Abstract
Purpose:
Unplanned readmission of post-operative brain tumor patients is often attributed to hospital and patient characteristics and is associated with higher mortality and cost. Previous studies demonstrate multiple patient outcome disparities in safety net hospitals (SNHs) when compared to non-SNHs. This study uses the Nationwide Readmissions Database (NRD) to determine if initial brain tumor resection at SNHs is associated with increased 30-day non-elective readmission rates.
Methods:
Patients with benign or malignant primary or metastatic brain tumor undergoing craniotomy for surgical resection were retrospectively identified in the NRD from 2010–2014. SNHs were defined as hospitals with Medicaid and uninsured patient burden in the top quartile. Descriptive and multivariate analyses employing survey-adjusted logistic regression evaluated patient and hospital level factors influencing 30-day readmissions.
Results:
During the study period, 83367 patients met inclusion criteria. 44.7% of patients had a benign tumor, and 55.3% had a malignant tumor. Secondary CNS neoplasm (5.99%), post-operative infection (5.96%), and septicemia (4.26%) caused most readmissions within 30 days. Patients had increased unplanned readmission rates if they underwent craniotomy for tumor resection at a SNH in a small metropolitan area (OR 1.11, 95% CI 1.02–1.21, p=0.01), but not at a SNH in a large metropolitan area (OR 0.99, 95% CI 0.93–1.05, p=0.73).
Conclusion:
This finding may reflect differences in access to care and disparities in neurosurgical resources between small and large metropolitan areas. Inequities in expertise and capacity are relevant as surgical volume was also related to readmission rates. Further studies may be warranted to address such disparities.
Keywords: Nationwide Readmissions Database, Craniotomy, Brain Tumor, Readmission, Safety Net Hospital, Metropolitan
Introduction
The incidence of benign and malignant brain and central nervous system tumors in the United States is 28.57 people per 100,000 per year, with malignant brain tumors often defined by both primary and secondary (metastatic) tumors [1, 2]. While craniotomy for tumor resection is commonly the first step in management for patients with brain tumors, this patient population may be at increased risk of readmission due to post-operative complications, recurrence or persistence of tumor, and overall poor health due to underlying disease [2].
30-day readmission rates are a measure of patient outcomes and hospital performance that have garnered particular interest. Prior studies have reported a 30-day readmission rate of 7.5–17.3% among patients that have undergone surgical resection of a brain tumor with the most common causes for readmission being seizures, surgical site infection, and new motor deficits [3–7]. Patient readmissions are costly and often indicate a poor prognosis [2]. Studies have estimated that patients readmitted after resection of glioblastoma live 1.6 fewer months and have twice the mortality risk compared to non-readmitted patients [3, 6]. In addition to this temporal cost, it is estimated that each readmission costs these patients an extra $20,296 in hospital charges [4]. Identifying factors associated with unplanned readmission could provide an opportunity to modify these risks. Prior studies have found increased 30-day readmission rates following craniotomy for patients with brain tumors who are publicly insured or treated at non-academic hospitals [2, 4]. However, prior studies have not assessed the relationship between the safety net status of the treating hospital and the readmission rate of patients with brain tumors following craniotomy.
Safety net hospitals (SNHs), by mission or legal mandate, provide medical care for a disproportionately large number of patients who are uninsured, unable to pay, or who have Medicaid [8, 9]. SNHs comprise 25% of hospitals in the United States, but are responsible for nearly 33% of all inpatient stays, indicating that they provide a disproportionate amount of care [8]. Non-safety net hospitals (non-SNHs) are more likely to be teaching hospitals, have higher surgical volumes, and be located in large and small metropolitan areas [8]. The safety net patient population may represent a more vulnerable population, with increased risk for adverse outcomes following medical or surgical treatment. Studies have found multiple disparities in outcomes between patients receiving care at SNHs versus non-SNHs, with large database analyses noting that SNH patients experience lower standard-of-care treatment rates, increased readmission rates, and decreased survival rates compared to those treated at non-SNHs [10–13]. The Patient Protection and Affordable Care Act, through the Hospital Readmissions Reduction Program, allows the Centers for Medicare and Medicaid Services to penalize hospitals with higher than average 30-day readmission rates, which has resulted in reduced readmission rates, overall [14]. However, SNHs continue to have higher than average readmission rates [15].
While SNHs have been associated with an elevated likelihood of readmission in a general patient population, studies have also reported increased perioperative complications in patients treated at SNHs following resection of glioblastoma and metastatic brain tumors [15–18]. Taken together, this suggests the possibility that patients receiving craniotomy for tumor resection may experience increased readmission rates following treatment at a SNH. As craniotomies are a commonly performed procedure with a 30-day overall complication rate of 16.1%, readmission after craniotomy for tumor resection may serve as an effective metric to compare the quality of care these patients receive at SNHs versus non-SNHs [19]. It is therefore important to understand characteristics of SNHs, health system factors, and disease processes that may make certain brain tumor patient populations more at risk for readmission.
This study leveraged the Nationwide Readmissions Database, a publicly available dataset including >45% of inpatient admissions with longitudinal follow-up, to evaluate national readmission trends following craniotomy for brain tumor resection. We hypothesize that initial treatment at a SNH results in increased non-elective readmission rates among patients following craniotomy for surgical resection of benign and malignant brain tumors.
Methods
Data Source
The 2010–2014 cohorts of the Nationwide Readmissions Database (NRD) were queried for this study. The NRD is a publicly accessible dataset featuring discharge information related to 49.3% of all hospital inpatient stays for all-payers across 22 geographically diverse states in the United States. It is a component of the Healthcare Cost and Utilization Project (HCUP) by the Agency for Healthcare Research and Quality (AHRQ). Information in the NRD represents a compendium of constituent State Inpatient Databases and contains verified and de-identified patient linkage variables to track patients through hospitalizations in a given state for a given year.
Study Population
International Classification of Diseases, Ninth Edition (ICD-9) diagnostic and procedure codes were used to identify patients for inclusion in this study. Patients older than 18 years old with a primary diagnosis of benign (192.1, 225.0–225.2, 237.6, 237.0) or malignant primary and metastatic brain tumor (191.0–191.9, 198.3) with a concomitant tumor resection procedure (01.51, 01.53, 01.59, 04.01, 07.61, 07.64) were included. Patients receiving stereotactic or open biopsy for tumor were not included. Because the NRD only encompasses all readmissions for a patient within a single calendar year, patients with an index admission between January and November for a given year were included to assess 30-day readmission. Once a patient was readmitted, they were not followed for subsequent readmissions. Patients who died during their index admission were excluded. Figure 1 depicts the strategy used to query the NRD and the number of patients that satisfy all inclusion and exclusion criteria.
Fig. 1.
