Abstract
West African tick-borne relapsing fever (TBRF) is difficult to diagnose due to the low number of spirochetes in the bloodstream of patients. Previously, the causative microorganism, Borrelia crocidurae, had never been cultured in vitro. TBRF was rapidly diagnosed for two patients returning from western Africa with fever of unknown origin by quantitative buffy coat (QBC) analysis. Diagnosis was confirmed by intraperitoneal inoculation of blood specimens from patients into laboratory mice. In vitro experiments showed that QBC analysis may be as much as 100-fold more sensitive than thick smear. Spirochetes were also cultured from blood samples from both patients in modified Kelly’s medium and were identified as B. crocidurae by partial sequencing of the PCR-amplified rrs gene.
Relapsing fever, an infectious disease with a sudden onset of high fever with septicemic signs and symptoms, is characterized by the occurrence of one or more spells of fever after the subsidence of the primary febrile attack. There are two forms of relapsing fever, both caused by Borrelia species. Louse-borne or epidemic relapsing fever, caused by Borrelia recurrentis, is transmitted from person to person by the human body louse. Tick-borne or endemic relapsing fever (TBRF) is due to at least 16 distinctive Borrelia species harbored in soft ticks of the genus Ornithodoros (Alectorobius). Clinically, the manifestations of louse-borne relapsing fever and TBRF are quite similar (13). TBRF is a serious disease with, if untreated, a mortality rate of up to 5% (13). TBRF acquired during pregnancy poses a high risk of loss of pregnancy, up to 50% (7). Neurological symptoms have been reported for 9% of the patients with TBRF (13). Such findings were reported previously among TBRF patients in Senegal (2) and TBRF patients returning from Senegal to Europe (4). Tetracycline or doxycycline effectively eliminates the spirochetemia (4, 13).
In West Africa, the incidence of TBRF due to Borrelia crocidurae is rising (14). The mainstay of diagnosis of relapsing fever Borrelia is demonstration of the spirochetes in Giemsa-stained thick blood smears (11). However, thick smears from patients with B. crocidurae spirochetemia are often negative due to a low number of spirochetes in the bloodstream (6, 14). Since B. crocidurae could not be cultured in vitro up to now, intraperitoneal inoculation of mice with blood from patients with TBRF is the only more sensitive diagnostic alternative (6, 14). Since this method is laborious and seldom performed routinely, TBRF is most likely underdiagnosed frequently. Patients with undiagnosed TBRF are commonly at first treated with antimalarial agents (2, 4). Therefore, there is a strong need for simple and fast diagnostic techniques. We diagnosed TBRF in two patients with fever of unknown origin returning from West Africa. Initially, both patients were suspected of having malaria. The diagnosis was obtained rapidly by quantitative buffy coat (QBC) analysis of blood samples from the patients. In addition, B. crocidurae was cultured in vitro from blood samples from both patients.
Patient 1, a 32-year-old nonpregnant Dutch woman, was referred to our hospital on 30 May 1997 because of fever of unknown origin and severe headache. She had been working in a development project in a rural area in the northern part of The Gambia for 6 years. Five and two weeks before presentation, she had experienced episodes of fever. The first episode was treated with Fansidar, and the second one was treated with amoxycillin. After both episodes, she had a full recovery. On 20 May, she experienced a new episode of fever, complicated by a severe headache, a stiff neck, and vomiting. Emergency repatriation was scheduled on 28 May, but due to her severe illness, she was admitted to a hospital in Dakar, Senegal. A lumbar puncture was performed. The cerebrospinal fluid (CSF) specimen contained 130 leukocytes/μl and had a decreased glucose and an increased protein concentration. No bacteria were seen or cultured. A thick blood smear showed no malaria parasites. Intravenous treatment with quinine did not improve her clinical condition. The next day, she was repatriated to The Netherlands and admitted to our hospital. The body temperature was 36.4°C; pulse, respiratory rate, and blood pressure were normal. She had no hepatosplenomegaly, no skin abnormalities, and no enlarged lymph nodes. Erythrocyte sedimentation rate was 61 mm/h (normal value, <12 mm/h). Hemoglobin, leukocyte, platelet, creatinine, and liver enzyme levels were within the normal ranges. CSF contained 680 leukocytes/μl (normal, < 5/μl) (differentiation: 60% lymphocytes, 19% monocytes, 1% granulocytes), 2.4 mmol of glucose (blood glucose, 5.2 mmol/liter; CSF glucose slightly decreased [normal value is 50 to 70% of blood glucose]), and 1.20 g of protein per liter (normal value, <0.50 g/liter). Examinations of Gram- and Ziehl-Nelsen-stained CSF sediments were negative. Routine blood cultures in BactAlert FA growth medium (Organon Teknika, Durham, N.C.) as well as bacterial and viral CSF cultures remained negative.
