Abstract
Objectives:
Neutrophil-to-lymphocyte ratio (NLR) has been reported as prognostic in pancreatic ductal adenocarcinoma (PDAC). Data about NLR changes during neoadjuvant therapy (NAT) and its relationship with pathological tumor response and survival are lacking.
Methods:
PDAC patients with NAT followed by resection between 2009–2015 were identified from a prospective database. NLR was collected prior to NAT (baseline), on chemotherapy (prior to cycle 3), and prior to surgery. Baseline NLR, and changes in NLR between baseline and on chemotherapy (delta 1) and between baseline and surgery (delta 2) were compared with pathologic response (<90% and ≥90% defined as poor and good), overall (OS), and disease-free survival (DFS) using Wilcoxon-rank sum and Cox proportional hazards models.
Results:
Of 93 patients, 17% had good pathological response. Median (interquartile range) NLR at baseline, third cycle, and surgery were 2.7 (2.0–3.7), 2.5 (1.9–4.1), and 3.1 (2.1–5.3), respectively. Median change in NLR from baseline to third cycle was 0.06 (p=0.72), and 0.6 from baseline to surgery (p<0.01). Baseline NLR, delta 1, and delta 2 were not associated with pathological response, OS, or DFS.
Conclusions:
NLR increased after NAT, but a significant association between NLR and pathological response, OS, and DFS in resected PDAC patients was not observed.
Keywords: Pancreatic Cancer, Pancreatic Ductal Adenocarcinoma, Neutrophil-to-Lymphocyte Ratio
INTRODUCTION
Pancreatic adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the United States, and the 5-year overall survival (OS) rate remains <10%.1 For borderline resectable or locally advanced PDAC, neoadjuvant therapy (NAT) with chemotherapy or chemoradiation is the preferred treatment modality. However, there is currently no way to predict response to NAT, and new biomarkers are needed to improve treatment selection.
Neutrophil-to-lymphocyte ratio (NLR) is a marker of systemic inflammation that is believed to reflect antitumor inflammation capacity. NLR has been shown to have prognostic significance for many types of cancer, including gastric, colon, and breast cancer.2–4 In PDAC patients, data are mixed regarding the relationship between NLR and outcomes. Most studies have found lower preoperative NLR to be associated with improved OS and disease free survival (DFS),5–9 although others have found no relationship.10 Studies have also shown an association between inflammatory response in the tumor microenvironment and oncological outcome; specifically, patients with neutrophil infiltration around the tumor may have a worse prognosis, whereas those with lymphocytic infiltration may have a better prognosis.11 Given these findings and the association between pathological tumor response and survival in PDAC,12–14 we hypothesized that NLR may associate with tumor response to NAT.
To date, knowledge about the natural history of NLR from diagnosis and in response to NAT is lacking. The aim of this study was to evaluate NLR changes over time after NAT in patients with resectable PDAC and determine whether changes in NLR are associated with pathological tumor response, OS, or DFS.
METHODS
Study population
All patients treated for PDAC at Memorial Sloan Kettering Cancer Center are included in a prospectively maintained institutional database. After IRB approval, patients with primary, non-metastatic, PDAC who underwent resection with curative intent between 2009 and 2015 and received NAT with chemotherapy or chemoradiation were identified. Patients were included if they had blood work performed at our institution prior to the initiation of NAT. Patients were excluded if they had a total bilirubin >3.0 gm/dL or a white blood cell count >12.0 × 109/L prior to initiation of NAT, if they received <2 cycles of chemotherapy, or if complete blood count (CBC) prior to surgery was not available. Clinical, pathological, treatment characteristics, and follow-up data were extracted from the electronic medical record.
Neutrophil-to-Lymphocyte Ratio
Laboratory values were collected from a CBC prior to initiation of NAT (t=0), prior to the third cycle of chemotherapy (t=1), and prior to surgery (t=2). To calculate NLR, the absolute neutrophil count (K/μL) was divided by the absolute lymphocyte count (K/μL). The change in NLR was calculated for two different intervals: between baseline (t=0) and prior to the third cycle of chemotherapy (t=1) (defined as delta 1), and between baseline (t=0) and prior to surgery (t=2) (defined as delta 2).