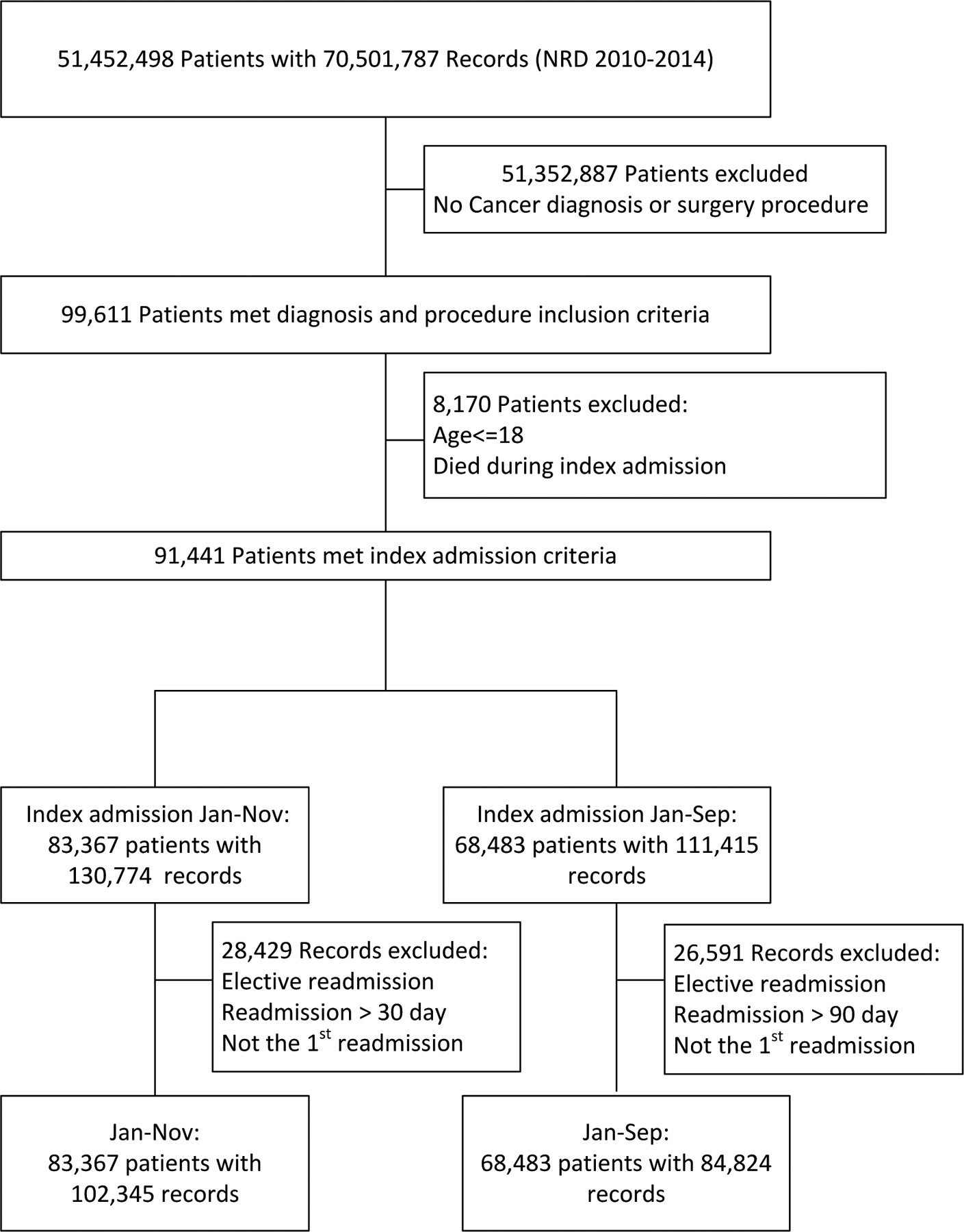
Diagram showing stepwise patient search, grouping, and counts after applying inclusion and exclusion criteria to the NRD for 2010–2014 grouped in 30- and 90-day readmission cohorts.
Patient and Hospital Characteristics
Univariate and multivariate analyses were used to evaluate the association between patient and hospital characteristics, and an outcome of non-elective readmission. Patient factors included gender (female/male), age (categorized as 18–44, 45–59, 60–74, ≥75 years old), admission from emergency department (yes/no), index length of stay (0–3 days, 4–5 days, 6–10 days, >10 days), All Patient Refined Diagnosis-Related Group (APR-DRG): Severity of Illness Subclass (minor, moderate, major, extreme), and discharge disposition (routine/other). APR-DRG is a well-characterized and widely implemented measure of disease severity developed by 3M Health Information Systems, incorporating principal diagnosis, age, secondary diagnoses (including complications and comorbid conditions), and procedures to assign a severity of illness subclass [20]. Neurological complications defined as presence of any of the following: intracerebral hemorrhage (431, 998.11–12), seizures (345.0–345.91), and neurological complications after procedure (997.01–997.09); and major complications defined as any of the following: pneumonia (481–482, 482.1–482.3, 482.30–482.32, 482.39–482.41, 482.49, 482.80–482.84, 482.89, 482.90, 483.0, 483.1, 485–487.0, 997.3, 507.0) pulmonary embolism (415.1–415.9), renal failure (584, 584.5–584.9), cerebrovascular accident (433.01, 433.11, 433.21, 433.31, 433.81, 433.91), myocardial infarction (410.00–410.90, 410.01, 410.11–410.91), cardiac arrest (427.5), sepsis (995.91), and septic shock (995.92) were identified by relevant ICD-9 codes and included in analysis. Neurologic and major complication ICD-9 codes included have been previously published [21]. Median household income for a patient’s zip code for a given year (0–25, 26–50, 51–75, 76–100 percentiles) was also included. In post-hoc analysis we also sought to also assess the influence of specific patient comorbidities upon unplanned readmission. We therefore included additional risk factors in our univariate and multivariate models, including Elixhauser comorbidity score, as well as the specific comorbidities of diabetes mellitus (controlled and uncontrolled), coronary artery disease, chronic kidney disease, peripheral vascular disease, and cerebrovascular accident in additional analyses. The Elixhauser comorbidity index is an established and widely employed measure that includes 29 predetermined comorbidities (categorized as present/absent) to produce a weighted sum that has been demonstrated to correlate with patient outcomes such as length of stay and mortality [22].
Hospital characteristics included large metropolitan (≥1 million residents), small metropolitan (<1 million residents) or non-metropolitan county location, control or ownership of hospitals (government, nonfederal; private, non-profit; private, investor-owned), teaching status (teaching or non-teaching), number of beds (small, medium, large), and procedure volume (above or below 90th percentile).
All hospital records were used to calculate percentage of Medicaid and uninsured patients in each year, then stratified into quartiles. If the sum of Medicaid and uninsured patients placed a hospital in the top quartile, then it was designated as a safety net hospital, as similarly employed by Brandel et al. and Bakhsheshian et al. [16,23]. This enabled the creation of a dichotomous variable of either safety net hospital or non-safety net hospital.
The primary outcome measure was first non-elective readmission. Time to first non-elective readmission and reason for unplanned readmission were also determined.
Statistical Analysis
Descriptive statistics used means and standard deviation for normally distributed continuous variables, medians and interquartile ranges (IQR) for non-normal continuous variables and counts, and percentages for categorical variables. To evaluate factors, including safety net hospital status, associated with non-elective readmission, variables were first evaluated in bivariate analyses. Variables with significant association with readmission were then included in multivariate analyses. Generalized equation modeling was used for multivariable logistic regression adjusting for hospital clustering, using a stepwise approach. An interaction analysis between the population size where a hospital was located and safety net status designation was also performed to determine if an interaction between these two variables influenced the outcome of readmission within 30 days. All variables were checked for confounders, and possible collinearity. Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used to compare different models. Hosmer–Lemeshow goodness of fit was used for general model fitting. Analysis was conducted using SAS 9.4 (Cary, NC). Significance levels were denoted with a p<0.05.