Patient 2, a 30-year-old nonpregnant woman, born in Senegal but living in The Netherlands, was admitted to our hospital on 19 June 1997. She had visited her relatives in Senegal. Five days after her return, she had developed fever, rigors, arthralgia, myalgia, and abdominal pain. Her body temperature was 40.2°C. Physical examination revealed no abnormalities. On her chest, signs of a possible insect bite were seen. Hemoglobin was 6.6 mmol/liter (normal value, 7.5 to 9.0 mmol/liter); leukocyte, platelet, creatinine, and liver enzyme levels were normal. The differential diagnosis in both patients included malaria, septicemia, typhoid fever, leptospirosis, arboviral or other viral infections, and relapsing fever.
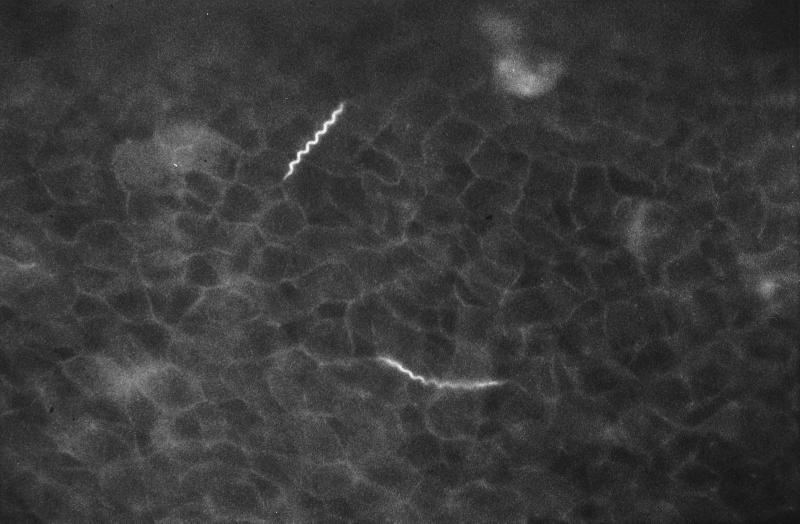
Peripheral blood specimens collected from both patients were used to prepare thick smears and to perform QBC analysis, mouse inoculation, and culture of spirochetes. QBC tubes (Becton Dickinson, Franklin Lakes, N.J.) were filled with blood (approximately 55 to 65 μl) and mixed with acridine-orange dye, which coats the interior of the tube (3). The tubes were stoppered, and the plastic float was inserted. The tubes were centrifuged in a centrifuge for capillary tubes (Becton Dickinson) at 12,000 rpm for 5 min and observed by fluorescence microscopy (Olympus BH-2) with a 50× oil immersion objective. Directly after centrifugation, the entire plasma-leukocyte interface was examined microscopically by turning the tube around and examining all sections. By QBC analysis of blood from both patients, brightly luminescent spirochetes were observed (Fig. 1), concentrating at the plasma-leukocyte interface. Thick blood smears were stained with Giemsa’s stain, and 200 oil immersion fields (×1,000) were systematically examined. In thick smears from the second patient, spirochetes were visible, whereas thick smears from the first patient were negative, even after extensive reexamination. Relapsing fever was diagnosed for both patients. After treatment with tetracycline (500 mg four times daily for 7 days), both patients made an uneventful recovery. Jarisch-Herxheimer reactions were not observed. For our patients, the diagnosis of relapsing fever was confirmed by animal inoculation and in vitro culture of spirochetes. For animal inoculation, 250 μl of blood was injected intraperitoneally into six Swiss mice. Four to six days after inoculation, mice were exsanguinated and the presence of spirochetes in their blood was studied by microscopic examination of Giemsa-stained thick smears, QBC, and in vitro culture. Four to six days after intraperitoneal injection of blood specimens from patients into six Swiss mice, all mice developed spirochetemia, detectable with Giemsa-stained thick smears and QBC analysis.
FIG. 1.
Acridine-orange-stained spirochetes as seen in QBC analysis of blood from patient 2. Magnification, ×500.
