Abstract
Background
Pulmonary hypertension is a condition of complex aetiology that culminates in right heart failure and early death. Soluble guanylate cyclase (sGC) stimulators are a promising class of agents that have recently gained approval for use.
Objectives
To evaluate the efficacy of sGC stimulators in pulmonary hypertension.
Search methods
We searched CENTRAL (Cochrane Central Register of Controlled Trials), MEDLINE, EMBASE and the reference lists of articles. Searches are current as of 12 February 2016.
Selection criteria
We selected randomised controlled trials (RCTs) involving participants with pulmonary hypertension of all ages, severities and durations of treatment.
Data collection and analysis
AW, MS and RW independently selected studies, assessed evidence quality and extracted data. This process was overseen by RT and SG. All included studies were sponsored by the drug manufacturer.
Main results
Five trials involving 962 participants are included in this review. All trials were of relatively short duration (< 16 weeks). Due to the heterogenous aetiology of pulmonary hypertension in participants, results are best considered according to each pulmonary hypertension subtype.
Pooled analysis shows a mean difference (MD) increase in six‐minute walking distance (6MWD) of 30.13 metres (95% CI 5.29 to 54.96; participants = 659; studies = 3). On subgroup analysis, for pulmonary arterial hypertension (PAH) there was no effect noted (6MWD; MD 11.91 metres, 95% CI −44.92 to 68.75; participants = 398; studies = 2), and in chronic thromboembolic pulmonary hypertension (CTEPH) sGC stimulators improved 6MWD by an MD of 45 metres (95% CI 23.87 to 66.13; participants = 261; studies = 1). Data for left heart disease‐associated PH was not available for pooling. Importantly, when participants receiving phosphodiesterase inhibitors were excluded, sGC stimulators increased 6MWD by a MD of 36 metres in PAH. The second primary outcome, mortality, showed no change on pooled analysis against placebo (Peto odds ratio (OR) 0.57, 95% CI 0.18 to 1.80).
Pooled secondary outcomes include an increase in World Health Organization (WHO) functional class (OR 1.53, 95% CI 0.87 to 2.72; participants = 858; studies = 4), no effect on clinical worsening (OR 0.45, 95% CI 0.17 to 1.14; participants = 842; studies = 3), and a reduction in mean pulmonary artery pressure (MD −2.77 mmHg, 95% CI −4.96 to −0.58; participants = 744; studies = 5). There was no significant difference in serious adverse events on pooled analysis (OR 1.12, 95% CI 0.66 to 1.90; participants = 818; studies = 5) or when analysed at PAH (MD −3.50, 95% CI −5.54 to −1.46; participants = 344; studies = 1), left heart disease associated subgroups (OR 1.56, 95% CI 0.78 to 3.13; participants = 159; studies = 2) or CTEPH subgroups (OR 1.29, 95% CI 0.65 to 2.56; participants = 261; studies = 1).
It is important to consider the results for PAH in the context of a person who is not also receiving a phosphodiesterase‐V inhibitor, a contra‐indication to sGC stimulator use. It should also be noted that CTEPH results are applicable to inoperable or recurrent CTEPH only.
Evidence was rated according to the GRADE scoring system. One outcome was considered high quality, two were moderate, and eight were of low or very low quality, meaning that for many of the outcomes the true effect could differ substantially from our estimate. There were only minor concerns regarding the risk of bias in these trials, all being RCTs largely following the original protocol. Most trials employed an intention‐to‐treat analysis.
Authors' conclusions
sGC stimulators improve pulmonary artery pressures in people with PAH (who are treatment naive or receiving a prostanoid or endothelin antagonist) or those with recurrent or inoperable CTEPH. In these settings this can be achieved without notable complication. However, sGC stimulators should not be taken by people also receiving phosphodiestase‐V inhibitors or nitrates due to the risks of hypotension, and there is currently no evidence supporting their use in pulmonary hypertension associated with left heart disease. There is no evidence supporting their use in children. These conclusions are based on data with limitations, including unavailable data from two of the trials.
Plain language summary
Soluble guanylate cyclase stimulators for raised blood pressure within the lungs
Review question
We reviewed the use of a set of drugs, soluble guanylate cyclase stimulators, for the improvement of symptoms in participants with pulmonary hypertension (PH). This was in comparison to current treatment or no treatment.
Background
PH involves high blood pressure in the blood vessels of the lungs. This causes shortness of breath and reduces the ability to exercise, leading to faints and dizziness. PH can cause the heart to fail, leading to a shortened life. PH is not a single disease, but includes a group of diseases. Key PH types for this review include pulmonary arterial hypertension (PAH), chronic thromboembolic pulmonary hypertension (CTEPH) and PH due to left heart disease.
Study characteristics
This evidence is current to February 2016. Males and females of all ages diagnosed with PH were included in this review. We selected only randomised clinical trials. All trials used a comparison to no treatment. Trial durations ranged from 12 to 16 weeks. This review involves five trials on 962 participants. All included studies were sponsored by the maker of the drug.
Key results
Soluble guanylate cyclase stimulators appear to reduce lung pressures and improve exercise capacity in PAH and recurrent or inoperable CTEPH, but not in PH due to left heart disease. It is uncertain if these drugs have an effect on death rates and general health decline, or if they may be associated with serious side effects. There is evidence that suggests these drugs should not be taken at the same time as phosphodiesterase‐V inhibitors.
Quality of the evidence
One outcome was considered to be high quality according to the GRADE scoring system. Two were considered moderate strength and eight outcomes were considered low or very low strength. This means the results reported may not represent the true effect.
Summary of findings
Summary of findings for the main comparison. Summary of findings table.
| Riociguat compared to placebo for pulmonary hypertension | |||||||
| Patient or population: Adults (>18 years) with pulmonary hypertension of varied aetiology (PAH, CTEPH and left heart disease) Setting: Randomised controlled trials of 16 weeks duration or less Intervention: Ricociguat Comparison: Placebo | |||||||
| Outcomes | Aetiology | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Ricociguat | ||||||
| Change in Exercise Capacity (6MWD) | Group 1: PAH | ‐ | The mean change in Exercise Capacity (6MWD) ‐ Group 1: PAH in the intervention group was 12 m higher (45 fewer to 69 more) | ‐ | 398 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1,2,3,4 | |
| Group 2: Left heart disease | ‐ | ‐ | ‐ | (0 studies) | ‐ | ||
| Group 4: CTEPH | ‐ | The mean change in Exercise Capacity (6MWD) ‐ Group 4: CTEPH in the intervention group was 45 m higher (24 m more to 66 m more) | ‐ | 261 (1 RCT) | ⊕⊕⊕⊕ HIGH | Note small effect size | |
| Mortality | Group 1: PAH | 23 per 1000 | 7 per 1000 (1 to 43) | OR 0.29 (0.05 to 1.91) | 398 (2 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | |
| Group 2: Left heart disease | 0 per 1000 | 0 per 1000 (0 to 0) | OR 4.57 (0.42 to 50.06) | 240 (1 RCT) | ⊕⊕⊝⊝ LOW 2 | ||
| Group 4: CTEPH | 34 per 1000 | 10 per 1000 (2 to 65) | OR 0.30 (0.05 to 1.96) | 261 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | ||
| Improvement in WHO Functional Class | Group 1: PAH | 164 per 1000 | 235 per 1000 (128 to 315) | OR 1.53 (0.87 to 2.71) | 397 (2 RCTs) | ⊕⊕⊝⊝ LOW 1,2 | |
| Group 2: Left heart disease | 217 per 1000 | 189 per 1000 (102 to 325) | OR 0.84 (0.41 to 1.73) | 201 (1 RCT) | ⊕⊕⊝⊝ LOW 1,2 | ||
| Group 4: CTEPH | 149 per 1000 | 330 per 1000 (201 to 490) | OR 2.80 (1.43 to 5.46) | 260 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| Serious Adverse Events (Participants with one or more event) | Group 1: PAH | 189 per 1000 | 179 per 1000 (49 to 472) | OR 0.93 (0.22 to 3.83) | 398 (2 studies) | ⊕⊝⊝⊝ VERY LOW 1,2,3 | |
| Group 2: Left heart disease | 244 per 1000 | 335 per 1000 (201 to 502) | OR 1.56 (0.78 to 3.13) | 159 (2 studies) | ⊕⊕⊝⊝ LOW 1,2 | ||
| Group 4: CTEPH | 159 per 1000 | 196 per 1000 (110 to 326) | OR 1.29 (0.65 to 2.56) | 261 (1 study) | ⊕⊕⊕⊝ MODERATE 1 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CTEPH: Chronic Thromboembolic Pulmonary Hypertension CI: Confidence interval; PAH: Pulmonary arterial hypertension; RR: Risk ratio; OR: Odds ratio; WHO: World Health Organization; 6MWD: 6‐minute walk distance | |||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1 Downgraded due to indirectness (variable treatment regimens in included studies)
2 Downgraded due to imprecision in outcome
3 Downgraded due to heterogeneity (I² > 50%)
4 Note that the pooled outcome includes data from trials with contra‐indicated drugs (PDE5is)
Background
Description of the condition
Pulmonary hypertension (PH) is an important cause of morbidity and mortality, affecting up to 100 million people worldwide (Schermuly 2011). It leads to a substantial loss of exercise capacity, as well as causing right ventricular overload, resulting in heart failure and early mortality. Current three year survival ranges from 58.2% to 73.3% (Ling 2012).
PH is diagnosed when resting mean pulmonary arterial pressure (pPA) is 25 mmHg or more at cardiac catheterization. The clinical phenotype includes dyspnoea (Table 2), syncope (Table 2), chest pain and fatigue, whilst examination may reveal cyanosis (Table 2), right ventricular heave, a loud pulmonary second heart sound, and in late disease, signs of right heart failure. The degree of physical limitation can be categorised by the 6‐minute walking distance (6MWD) or the results at cardiopulmonary exercise testing (Miyamoto 2000; Abumehdi 2015). PH symptoms are classified clinically according to the World Health Organization (WHO) functional class system (1 = no limitation, 4 = symptomatic at rest; Galiè 2013).
1. Glossary of Terms.
| Term | Explanation |
| Cyanosis | A blue discolouration of the skin secondary to poor circulation or blood oxygenation |
| Dyspnoea | Difficulty in breathing |
| Syncope | Transient loss of consciousness and posture due to inadequate perfusion of the brain |
PH is divided into five aetiological categories according to the WHO criteria (Simonneau 2013b), of which three categories are relevant to our review. Group 1 is pulmonary arterial hypertension (PAH). This is subdivided further into groups including PH associated with congenital heart disease (CHD), connective tissue disease, heritable PH and, the most common group, idiopathic PH. PAH treatment options include calcium channel blockers, phosphodiesterase‐V inhibitors (PDE5is), dual‐endothelin receptor antagonists and prostanoids. More recently soluble guanylate cyclase (sGC) stimulators have gained approval. Despite this, annual mortality remains 15% (Thenappan 2010). Group 2 is PH associated with left heart disease, the most common form of PH worldwide, affecting up to 30% of people with heart failure. There is no proven treatment that specifically targets PH in this condition (Damy 2010). The other major class relevant to this review is group 4, chronic thromboembolic PH (CTEPH). This entails vessel occlusion secondary to thromboses or emboli, alongside a vasoconstrictive component, which leads to significant increases in vascular resistance. PH is associated with poorer outcomes in pulmonary embolism unless early diagnosis is made (Riedel 1982). Pulmonary endartectomy (PEA) is the gold‐standard management for CTEPH. However, up to 63% of candidates are ineligible for surgery due to co‐morbidities, unsuitable morphology, surgical centre access or declining consent (Mayer 2011). Balloon angioplasty has also been used with some effect for inoperable cases, although there is currently no randomised data underlying this (Tatebe 2016). Furthermore, the condition either recurs or is refractory to surgery in 5% to 35% of cases (Thistlesthwaite 2006; Bonderman 2007). This leaves substantial unmet medical need.