Statistical Analysis
Continuous data were expressed as median and interquartile range (IQR), and categorical variables were reported as number and percentages (%). Pathological treatment response was reported by the pathologist in percentages. Treatment response was dichotomized as <90% and ≥90% and defined as poor and good response, respectively, based on a simplified version of the frequently used Evans score, where grade I and II represent between 0–90% of tumor cell destruction, and grade III and IV represent 90–100% of tumor cell destruction.15 Chi-square and Wilcoxon-rank sum tests were used to compare delta 1, delta 2, and other covariate differences between poor and good responders. The Fisher’s exact test was used for subgroups with numbers <5. Differences in median delta 1 and delta 2 were evaluated by Wilcoxon signed-rank test. OS and DFS were calculated from date of surgery until date of first recurrence or death (for DFS) whichever occurred first, or to date of death (for OS). Patient data were censored at the date of last follow-up. OS and DFS were estimated using Kaplan-Meier methods. Cox proportional hazards model was used to examine the impact of baseline NLR, change in NRL between baseline and the third cycle (delta 1), and change in NRL between baseline and surgery (delta 2) on survival outcomes. All p values were based on 2-tailed statistical analysis, and p<0.05 was considered statistically significant. All analyses were performed using R version 3.6.0 (R Foundation for Statistical Computing Vienna, Austria).
RESULTS
A total of 93 patients were included in the analysis. After NAT, 77 (83%) patients had a poor pathological response, and 16 (17%) had a good response (Table 1). Median age was 64 (IQR 59–69) years, and there was no age difference between the poor and good responders (p = 0.4). There was no difference in sex (47% vs. 25%, p = 0.2), history of diabetes (26% vs. 31%, p = 0.8), or history of smoking (48% vs. 44%, p > 0.9) between the poor and good responder groups. Median baseline white blood cell, neutrophil, and lymphocyte counts in the poor responder group were 6.7, 4.2, and 1.5 × 109/L, respectively, and similar values were found in the good responder group (all p > 0.5). Overall, 50% (46/93) of patients received FOLFIRINOX, and 20/93 (22%) had gemcitabine with oxaliplatin, as that was the regimen of choice in an ongoing trial at our institution.16 In the group of patients who received other regimens, 3 received gemcitabine with abraxane and 9 had a single drug only (gemcitabine or capecitabine). The type of NAT chemotherapy was not associated with pathological response (p = 0.5). The median duration of NAT chemotherapy was not different between the groups (12 [IQR 6–18] weeks in the poor responders vs. 22 [IQR 11–32] weeks in the good responders, p = 0.018). Neoadjuvant radiation therapy was used in 28/77 (36%) of the poor responders and 14/16 (88%) of the good responders (p < 0.001).
Table 1.