Results
Study Participants and Demographic Data
A total of 2953 hospitals were identified in our cohort from 2010–2014, with 741 (25.1%) being safety net hospitals (SNHs) and 2212 (74.9%) being non-SNHs. 83367 patients underwent craniotomy for tumor resection and met inclusion criteria. Of these, 17791 (21.3%) patients were initially treated at a SNH, while 65576 (78.7%) were initially treated at a non-SNH. 40.6% of patients had resection of a benign tumor, and 59.4% of patients underwent resection of a malignant tumor. 18.6% of patients were aged 18–44 years old, 33.9% were 45–59 years old, 36.2% were 60–74 years old, and 11.2% were ≥75 years old. 54.3% of patients were female (Table 1). A total of 18978 patients were readmitted (unplanned) following tumor resection, with 4176 (23.5%) occurring after treatment at SNHs, and 14802 (22.6%) occurring following treatment at non-SNHs. The median time to readmission was 24 days. The most common specific reasons for unplanned readmission were post-operative infection (5.96%), septicemia (4.26%), and pulmonary embolism (4.19%) (Table 2).
Table 1.
Patient and hospital characteristics at safety net and non-safety net hospital admission: 30-day readmission cohort
| Total (n=83367) | Safety Net (n=17791) | Non-Safety Net (n=65576) | p-value | |
|---|---|---|---|---|
| Cancer, n (%) | ||||
| Benign | 33886 | 7958 (44.73) | 25928 (39.54) | <0.0001 |
| Malignant | 49481 | 9833 (55.27) | 39648 (60.46) | |
| Age, n (%) | ||||
| 18–44 | 15521 | 3637 (20.44) | 11884 (18.12) | |
| 45–59 | 28293 | 6200 (34.85) | 22093 (33.69) | |
| 60–74 | 30184 | 6261 (35.19) | 23923 (36.48) | |
| ≥75 | 9369 | 1693 (9.52) | 7676 (11.71) | |
| Male | 38124 | 8034 (45.16) | 30090 (45.89) | |
| Female | 45243 | 9757 (54.84) | 35486 (54.11) | |
| Medicare | 29323 | 5926 (33.31) | 23397 (35.68) | |
| Medicaid | 8767 | 3513 (19.75) | 5254 (8.01) | |
| Private insurance | 39309 | 6355 (35.72) | 32954 (50.25) | |
| Self-pay | 2472 | 905 (5.09) | 1567 (2.39) | |
| No charge | 307 | 129 (0.73) | 178 (0.27) | |
| Other | 2966 | 914 (5.14) | 2052 (3.13) | |
| Missing | 223 | 49 (0.28) | 174 (0.27) | |
| Median Household Income*, n (%) | ||||
| 0–25 percentile | 17077 | 5597 (31.46) | 11480 (17.51) | |
| 26–50 percentile | 18402 | 4309 (24.22) | 14093 (21.49) | |
| 51–75 percentile | 21217 | 4250 (23.89) | 16967 (25.87) | |
| 76–100 percentile | 25026 | 3254 (18.29) | 21772 (33.20) | |
| Missing | 1645 | 381 (2.14) | 1264 (1.93) | |
| All Patient Refined DRG: Severity of Illness Subclass, n (%) | ||||
| No class | DS** | DS** | DS** | |
| Minor | 20722 | 4249 (23.88) | 16473 (25.12) | |
| Moderate | 33193 | 6860 (38.56) | 26333 (40.16) | |
| Major | 22062 | 4883 (27.45) | 17179 (26.20) | |
| Extreme | 7388 | 1798 (10.11) | 5590 (8.52) | |
| 0–3 days | 27537 | 4811 (27.04) | 22726 (34.66) | |
| 4–5 days | 16357 | 3294 (18.51) | 13063 (19.92) | |
| 6–10 days | 20402 | 4619 (25.96) | 15783 (24.07) | |
| >10 days | 19071 | 5067 (28.48) | 14004 (21.36) | |
| Yes | 3532 | 853 (4.79) | 2679 (4.09) | |
| No | 79835 | 16938 (95.21) | 62897 (95.91) | |
| Yes | 16724 | 3621 (20.35) | 13103 (19.98) | |
| No | 66643 | 14170 (79.65) | 52473 (80.02) | |
| Routine | 53686 | 11715 (65.85) | 41971 (64.00) | <0.0001 |
| Other | 29639 | 6059 (34.06) | 23580 (35.96) | |
| Missing | 42 | 17 (0.10) | 25 (0.04) | |
| Small | 4535 | 265 (1.49) | 4270 (6.51) | |
| Medium | 12350 | 2524 (14.19) | 9826 (14.98) | |
| Large | 66482 | 15002 (84.32) | 51480 (78.50) | |
| Teaching | 67093 | 15992 (89.89) | 51101 (77.93) | |
| Non-teaching | 16274 | 1799 (10.11) | 14475 (22.07) | |
| >90th percentile | 42044 | 8945 (50.28) | 33099 (50.47) | |
| ≤90th percentile*** | 41323 | 8846 (49.72) | 32477 (49.53) | |
| Yes | 25170 | 6418 (36.07) | 18752 (28.60) | <0.0001 |
| No | 58197 | 11373 (63.93) | 46824 (71.4) | |
| Hospital Urban-Rural Designation, n (%) | ||||
| Metropolitan | 57814 | 10883 (61.17) | 46931 (71.57) | |
| Other | 25553 | 6908 (38.83) | 18645 (28.44) | |
| Control/Ownership of Hospital, n (%) | ||||
| Government, non-federal | 14477 | 6873 (38.63) | 7604 (11.60) | |
| Private, non-profit | 62819 | 9800 (55.08) | 53019 (80.85) | |
| Private, investor-owned | 6071 | 1118 (6.28) | 4953 (7.55) | |
For patient’s ZIP code, based on current year.
Data suppressed for patient confidentiality
103 procedures / year
Table 2.
Most frequent reasons for 30-day readmissions for all patients at all hospitals
| ICD-9 Diagnosis | n (%) |
|---|---|
| 198.3 Secondary malignant neoplasm of the brain and spine | 1143 (5.99) |
| 998.59 Post-operative infection | 1137 (5.96) |
| 038.9 Septicemia | 813 (4.26) |
| 415.19 Pulmonary embolism and infarction | 799 (4.19) |
| 486 Pneumonia | 584 (3.06) |
| 997.09 Nervous system complication | 556 (2.91) |
| 191.9 Malignant neoplasm of the brain | 450 (2.36) |
| 191.1 Malignant neoplasm of the frontal lobe | 380 (1.99) |
| 599.0 Urinary tract infection | 338 (1.77) |
| 780.39 Convulsions | 324 (1.70) |
| 453.41 Deep vein thrombosis of the lower extremity | 307 (1.61) |
| 997.01 Central nervous system complication | 286 (1.50) |
| 345.90 Epilepsy | 279 (1.46) |
| 191.2 Malignant neoplasm of the temporal lobe | 258 (1.35) |
| 331.4 Obstructive hydrocephalus | 250 (1.31) |
| 191.3 Malignant neoplasm of the parietal lobe | 199 (1.04) |
Hospital Characteristics: Readmission after Treatment at Safety Net or Non-Safety Net Hospital
While initial analysis of the entire cohort showed that safety net burden of the initial treating hospital was not associated with 30-day readmission (OR 1.03, 95% CI 0.98–1.08, p=0.21), in multivariate analysis (Table 3), we noted that SNH status showed significant interaction with metropolitan location of the treating hospital. Following interaction analysis, safety net hospitals in small metropolitan areas (<1 million residents), specifically, had higher 30-day readmission rates compared to non-safety net hospitals in small metropolitan areas (OR 1.11, 95% CI 1.02–1.21, p=0.01) (Table 4). Of note, a higher percentage of patients were treated at safety net hospitals in small metropolitan regions (27%) when compared to patients treated at SNHs in large metropolitan regions (19%). Hospital procedure volume below the 90th percentile (OR 1.08, 95% CI 1.03–1.13, p=0.0005) was also associated with increased rate of readmission, while rural location (OR 0.89, 95% CI 0.85–0.93, p<0.0001) was associated with decreased rate of readmission.