Description of the intervention
As our understanding of PH has evolved, numerous medications have been developed with the hope of improving clinical outcomes (Galiè 2013). Historically calcium channel blockers are the first line treatment but only for those responding positively at vaso reactivity tests. Prostanoids were the first real breakthrough, and are still widely considered the gold standard due to their superior efficacy but have a significant side effect profile. However, the most common form requires continuous intravenous infusion, with associated complications limiting utility. More recent developments include both selective and dual endothelin receptor antagonists (e.g. ambrisentan, bosentan, macitentan) and phosphodiesterase‐V inhibitors (e.g. sildenafil, tadalafil) (Wardle 2013b). The decision of which PAH regimen to use is determined by WHO functional class amongst other factors (Galiè 2013). Studies have suggested and it is widely accepted that 6MWD greater than 400 m is associated with improved survival; however, this is yet to be proved. There is a lack of randomised controlled trial (RCT) data in paediatric groups overall, meaning drug treatment is based largely upon adult evidence (Ivy 2013).
In 2013, the Food and Drug Administration (FDA) approved riociguat, a sGC stimulator, the first in a novel class of PH treatments (Guha 2013), which now also has European approval (Dowdall 2014). This license pertained not only to PAH, but to CTEPH also. This makes sGC stimulators the first specific treatment for inoperable or recurrent CTEPH. Inoperable CTEPH is that determined to be unsuitable for PEA by a multi‐disciplinary surgical team, whilst recurrent CTEPH is that occurring after PEA has been performed. Medical therapy is not intended as a replacement for surgery in people suitable for PEA (Archer 2013) and any attempt at this should be discouraged. Furthermore, surgery should not be delayed for trials of medical therapy (Keogh 2009).
When considering the clinical use of riociguat, the price implications are still poorly defined, but should be in line with rival PAH agents at GBP 25,931 per year (Bayer 2014). Riociguat comes in the form of an oral tablet (up to 2.5 mg as tolerated), taken three times daily to aid patient compliance.
How the intervention might work
sGC stimulators are a novel class of PH medication that manipulate endogenous mechanisms controlling pulmonary pressures. In normal physiology, the body regulates pulmonary flow, and therefore resistance, through a unique set of endocrine and paracrine effectors. One of these is the ventilation‐induced release of endothelial nitric oxide (NO) to stimulate vasodilatation via smooth muscle cells. NO does this by stimulating sGC to produce cyclic guanosine monophosphate (cGMP). cGMP activates kinases that lead to vasodilatation and the inhibition of inflammation and thrombosis (Denninger 1999; Ghofrani 2004). In addition, the potential for increasing cGMP as a therapeutic target for pulmonary vasodilatation is demonstrated by PDE5is (Wardle 2013a; Wardle 2013b). However, it is hoped that sGC stimulators may display greater efficacy than PDE5is due to their independence from NO bioavailability, relative lack of adverse effects, and actions as a NO sGC sensitiser and stimulator (Stasch 2011).
Why it is important to do this review
There is already significant controversy over the most effective medication for PAH and this will deepen with the introduction of sGC stimulators. It also remains to be seen how sGC stimulators should be used in the context of CTEPH — whether they are a replacement for PEA in people at high surgical risk, an adjunct, or a measure of last resort. By collating the evidence of these agents in PH it is hoped that we will address these issues whilst also increasing the evidence base for adverse effects — an area still lacking sufficient data. Finally, this work will also act as a protocol capable of being repeated in the future as the evidence base for these agents continues to evolve, and therefore begin to act as basis for comparison between different available treatments.
Objectives
To evaluate the efficacy of sGC stimulators in pulmonary hypertension.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). This included studies reported as full‐text, abstract only, and unpublished data. We excluded all studies not conforming to the RCT format.
Types of participants
We included both adults and children (0 to 18 years) diagnosed with PH.
Types of interventions
We included trials comparing sGC stimulators with usual care and placebo. We also sought to compare head‐to‐head trials comparing different sGC stimulators as a separate comparison. We included the following co‐interventions.
PDE5is
Endothelin receptor antagonists
Prostanoids
Nitrates
Calcium channel blockers
Non‐PH specific medications including diuretics, anticoagulants and oxygen.
Types of outcome measures
Primary outcomes
Change in exercise capacity, measured by 6MWD.
Mortality
Secondary outcomes
Change in WHO functional class
Time to clinical worsening
Change in pulmonary arterial pressure (mmHg)
Serious adverse events
Reporting one of more of the outcomes listed here in the trial was not an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified trials from searches of the following databases:
The Cochrane Airways Group Register of Trials – all years.
The Cochrane Central Register of Controlled Trials (CENTRAL), issue 2, 2016 (The Cochrane Library).
MEDLINE (Ovid) 1950 to February 2016.
EMBASE (Ovid) 1974 to February 2016.
Trials registries (ClinicalTrials.gov and the WHO trials portal).
The search strategy is detailed in Appendix 1. We searched all databases from inception to present day, and did not impose any restriction on language or type of publication. We searched for conference abstracts and grey literature through the CENTRAL database. The search date was 12 February 2016.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
We searched for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed). This was done on 12 February 2016.
Data collection and analysis
Selection of studies
Two review authors (MS and RW) independently screened titles and abstracts for inclusion and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved all available full‐text study reports and publications, and two review authors (MS and RW) independently screened for inclusion or identified and recorded reasons for exclusion. We resolved any disagreement through discussion or, when required, through a third review author (AW). One final review author (RT) analysed the included and excluded texts to ensure uniform enforcement of the study protocol. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and 'Characteristics of excluded studies' table.
1.
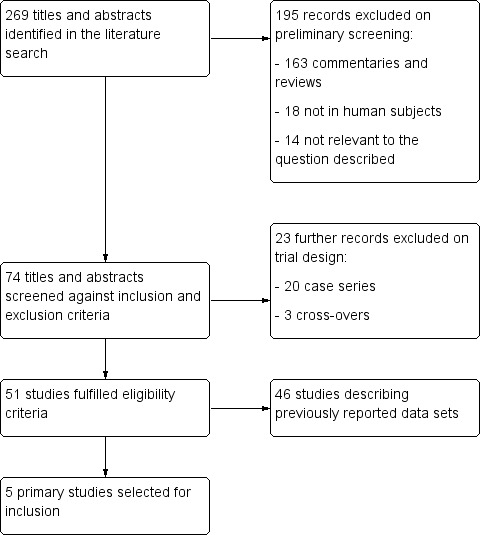
Study Selection Flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data which had been piloted on at least one study in the review. One review author (MS) extracted study characteristics from included studies and this was duplicated by a second review author (RW). We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, and date of study.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (MS and RW) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third review author (AW). One review author (AW) transferred data into the Review Manager (RevMan 2012) file. We double‐checked that data was entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (RW) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (MS and RW) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (AW or RT). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We graded each potential source of bias as 'high', 'low' or 'unclear' and provided a quote from the study report together with a justification for our judgment in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). Where information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assesment of bias in conducting the systematic review
We conducted the review according to the published protocol (Wardle 2014) and have reported any deviations from it in the "Differences between protocol and review" section below.
Measures of treatment effect
We analysed dichotomous data as risk ratios and continuous data as the mean difference or standardised mean difference. We entered data as a scale with consistent direction of effect. Meta‐analyses were performed only where it was meaningful i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. Any skewed data is described in narrative using medians and interquartile ranges. Where multiple trial arms were reported in a single trial, we included only the relevant arms in the data set. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting. When trials were taking measurements of treatment effect at different time points, we took the data point nearest to the predetermined set time point. Set time points included results at four weeks, 12 weeks, 26 weeks and 52 weeks post‐initiation of treatment.
Unit of analysis issues
The unit of analysis was the individual participant. The eligibility of non‐standard randomisation study designs was questioned and taken into account during subsequent analysis of bias. We excluded cross‐over study designs due to the large variability in PH disease course, the potential for previous treatment to modify future outcomes, and the risk of pharmacodynamic crossover.
Dealing with missing data
We contacted investigators and study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). When this was not possible, and the missing data was thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results using a sensitivity analysis.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity amongst the trials in each of the analyses. When we identified substantial heterogeneity we reported it and explored possible causes using prespecified subgroup analyses.
Assessment of reporting biases
If we had been able to pool more than 10 trials, we would have created a funnel plot to explore possible small study and publication biases.
Data synthesis
We used a random‐effects model throughout. We made this decision on the basis that the inherent heterogeneity of this population should not affect the outcome measures of interest in this work.
Summary of findings table
We created a 'Summary of findings' table aligned to the following outcomes:
Change in exercise capacity, measured by 6MWD
Mortality
Change in WHO functional class
Serious adverse events
We used the eight GRADE considerations (risk of bias, inconsistency, indirectness, imprecision, publication bias, large effect, plausible confounding and dose response gradient) to assess the quality of evidence as it relates to the studies that contribute data to the relevant meta‐analysis. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (GRADEpro GDT). All decisions to down‐grade or up‐grade the quality of studies is justified within footnotes and comments to aid the reader's understanding of the review as necessary. Data on 6MWD, mortality and change in WHO functional class was broken down according to PH classification group.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses.
Dose (riociguat: less than 1 mg three times daily, 1 to 2.4 mg three times daily, or 2.5 mg or greater three times daily). This was not performed due to inadequate data volumes and detail in results.
Frequency of medication administration. All included studies employed the same frequency of administration.
Duration of treatment (< 24 weeks or > 24 weeks). No studies exceeded 24 weeks.
WHO functional class. There was inadequate study detail available to subgroup according to WHO functional class.
Combination with alternative PH therapies (phosphodiesterase‐V inhibitors, endothelin receptor antagonists, prostanoids, nitrates, calcium channel blockers, non‐PH specific medications including diuretics, anti‐coagulants and oxygen). This was performed for exercise and serious adverse event outcomes.
Underlying aetiology of pulmonary hypertension, including WHO classification grouping and then subgrouping. This was performed for exercise and serious adverse event outcomes.
We used the following outcomes in subgroup analyses.
Change in 6MWD
Serious adverse event
During this, we applied the formal test for subgroup interactions in Review Manager 5 (RevMan 2012). In addition to these subgroup analyses, we also analysed data together as a single complete set to allow subgroup analyses to infer causes of heterogeneity.
Sensitivity analysis
We carried out the following sensitivity analyses when appropriate.
Selection bias i.e. RCT versus quasi‐RCT
Performance and assessment bias e.g. blinding when applicable
Attrition bias (rate of drop‐out less than 20% versus 20% or higher)
Results
Description of studies
See Included studies; Excluded studies.
Results of the search
The literature search identified 259 abstracts and titles. After applying the aforementioned inclusion and exclusion criteria, we identified five RCTs suitable for inclusion within this review (Bonderman 2013; Ghofrani 2013a; Ghofrani 2013b; Bonderman 2014; Galiè 2015). This process is summarised in a flow diagram (Figure 1). Full text publications were obtained from the websites of the original source of publication. When studies were referenced more than once, data were combined in order to give as complete an interpretation as possible of available data.
Included studies
All studies were randomised and controlled in a parallel design and their characteristics are summarised in Table 3 and Table 4.
