Abstract
Islands are useful model systems for examining human–environmental interactions. While many anthropogenic effects visible in the archaeological and paleoecological records are terrestrial in nature (e.g., clearance of tropical forests for agriculture and settlement; introduction of nonnative flora and fauna), native peoples also relied heavily on marine environments for their subsistence and livelihood. Here we use two island case studies—Palau (Micronesia) and the Lesser Antilles (Caribbean)—and approach their long-term settlement history through a “ridge-to-reef” perspective to assess the role that human activity played in land- and seascape change over deep time. In particular, we examine the entanglement of terrestrial and marine ecosystems resulting from anthropogenic effects and cultural responses to socio-environmental feedback. We suggest that on the humanized tropical islands of the Anthropocene, mangroves, near shore and littoral areas, and coral reefs were major sites of terrestrial–marine interface chronicling and modulating anthropogenic effects.
Keywords: historical ecology, archaeology, Micronesia, Lesser Antilles, traditional ecological knowledge
Oceanic islands are useful model systems for examining how humans may have impacted pristine ecologies (1–3). They are aquatically bounded, relatively (or completely) isolated, often remote, and required specific dispersal mechanisms for biota to colonize. Although some scholars have challenged this approach as being too broad and see islands as no different from larger continental land masses (4), many archaeologists have found it appropriate because of islands’ scale (size) and sensitivity to change (both internal and external) (5). Over evolutionary time, this has resulted in high levels of biodiversity and endemism, fostering a dizzying array of unique biotic communities. As a result, islands have played an increasingly important role in debates surrounding the Anthropocene—the proposed geologic epoch or age defined by human alteration of Earth’s systems—and its conceptualization, temporal framework, and the degree to which anthropogenic processes have affected environments that were for millennia devoid of human intervention (6–8). This research contributes to the Anthropocene debate by highlighting how humans on islands shaped ecological processes linking terrestrial and marine environments prior to the modern era (9).
Archaeological and paleoecological research demonstrates that humans have been among the most significant drivers of Holocene ecological change not just on islands but globally (10–12). Homo sapiens are essentially a volatile mix of culturally driven, efficient predators, technological savants, and curious, highly adaptable migrants. These combined qualities allowed humans to settle five of the six continents by at least approximately 16 ka, and over the last few thousand years of the Holocene, reach the most remote patches of land on Earth by deftly crossing the world’s seas and oceans. It was during these forays to islands that humans brought what has often been termed a “transported landscape” (13)—consisting of nonnative plants and animals and notions of how to modify the landscape for agriculture. This eventually led to dramatic environmental changes to island ecosystems.
Although much research has focused on how these colonizers and subsequent generations modified terrestrial environments through land clearance for settlement and food production, long-term survival was inextricably linked to the marine realm for subsistence, material resources, and passage between communities. Here, we use two case studies from the Pacific and Caribbean—Palau in western Micronesia and the Lesser Antilles in the Caribbean—to highlight how these islands can serve as ideal model systems for examining human intervention from a deep-time perspective (Fig. 1). We use a holistic, ridge-to-reef approach that integrates terrestrial and marine systems to understand the interconnectedness of their historical ecology and the role of humans in this process. Our primary focus is on examining the interconnectivity of the land and sea and how the confluence of these two ecotones—particularly mangroves, coral reefs, and nearshore or littoral environments—were pivotal for human occupation, but also distinct markers for capturing and modulating anthropogenic effects. By integrating data from archaeology, history, and ecology, we identify the ways in which human societies on these islands have modified the land- and seascape and also in the case of Palau, similar to other Pacific Islands, established socio-environmental feedback loops structured within customary marine tenure frameworks that may have served as buffer mechanisms to prevent long-term degradation and ensure resource productivity.
Fig. 1.
(Left) Map of the Palauan archipelago showing location of sites mentioned in text. (Right) Map of the Eastern Caribbean with insets of Lesser Antilles and the Grenadines showing marine and coral reef banks.
Archaeological and Ecological Background
The archaeological chronologies presented below employ years before present (BP). Temporal ranges given in approximate years BP amalgamate multiple radiocarbon assays for relevant sites/phenomena and in some cases incorporate Bayesian modeling of radiocarbon dates (14, 15). Calibrated single radiocarbon determinations are presented as years cal. BP.
Palau.
The Palauan archipelago comprises several hundred islands that stretch for more than 150 km in the northwest tropical Pacific (Fig. 1). Palau is part of the Caroline Islands chain in western Micronesia and is geologically varied, ranging from the larger volcanic island of Babeldaob (approximately 331 km2) to atolls (e.g., Kayangel) and uplifted limestone (both low-platform and high-carbonate outcrops), the latter of which are the most common and locally referred to as the “Rock Islands” (SI Appendix, Fig. S1 A–D). Around 80% of land cover in Palau is tropical moist forest, the dominant of which are mangroves, swamps, and upland evergreen broadleaf, while the remaining 20% is savanna and grassland, most of which is found on Babeldaob (SI Appendix, Fig. S1 E and F). A large swathe of Palau today is agro- or secondary forest due to repeated human burning over the millennia in which more than half of Babeldaob’s savanna vegetation is the result of anthropogenic forces (16).
The main Palauan archipelago is largely surrounded by a barrier reef along with a mix of outer fringing, sunken, sheltered, and island-fringing reef systems (17, 18). These are effective at blocking ocean swells, creating protective lagoons, and have greatly influenced the development and growth of Palau’s marine environments. Given the archipelago’s position along the margin of the Coral Triangle and within the Polynesia–Micronesia Biodiversity Hotspot—which has the highest known species diversity within the world’s shallow water areas—Palau is exceptional and the most biodiverse in Micronesia (17). Due to the variety of island types and geomorphological processes, a wide array of complex marine habitats are found in Palau, including reef passages, channels, shallow lagoon-fringing flats, nearshore and lagoon patch reefs, lagoon sediment bottoms, seagrass areas, marine lakes, lagoon pelagic environments, mangroves, and estuaries (17, 18), all of which were exploited by Palauans in the past and today.
Archaeologically, the earliest evidence for human occupation in Palau dates to approximately 3300 to 2700 BP and comes from several interior sites on Babeldaob with pottery (19) and three Rock Island sites: Ulong (approximately 3300 to 3000 BP), Ucheliungs (approximately 3000 to 2800 BP), and Chelechol ra Orrak (approximately 3000 to 2700 BP), the latter two of which contain both human burials and subsistence activity (14, 20, 21) (SI Appendix, Fig. S1C). All of these sites are roughly contemporaneous with the first human incursions into Remote Oceania, including the Mariana Islands (22, 23) and Lapita expansion (24).
During the initial stages of Palauan occupation approximately 3300 to 3000 BP, peoples were using the interior of the larger islands of Babeldaob and Koror, coinciding with the use of limestone caves for burying the dead (14) and sporadic human occupation of the Rock Islands (14, 20, 25). Around 2400 BP, the construction of nearly 30 earthwork formations is evident during the “Earthwork Era” (19). Radiocarbon dating indicates that construction of these earthwork complexes, which included stepped terraces, ditches, gullies, and other features, were not agricultural but symbolic and defensive (26) and reflect growing social complexity that accelerated between approximately 2400 to 1200 BP (SI Appendix, Fig. S1 E and F). The use of these earthworks, most of which are found within the interior, appears to gradually decline over the course of two millennia and habitation inexplicably begins to shift to the coastal margins between approximately 1200 to 700 BP. Palauan occupation then focuses on the construction of numerous traditional (stonework) coastal village sites with an extensive array of architectural features, such as residential platforms, pathways and other pavements, docks, bathing areas, and shrines (SI Appendix, Fig. S1 G and H). These were situated behind mangrove forests, perhaps as a defensive measure, with channels cut through to monitor and provide access to the sea (Fig. 2A). Many of these villages were recorded historically after initial contact when the British packet the Antelope wrecked off of Ulong Island in 1783 and are still used today.