Patient, tumor, and treatment characteristics according to pathological tumor response
| Characteristic1 | Overall, N = 93 | Poor responders, n = 77 | Good responders, n = 16 | P-value2 |
|---|---|---|---|---|
| Age, y | 64 (59, 69) | 64 (59, 68) | 67 (60, 70) | 0.4 |
| Sex | 0.2 | |||
| - Female | 40 (43%) | 36 (47%) | 4 (25%) | |
| - Male | 53 (57%) | 41 (53%) | 12 (75%) | |
| Body mass index | 25.9 (23.4, 28.6) | 25.9 (23.2, 28.6) | 26.2 (24.4, 28.6) | 0.5 |
| History of diabetes | 25 (27%) | 20 (26%) | 5 (31%) | 0.8 |
| History of smoking | 44 (47%) | 37 (48%) | 7 (44%) | >0.9 |
| Baseline laboratory values | ||||
| - white blood cell count | 6.70 (5.40, 8.00) | 6.50 (5.30, 7.90) | 6.75 (6.05, 8.75) | 0.5 |
| - absolute neutrophils count | 4.20 (3.20, 5.50) | 4.20 (3.20, 5.50) | 4.50 (3.30, 5.75) | 0.7 |
| - absolute lymphocyte count | 1.50 (1.20, 1.90) | 1.50 (1.20, 1.90) | 1.40 (1.28, 2.28) | 0.8 |
| - NLR | 2.73 (2.00, 3.67) | 2.69 (2.00, 3.67) | 2.77 (1.91, 3.65) | >0.9 |
| Tumor location | >0.9 | |||
| - Head | 76 (82%) | 63 (82%) | 13 (81%) | |
| - Body | 8 (8.6%) | 7 (9.1%) | 1 (6.2%) | |
| -Tail | 9 (9.7%) | 7 (9.1%) | 2 (12%) | |
| Neoadjuvant chemotherapy regimen | 0.6 | |||
| - FOLFIRINOX | 46 (50%) | 37 (48%) | 9 (56%) | |
| - Gemcitabine & Oxaliplatin | 20 (22%) | 17 (22%) | 3 (19%) | |
| - Other | 27 (29%) | 23 (30%) | 4 (25%) | |
| Neoadjuvant chemotherapy duration (weeks) | 12 (7, 20) | 12 (6, 18) | 22 (11, 32) | 0.018 |
| Neoadjuvant radiation | 42 (45%) | 28 (36%) | 14 (88%) | <0.001 |
| Pathological tumor stage | 0.7 | |||
| - 0 | 3 (3.2%) | 0 (0%) | 3 (19%) | |
| - 1 | 3 (3.2%) | 2 (2.6%) | 1 (6.2%) | |
| - 2 | 2 (2.2%) | 2 (2.6%) | 0 (0%) | |
| - 3 | 85 (91%) | 73 (95%) | 12 (75%) | |
| Positive pathologic lymph nodes | 38 (41%) | 38 (49%) | 0 (0%) | <0.001 |
Data are median (IQR) or n (%)
Wilcoxon rank-sum test; chi-square test of independence; Fisher’s exact test
Abbreviation: NLR = neutrophil-to-lymphocyte ratio
Pathological response
In the entire cohort, the median NLR at initiation of NAT (t=0), prior to third cycle (t=1), and prior to surgery (t=2) were 2.7 (IQR 2.0–3.7), 2.5 (IQR 1.9–4.1), and 3.1 (IQR 2.1–5.3), respectively (Figure 1). From baseline to prior to the third cycle, NLR did not significantly change (median delta 1: 0.06, IQR −1.0–1.1, p = 0.719), but it did significantly increase between baseline and surgery (median delta 2: 0.6, IQR 0.8–2.0, p < 0.01). Comparing poor and good responders, baseline NLR was not different (2.7 vs. 2.8, p = 0.9). In addition, the change in NLR from baseline to third cycle did not differ between poor and good responders, with a median delta 1 of 0.06 and −0.02, respectively (p = 0.8). There was also no difference from baseline to surgery (median delta 2: poor 0.6 vs. good 0.8, p = 0.8) (Table 2). Change in NLR was also not associated with the type of NAT chemotherapy given (delta 1: p = 0.810; delta 2: p = 0.626).
Figure 1. Neutrophil-to-lymphocyte ratio at each time point in all patients.