2. Characteristics of included studies summary.
| Study | PH Type | Number of Countries | Number Randomised | Number Completed | Intervention Group PH Treatment | Control Group PH Treatment | Duration | Trial Phase |
| Bonderman 2013 | Group 2: left heart disease | 18 | 202 | 172 | Riociguat, dose titrated up to 2 mg three times daily. | Placebo | 16 weeks | IIb |
| Bonderman 2014 | Group 2: left heart disease | 3 | 39 | 37 | Riociguat 0.5 mg, 1 mg, or 2 mg. | Placebo | Single dose | IIa |
| Galiè 2015 | Group 1: PAH | 5 | 18 | 17 | Riociguat, dose titrated up to 2 mg three times daily. People on stable sildenafil therapy at point of selection. | Placebo with sham titrations. People on stable sildenafil therapy at point of selection. | 12 weeks | II |
| Ghofrani 2013a | Group 1: PAH | 30 | 445 | 405 | Riociguat, dose titrated up to 2.5 mg three times daily. | Placebo with sham titrations. | 12 weeks | III |
| Ghofrani 2013b | Group 4: CTEPH | 26 | 262 | 243 | Riociguat, dose titrated up to 2.5 mg three times daily. | Placebo with sham titrations. | 16 weeks | III |
CTEPH: Chronic Thromboembolic Pulmonary Hypertension; PAH: Pulmonary arterial hypertension
3. Parameters relevant to protocol studied.
| Study | Change in 6MWD | Mortality | Improvement in WHO functional class | Clinical Worsening | Serious Adverse Events | Change in mean PA pressure |
| Bonderman 2013 | 3 | 3 | 3 | 3 | 3 | 1 |
| Bonderman 2014 | ‐ | 3 | ‐ | ‐ | 2 | 3 |
| Galiè 2015 | 3 | 3 | 3 | ‐ | 3 | 3 |
| Ghofrani 2013a | 1 | 3 | 2 | 2 | 3 | 3 |
| Ghofrani 2013b | 1 | 3 | 2 | 2 | 3 | 3 |
"1" = Primary outcome measure of study. "2" = Secondary outcome measure of study. "3" = Parameter measured but not explicitly as a primary or secondary outcome. "‐" = measure not included in study.
Out of the five included studies, a total of 966 participants were randomised, with 874 of these participants completing the study; this is made up of Bonderman 2013 (202 randomised, 172 completed), Bonderman 2014 (39 randomised, 37 completed), Galiè 2015 (18 randomised, 17 completed), Ghofrani 2013a (445 randomised, 405 completed), and Ghofrani 2013b (262 randomised, 243 completed). Four of these were controlled against placebo only (Bonderman 2013; Ghofrani 2013a; Ghofrani 2013b; Bonderman 2014). One study used stably managed sildenafil treatment in the control group as well the interventional group (Galiè 2015). All studies chose riociguat as their interventional agent.
All participants were adults (> 18 years old) and diagnosed with pulmonary hypertension. The aetiology of pulmonary hypertension varied and included PAH (Ghofrani 2013a; Galiè 2015), CTEPH (Ghofrani 2013b) and left‐sided heart failure (Bonderman 2013; Bonderman 2014).
6MWD was the primary outcome measure in two trials (Ghofrani 2013a; Ghofrani 2013b). Change in pPA was the primary outcome in two studies (Bonderman 2013; Bonderman 2014). Adverse events were the primary outcome in one study (Galiè 2015). Additional outcome measures included change in pulmonary vascular resistance, N‐terminal pro‐brain natriuretic peptide, WHO functional class, time to clinical worsening, Borg dyspnoea scale score, quality of life, haemodynamic and echocardiographic cardiac parameters and pharmacokinetics.
Further details including dosing and baseline exercise capacity can be found in the 'Characteristics of included studies' table.
Excluded studies
Twenty‐four references were excluded, and the reasons for this are outlined in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Allocation
All works reported are international, multicentre, double‐blind, randomised, placebo‐controlled trials (Bonderman 2013; Ghofrani 2013a; Ghofrani 2013b; Bonderman 2014; Galiè 2015). All studies utilised computer‐generated random numbers alongside adequate concealment of allocation.
Blinding
All studies are self‐described as "double‐blinded". However, none of the studies provided further information on the blinding process, therefore all studies were judged to have an unclear risk of bias.
Incomplete outcome data
Details of dropouts and withdrawals were described by all studies. Two studies employed an intention‐to‐treat analysis (Ghofrani 2013a; Ghofrani 2013b). Two studies employed a per‐protocol analysis (Bonderman 2014; Galiè 2015). One study used a combination of intention‐to‐treat and per protocol analyses depending on the variable (Bonderman 2013). No study had a dropout rate exceeding 20%. The risk of bias is more completely described in the 'Characteristics of included studies' tables and Figure 2.
2.
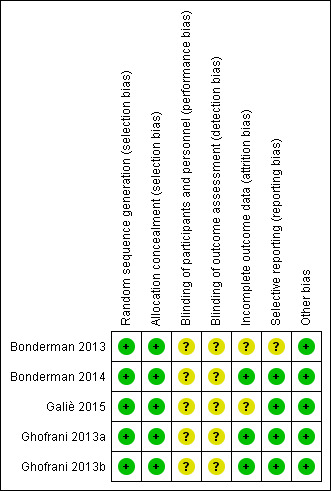
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Selective reporting
A low risk of reporting bias was found in four studies (Ghofrani 2013a; Ghofrani 2013b; Bonderman 2014; Galiè 2015), with outcomes reported in published works consistent with those stated in the protocols published beforehand. One study published all of the outcomes mentioned in the original protocol, as well as some further data not previously mentioned (Bonderman 2013). One study reported important outcomes for this meta‐analysis (6MWD and change in WHO functional class) as exploratory variables only (Galiè 2015). Galiè 2015 also had a further extension study that was stopped early. There is no data on this study that is publicly available and therefore this represents a potentially significant source of bias.
Other potential sources of bias
It should be noted that all included studies were sponsored by the drug manufacturer.
Effects of interventions
See: Table 1
Riociguat versus placebo
On theoretical discussion and preliminary analysis it was agreed that PH pathophysiology varies widely and is likely to drive heterogeneity in results. As such, we have presented all results broken down according to PH diagnostic classification. Any further analyses that were run are commented on separately within the text but not within forest plots. It should be noted that these other subgroups do not take into account PH aetiology.
Throughout all studies there were no differences in the daily frequency of dose administration reported. All reported trials were of less than 24 weeks total duration and therefore no further subgroup analysis was required. Futher subgroup analysis is detailed alongside pooled data analysis in the appropriate results.
Primary outcomes
Exercise capacity
Four trials of 819 participants assessed change from baseline in 6MWD (Bonderman 2013; Ghofrani 2013a; Ghofrani 2013b; Galiè 2015). That stated, only Ghofrani 2013a, Ghofrani 2013b and Galiè 2015 presented data suitable for pooling to the appropriate outcome. This totaled 659 participants. The pooled data demonstrate a mean difference (MD) versus placebo of 30.13 m (95% CI 5.29 to 54.96; participants = 659; studies = 3; I² = 64%) with potential substantial heterogeneity (Analysis 1.1). All studies used dose titration of riociguat up to 2.5 mg three times daily and so further subgroup analysis of dosing was not applicable. Authors were contacted for data regarding WHO functional class at baseline and changes in exercise capacity; unfortunately no further data was obtained. Only one trial explicitly dealt with a combination of riociguat with other named PH therapies (Galiè 2015). Data were presented according to PH aetiology.
1.1. Analysis.
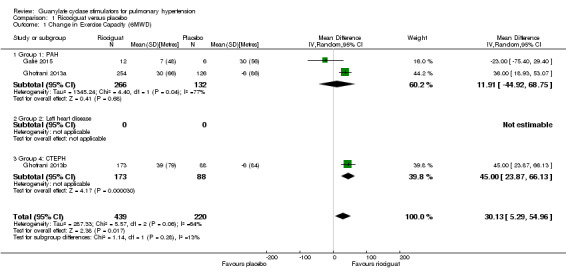
Comparison 1 Ricociguat versus placebo, Outcome 1 Change in Exercise Capacity (6MWD).
When looking at the PAH group alone, MD was 12 m (95% CI −44.92 to 68.75; participants = 398; studies = 2; I² = 77%).
Bonderman 2013 presented 6MWD change data relative to the control group only and therefore was not suitable for pooled analysis. This trial reported a placebo‐corrected least‐squares MD of 10 m (95% CI −18 to 39; P = 0.48; participants = 202) in favour of riociguat 2 mg.
Looking at CTEPH alone, the MD was 45 m (95% CI 23.87 to 66.13; participants = 261; studies = 1; I² = 0%).
Mortality
Fourteen deaths were reported from a total 899 participants in all trials (Analysis 1.2). The pooled analysis shows no significant difference in mortality (Peto OR 0.57, 95% CI 0.18 to 1.80; participants = 899; studies = 5; I² = 22%) and did not display significant heterogeneity.
1.2. Analysis.
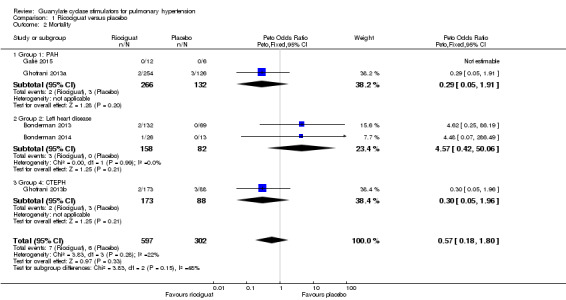
Comparison 1 Ricociguat versus placebo, Outcome 2 Mortality.
Secondary outcomes
Change in WHO functional class
Four studies (Bonderman 2013; Ghofrani 2013a; Ghofrani 2013b; Galiè 2015), including 858 participants, measured changes in exercise capacity according to the WHO functional class system (Analysis 1.3). Pooled analysis shows an odds ratio of 1.53 (95% CI 0.87 to 2.72; participants = 858; studies = 4; I² = 49%) in favour of riociguat for the improvement of WHO functional class. I² for this outcome is 49% and could represent substantial heterogeneity.
1.3. Analysis.
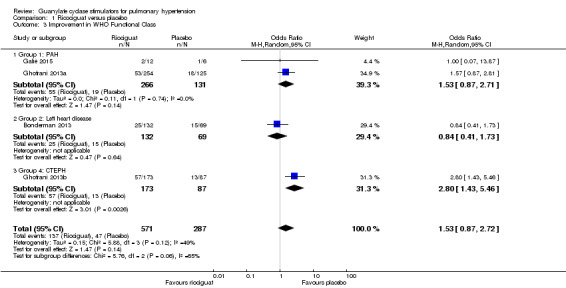
Comparison 1 Ricociguat versus placebo, Outcome 3 Improvement in WHO Functional Class.
Time to clinical worsening
Three studies (Bonderman 2013; Ghofrani 2013a; Ghofrani 2013b), including 842 participants, reported events of clinical worsening during the trial period (Analysis 1.4). Pooled analysis shows an OR of 0.45 (95% CI 0.17 to 1.14; participants = 842; studies = 3; I² = 54%) towards clinical worsening in the placebo group. I² for this outcome is 54% and could represent substantial heterogeneity.
1.4. Analysis.
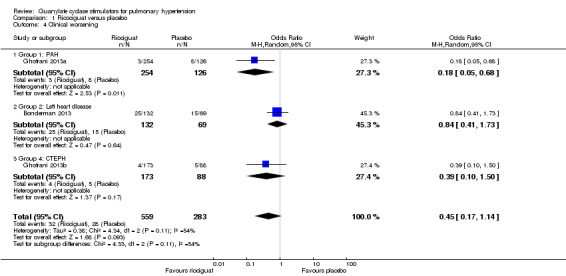
Comparison 1 Ricociguat versus placebo, Outcome 4 Clinical worsening.
Serious adverse events
Due to variability in reporting, only serious adverse events were analysed in this review and this has been measured as the number of people experiencing one or more events rather than total number of events. From 818 participants, pooled data indicates an OR of 1.12 (95% CI 0.66 to 1.90; participants = 818; studies = 5; I² = 39%) for serious adverse events being recorded between placebo and riociguat groups, with potential for moderate heterogeneity (Analysis 1.5).
1.5. Analysis.
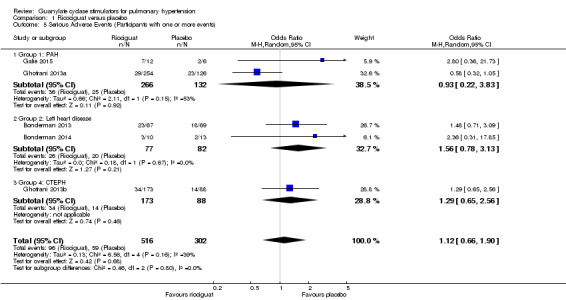
Comparison 1 Ricociguat versus placebo, Outcome 5 Serious Adverse Events (Participants with one or more events).