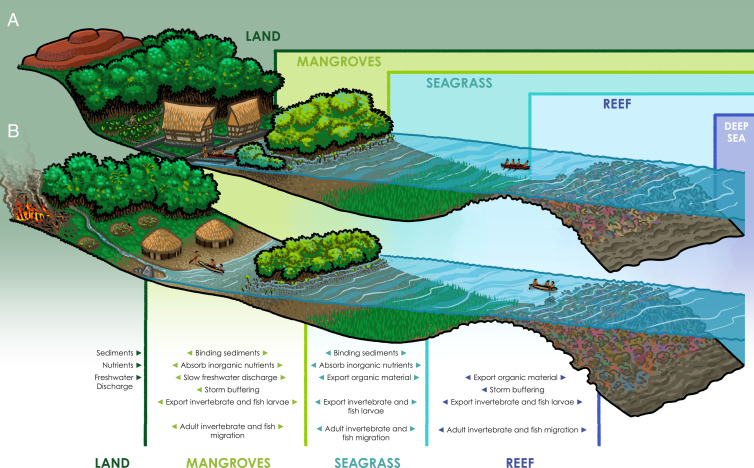
Fig. 2.
Schematic showing the interplay between land and sea for Indigenous peoples across major ecotones in (A) Palau and (B) the Caribbean Lesser Antilles. Note the prevalence of nutrient export into and through each respective habitat and the ecosystem services that each provides to humans (e.g., agricultural products, marine resources). Image credit: John Gordon Swogger (artist).
Subsistence practices in Palau included a mix of dryland and wetland agriculture for various cultigens like taro (the predominant crop) and breadfruit, along with pandanus and coconut, for example (27, 28), limited harvesting of native terrestrial fauna (e.g., birds, bats); a single domesticated animal (pig; Sus scrofa) for about 1,000 y (approximately 1400 to 400 BP), and heavy exploitation of marine resources. Data from past marine subsistence use in Palau spans the entire range of known human occupation approximately 3000 BP to the present, with substantial assemblages coming primarily from vertebrates and invertebrates recovered at several archaeological sites in the Rock Islands, including Chelechol ra Orrak (29–31), Ulong (32), and several others in the southern lagoon such as Ngemelis and Uchularois (33, 34). Prehistoric faunal material has also been recovered sporadically throughout the Palauan archipelago, primarily from contract projects. However, sample sizes are typically small due to poor preservation or other factors, or lack sufficient associated radiocarbon dates, suitable recovery methods, and quantification measures, and so are not considered here.
Onshore paleoenvironmental data derive from cores taken in Babeldaob and the Ngerekebesang islet (28, 35) that span the Middle to Late Holocene and cover the range of geographical features for the islands and include abandoned taro pondfields. Modern references were also used to examine more recent land-use changes (16, 36, 37), primarily on Babeldaob, which has the most extensive watersheds and where the majority of agricultural production takes place. Studies examining changes to reefs and other marine habitats historically and in the modern era were also integrated into our overall assessment (17, 38–40).
Lesser Antilles.
The Lesser Antilles extends 800 km from Anguilla in the north to Grenada in the south, marking the eastern boundary of Caribbean Sea (Fig. 1). The chain is composed of limestone forearc and volcanic backarc islands that formed through Cenozoic era subduction of the South American plate beneath the Caribbean plate. The larger volcanic islands are characterized by mountainous topography, higher precipitation, and moist and dry tropical forests (SI Appendix, Fig. S1 I and K). Small, low-relief, and limestone islands (e.g., The Grenadines and La Désirade) experience arid conditions due to limited orographic rainfall and highly permeable ground surfaces, and tend to support dry forests and xeric scrub and shrubland (SI Appendix, Fig. S1 L and M). Except for the steeper mountain reaches, most remaining forest in the region is secondary growth, the product of millennia of anthropogenic landscape transformation that culminated in the large-scale sugar monocropping of the 17th to 19th centuries (41, 42).
Surrounding marine environments are biodiverse and have been economically important to humans through time. The most significant habitats exploited by past peoples are found near coasts and include shallow, inshore waters comprising seagrass meadows, sandy bottoms, and mixed substrate; coral reefs; and pelagic waters of the neritic zone (41, 43) (SI Appendix, Fig. S1 J and N). Lesser Antillean reefs consist predominantly of patch and fringing reefs, but barrier reef development is notable within The Grenadines microarchipelago, which forms part of the Grenadine Bank (4,000 km2) of the southern Lesser Antilles (Fig. 1 and SI Appendix, Fig. S1J). Extensive barrier reefs also occur within the historically productive, shallow Anguilla Bank (3,400 km2) encompassing the island shelves of Anguilla, St. Barthélemy, and St. Martin (44, 45) (Fig. 1). Mangroves grow on sedimentary shorelines of wetlands, sheltered bays, and estuaries, providing nursery and feeding habitat for juvenile fish, including members of the parrotfish (Labridae: Scarinae), snapper (Lutjanidae), and grunt (Haemulidae) families (46–50), which are among the most frequently occurring taxa in archaeological fish assemblages (42) (SI Appendix, Fig. S1O).
Archaeological evidence indicates that the Lesser Antilles were colonized approximately 5000 BP by Archaic peoples likely originating in South America (15). Archaic sites occur almost exclusively north of Dominica and typically number only a few per island, suggesting low population densities at this time. The presence of seasonally occupied camps is consistent with hunter-gather lifeways (51), but increasing evidence suggests Archaic peoples engaged in plant cultivation and were more sedentary than once believed (52). Beginning approximately 2500 BP, Arawak groups from northeastern South America moved into Puerto Rico and the northern Lesser Antilles. Paleogenetic data suggest they largely replaced the preexisting Archaic population (53). The settlement locations of these new Ceramic Age peoples are highly visible archaeologically based on a horizon of copious, distinctive pottery. Overall, archaeological trends during the Ceramic Age (approximately 2500 to 500 BP) suggest demographically driven settlement expansion (54). Compared to the Archaic, the number of sites per island increases by one or two orders of magnitude, with many residential sites occupied continuously for centuries or longer. Between approximately 2000 and 1600 BP populations expanded west and south. Reliably dated archaeological evidence for human occupation now occurs south of Guadeloupe for the first time (15, 55). After approximately 1550 BP, Ceramic Age peoples moved into the Greater Antilles and eventually the Bahama Archipelago (15), with most habitable islands occupied before European arrival.