For culture, 300 μl of human blood or 100 μl of mouse blood was inoculated into 7 ml of modified Kelly’s medium (MKM) (9). MKM consisted of 0.7× CMRL-1066 medium (Gibco, Paisley, United Kingdom) to which had been added Neopepton (Difco, Detroit, Mich.) (2.1 g/liter), HEPES (4.2 g/liter), Na-citrate (0.5 g/liter), glucose (3.5 g/liter), Na-pyruvate (0.56 g/liter), N-acetylglucosamine (0.28 g/liter), NaHCO3 (1.5 g/liter), 5% heat-inactivated normal rabbit serum (Gibco), bovine serum albumin (Sigma, St. Louis, Mo.) (34 g/liter), and gelatin (Oxoid, Basingstoke, United Kingdom) (10 g/liter). Cultures were incubated at 33°C and examined twice weekly for the presence of spirochetes. Negative cultures were held up to 4 weeks. Direct culture of blood specimens from both patients in MKM resulted in growth of spirochetes (strain A124B from patient 1 and strain A125B from patient 2) after 5 to 7 days. Spirochetes could also be cultured from blood samples from four of the six spirochetemic mice. Subcultures in MKM could be made at least four times without detectable loss of viability of the isolates.
In order to compare the sensitivities of thick smear and QBC analysis, 1 ml of MKM containing 107 B. crocidurae spirochetes quantitated by dark-field microscopy was mixed with 10 ml of EDTA-anticoagulated whole blood from a healthy person. Subsequently, 10-fold serial dilutions of this mixture were made in EDTA-anticoagulated blood in duplicate. All dilutions were examined by Giemsa-stained thick smears and QBC analysis for the presence of spirochetes. QBC analysis of blood samples containing 103 spirochetes/ml of blood, corresponding to 50 spirochetes in the QBC sample, was always positive for both isolates (Table 1). One of four samples containing 102 spirochetes/ml of blood was also positive. The Giemsa-stained thick smears were consistently positive at 105 spirochetes/ml of blood, and only one of four samples containing 104 spirochetes/ml was positive. All samples with less than 104 spirochetes/ml were negative in thick smear analysis.
TABLE 1.
Detection of B. crocidurae in EDTA-blood by thick smear analysis and QBCa
| Method | B. crocidurae strain | Result for no. of spirochetes/ml of EDTA-blood
|
|||||
|---|---|---|---|---|---|---|---|
| 0 | 102 | 103 | 104 | 105 | 106 | ||
| Thick smear | A124B | −, − | −, − | −, − | +, − | +, + | +, + |
| A125B | −, − | −, − | −, − | +, + | +, + | ||
| QBC | A124B | −, − | +, − | +, + | +, + | +, + | +, + |
| A125B | −, − | +, + | +, + | +, + | +, + | ||
Tenfold serial dilutions of spirochetes (strains A124B and A125B) in EDTA-anticoagulated blood were examined by QBC and thick smear analysis. The table shows results from samples from duplicate serial dilution series. +, positive; −, negative.
Sequence analysis of the gene encoding the 16S rRNA (the rrs gene) can discriminate between different relapsing fever spirochetes, although these genes are very similar to each other. As an example, the sequence difference between B. crocidurae and Borrelia duttonii is only one nucleotide in the complete rrs gene. However, this sequence difference was conserved between nine B. crocidurae strains and three B. duttonii strains (10). For further characterization of our cultured spirochetes, part of the rrs gene was PCR amplified and sequenced. Obtained sequences were compared with rrs sequences from various other relapsing fever Borrelia species. DNA from cultured strains was extracted as described by Wilson (16), and 1 μg of DNA was used as input in PCR. For PCR, primers BBRNA8 (ACGCTGGCAGTGAGTCTTA) and BBRNA14 (ATATCAACAGATTCCACCC), corresponding to nucleotides 33 to 51 and 702 to 684 of the rrs gene of relapsing fever spirochetes (10), were used. PCR was performed in a final volume of 100 μl of buffer containing 50 mM KCl, 10 mM Tris-HCl, 2.5 mM MgCl2, 100 μg of gelatin per ml, 200 μM (each) deoxynucleoside triphosphate, and 0.5 μM (each) primer. After 40 cycles of 1 min at 94°C, 1 min at 46°C, and 1 min 30 s at 72°C, followed by a final extension step of 10 min at 72°C, 5 μl of PCR product was analyzed on agarose gels. An amplification product of the expected size of 668 bp was obtained from both isolates. Sequencing of PCR products was done with a dye terminator kit (Perkin-Elmer, Gouda, The Netherlands) on an automated sequencer (Pharmacia) with primers BBRNA8, BBRNA14, BBRNA15 (CTGCTGCCTCCCGTAGGAG, 352 to 333), and BBRNA13 (TTTATAATGAGGAATAAGC, 432 to 451). Sequence analysis showed that the rrs genes from both strains (GenBank accession no. AF116917 and AF116918) were identical to each other. Sequences were compared with published sequences from B. crocidurae (U42283 and eight other identical sequences [10]), B. duttonii (U42288 and two other identical sequences [10]), Borrelia hispanica (U42294), B. recurrentis (U42300), Borrelia persica (U42297), and Borrelia hermsii (U42292) (Table 2). Except for an insertion of a C nucleotide at position 381 in our sequences, which was not found in any sequence published by Marti Ras et al. (10), our sequences were identical to those of the nine B. crocidurae sequences (10). Therefore, we conclude that both strains were B. crocidurae.