Two studies dealt with participants with PAH (Ghofrani 2013a; Galiè 2015). The pooled data did not show a difference in serious adverse events (OR 0.93, 95% CI 0.22 to 3.83; participants = 398; studies = 2; I² = 53%) and potential substantial heterogeneity. We believe this is likely due to the use of PDE5is in Galiè 2015. This study presented data descriptively due to the small cohort size, and did not show a difference in serious adverse events. However, caution should be taken when reviewing these results as the longer‐term extension of the trial was stopped due to a high incidence of adverse events. Two studies analysed effects in PH associated with left heart disease (Bonderman 2013; Bonderman 2014). This showed an odds ratio of 1.56 (95% CI 0.78 to 3.13; participants = 159; studies = 2; I² = 0%) without evidence of heterogeneity. One study analysed effects in CTEPH: this reported an odds ratio of 1.29 (95% CI 0.65 to 2.56; participants = 261; studies = 1; I² = 0%). Of note, 2% of riociguat recipients developed haemoptysis in this study.
Change in pulmonary artery pressure
Four studies (Bonderman 2013; Ghofrani 2013a; Ghofrani 2013b; Bonderman 2014), involving 744 participants, directly measured changes in pulmonary artery pressure at baseline and study endpoint (Analysis 1.6). Pooled analysis shows a MD of −2.77 mmHg (95% CI −4.96 to −0.58; participants = 744; studies = 3; I² = 49%) in the riociguat group versus placebo. I² for this outcome is 49% and could represent substantial heterogeneity.
1.6. Analysis.
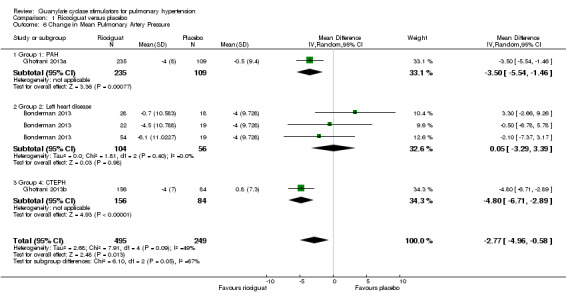
Comparison 1 Ricociguat versus placebo, Outcome 6 Change in Mean Pulmonary Artery Pressure.
Bonderman 2014 presented pulmonary artery pressure relative to the control group only and therefore was not suitable for pooled analysis. This trial reported a mean treatment difference of −8.0 mmHg (95% CI −15.5 to −0.6; P = 0.04; participants = 36) in favour of riociguat 2 mg.
Galiè 2015 measured pulmonary artery pressure as an exploratory variable but did not publish relevant data. The study does, however, state that there were "no between‐group differences".
Discussion
Summary of main results
Pooled data show that sGC stimulators can increase exercise capacity (6MWD) and cause reductions in pulmonary artery pressure in adults with PH. However, the significant levels of statistical heterogeneity should be noted when reviewing pooled results and broadly speaking, treatment effects should be looked at with respect to PH aetiology. There is currently no evidence for use in children and studies assessing use in left heart disease have been negative thus far. Furthermore, evidence suggests that use in the context of nitrates or PDE5is should be avoided and there is no evidence for the use of riociguat in people suitable for PEA.
PH Group 1: PAH
PAH is an important cause of mortality. Until recently treatments that decrease pulmonary pressures and improve exercise capacity were restricted to prostanoids, endothelin receptor antagonists and phosphodiesterase inhibitors. This systematic review supports the addition of sGC stimulators to improve exercise capacity. This is true for those not receiving PDE5is only and that is how results are reviewed. sGC stimulators improve 6MWD, a marker of prognosis (McLaughlin 2013), by 30 m when taken three times a day. This is very similar to the 28 m achieved with the addition of sildenafil to people receiving prostanoids (Simonneau 2008). However, this is yet to translate into improvements in mortality. Prostanoids have shown mortality benefit at 6MWD improvement of 47 metres in idiopathic PAH (Barst 1996). Riociguat shows a similar improvement in 6MWD, especially in the 1 year extension study (+ 48 m, SD 72 m) (Rubin 2013), but is yet to show associated mortality benefit. This same group, excluding PDE5is, showed improvements in clinical worsening, WHO functional class and pulmonary artery pressures.
The above results have been achieved without clear evidence of harm in treatment‐naive participants, although the occurrence of haemoptysis means it is now a relative contraindication to treatment. PAH combination treatment is becoming increasingly common and often considered desirable as emphasised by its superiority in the recent Ambition study (Galiè 2014). Riociguat has shown this to be possible with people on stable prostanoid or endothelin receptor antagonist therapy. There is currently no data on which combination of these agents may be most desirable. However, riociguat cannot be combined with PDE5is. Galiè 2015 showed that the long‐term addition of riociguat to people already on stable sildenafil is potentially dangerous with elevated incidences of adverse events, serious adverse events including hypotension, participant dropout and mortality. It has therefore been recommended that riociguat be contraindicated in people already receiving sildenafil or any other PDE5i. It should be noted that these studies are largely based upon idiopathic PAH and results may differ between PAH subgroups
PH Group 2: Left heart disease
PH associated with heart failure with preserved or reduced ejection fraction is an important cause of mortality and morbidity in a very large and important patient group, affecting up to 30% of people with reduced ejection fraction (Damy 2010). That acknowledged, there remains controversy on how best to manage PH in this setting. Current treatment includes optimisation of heart failure medication including beta‐blockers and angiotensin‐converting enzyme inhibitors, the use of diuretics for careful fluid management and, when appropriate, biventricular pacing. There has been some investigation into the use of PAH therapies such as PDE5is in this setting but no agent has yet to demonstrate adequate efficacy for widescale use.
Thus far, two trials have been completed on the use of riociguat in left heart disease‐associated PH (Bonderman 2013; Bonderman 2014). This included left ventricular systolic dysfunction (Bonderman 2013) and preserved left ventricular ejection fraction heart failure (Bonderman 2014). The results from these works do not show benefit due to the addition of riociguat in terms of exercise capacity or mortality and both miss their primary endpoint of achieving a reduction in PAP. These studies do report favourable haemodynamics (e.g. systemic vascular resistance, cardiac index, pulmonary vascular resistance) and improvements in quality of life scales, but do not reach the primary outcomes of the studies. Based on this, there is currently no evidence to support the introduction of sGC stimulators to this form of PH.
PH Group 4: CTEPH
CTEPH is an important cause of mortality with limited treatment options. Gold‐standard treatment is PEA; however, in a group of patients this is either ineffective (recurrent CTEPH) or not feasible (inoperable CTEPH). Before riociguat's approval, management for these people was supportive and held a poor prognosis. The only other pharmacological intervention investigated has been bosentan, and despite reducing pulmonary artery pressures, it did not improve 6MWD over 16 weeks (Jais 2008).
Riociguat has been evaluated in recurrent and inoperable CTEPH, and it is only in these groups that it can be recommended. In this setting, riociguat improves exercise tolerance and WHO functional class whilst reducing the incidence of clinical worsening and lowering pulmonary artery pressures. Evidence regarding serious adverse events remains inconclusive. Current evidence has not been designed to demonstrate an effect on mortality. That stated, the improvements in pulmonary haemodynamics achieved are significant, and higher pressures are a proven marker of poor prognosis (Jamieson 2003). There is no evidence for the use of riociguat in CTEPH suitable for PEA. CTEPH should be considered a surgically curable condition before reviewing medical therapy. PEA remains the gold standard treatment for CTEPH as it not only improves haemodynamics and exercise capacity, but also has a demonstrable impact on survival (Scholzel 2012). As such, people with CTEPH who are potential PEA candidates should still be assessed at a centre capable of achieving this for complete exclusion of a PEA strategy before the consideration of riociguat.
Overall completeness and applicability of evidence
All studies in this review collected data on one of the stated primary outcomes of this work (6MWD or mortality). All relevant participants, interventions and outcomes have been investigated. Overall, the evidence collected is highly applicable to this review. The evidence demonstrates that sGC stimulators have an important utility in PH. That stated, further data looking at different PH subgroups is required for a more complete guide as to their utility.
Since the publication of initial PATENT and CHEST trials both have been subject to open‐label extension. In PATENT‐2 people receiving either monotherapy or combination with endothelin receptor antagonists or prostanoids showed sustained improvements in 6MWD, WHO‐FC, brain natriuretic peptide and survival at two years (Ghofrani 2016). CHEST‐2 showed overall survival of 93% and clinical worsening‐free survival of 82% at two years (Simonneau 2016). Both studies add weight to the notion of long‐term use of sGC‐stimulators.
Quality of the evidence
All studies included in this meta‐analysis were parallel, double‐blind, RCTs. Trials reported data using a mixture of per protocol and intention‐to‐treat analyses. The quality of the current evidence can be considered to be low overall (one high quality outcome, two moderate quality outcomes and eight low or very low quality outcomes). Downgrades were associated with imprecision and indirectness within the evidence base and this was sometimes associated with heterogeneity as explored more fully in Table 1.
Potential biases in the review process
There are no known biases to disclose in the implementation of this review. It should be noted that all works included in this review were, however, sponsored by the pharmaceuticals company that launched the agent.
Agreements and disagreements with other studies or reviews
At the time of writing, this is the only known meta‐analysis of data on the use of sGC stimulators in PH. Published collative work so far has been in the form of literature reviews and editorials (Archer 2013; Hoeper 2015; Humbert 2015). These generally advocate the use of sGC stimulators in inoperable or recurrent CTEPH and in adults with PAH. The findings of this analysis are in agreement with these works.
Ongoing and future research
A wide scope remains to investigate the role of sGC stimulators in PAH and CTEPH amongst other conditions. This is demonstrated by the ongoing long‐term extension studies PATENT‐2 (NCT00863681) and CHEST‐2 (NCT00910429). Furthermore, there is currently an ongoing recruitment process for an uncontrolled open‐label phase 3b investigation into the use of riociguat in people with PAH who are negative responders to phosphodiesterase‐V inhibitors (RESPITE; NCT02007629). This study will allow a cross‐over insight into the effects of sildenafil and riociguat.
The efficacy and safety of riociguat in other forms of PH is also being investigated. This includes people with PH associated with COPD (NCT00640315), systemic sclerosis (NCT02283762), idiopathic interstitial pneumonia (NCT02138825) and cystic fibrosis. In addition, despite thus far disappointing results in the use of riociguat in hearts with preserved systolic function, there are plans for a further parallel randomised controlled phase II trial (SOCRATES) investigating the use for associated PH (Pieske 2014) as well as similar trials for verciguat. Results from well‐designed RCTs in these areas are strongly needed. There is also a call for studies providing greater detail on effects in children, and the effect of sGC stimulators relative to alternative treatment options in both PAH (e.g. parallel trials vs. PDE5is) and CTEPH (e.g. parallel trials vs. balloon angioplasty).
Authors' conclusions
Implications for practice.
In PAH, sGC stimulators reduce mean pulmonary artery pressures and, in the absence of concomitant phosphodiesterase‐V inhibitors, improve exercise capacity. Based on this, sGC stimulators are recommended for use in PAH in people not receiving PDE5is. That stated, the exact role they should play in treatment algorithms requires further head‐to‐head and treatment‐naive studies. Given their contra‐indication with PDE5is and similar positioning in treatment pathways to these agents, patient‐specific factors including the balance of evidence for specific PAH aetiologies and any history of adverse effects should be considered when deciding between these agents. The RESPITE study should add further granularity to this decision‐making process.
For PH associated with left heart disease, there is currently no evidence to support their use.
In inoperable or recurrent CTEPH, sGC stimulators can improve exercise capacity and WHO functional class whilst reducing mean pulmonary pressures. sGC stimulators should be used in people with inoperable or recurrent CTEPH as a second line treatment to endarterectomy only. CTEPH should always be considered a surgically curable condition before reviewing medical therapy.
Data trends towards reduced mortality in both PAH and CTEPH; however, no significant findings have yet been made. Further information is also required regarding the significance of serious adverse events. There is also currently no evidence supporting the use of sGC stimulators in children.
Implications for research.
