The infiltration of autoreactive encephalitogenic adaptive immune cells into the central nervous system (CNS) and pursuant inflammation of the brain and spinal cord are thought to be critical events in initiating and perpetuating disease activity in the human autoimmune disorder multiple sclerosis (MS) (1). Immune cell trafficking into the CNS is regulated by numerous soluble molecules and adhesion molecules (2), and α4-integrin has been identified as being crucial in that regard (3, 4). While there is an ongoing debate on whether or not MS is initially triggered from within the CNS compartment (“inside → out” dogma), most scientific data suggest a breakdown of immune tolerance to CNS autoantigens that lead to the activation and differentiation of encephalitogenic autoimmune lymphocytes (“outside → in” dogma) (1). A pivotal immunological experiment that corroborates the “outside → in” pathogenic dogma of MS is the effectiveness of natalizumab, a humanized monoclonal antibody (mAb) that prevents the interaction of α4-integrin on leukocytes with its ligands on the blood–brain barrier (BBB) and within the extracellular matrix (4), namely vascular adhesion molecule (VCAM)-1 and fibronectin. Natalizumab substantially reduces the migration of leukocytes into the CNS (4, 5) and significantly reduces clinical and paraclinical disease activity in patients with relapsing forms of MS (6, 7).
Recently, Fleischer et al. proposed that choroid plexus (ChP), highly vascularized organoids situated within the brain ventricles at the intersection of the systemic blood circulation and the cerebrospinal fluid (CSF) compartment, may be one anatomical area to serve as a surrogate marker for MS disease activity (8). In their study, Fleischer et al. show that MRI volumetric assessments depict a significant enlargement of ChP among patients with relapsing remitting MS (RRMS) compared to healthy controls. Interestingly, there was a positive correlation between ChP volume and disease severity and neurological disability. When patients were evaluated with regard to their burden of tissue signal changes using conventional T1- or T2-weighted MRI images or ChP volumetrics the latter proved to be a more sensitive correlate. Furthermore, the authors show that, despite a positive correlation between disease duration and ChP volume in treatment-naïve patients, treatment with dimethyl fumarate (DMF), which has no known direct effect of leukocyte migration across the BBB (9), did not attenuate ChP volume. Instead, ChP in patients treated with natalizumab did not further enlarge. This observation supports the assumption that ChP volume may be an imaging correlate of immune cell trafficking into the CNS rather than a mere readout of clinical improvement. Consistent with the findings made in MS patients, ChP enlargement positively correlated with longevity and severity in the experimental autoimmune encephalomyelitis (EAE) model of MS. Enlarged ChP in experimental animals exhibited higher cellularity and glial activation along with up-regulation of gene expression pathways related to T cell activation and adhesion.
ChP have not been on the frontline of neurological research (10). Each plexus constitutes an involuted region of the pia-arachnoid and is located within the ventricles. Interstitial fluid (ISF) in the brain exchanges bidirectionally with CSF via the “brain glymphatic,” a recently described glial cell–supported lymphatic system (11). Depending on CSF pressure, brain ISF that originates from the blood flows along glymphatic perivascular spaces into the CSF-containing subarachnoid space, and then via the subarachnoid granulations into the dural venous sinuses or peridural lymphatics. Alternatively, brain ISF may also return to the blood by reverse flow across the BBB. Thus, there are various scenarios in which one can envision the extravasation of immune cells into the brain through one or more ChP during CNS autoimmunity. Leukocytes express integrins and other adhesion molecules (12). As mentioned above, the approved agent natalizumab reduces the access of lymphocytes to the CNS by blocking α4-integrin. Another adhesion molecule, mucosal vascular addressin cell adhesion molecule (MAdCAM)-1, is up-regulated in ChP epithelium during EAE (13) and likely facilitates the entry of leukocyte subsets into this compartment. Interestingly, it was recently demonstrated that MAdCAM-1–deficient (−/−) mice are relatively resistant to actively induced EAE (14). MAdCAM-1 predominantly binds to α4β7-integrin (15).