Ceramic Age peoples engaged in a mixed economy of food production, fishing, and hunting (41). Zooarchaeological assemblages spanning the Ceramic Age Lesser Antilles are dominated by fish and molluscan remains, with variable amounts of sea turtle (Cheloniidae), indicating heavy focus on marine and littoral resources (e.g., refs. 41 and 56–59). Island- and regional-scale syntheses provide evidence for long-term trends in pre-Columbian fisheries and heterogeneous anthropogenic effects between approximately 2500 and 500 BP (43, 44, 60–64). Terrestrial vertebrate taxa are typically less abundant than marine vertebrates or invertebrates in zooarchaeological contexts, with mammal exploitation largely restricted to endemic rice rats (Oryzomyini spp.), agouti (Dasyprocta sp.), and in the southern Lesser Antilles, opossum (Didelphis marsupialis) (41, 57, 65). The latter two taxa were introduced by humans, likely to compensate for an impoverished insular vertebrate fauna (65).
Pollen, phytolith, and starch grain data (42, 52), supported by stable isotope analyses of diet on human skeletal remains (66), provide evidence for cultivation of wild and introduced domestic cultigens, such as manioc (Manihot esculenta) and maize (Zea mays) in the Caribbean. Ceramic Age agricultural techniques are archaeologically not well evidenced, but ethnohistoric accounts and allusion to culturally affiliated lowland South American groups suggest use of swidden practices, with cultigens grown on mounded fields and intercropped forest gardens (41) (Fig. 2B). Paleoenvironmental cores from Grenada, Barbados, Martinique, Marie-Galante, Antigua, and Barbuda (42, 67) provide region-wide evidence for vegetation, landscape burning, erosion, and changing land use from the prehuman period to the present. We employed studies of historical and recent environmental change in coral reef, mangrove, and coastal habitats (46, 47, 68–70) of the Caribbean and elsewhere to illuminate the ecological dynamics of past human–environment entanglements.
From Ridge-to-Reef: Terrestrial and Marine Entanglements through Time
Palau.
Paleoenvironmental data from coring regimes in Palau (28) demonstrate an increase in erosion and a transition from mostly peaty sediments (characteristic of anerobic and acid soil conditions) to organically rich clay and loam on the larger volcanic islands commensurate with earthwork construction (16, 19). This seems to also be reflected in changes to material culture, where grog replaces beach sand as the preferred temper in pottery approximately 2400 BP, presumably due to upland erosion from vegetation clearance and land clearance for earthworks that led to increased sedimentation of coastal embayments and reduced beach sand availability (25). Past expansion of anthropogenic savanna-like vegetation, covering much of Babeldaob, appears directly linked to anthropogenic forest clearance over the last few millennia (16).
Zooarchaeological analyses of assemblages recovered from several sites in Palau’s Rock Islands record three millennia of fish and mollusk exploitation, reinforcing the long-term economic importance of marine resources to Palauans (25, 29, 31, 32) also evidenced in paleodietary data from human remains dating to within the last 3,000 y (21). Research suggests overharvesting of mollusks, such as Tridacna sp., Strombus sp., and Lambis sp. on Ulong between approximately 3100 and 500 BP (32). Changes in fish assemblages are evident at Ngemelis between approximately 800 and 400 BP, where wrasses and parrotfishes increase while emperors, snappers, and other taxa decline over a 400-y period. Contrasting trends in marine resource use are observed farther north at Chelechol ra Orrak, however, where there is a marked decline in fishing and uptick in mollusk collection between approximately 3000 BP to the present (29, 30) (Fig. 1). The most heavily exploited gastropod species at Orrak, Gibberulus gibberulus, becomes larger through time, despite increasing human harvest pressure (30). Because of its proximity (∼0.4 km) to Babeldaob, where human populations were concentrated, Orrak possesses the only substantial zooarchaeological assemblage in the Rock Islands that would have captured the effects on faunal exploitation of large-scale landscape modification (earthwork construction, forest clearance), agricultural intensification, and population growth. These data then raise intriguing questions about the extent to which earthwork construction, agricultural production on Babeldaob and nearby traditional coastal villages, and traditional conservation practices—coupled with the surrounding habitat structure (sand flats, shallow coral reefs, barrier reef)—influenced the taxonomic composition of Orrak’s faunal assemblages. What were the ridge-to-reef land management activities exercised in the past, and how might more recent studies serve as analogs for early agricultural practices and their mitigating effects?
Recent land-use research, primarily on Babeldaob, indicates land cover changed dramatically following human colonization approximately 3300 to 3000 BP. Prior to settlement, Palau was dominated by a forested landscape with few open areas; some estimates suggest at least 39% of Babeldaob’s 278-km2 land area has been disturbed over time, leading to significant declines in endemic flora and overall habitat quality (16). Studies examining sedimentation rates on Babeldaob’s watersheds clearly show the negative influence of land-based development activities (e.g., roads, housing) on coral reefs and associated resources (36), which have been exacerbated by episodical natural events, such as El Niño. An exceptionally devastating one in 1997/1998 led to mass coral bleaching, a drought where virtually no rain fell between January and May 1998, and bush fires that likely augmented inland erosion, resulting in near-shore sedimentation once the rains returned (17).
Despite these issues, research in Palau clearly demonstrates the positive effect that mangroves have on trapping sediment. In a study of two major rivers on Babeldaob (Ngerdorch and Ngerikiil) that flow through mangrove swamps into coral-fringed lagoons, the estuaries collectively trapped more than 40% of the fine sediment from riverine discharge (71). In mangroves where there has been a long-established agricultural focus on wetland taro (Colocasia esculenta) cultivation, these gardens were strategically placed where freshwater influx affords ideal growing conditions and work in tandem to trap up to 90% of the sediment that flows from inland areas before reaching the coastal margins (72).
This traditional form of agriculture has for millennia provided Palauans with an important source of starch and food security. Given the heavy dependency on marine resources for protein, it is quite plausible that early Palauans, relying on taro for a major part of their subsistence—and recognizing the deleterious effects that land clearance and cultivation had on marine taxa during the first phase of occupation—began moving to the coast between 1200 and 700 BP to establish traditional (stonework) villages. Archaeological evidence from Orrak shows that the decline of fishing is temporally staggered, with the first dramatic decline (an order of magnitude) in fish observed between phases 1 (approximately 1400 to 1240 BP) and 2 (approximately 1290 to 720 BP) (29) that roughly coincides with this movement of settlements to the coast. As noted previously, people began investing more time in taro cultivation in this earlier phase, which decreased fishing opportunities. Protein shortfalls would have been made up by an increase in mollusk foraging, which explains why evidence of resource depression at Orrak is absent despite the decline in fish abundance.
Historically, Palauans have subscribed to a sacred and traditional form of conservation management known as bul, in which the council of chiefs (klobak) enact official moratoria on resource use for both land and sea (40, 73, 74). These restrictions cover hunting, fishing, collecting betelnut, cutting of timber for fuel and construction, and many other activities in specific areas to ensure that resources can be harvested when needed; this also includes seasonal restrictions (e.g., harvesting certain species of nearshore fish only during stormier months when offshore fishing is too dangerous). Is it possible that this formalized conservation ethic developed out of the need to aggregate in coastal villages and protect the spectrum of resource use in which Palauans have depended on so heavily for millennia?
