Abstract
The new genus Lacazia P. Taborda, V. Taborda, et McGinnis is proposed to accommodate Lacazia loboi (O. M. Fonseca et Lacaz) P. Taborda, V. Taborda, et McGinnis, the obligate pathogen that causes lobomycosis in mammals. The continued placement of that fungus in the genus Paracoccidioides Almeida as Paracoccidioides loboi is taxonomically inappropriate. Loboa loboi Ciferri et al. is a synonym of Paracoccidioides brasiliensis.
In 1930, Jorge Lobo (14) described a previously unknown infection in human cutaneous and subcutaneous tissue. He believed that the etiologic agent (15) was a fungus similar to Paracoccidioides brasiliensis (Splendore) Almeida, in that the fungus consisted of solitary, budding yeast cells and pseudohyphae in tissue. It was reported that tissue taken from the original patient’s lesion was placed upon Sabouraud glucose agar and a fungus was isolated. This isolate was described as Glenosporella loboi O. Fonseca f. et Leão 1940 (9) but was, in fact, P. brasiliensis (3). The current consensus is that this etiologic agent of mycotic infection is an obligate pathogen of humans and other mammals which has not been cultivated in vitro.
In 1971, Fonseca and Lacaz (10) proposed the new species name Paracoccidioides loboi for the etiologic agent of lobomycosis. The proposal included the designation of neotype material in the form of human tissue sections that were distributed to several culture collections and herbaria throughout the world. No single slide was designated as a holotype. The proposed name of the agent was invalidly published, because a Latin description was not included (article 36.1 of reference 11). To validate the name (article 45 of reference 11), in 1996, Lacaz provided the required Latin description (12), restated the facilities to which the “neotypus” (actually syntype) had been distributed, and gave the reference to the proposed new species name.
Lacaz informed us that the syntype tissue section that had been maintained at the Instituto de Medicina Tropical de São Paulo (IMTSP) has been lost. Based upon our study of the lectotype (BPI 792295, U.S. National Fungus Collections) and tissue sections from 35 patients living in the Amazon region of Brazil, we conclude that no existing genus can accommodate this taxon. We propose a new genus and binomial for the obligate pathogen that causes lobomycosis.
Taxonomy of Lacazia P. Taborda, V. Taborda, et McGinnis gen. nov. (i) Type species.
Paracoccidioides loboi O. M. Fonseca et Lacaz (12) (synonymous with L. loboi [O. M. Fonseca et Lacaz] comb. nov.).
(ii) English description.
In vivo, globose to subglobose cells producing blastoconidia connected by a narrow tubular connector, solitary, catenulate, or both, with the latter variable in branching.
(iii) Latin description.
In vivo, globosae et subglobosae cellulae producentes blastoconidia coniuncta ab angustaligatione in specie tubi, solae, catenulatae vel ambo sed his variabilibus pro summa ramorum.
(iv) Etymology.
The generic name Lacazia has been selected to acknowledge the major contributions to our understanding of lobomycosis made by Carlos da Silva Lacaz.
Taxonomy of Lacazia loboi.
(O. M. Fonseca et Lacaz), comb. nov.
(i) Basionym.
Paracoccidioides loboi O. M. Fonseca et Lacaz (12), illustrated in Fig. 9 of reference 10.
(ii) Lectotypus.
Slide BPI 792295 in the U.S. National Fungus Collections, human cutaneous-subcutaneous lesion, IMTSP.
(iii) Syntypes.
Deposited by Lacaz in 1971 at the Mycological Herbarium (DAOM), Agriculture Canada, Ottawa, Canada; the Commonwealth Mycological Institute (CMI), Surrey, United Kingdom; the Laboratory of Cryptogamy (LCP), National Museum of Natural History, Paris, France; and the University of Uppsala (UPS), Uppsala, Sweden.
(iv) English description.
In vivo, globose to subglobose cells producing blastoconidia connected by narrow connectors, cells 7.6 to 7.9 μm (range 5 to 11 μm). Catenulate, chains of various lengths and degrees of branching. Constitutive melanin present in yeast cell walls. Cell walls of older cells increase in thickness, resulting in the separation of adjacent cells.
(v) Etymology.
The specific epithet loboi was proposed by Fonseca and Leão (9) and validly published by Lacaz in 1996 (12) to honor the Brazilian dermatologist Jorge O. Lobo because of his discovery of this mycosis.
(vi) Disease nomenclature.
We support the disease name lobomycosis accepted by the Nomenclature Committee of the International Society for Human and Animal Mycology (17).
(vii) Materials examined.