This meta‐analysis partially answers the question of the utility of sGC stimulators in PH, specifically regarding PAH, inoperable or recurrent CTEPH and left heart disease. Further trials are required to evaluate their use in other PH forms, other population groups such as children, and to clarify positioning in treatment algorithms. sGC stimulators' effects on mortality require further investigation in the long term.
Acknowledgements
Haydn Walters was the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Database search strategies
Airways Register (via Cochrane Register of Studies)
#1 PULM:MISC1 #2 MeSH DESCRIPTOR Hypertension, Pulmonary Explode All #3 MeSH DESCRIPTOR Pulmonary Heart Disease #4 "pulmonary vascular disease":TI,AB #5 #1 or #2 or #3 or #4 #6 MeSH DESCRIPTOR Guanylate Cyclase Explode All #7 guanylate NEAR3 stimulator* #8 riociguat* #9 BAY63‐2521 #10 Adempas #11 #6 OR #7 OR #8 OR #9 OR #10 #12 #5 AND #11
CENTRAL (the Cochrane Library)
#1 MESH DESCRIPTOR Hypertension, Pulmonary EXPLODE ALL TREES #2 MESH DESCRIPTOR pulmonary heart disease EXPLODE ALL TREES #3 (pulmonary NEAR3 hypertensi*):TI,AB,KY #4 (pulmonary NEAR3 embolism*):TI,AB,KY #5 #1 OR #2 OR #3 OR #4 #6 MESH DESCRIPTOR Guanylate Cyclase EXPLODE ALL TREES #7 (guanylate NEAR3 stimulator*):TI,AB,KY #8 riociguat*:TI,AB,KY #9 BAY63‐2521:TI,AB,KY #10 Adempas:TI,AB,KY #11 #6 OR #7 OR #8 OR #9 OR #10 #12 #5 AND #11
MEDLINE (Ovid)
1. exp Hypertension, Pulmonary/ 2. Pulmonary Heart Disease/ 3. (pulmonary adj3 hypertensi$).tw. 4. (pulmonary$ adj3 embolism$).tw. 5. or/1‐4 6. exp Guanylate Cyclase/m 7. (guanylate adj3 stimulator$).tw. 8. riociguat$.tw. 9. BAY63‐2521.tw. 10. Adempas.tw. 11. or/6‐10 12. 5 and 11 13. (controlled clinical trial or randomised controlled trial).pt. 14. (randomised or randomised).ab,ti. 15. placebo.ab,ti. 16. dt.fs. 17. randomly.ab,ti. 18. trial.ab,ti. 19. groups.ab,ti. 20. or/13‐19 21. Animals/ 22. Humans/ 23. 21 not (21 and 22) 24. 20 not 23 25. 12 and 24
EMBASE (Ovid)
1. exp pulmonary hypertension/ 2. (pulmonary adj3 hypertensi$).tw. 3. (pulmonar$ adj3 embolism$).tw. 4. or/1‐3 5. exp guanylate cyclase/ 6. (guanylate adj3 stimulator$).tw. 7. riociguat$.tw. 8. BAY63‐2521.tw. 9. Adempas.tw. 10. or/5‐9 11. 4 and 10 12. Randomized Controlled Trial/ 13. randomization/ 14. controlled clinical trial/ 15. Double Blind Procedure/ 16. Single Blind Procedure/ 17. Crossover Procedure/ 18. (clinica$ adj3 trial$).tw. 19. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw. 20. exp Placebo/ 21. placebo$.ti,ab. 22. random$.ti,ab. 23. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw. 24. (crossover$ or cross‐over$).ti,ab. 25. or/12‐24 26. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 27. human/ or normal human/ or human cell/ 28. 26 and 27 29. 26 not 28 30. 25 not 29 31. 11 and 30
Data and analyses
Comparison 1. Ricociguat versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in Exercise Capacity (6MWD) | 3 | 659 | Mean Difference (IV, Random, 95% CI) | 30.13 [5.29, 54.96] |
| 1.1 Group 1: PAH | 2 | 398 | Mean Difference (IV, Random, 95% CI) | 11.91 [‐44.92, 68.75] |
| 1.2 Group 2: Left heart disease | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Group 4: CTEPH | 1 | 261 | Mean Difference (IV, Random, 95% CI) | 45.0 [23.87, 66.13] |
| 2 Mortality | 5 | 899 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.57 [0.18, 1.80] |
| 2.1 Group 1: PAH | 2 | 398 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.05, 1.91] |
| 2.2 Group 2: Left heart disease | 2 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.57 [0.42, 50.06] |
| 2.3 Group 4: CTEPH | 1 | 261 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.05, 1.96] |
| 3 Improvement in WHO Functional Class | 4 | 858 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.87, 2.72] |
| 3.1 Group 1: PAH | 2 | 397 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.87, 2.71] |
| 3.2 Group 2: Left heart disease | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.41, 1.73] |
| 3.3 Group 4: CTEPH | 1 | 260 | Odds Ratio (M‐H, Random, 95% CI) | 2.80 [1.43, 5.46] |
| 4 Clinical worsening | 3 | 842 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.17, 1.14] |
| 4.1 Group 1: PAH | 1 | 380 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.05, 0.68] |
| 4.2 Group 2: Left heart disease | 1 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.41, 1.73] |
| 4.3 Group 4: CTEPH | 1 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.10, 1.50] |
| 5 Serious Adverse Events (Participants with one or more events) | 5 | 818 | Odds Ratio (M‐H, Random, 95% CI) | 1.12 [0.66, 1.90] |
| 5.1 Group 1: PAH | 2 | 398 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.22, 3.83] |
| 5.2 Group 2: Left heart disease | 2 | 159 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [0.78, 3.13] |
| 5.3 Group 4: CTEPH | 1 | 261 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.65, 2.56] |
| 6 Change in Mean Pulmonary Artery Pressure | 3 | 744 | Mean Difference (IV, Random, 95% CI) | ‐2.77 [‐4.96, ‐0.58] |
| 6.1 Group 1: PAH | 1 | 344 | Mean Difference (IV, Random, 95% CI) | ‐3.5 [‐5.54, ‐1.46] |
| 6.2 Group 2: Left heart disease | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐3.29, 3.39] |
| 6.3 Group 4: CTEPH | 1 | 240 | Mean Difference (IV, Random, 95% CI) | ‐4.8 [‐6.71, ‐2.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bonderman 2013.
| Methods | Multicentre randomised placebo‐controlled, parallel, double‐blind trial. | |
| Participants | Adults with PH due to left systolic dysfunction heart failure. Adults not on any other PH medication. Total randomised n = 202 (69 placebo, 132 riociguat, 1 missing). Included 172 males. Mean age 58.1 years (range 25 to 79). Baseline 6MWD 382.1 m in placebo and 394.8 m in treated groups. |
|
| Interventions | Intervention groups: (1) riociguat 2 mg, (2) riociguat 1 mg, (3) riociguat 0.5 mg three times daily. Control: placebo. All taken orally for a total 16 weeks' duration three times daily with dose titration at weeks 2 and 4 according to systolic blood pressure. |
|
| Outcomes | Primary outcome: Change from baseline in pPA at week 16. Secondary outcomes: systolic pulmonary artery pressure, left ventricular ejection fraction, E‐wave deceleration time, E‐wave/A‐wave ratio, tricuspid annular plane systolic excursions, venous oxygen saturation, pVR, systemic vascular resistance, transpulmonary pressure gradient, pulmonary capillary wedge pressure, 6MWD, quality of life, brain natriuretic peptide, troponin T. |
|
| Notes | LEPHT, clinical trial identifier NCT01172756. Funding from Bayer HealthCare Pharmaceuticals (Berlin, Germany). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised" Comment: Probably done. Computer generated random number process. |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised 2:1:1:2 to four treatment arms" Comment: Probably done. Assumed adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: Probably done. Stated to be double‐blind. No further information provided as to process. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: Probably done. Stated to be double‐blind. No further information provided as to process. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Key outcome variables are reported as per protocol analysis with intention‐to‐treat analysis variable performed. Rates and reasons for dropout clearly stated and not significantly different between the groups. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome is consistent with protocol, however several secondary outcome variables mentioned in initial protocol (Ghio 2012 Aug) are not detailed in final analysis. |
| Other bias | Low risk | None found |
Bonderman 2014.
| Methods | Multicentre randomised placebo‐controlled, parallel, double‐blind trial. | |
| Participants | Adults with pulmonary hypertension associated with preserved left systolic function heart failure. People not on any other PH medication. Total randomised n = 39 (13 placebo, 26 riociguat). Included 13 males. Mean age 71.0 years (range 48.0 to 86.0). Baseline 6MWD unavailable. |
|
| Interventions | Intervention groups: (1) riociguat 2 mg, (2) riociguat 1 mg, (3) riociguat 0.5 mg three times daily Control: placebo. All taken orally as a single dose with all variables measured at six hours except for adverse events (30 days). |
|
| Outcomes | Primary outcome: Change from baseline in pPA at 6 hours. Secondary outcomes: cardiac index, cardiac output, blood pressure, heart rate, pulmonary vascular resistance, adverse events. |
|
| Notes | DILATE‐1, clinical trial identifier NCT01172756 Funding from Bayer HealthCare Pharmaceuticals (Berlin, Germany). Editorial assistance was provided by Adelphi Communications Ltd (Bollington, England), supported by Bayer HealthCare Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised" Comment: Probably done. Computer generated random number process. |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised" Comment: Probably done. Assumed adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: Probably done. Stated to be double blind. No further information provided as to process. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: Probably done. Stated to be double‐blind. No further information provided as to process. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per‐protocol analysis performed. Clearly cited rates of drop‐out and withdrawal with adequate explanation and no significant difference between groups. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes include all those mentioned in the trial protocol. Clinicaltrials.gov, NCT01172756 |
| Other bias | Low risk | None found. |
Galiè 2015.
| Methods | Multicentre randomised placebo‐controlled, parallel, double‐blind trial. | |
| Participants | Adults (ages 18 to 75) diagnosed with PAH and on a stable treatment regimen (> 90 days) including sildenafil 20 mg three times daily. 18 participants randomised (12 riociguat, 6 placebo). Mean age 59.3 years (standard deviation 10.3). 6 males in total. | |
| Interventions | Intervention: Riociguat 1mg three times daily Control: Placebo Treatement was administered orally for 12 weeks. Doses of riociguat were started at 1 mg and titrated in 0.5 mg increments according to systemic systolic blood pressure for a final range of 0.5 mg to 2.5 mg three times daily. Baseline 6MWD 426 m in placebo and 359 m in treated groups. |
|
| Outcomes | Primary outcome: change from baseline in systolic blood pressure at 4 hours post dose Secondary outcome: changes in diastolic blood pressure, heart rate, adverse events Exploratory outcomes: 6MWD and change in WHO functional class |
|
| Notes | PATENT PLUS, clinical trials identifier NCT01179334 Besides the 12‐week study period, there was a longer term extension study that was terminated by the sponsor due to increased rates of adverse events. Information on this extension is incomplete and therefore not included in this analysis. Funded by Bayer HealthCare Pharmaceuticals (Berlin, Germany). Editorial assistance was provided by Adelphi Communications Ltd (Bollington, UK), sponsored by Bayer HealthCare Pharmaceuticals. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised" Comment: Probably done. Computer generated random number process. |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised" Comment: Probably done. Assumed adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: Probably done. Stated to be double‐blind. No further information provided as to process. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double‐blind" Comment: Probably done. Stated to be double‐blind. No further information provided as to process. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data is presented descriptively in a per protocol fashion. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes include all those mentioned in the trial protocol. Clinicaltrials.gov, NCT01179334 |
| Other bias | Low risk | None |
Ghofrani 2013a.