In conjunction with bul conservation tactics—and having integrated these villages adjacent to, but behind mangrove forests (perhaps as both a buffer for storm surges, a source of food and fuel, and a defensive measure)—the coastal positioning of these villages appears to have been an effective way to maximize caloric return by managing soil runoff through a unique and integrated agricultural system that included the placement of taro gardens in anthropogenically constructed swampy areas situated in lowland areas upstream from mangroves. This strategy is in contrast to other island groups in Micronesia where taro is usually cultivated in well-drained ditches or mixed gardens and which lack the diverse combination of island types, barrier reef system, and associated marine ecotones seen in Palau (72).
Lesser Antilles.
Overall, paleoenvironmental data for the Lesser Antilles reveal trends for escalating intensity of land use, terrestrial and marine habitat change, and fisheries declines in association with human settlement of the region. Here, we focus on the role of humans as active mediators of terrestrial–marine feedback systems between approximately 2500 and 500 BP, during the Ceramic Age—the most archaeologically well-evidenced period in Caribbean prehistory—but also consider anthropogenic effects in the several centuries after European contact.
Collective data from 146 zooarchaeological assemblages on 13 Lesser Antillean islands, Puerto Rico, and St. Thomas exhibit reductions over time in the average weight, size, trophic level, and catch biomass of reef fish, such as snappers (Lutjanidae) and parrotfish (Labridae: Scarinae) (41, 43, 63, 64). Osteometric data show the mean size of fish from combined mangrove, sea grass, coral reef, and deep-water pelagic habitats decreases after approximately 1500 to 500 BP and again during the Colonial period (17th to 19th centuries) (43). These trends are consistent with the effects of growth overfishing driven by human predation pressure and the modern phenomenon of “fishing down marine food webs” (75) as higher-yield fish become depleted.
As detailed below, zooarchaeological, proxy sediment core, and human isotopic data suggest islanders intensified terrestrial resource use in various ways after approximately 1500 BP, possibly to offset diminished marine subsistence. One means to recapture lost protein may have been through the captive management of introduced species (60, 65). Although unclear for the Lesser Antilles, animal management is indicated post-900 BP for the Bahama Archipelago to the north based on isotopic and morphometric studies of hutia (Echimyidae: Capropmyinae) (76). Intensification of wild protein sources is evident on Carriacou and Nevis where collection of low-yield, rocky littoral nerite snails (Nerita spp.) increased significantly after approximately 1200 to 1000 BP, although based on zooarchaeological data this did not depress snail populations (SI Appendix, Fig. S1N) (61, 62).
Increased food production may have also occurred on islands where soil and water conditions made this viable. Paleoenvironmental cores from Grenada (67, 77), Barbados (78), Marie-Galante (79), and Antigua (80) exhibit spikes after approximately 1400 BP in charcoal and fossil pollen markers for open habitat and disturbance that are indicative of landscape burning, population growth, and intensified land management associated with food production. Increased sedimentation rates are also evident in cores, although in some cases the onset cannot be conclusively assigned to the pre- versus postcontact period. Agricultural intensification is also apparent in the Bahamas, where δ13C and δ15N isotopic studies of diet in human skeletons reveal increased reliance on root crops between about 1000 and 400 y ago, following disappearances and declines of prey taxa in the zooarchaeological record (66).
Mangroves and coral reefs are sensitive to runoff from land disturbance, which can contribute to mortality through processes of sedimentation and eutrophication (46, 81). In the southwestern Caribbean, significant mangrove deforestation is recorded after 1850 in core sediments from San Andrés, linked to clearance for extensive coconut cultivation (82). Across different catchments and islands in the Lesser Antilles, paleoenvironmental cores exhibit varying mangrove responses to the land clearance noted above, including contraction, expansion, and stability (42). This response variability is likely due to complex interactions between species-specific tolerances and geomorphology, sea-level, tidal flow, aggradation, sediment properties, and salinity (46, 47). Sedimentation, for example, may expand substrate available for mangrove propagule colonization, but overly rapid vertical accretion can smother pneumatophores causing mortality (46). In addition, fuelwood collection may have contributed to prehistoric mangrove deforestation (41), as it did historically (83).
The pre-20th century extent of Caribbean mangroves is not well known, making overall post-2500 BP losses challenging to assess (83). Recent impacts are more firmly established, with mangrove cover declining by an estimated 24% between 1980 and 2005 due primarily to anthropogenic causes (84). Importantly, regional- and island-scale analyses demonstrate correspondence between modern Caribbean mangrove expanse and the density and abundance on adjacent coral reefs of fish that depend on mangrove habitat in juvenile stages (49, 50, 85). This relationship indicates a mechanism by which instances of premodern mangrove loss could have operated jointly with overfishing to alter fish abundance and community structure.
There are currently limited data on (pre)historic Lesser Antillean reef health that could be used to assess the direct impact of land runoff prior to the modern era. Reef cores from Panama’s Caribbean coast, however, reveal the potential effects of preindustrial runoff (70). Here, declining coral reef accretion beginning in the 18th century is associated with increases in infaunal bivalves and declines in foraminifera indicators for high water clarity, suggesting the loss of hard substrate and an increase in suspended solids. These effects are attributed to terrestrial runoff from a nearby site of long-term, intensive agriculture.
While not tested archaeologically, it is surmisable that (pre)historic mangrove and coral reef loss, where this occurred, would have created a positive feedback effect in which reduced fish recruitment and habitat availability diminished fishing success, driving further agricultural intensification, forest clearance, and terrestrial runoff (Fig. 3B). These effects may have compounded the existing overfishing of some islands.
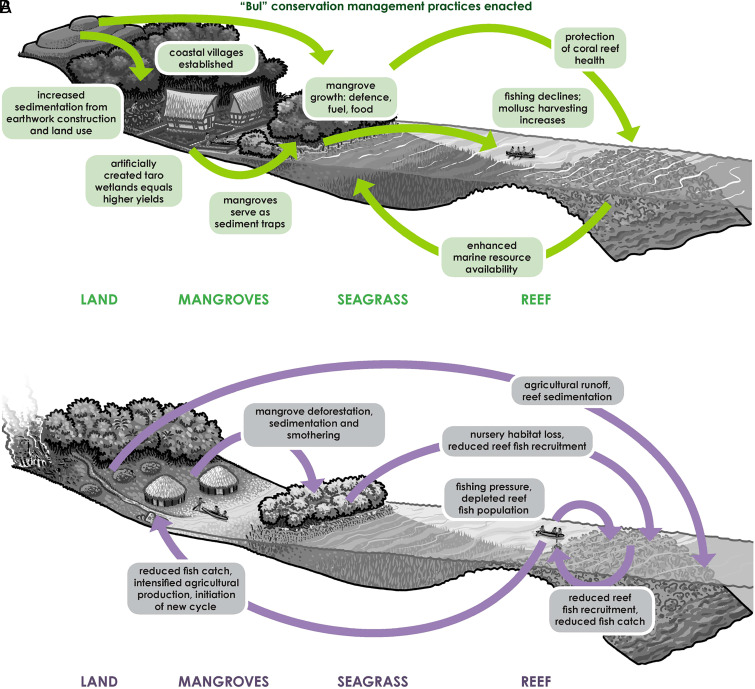
Fig. 3.