Table 3.
Summary of associations with 30-day readmissions
| OR | 95% CI | p-value | |
|---|---|---|---|
| Safety Net Burden | |||
| Yes (≥28.6%, top quartile) | 1.03 | 0.98–1.08 | 0.21 |
| No | Ref | ||
| Cancer | |||
| Benign | Ref | ||
| Malignant | 1.92 | 1.84–2.00 | <0.0001 |
| Age | |||
| 18–44 | Ref | ||
| 45–59 | 1.19 | 1.13–1.26 | <0.0001 |
| 60–74 | 1.27 | 1.20–1.34 | <0.0001 |
| ≥75 | 1.34 | 1.24–1.44 | <0.0001 |
| Gender | |||
| Male | 1.14 | 1.11–1.18 | <0.0001 |
| Female | Ref | ||
| Primary Insurance | |||
| Medicare | 0.93 | 0.87–1.00 | 0.04 |
| Medicaid | Ref | ||
| Private insurance | 0.73 | 0.69–0.78 | <0.0001 |
| Self-pay | 0.70 | 0.62–0.79 | <0.0001 |
| No charge | 0.95 | 0.73–1.25 | 0.72 |
| Other | 0.76 | 0.68–0.85 | <0.0001 |
| Median Household Income* | |||
| 0–25 percentile | Ref | ||
| 26–50 percentile | 0.98 | 0.93–1.04 | 0.53 |
| 51–75 percentile | 0.95 | 0.90–1.00 | 0.04 |
| 76–100 percentile | 0.92 | 0.87–0.97 | 0.0012 |
| All Patient Refined DRG: Severity of Illness Subclass | |||
| Minor | Ref | ||
| Moderate | 1.14 | 1.09–1.20 | <0.0001 |
| Major | 1.35 | 1.27–1.43 | <0.0001 |
| Extreme | 1.42 | 1.32–1.54 | <0.0001 |
| Index Length of Stay | |||
| 0–3 days | Ref | ||
| 4–5 days | 1.14 | 1.08–1.21 | <0.0001 |
| 6–10 days | 1.26 | 1.19–1.33 | <0.0001 |
| >10 days | 1.42 | 1.34–1.51 | <0.0001 |
| Disposition | |||
| Routine | Ref | ||
| Other | 1.32 | 1.27–1.38 | <0.0001 |
| Hospital Volume | |||
| Above 90th percentile | Ref | ||
| ≤90th percentile** | 1.08 | 1.03–1.13 | 0.0005 |
| HCUP Used Emergency | |||
| Yes | 1.09 | 1.04–1.13 | 0.0001 |
| No | Ref | ||
| Hospital Urban-Rural Designation | |||
| Metropolitan | Ref | ||
| Other | 0.89 | 0.85–0.93 | <0.0001 |
For patient’s ZIP code, based on current year.
103 procedures / year
Table 4.
30-day readmission odds of safety net and non-safety net hospitals in metropolitan areas
| OR | 95% CI | p-value | |
|---|---|---|---|
| Small Metropolitan | |||
| Safety Net Hospitals | 1.11 | 1.02–1.21 | 0.01 |
| Non-Safety Net Hospitals | Ref | ||
| Large Metropolitan | |||
| Safety Net Hospitals | 0.99 | 0.93–1.05 | 0.73 |
| Non-Safety Net Hospitals | Ref |
Patient Traits: Demographic and Disease Influences on 30-day Readmission Rate
A diagnosis of malignant tumor (OR 1.92, 95% CI 1.84–2.00, p<0.0001) was associated with increased likelihood of readmission (Table 3). Male gender (OR 1.14, 95% CI 1.11–1.18, p<0.001), and age [in the 45–59 (OR 1.19, 95% CI 1.13–1.26, p<0.0001), 60–74 (OR 1.27, 95% CI 1.20–1.34, p<0.0001), and ≥75 (OR 1.34, 95% CI 1.24–1.44, p<0.0001) year-old groups] were also associated with increased likelihood of unplanned readmission. Increased likelihood of non-elective readmission was associated with increased index length of stay [4–5 days (OR 1.14, 95% CI 1.08–1.21, p<0.0001), 6–10 days (OR 1.26, 95% CI 1.19–1.33, p<0.0001), and >10 days (OR 1.42, 95% CI 1.34–1.51, p<0.0001)]. Increased APR-DRG illness severity class [moderate (OR 1.14, 95% CI 1.09–1.20, p<0.0001), major (OR 1.35, 95% CI 1.27–1.43, p<0.0001, and extreme (OR 1.42, 95% CI 1.32–1.54, p<0.0001)] was also associated with increased likelihood of readmission. Finally, increased likelihood of unplanned readmission was also associated with non-routine discharge (OR 1.32, 95% CI 1.27–1.38, p<0.0001), and emergency room visit (OR 1.09, 95% CI 1.04–1.13, p=0.0001).
Decreased likelihood of non-elective readmission was associated with higher median household income [51–75 (OR 0.95, 95% CI 0.90–1.00, p=0.04) and 76–100 (OR 0.92, 95% CI 0.87–0.97, p=0.0012) percentiles]. Private insurance (OR 0.73, 95% CI 0.69–0.78, p<0.0001) was also associated with decreased likelihood of unplanned readmission.
Our post hoc analysis revealed Elixhauser comorbidity index was independently associated with increased likelihood of non-elective readmission within 30 days in multivariate analysis [score of 1 (OR 1.22, 95% CI 1.15–1.29, p<0.0001), 2 (OR 1.41, 95% CI 1.33–1.50, p<0.0001), and 3 or more (OR 1.60, 95% CI 1.51–1.70, p<0.0001)]. When including Elixhauser comorbidity index into the multivariate model, tumor resection at SNHs remained significantly associated with increased likelihood of unplanned readmission in small metropolitan areas [small metropolitan (OR 1.12, 95% CI 1.03–1.21, p=0.009)]. Furthermore, inclusion of specific comorbidities [diabetes mellitus (OR 1.17, 95% CI 1.11–1.22, p<0.0001), coronary artery disease (OR 1.12, 95% CI 1.08–1.17, p<0.0001), and chronic kidney disease (OR 1.19, 95% CI 1.09–1.31, p=0.0002)] were also each linked with increased likelihood of non-elective readmission within 30-days. Peripheral vascular disease (OR 1.08, 95% CI 0.98–1.20, p=0.12) or a cerebrovascular accident (OR 0.42, 95% CI 0.12–1.51, p=0.18) had no statistical influence on readmission. Again, however, inclusion of each of these specific risk factors in a multivariate model did not alter our original findings: tumor resection at SNHs remained significantly associated with increased likelihood of unplanned readmission in a small metropolitan area [small metropolitan (OR 1.11, 95% CI 1.02–1.20, p=0.014).
