Abstract
Background
Clostridium perfringens-associated necrotic enteritis (NE) is a serious problem affecting broiler production. A major global challenge is to reduce the use of antibiotics in poultry industry due to their negative impacts on public health. One alternative is to use nanoparticles (NPs) to overcome bacterial resistance to antibiotics. Silver nanoparticles (Ag NPs) showed strong antimicrobial activity.
Methods
A total of 120 Cobb broiler chicks (1-day old) were obtained for this study and were divided into 4 equal groups at age of 14 days (30 birds each); each group was subdivided into 3 equal replicates (10 birds each). The groups were designated as follows: G1, infected; G2, infected and treated with Ag NPs; G3, treated with Ag NPs; and G4, negative control. Birds were infected with 4×108 colony forming unit (CFU)/mL/bird C. perfringens type A for 2 successive days. In the treated groups, Ag NPs (mean diameter 15 nm; total dose 150 µg/bird) were administered via crop gavage. During the observation period (5 weeks), bird performance and immune organ indexes were recorded. Serum samples were collected for immunological evaluation, and tissue samples were collected for histopathology and estimation of Ag NPs residues.
Results
Treatment with Ag NPs reduced the colonization of C. perfringens in the intestine and ceca, decreased the severity of clinical signs and reduced mortalities in comparison with infected non-treated group. Ag NPs treatment alleviated pathological lesions in the intestine and liver, but their residues were found in the muscles.
Conclusion
Ag NPs have a positive impact on gut health integrity while having no impact on immune organs. Ag NPs have some residues in muscles; therefore, further studies are needed on the concentration and size of Ag NPs, the route of administration, and withdrawal time to ensure the safety of chicken meat for human consumption.
Keywords: broiler chickens, Clostridium perfringens, FCR, histopathology, necrotic enteritis, residues, silver nanoparticles
Introduction
The broiler chicken industry is significant in Egypt, but it is currently facing the challenge of diarrhea infection, a prevalent condition that threatens poultry production.1 Necrotic enteritis (NE) in chickens is caused by Clostridium perfringens, an anaerobic gram-positive, spore-forming, rod-shaped bacterium.2,3 C. perfringens is classified into five toxinogenic types (A–E) according to the production of four different major toxins (alpha, beta, epsilon, and iota).4,5 Recently, novel toxins (Beta2, NetB, and TpeL) have been discovered, necessitating an augmented scheme of classification.6 Necrotic enteritis is usually caused by C. perfringens type A and sometimes by type C.7 C. perfringens is commonly found in the intestines of healthy poultry, normally at levels less than 102–104 CFU/g of intestine content, compared with 107–109 CFU/g in birds with disease.8 The effects of clinical and subclinical NE have been estimated to cost the industry $6 billions per year.9 Acute NE affects broilers between 2 and 6 weeks old, causing 1% mortality daily, with cumulative mortality reaching 10–40%.3,10 Subclinical NE leads to intestinal mucosal damage resulting in reduced digestion and absorption, decreased body weight gain, and increased feed conversion ratio (FCR). It may also cause hepatitis or cholangiohepatitis.11,12 C. perfringens causes serious foodborne enteritis in humans,13 and its presence in human foods, such as chicken meat, may be inevitable.14 Histopathologically, NE is characterized by severe enterocyte necrosis, extensive villous fusion, and severe inflammatory reaction in the lamina propria with severe dilatation of blood capillaries associated with minute hemorrhages in most of the villi, especially in the duodenum and jejunum. In the liver, NE induces severe congestion, portal hepatitis associated with hepatocellular degeneration, and necrosis. In cecal tonsils, it results in severe depletion of lymphoid tissue and lymphocytolysis.15,16 Antibiotic feed additives cause an increased incidence of resistance among the enteric bacteria in broiler chickens, resulting in antibiotic resistance in zoonotic enteropathogens, especially C. perfringens.17 In a previous study, 125 isolates of C. perfringens obtained from clinical broiler chickens infected with NE exhibited complete multidrug resistance to streptomycin, gentamicin, lincomycin, erythromycin, spiramycin, and oxolinic acid and partial resistance to spectinomycin, tylosin–fosfomycin, ciprofloxacin, rifampicin, chloramphenicol, enrofloxacin, neomycin, colistin, pefloxacin, doxycycline, norfloxacin, oxytetracycline, flumequine, and trimethoprim–sulfamethoxazole.17 Nanotechnology is now providing new tools to aid improvements in animal health and productivity and to overcome the problem of multidrug resistance.18 Silver nanoparticles (Ag NPs) have various applications in poultry production, for example, they are used in disinfectant preparations in hatcheries,19 and they are exploited for their antibacterial effect on many gram-negative and gram-positive bacteria;19–24 moreover, they exhibit antibacterial activity against Campylobacter jejuni, Escherichia coli (E. coli), Bacillus spp., Klebsiella pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa.25,26 Ag NPs have also been used as a growth promoter in drinking water for broiler chickens27 and have been reported to improve their health and performance.28 This study aimed to evaluate the antibacterial effect of Ag NPs on experimentally induced NE in broiler chickens and to measure bird performance and immune response.
Materials and Methods
Silver Nanoparticle Preparation and Characterization
Ag NPs were prepared using the chemical reduction method. Silver nitrate was reduced with sodium borohydride and sodium citrate in the presence of polyvinylpyrrolidone as a capping agent.18
Synthesized NPs were characterized by studying the absorption spectra of Ag NPs in solution via UV–Vis spectroscopy (NanoDrop 2000) and by imaging their morphological features via transmission electron microscopy (TEM) (FEI Tecnai G20, the Netherlands) at an acceleration voltage of 200 kV.
Clostridium perfringens Strain Preparation
The pathogenic strain of C. perfringens type A used in this study was obtained from the strain bank at the Department of Poultry Diseases, Faculty of Veterinary Medicine, Cairo University. Under aseptic conditions, the C. perfringens type A strain was inoculated into cooked meat media and anaerobically incubated at 37°C for 18 h. The bacterial cells obtained were then resuspended into phosphate buffered saline (PBS), and the colony count was adjusted using McFarland tube. At 14 days of age, birds in the positive control group and those in the infected group treated with Ag NPs were infected via crop gavage with 4×108 CFU/mL/bird of freshly prepared C. perfringens in PBS on 2 successive days in accordance with the method described by Awaad et al.29
Experimental Design
A total of 120 Cobb broiler chicks (1-day old) were obtained from Cairo Poultry Company, Egypt, and reared on a deep litter system with fresh wood shavings as bedding at a thickness of ~10 cm on a concrete floor. The chicks were housed in optimal conditions of temperature, humidity, and ventilation and maintained under a 24-h constant-light system during the observation period (5 weeks). The birds received balanced ration (including starter, grower, and finisher ration) without any additives and fresh clean water ad libitum. At 14 days of age, 120 chicks were randomly divided into 4 equal groups (30 birds each). Each group was subdivided into three equal replicates (10 birds/replicate). The groups were designated as follows: positive control infected (G1), infected and treated with Ag NPs (G2), treated with Ag NPs (G3), and blank negative control (G4). The vaccination program for all birds included ocular installation of Hitchner B1+H120 vaccine at 7 days old, subcutaneous injection of avian influenza-inactivated H5N2 vaccine at 10 days old, ocular installation of LaSota vaccine at 14 days old, ocular installation of 228E IBDV vaccine at 18 days old, and finally administration of LaSota vaccine in drinking water at 28 days old, following previously published protocols.16,29
Each bird in G2 and G3 received 1 mL of Ag NP suspension (30 µg/mL) via crop gavage for 5 successive days post infection (PI) starting at 14 days old, resulting in a total dose for each bird of 150 µg of Ag NPs.