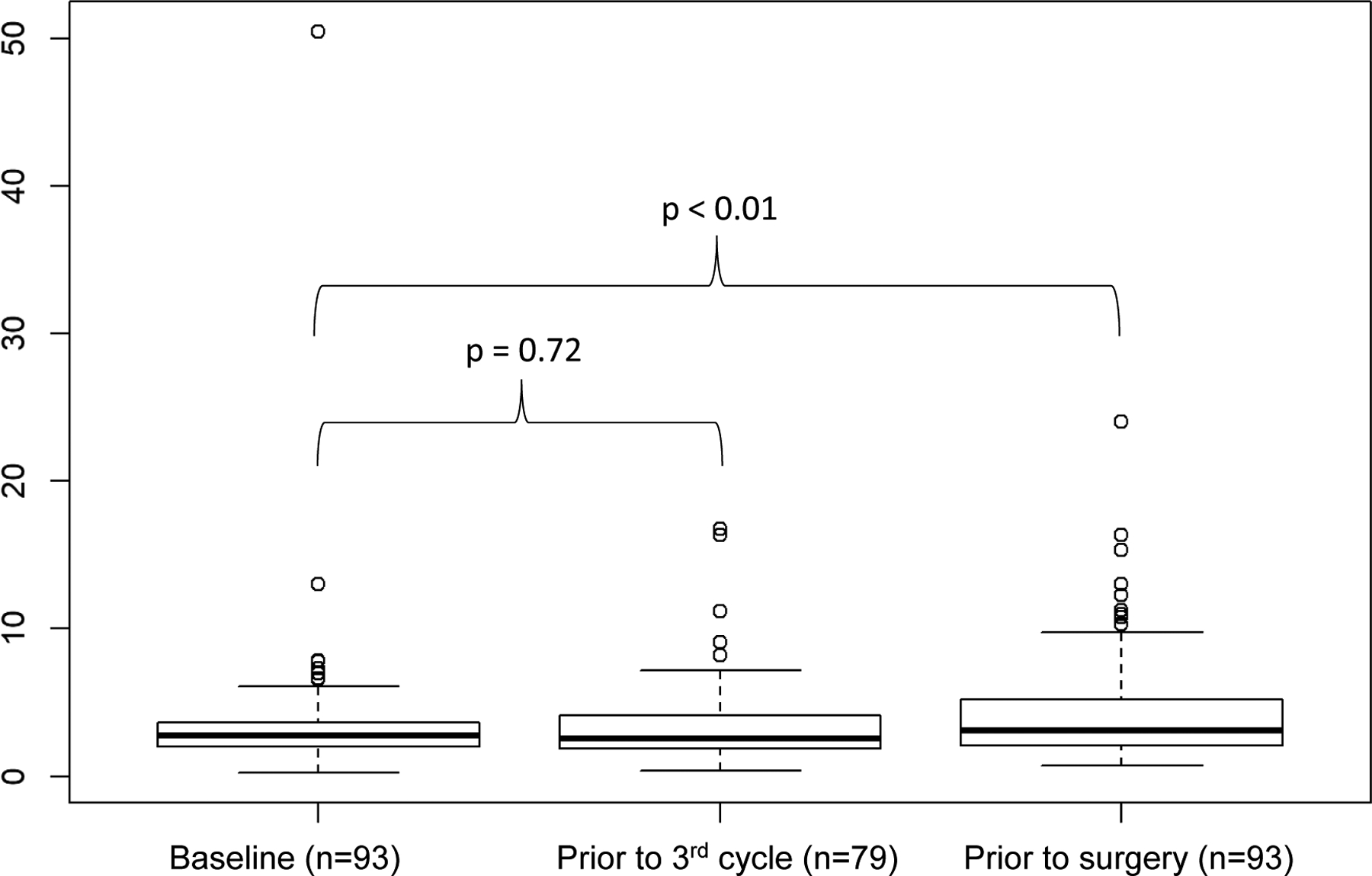
The dark vertical line indicates the median. NAT: neoadjuvant treatment.
Table 2.
Relationship between baseline NLR and changes in NLR on pathological tumor response
| Characteristic1 | Poor responders, n = 77 | Good responders, n = 16 | P-value2 |
|---|---|---|---|
| Baseline NLR | 2.69 (2.00, 3.67) | 2.77 (1.91, 3.65) | >0.9 |
| Delta 1 | 0.06 (−0.88, 1.08) | −0.02 (−1.09, 1.47) | 0.8 |
| - NLR unknown | 12 | 2 | |
| Delta 2 | 0.61 (−0.75, 1.93) | 0.81 (−0.54, 1.88) | 0.8 |
Data are median (IQR)
Wilcoxon rank-sum test
Abbreviations: NLR = neutrophil-to-lymphocyte ratio, NAT = neoadjuvant treatment; delta 1 = baseline to cycle 3; delta 2 = baseline to surgery
Survival
With a median follow up of 50 (range: 4.4–110) months among survivors, we observed total of 62 deaths and 72 recurrences after surgery. Baseline NLR, change in NLR from baseline to third cycle (delta 1), and the change in NLR from baseline to surgery (delta 2) were not associated with OS, with hazard ratios (HR) of 0.98 (95%CI 0.91–1.07), 1.08 (95%CI 0.98–1.19), and 1.00 (95%CI 0.96–1.05), respectively (Table 3). Additionally, baseline NLR, delta 1, and delta 2 were not associated with DFS (HR 1.02 [95%CI 0.98–1.06], 1.00 [95%CI 0.94–1.07], and 0.98 [95%CI 0.95–1.02], respectively).