Materials containing L. loboi included lectotypus BPI 792295 from the U.S. National Fungus Collections and samples from 35 cases from Instituto L.S. Lima (ILSL): ILSL B95-1480 and B96-1071, patient 1, 76-year-old male, diffuse lesions, Acre (AC), Brazil (BR); ILSL B95-1423, patient 2, 55-year-old male, left ear, AC, BR; ILSL B95-1484, patient 3, 54-year-old male, right ear, AC, BR; ILSL B95-1485, patient 4, 52-year-old male, diffuse, AC, BR; ILSL B95-1488, patient 5, 29-year-old female, right leg, AC, BR; ILSL B95-1575 and B96-263, patient 6, 34-year-old male, left ear, AC, BR; ILSL B95-1490, patient 7, 72-year-old male, diffuse, AC, BR; ILSL B95-1491, patient 8, 29-year-old female, right leg, AC, BR; ILSL B96-1082, patient 9, 77-year-old male, right cheek, AC, BR; ILSL B95-1492 and B95-1493, patient 10, 74-year-old male, face, AC, BR; ILSL B95-1494, patient 11, 70-year-old male, left ear, AC, BR; ILSL B95-1495 and B96-1080, patient 12, 72-year-old male, right ear, AC, BR; ILSL B95-1496 and B96-1072, patient 13, 72-year-old male, left ear, AC, BR; ILSL B95-1497 and B96-1078, patient 14, 64-year-old male, right ear, AC, BR; ILSL B95-1498 and B96-914, patient 15, 20-year-old male, right ear, AC, BR; ILSL B95-2088, patient 16, 48-year-old male, right foot and leg, AC, BR; ILSL B95-2089, patient 17, 35-year-old male, right elbow, AC, BR; ILSL B95-2090, patient 18, 35-year-old male, right ear, AC, BR; ILSL B95-2025 and B96-1079, patient 19, 59-year-old male, left leg, AC, BR; ILSL B95-2091, patient 20, 53-year-old male, left leg, AC, BR; ILSL B95-2092 and B96-1077, patient 21, 51-year-old male, left thigh, AC, BR; ILSL B95-2093 and B96-1083, patient 22, 46-year-old male, right ear, AC, BR; ILSL B95-2024, patient 23, 43-year-old male, right ear, AC, BR; ILSL B95-2094 and B96-1073, patient 24, 65-year-old male, legs, AC, BR; ILSL B96-264, patient 25, 56-year-old male, right elbow, AC, BR; ILSL B95-2096, patient 26, 29-year-old male, left ear, AC, BR; ILSL B95-2040, patient 27, 40-year-old male, chest, AC, BR; ILSL B95-2553, patient 28, 48-year-old female, diffuse, AC, BR; ILSL B95-2098, patient 29, 53-year-old male, right thigh, AC, BR; ILSL B95-2099 and B96-1074, patient 30, 78-year-old male, left ear, AC, BR; ILSL B95-2100 and B96-1081, patient 31, 62-year-old male, left ear, AC, BR; ILSL B96-1075, patient 32, 52-year-old male, right ear, AC, BR; ILSL B96-1471, patient 33, 42-year-old male, right ear, AC, BR; ILSL B86-755 and B87-1025, patient 34, 78-year-old male, ears, BR; and ILSL B95-2097, patient 35, 44-year-old male, right arm, AC, BR. Materials yielding P. brasiliensis were from five culture-proven human cases from the ILSL: ILSL 95-042, patient 36, 56-year-old female, cervical lymph node, São Paulo (SP), BR; ILSL B95-170, patient 37, 42-year-old male, right foot, SP, BR; ILSL B95-940, patient 38, 40-year-old male, mouth, SP, BR; ILSL B95-1762, patient 39, 36-year-old female, cervical lymph node, SP, BR; and ILSL B96-2772, patient 40, 49-year-old male, face, SP, BR.
Discussion.
It is clearly recognized that P. brasiliensis is a fungus that can be isolated from human tissue, as well as from animals inoculated with environmental material. The isolate that was originally thought to be the agent of lobomycosis probably represents a laboratory isolate from a different patient, because the original patient’s condition did not have the features of an infection caused by P. brasiliensis (2, 15). The binomial Glenosporella loboi O. Fonseca f. et Leão 1940 was based upon an isolate now known to be P. brasiliensis (3, 10). Another isolate believed to be the etiologic agent of lobomycosis was described as Glenosporopsis amazonica O. Fonseca f. 1943 (8). This fungus (18) was most likely an environmental contaminant that is identifiable as Aspergillus penicillioides Spegazzini.