| Methods | Multicentre randomised placebo‐controlled, parallel, double‐blind trial. | |
| Participants | Adults (> 18 years) with pulmonary arterial hypertension and on a stable treatment plan (stable prostanoids or endothelin receptor antagonists or treatment‐naive). Total randomised n = 443 (126 placebo, 317 riociguat). Included 93 males. Mean age 51.0 years (standard deviation 17). Baseline 6MWD 368 m in placebo and 361 m in treated groups. |
|
| Interventions | Intervention groups: (1) riociguat 2.5 mg, (2) riociguat 1.5 mg three times daily Control: placebo. All taken orally over 12 weeks three times daily. Dose adjustments made according to systemic blood pressure until week 8 at which point dose adjustments were stopped. |
|
| Outcomes | Primary outcome: Change from baseline in 6MWD. Secondary outcomes: Changes in pulmonary vascular resistance, brain natriuretic peptide, WHO functional class, clinical worsening, Borg dyspnoea score, quality of life and adverse events including mortality. |
|
| Notes | PATENT 1, clinical trial identifier NCT00810693 Funded by Bayer HealthCare Pharmaceuticals (Berlin, Germany). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned" Comment: Probably done. Computer generated random number process. |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly assigned, in a 2:4:1 ratio, to one of three regimens" Comment: Probably done. Assumed adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". Comment: Probably done. No further information provided as to process. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". Comment: Probably done. Stated to be double‐blind. No further information provided as to process. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed and reasons for patient exclusions are clearly stated. |
| Selective reporting (reporting bias) | Low risk | All expected results reported by the trial protocol NCT00810693 are detailed within the final published study report. |
| Other bias | Low risk | None noted |
Ghofrani 2013b.
| Methods | Multicentre randomised placebo‐controlled, parallel, double‐blind trial. | |
| Participants | Adults (> 18 years) with in‐operable, persistent or recurrent CTEPH. Total randomised n = 261 (88 placebo, 173 riociguat). Included 89 males. Mean age 59 years (standard deviation 14). People on no other treatment for PAH, or on prostanoids or endothelin receptor antagonists. Baseline 6MWD 356m in placebo and 342m in treated groups. |
|
| Interventions | Intervention group: riociguat 1 mg three times daily Control: placebo. All taken orally over 16 weeks three times daily. Riociguate started at 1 mg and then dose titrated to systemic blood pressure until week 8 at which point dose adjustment was stopped. Final dosing range 0.5 mg to 2.5 mg three times daily. |
|
| Outcomes | Primary outcome: Change from baseline in 6MWD. Secondary outcomes: Changes in pulmonary vascular resistance, brain natriuretic peptide, WHO functional class, clinical worsening, Borg dyspnoea score, quality of life and adverse events including mortality. |
|
| Notes | CHEST‐1, NCT00855465 Funded by Bayer HealthCare Pharmaceuticals (Berlin, Germany). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned" Comment: Probably done. Computer‐generated random number process. |
| Allocation concealment (selection bias) | Low risk | Quote: "eligible patients were randomly assigned in a 1:2 ratio" Comment: Probably done. Assumed adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "double blind". Comment: Probably done. Stated to be double blind. No further information provided as to process. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "double blind". Comment: Probably done. Stated to be double‐blind. No further information provided as to process. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed and reasons for patient exclusions are clearly stated. |
| Selective reporting (reporting bias) | Low risk | All expected results reported by the trial protocol NCT00855465 are detailed within the final published study report. |
| Other bias | Low risk | None |
CTEPH: chronic thromboembolic pulmonary hypertension; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; pPA: pulmonary artery pressure; pVR: pulmonary vascular resistance; WHO: World Health Organization; 6MWD: 6‐minute walk distance.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aschauer 2015 | sGC stimulator not compared with usual care or placebo |
| Aschauer 2015b | sGC stimulator not compared with usual care or placebo |
| Becker 2013 | sGC stimulator not compared with usual care or placebo |
| Denton 2015 | sGC stimulator not compared with usual care or placebo |
| Egenlauf 2015 | sGC stimulator not compared with usual care or placebo |
| Frey 2008 | Interaction study, outcomes not as per inclusion criteria |
| Frey 2009 | Interaction study, outcomes not as per inclusion criteria |
| Frey 2011 | Interaction study, outcomes not as per inclusion criteria |
| Ghofrani 2009 | sGC stimulator not compared with usual care or placebo |
| Ghofrani 2010 | sGC stimulator not compared with usual care or placebo |
| Ghofrani 2011 | sGC stimulator not compared with usual care or placebo |
| Grimminger 2009 | sGC stimulator not compared with usual care or placebo |
| Haddad 2015 | sGC stimulator not compared with usual care or placebo |
| Halank 2015 | sGC stimulator not compared with usual care or placebo |
| Hoeper 2010 | sGC stimulator not compared with usual care or placebo |
| Hoeper 2013 | sGC stimulator not compared with usual care or placebo |
| Parsley 2013 | Interaction study, outcomes not as per inclusion criteria |
| Sulica 2015 | sGC stimulator not compared with usual care or placebo |
sGC: soluble guanylate cyclase
Differences between protocol and review
Due to the variable reporting format of what constitutes an adverse event and the limited ability to access complete raw data, the second primary outcome measure stated in the protocol 'Serious adverse events including mortality' was replaced with a measures of 'Mortality' (Analysis 1.2) and 'Serious Adverse Events' (Analysis 1.5). Adverse events that were not serious or did not lead to a death are not reported on here due to insufficient data granularity despite contacting author groups. Furthermore, due to the distinct pathologies of the varying forms of PH and the heterogeneity this causes in pooled analysis, data in this work has been presented as subgroups according to PH diagnostic class throughout the Results and Discussion sections. Subsequently, data on pulmonary artery pressure changes and time to clinical worsening were excluded from the 'Summary of findings' table for reasons of clarity and their lacking significant data to bring together in meta‐analysis.
Contributions of authors
Both AW and RT contributed to the protocol in terms of conception, drafting, reviewing and finalisation. MS, RW and AW were involved in the selection of papers, extraction of data and completion of the review and analysis. RT and SG overviewed the process with input throughout.
Sources of support
Internal sources
The authors declare that no such funding was received for this systematic review, Other.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
AW, MS, RW: none known. SG: has received payments for consultancy and lecture fees from Actelion, Bayer, Gilead, GSK, Novartis, Pfizer and United Therapeutics and is a Trustee/Company Director of Breathing PLC and the Preventive Cardiology Trust. Professor Tulloh has received lecture fees / honoraria from companies that supply other pharmaceutical agents in pulmonary hypertension, including Pfizer, GSK and Actelion.
New
References
References to studies included in this review
Bonderman 2013 {published data only}
- Bonderman D, Ghio S, Felix SB, Ghofrani H‐A, Michelakis E, Mitrovic V, et al. Riociguat for HF with pulmonary hypertension (PH): Post‐hoc analysis of LEPHT by baseline pulmonary vascular resistance (PVR) and pulmonary vascular gradient (PVG). Journal of Cardiac Failure 2014;20(8):S9. [Google Scholar]
- Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis E, Mitrovic V, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double‐blind, randomized, placebo‐controlled, dose‐ranging hemodynamic study. Circulation 2013;128(5):502‐11. [DOI] [PubMed] [Google Scholar]
- Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis ED, Mitrovic V, et al. Riociguat improves pulmonary arterial compliance in patients with pulmonary hypertension due to systolic left ventricular dysfunction: Results from a post‐hoc analysis of the phase IIB double‐blind, randomized, placebo‐controlled, dose‐ranging hemodynamic LEPHT study. American Heart Association Scientific Sessions; 2013 Nov 16‐20; Dallas. 2013; Vol. 128:A17234.
- Ghio S, Bonderman D, Felix SB, Ghofrani HA, Michelakis ED, Mitrovic V, et al. Left ventricular systolic dysfunction associated with pulmonary hypertension riociguat trial (LEPHT): rationale and design. European Journal of Heart Failure 2012;14(8):946‐53. [DOI] [PubMed] [Google Scholar]
- Semigran M, Bonderman D, Ghio S, Felix SB, Ghofrani HA, Michelakis ED, et al. Left ventricular systolic dysfunction associated with pulmonary hypertension riociguat trial (LEPHT). Circulation 2012;126(23):2789‐90. [DOI] [PubMed] [Google Scholar]
Bonderman 2014 {published data only}
- Bonderman D, Pretsch I, Steringer‐Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension. Chest 2014;146(5):1274‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondermann D, Pretsch I, Steringer‐Mascherbauer R, Rosenkranz S, Tufaro C, Frey R, et al. Acute hemoDynamic effects of rIociguat in patients with puLmonary hypertension Associated with diasTolic heart failurE (DILATE‐1): A randomized, double‐blind, placebo‐controlled, single‐dose study. European Heart Journal 2013;34(suppl 1):620 [P3321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galiè 2015 {published data only}
- Galile N, Muller K, Scalise AV, Grunig E. PATENT PLUS: A blinded, randomised and extension study of riociguat plus sildenafil in PAH. European Respiratory Journal 2015;45(5):1314‐22. [DOI] [PubMed] [Google Scholar]
- Galiè N, Neuser D, Muller K, Scalise A‐V, Grünig E. A placebo‐controlled, double‐blind phase II interaction study to evaluate blood pressure following addition of riociguat to patients with symptomatic pulmonary arterial hypertension (PAH) receiving sildenafil (PATENT PLUS). American Journal of Respiratory and Critical Care Medicine 2013;187:A3530. [Google Scholar]
- Humbert MJC, Galie N, Ghofrani HA, Jansa P, Keogh AM, Langleben D, et al. Efficacy of riociguat in pretreated versus treatment‐naive patients with pulmonary arterial hypertension (PAH) in the phase III PATENT‐1 study. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A3534. [Google Scholar]
Ghofrani 2013a {published data only}
- Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z‐C, Keogh AM, et al. Correlation of improvements in hemodynamics and exercise capacity in patients with PAH: Results from the phase III PATENT‐1 study [Abstract]. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42:346s [1784].
- Ghofrani H, Galie N, Grimminger F, Humbert M, Keogh A, Langleben D, et al. Riociguat for the treatment of pulmonary arterial hypertension: a randomized, double‐blind, placebo‐controlled study (PATENT‐1) [Abstract]. Chest 2012;142(4):1027A. [Google Scholar]
- Ghofrani HA, Galie N, Grimminger F, Grunig E, Humbert M, Jing ZC, et al. Riociguat for the treatment of pulmonary arterial hypertension. New England Journal of Medicine 2013;369(4):330‐40. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Hoeper MM, Halank M, Meyer FJ, Stahler G, Behr J, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: long‐term safety, tolerability, and efficacy [Abstract]. American Journal of Respiratory and Critical Care Medicine 2012;185:A2370. [Google Scholar]
- Grunig E, Gaile N, Humbert M, Keogh AM, Langleben D, Rubin LJ, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): A responder analysis from the phase III PATENT‐1 study. European Respiratory Society Annual congress, 2013 Sept 7‐11, Barcelona, Spain. 2013.
- Grünig E, Galiè N, Humbert M, Keogh AM, Langleben D, Rubin LJ, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): A responder analysis from the phase III PATENT‐1 study [Abstract]. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona 2013;42(Suppl 57):346s [1783]. [Google Scholar]
- Grünig E, Sikirica M, Curram J, Davie N, Gohfrani HA. Riociguat for the treatment of patients with pulmonary arterial hypertension (PAH): Responder analysis of who functional class iii patients from the patent‐1 study. Value in Health. Conference: ISPOR 16th Annual European Congress Dublin Ireland. Conference Start: 20131102 Conference End: 20131106. November 2013.