Synthesis of ridge-to-reef feedback loops. (A) In Palau (from left to right): land use activities that begin approximately 3000 BP, and intensify between approximately 2400 to 1200 BP through earthwork construction, lead to erosion and sedimentation infill of lagoons and coral reefs. Dependence on marine resources for protein is threatened and creates a positive feedback loop. Movement to the coast and construction of traditional (stonework) villages situated behind mangrove forests along with artificially built swamps for taro cultivation mitigate these effects, providing inland sediment traps and sustainable subsistence practices which are also more easily defendable. The implementation of bul conservation practices—perhaps in recognition of these issues—provide the cultural framework for ensuring responsible resource use over the long term. (B) In the Lesser Antilles (from left to right): burning and land clearance for agriculture that begins approximately 2500 BP leads to increased terrestrial runoff and sedimentation of mangrove and coral reef habitats, depressing recruitment of economically important fish and invertebrates. Positive feedback leads to intensification of terrestrial resources after approximately 1500 BP to offset loss of marine protein; land clearance for agriculture increases, accelerating sediment and nutrient runoff, contributing to further declines in reef and mangrove habitat and biota. This process is interrupted by negative feedback, where limited arable land and fresh water inhibit potential agricultural intensification and population growth. Sustainable marine resource extraction may be realized in these locations through a combination of fisheries management strategies, low population density, and the relative productivity of surrounding marine banks. Image credit: John Gordon Swogger (artist).
Parrotfish may have played a key role in this feedback system. The density of parrotfish, which are significant grazers of coral algae, is positively linked to hard coral cover, and phase shifts from Acropora coral to macroalgal dominance in Caribbean reefs over the last four decades are attributed to their overfishing (81, 86). Notably, several studies (68–70) found these shifts were underway in the wider Caribbean at least two to three decades prior to the headline-grabbing coral disease outbreaks and bleaching events of the 1980s and 1990s. Sediment and subfossil core data further indicate parrotfish populations and acroporid coral accretion were anthropogenically depressed by the mid-18th to mid-19th centuries in Caribbean Panama (68, 70). Archaeologically, parrotfish are among the most heavily exploited fish in prehistory (41, 43) and consistently among the taxa which exhibit fishing-induced body size declines in the Lesser Antilles (64). Top-down (human fishing) and bottom-up (anthropogenic reduction of mangroves) control over parrotfish abundance may have impacted coral reef health in deeper antiquity, with the human compensatory response to intensify agricultural production amplifying this effect (Fig. 3B).
Each link of this feedback scenario (i.e., overfishing, parrotfish decline due to mangrove reduction, etc.) is individually evidenced for the Caribbean at different locations across the region. Demonstrating a continuous sequence of cause and effect in the past at a specific location, however, has not yet been possible because, as far as we are aware, no single locale has been assessed for all the relevant data. The positive feedback system described here need not have occurred on all islands in the past, but empirically determining its extent requires diachronic, catchment-specific archaeological, zooarchaeological, sedimentological, palynological, and paleoreef records.
A relevant caveat is that patterns for prehistoric overfishing in the region are not universal. Studies of taxonomic composition, body size, trophic structure, abundance-biomass comparisons, and habitat representation also demonstrate sustainability or negative evidence for overfishing at sites on Anguilla (44, 87), Basse-Terre (60), and Grand-Terre (56) in Guadeloupe, and Carriacou (61) in the Grenadines. Along with similar findings for mollusks at several sites (61, 62) these data offer a cautionary reminder that despite their ecological fragility, detrimental anthropogenic effects are not inevitable for islands. The basis for the common finding of fisheries sustainability among these sites remains unclear, although for Anguilla (91 km2) and Carriacou (34 km2) it may relate to checks on the potential for population growth and agricultural intensification due to limited land area, thin soils (Anguilla), and the absence of surface freshwater (i.e., negative feedback), combined with the high marine productivity of the surrounding Anguilla and Grenadines Banks. Morisita-Horn and corrected Forbes indices of similarity between the islands’ zooarchaeological assemblages demonstrate strong correspondences in taxonomic composition and abundance despite approximately 800 km of geographic separation and differing marine habitat structure (88), raising the possibility that strategic human decisions about which taxa to target and at what intensity to fish these may also underlie sustainable prehistoric exploitation.
Discussion and Conclusions
Archaeological, paleoecological, historical, and modern research on landscape use in the archipelagoes of Palau and the Lesser Antilles provide important insights into the nature of human–environmental interactions over the course of several millennia. Because islands serve as useful model systems for examining the impact that humans may have had on novel landscapes (1, 2, 5), they can more readily capture the ways in which populations have adapted to, modified, and responded to natural and cultural processes. Through time, various socio-environmental feedback loops have prompted changes in human behavior—both positive and negative—which appear to have influenced how resources were extracted, managed, and cultivated (Fig. 3).
For Palau, there is some evidence of potential overharvesting of marine resources (fish, mollusks) in the southern Rock Islands over the last 2,000 to 3,000 y. However, at Orrak, which lies along the southeastern margin of Babeldaob, escalating exploitation of at least one species—the humpbacked conch—led to the species becoming larger over time, contra expectations for an overharvested population, and is associated with sustainable fishing (30, 61). Over this same period, peoples in Palau began moving from inland areas and ceasing construction and use of the extensive network of earthworks and turning to intensively settling the coastal margins between approximately 1200 and 700 BP (19). The development of these elaborate traditional (stonework) villages throughout the archipelago point to territorial circumscription, the consolidation of power, and the need to feed a growing population. Given evidence for increased erosion, sedimentation, and changing flora regimes in the interior of Babeldaob, coupled with heavy reliance on marine resources for subsistence and other purposes, it is quite possible that the placement of these villages conterminous with mangroves and artificially created wetland swamps for growing taro, were instrumental in maintaining coral reef health and preserving resources critical for their long-term survival. These efforts may have been the impetus for the eventual formation of bul conservation management practices that have been a fundamental part of Palauan lifeways for centuries (Fig. 3A).
In the Lesser Antilles, the sum evidence indicates prehistoric and historic precursors for today’s diminished terrestrial and marine ecosystems, including fisheries and coral reef declines, altered forest cover, and increased land runoff. While lesser in magnitude than modern impacts, these earlier effects may have nonetheless initiated a “slow burn” of ecosystem resilience in the Lesser Antilles, fueled in the last few centuries by the escalating stressors that have culminated in dramatic ecological change, like recent mass coral mortality. In the humanized islands of the past, marine and terrestrial systems were linked through feedback mechanisms mediated by the intensity of human fishing pressure and food production. Evidence for sustainable fishing and mollusk collection on some islands suggests the outcomes of human settlement were contingent on both local environmental conditions and human behavior, with the possibility that the latter could involve conscious and strategic actions to safeguard long-term resource availability (Fig. 3B). As in the case of Palau, above, the potential enactment of practices aimed specifically at sustaining resources recognizes the agency of past humans and their ability to respond to changing environmental conditions based on traditional ecological knowledge accrued over generations. In the Caribbean, European colonization after AD 1492 catalyzed rapid Indigenous depopulation and social disruption (89), limiting opportunities for traditional practices to be chronicled before they were affected by colonial institutions. Assessment of precontact resource management and conservation intent must thus be based almost wholly on archaeological and paleoenvironmental data (e.g., refs. 90 and 91). In contrast, Palau’s well-documented history of harnessing traditional ecological knowledge in the form of bul and other resource management practices—coupled with its relatively late contact with Europeans at the end of the 18th century—likely facilitated the perpetuation of these cultural behaviors, which are still used today. The Palauan government, in fact, is considered a world leader in marine conservation efforts that have included, among other things, the establishment of the 70 (Ngerukewid) Islands Wildlife Preserve (1956), the world’s first shark sanctuary (2009), the “Palau Pledge” (2017) that requires visitors to sign a statement upon entry to be sensitive and act responsibly to Palau’s culture and environment, and the recent Palau National Marine Sanctuary (2020), covering 80% of Palau’s national waters, in which all extractive activities (e.g., mining, fishing) are now prohibited.