Discussion
This study leveraged the Nationwide Readmissions Database (NRD) from 2010–2014 to determine patient, hospital, and health system level factors associated with unplanned 30-day readmission following craniotomy and tumor resection in patients with benign and malignant brain tumors. Readmission is associated with increased cost and poor patient outcomes generally, and following neurosurgery specifically [2]. In this cohort, a total of 18,978 (22.8%) of 83,367 patients were readmitted within 30 days. Common reasons for readmission included surgical complications such as post-operative infection and nervous system complication, as well as indicators of underlying disease including septicemia, pulmonary embolism, and pneumonia. These are similar to other reported reasons for readmission in this patient population [2, 4]. The all patient 30-day non-elective readmission rate of 22.8% is slightly higher than the previous single institution and large scale study measurements of 30-day readmission rates following craniotomy for brain tumor that range from 7.5%−17.3% [2–4, 6, 7]. Moreover, a similar large scale database study of 43,356 patients undergoing cranial surgery found that 30-day readmission rates varied for seizure (13.9%), neoplasm (17.3%), trauma (19.8%), and vascular (23.9%) indications [7]. While that comparison is specific to cranial surgery, other large database studies report 30-day readmission rates of 3.8% after orthopedic surgeries and 14.2% after major cancer surgery [24, 25]. Here we report a higher 30-day unplanned readmission rate following brain tumor resection relative to most other estimates for non-tumor cranial surgeries, orthopedic surgeries, and complex cancer operations. This suggests that patients with brain tumors may face specific patient, hospital, and health system level factors that make them particularly susceptible to increased readmission. For example, malignant brain tumor, hospital craniotomy volume below the 90th percentile, and non-routine discharge were traits associated with an increased rate of unplanned 30-day readmission in the current study.
Readmission rates are widely accepted as a metric for patient outcomes [2, 3–7, 14]. In the present study, prior to accounting for the location of the SNH, there is no difference in unplanned 30-day readmission rates between patients with benign and malignant brain tumors treated with craniotomy for tumor resection at SNHs compared to non-SNHs. Other database studies have characterized outcomes after neurosurgical treatment at SNHs with mixed results. Treatment at a SNH was not associated with worse survival outcomes in patients with subarachnoid hemorrhage [26]. However, it was associated with lower standard-of-care treatment rates and worse survival for patients with glioblastoma, and higher complication and mortality rates in cases of severe traumatic brain injury, when compared to treatment at non-SNHs [16, 23]. Pre-existing disparities between SNHs and non-SNHs with regard to institutional characteristics such as teaching status, bed size, location, and readmission rates for other conditions may influence patient outcomes [8, 13]. It is because of these factors that we sought to determine if underlying institutional and contextual characteristics were associated with readmission rates in our study population.
Although there was no difference in unadjusted overall 30-day readmission rates between SNHs and non-SNHs, interaction analysis revealed safety net hospital status showed significant interaction with metropolitan area status. Upon accounting for this critical interaction, treatment at a SNH in a small metropolitan area (<1 million residents) was associated with an 11% increased likelihood of readmission when compared to those treated at non-SNHs in these areas. Prior research has focused on the effect of geographic location on outcomes, including readmission. For example, it has been estimated that 58% of the total variation in nationwide publicly reported 30-day readmission rates is attributable to the county context in which a hospital is located [27]. Variations in readmission rates based on geography and population size of the county where a hospital is located have been reported; however, in these studies, small metropolitan areas tend to have lower readmission rates compared to large metropolitan areas and even rural areas [13, 28–30]. These studies differ from ours with regards to patient population as none of them include neurosurgical patients, only study Medicare patients or evaluate data in only a small subset of states [13, 28–30]. While other studies have evaluated outcomes after treatment at SNHs compared to non-SNHs, few have sub-categorized these hospitals based on geographic location. Here we compared readmission rates between SNHs and non-SNHs in small metropolitan areas, whereas prior studies compared SNHs and non-SNHs in all areas, or compared metropolitan and rural hospitals.
Small metropolitan areas are defined as counties with a population of less than one million people. In our study, 19% of hospitals in small metropolitan areas were SNHs and 81% were non-SNHs. However, 27% of patients treated in small metropolitan areas were treated at SNHs. In contrast, 29% of hospitals in large metropolitan areas were SNHs, but only 19% of qualifying patients were treated at a SNH. This suggests a disproportionate burden on SNHs in small metropolitan areas to treat relatively more patients. Small metropolitan areas tend to have limited neuro-oncology resources due to geographic and financial-based maldistributions of medical- and radiation-oncology equipped hospitals [31, 32]. For example, radiation oncologists tend to be more prevalent in metropolitan and higher income areas, and their geographic distribution is significantly more skewed when compared to primary care physicians or physicians in general [31]. Further, the density of hospitals with radiation oncology services in a given health service area, which is a county or cluster of contiguous counties, was a predictor of patient receipt of post-operative radiation therapy [32]. SNHs in these areas may bear the brunt of these resource limitations. Brandel et al. found that glioblastoma patients treated at SNHs are less likely to receive standard-of-care therapies and have increased short-term and long-term mortality [16]. They posit that the critical technological advances of neuronavigation, intraoperative awake mapping, and intraoperative magnetic resonance imaging may not be affordable for SNHs [16]. The disparity in resources may be compounded by the socioeconomic status of the surrounding geographic area. Indeed, it has been shown that small metropolitan areas have higher poverty rates than large metropolitan areas [33, 34]. Further, lower median income of a health service area is associated with a lower likelihood that glioblastoma patients underwent successful maximal tumor resection, had access to oncology departments, and received standard-of-care therapy [31, 35]. Importantly, receipt of standard-of-care treatment is positively correlated with favorable outcomes such as longer survival [16, 35,36]. Geographic variations in socioeconomic status and hospital infrastructure and resources may account for disparities in brain tumor treatment, and could also be influencing the higher readmission rate at SNHs in small metropolitan areas [31, 32, 37, 38].
Conversely, there was no significant difference in the readmission rates between SNHs and non-SNHs in large metropolitan areas. SNHs in these areas may have access to more resources and care networks that can prevent unplanned readmissions. These areas tend to have a higher density of surgical as well as medical and radiation oncological resources, which facilitate the provision of optimal therapy, thereby improving survival and likely limiting the risk of readmission in large metropolitan areas [31, 32, 34, 36]. Indeed, SNHs and non-SNHs in urban areas have been shown to have similar outcomes with regards to mortality and readmission for patients with acute myocardial infarction, heart failure, and pneumonia [39]. Therefore, SNHs in large metropolitan areas may be better equipped to treat medically and surgically complex patients with brain tumors, and may have better access to services that assist in reducing readmissions [40].