- Jing JC, Gaile N, Ghofrani HA, Humbert M, Langleben D, Rubin LJ, et al. Comparison of hemodynamic parameters in treatment‐naive and pretreated patients with pulmonary arterial hypertension (PAH) in the Phase III PATENT‐1 study. European Heart Journal. 2013;34(1):44‐6. [DOI] [PubMed] [Google Scholar]
- Langleben D, Galie N, He J, Huang Y, Humbert M, Keogh AM, et al. Baseline characteristics and response to treatment in pretreated versus treatment‐naive patients with pulmonary arterial hypertension (PAH) in the phase III PATENT‐1 study. American Journal of respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A3532. [Google Scholar]
- Langleben D, Galiè N, He J, Huang Y, Humbert M Keogh A, et al. Use of clinically relevant responder threshold criteria to evaluate the response to treatment in the phase III PATENT‐1 study. Journal of Heart and Lung Transplantation 2015;34(3):338‐47. [DOI] [PubMed] [Google Scholar]
- Meyer G, Gaile N, Grimminger F, Grunig E, Humbert M, Jing Z‐C. Long‐term riociguat treatment in PAH patients in WHOfunctional Class (FC) I/II Versus FC III/IV at baseline: results from the 12‐week phase III PATENT‐1 study and PATENT‐2open‐label extension. Chest 2013;145((3 Suppl)):513A. [Google Scholar]
- Meyer G, Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z‐C, et al. Long‐term riociguat treatment in PAH patients in WHO functional class (FC) I/II versus FC III/IV at baseline: results from the 12‐week phase III PATENT‐1 study and PATENT‐2 open‐label extension [Abstract]. American College of Chest Physicians World Congress; 2014 Oct 25‐30; Austin. 2014; Vol. 145:513A.
- Rubin L, Gaile N, Grimminger F, Grunig E, Humbert M, Jing Z‐C. Riociguat for the treatment of pulmonary arterial hypertension(PAH): 1‐Year results from the PATENT‐2 long‐term extension study. Chest 2013;144(4):1024A. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Galiè N, Grimminger F, Grunig E, Humbert M, Zing ZC, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long‐term extension study (PATENT‐2). European Respiratory Society 2015;45(5):1303‐13. [DOI] [PubMed] [Google Scholar]
- Rubin RJ, Gaile N, Grimminger F, Grunig E, Humbert MJ, Jing Z‐C, et al. Riociguat for the treatment of pulmonary arterial hypertension (pah): a phase Iii long‐term extension study (patent‐2). American Thoracic Society International Conference, May 17‐22, 2013, Philadelphia, Pennsylvania, USA. 2013.
Ghofrani 2013b {published data only}
- D'Armini AM, Ghofrani H‐A, Kim NH, Mayer E, Simonneau G, Wilkins MR, et al. Riociguat for the treatment of inoperable CTEPH or persistent/recurrent PH after pulmonary endarterectomy (PEA): A responder analysis from the phase III CHEST‐1 study [Abstract]. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42:543s [P2598].
- D'Armini AM, Ghofrani HA, Kim NH, Mayer E, Morsolini M, Pulido‐Zamudio T. Use of responder threshold criteria to evaluate the response to treatment in the phase III CHEST‐1 study. Journal of Heart and Lung Transplantation 2015;34(3):348‐55. [DOI] [PubMed] [Google Scholar]
- Ghofrani H, Grimminger F, Hoeper M, Kim N, Mayer E, Neuser D, et al. Riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension: a randomized, double‐blind, placebo‐controlled study (CHEST‐1) [Abstract]. Chest 2012;142(4):1023A. [Google Scholar]
- Ghofrani H‐A, Grimminger F, Hoeper MM, Kim NH, Mayer E, Simonneau G, et al. Impact of riociguat on health‐related quality of life (HRQoL) in patients with chronic thromboembolic pulmonary hypertension (CTEPH)[Abstract]. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013; Vol. 42, issue Suppl 57:701s [P3418].
- Ghofrani HA, D'Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. New England Journal of Medicine 2013;369(4):319‐29. [DOI] [PubMed] [Google Scholar]
- Ghofrani HA, Grimminger F, Hoeper M, Kim N, Mayer E, Simonneau G, et al. Impact of riociguat on health‐related quality of life (HRQoL) in patients with chronic thromboembolic pulmonary hypertension (CTEPH). European Respiratory Society Annual Congress, 2013 Sept 7‐11, Barcelona, Spain. 2013.
- Ghofrani HA, Hoeper MM, Halank M, Meyer FJ, Staehler G, Behrf J, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. European Respiratory Journal 2010;36(4):792‐9. [DOI] [PubMed] [Google Scholar]
- Jansa P, Ghofrani H‐A, Hoeper MM, Kim NH, Mayer E, Neurohr C, et al. Comparison of hemodynamic parameters in patients with inoperable and persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH) in the Phase III CHEST‐1 study. European Heart Journal 2013;34(suppl 1):187. [Google Scholar]
- Jansa P, Ghofrani HA, Kim NH, Mayer E, Neurohr C, Simonneau G, et al. Comparison of hemodynamic parameters in patients with inoperable and persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH) in the Phase III CHEST‐1 study. European Heart Journal. 2013;34(1):187. [Google Scholar]
- Simonneau G, D'Armini AM, Ghofrani HA, Grimminger F, Hoeper MM, Jansa P, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension (CTEPH): 1‐year results from the CHEST‐2 long‐term extension study [Abstract]. Chest 2013;144(4_MeetingAbstracts)):1023A. [Google Scholar]
- Simonneau G, D'Armini AM, Ghofrani HA, Grimminger F, Hoeper MM, Jansa P, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long‐term extension study (CHEST‐2). European Respiratory Journal 2015;45(5):1293‐302. [DOI] [PubMed] [Google Scholar]
- Simonneau G, D’Armini A, Ghofrani H, Grimminger F, Hoeper M, Jansa P, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension (CTEPH): 1‐year results from the CHEST‐2 long‐term extension study [Abstract]. Chest 2013;144(4 Meeting Abstracts):1023A. [Google Scholar]
- Wang C, D'Armini AM, Ghofrani HA, Grimminger F, Hoeper M, Jansa P. Long‐term riociguat treatment in inoperable and persistent/recurrent CTEPH patients in WHO functional class (FC) I/II versus FC III/IV at baseline: results from the 16‐week phase III CHEST‐1 study and CHEST‐2 open‐label extension [Abstract]. Chest 2014;145(3 Suppl):535B. [Google Scholar]
References to studies excluded from this review
Aschauer 2015 {published data only}
- Aschauer S, Duca F, Bachmnn A, Kammerlander A, Zotter‐Tufaro C, Mascherbauer J, et al. Riociguat treatment for patients with pulmonary hypertension due to heart failure with preserved ejection fraction. European Heart Journal. Conference: European Society of Cardiology, ESC Congress 2015 London United Kingdom. Conference Start: 20150829 Conference End: 20150902. 01 Aug 2015:Conference Publication: (var.pagings). 36 (pp 315).
Aschauer 2015b {published data only}
- Aschauer SS, Duca F, Bachmann A, Kammerlander A, Zotter‐Tufaro C, Mascherbauer J, et al. Riociguat treatment for patients with pulmonary hypertension due to heart failure with preserved ejection fraction. European Journal of Heart Failure. Conference: Heart Failure 2015 and the 2nd World Congress on Acute Heart Failure Seville Spain. Conference Start: 20150523 Conference End: 20150526.. May 2015; Vol. Mascherbauer J, issue Bonderman D:Conference Publication: (var.pagings). 17 (pp 278‐279.
Becker 2013 {published data only}
- Becker C, Frey R, Unger S, Thomas D, Reber M, Weimann G, et al. Pharmacokinetic (PK) interaction of ketoconazole (KC), clarithromycin (CM) and midazolam (MZ) with riociguat. European Respiratory Journal 2013;42:4068. [Google Scholar]
Denton 2015 {published data only}
- Denton C, Coghlan JG, Ghofrani HA, et al. Efficacy and safety of riociguat in patients with pulmonary arterial hypertension (PAH) associated with connective tissue disease (CTD). Annals of the Rheumatic Diseases. Conference: Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2015 Rome Italy. Conference Start: 20150610 Conference End: 20150613. June 2015.;74:Conference Publication: (var.pagings) (pp 588‐589). [Google Scholar]
Egenlauf 2015 {published data only}
- Egenlauf B, Marra A, Ehiken N, et al. Change of right heart size and function by long‐term therapy with riociguat in patients with PAH and CTEPH. European Respiratory Journal. Conference: European Respiratory Society Annual Congress 2015 Amsterdam Netherlands. Conference Start: 20150926 Conference End: 20150930. 01 Sep 2015;Conference Publication:46. [Google Scholar]
Frey 2008 {published data only}
- Frey R, Muck W, Unger S, Artmeier‐Brandt U, Weimann G, Wensing G. Single‐Dose Pharmacokinetics, Pharmacodynamics, Tolerability, and Safety of the Soluble Guanylate Cyclase Stimulator BAY 63‐2521: An Ascending‐DoseStudy in Healthy Male Volunteers. Journal of Clinical Pharmacology 2008;48(8):926‐34. [DOI] [PubMed] [Google Scholar]
Frey 2009 {published data only}
- Frey R, Muck W, Kirschbaum N, Kratzschmar J, Weimann G. Warfarin pharmacodynamics and pharmacokinetics are not affected by the soluble guanylate cyclase stimulator riociguat (bay 63‐2521). BMC Pharmacology 2009;9(suppl 1):P15. [Google Scholar]
- Frey R, Muck W, Kirschbaum N, Kratzschmar J, Weimann G, Wensing G. Riociguat (BAY 63‐2521) and warfarin: a pharmacodynamic and pharmacokinetic interaction study. Journal of Clinical Pharmacology 2011;51(7):1051‐60. [DOI] [PubMed] [Google Scholar]
- Frey R, Muck W, Kirschbaum N, Kratzschmar J, Weimann G, Wensing G. Warfarin pharmacodynamics and pharmacokinetics are not affected by the soluble guanylate cyclase stimulator riociguat (BAY 63‐2521): results of a randomized, controlled trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A3349. [Google Scholar]
Frey 2011 {published data only}
- Frey R, Muck W, Unger S, Reber M, Kratzschmar J, Becker C, et al. No pharmacodynamic (PD) and pharmacokinetic (PK) interaction of riociguat (BAY 63‐2521) and aspirin. 5th International Conference on cGMP: Generators, Effectors and Therapeutic Implications; 2011 24‐26 June; Halle. 2011; Vol. 11:P25.
- Frey R, Mück W, Unger S, Reber M, Krätzschmar J, Becker C, et al. No pharmacodynamic (PD) and pharmacokinetic (PK) interaction of riociguat (BAY 63‐2521) and aspirin [Abstract]. European Respiratory Society 21st Annual Congress; 2011 Sep 24‐28; Amsterdam. 2011; Vol. 38:724s [P3977].
Ghofrani 2009 {published data only}
- Ghofrani HA, Hoeper MM, Hoeffken G, Halank M, Weimann G, Grimminger F. Riociguat dose titration in patients with chronic thromboembolic pulmonary hypertension (CTEPH) or pulmonary arterial hypertension (PAH). BMC Pharmacology 2009;9((Suppl 1)):S14. [Google Scholar]
Ghofrani 2010 {published data only}
- Ghofrani HA, Hoeper MM, Halank M, Meyer FJ, Staehler G, Behr J, et al. A phase 2 study of the soluble guanylate cyclase stimulator riociguat in patients with chronic thromboembolic pulmonary hypertension or pulmonary arterial hypertension. Circulation. Conference: World Congress of CardiologyScientific Sessions 2010, WCC 2010 Beijing China. Conference Start: 20100616 Conference End: 20100619.. Jul 2010; Vol. 122:2.
- Ghofrani HA, Hoeper MM, Halank M, Meyer FJ, Staehler G, Behr J, et al. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. European Respiratory Journal 2010;36:792‐9. [DOI] [PubMed] [Google Scholar]
Ghofrani 2011 {published data only}
- Ghofrani HA, Staehler G, Gruenig E, Halank M, Mitrovic V, Unger S, et al. The Effect Of The Soluble Guanylate Cyclase Stimulator Riociguat On Hemodynamics In Patients With Pulmonary Hypertension Due To Chronic Obstructive Pulmonary Disease [Abstract]. American Journal of Respiratory and Critical CareMedicine. Conference: American Thoracic Society InternationalConference, ATS 2011 Denver, CO United States. ConferenceStart: 20110513 Conference End: 20110518. May 2011; Vol. 183.
