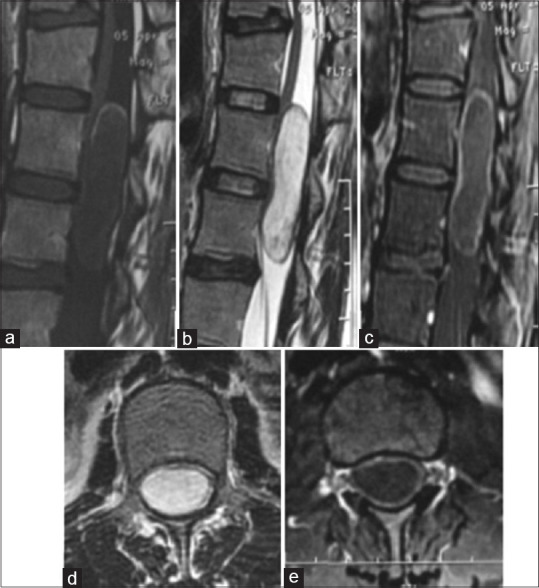
Spinal magnetic resonance imaging: Dermoid cyst of the medullary conus. Sagittal section on T1 sequence (a), T2 sequence (b), T1 fat sat after gadolinium enhancement (c); Axial sections on T2 sequence (d) and T1 fat sat after gadolinium enhancement (e): a tumoral process on the medullary conus with heterogeneous signal on T1 and T2 sequences with an apical crown. Enhancement is peripheral and intense
Epidermoid cyst
Intramedullary epidermoid cysts are very rare, and they represent <1% of all intramedullary cases. We could find only sixty cases prior to our series which contained five cases on its own. They represent in our case 4.2% of intramedullary tumors. Like dermoid cysts, they can be congenital or acquired, and they are mostly thoracic. They show on the MRI as well limited lesion hyperintense on T1 and T2 with peripheral gadolinium enhancement. On the cranial level, these lesions show as hyperintense on diffusion sequences; this is not the case of intramedullary lesions[28,29] [Figure 6].
Figure 6.
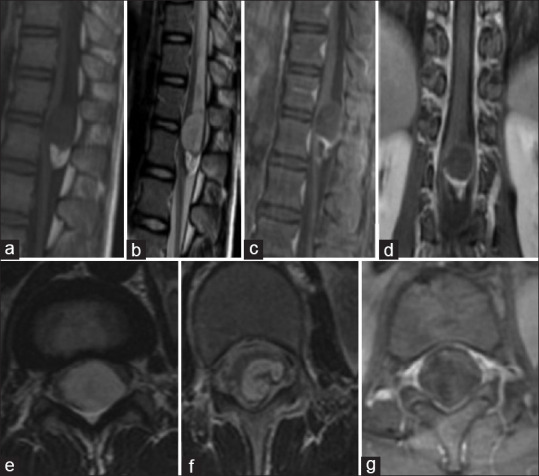
Spinal MRI: Epidermoid cyst of the medullary conus. Sagittal section on T1 sequence (a), T2 sequence (b), T1 Fatsat after gadolinium enhancement (c); Coronal section on T1 fatsat with gadolinium enhancement (d) Axial sections on T2 sequence(e) T1 Fatsat after Gadolinium enhancement (f)and axial T2 sequence: A tumoral well limited process of the medullary conus heterogenous on all sequences with a peripheral enhancement. This lesion has a double component, one polar and superior hypointense on T1 and hyperintense and heterogenous on T2 the second is of lipidic nature presenting as hyperintense on T1 and disappearing on Fatsat sequences
Neurinoma
In our series, we had seven cases of intramedullary neurinomas (5.83%) which is much larger than what is reported in the literature (0.3%–1.5%).[30] The presence of intramedullary neurinomas is not well explained because normally there is no Schwann cells in the spinal cord, but some theories have been advanced such as malformities, their development from perivascular nervous cells (anterior spinal artery), the presence of aberrant Schwann cells in the spinal cord, or glial transformation on Schwann cells.[31] They show as hypo- or isointense on T1 sequences and hyperintense on T2 with a homogeneous gadolinium enhancement[30] [Figure 7].