Table 3.
Relationship between baseline NLR and changes in NLR on survival outcomes
| Overall Survival | Disease Free Survival | ||||||
|---|---|---|---|---|---|---|---|
| N | HR | 95%CI | P-value | HR | 95%CI | P-value | |
| Baseline NLR | 93 | 0.98 | 0.91–1.07 | 0.7 | 1.02 | 0.98–1.06 | 0.2 |
| Delta 1 | 79 | 1.08 | 0.98–1.19 | 0.1 | 1.00 | 0.94–1.07 | 0.9 |
| Delta 2 | 93 | 1.00 | 0.96–1.05 | 0.9 | 0.98 | 0.95–1.02 | 0.3 |
Abbreviations: NLR = neutrophil-to-lymphocyte ratio, HR = hazard ratio; delta 1 = baseline to cycle 3; delta 2 = baseline to surgery
DISCUSSION
We studied the association between clinical outcomes and baseline NLR and changes in NLR during treatment in 93 PDAC patients that underwent NAT followed by resection. We found that NLR did not change during the early phase of NAT, and it slightly increased from a median of 2.7 to 3.1 prior to surgery. However, baseline NLR and changes in NLR were not associated with a pathological response of the tumor, nor with OS or DFS.
The prognostic role of preoperative NLR for OS and DFS in PDAC patients has been studied extensively. In contrast to our findings, a recent meta-analysis found significant correlations between high NLR and poor survival rates.9 Interestingly, NLR had no prognostic value in patients who underwent chemoradiation.9 Only two studies have investigated the prognostic role of changes in NLR during NAT. Both found that a high baseline NLR and an increase in NLR after one and two cycles of chemotherapy were associated with worse survival in Asian patients with locally advanced (n=132) and metastatic (n=403) PDAC, respectively.17,18 Neither study excluded patients with high white blood cell count, high bilirubin, biliary obstruction, cholangitis, or pancreatitis in contrast to our study. Moreover, we included PDAC patients that successfully underwent NAT and were selected for resection with curative intent. As such, we studied a selected group with relatively good prognosis. The differences in baseline NLR and NLR changes during NAT may be subtle in a more homogenous study population, such as ours. Another reason for the discrepancy in findings might be that the previous studies approached NLR as a discrete variable (high vs. low at baseline, or increased vs. decreased during NAT), and the cutoffs used varied. In contrast, given that dichotomizing variables results in the loss of information and statistical power and increases the risk of false positive results,19 we approached NLR as a continuous variable. Although there is lack of understanding of the biological implications of NLR, we believe that a continuous relationship between NLR and prognosis is more likely than a discrete relationship with a hard cut-off.
Previously, the mean NLR in a young adult healthy population was found to be 1.7 (95%CI 0.7–3.5).20 Although our median NLR of 2.7 was higher than the reported mean in healthy young adults, it was still within the confidence interval. This suggests that there may be normal fluctuations either within or between patients that is difficult to differentiate from a prognostic effect. In addition, aside from age, smoking, and diabetes, we did not look at other comorbid conditions that may influence NLR. It is also possible that there truly is no relationship between NLR and prognosis in Western PDAC patients. Publication bias may also explain why a significant association between NLR and prognosis is more commonly reported than a lack of association.
Limitations of this study include the small sample size; it is possible that a larger study population would have allowed us to detect a small predictive or prognostic value for baseline NLR or NLR changes during NAT. Also, the retrospective nature of this study comes with disadvantages, and multiple patients were excluded due to a lack of laboratory values available in the electronic medical record if NAT occurred outside of our institution. Further, we studied a heavily selected study population, and more advanced PDAC patients with NAT who progressed and never underwent surgery were not included. However, a more homogenous study population could provide more clinically meaningful biomarker information. As far as we know, we are the first to study pathological treatment response as outcome in relation to NLR. Another strength of this study is the investigation of NLR across time. Since we did find a significant difference in median NLR between initiation of NAT and prior to surgery, we advise the standardized use of one of those two time points within the same cohort in future studies and meta-analyses. In addition, for statistical reliability, we suggest studying NLR as a continuous outcome rather than a dichotomous variable.