All the experimental procedures and bird handling were approved from the institutional animal care and use committee of the Faculty of Veterinary Medicine, Cairo University.
Evaluation of Bird Performance
Morbidity and Mortality Rates
General health condition, clinical signs and mortalities were recorded daily.
Body Weight and Feed Conversion Rate (FCR)
For body weight, 15 birds/group (5 birds/replicate) were randomly selected and weighed individually as birds were weighted at week 2, 3, 4, 5. Feed consumption was measured on the same days of weighing birds. FCR is determined by the formula (g feed/g live body weight gain) according to Timmerman et al.30
Sampling for C. perfringens Counting
Three birds were randomly selected and ethically slaughtered from each group (one bird/replicate) at 21, 28 and 35-days post infection (PI).
C. perfringens intestinal and cecal counts; At 7 days PI, 0.2 g from intestinal and cecal contents were collected separately from each bird (3 birds/group) (one bird/replicate) and serially diluted in sterile PBS to 1:100, 1:1000, and 1:10,000; then, 0.1 mL of each dilution was poured onto the surface of sheep blood agar plates and tryptose sulfite cycloserine (TSC) agar (supplemented by D-cycloserine) with egg yolk emulsion and incubated anaerobically using GasPak anaerobic jar and GasPak anaerobic kits for 24 h at 37°C. Typical C. perfringens colonies (black colonies) on the TSC agar, or large dome-shaped colonies with a double zone of hemolysis on the blood agar plates, were counted and reported as CFU/g. The colonies were chosen and confirmed using the criteria of Harmon31 and Garrido et al.32
Macroscopic Lesion Score
Macroscopic lesions were scored according to the six-point system of Keyburn et al33 and Shojadoost et al.34
Histopathological Examination
Intestine, liver, kidneys, and immune organs (thymus glands, bursa of Fabricius (BF), and spleen) were collected and fixed in 10% buffered formalin and then processed, cut, and HE-stained according to the methods described by Bancroft and Gamble.35
Immunological Evaluation
Immune Organ (BF, Thymus Gland and Spleen) Indexes
Immune organs were collected and weighed to calculate their indexes (%) as follows:
Immune organ index = weight of immune organ (g) × 100/live body weight (g).
Humoral Immunity Estimation
To assess the role of Ag NPs on the bird’s immunity, Anti-ND vaccine antibody titers were estimated by collecting blood samples at 0 and 7 days post ND vaccination from the wing veins of 5 birds/group randomly selected, and the sera were subjected to the hemagglutination inhibition (HI) test.36
Determination of Ag NP Tissue Residues
Ag NP residues in muscles were determined via inductively coupled plasma optical emission spectrometry (ICP-OES).21 Briefly, muscle tissue samples (5 g) from both groups treated with Ag NPs (G2 and G3) were sampled at the end of the experiment (day 35). The samples were subjected to microwave digestion under heat and pressure (temperature 200°C; pressure 40 bar) with the addition of 2 mL of nitric acid (30%) before analysis via ICP-OES (Thermo Fisher Scientific, England).
Statistical Analysis
One-way analysis of variance was used to compare the effects of different treatments on growth performance parameters, immune organ indexes, and intestinal clostridial count of the broiler chickens. The Kruskal–Wallis test was employed when data were not normally distributed (lesion scores and HI titers). Data were expressed as means and standard error. Fisher’s exact test was used to assess the relationship between mortality rates and treatment groups. Statistical significance was set to P < 0.05. The PASW Statistics Version 18.0 software (SPSS, Chicago, IL, USA) was used to conduct statistical analyses. The R 3.6.1 software (http://www.r-project.org/) was used to create the chart graphics.
Results
Silver Nanoparticle Characterization
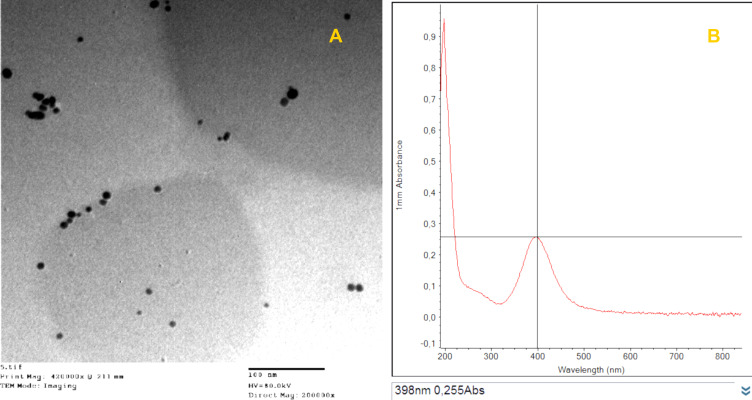
TEM imaging showed dispersed, spherical Ag NPs with a mean diameter of 15 nm (Figure 1A). UV–Vis absorption spectra revealed maximum absorbance of the prepared Ag NPs at 398 nm (Figure 1B).
Figure 1.
Characterization of silver nanoparticles. (A) Transmission electron microscopy photo shows spherical-shaped silver nanoparticles with 15 nm mean diameter (scale bar 100 nm). (B) UV-vis absorption spectra show maximum absorbance peak at 398 nm.
Clinical Signs and Morbidity and Mortality Rates
After experimental infection, clinical signs were clearly observed at 1 week PI in G1, manifesting as general depression, ruffled feathers, and presence of dark orange droppings in 40% of infected birds. In G2, the observed clinical signs were manifest in ~15% of birds, whereas the other 2 groups showed no clinical signs.
The mortality rates in G1 and G2 at 1 week PI were 20% (6/30) and 3.3% (1/30), respectively, whereas the other two groups recorded no mortalities (Table 1). Compared with the positive control group (G1), the reductions in mortality rates for the other groups were significant (P = 0.004).
Table 1.