Figure 7.
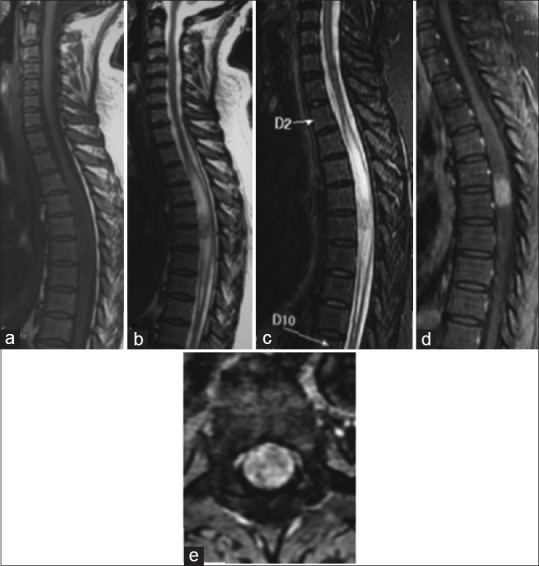
Spinal MRI: Dorsal Neurinoma. Sagittal section on T1 sequence (a), T2 sequence (b), STIR (Short Tau Inversion Recovery) sequence) (c) T1 Fatsat after gadolinium enhancement (d); Axial sections on T1 Fatsat after Gadolinium enhancement (e):There is a intromedullary dorsal tumoral process on D5-D6, It is hypointense on T1 and has a heterogenous T2 hypersignal with a central hypointense signal. The enhancement is intense and homogenous
Teratoma
Spinal teratoma is very rare; the first case of spinal mature teratoma was reported by Ak et al. in 1931.[32] Although they are very rare, our series contains four cases of intramedullary teratomas meaning 3.3%. They can be mature containing cartilaginous, epithelial, glandular structures. They can be immature or malignant[33] [Figure 8].
Figure 8.
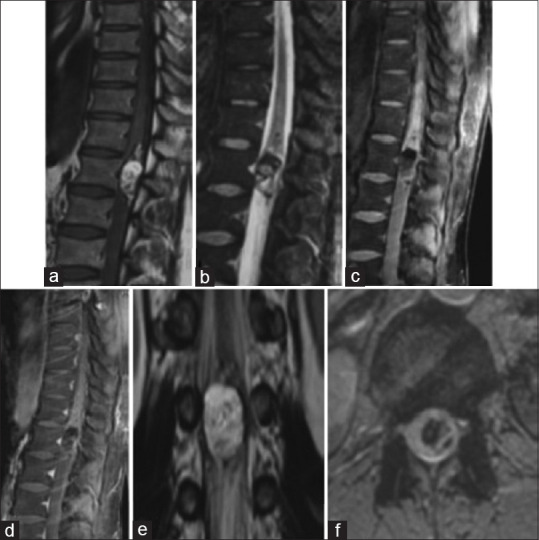
Spinal magnetic resonance imaging: Teratoma of the medullary conus. Sagittal section on T1 sequence (a), STIR sequence (b), T1 fat sat before (c) after gadolinium enhancement (d); Coronal section on T2 sequence (e); Axial section on gradient echo (f): a tumoral process with triple component, one tissular with nodular enhancement, one apical with lipidic consistence, and a third that is hemorrhagic
Functional results
In the literature, age is a controversial factor in functional results; for some authors, adults have a poorer prognosis than children,[34] and for others, age has no real significance.[35] In our series, comparing both tranches of age showed better results for children but with no statistical significance.
Comparing functional results to the starting McCormick score showed in most series that patients usually do not show improvement more than their score just before surgery whatever the histological type which encourages to operate these tumors at diagnosis.[36]
Dorsal localization is correlated to poorer functional prognosis, and the presence of cysts and syringomyelia predicts better results because they are associated with better cleavage plane during resection.[37] In our series cervical and tumors of the cervico dorsal junction had better functional results. Dorsal localization confirmed to be of worse functional result. Tumors of the cervico occipital junction had poorer vital prognosis. All of these results have statistical significance.