The observations made by Fleischer et al. (8) are plausible and intriguing, and they lead to many unanswered questions. As stated above, ChP are localized within the ventricular system (Fig. 1). Most MS lesions are also localized around the lateral ventricles and in the brainstem and can be visualized by MR imaging (Fig. 1), suggesting that there are spatial factors that drive them. However, other MS lesions are situated in the cerebral cortex or the spinal cord, sometimes exclusively. Thus, one has to wonder whether there are different mechanisms of leukocyte entry in the latter patients that are independent or less dependent on ChP. Further, do only certain leukocyte subsets use the ChP as an entry point into the brain? Would those differences in brain access explain different MS disease manifestations? The EAE model shows that differential usage of adhesion molecules can lead to anatomically and clinically distinct disease phenotypes. For instance, there is a differential requirement for α4-integrin among CD4+ T helper (Th) cell subsets to enter the CNS during EAE. Rothhammer et al. showed that antibody-mediated inhibition of α4-integrins prevents adoptive transfer EAE by interferon gamma-expressing Th1 cells (16). In contrast, interleukin (IL)-17–expressing Th17 cells were able to enter irrespective of α4-integrin blockade. CD4+ T cell lineage α4-integrin–deficient mice were susceptible to actively induced EAE but displayed an atypical ataxic syndrome with predominantly supraspinal infiltrates of IL-23R+CCR6+CD4+ T cells. Glatigny et al. also demonstrated that selective deletion of α4-integrin in donor CD4+ T cells does not prevent the development of adoptive transfer EAE mediated by Th17 cells (17). Based on these observations and on the selective binding of integrins to specific ligands, one can envision use of disease-modifying therapies (DMT) for MS to be more discriminatory in the future. For instance, whereas MS patients with MS lesions that disseminated throughout the CNS might be treated with natalizumab, patients with lesion that are exclusively located in periventricular areas may be candidates for anti–α4β7-integrin (18) or anti-MAdCAM mAb therapies (19).
Fig. 1.
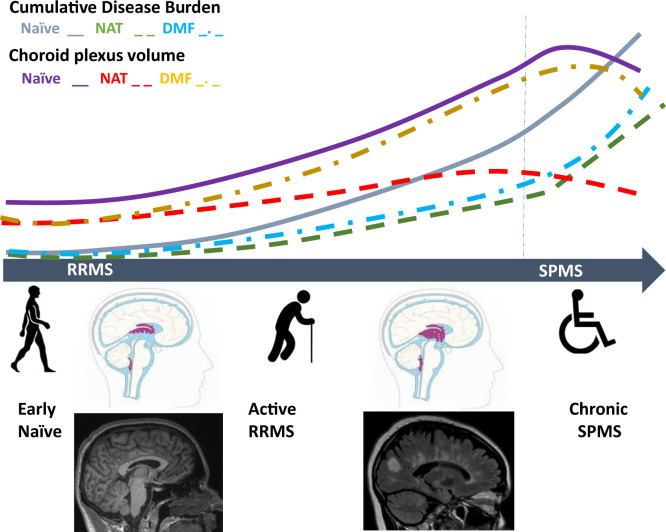
The association of ChP volume with MS cumulative disease burden and treatment responses. The vast majority of patients who are diagnosed with MS have a relapsing remitting disease course. These patients experience clinical relapses and accumulate new lesions on brain MRI, two events that are often termed “disease activity” and, for the purpose of this illustration, “cumulative disease burden.” All currently approved DMT for MS reduce this cumulative disease burden. In the study by Fleischer et al. (8) there was a positive correlation between ChP volume and cumulative disease burden. Furthermore, the authors show that, despite a positive correlation between disease duration and ChP volume in treatment-naïve patients, treatment with DMF was not a predictor for ChP volume attenuation in follow-up assessments, whereas treatment with natalizumab was. Natalizumab is a humanized mAb that prevents the interaction of α4-integrin on leukocytes with its ligands on the BBB and within the extracellular matrix, reducing the adhesion and diapedesis into the brain. In contrast, DMF has no known direct effect of leukocyte migration across the BBB. This observation supports the assumption that ChP volume may be an imaging correlate of immune cell trafficking into the CNS rather than a mere readout of clinical improvement or treatment responses. Eventually, many MS patients no longer have clinical relapses, and they transition to a disease phenotype called SPMS. In patients with SPMS, currently approved DMT are no longer effective, including natalizumab. This suggest that 1) leukocyte traffic into the CNS no longer occurs through the ChP in patients with SPMS, 2) the composition of leukocytes changes at that disease stage, or 3) adhesion molecules other than α4-integrin are utilized for adhesion and diapedesis by those leukocytes in SPMS. This may further suggest that ChP volumetrics would no longer be a good imaging marker in SPMS. Perhaps most importantly, changes in ChP volume dynamics may serve as a marker of transition from RRMS to SPMS, a meaningful biological event for which there is currently no biomarker.
The vast majority of patients who are diagnosed with MS have a relapsing remitting disease course (RRMS), which suggests a dynamic rather than continuous extravasation of immune cells from the blood. These patients experience clinical relapses and accumulate new lesions on brain MRI, two events that are often termed “disease activity.” Currently approved DMT for MS reduce this disease activity. Eventually, many patients no longer have clinical relapses, and they transition to a disease phenotype called secondary progressive MS (SPMS), when they start to accumulate neurological disability more rapidly. In patients with SPMS, currently approved DMT are no longer effective (20), including natalizumab (21). A subset of MS patients never experience clinical relapses, and they are categorized as primary progressive MS (PPMS) patients. In PPMS patients without MRI disease activity, currently approved DMT also do not provide a meaningful benefit (22). Might this suggest that leukocyte traffic into the CNS no longer occurs through the ChP in patients with SPMS or PPMS, or that the composition of leukocytes changes at that disease stage, or that adhesions molecules other than α4-integrin are utilized for adhesion and diapedesis by those leukocytes? Consequently, would the above-mentioned observations on disease course and treatment responses also suggest that ChP volumetrics would no longer be a good imaging marker in SPMS and PPMS? Perhaps most importantly, could changes in ChP volume dynamics serve as a marker of transition from RRMS to SPMS, a meaningful biological event for which there is currently no biomarker?