Effect of Ag NPs on Broiler Growth Performance and Mortality
| Treatmentd | Body Weight (g) | Weight Gain (g) | Feed Intake (g) | FCR (g/g) | Mortality (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D 21 | D 28 | D 35 | D 21 | D 28 | D 35 | D 21 | D 28 | D 35 | D 21 | D 28 | D 35 | ||
| G1 | 787 | 1124 b | 1868 | 388 | 337 c | 744 | 587 | 667 | 1233 | 1.53 ab | 2.00 a | 1.71 | 6/30 (20%) a |
| G2 | 822 | 1232 a | 1955 | 423 | 410 ab | 723 | 560 | 653 | 1227 | 1.34 bc | 1.60 bc | 1.73 | 1/30 (3.3%) ab |
| G3 | 828 | 1265 a | 1987 | 425 | 438 a | 779 | 527 | 647 | 1223 | 1.26 c | 1.49 c | 1.65 | 0/30 (0.0%) b |
| G4 | 787 | 1186 ab | 1874 | 387 | 399 b | 688 | 630 | 667 | 1227 | 1.66 a | 1.69 b | 1.81 | 0/30 (0.0%) b |
| SEM | 10.34 | 14.46 | 32.58 | 7.18 | 6.90 | 17.53 | 0.03 | 0.03 | 0.04 | ||||
| p- value | NS | 0.002 | NS | NS | <0.0001 | NS | <0.0001 | <0.0001 | NS | 0.004 | |||
Notes: a,b,cMean values or percentages with different superscripts in the same column are significantly different (P < 0.05). dTreatments: G1, chicks infected with Clostridium perfringens but not treated with Ag NPs; G2, chicks infected with C. perfringens and treated with Ag NPs; G3, uninfected chicks treated with Ag NPs; G4, chicks neither infected nor treated with Ag NPs.
Abbreviations: FCR, feed conversion ratio (g of feed/g of body weight gain); SEM, standard error of mean; NS, not significant.
Body Weight and FCR
The performance parameters for growth are presented in Table 1. At 28 days of age, the birds that had been treated with Ag NPs (G2 and G3) exhibited significantly higher body weight (P = 0.002) and weight gain (P < 0.0001) than the control birds (G1 and G4). Significant improvements were observed in the FCR of the birds that received Ag NPs (G2 and G3) at days 14 to 28 (P < 0.0001).
C. perfringens Intestinal and Cecal Counts
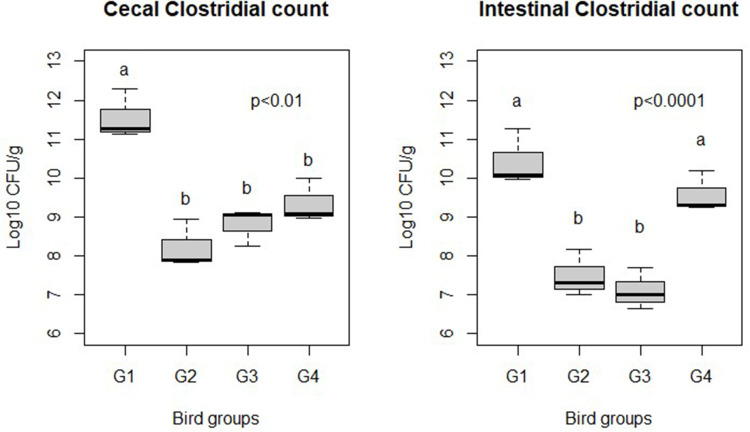
Cecal and intestinal loads of C. perfringens are illustrated in Figure 2. At 7 days PI, G2, G3, and G4 exhibited significant reductions in cecal C. perfringens counts (P = 0.001) compared with G1. Furthermore, both G2 and G3 showed significantly lower intestinal C. perfringens counts compared with G1 and G4 (P < 0.0001).
Figure 2.
Effect of Ag NPs on broiler chicken cecal and intestinal C. perfringens counts (log10 CFU/g). G1: Control positive infected group; G2: Infected and Ag NPs treated group; G3: Ag NPs treated group; G4: Control negative group. a,bDifferent superscripts indicate significant difference (Tukey’s test; P < 0.05).
Immunological Evaluation
Immune Organ (BF, Thymus Gland, and Spleen) Body Indexes
Immune organ weight/body weight ratios are presented in Table 2. There were no differences in the BF index for all treatments. At 21 days of age, G2 demonstrated the lowest thymus weight (P = 0.016), whereas G1 had the lowest spleen weight (P < 0.0001). At 28 days of age, the highest spleen weight was recorded for G3 and the lowest for G1 (P < 0.0001). At day 35, there were no significant differences between different groups in immune organ indexes.
Table 2.
Effect of Ag NPs on Immune Organ Indexes (BF, Thymus Gland, and Spleen)
| Treatmentd | Bursa of Fabricius | Thymus | Spleen | ||||||
|---|---|---|---|---|---|---|---|---|---|
| D 21 | D 28 | D 35 | D 21 | D 28 | D 35 | D 21 | D 28 | D 35 | |
| G1 | 0.138 | 0.127 | 0.080 | 0.884ab | 0.826 | 0.670 | 0.071b | 0.065c | 0.069 |
| G2 | 0.127 | 0.112 | 0.071 | 0.719b | 0.713 | 0.531 | 0.135a | 0.066c | 0.063 |
| G3 | 0.126 | 0.135 | 0.068 | 1.040a | 0.841 | 0.529 | 0.148a | 0.110a | 0.063 |
| G4 | 0.138 | 0.126 | 0.075 | 1.061a | 0.800 | 0.573 | 0.136a | 0.086b | 0.065 |
| SEM | 0.004 | 0.004 | 0.002 | 0.049 | 0.028 | 0.024 | 0.009 | 0.006 | 0.002 |
| p- value | NS | NS | NS | 0.014 | NS | NS | <0.0001 | <0.0001 | NS |
Notes: a,b,cMean values with different superscripts in the same column are significantly different (P < 0.05). dTreatments: G1, chicks infected with Clostridium perfringens but not treated with Ag NPs; G2, chicks infected with C. perfringens and treated with Ag NPs; G3, uninfected chicks treated with Ag NPs; G4, chicks neither infected nor treated with Ag NPs.
Abbreviations: SEM, standard error of mean; NS, not significant.
Humoral Immunity Estimation
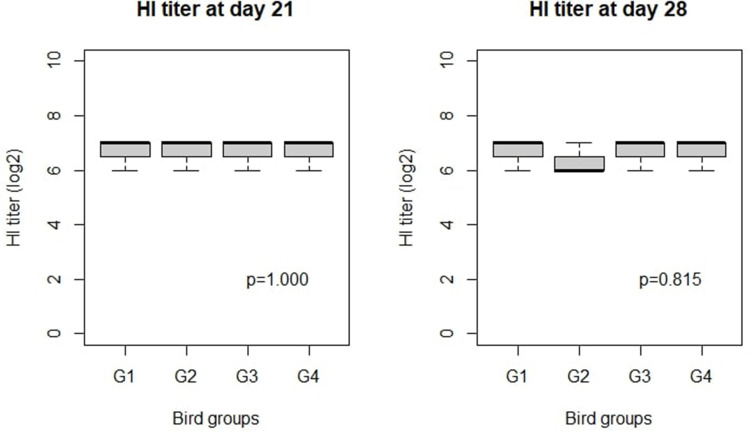
The observed HI titer values after ND vaccination are illustrated in Figure 3. There were no differences in HI titers among the groups at 7 or 14 days PI.
Figure 3.
Effect of Ag NPs on broiler chicken HI titer against NDV. G1: Control positive infected group; G2: Infected and Ag NPs treated group; G3: Ag NPs treated group; G4: Control negative group. Statistical comparison was done by Kruskal–Wallis Test, at significance level P < 0.05.