Histological type of the tumor is reported in the literature as a known and most important prognosis factor.[37] We discussed it comparing histological groups as nonglial tumors, astrocytomas, and ependymomas than high- and low-grade glial tumors. It is statistically significant (P = 0.03) in our series that nonglial tumors are of better function prognosis than glial tumors and that ependymomas show better results than astrocytomas. High-grade astrocytomas and ependymomas have bad results compared to low-grade tumors. Complete surgical resection shows better results.
Tumoral progression
We compared tumoral progression based on histological type; nonglial tumors had lesser chance of relapse or progression (5.8%) compared to glial (17.5%) which joins the literature but has no statistical significance. We found some discordance with published data concerning ependymomas which have 5% chance of progressing after resection in the literature.[38] In our series, they presented more percentage of progression than astrocytomas (29.7%–14.3%), whereas series like Abdel-Wahab et al.'s showed that astrocytomas have a higher risk of tumoral progression.[35] They had 61% of progression with surgery alone and 56.4% of progression with surgery and adjuvant radiotherapy.[35]
High-grade gliomas showed a very high risk of tumoral progression compared to low-grade ones which joins the literature. Abdel-Wahab et al. showed also that total resection is compatible with lower risk of tumoral progression;[35] we had the same results with our series with 37.8% progression with partial resection compared to 15.8% with total resection.
Comparing histological type and quality of resection with tumoral progression, we had some important conclusions: ependymomas are more correlated to total resection than astrocytomas probably due to a less infiltrative evolution and better cleavage plane. While total resection has an important effect on tumoral progression of ependymomas, it is lesser effective on astrocytomas probably due to the persistence of residual cells.
CONCLUSION
We conclude this work with some statements. Surgery is the major therapeutic weapon against intramedullary tumors. Functional results and tumoral progression after resection are the main studied parameters. In terms of functional results, age is not a significant factor. Presurgical functional state, the histological type (ependymomas are of better prognosis), and the extent of surgical resection are the important factors. In terms of tumoral progression, total resection is more feasible for ependymomas than astrocytomas. Total resection is a significantly good factor for tumoral progression for ependymomas. It is very controversial concerning astrocytomas.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ottenhausen M, Ntoulias G, Bodhinayake I, Ruppert FH, Schreiber S, Förschler A, et al. Intradural spinal tumors in adults – Update on management and outcome. Neurosurg Rev. 2019;42:371–42. doi: 10.1007/s10143-018-0957-x. [DOI] [PubMed] [Google Scholar]
- 2.Campello C, Parker F, Slimani S, Le Floch A, Herbrecht A, Aghakhani N, et al. Adult intramedullary gliomas. Neurochirurgie. 2017;63:381–90. doi: 10.1016/j.neuchi.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Chandy MJ, Babu S. Management of intramedullary spinal cord tumours: Review of 68 patients. Neurol India. 1999;47:224–8. [PubMed] [Google Scholar]
- 4.Hausmann ON, Kirsch EC, Tolnay M, Gratzl O. Intramedullary spinal cord tumours: A clinical outcome and radiological follow-up study. Swiss Med Wkly. 2001;131:582–7. [PubMed] [Google Scholar]