In addition to differential access to well-equipped hospitals based on geography, access to high-volume hospitals also impacts readmission rates. This study found that patients with brain tumors treated at low-volume (≤90th percentile (103 craniotomies per year)) hospitals were more likely to be readmitted within 30 days. Prior research has shown that patients with brain tumors who undergo treatment at high-volume hospitals and centers or are treated by neurosurgeons who see a high volume of brain tumor patients experience fewer complications, have shorter hospitalizations, are less likely to be readmitted within 30 days, and have lower 30- and 90-day mortality rates [3, 41–46]. Lopez Ramos et al. compared 30-day readmission rates of glioblastoma patients who traveled short distances and received care at low-volume hospitals against those who traveled long distances to undergo care at high-volume hospitals. They found that those treated at high-volume hospitals were less likely to be readmitted within 30 days despite traveling a longer distance, which may typically be thought of as an obstacle to care [37, 43, 47]. Interestingly, patients who were able to travel long distances to receive care at a high-volume hospital were less likely to be underinsured [43]. Access to high-volume centers may be important to overcoming other barriers to care, such as geographic or resource limitations.
Additionally, indicators of socioeconomic status, such as median household income and primary insurance type, were associated with readmission. This relationship between socioeconomic status and readmission has been observed generally, and in neurosurgical contexts specifically [2, 4, 7]. In the current study, higher median household income was associated with lower likelihood of unplanned readmission. Similarly, patients with private insurance were less likely to be readmitted within 30 days relative to patients whose care was covered by Medicaid or uncompensated for, further underscoring the influence of insurance status on both the ability to receive care and health outcomes. Another study using the NRD also found that patients with malignant brain tumors with Medicaid or Medicare insurance were more likely to be readmitted within 30- and 90-days [2].
Severity of disease, as indicated by neurological loss of function and number of comorbidities at presentation, has been shown to influence the likelihood of unplanned 30-day readmission in our study and in others [2–4, 7]. Patients with malignant brain tumors are more likely to be readmitted within 30 days compared to patients with benign tumors. Moreover, higher All Patient Refined Diagnosis Related Groups (APR-DRG) severity of illness subclass, higher Elixhauser comorbidity index score, and presence of the select comorbidities of diabetes mellitus, coronary artery disease, and chronic kidney disease were associated with increased likelihood of unplanned readmission within 30 days. Initial presentation to the emergency department may indicate more severe symptoms and disease, and this was also associated with increased likelihood of non-elective 30-day readmission [7]. Patients presenting with more severe disease may represent a medically complex patient population. This may lead to longer initial hospital stays and non-routine discharges to health care facilities other than home [2–4]. Indeed, we found that length of stay greater than 3 days was associated with increased odds of 30-day non-elective readmission. Non-routine discharge was also associated with greater likelihood of unplanned readmission within 30 days. Other studies have similarly identified multiple medical comorbidities and discharge to nursing facilities as predictors of non-elective readmission within 30 and 90 days [2, 4]. Finally, treatment at a SNH versus a non-SNH in a small metropolitan area remained significantly associated with increased likelihood of unplanned readmission in post-hoc analysis, even when including Elixhauser comorbidity scores and select comorbidities (diabetes, coronary artery disease, chronic kidney disease) into multivariate models. The persistence of the main finding of increased odds of readmission within 30 days if treated at a small metropolitan SNH despite adjusting for these comorbidity metrics further evidences disparities in the ability for hospitals and health systems to manage medically complex patients in small versus large counties. Studies have highlighted that readmission in such populations may be a result of healthcare systems that may be unable to discharge patients with complete understanding of their pathology, without conveying information on symptom management, medication use and follow-up care [48]. These issues may be compounded at SNHs in small metropolitan areas due to geographic location and resource limitations. Therefore, addressing these underlying factors may provide an opportunity to improve readmission rates at SNHs in small metropolitan areas.
Risk models employed by the Centers for Medicare and Medicaid Services (CMS) evaluating readmission do not focus on specific populations with higher risk of readmission, and unfortunately may penalize hospitals, such as SNHs caring for particularly vulnerable populations [49]. Because 58% of the national variation in readmission rate is influenced by geography and county level factors [27], suggestions to improve unplanned readmission rate have thus included increasing the funding of these SNHs through an adjusted reimbursement rate that accounts for county level health and readmission risk factors Through these steps, prior studies have suggested that SNHs in small metropolitan areas could have more capital to invest in technology and staff to reduce the disparity in resources and patient follow-up and education.
Limitations
As with any large database study there are limitations inherent to using the Nationwide Readmissions Database (NRD). The NRD relies on accurate documentation, data entry, and linkage number assignment, therefore any error of coding and data handling imposes a limitation. The coding accuracy of discharges generally is estimated to be 80% [50]. Further, the analysis is limited by the data and codes provided. For example, patient diagnosis and procedure variables are limited to available International Classification of Diseases, Ninth Edition (ICD-9) codes. This coding restriction prevents the ability to tell if a malignant tumor was primary or secondary, impairs the differentiation between different types of malignant tumors such as glioblastoma multiforme from other metastatic tumors, and denies insight into whether or not a given patient’s tumor operation was an initial operation or a re-operation [2]. Coding restrictions further do not provide information to assess duration of the operation or complexity of the surgical procedure and approach.
Additional limitations specific to the NRD include the censorship of patient race, hospital identifiers, and the state in which the hospital is located, which limits the ability to conduct additional socioeconomic and geographic comparisons. Since the NRD also does not keep track of patients who travel across state lines for their care, the data fails to include patients who were readmitted in a state that is not included in the NRD. This may be relevant as many patients with brain tumor may choose to travel or be transferred across state lines to receive care at tertiary care centers given their complex medical condition. The NRD also only tracks patients for a single calendar year, so 30-day readmissions data excludes data related to admissions beyond November for a given year. It is also important to acknowledge that the NRD hospital identifier number changes yearly, so the total number of hospitals identified over the five-year period (2010–2014) is about five times the actual number of hospitals in the NRD. Therefore, we focused on the proportions of hospitals, rather than counts. Finally, the publicly available subsequent years of NRD data, 2015–2016, were not included, as coding was significantly altered via ICD-10 coding, potentially creating a more heterogenous cohort for analysis. Despite these limitations, the NRD has been widely utilized to explore readmission trends across a variety of subspecialties [2, 25].
Conclusion
Using the Nationwide Readmissions Database, we estimated the unplanned 30-day readmission rate for patients who underwent craniotomy for resection of a brain tumor, and evaluated patient and hospital level factors associated with readmission. The all patient 30-day readmission rate was 22.8% with the most frequent causes of readmission being secondary malignant neoplasm of the brain or spine, post-operative infection, and septicemia. In small metropolitan areas, patients treated at SNHs were more likely to be readmitted within 30 days compared to patients treated at non-SNHs. Multiple health system factors potentially drive this finding. Safety net hospitals play a significant role in the U.S. health care system by providing care to many underserved patients nationwide. Further study may be directed to evaluate factors that contribute to the disparity between SNHs and non-SNHs in small metropolitan areas to determine if this difference in readmission rate exists in other subspecialty contexts, and evaluate interventions to remedy this inequity in unplanned readmission rates.
Acknowledgements:
UL1TR001855 and UL1TR000130 grants from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health for Dr. Li Ding. National Institutes of Health Southern California Clinical and Translational Science Institute KL2 Clinical and Translational Research Scholar Award to Dr. Frank J. Attenello. Southern California Clinical and Translational Science Institute Research Career Development Award to Dr. Frank J. Attenello.