Macroscopic Lesion Score
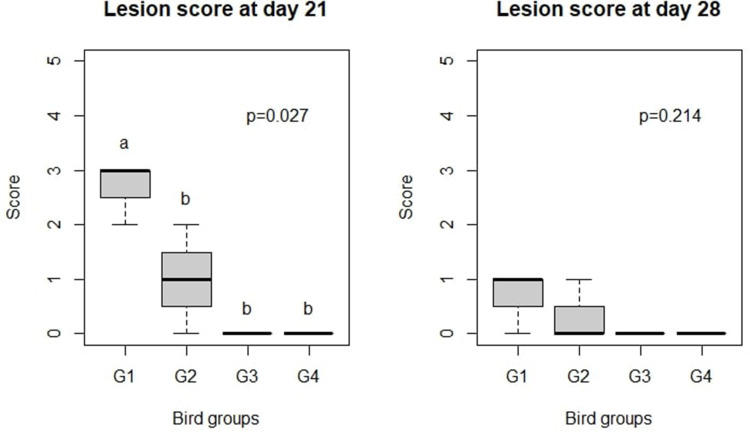
Differences in lesion scores were observed only at day 21 (7 days PI). C. perfringens infection induced higher intestinal lesions (P = 0.027) in G1 compared with G2 (Figure 4).
Figure 4.
Effect of Ag NPs on broiler chicken macroscopic lesion score. G1: Control positive infected group; G2: Infected and Ag NPs treated group; G3: Ag NPs treated group; G4: Control negative group. a,bDifferent superscripts indicate significant difference (Kruskal–Wallis Test; P < 0.05).
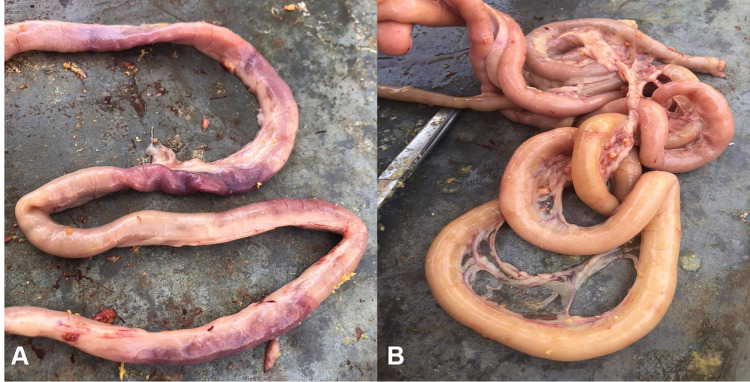
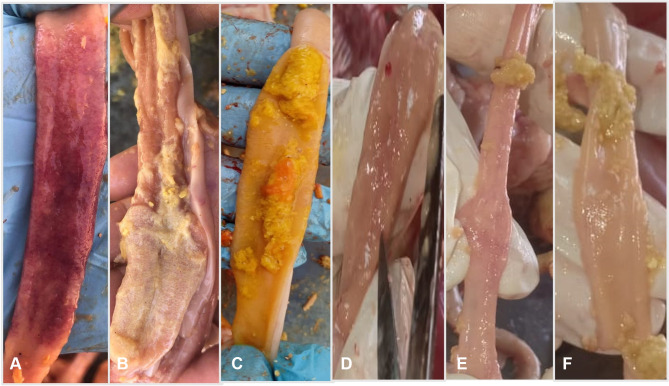
Macroscopic intestinal lesions were severe in G1; this group exhibited ballooning of the intestine and severe intestinal congestion from the serosal surface in some cases (Figure 5). Upon dissection, the opened intestine revealed the presence of frothy intestinal content mixed with undigested feed particles (Figure 6). The intestinal mucosa showed varying degrees of intestinal necrosis with the presence of hemorrhagic patches and with intestinal blood vessel engorgement. Lesions were commonly seen in duodenal loops and the jejunum (Figure 6).
Figure 5.
(A) Small intestine of Group 1 showing severe congestion of intestine. (B) Small intestine of group 1 showing ballooning of intestine with gases.
Figure 6.
(A) Opened small intestine of Group 1 showing severe necrosis, congestion of intestinal mucosa with presence of large hemorrhagic patches. (B) Opened small intestine of group 1 showing necrosis with velvet appearance in intestinal mucosa. (C) Opened intestine of Group 1 showing presence of undigested feed particles mixed with Orange color contents. (D) Opened intestine of Group 2 showing slight congestion of intestinal mucosa. (E) Opened intestine showing normal intestinal mucosa of Group 3. (F) Opened intestine showing normal intestinal mucosa of Group 4.
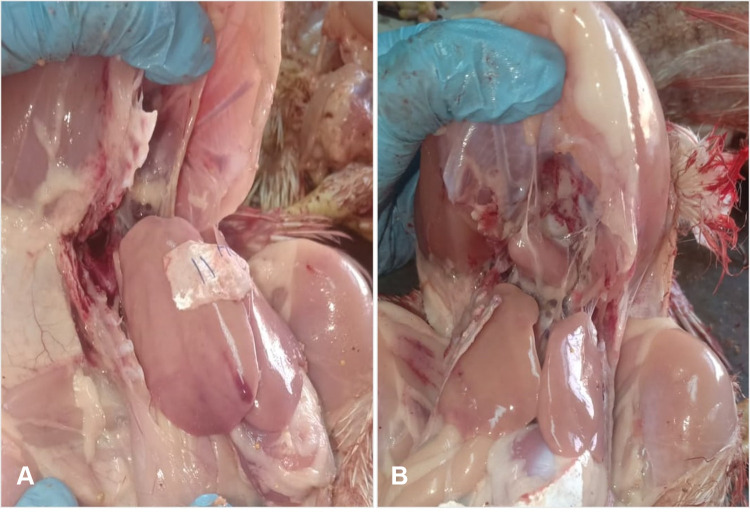
The liver of experimentally infected birds (G1) showed subcapsular hemorrhage, especially at the liver margin, whereas the intestine of birds in G2 appeared congested with apparently normal liver appearance (Figure 7). No macroscopic lesions were observed in either G3 or G4.
Figure 7.
(A) Opened chicken carcass showing pale liver and subcapsular hemorrhage in liver of Group 1. (B) Opened chicken carcass showing apparently normal liver of Group 2.