Fonseca and Lacaz (10) proposed the new species name P. loboi, without the required Latin description, based upon the fungus in human tissue sections that they studied and designated as “neotypus.” In actuality, the tissue sections are not a neotype, because these authors were specifically establishing a new species for the agent of lobomycosis independent of the use of cultures. Lacaz has informed us that the original slide maintained at IMTSP has been lost. Therefore, we designate BPI 792295 as lectotypus (article 9.2 of reference 11) for this taxon and designate the other tissue sections they sent to various collections throughout the world as syntypes (article 9.4 of reference 11).
Validation of the new species name P. loboi was completed in 1996 when the required Latin description was provided (article 45.1 of reference 11). According to recommendation 32C.1 of reference 11, adoption of a binomial previously but not validly published for a different taxon should be avoided. Such recommendation did not invalidate the specific epithet loboi as suggested by Borelli (4).
Additional confusion resulted when the new generic name and combination Loboa loboi Ciferri, Azevedo, et Carneiro 1956 (5) was proposed. This proposal was based upon the culture identified as Glenosporella loboi, a fungus known to be identical with P. brasiliensis. Even though this name is popular, L. loboi Ciferri, Azevedo, et Carneiro is a synonym of P. brasiliensis and taxonomically not related to the agent of lobomycosis.
In 1952, Langeron and Vanbreuseghem (13) used the name Blastomyces loboi, which is an invalidly described binomial (article 32 of reference 11), as their recommendation for the appropriate name to be used for the causal agent of lobomycosis. In 1968, Borelli (4) proposed the generic name Lobomyces for the fungus in the tissue. This proposal was made as a suggestion in passing and is therefore invalid (article 34 of reference 11).
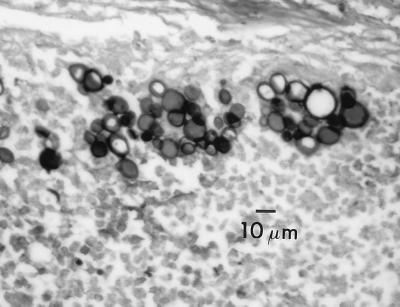
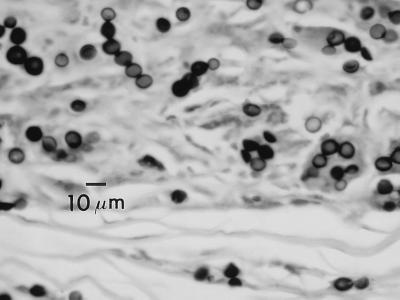
The genus Paracoccidioides Almeida 1930 (1) is characterized in part by the formation of a globose to subglobose solitary yeast cell that forms a nucleus from which multiple daughter blastoconidia are formed. The central cells become extremely large with thickened cell walls (Fig. 1). In contrast, L. loboi forms yeast cells that remain consistent in diameter, giving rise to branching chains of blastoconidia (Fig. 2). In addition, the cell wall of L. loboi contains constitutive melanin that is readily detectable by the use of the Fontana-Masson histologic stain (21). An apparent difference in temperature tolerance in vivo exists between these two species. P. brasiliensis is a pulmonary pathogen that can disseminate by hematogenous means, including associating with tegumentary surfaces, whereas L. loboi is known to be associated only with cutaneous-subcutaneous tissue, especially tissue with a lower temperature, such as the skin of ears, elbows, the anterior aspect of legs, and ankles.
FIG. 1.
Lacazia loboi. Human subcutaneous tissue, Gomori methenamine silver stain.
FIG. 2.
Paracoccidioides brasiliensis. Human lung tissue, Gomori methenamine silver stain.
In essence, owing to the fact that L. loboi has not been cultured in vitro, the generic name Lacazia circumscribes a fungus known only from mammalian tissue where it causes lobomycosis. Lesions of the skin and subcutaneous tissue of humans, marine dolphins (Tursiops truncatus Montagu 1821) (6, 20), and marine-freshwater dolphins (Sotalia fluviatilis Gervais 1853) (7) are predominantly localized and keloid-like (solitary or multiple) but can be extensive and either multifocal or diffuse and can combine keloid-like lesions with macular, gummatous, ulcerative, or verrucous surfaces.
Human cases in most Latin American countries have been reported as well as isolated cases in Holland (20) and Bangladesh (19), although in our opinion, the Bangladesh case may not have been lobomycosis. Dolphins with lobomycosis have been found along the Florida and Texas coasts (6), the Spanish-French coast (20), the South Brazilian coast (16), and the Suriname river estuary (7).
Acknowledgments
We thank Carlos da Silva Lacaz, Libero Ajello, and Richard Korf for reviewing the mycological aspects of the manuscript and Diltor A. Opromolla and Raul N. Fleury from the ILSL for their encouragement to conduct this research. Lester L. Pasarell is owed special appreciation for his technical help and stimulating discussions.