Taken together, the Palauan and Lesser Antillean cases suggest past anthropogenic impacts were heterogeneous: both sustainable and unsustainable. This finding is significant for highlighting both the complexities of coupled human–environment systems on islands and the importance of considering local conditions across terrestrial and marine gradients to understand these complexities. Coastal zones are not boundaries, but interfaces between land and sea. Because they and the human societies they support belong wholly to neither, holistic, diachronic treatment of marine and terrestrial socio-ecological phenomena may better reflect historical reality for the majority of the world’s islands and coasts. As demonstrated here, a ridge-to-reef approach that transcends ecotones can provide a more robust context for understanding instances where prehistoric human activity did not result in the negative impacts observed in similar contexts and whether apparent sustainable practices may be rooted in intimate traditional ecological knowledge of the whole environment. More broadly, by acknowledging the interconnectedness of marine and terrestrial systems and integrating effects operating at different temporal and spatial scales, a ridge-to-reef perspective may better capture the complexities of direct and indirect consequences, offering more powerful explanations of socio-ecological change and the role of humans in these processes.
Equifinality and the challenges of demonstrating causal relationships in prehistoric and historic feedback systems mean that for both Palau and the Lesser Antilles, future scholarship should seek to build high-resolution, diachronic records for human land use, marine resource exploitation, and mangrove and reef dynamics at local scales that show direct cause and effect. Using a model-systems approach provides the framework for doing so, but achieving this will require integrative collaboration across multiple disciplines. Local data may then be marshalled for region-wide meta-analyses that establish the scope and magnitude of past anthropogenic influences, their relationship to modern phenomena, and the potential relevance of traditional sustainability solutions to the present.
Supplementary Material
Acknowledgments
We thank Patrick Roberts for the kind invitation to contribute to this special issue; Evan Levine who helped draft the maps in Fig. 1; John Swogger, who drafted Figs. 2 and 3, and the Editor and three reviewers who all provided useful comments on earlier drafts of this paper.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. R.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022209118/-/DCSupplemental.
Data Availability
All study data are included in main text and SI Appendix.
References
- 1.Vitousek P. M., Oceanic islands as model systems for ecological studies. J. Biogeogr. 29, 573–582 (2002). [Google Scholar]
- 2.Kirch P. V., Hawaii as a model system for human ecodynamics. Am. Anth. 109, 8–26 (2007). [Google Scholar]
- 3.Fitzpatrick S. M., Erlandson J. M., Island archaeology, model systems, the Anthropocene, and how the past informs the future. J. Island Coast. Archaeol. 13, 283–299 (2018). [Google Scholar]
- 4.Terrell J. E., Metaphor and theory in island archaeology. J. Island Coast. Archaeol, 10.1080/15564894.2020.1830892 (2020). [DOI] [Google Scholar]
- 5.Leppard T. P., et al., The premise and potential of model-based approaches to island archaeology: A response to Terrell. J. Island Coast. Archaeol., 10.1080/15564894.2021.1904463 (2021). [DOI] [Google Scholar]
- 6.Braje T., et al., Archaeology, historical ecology and anthropogenic island ecosystems. Environ. Conserv. 44, 286–297 (2017). [Google Scholar]
- 7.Norder S. J., et al., Global change in microcosms: Environmental and societal predictors of land cover change on the Atlantic Ocean Islands. Anthropocene 30, 100242 (2020). [Google Scholar]
- 8.Rick T. C., Kirch P. V., Erlandson J. M., Fitzpatrick S. M., Archeology, deep history, and the human transformation of island ecosystems. Anthropocene 4, 33–45 (2013). [Google Scholar]
- 9.Nogué S., et al., The human dimension of biodiversity changes on islands. Science 372, 488–491 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Ellis E. C., et al., People have shaped most of terrestrial nature for at least 12,000 years. Proc. Natl. Acad. Sci. U.S.A. 118, e2023483118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long J. L., Introduced Mammals of the World: Their History, Distribution, and Influence (CSIRO, 2003). [Google Scholar]
- 12.Stephens L., et al., Archaeological assessment reveals Earth’s early transformation through land use. Science 365, 897–902 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Kirch P. V., Transported landscapes. Nat. Hist. 91, 32–35 (1982). [Google Scholar]
- 14.Fitzpatrick S. M., Jew N. P., Radiocarbon dating and Bayesian modelling of one of Remote Oceania’s oldest cemeteries at Chelechol ra Orrak, Palau. Antiquity 92, 149–164 (2018). [Google Scholar]
- 15.Napolitano M. F., et al., Reevaluating human colonization of the Caribbean using chronometric hygiene and Bayesian modeling. Sci. Adv. 5, eaar7806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costion C. M., et al., Using the ancient past for establishing current threat in poorly inventoried regions. Biol. Conserv. 147, 153–162 (2012). [Google Scholar]
- 17.Colin P. L., Marine Environments of Palau (Coral Reef Research Foundation, Koror, 2009). [Google Scholar]
- 18.Kayanne H., et al., Eds., Coral Reefs of Palau (Palau International Coral Reef Center, 2007). [Google Scholar]
- 19.Liston J., Cultural chronology of earthworks in Palau, western Micronesia. Archaeol. Ocean. 44, 56–73 (2009). [Google Scholar]
- 20.Clark G. R., Anderson A., Wright D., Human colonization of the Palau Islands, western Micronesia. J. Island Coast. Archaeol. 1, 215–232 (2006). [Google Scholar]
- 21.Stone J. H., Fitzpatrick S. M., Krigbaum J., Stable isotope analysis of human diet at Chelechol ra Orrak, Palau. Bioarchaeol. Intl. 3, 142–156 (2019). [Google Scholar]
- 22.Carson M. T., Peopling of Oceania: Clarifying an initial settlement horizon in the Mariana Islands at 1500 BC. Radiocarbon 62, 1733–1754 (2020). [Google Scholar]
- 23.Rieth T. M., Athens J. S., Late Holocene human expansion into Near and Remote Oceania: A Bayesian model of the chronologies of the Mariana Islands and Bismarck Archipelago. J. Island Coast. Archaeol. 14, 5–16 (2019). [Google Scholar]
- 24.Bedford S., Spriggs M., Eds., Debating Lapita: Distribution, Chronology, Society and Subsistence (ANU Press, Canberra, 2019), vol. 52. [Google Scholar]
- 25.Clark G. R., A 3000-year culture sequence from Palau, western Micronesia. Asian Perspect. 44, 349–380 (2005). [Google Scholar]
- 26.Liston J., Tuggle H. D., “Prehistoric warfare in Palau” in The Archaeology of Warfare: Prehistories of Raiding and Conquest, Arkush E., Allen M. W., Eds. (University Press of Florida, 2006), pp. 148–183. [Google Scholar]
- 27.Krämer A., Results of the South Seas Expedition, 1908-1910. II. Ethnography: B: Micronesia (Hamburg Scientific Foundation, L. Friederichsen & Co, 1917). [Google Scholar]
- 28.Athens J. S., Ward J. V., Compact Road Archaeological Investigations, Babeldaob Island. Palau. Volume IV: The Holocene Paleoenvironment of Palau. (Drafts 1998, 1999). Prepared for U.S. Army Corps of Engineers, Pacific Ocean Division, Hawai'i (International Archaeological Research Institute, Inc., Honolulu, 2005). [Google Scholar]
- 29.Fitzpatrick S. M., Giovas C. M., Kataoka O., Temporal trends in prehistoric fishing in Palau, Micronesia over the last 1500 years. Archaeol. Ocean. 46, 6–16 (2011). [Google Scholar]
- 30.Giovas C. M., Fitzpatrick S. M., Clark M., Abed M., Evidence for size increase in an exploited mollusc: Humped conch (Strombus gibberulus) at Chelechol ra Orrak, Palau from ca. 3000-0 BP. J. Archaeol. Sci. 37, 2788–2798 (2010). [Google Scholar]
- 31.Giovas C. M., Fitzpatrick S. M., Kataoka O., Clark M., Prey body size and anthropogenic resource depression: The decline of prehistoric fishing at Chelechol ra Orrak, Palau. J. Anthropol. Archaeol. 41, 132–146 (2016). [Google Scholar]
- 32.Ono R., Clark G., A 2500‐year record of marine resource use on Ulong Island, Republic of Palau. Int. J. Osteoarchaeol. 22, 637–654 (2012). [Google Scholar]
- 33.Carucci J., “Cultural and natural patterning in prehistoric marine foodshell from Palau, Micronesia,” PhD dissertation. University of Southern Illinois, Carbondale, IL. (1993).