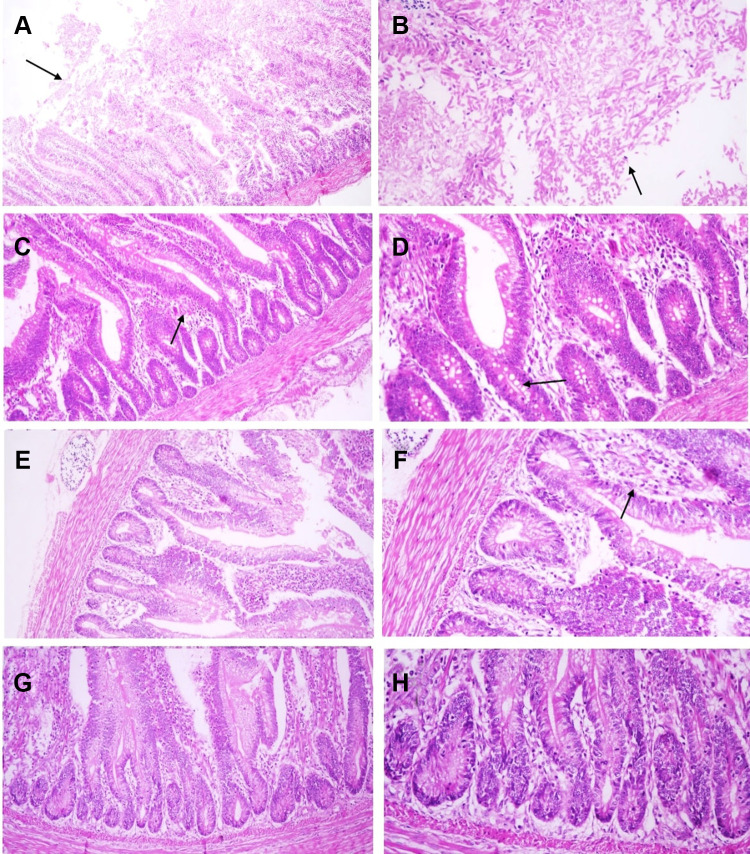
Histopathological Examination
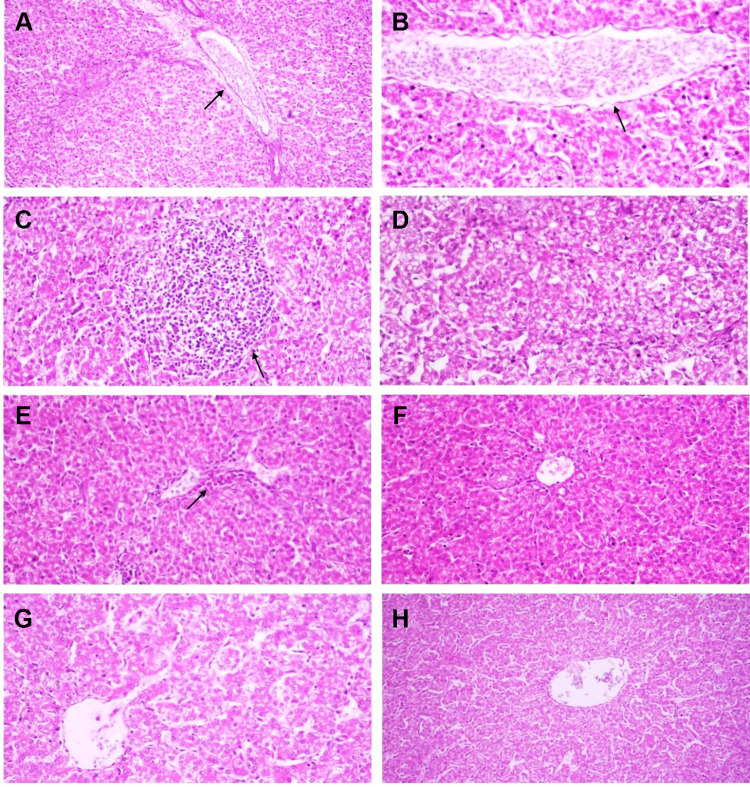
Histopathological lesions were mostly prominent in the experimentally infected birds (G1, positive control group). The epithelial lining of intestinal villi was desquamated with massive necrosis of the intestinal villi and intestinal glands. Inflammatory cells mixed with cell debris and C. perfringens bacilli were observed (Figure 8A and B). In the liver, the portal vein demonstrated severe dilatation and engorgement with red blood cells. The portal area showed portal edema and inflammatory cell infiltration (Figure 9A); the central vein was also congested (Figure 9B). There was a focal aggregation of mononuclear inflammatory cells in the hepatic parenchyma, dilatation of hepatic sinusoids, and degeneration of hepatocytes (Figure 9C and D).
Figure 8.
Microphotographs of chicken intestine sections: (A and B) Clostridium perfringens- experimentally infected group (Group 1), (C and D) C. perfringens infected group and treated with silver nanoparticles (Group 2), (E and F) Silver nanoparticles treated group (Group 3), (G and H) Negative control group (Group 4). (A) Massive and diffuse necrosis of intestinal villi with complete necrosis and sloughing of enterocytes (arrow) (HE, x40), (B) Necrosed and sloughed enterocytes mixed with clostridial bacilli and inflammatory cells (arrow) (HE, x200), (C) Intestinal villi are maintaining their integrity while lamina propria is infiltrated by inflammatory cells (arrow) (HE, x100), (D) Multiple vacuolations in the enterocytes and epithelial lining of intestinal glands (arrow) (HE, x200), (E) Normal structure of intestinal villi (HE, x100), (F) mild infiltration of lamina propria with inflammatory cells (arrow) (HE, x200), (G) Normal histological structure of intestinal villi (HE, x100), (H) Higher magnification of previous microphotograph reveals normal structure of enterocytes and intestinal glands (HE, x200).
Figure 9.
Microphotographs of chicken liver: (A–D) Clostridium perfringens- experimentally infected group (Group 1), (E and F) C. perfringens infected group and treated with silver nanoparticles (Group 2), (G) Silver nanoparticles treated group (Group 3), (H) Negative control group (Group 4). (A) Portal vein is severely dilated and engorged with RBCs and surrounded with portal edema and inflammatory cells infiltration (arrow) (HE, x100), (B) Congestion of the hepatic central vein (arrow) (HE, x200), (C) Focal aggregation of mononuclear inflammatory cells in hepatic parenchyma (arrow) (HE,x200), (D) Hepatocytes are showing degenerative changes and dilatation of hepatic sinusoids (HE, x200), (E) Central vein is surrounded with inflammatory cells (arrow) (HE, x200), (F) Hepatic lobule and portal area is showing normal histological pattern (G and H) Normal histological structure of hepatic lobule (HE, x200).
In the infected and Ag NP-treated groups, the intestine showed intact intestinal villi and glands, whereas the lamina propria was mildly infiltrated by inflammatory cells with multiple vacuolations in the enterocytes and epithelial lining of intestinal glands (Figure 8C and D). The liver showed normal histology with only a few central veins surrounded with inflammatory cells (Figure 9E and F).
The Ag NP-treated group exhibited a normal histological structure of intestinal villi, enterocytes, and intestinal glands, with few inflammatory cells infiltrating the lamina propria (Figure 8E and F), whereas the negative control group showed normal histology of the intestine (Figure 8G and H).
Both the Ag NP-treated groups and the negative control group showed normal liver histology (Figure 9G and H), and the immune organs examined show no pathological lesions.
Determination of Ag NP Tissue Residues
ICP-OES analysis at the age of 35 days revealed the presence of residual Ag in the muscles in G2 and G3 at concentrations of 413 and 501 ng/g (average = 457 ± 44 ng/g), respectively. No residual Ag was detected in G1 or G4.
Discussion
Nanotechnology has many applications in the poultry industry.24 In this study, the characterization of Ag NP via UV–Vis spectroscopy revealed a maximum absorption peak at 398 nm, which corresponds with the plasmonic absorption of Ag NPs37 and confirms their successful synthesis. TEM also confirmed the formation of Ag NPs and revealed their morphological properties with regard to size and shape. In this study, the treated birds were administered a total dose of 150 µg of Ag NPs spread over 5 days. At the concentrations used, the Ag NPs had an antibacterial effect on C. perfringens (as shown by reduced counts) and improved the general health condition of the tested birds, although there were no effects observed on the examined immunological parameters. In a parallel study by Al-Saeedi,38 Ag NPs were supplied in the drinking water of broiler chickens at various concentrations (20, 30, 40, and 50 ppm) for the entire rearing time. In that study, the authors found that Ag NPs at 50 ppm resulted in significantly (P < 0.05) high values for the BF index, as well as a significant (P < 0.05) improvement in the number of beneficial bacteria (Lactobacilli) in the jejunum and reduced numbers of harmful bacteria (E. coli).