Future studies should address many of the questions raised above and need to elucidate whether volumetric imaging of ChP can be utilized as a biomarker in clinical trials and in clinical practice. If the promises of findings made by Fleischer et al. (8) hold true, longitudinal assessments of ChP in patients with MS may substantially further our understanding of MS pathogenesis, phenotypes, and therapies.
Acknowledgments
O.S. is funded by a Merit Review grant [federal award document number (FAIN) BX005664-01] from the US Department of Veterans Affairs, Biomedical Laboratory Research and Development.
Footnotes
Competing interest statement: O.S. serves on the editorial boards of Therapeutic Advances in Neurological Disorders; has served on data monitoring committees for Genentech-Roche, Pfizer, Novartis, and TG Therapeutics without monetary compensation; has advised EMD Serono, Celgene, Genentech, TG Therapeutics, and Genzyme; and currently receives grant support from EMD Serono and Exalys.
See companion article, “Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans,” 10.1073/pnas.2025000118.
References
- 1.Milo R., Korczyn A. D., Manouchehri N., Stuve O., The temporal and causal relationship between inflammation and neurodegeneration in multiple sclerosis. Mult. Scler. 26, 876–886 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Ransohoff R. M., Schafer D., Vincent A., Blachère N. E., Bar-Or A., Neuroinflammation: Ways in which the immune system affects the brain. Neurotherapeutics 12, 896–909 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yednock T. A., et al., Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 356, 63–66 (1992). [DOI] [PubMed] [Google Scholar]
- 4.Stüve O., et al., Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann. Neurol. 59, 743–747 (2006). [DOI] [PubMed] [Google Scholar]
- 5.del Pilar Martin M., et al., Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch. Neurol. 65, 1596–1603 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Polman C. H.et al.; AFFIRM Investigators , A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Rudick R. A.et al.; SENTINEL Investigators , Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N. Engl. J. Med. 354, 911–923 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Fleischer V., et al., Translational value of choroid plexus imaging for tracking neuroinflammation in mice and humans. Proc. Natl. Acad. Sci. U.S.A. 118, e2025000118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey D., et al., Dimethyl fumarate in relapsing-remitting multiple sclerosis: Rationale, mechanisms of action, pharmacokinetics, efficacy and safety. Expert Rev. Neurother. 15, 339–346 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Lun M. P., Monuki E. S., Lehtinen M. K., Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 16, 445–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jessen N. A., Munk A. S., Lundgaard I., Nedergaard M., The glymphatic system: A beginner’s guide. Neurochem. Res. 40, 2583–2599 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vajkoczy P., Laschinger M., Engelhardt B., Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J. Clin. Invest. 108, 557–565 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steffen B. J., Breier G., Butcher E. C., Schulz M., Engelhardt B., ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am. J. Pathol. 148, 1819–1838 (1996). [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhbandner K., et al., MAdCAM-1-mediated intestinal lymphocyte homing is critical for the development of active experimental autoimmune encephalomyelitis. Front. Immunol. 10, 903 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altevogt P., et al., The alpha 4 integrin chain is a ligand for alpha 4 beta 7 and alpha 4 beta 1. J. Exp. Med. 182, 345–355 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothhammer V., et al., Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J. Exp. Med. 208, 2465–2476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glatigny S., Duhen R., Oukka M., Bettelli E., Cutting edge: Loss of α4 integrin expression differentially affects the homing of Th1 and Th17 cells. J. Immunol. 187, 6176–6179 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi T., et al., Ulcerative colitis. Nat. Rev. Dis. Primers 6, 74 (2020). [DOI] [PubMed] [Google Scholar]
- 19.D’Haens G., et al., Effect of PF-00547659 on central nervous system immune surveillance and circulating β7+ T cells in Crohn’s disease: Report of the TOSCA Study. J. Crohn’s Colitis 12, 188–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manouchehri N., Stüve O., Trials and therapies in secondary progressive MS, simplified. Nat. Rev. Neurol. 15, 431–432 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Kapoor R.et al.; ASCEND investigators , Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): A phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 17, 405–415 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Manouchehri N., Stüve O., Should ocrelizumab be used in non-active primary progressive multiple sclerosis? Time for a re-assessment. Ther. Adv. Neurol. Disord. 14, 1756286421990500 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]