TABLE 2.
Comparison of sequences of the partial rrs genes (nucleotides 58 to 679) of strains A124B and A125B (identical to each other [this study]) and other relapsing spirochetes (10)a
| Sequence | Nucleotide at position no.:
|
Total no. of nucleotide substitutions | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 63 | 70 | 175 | 181 | 185 | 214 | 252 | 259 | 381 | 469 | 495 | 562 | 595 | 624 | 625 | 626 | 633 | ||
| A124B-A125B | G | G | G | A | C | A | A | G | C | G | C | G | G | A | G | C | A | |
| B. crocidurae | - | - | - | - | - | - | - | - | x | - | - | - | - | - | - | - | - | |
| B. duttonii | A | - | - | - | - | - | - | - | x | - | - | - | - | - | - | - | - | 1 |
| B. hispanica | - | - | - | C | - | - | - | - | x | - | - | - | A | - | - | - | - | 2 |
| B. recurrentis | A | - | - | - | - | T | - | - | x | - | T | - | - | - | - | - | - | 2 |
| B. persica | - | - | - | - | - | - | G | - | x | - | - | - | A | - | - | - | G | 3 |
| B. hermsii | - | A | A | - | T | - | - | A | x | A | C | C | - | G | A | A | G | 11 |
Nucleotides are numbered according to Escherichia coli numbering starting at the 5′ end of the sequence. At position 381, an insertion of a C nucleotide in A124B and A125B was found (not included in the numbering of subsequent nucleotides). Only nucleotides showing differences among the species are shown. A hyphen indicates identity with the sequences of A124B and A125B.
The incidence of TBRF in Senegal is high (14). In Senegal, TBRF is considered to be caused by B. crocidurae, based on the spread of the tick vector Ornithodoros sonrai (Alectorobius sonrai). Throughout Senegal, the sub-Saharan drought has facilitated southbound spread of this vector (15). Southward spread of the vector from adjacent Senegal may explain why one of our patients acquired TBRF in The Gambia, a country in which the disease had not previously been reported.
Our study showed that smear-negative spirochetemia can be diagnosed effectively with the QBC technique. QBC is easy to perform, and results are available within 10 min after blood sampling. In comparison to thick smear analysis, QBC examination could be as much as 100-fold more sensitive. The QBC technique is in common use for diagnosis of malaria, but the presence of microfilariae and trypanosomes in the bloodstream can also be effectively detected by QBC (3). As with trypanosomes, spirochetes concentrate in the QBC just above the buffy coat layer. After centrifugation, immediate microscopic examination of this layer with examination of multiple fields is important because the spirochetes rapidly migrate into the plasma layer. The use of acridine-orange staining for identification of spirochetes in blood smears has been described earlier (12). However, spirochetes were not seen in acridine-orange-stained thick blood smears made as described previously (12). Concentration of spirochetes as done by QBC apparently improves the detection of spirochetemia in patients with B. crocidurae TBRF.
Some African relapsing fever spirochetes have been cultured successfully in vitro. B. duttonii was cultured in cell culture medium containing SflEp cells (8), and B. recurrentis was grown in Kelly’s growth medium (5). A Borrelia species isolated from blood from patients from Spain grew in BSK-II medium (1). In vitro culture of B. crocidurae had not previously been reported. MKM proved highly suitable for recovering B. crocidurae from human and murine blood samples and for subculture of the isolates, which enabled us to identify the isolates by PCR and sequence analysis of the rrs gene of spirochetal DNA from MKM-grown spirochetes. In conclusion, QBC and use of in vitro cultivation with MKM are promising new tools for the diagnosis of TBRF and identification of the causative Borrelia species.
Acknowledgments
We thank Ellen Lommerse, Anneke Oei, and Karin Wolbers for excellent technical assistance and Jan Weel for his help in DNA purification.