In this study, the observed clinical signs were general depression, ruffled feathers, and presence of orange frothy droppings in G1 and G2. Similar findings (variable degrees of diarrhea) have been reported in chickens experimentally infected with C. perfringens.16
The mortality rates were 20% and 3.3% in G1 and G2, respectively. A cumulative mortality of 10%–40% due to NE in broiler chickens has been reported.2,10 Awaad et al16 recorded lower mortality (7.41%) in chickens experimentally infected with C. perfringens, though this variation may be due to differences in bacterial strain, dose, or experimental conditions. The significant reduction in mortality in G2 (P = 0.004) may be attributed to the antibacterial effect of Ag NPs on C. perfringens infection.
Differences in lesion scores were observed at 7 days PI. Untreated infected chickens showed an increased number of intestinal lesions (P = 0.027; Figure 4) compared with the groups treated with Ag NPs. Our results for the positive control (infected) group are in line with those reported by Awaad et al.16,29
The performance parameters for growth at 28 days of age showed that birds treated with Ag NPs (G2 and G3) exhibited significantly higher body weight (P = 0.002) and weight gain (P < 0.0001) than control birds (G1 and G4). Significant improvements were observed in the FCR of birds that received Ag NPs (G2 and G3) at days 14 and 28 (P < 0.0001). Małaczewska28,39 concluded that Ag NPs improved the health of birds and boosted their growth performance.
In this study, the cecal and intestinal loads of C. perfringens at 7 days PI for G2, G3, and G4 were significantly lower (P = 0.001) than those for the control infected group (G1). Furthermore, both G2 and G3 had significantly lower intestinal C. perfringens counts compared with G1 and G4 (P < 0.0001). These results are consistent with those of a previous study in which Ag NPs exhibited antimicrobial activity in vitro against C. botulinum type A.40 The antibacterial effect of metal NPs could result from the disruption and penetration of the microorganism’s cell membrane, causing damage to the cell wall leading to leakage of cytoplasm contents.41 Ag NPs have been shown to exhibit antibacterial inhibitory effects through their close interaction with thiol groups present in the main respiratory enzymes.42
After Ag NP treatment, there were no significant effects on the immune response of the chickens, with no changes observed in BF indexes. After 35 days, no significant difference was noted in all immune organ indexes between all treated groups. This could be attributed to administration of Ag NPs over a 5-day period (14th to 19th day), the improvement in chicken performance was mainly observed over the following 14 days (from 14th to 28th day). Also, there were no significant differences in HI titers among the groups at 7 or 14 days PI. Kulak et al43 found that oral administration of Ag NPs (2.87 and 12.25 mg/bird) on a regular basis improved HI (as demonstrated by increased levels of immunoglobulins and interleukin 6), and they confirmed that the effects of Ag NPs on the immune status of the birds was dependent on the size and the concentration of Ag NPs. Contrarily, Ognik et al44 found that administration of Ag NPs to chickens caused a disruption in protein catabolism, as demonstrated by reduced liver enzyme activity and lower concentrations of the key protein metabolism products (creatinine and urea). Ahmadi et al45 used Ag NPs as feed additives at different concentrations (4, 8, and 12 mg Ag NPs per kg of diet) in broiler chickens, finding that all concentrations had a negative impact on performance, health, and immune response.
Observations of histological changes in chicken intestine and liver between the experimentally infected control group and the groups treated with Ag NPs indicated variable differences. In the intestine, the infected group showed severe necrotic changes in the intestinal villi and glands with the presence of massive numbers of bacteria associated with the necrotic area, following the usual pathological picture for C. perfringens.46 With the Ag NP treatment, the condition of intestinal villi was significantly improved, with the structure maintained and inflammatory reaction reduced. Enterocytes showed some vacuolation but not reaching the stage of necrosis. Hepatic lesions induced by experimental infection were prominent in G1 and included congestion of the portal and central veins, inflammatory cell infiltration, and degenerative changes in hepatocytes. The appearance of hepatic lesions in C. perfringens infections is attributed to the movement of bacteria through damaged intestine to the liver via the portal hepatic vein.47 The treated group demonstrated a normal histological pattern of hepatocytes and hepatic vessels with only a few inflammatory cells around the central vein. Ahmadi et al48 reported that different levels of Ag had no significant impact on cell changes in the liver tissue of broiler chickens.
The improvement in clinical signs combined with bacterial load decrease and lack of negative histopathological changes in the intestine and liver confirmed the antibacterial activity of orally administered Ag NPs against C. perfringens. These results agree with previous reports of the antibacterial effects of Ag NPs on E. coli in chickens,49 Pseudomonas aeruginosa and Flavobacterium johnsoniae in fish,50,51 Salmonella spp. in sheep and goats,21 and Staphylococcus aureus and E. coli in in vitro studies.52 In addition, Sawosz et al53 found that oral administration of water containing 25 mg/kg of Ag NPs increased the population of lactic acid bacteria in quail intestine and improved intestinal health.
The accumulation of Ag NPs in organs affects their biological function and weight.43 Our findings confirmed that the administration of Ag NPs to chickens leaves residual Ag in the muscles at the age of 35 days (average, 457 ng/g), which could lead to human exposure to Ag NPs through consumption of edible parts of treated chicken. Our findings agree with those of Ahmadi and Rahimi;54 they incorporated Ag NPs in the drinking water of broiler chickens at different concentrations (4, 8, and 12 ppm) and found residues in the edible parts of broiler muscle at all concentrations. However, in one study, oral administration of six doses of approximately 1 mg/kg of Ag NPs (diameter, 20 nm) did not leave any residues in the muscles of hens but only accumulated in the liver and eggs.55 Kulak et al43 found that oral administration of Ag NPs in broiler chickens resulted in residues in the small intestine and liver but not in the heart or breast muscle. It is considered that up to 100 µg Ag/L in drinking water is safe for human consumption without inducing adverse effects.56 We intend to perform further studies to determine the necessary withdrawal time after oral treatment with Ag NPs.
Conclusion
We conclude that Ag NPs are able to reduce C. perfringens colonization in broiler chicken intestine and that they have a positive impact on performance, general health, and gut health integrity while having no impact on immune organs and immune response. Ag NPs have a cumulative residual effect in chicken meat; therefore, further studies are needed on the concentration and size of Ag NPs, the route of administration, and withdrawal time to ensure the safety of treated chicken meat for human consumption.