REFERENCES
- 1.Almeida F. Estudos comparativos do granuloma coccidióidico nos Estados Unidos e no Brasil. Novo gênero para o parasito brasileiro. An Fac Med Sao Paulo. 1930;5:125–141. [Google Scholar]
- 2.Almeida F, Lacaz C S. -1949. Blastomicose “tipo Jorge Lobo.”. An Fac Med Univ Sao Paulo. 1948;24:5–37. [Google Scholar]
- 3.Artagaveytia-Allende R C, Montemayor L. Estudio comparativo de varias cepas de Paracoccidioides brasiliensis y especies afines. Mycopathologia. 1949;4:356–366. [Google Scholar]
- 4.Borelli D. Lobomicose: nomenclatura de su agente (revisión critica) Med Cutanea. 1968;3:151–156. [Google Scholar]
- 5.Ciferri R, Azevedo P C, Campos S, Carneiro L S. Taxonomy of Jorge Lobo’s disease fungus. Inst Micol Univ Recife. 1956;53:1–21. [PubMed] [Google Scholar]
- 6.Cowen D F. Lobo’s disease in a bottlenose dolphin (Tursiops truncatus) from Matagorda Bay, Texas. J Wildl Dis. 1993;29:488–489. doi: 10.7589/0090-3558-29.3.488. [DOI] [PubMed] [Google Scholar]
- 7.De Vries G A, Laarman J J. A case of Lobo’s disease in the dolphin Sotalia guianensis. Aquat Mamm. 1973;1:26–33. [Google Scholar]
- 8.Fonseca O F. Parasitologia médica. Parasitos e doenças parasitárias do homem. Rio de Janeiro, Brazil: Guanabara-Koogan; 1943. pp. 703–725. [Google Scholar]
- 9.Fonseca O F, Leão A E A. Contribuição para o conhecimento das granulomatoses blastomycoides. O agente etiológico da doença de Jorge Lobo. Rev Med Cir Bras. 1940;48:147–158. [Google Scholar]
- 10.Fonseca O J M, Lacaz C S. Estudo de culturas isoladas de blastomicose queloidiforme (doença de Jorge Lobo). Denominação ao seu agente etiológico. Rev Inst Med Trop Sao Paulo. 1971;13:225–251. [PubMed] [Google Scholar]
- 11.Greuter W, editor. International code of botanical nomenclature (Tokyo code). Koenigstein, Germany: Koeltz Scientific Books; 1994. [Google Scholar]
- 12.Lacaz C S. Paracoccidioides loboi (Fonseca Filho et Area Leão, 1940) Almeida e Lacaz, 1948–1949. Description of the fungus in Latin. Rev Inst Med Trop Sao Paulo. 1996;38:229–231. doi: 10.1590/s0036-46651996000300013. [DOI] [PubMed] [Google Scholar]
- 13.Langeron M, Vanbreuseghem R. Précis de mycologie. Mycologie generale: mycologie humaine et animale techniques. Paris, France: Masson et Cie; 1952. pp. 490–491. [Google Scholar]
- 14.Lobo J O. Nova espécie de blastomycose. Bras-Med. 1930;44:1227. [Google Scholar]
- 15.Lobo J O. Um caso de blastomicose produzido por uma espécie nova, encontrada em Recife. Rev Med Pernambuco. 1931;1:763–765. [Google Scholar]
- 16.Lopes P C S, Paula G S, Both M C, Xavier F M, Scaramello A C. First case of lobomycosis in a bottlenose dolphin from Southern Brazil. Mar Mamm Sci. 1993;9:329–331. [Google Scholar]
- 17.Odds F C, Rinaldi M G. Nomenclature of fungal diseases. Curr Top Med Mycol. 1995;6:40–41. [PubMed] [Google Scholar]
- 18.Raper K B, Fennell D I. The genus Aspergillus. Baltimore, Md: Williams and Wilkins; 1965. p. 234. [Google Scholar]
- 19.Rumi T K, Kapkaev R A. Keloid blastomycosis (Lobo’s disease) Vestn Dermatol Venerol. 1988;62:41–43. [PubMed] [Google Scholar]
- 20.Symmers W S. A possible case of Lobo’s Disease acquired in Europe from a bottle-nosed dolphin (Tursiops truncatus) Bull Soc Pathol Exot. 1983;77:777–784. [PubMed] [Google Scholar]
- 21.Taborda V B A, Taborda P R O, McGinnis M R. Constitutive melanin in the cell wall of the etiologic agent of lobomycosis. Rev Inst Med Trop Sao Paulo. 1999;41:9–12. doi: 10.1590/s0036-46651999000100003. [DOI] [PubMed] [Google Scholar]