REFERENCES
- 1.Anda P, Sanchez-Yebra W, del Mar Vitutia M, Perez Pastrana E, Rodríguez I, Miller N S, Backenson P B, Benach J L. A new Borrelia species isolated from patients with relapsing fever in Spain. Lancet. 1996;348:162–165. doi: 10.1016/s0140-6736(96)02332-x. [DOI] [PubMed] [Google Scholar]
- 2.Aubry P, Renambot J, Teyssier J, Buisson Y, Granic G, Brunetti G, Dano P, Bauer P. Les borrélioses à tiques au Sénégal à propos de 23 observations. Dakar Méd. 1983;28:413–420. [PubMed] [Google Scholar]
- 3.Bailey J W, Smith D H. The quantitative buffy coat for the diagnosis of trypanosomes. Trop Dr. 1994;24:54–56. doi: 10.1177/004947559402400204. [DOI] [PubMed] [Google Scholar]
- 4.Colebunders R, De Serrano P, Van Gompel A, Wynants H, Blot K, Van den Enden E, Van den Ende J. Imported relapsing fever in European tourists. Scand J Infect Dis. 1993;25:533–536. doi: 10.3109/00365549309008539. [DOI] [PubMed] [Google Scholar]
- 5.Cutler S J, Fekade D, Hussein K, Knox K A, Melka A, Cann K, Emilianus A R, Warrell D A, Wright D J M. Successful in-vitro cultivation of Borrelia recurrentis. Lancet. 1994;343:242. doi: 10.1016/s0140-6736(94)91032-4. [DOI] [PubMed] [Google Scholar]
- 6.Diatta G, Trape J F, Legros F, Rogier C, Duplantier J M. A comparative study of three methods for detection of Borrelia crocidurae in wild rodents in Senegal. Trans R Soc Trop Med Hyg. 1994;88:423–424. doi: 10.1016/0035-9203(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 7.Jongen V H W M, Van Roosmalen J, Tiems J, van Holten J, Wetsteyn J. Tick-borne relapsing fever and pregnancy outcome in rural Tanzania. Acta Obstet Gynecol Scand. 1997;76:834–838. doi: 10.3109/00016349709024361. [DOI] [PubMed] [Google Scholar]
- 8.Konishi H, Morshed M G, Akitomi H, Nakazawa T. In vitro cultivation of Borrelia duttonii on cultures of SflEp cells. Microbiol Immunol. 1993;37:229–232. doi: 10.1111/j.1348-0421.1993.tb03204.x. [DOI] [PubMed] [Google Scholar]
- 9.Preac-Mursic V, Wilske B, Schierz G. European Borrelia burgdorferi isolated from humans and ticks: culture conditions and antibiotic susceptibility. Zentbl Bakteriol A. 1986;263:112–118. doi: 10.1016/s0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- 10.Ras N M, Lascola B, Postic D, Cutler S J, Rodhain F, Baranton G, Raoult D. Phylogenesis of relapsing fever Borrelia spp. Int J Syst Bacteriol. 1996;46:859–865. doi: 10.1099/00207713-46-4-859. [DOI] [PubMed] [Google Scholar]
- 11.Schwan T G, Burgdorfer W, Rosa P A. Borrelia. In: Murray P A, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 626–635. [Google Scholar]
- 12.Sciotto C G, Lauer B A, White W L, Istre G R. Detection of Borrelia in acridine orange-stained blood smears by fluorescence microscopy. Arch Pathol Lab Med. 1983;107:384–386. [PubMed] [Google Scholar]
- 13.Southern P M, Jr, Sanford J P. Relapsing fever. A clinical and microbiological review. Medicine. 1969;48:129–149. [Google Scholar]
- 14.Trape J F, Duplantier J M, Bouganali H, Godeluck B, Legros F, Cornet J P, Camicas J L. Tick-borne borreliosis in West Africa. Lancet. 1991;337:473–475. doi: 10.1016/0140-6736(91)93404-w. [DOI] [PubMed] [Google Scholar]
- 15.Trape J F, Godeluck B, Diatta G, Rogier C, Legros F, Albergel J, Pepin Y, Duplantier J M. The spread of tick-borne borreliosis in West Africa and its relationship to sub-Saharan drought. Am J Trop Med Hyg. 1996;54:289–293. doi: 10.4269/ajtmh.1996.54.289. [DOI] [PubMed] [Google Scholar]
- 16.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel F M, Brent R, Kingston R E, editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1988. pp. 2.4.1–2.4.5. [DOI] [PubMed] [Google Scholar]