Funding:
FJA is supported by a NIH SC CTSI KL2 Clinical and Translational Research Scholar Award. FJA is supported by a SC CTSI Research Career Development Award. LD is supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health.
Footnotes
Conflicts of interest/Competing interests: The authors declare that they have no conflicts of interest.
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Availability of data and material:
Data deposition does not apply as data was derived from and is available within the Nationwide Readmissions Database, which is publicly available, as it is part of the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality.
References
- 1.Ostrom Quinn T., et al. “CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012.” Neuro-Oncology, vol. 17, no. suppl 4, 26 October. 2015, pp. iv1–iv62., doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donoho Daniel A., et al. “Predictors of 30- and 90-Day Readmission Following Craniotomy for Malignant Brain Tumors: Analysis of Nationwide Data.” Journal of Neuro-Oncology, vol. 136, no. 1, 7 October. 2017, pp. 87–94., doi: 10.1007/s11060-017-2625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuño M, Ly D, Ortega A, Sarmiento JM, Mukherjee D, Black KL, Patil CG (2014) Does 30-day readmission affect long-term outcome among glioblastoma patients? Neurosurgery 74(2):196–205 [DOI] [PubMed] [Google Scholar]
- 4.Marcus LP, McCutcheon BA, Noorbakhsh A, Parina RP, Gonda DD, Chen C, Chang DC, Carter BS (2014) Incidence and predictors of 30-day readmission for patients discharged home after craniotomy for malignant supratentorial tumors in California (1995–2010) Clinical article. J Neurosurg 120(5):1201–1211 [DOI] [PubMed] [Google Scholar]
- 5.Taylor BE, Youngerman BE, Goldstein H, Kabat DH, Appelboom G, Gold WE, Connolly ES Jr (2016) Causes and timing of unplanned early readmission after neurosurgery. Neurosurgery 79(3):356–369 [DOI] [PubMed] [Google Scholar]
- 6.Dickinson H, Carico C, Nuño M, Mukherjee D, Ortega A, Black KL, Patil CG (2015) Unplanned readmissions and survival following brain tumor surgery. J Neurosurg 122(1):61–68 [DOI] [PubMed] [Google Scholar]
- 7.Moghavem Nuriel, et al. “Cranial Neurosurgical 30-Day Readmissions by Clinical Indication.” Journal of Neurosurgery, vol. 123, no. 1, 6 February. 2015, pp. 189–197., doi: 10.3171/2014.12.jns14447. [DOI] [PubMed] [Google Scholar]
- 8.Sutton JP (Social and Scientific Systems), Washington RE (Council for Affordable Quality Healthcare), Fingar KR (Truven Health Analytics), Elixhauser A (AHRQ). Characteristics of Safety-Net Hospitals, 2014. HCUP Statistical Brief #213. October 2016. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb213-Safety-Net-Hospitals-2014.pdf. [PubMed] [Google Scholar]
- 9.Institute of Medicine (US) Committee on the Changing Market, Managed Care, and the Future Viability of Safety Net Providers; Ein Lewin M, Altman S, editors. America’s Health Care Safety Net: Intact but Endangered. Washington (DC): National Academies Press (US); 2000. [PubMed] [Google Scholar]
- 10.Mokdad AA, Murphy CC, Pruitt SL, Mansour JC, Marrero JA, Singal AG, et al. Effect of hospital safety net designation on treatment use and survival in hepatocellular carcinoma. Cancer. 2018;124(4):743–51. 10.1002/cncr.31066 [DOI] [PubMed] [Google Scholar]
- 11.Salerno Amy M et al. “Trends in readmission rates for safety net hospitals and non-safety net hospitals in the era of the US Hospital Readmission Reduction Program: a retrospective time series analysis using Medicare administrative claims data from 2008 to 2015.” BMJ open vol. 7,7 e016149. 13 July. 2017, doi: 10.1136/bmjopen-2017-016149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyer Erik H et al. “Patterns of Hospital Performance on the Hospital-Wide 30-Day Readmission Metric: Is the Playing Field Level?” Journal of general internal medicine vol. 33,1 (2017): 57–64. doi: 10.1007/s11606-017-4193-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talutis SD, Chen Q, Wang N, Rosen AK. Comparison of Risk-Standardized Readmission Rates of Surgical Patients at Safety-Net and Non–Safety-Net Hospitals Using Agency for Healthcare Research and Quality and American Hospital Association Data. JAMA Surg. Published online January 16, 2019. doi: 10.1001/jamasurg.2018.5242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the Hospital Readmissions Reduction Program. N Engl J Med. 2016;374(16):1543–1551. doi: 10.1056/NEJMsa1513024 [DOI] [PubMed] [Google Scholar]
- 15.Gilman Matlin, et al. “California Safety-Net Hospitals Likely To Be Penalized By ACA Value, Readmission, And Meaningful-Use Programs.” Health Affairs, vol. 33, no. 8, August. 2014, pp. 1314–1322., doi: 10.1377/hlthaff.2014.0138. [DOI] [PubMed] [Google Scholar]
- 16.Brandel Michael G., et al. “Management of Glioblastoma at Safety-Net Hospitals.” Journal of Neuro-Oncology, vol. 139, no. 2, 24 April. 2018, pp. 389–397., doi: 10.1007/s11060-018-2875-8. [DOI] [PubMed] [Google Scholar]
- 17.Dupree JM, Neimeyer J, McHugh M. An advanced look at surgical performance under Medicare’s hospital-inpatient value-based purchasing program: who is winning and who is losing? J Am Coll Surg. 2014;218:1–7. [DOI] [PubMed] [Google Scholar]
- 18.Diao K, Sun Y, Yoo SK, et al. Safety- net versus private hospital setting for brain metastasis patients treated with radiosurgery alone: Disparities in follow- up care and outcomes. Cancer 2018;124:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cote DJ, Karhade AV, Larsen AMG, Burke WT, Castlen JP, Smith TR. United States neurosurgery annual case type and complication trends between 2006 and 2013: an American College of Surgeons National Surgical Quality Improvement Program analysis. J Clin. Neurosci 2016;31:106–111. [DOI] [PubMed] [Google Scholar]
- 20.3M Health Information Systems: Averill RF, Goldfield, Hughes JS, Bonazelli J, McCullough EC, Steinbeck BA, Mullin R, Tang AM; National Association of Children’s Hospitals and Related Institutions, Inc.: Muldoon J, Turner L; Medical Advisory Committee for NACHRI APR-DRG Research Project: Gay J. (2003). All Patient Refined Diagnosis Related Groups (APR-DRGs) Version 20.0 Methodology Overview [PDF file]. Retrieved from: https://hcup-us.ahrq.gov/db/nation/nis/APR-DRGsV20MethodologyOverviewandBibliography.pdf. [Google Scholar]
- 21.Tang Austin M., et al. “Nonindex Readmission After Ruptured Brain Aneurysm Treatment Is Associated with Higher Morbidity and Repeat Readmission.” World Neurosurgery, 5 July 2019, doi: 10.1016/j.wneu.2019.06.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser A, Steiner C, Harris DR, Coffey RM. “Comorbidity measures for use with administrative data.” Medical Care, 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 23.Bakhsheshian Joshua, et al. “Safety-Net Hospitals Have Higher Complication and Mortality Rates in the Neurosurgical Management of Traumatic Brain Injuries.” World Neurosurgery, vol. 119, November. 2018, doi: 10.1016/j.wneu.2018.07.134. [DOI] [PubMed] [Google Scholar]