Ethical Compliance
All the experimental procedures and bird handling were performed according to International Council for Laboratory Animal Science (ICLAS) guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) at the Faculty of Veterinary Medicine, Cairo University.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Salem HM, Attia MM. Accidental intestinal myiasis caused by Musca domestica L. (Diptera: muscidae) larvae in broiler chickens: a field study. Int J Trop Insect Sci. 2021:1–6. doi: 10.1007/s42690-021-00492-w34518770 [DOI] [Google Scholar]
- 2.Baba E, Ikemoto T, Fukata T, Sasai K, Arakawa A, McDougald LR. Clostridial population and the intestinal lesions in chickens infected with Clostridium perfringens and Eimeria necatrix. Vet Microbiol. 1997;54(3–4):301–308. doi: 10.1016/S0378-1135(96)01289-8 [DOI] [PubMed] [Google Scholar]
- 3.Cooper KK, Songer JG. Virulence of Clostridium perfringens in an experimental model of poultry necrotic enteritis. Vet Microbiol. 2010;142(3–4):323–328. doi: 10.1016/j.vetmic.2009.09.065 [DOI] [PubMed] [Google Scholar]
- 4.Khelfa DE, Abd El-Ghany WA, Salem HM. Serological and molecular typing of Clostridium perfringens and its toxins recovered from weaned Rabbit’s flocks in Egypt. Life Sci J. 2012;9(4):2263–2271. [Google Scholar]
- 5.Khelfa DE, Abd El-Ghany WA, Salem HM. Recent status of Clostridial Enteritis affecting early weaned Rabbits in Egypt. Life Sci J. 2012;9(4):2272–2279. [Google Scholar]
- 6.Keyburn AL, Boyce JD, Vaz P, et al. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008;4(2):e26. doi: 10.1371/journal.ppat.0040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engström BE, Fermer C, Lindberg A, Saarinen E, Båverud V, Gunnarsson A. Molecular typing of isolates of Clostridium perfringens from healthy and diseased poultry. Vet Microbiol. 2003;94(3):225–235. doi: 10.1016/S0378-1135(03)00106-8 [DOI] [PubMed] [Google Scholar]
- 8.Craven SE, Cox NA, Stern NJ, Mauldin JM. Prevalence of Clostridium perfringens in commercial broiler hatcheries. Avian Dis. 2001;45(4):1050–1053. doi: 10.2307/1592887 [DOI] [PubMed] [Google Scholar]
- 9.Wade B, Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31(7):16–17. [Google Scholar]
- 10.McDevitt RM, Brooker JD, Acamovic T, Sparks NHC. Necrotic enteritis; a continuing challenge for the poultry industry. Worlds Poult Sci J. 2006;62(2):221–247. doi: 10.1079/WPS200593 [DOI] [Google Scholar]
- 11.Løvland A, Kaldhusdal M. Liver lesions seen at slaughter as an indicator of necrotic enteritis in broiler flocks. FEMS Immunol Med Microbiol. 1999;24(3):345–351. doi: 10.1016/S0928-8244(99)00052-8 [DOI] [PubMed] [Google Scholar]
- 12.Hofacre CL, Beacorn T, Collett S, Mathis G. Using competitive exclusion, mannan-oligosaccharide and other intestinal products to control necrotic enteritis. J Appl Poult Res. 2003;12(1):60–64. doi: 10.1093/japr/12.1.60 [DOI] [Google Scholar]
- 13.Brynestad S, Granum PE. Clostridium perfringens and foodborne infections. Int J Food Microbiol. 2002;74(3):195–202. doi: 10.1016/S0168-1605(01)00680-8 [DOI] [PubMed] [Google Scholar]
- 14.Ghadban G, Kabakchiev M, Angelov A. Efficacy of different methods of probiotic treatment in preventing infection of broiler chicks with Salmonella typhimurium and E. coli O7. Proc 10th EPC. 1998;26:305–310. [Google Scholar]
- 15.Lee SH, Lillehoj HS, Jang SI, Lillehoj EP, Min W, Bravo DM. Dietary supplementation of young broiler chickens with Capsicum and turmeric oleoresins increases resistance to necrotic enteritis. Br J Nutr. 2013;110(5):840–847. doi: 10.1017/S0007114512006083 [DOI] [PubMed] [Google Scholar]
- 16.Awaad MH, Elmenawey M, Shalaby B, et al. Opposing of necrotic enteritis by phytonutrients and/or acidifiers in broiler chickens. IOSR JAVS. 2019;12:12–21. [Google Scholar]
- 17.Osman KM, Elhariri M. Antibiotic resistance of Clostridium perfringens isolates from broiler chickens in Egypt. Rev Sci Tech. 2013;32(3):841–850. doi: 10.20506/rst.32.2.2212 [DOI] [PubMed] [Google Scholar]
- 18.Bai DP, Lin XY, Huang YF, Zhang XF. Theranostics aspects of various nanoparticles in veterinary medicine. Int J Mol Sci. 2018;19(11):3299. doi: 10.3390/ijms19113299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banach M, Tymczyna L, Chmielowiec-Korzeniowska A, Pulit-Prociak J. Nanosilver biocidal properties and their application in disinfection of hatchers in poultry processing plants. Bioinorg Chem Appl. 2016;2016:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28. doi: 10.1016/j.jare.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farouk MM, El-Molla A, Salib FA, Soliman YA, Shaalan M. The role of silver nanoparticles in a treatment approach for multidrug-resistant salmonella species isolates. Int J Nanomedicine. 2020;15:6993. doi: 10.2147/IJN.S270204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra M,N, Mithun PR, Rao HCY, Mahendra C, Satish S. Nanoparticle applications in sustainable agriculture, poultry, and food: trends and perspective. Nanotoxicity. 2020;7:341–353. [Google Scholar]
- 23.Patra AK, Lalhriatpuii M. Progress and prospect of essential mineral nanoparticles in poultry nutrition and feeding a review. Biol Trace Elem Res. 2020;197(1):233–253. doi: 10.1007/s12011-019-01959-1 [DOI] [PubMed] [Google Scholar]
- 24.Abd El-Ghany WA, Shaalan M, Salem HM. Nanoparticles applications in poultry production: an updated review. Worlds Poult Sci J. 2021. doi: 10.1080/00439339.2021.1960235 [DOI] [Google Scholar]
- 25.Saraniya Devi J, Valentin B. Antibacterial and antifungal activity of silver nanoparticles synthesized using Hypnea muciformis. Biosci Biotechnol Res Asia. 2014;11(1):235–338. doi: 10.13005/bbra/1260 [DOI] [Google Scholar]
- 26.Vadalasetty KP, Lauridsen CH, Engberg RM, et al. Influence of silver nanoparticles on growth and health of broiler chickens after infection with Campylobacter jejuni. BMC Vet Res. 2018;14(1):1. doi: 10.1186/s12917-017-1323-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pineda L, Chwalibog A, Sawosz E, et al. Effect of silver nanoparticles on growth performance, metabolism and microbial profile of broiler chickens. Arch Anim Nutr. 2012;66(5):416–429. doi: 10.1080/1745039X.2012.710081 [DOI] [PubMed] [Google Scholar]
- 28.Małaczewska J. Cytotoxicity of silver nanoparticles. Med Weter. 2010;66(12):833–838. [Google Scholar]