- 5.Fisher CG, Goldschlager T, Boriani S, Varga PP, Fehlings MG, Bilsky MH, et al. A novel scientific model for rare and often neglected neoplastic conditions. Evid Based Spine Care J. 2013;4:160–2. doi: 10.1055/s-0033-1357365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Wang Z, Li Z, Liu B. Microsurgical treatment and functional outcomes of multi-segment intramedullary spinal cord tumors. J Clin Neurosci. 2009;16:666–71. doi: 10.1016/j.jocn.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Bouffet E, Pierre-Kahn A, Marchal JC, Jouvet A, Kalifa C, Choux M, et al. Prognostic factors in pediatric spinal cord astrocytoma. Cancer. 1998;83:2391–9. doi: 10.1002/(sici)1097-0142(19981201)83:11<2391::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Porter A, Lyons MK, Wingerchuk DM, Bosch EP. Spinal cord astrocytoma presenting as “idiopathic” intracranial hypertension. Clin Neurol Neurosurg. 2006;108:787–10. doi: 10.1016/j.clineuro.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Chang UK, Choe WJ, Chung SK, Chung CK, Kim HJ. Surgical outcome and prognostic factors of spinal intramedullary ependymomas in adults. J Neurooncol. 2002;57:133–9. doi: 10.1023/a:1015789009058. [DOI] [PubMed] [Google Scholar]
- 10.Rennie AT, Side L, Kerr RS, Anslow P, Pretorius P. Intramedullary tumours in patients with neurofibromatosis type 2: MRI features associated with a favourable prognosis. Clin Radiol. 2008;63:193–200. doi: 10.1016/j.crad.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Parsa AT, Ames CP, McCormick PC. Clinical management of intramedullary spinal ependymomas in adults. Neurosurg Clin N Am. 2006;17:21–7. doi: 10.1016/j.nec.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Sun B, Wang C, Wang J, Liu A. MRI features of intramedullary spinal cord ependymomas. J Neuroimaging. 2003;13:346–51. [PubMed] [Google Scholar]
- 13.Kahan H, Sklar EM, Post MJ, Bruce JH. MR characteristics of histopathologic subtypes of spinal ependymoma. AJNR Am J Neuroradiol. 1996;17:143–50. [PMC free article] [PubMed] [Google Scholar]
- 14.Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: A review. Curr Neurol Neurosci Rep. 2011;11:320–8. doi: 10.1007/s11910-011-0190-2. [DOI] [PubMed] [Google Scholar]
- 15.Ogunlade J, Wiginton JG, Elia C, Odell T, Rao SC. Primary Spinal Astrocytomas: A Literature Review. Cureus. 2019. Juill 26, [Last accessed on 2021 Jan 11]. Available from: https://www.cureus.com/articles/17694-primary-spinal-astrocytomas-a-literature-review . [DOI] [PMC free article] [PubMed]
- 16.Das JM, Hoang S, Mesfin FB. StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. [Last accessed on 2021 Jan 11]. Intramedullary spinal cord tumors. Available from: http://www.ncbi.nlm.nih.gov/books/NBK442031/ [PubMed] [Google Scholar]
- 17.Houten JK, Cooper PR. Spinal cord astrocytomas: Presentation, management and outcome. J Neurooncol. 2000;47:219–24. doi: 10.1023/a:1006466422143. [DOI] [PubMed] [Google Scholar]
- 18.Epstein FJ, Farmer JP, Freed D. Adult intramedullary astrocytomas of the spinal cord. J Neurosurg. 1992;77:355–9. doi: 10.3171/jns.1992.77.3.0355. [DOI] [PubMed] [Google Scholar]
- 19.Seo HS, Kim JH, Lee DH, Lee YH, Suh SI, Kim SY, et al. Nonenhancing intramedullary astrocytomas and other MR imaging features: A retrospective study and systematic review. Am J Neuroradiol. 2010;31:498–31:4. doi: 10.3174/ajnr.A1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen CX, Wu JF, Zhao W, Cai ZW, Cai RZ, Chen CM. Primary spinal glioblastoma multiforme: A case report and review of the literature. Medicine (Baltimore) 2017;96:e6634. doi: 10.1097/MD.0000000000006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong AP, Dahdaleh NS, Fessler RG, Melkonian SC, Lin Y, Smith ZA, et al. Risk factors and long-term survival in adult patients with primary malignant spinal cord astrocytomas. J Neurooncol. 2013;115:493–503. doi: 10.1007/s11060-013-1251-y. [DOI] [PubMed] [Google Scholar]