- 24.Minhas Shobhit V., et al. “Nationwide 30-Day Readmissions After Elective Orthopedic Surgery.” Journal for Healthcare Quality, vol. 39, no. 1, January. 2017, pp. 34–42., doi: 10.1097/jhq.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 25.Zafar SN, Shah AA, Channa H, Raoof M, Wilson L, Wasif N. Comparison of Rates and Outcomes of Readmission to Index vs Nonindex Hospitals After Major Cancer Surgery. JAMA Surg. 2018;153(8):719–727. doi: 10.1001/jamasurg.2018.0380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos Christian Lopez, et al. “The Effect of Hospital Safety-Net Burden on Outcomes, Cost, and Reportable Quality Metrics after Emergent Clipping and Coiling of Ruptured Cerebral Aneurysms.” Journal of Neurosurgery, 22 February. 2019, pp. 1–9., doi: 10.3171/2018.10.jns18103. [DOI] [PubMed] [Google Scholar]
- 27.Herrin J, St Andre J, Kenward K, Joshi MS, Audet AM, Hines SC. Community factors and hospital readmission rates. Health Serv Res. 2015;50(1):20–39. doi: 10.1111/1475-6773.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horwitz LI, Bernheim SM, Ross JS, et al. Hospital characteristics associated with risk-standardized readmission rates. Med Care. 2017;55(5):528–534. doi: 10.1097/MLR.0000000000000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3(5):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daras LC, Ingber MJ, Deutsch A, Hefele JG, Perloff J. Geographic region and profit status drive variation in hospital readmission outcomes among in- patient rehabilitation facilities in the United States. Arch Phys Med Rehabil. 2018;99(6):1060–1066. [DOI] [PubMed] [Google Scholar]
- 31.Aneja S, Khullar D, Yu JB. The influence of regional health system characteristics on the surgical management and receipt of post operative radiation therapy for glioblastoma multiforme. J Neurooncol. 2013;112(3):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aneja S, Smith BD, Gross CP, Wilson LD, Haffty BG, Roberts K, Yu JB. Geographic analysis of the radiation oncology workforce. Int J Radiat Oncol Biol Phys. 2011. doi: 10.1016/j.ijrobp.2011.01.070. [DOI] [PubMed] [Google Scholar]
- 33.Stone LC, Boursaw B, Bettez SP, Larzelere Marley T, Waitzkin H. Place as a predictor of health insurance coverage: A multivariate analysis of counties in the United States. Health Place. 2015;34:207 14. doi: http://dx.doi.org.libproxy2.usc.edu/10.1016/j.healthplace.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz Adrian, et al. “Geographic Distribution of Adult Inpatient Surgery Capability in the USA.” Journal of Gastrointestinal Surgery, vol. 23, no. 8, 7 January. 2019, pp. 1652–1660., doi: 10.1007/s11605-018-04078-9. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence YR, Mishra MV, Werner-Wasik M, Andrews DW, Showalter TN, Glass J, Shen X, Symon Z, Dicker AP. Improving prognosis of glioblastoma in the 21st century: who has benefited most? Cancer. 2012;118:4228–4234. doi: 10.1002/cncr.26685. [DOI] [PubMed] [Google Scholar]
- 36.Stupp R, Mason WP, van den Bent MJ et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 37.Probst JC, Laditka SB, Wang JY, Johnson AO (2007) Effects of residence and race on burden of travel for care: cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Serv Res 7:40. 10.1186/1472-6963-7-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherwood PR, Dahman BA, Donovan HS, Mintz A, Given CW, Bradley CJ. Treatment disparities following the diagnosis of an astrocytoma. J Neurooncol. 2011;101(1):67–74. doi: 10.1007/s11060-010-0223-8. [DOI] [PubMed] [Google Scholar]
- 39.Ross JS, Bernheim SM, Lin Z, et al. Mortality and Readmission at Safety Net and Non-Safety Net Hospitals for Three Common Medical Conditions. Health Aff (Millwood). 2012;31(8):1739–1748. doi: 10.1377/hlthaff.2011.1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Figueroa JF, Joynt KE, Zhou X, Orav EJ, Jha AK. Safety-Net Hospitals Face More Barriers Yet Use Fewer Strategies to Reduce Readmissions. Med Care. 2017;55(3):229–235. doi: 10.1097/MLR.0000000000000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauser A, Dutta SW, Showalter TN, Sheehan JP, Grover S, Trifiletti DM. Impact of academic facility type and volume on post-surgical outcomes following diagnosis of glioblastoma. J Clin Neurosci. 2018;47:103–110. [DOI] [PubMed] [Google Scholar]
- 42.Ramakrishna Rohan, Hsu Wei-Chun, Mao Jialin, Art Sedrakyan, Surgeon Annual and Cumulative Volumes Predict Early Postoperative Outcomes After Brain Tumor Resection, World Neurosurgery, Volume 114, 2018, Pages e254–e266, 10.1016/j.wneu.2018.02.172. [DOI] [PubMed] [Google Scholar]
- 43.Ramos Christian Lopez, et al. “The Impact of Traveling Distance and Hospital Volume on Post-Surgical Outcomes for Patients with Glioblastoma.” Journal of Neuro-Oncology, vol. 141, no. 1, 20 November. 2018, pp. 159–166., doi: 10.1007/s11060-018-03022-w. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Ping, et al. “Improved Survival of Glioblastoma Patients Treated at Academic and High-Volume Facilities: a Hospital-Based Study from the National Cancer Database.” Journal of Neurosurgery, 15 February. 2019, pp. 1–12., doi: 10.3171/2018.10.jns182247. [DOI] [PubMed] [Google Scholar]
- 45.Barker FG 2nd, Curry WT Jr, Carter BS (2005) Surgery for primary supratentorial brain tumors in the United States, 1988 to 2000: the effect of provider caseload and centralization of care. Neuro Oncol 7:49–63. 10.1215/s1152851704000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cowan JA Jr, Dimick JB, Leveque JC, Thompson BG, Upchurch GR Jr, Hoff JT (2003) The impact of provider volume on mortality after intracranial tumor resection. Neurosurgery 52:48–53 [DOI] [PubMed] [Google Scholar]
- 47.Syed ST, Gerber BS, Sharp LK (2013) Traveling towards disease: transportation barriers to health care access. J Community Health 38:976–993. 10.1007/s10900-013-9681-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Epstein AM. Revisiting readmissions—changing the incentives for shared accountability. N Engl J Med 2009;360:1457–9. 10.1056/NEJMe0901006 [DOI] [PubMed] [Google Scholar]
- 49.Bhalla R, Kalkut G. Could Medicare readmission policy exacerbate health care system inequity? Ann Intern Med. 2010;152(2):114–117. doi: 10.7326/0003-4819-152-2-201001190-00185 [DOI] [PubMed] [Google Scholar]
- 50.Burns EM, Rigby E, Mamidanna R, Bottle A, Aylin P, Ziprin P, Faiz O (2012) Systematic review of discharge coding accuracy. Journal of Public Health 34(1):138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data deposition does not apply as data was derived from and is available within the Nationwide Readmissions Database, which is publicly available, as it is part of the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality.