- 34.Masse W. B., “The Archaeology and Ecology of Fishing in the Belau islands, Micronesia”, PhD dissertation, University of Southern Illinois, Carbondale, IL. (1989).
- 35.Dickinson W. R., Athens J. S., Holocene paleoshoreline and paleoenvironmental history of Palau: Implications for human settlement. J. Island Coast. Archaeol. 2, 175–196 (2007). [Google Scholar]
- 36.Golbuu Y., et al., Effects of land-use change on characteristics and dynamics of watershed discharges in Babeldaob, Palau, Micronesia. J. Mar. Biol. 2011, 1–17 (2011). [Google Scholar]
- 37.Povak N. A., et al., A decision support tool for the conservation of tropical forest and nearshore environments on Babeldaob Island, Palau. For. Ecol. Manage. 476, 118480 (2020). [Google Scholar]
- 38.Bejarano S., Golbuu Y., Sapolu T., Mumby P. J., Ecological risk and the exploitation of herbivorous reef fish across Micronesia. Mar. Ecol. Prog. Ser. 482, 197–215 (2013). [Google Scholar]
- 39.Immordino F., et al., Application of Sentinel-2 multispectral data for habitat mapping of Pacific Islands: Palau Republic (Micronesia, Pacific Ocean). J. Mar. Sci. Eng. 7, 1–16 (2019). [Google Scholar]
- 40.Johannes R., Words of the Lagoon: Fishing and Marine Lore in the Palau District of Micronesia (University of California Press, Los Angeles, 1981). [Google Scholar]
- 41.Newsom L. A., Wing E. S., On Land and Sea: Native American Uses of Biological Resources in the West Indies (University of Alabama Press, Tuscaloosa, 2004). [Google Scholar]
- 42.Siegel P. E., Ed., Island Historical Ecology: Socionatural Landscapes of the Eastern and Southern Caribbean (Berghahn Books, New York, 2018). [Google Scholar]
- 43.Grouard S., et al., Size estimation of pre‐Columbian Caribbean fish. Int. J. Osteoarchaeol. 29, 452–468 (2019). [Google Scholar]
- 44.Carder N., Reitz E. J., Crock J. G., Fish communities and populations during the post-Saladoid period (AD 600/800–1500), Anguilla, Lesser Antilles. J. Archaeol. Sci. 34, 588–599 (2007). [Google Scholar]
- 45.Hoggarth D., Management Plan for the Marine Parks of Anguilla (Organisation of Eastern Caribbean States Natural Resources Management Unit, St. Lucia, 2001). [Google Scholar]
- 46.Ellison J. C., Impacts of sediment burial on mangroves. Mar. Pollut. Bull. 37, 420–426 (1999). [Google Scholar]
- 47.Imbert D., Rousteau A., Scherrer P., Ecology of mangrove growth and recovery in the Lesser Antilles: State of knowledge and basis for restoration projects. Restor. Ecol. 8, 230–236 (2000). [Google Scholar]
- 48.Mumby P. J., et al., Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427, 533–536 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Nagelkerken I., et al., Dependence of Caribbean reef fishes on mangroves and seagrass beds as nursery habitats: A comparison of fish faunas between bays with and without mangroves/seagrass beds. Mar. Ecol. Prog. Ser. 214, 225–235 (2001). [Google Scholar]
- 50.Nagelkerken I., et al., How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale. Mar. Ecol. Prog. Ser. 244, 299–305 (2002). [Google Scholar]
- 51.Hofman C. L., Hoogland M. L., Plum piece: Evidence for Archaic seasonal occupation on Saba, northern lesser Antilles around 3300 BP. J. Caribbean Archaeol. 4, 12–27 (2003). [Google Scholar]
- 52.Pagán-Jiménez J. R., “Human–plant dynamics in the precolonial Antilles” in The Oxford Handbook of Caribbean Archaeology, Keegan W. F., Hofman C. L., Rodríguez Ramos R., Eds. (Oxford University Press, 2013), pp. 391–406. [Google Scholar]
- 53.Fernandes D. M., et al., A genetic history of the pre-contact Caribbean. Nature 590, 103–110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bérard B., “The Saladoid” in The Oxford Handbook of Caribbean Archaeology, Keegan W., Hofman C., Rodríguez Ramos R., Eds. (Oxford University Press, 2013), pp. 184–197. [Google Scholar]
- 55.Grouard S., Faunal remains associated with Late Saladoïd and Post-Saladoïd occupations at Anse à la Gourde, Guadeloupe, West Indies: Preliminary results. Archaeofauna 10, 71–98 (2001). [Google Scholar]
- 56.Krigbaum J., Fitzpatrick S. M., Bankaitis J., Human paleodiet at grand bay, Carriacou, Lesser Antilles. J. Island Coast. Archaeol. 8, 210–227 (2013). [Google Scholar]
- 57.Grouard S., “Chasses, pêches et captures des faunes vertébrées et crustacées des occupa-tions côtieres céramiques récentes du sud dela Martinique (Saladoïde récent, Vè siècle ap. J.-C.—Suazoïde récent XVè ap. J.C.)” in Martinique, Terre Amérindienne: Une Approche Pluridisciplinaire, Bérard B., Ed. (Sidestone Press, 2013), pp. 115–161. [Google Scholar]
- 58.LeFebvre M. J., Zooarchaeological analysis of prehistoric vertebrate exploitation at the Grand Bay Site, Carriacou, West Indies. Coral Reefs 26, 931–944 (2007). [Google Scholar]
- 59.Serrand N., Bonnissent D., Interacting Pre-Columbian Amerindian societies and environments: Insights from five millennia of archaeological invertebrate record on the Saint-Martin Island (French Lesser Antilles). Environ. Archaeol. 26, 99–114 (2021). [Google Scholar]
- 60.Bochaton C., et al., The pre-Columbian site of Roseau (Guadeloupe, FWI): Intra-site chronological variability of the subsistence strategies in a Late Ceramic archeological vertebrate assemblage. Archaeol. Anthropol. Sci. 13, 1–17 (2021). [Google Scholar]
- 61.Giovas C. M., Though she be but little: Resource resilience, Amerindian foraging, and long-term adaptive strategies in the Grenadines, West Indies. J. Island Coast. Archaeol. 11, 238–2635 (2016). [Google Scholar]
- 62.Giovas C. M., Clark M., Fitzpatrick S. M., Stone J., Intensifying collection and size increase of the tessellated nerite snail (Nerita tessellata) at the Coconut Walk site, Nevis, northern Lesser Antilles, AD 890–1440. J. Archaeol. Sci. 40, 4024–4038 (2013). [Google Scholar]
- 63.Wing E. S., The sustainability of resources used by Native Americans on four Caribbean islands. Int. J. Osteoarchaeol. 11, 14–23 (2001). [Google Scholar]
- 64.Wing E. S., Wing S. R., Prehistoric fisheries in the Caribbean. Coral Reefs 20, 1–8 (2001). [Google Scholar]
- 65.Giovas C. M., The beasts at large—Perennial questions and new paradigms for Caribbean translocation research. Part I: Ethnozoogeography of mammals. Environ. Archaeol. 24, 182–198 (2019). [Google Scholar]
- 66.Schulting R. J., et al., Six centuries of adaptation to a challenging island environment: AMS 14C dating and stable isotopic analysis of pre-Columbian human remains from the Bahamian archipelago reveal dietary trends. Quat. Sci. Rev. 254, 106780 (2021). [Google Scholar]
- 67.Benz E. J., “A paleoenvironmental reconstruction from the Island of Grenada, Caribbean environments during the time of human occupation,” PhD dissertation, Washington State University, Pullman, WA (2010).