- 22.Singh PK, Singh VK, Tomar J, Azam A, Gupta S, Kumar S. Spinal glioblastoma multiforme: Unusual cause of post-traumatic tetraparesis. J Spinal Cord Med. 2009;32:583–6. doi: 10.1080/10790268.2009.11754565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami T, Koyanagi I, Kaneko T, Yoneta A, Keira Y, Wanibuchi M, et al. Intramedullary spinal cord ganglioglioma presenting as hyperhidrosis: Unique symptoms and magnetic resonance imaging findings: Case report. J Neurosurg Spine. 2013;18:184–8. doi: 10.3171/2012.11.SPINE12530. [DOI] [PubMed] [Google Scholar]
- 24.Chu BC, Terae S, Hida K, Furukawa M, Abe S, Miyasaka K. MR findings in spinal hemangioblastoma: Correlation with symptoms and with angiographic and surgical findings. Am J Neuroradiol. 2001;22:206–17. [PMC free article] [PubMed] [Google Scholar]
- 25.Chen CY, Chen PH, Yao MS, Chu JS, Chan WP. MRI of hemangioblastoma in the conus medullaris. Comput Med Imaging Graph. 2008;32:78–81. doi: 10.1016/j.compmedimag.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Baker KB, Moran CJ, Wippold FJ, 2nd, Smirniotopoulos JG, Rodriguez FJ, Meyers SP, et al. MR imaging of spinal hemangioblastoma. Am J Roentgenol. 2000;174:377–82. doi: 10.2214/ajr.174.2.1740377. [DOI] [PubMed] [Google Scholar]
- 27.Sanaullah M, Mumtaz S, Memon AA, Hashim AS, Bashir S. Intramedullary dermoid cyst with relatively atypical symptoms: A case report and review of the literature. J Med Case Rep. 2013;7:104. doi: 10.1186/1752-1947-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fereydoonian NA, Bakhti S, Fereshtehnejad SM, Tabibkhooei AR. Intramedullary thoracic spine epidermoid cyst with myelopathic presentations: A report of a rare case. Clin Neurol Neurosurg. 2013;115:841–3. doi: 10.1016/j.clineuro.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Swamy MN. A case of intramedullar epidermoid cyst. Med J Armed Forces India. 2008;64:72–3. doi: 10.1016/S0377-1237(08)80155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K, Zhao J, Zhang Y, Su Y. Pediatric intramedullary schwannoma with syringomyelia: A case report and literature review. BMC Pediatr. 2018;18:374. doi: 10.1186/s12887-018-1341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhake RP, Chatterjee S. Recurrent thoracic intramedullary schwannoma: Report of two cases with long term follow up. Br J Neurosurg. 2019:1–4. doi: 10.1080/02688697.2019.1566516. doi: 10.1080/02688697.2019.1566516. [DOI] [PubMed] [Google Scholar]
- 32.Ak H, Ulu MO, Sar M, Albayram S, Aydin S, Uzan M. Adult intramedullary mature teratoma of the spinal cord: Review of the literature illustrated with an unusual example. Acta Neurochir (Wien) 2006;148:663–9. doi: 10.1007/s00701-006-0755-z. [DOI] [PubMed] [Google Scholar]
- 33.Acharya A, Grewal SS, Sobti S, John PS, Bind RK, Bhardwaj MK, et al. Intramedullary mature teratoma of spinal cord: A rare tumor with review of literature. Surg Neurol Int. 2020;11:266. doi: 10.25259/SNI_442_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milano MT, Johnson MD, Sul J, Mohile NA, Korones DN, Okunieff P, et al. Primary spinal cord glioma: A Surveillance, Epidemiology, and End Results database study. J Neurooncol. 2010;98:83–92. doi: 10.1007/s11060-009-0054-7. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Wahab M, Etuk B, Palermo J, Shirato H, Kresl J, Yapicier O, et al. Spinal cord gliomas: A multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys. 2006;64:1060–71. doi: 10.1016/j.ijrobp.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Berhouma M, Bahri K, Houissa S, Zemmel I, Khouja N, Aouidj L, et al. Management of intramedullary spinal cord tumors: Surgical considerations and results in 45 cases. Neurochirurgie. 2009;55:293–302. doi: 10.1016/j.neuchi.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 37.Sandalcioglu IE, Gasser T, Asgari S, Lazorisak A, Engelhorn T, Egelhof T, et al. Functional outcome after surgical treatment of intramedullary spinal cord tumors: Experience with 78 patients. Spinal Cord. 2005;43:34–41. doi: 10.1038/sj.sc.3101668. [DOI] [PubMed] [Google Scholar]
- 38.Angevine PD, McCormick PC. Spinal cord ependymomas. Oper Tech Neurosurg. 2003;6:9–14. [Google Scholar]