- 29.Awaad MHH, Salem HMM, Morsy EA, Mohamed FF, Awaad YMH, Abdel-Alim GA. Influence of bovine IGG-rich fraction on growth promotion, gut health and clostridium perfringens enumeration in necrotic enteritis infected broiler chickens. IOSR JAVS. 2019;12:78–85. [Google Scholar]
- 30.Timmerman HM, Veldman A, Van den Elsen E, Rombouts FM, Beynen AC. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult Sci. 2006;85(8):1383–1388. doi: 10.1093/ps/85.8.1383 [DOI] [PubMed] [Google Scholar]
- 31.Harmon S. Clostridium perfringens: enumeration and identification. In: FDA Bacteriological Analytical Manual. Association of Official Analytical Chemists. Arlington, VA; 1984: 1701–1710. [Google Scholar]
- 32.Garrido MN, Skjervheim M, Oppegaard H, Sørum H. Acidified litter benefits the intestinal flora balance of broiler chickens. Appl Environ Microbiol. 2004;70(9):5208–5213. doi: 10.1128/AEM.70.9.5208-5213.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keyburn AL, Sheedy SA, Ford ME, et al. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect Immun. 2006;74(11):6496–6500. doi: 10.1128/IAI.00806-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shojadoost B, Vince AR, Prescott JF. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet Res. 2012;43(1):1–12. doi: 10.1186/1297-9716-43-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. China: Elsevier health sciences; 2008. [Google Scholar]
- 36.Swayne DE. Laboratory Manual for the Isolation and Identification of Avian Pathogens. American Association of Avian Pathologists, University of Pennsylvania; 1998. [Google Scholar]
- 37.Mulfinger L, Solomon SD, Bahadory M, Jeyarajasingam AV, Rutkowsky SA, Boritz C. Synthesis and study of silver nanoparticles. J Chem Educ. 2007;84(2):322. doi: 10.1021/ed084p322 [DOI] [Google Scholar]
- 38.Al-Saeedi MKI, Dakhil HH, Fadhil Rasool Abbas Al-Khafaji FRA. Effect of adding silver nanoparticles with drinking water on some lymphatic organs and microflora in the intestinal for broiler chickens (ROSS 308). 1st International virtual conference of environmental sciences. IOP Conf Ser Earth Environ Sci. 2021;722:1200. doi: 10.1088/1755-1315/722/1/012004 [DOI] [Google Scholar]
- 39.Kout-Elkloub M, El Moustafa A, Ghazalah A, Rehan AAA. Effect of dietary nanosilver on broiler performance. Int J Poult Sci. 2015;14(3):177–182. doi: 10.3923/ijps.2015.177.182 [DOI] [Google Scholar]
- 40.Aminianfar M, Parvardeh S, Soleimani M. In vitro and in vivo assessment of silver nanoparticles against Clostridium botulinum type A botulinum. Curr Drug Discov Technol. 2019;16(1):113–119. doi: 10.2174/1570163815666180403163946 [DOI] [PubMed] [Google Scholar]
- 41.Hassan AA, Mansour MK, Oraby NH, Mohamed AM. The efficiency of using silver nanoparticles singly and in combination with traditional antimicrobial agents in control of some fungal and bacterial affection of buffaloes. Int J Curr Res. 2016;8(4):29758–29770. [Google Scholar]
- 42.Gordon O, Slenters TV, Brunetto PS, et al. Silver coordination polymers for prevention of implant infection: thiol interaction, impact on respiratory chain enzymes, and hydroxyl radical induction. Antimicrob Agents Chemother. 2010;54(10):4208–4218. doi: 10.1128/AAC.01830-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulak E, Ognik K, Stępniowska A, Sembratowicz I. The effect of administration of silver nanoparticles on silver accumulation in tissues and the immune and antioxidant status of chickens. J Animal Feed Sci. 2018;27(1):44–54. doi: 10.22358/jafs/84978/2018 [DOI] [Google Scholar]
- 44.Ognik K, Cholewińska E, Czech A, et al. Effect of silver nanoparticles on the immune, redox, and lipid status of chicken blood. Crech J Animal Sci. 2016;61(10):450–461. doi: 10.17221/80/2015-CJAS [DOI] [Google Scholar]
- 45.Ahmadi F, Khah MM, Javid S, Zarneshan A, Akradi L, Salehifar P. The effect of dietary silver nanoparticles on performance, immune organs, and lipid serum of broiler chickens during starter period. Int J Biosci. 2013;3:95–100. [Google Scholar]
- 46.Smyth JA. Pathology and diagnosis of necrotic enteritis: is it clear-cut? Avian Pathol. 2016;45(3):282–287. doi: 10.1080/03079457.2016.1158780 [DOI] [PubMed] [Google Scholar]
- 47.Immerseel FV, Buck JD, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33(6):537–549. doi: 10.1080/03079450400013162 [DOI] [PubMed] [Google Scholar]
- 48.Ahmadi J, Mehrdad I, Mahdi C. Pathological study of intestine and liver in broiler chickens after treatment with different levels of silver nanoparticles. World Appl Sci J. 2009;7:28–32. [Google Scholar]
- 49.Kumar I, Bhattacharya J. Assessment of the role of silver nanoparticles in reducing poultry mortality, risk and economic benefits. Appl Nanosci. 2019;9(6):1293–1307. doi: 10.1007/s13204-018-00942-x [DOI] [Google Scholar]
- 50.El‐Adawy MM, Eissa AE, Shaalan M, et al. Green synthesis and physical properties of Gum Arabic‐silver nanoparticles and its antibacterial efficacy against fish bacterial pathogens. Aquacult Res. 2021;52(3):1247–1254. doi: 10.1111/are.14983 [DOI] [Google Scholar]
- 51.Shaalan M, Sellyei B, El-Matbouli M, Székely C. Efficacy of silver nanoparticles to control flavobacteriosis caused by Flavobacterium johnsoniae in common carp Cyprinus carpio . Dis Aquat Org. 2020;137(3):175–183. doi: 10.3354/dao03439 [DOI] [PubMed] [Google Scholar]
- 52.Cho KH, Park JE, Osaka T, Park SG. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta. 2005;51(5):956–960. doi: 10.1016/j.electacta.2005.04.071 [DOI] [Google Scholar]
- 53.Sawosz E, Binek M, Grodzik M, et al. Influence of hydrocolloidal silver nanoparticles on gastrointestinal microflora and morphology of enterocytes of quails. Arch Anim Nutr. 2007;61(6):444–451. doi: 10.1080/17450390701664314 [DOI] [PubMed] [Google Scholar]
- 54.Ahmadi F, Rahimi F. The effect of different levels of nano silver on performance and retention of silver in edible tissues of broilers. World Appl Sci J. 2011;12:1–4. [Google Scholar]
- 55.Gallocchio F, Biancotto G, Cibin V, et al. Transfer study of silver nanoparticles in poultry production. J Agric Food Chem. 2017;65(18):3767–3774. doi: 10.1021/acs.jafc.7b00670 [DOI] [PubMed] [Google Scholar]
- 56.Giovanni M, Tay CY, Setyawati MI, et al. Toxicity profiling of water contextual zinc oxide, silver, and titanium dioxide nanoparticles in human oral and gastrointestinal cell systems. Environ Toxicol. 2015;30(12):1459–1469. doi: 10.1002/tox.22015 [DOI] [PubMed] [Google Scholar]