- 68.Cramer K. L., O’Dea A., Clark T. R., Zhao J. X., Norris R. D., Prehistorical and historical declines in Caribbean coral reef accretion rates driven by loss of parrotfish. Nat. Commun. 8, 14160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cramer K. L., et al., Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv. 6, eaax9395 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cramer K. L., O’Dea A., Leonard‐Pingel J. S., Norris R. D., Millennial‐scale change in the structure of a Caribbean reef ecosystem and the role of human and natural disturbance. Ecography 43, 283–293 (2020). [Google Scholar]
- 71.Victor S., et al., Fine sediment trapping in two mangrove-fringed estuaries exposed to contrasting land-use intensity, Palau, Micronesia. Wetlands Ecol. Manage. 12, 277–283 (2004). [Google Scholar]
- 72.Koshiba S., et al., Palau’s taro fields and mangroves protect the coral reefs by trapping eroded fine sediment. Wetlands Ecol. Manage. 21, 157–164 (2013). [Google Scholar]
- 73.Friedlander A. M., et al., Size, age, and habitat determine effectiveness of Palau’s marine protected areas. PLoS One 12, e0174787 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pilbeam V., van Kerkhoff L., Weir T., Conservation decision-making in Palau: An example of the parallel working of scientific and traditional ecological knowledge. Environ. Manage. 64, 564–579 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Pauly D., Christensen V., Dalsgaard J., Froese R., Torres F. Jr, Fishing down marine food webs. Science 279, 860–863 (1998). [DOI] [PubMed] [Google Scholar]
- 76.LeFebvre M. J., deFrance S. D., Kamenov G. D., Keegan W. F., Krigbaum J., The zooarchaeology and isotopic ecology of the Bahamian hutia (Geocapromys ingrahami): Evidence for pre-Columbian anthropogenic management. PLoS One 14, e0220284 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones J. G., et al., “Grenada” in Island Historical Ecology: Sociocultural Landscapes of the Eastern and Southern Caribbean, Siegel P. E., Ed. (Berghahn Books, 2018), pp. 129–154. [Google Scholar]
- 78.DiNapoli R. J., Leppard T. P., Islands as model environments. J. Island Coast. Archaeol. 13, 157–160 (2018). [Google Scholar]
- 79.Jones J. G., Dunning N. P., Pearsall D. M., Siegel P. E., “Marie-Galante” in Island Historical Ecology: Sociocultural Landscapes of the Eastern and Southern Caribbean, Siegel P. E., Ed. (Berghahn Books, 2018), pp. 226–238. [Google Scholar]
- 80.Jones J. G., et al., “Antigua” in Island Historical Ecology: Sociocultural Landscapes of the Eastern and Southern Caribbean, Siegel P. E., Ed. (Berghahn Books, 2018), pp. 239–269. [Google Scholar]
- 81.Jackson J. B. C., Donovan M. K., Cramer K. L., Lam V. V., Eds., Status and Trends of Caribbean Coral Reefs: 1970-2012 (Global Coral Reef Monitoring Network, IUCN, 2014). [Google Scholar]
- 82.González C., et al., Mangrove dynamics in the southwestern Caribbean since the ‘Little Ice Age’: A history of human and natural disturbances. Holocene 20, 849–861 (2010). [Google Scholar]
- 83.Ellison A. M., Farnsworth E. J., Anthropogenic disturbance of Caribbean mangrove ecosystems: Past impacts, present trends, and future predictions. Biotropica 28, 549–565 (1996). [Google Scholar]
- 84.Polidoro B. A., et al., The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS One 5, e10095 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Serafy J. E., Shideler G. S., Araújo R. J., Nagelkerken I., Mangroves enhance reef fish abundance at the Caribbean regional scale. PLoS One 10, e0142022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mumby P. J., Herbivory versus corallivory: Are parrotfish good or bad for Caribbean coral reefs? Coral Reefs 28, 683–690 (2009). [Google Scholar]
- 87.Carder N., Crock J. G., A pre-Columbian fisheries baseline from the Caribbean. J. Archaeol. Sci. 39, 3115–3124 (2012). [Google Scholar]
- 88.Giovas C. M., A simple method for quantifying compositional correspondence between zooarchaeological assemblages using paired similarity indices. J. Archaeol. Method Theory 10.1007/s10816-021-09512-y (2021). [DOI] [Google Scholar]
- 89.Koch A., Brierley C., Maslin M. M., Lewis S. L., Earth system impacts of the European arrival and Great Dying in the Americas after 1492. Quat. Sci. Rev. 207, 13–36 (2019). [Google Scholar]
- 90.Rivera-Collazo I. C., Sánchez-Morales L. M., “Domesticating the island: Anthropogenic soils and landform modification as components of subsistence-resource acquisition strategies in Puerto Rico” in The Archaeology of Caribbean and Circum-Caribbean Farmers (6000 BC-AD 1500), Reid B. A., Ed. (Routledge, 2018), pp. 252–272. [Google Scholar]
- 91.Masse W. B., Liston J., Carucci J., Athens J. S., Evaluating the effects of climate change on environment, resource depletion, and culture in the Palau Islands between AD 1200 and 1600. Quat. Int. 151, 106–132 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in main text and SI Appendix.