Conclusions
In non-metastatic, primary PDAC patients undergoing neoadjuvant chemotherapy or chemoradiation (NAT) followed by surgery with curative intent, NLR significantly increased from initiation of NAT until surgery. However, baseline NLR and changes in NLR during NAT were not associated with pathological response of the tumor, OS, or DFS. Our findings suggest that NLR may not be a predictive or prognostic value in this population, and that standardization of NLR assessment is essential for future research.
Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosures: None
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Wang SC, Chou JF, Strong VE, Brennan MF, Capanu M, Coit DG. Pretreatment Neutrophil to Lymphocyte Ratio Independently Predicts Disease-specific Survival in Resectable Gastroesophageal Junction and Gastric Adenocarcinoma. Ann Surg. 2016;263(2):292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rashtak S, Ruan X, Druliner BR, et al. Peripheral Neutrophil to Lymphocyte Ratio Improves Prognostication in Colon Cancer. Clin Colorectal Cancer. 2017;16(2):115–123 e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giakoustidis A, Neofytou K, Costa Neves M, et al. Identifying the role of neutrophil-to-lymphocyte ratio and platelets-to-lymphocyte ratio as prognostic markers in patients undergoing resection of pancreatic ductal adenocarcinoma. Ann Hepatobiliary Pancreat Surg. 2018;22(3):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang LP, Xu XY, Ji Y, Huang PW. The Prognostic Value of Preoperative Neutrophil-to-Lymphocyte Ratio in Resected Patients with Pancreatic Adenocarcinoma. World journal of surgery. 2018;42(11):3736–3745. [DOI] [PubMed] [Google Scholar]
- 7.Abe T, Nakata K, Kibe S, et al. Prognostic Value of Preoperative Nutritional and Immunological Factors in Patients with Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol. 2018;25(13):3996–4003. [DOI] [PubMed] [Google Scholar]
- 8.Sierzega M, Lenart M, Rutkowska M, et al. Preoperative Neutrophil-Lymphocyte and Lymphocyte-Monocyte Ratios Reflect Immune Cell Population Rearrangement in Resectable Pancreatic Cancer. Ann Surg Oncol. 2017;24(3):808–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh D, Pyo JS, Son BK. Prognostic Roles of Inflammatory Markers in Pancreatic Cancer: Comparison between the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio. Gastroenterol Res Pract. 2018;2018:9745601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla A, Huang TL, Ibrahim AM, Hardacre JM, Siegel C, Ammori JB. Pretherapy neutrophil to lymphocyte ratio and platelet to lymphocyte ratio do not predict survival in resectable pancreatic cancer. HPB (Oxford). 2018;20(5):398–404. [DOI] [PubMed] [Google Scholar]
- 11.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloyd JM, Wang H, Egger ME, et al. Association of Clinical Factors With a Major Pathologic Response Following Preoperative Therapy for Pancreatic Ductal Adenocarcinoma. JAMA Surg. 2017;152(11):1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macedo FI, Ryon E, Maithel SK, et al. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg. 2019;270(3):400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truty MJ, Kendrick ML, Nagorney DM, et al. Factors Predicting Response, Perioperative Outcomes, and Survival Following Total Neoadjuvant Therapy for Borderline/Locally Advanced Pancreatic Cancer. Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 15.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Archives of surgery. 1992;127(11):1335–1339. [DOI] [PubMed] [Google Scholar]
- 16.O’Reilly EM, Perelshteyn A, Jarnagin WR, et al. A single-arm, nonrandomized phase II trial of neoadjuvant gemcitabine and oxaliplatin in patients with resectable pancreas adenocarcinoma. Ann Surg. 2014;260(1):142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Yan H, Wang Y, Shi Y, Dai G. Significance of baseline and change in neutrophil-to-lymphocyte ratio in predicting prognosis: a retrospective analysis in advanced pancreatic ductal adenocarcinoma. Sci Rep. 2017;7(1):753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22(2):670–676. [DOI] [PubMed] [Google Scholar]
- 19.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]