Grimminger 2009 {published data only}
- Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, Bolkow D, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. European Respiratory Journal 2009;33(4):785‐92. [DOI] [PubMed] [Google Scholar]
Haddad 2015 {published data only}
- Haddad R, Mielniczuk L, Pugliese C, Chandy G, Stewart D, Contreras‐Dominguez V, et al. Riociguat for chronic thromboembolic pulmonary hypertension‐initial experience. Canadian Journal of Cardiology. 2015;31(10):S39‐40. [Google Scholar]
- Haddad R, Mielniczuk L, Pugliese C, Chandy G, Stewart D, Contreras‐Dominguez V, et al. Riociguat for chronic thromboembolic pulmonary hypertension‐initial experience. Journal of Cardiac Failure 2015;21(8):S71‐2. [Google Scholar]
Halank 2015 {published data only}
- Halank M, Humbert M, Sanchez M‐AG. Riociguat for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Real‐life data from the EXPERT registry. European Respiratory Journal 2015;46(suppl 59):OA4999. [Google Scholar]
Hoeper 2010 {published data only}
- Hoeper MM, Halank M, Wilkens H, Guenther A, Weimann G, Gebert I, et al. Riociguat for patients with pulmonary hypertension associated with interstitial lung disease. American Journal of Respiratory and Critical Care Medicine 2010;41(4):853‐60. [DOI] [PubMed] [Google Scholar]
Hoeper 2013 {published data only}
- Hoeper MM, Halank M, Wilkens H, Gunther A, Weimann G, Gebert I, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. European Respiratory Journal 2013;41(4):853‐60. [DOI] [PubMed] [Google Scholar]
Parsley 2013 {published data only}
- Parley EL, Masamune H, Hoye WL, Rubin LJ, Gladwin MT. Nebulized inhaled nitrite (AIR001) for pulmonary arterial hypertension: Studies to determine safety, pharmacokinetics, and maximum tolerated dose, lack of pharmacodynamic interaction with sildenafil and optimal nebulizer device. 5th International Meeting on the Role of Nitrite and Nitrate in Physiology, Pathophysiology, and Therapeutics; 2013 May 4‐5; Pittsburgh. 2013:S41‐2.
Sulica 2015 {published data only}
- Sulica E, Fenton R, Cefali F. Early observations on the use of riociguat in a large, metropolitan pulmonary arterial hypertension/chronic thromboembolic pulmonary hypertension treatment center. Cardiology and Therapy 2015;4(2):209‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abumehdi 2015
- Abumehdi MR, Wardle AJ, Nazzal R, Charalampopoulos A, Schulze‐Neick I, Derrick G, et al. Feasibility and safety of cardiopulmonary exercise testing in children with pulmonary hypertension. Cardiology in the Young 2015;26(6):1144‐50. [DOI] [PubMed] [Google Scholar]
Archer 2013
- Archer SL. Riociguat for pulmonary hypertension ‐ a glass half full. New England Journal of Medicine 2013;369(4):386‐8. [DOI] [PubMed] [Google Scholar]
Barst 1996
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The New England Journal of Medicine 1996;334(5):296‐302. [DOI] [PubMed] [Google Scholar]
Bayer 2014
- Bayer Pharmaceuticals. 2014 Price List. www.bayer.co.uk/html/pdf/Pharma/190614_Official_List_Prices.pdf (accessed 7 July 2014).
Bonderman 2007
- Bonderman D, Skoro‐Sajer N, Jakowitsch J, Adlbrecht C, Dunkler D, Taghavi S, et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation 2007;115(16):2153‐8. [DOI] [PubMed] [Google Scholar]
Damy 2010
- Damy T, Goode KM, Kallvikbacka‐Bennett A, Lewinter C, Hobkirk J, Nikitin NP, et al. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. European Heart Journal 2010;31(18):2280‐90. [DOI] [PubMed] [Google Scholar]
Denninger 1999
- Denninger JW, Marletta MA. Guanylate cyclase and the NO/cGMP signalling pathway. Biochimica et Biophysica Acta 1999;1411(2‐3):334‐50. [DOI] [PubMed] [Google Scholar]
Dowdall 2014
- Dowdall M. Riociguat recommended by CHMP for approval in the WU for use in two forms of pulmonary hypertension. Future Cardiology 2014;10(2):163. [PubMed] [Google Scholar]
Galiè 2013
- Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al. Updated treatment algorithm of pulmonary arterial hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D60‐72. [DOI] [PubMed] [Google Scholar]
Galiè 2014
- Galiè N. The AMBITION study: design and results. European Respiratory Journal 2014;44(Suppl.58):2916. [Google Scholar]
Ghofrani 2004
- Ghofrani HA, Pepke‐Zaba J, Barbera JA, Channick R, Keogh AM, Gomez‐Sanchez MA, et al. Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. Journal of the American College of Cardiology 2004;43(12 Suppl):68S‐72S. [DOI] [PubMed] [Google Scholar]
Ghofrani 2016
- Ghofrani HA, Grimminger F, Grünig E, Huang Y, Jansa P, Jing ZC, et al. Predictors of long‐term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT‐2 open‐label, randomised, long‐term extension trial. The Lancet Respiratory Medicine 2016;4(5):361‐71. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster. GRADEpro GDT. Version accessed 13 July 2016. Hamilton (ON): GRADE Working Group, McMaster, 2014.
Guha 2013
- Guha M. First‐in‐class guanylate cyclase stimulator approved for PAH. Nature Biotechnology 2013;31(12):1064. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoeper 2015
- Hoeper MM. Pharmacological therapy for patients with chronic thromboembolic pulmonary hypertension. European Respiratory Review 2015;24(136):272‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humbert 2015
- Humbert M, Ghofrani HA. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2016;71(1):73‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivy 2013
- Ivy DD, Abman SH, Barst RJ, Berger RM, Bonnet D, Fleming TR, et al. Pediatric pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D117‐26. [DOI] [PubMed] [Google Scholar]
Jais 2008
- Jais X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT(Bosentan effects in iNopErable forms of chronIc thromboembolic pulmonary hypertension), a randomized, placebo‐controlled trial. Journal of the American College of Cardiology 2008;52(25):2127‐34. [DOI] [PubMed] [Google Scholar]
Jamieson 2003
- Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Annals of Thoracic Surgery 2003;76(5):1462‐4. [DOI] [PubMed] [Google Scholar]
Keogh 2009
- Keogh AM, Mayer E, Benza RL, Corris P, Dartevelle PG, Frost AE, et al. Interventional and surgical modalities of treatment in pulmonary hypertension. Journal of the American College of Cardiology 2009;54(1 Suppl):S67‐77. [DOI] [PubMed] [Google Scholar]
Ling 2012
- Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2012;186(8):790‐6. [DOI] [PubMed] [Google Scholar]
Mayer 2011
- Mayer E, Jenkins D, Lindner J, D'Armini A, Kloek J, Meyns B, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Journal of Thoracic and Cardiovascular Surgery 2011;141(3):702‐10. [DOI] [PubMed] [Google Scholar]
McLaughlin 2013
- McLaughlin VV, Gaine SP, Howard LS, Leuchte HH, Mathier MA, Mehta S. Treatment goals of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D73‐81. [DOI] [PubMed] [Google Scholar]
Miyamoto 2000
- Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six‐minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. American Journal of Respiratory and Critical Care Medicine 2000;161(2 Pt 1):487‐92. [DOI] [PubMed] [Google Scholar]
NCT00640315
- Bayer. Single dose study in patients with chronic obstructive pulmonary disease (COPD) associated pulmonary hypertension. https://clinicaltrials.gov/ct2/show/NCT00640315 (accessed 13 July 2015).
NCT00863681
- Bayer. Long‐term extension study in patients with pulmonary arterial hypertension (PATENT‐2). https://clinicaltrials.gov/ct2/show/NCT00863681 (accessed 13 July 2015).
NCT00910429
- Bayer. Long‐term extension study in patients with chronic thromboembolic pulmonary hypertension (CHEST‐2). https://clinicaltrials.gov/ct2/show/NCT00910429 (accessed 13 July 2015).
NCT02007629
- Bayer. Riociguat clinical effects studied in patients with insufficient treatment response to phosphodiesterase‐5 inhibitor (RESPITE). https://clinicaltrials.gov/ct2/show/NCT02007629 (accessed 13 July 2015).
NCT02138825
- Bayer. Efficacy and safety of riociguat in patients with symptomatic pulmonary hypertension (PH) associated with idiopathic interstitial pneumonias (IIP) (13605RISE‐IIP). https://clinicaltrials.gov/ct2/show/NCT02138825 (accessed 13 July 2015).
NCT02283762
- Bayer. Efficacy and safety of riociguat in patients with systemic sclerosis. https://clinicaltrials.gov/ct2/show/NCT02283762 (accessed 13 July 2015).
Pieske 2014
- Pieske B, Butler J, Filippatos G, Lam C, Maggioni AP, Ponikowski P, et al. Rationale and design of the SOluble guanylate cyclase stimulatoR in heArT failurE studies (SOCRATES). European Journal of Heart Failure 2014;16(9):1026‐38. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riedel 1982
- Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow‐up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest 1982;81(2):151‐8. [DOI] [PubMed] [Google Scholar]
Rubin 2013
- Rubin L, Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z‐C, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): 1‐year results from the PATENT‐2 long‐term extension study [Abstract]. Chest. 2013; Vol. 144, issue 4 Meeting Abstracts:1024A.
Schermuly 2011
- Schermuly RT, Janssen W, Weissmann N, Stasch JP, Grimminger F, Ghofrani HA. Riociguat for the treatment of pulmonary hypertension. Expert Opinion on Investigational Drugs 2011;20(4):567‐76. [DOI] [PubMed] [Google Scholar]
Scholzel 2012
- Scholzel BE, Post MC, Thijs Plokker HW, Snijder RJ. Clinical worsening during long‐term follow‐up in inoperable chronic thromboembolic pulmonary hypertension. Lung 2012;190(2):161‐7. [DOI] [PubMed] [Google Scholar]
Simonneau 2008
- Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost AE, et al. Addition of sildenafil to long term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomised trial. Annals of Internal Medicine 2008;149(8):521‐30. [DOI] [PubMed] [Google Scholar]
Simonneau 2013b
- Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D34‐41. [DOI] [PubMed] [Google Scholar]
Simonneau 2016
- Simonneau G, D'Armini AM, Ghofrani HA, Grimminger F, Jansa P, Kim NH, et al. Predictors of long‐term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: data from the CHEST‐2 open‐label, randomised, long‐term extension trial. The Lancet Respiratory Medicine 2016;4(5):372‐80. [DOI] [PubMed] [Google Scholar]
Stasch 2011
- Stasch JP, Pacher P, Evgenov OV. Guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011;123(20):2263‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tatebe 2016
- Tatebe S, Sugimura K, Aoki T, Miura M, Nochioka K, Yaoita N, et al. Multiple Beneficial Effects of Balloon Pulmonary Angioplasty in Patients With Chronic Thromboembolic Pulmonary Hypertension. Circulation Journal: official journal of the Japanese Circulation Society 2016;80(4):980‐8. [DOI] [PubMed] [Google Scholar]
Thenappan 2010
- Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg‐Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. European Respiratory Journal 2010;35(5):1079‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thistlesthwaite 2006
- Thistlethwaite PA, Kemp A, Du L, Madani MM, Jamieson SW. Outcomes of pulmonary endarterectomy for treatment of extreme thromboembolic pulmonary hypertension. Journal of Thoracic and Cardiovascular Surgery 2006;131(2):307‐13. [DOI] [PubMed] [Google Scholar]
Wardle 2013a
- Wardle AJ, Wardle R, Luyt K, Tulloh R. The utility of sildenafil in pulmonary arterial hypertension: a focus on bronchopulmonary dysplasia. Archives of Disease in Childhood 2013;98(9):613‐7. [DOI] [PubMed] [Google Scholar]
Wardle 2013b
- Wardle A, Tulloh R. Evolving management of pediatric pulmonary arterial hypertension: impact of phosphodiesterase inhibitors. Pediatric Cardiology 2013;34(2):213‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wardle 2014
- Wardle AJ, Tulloh RMR. Guanylate cyclase stimulators for pulmonary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD011205] [DOI] [PMC free article] [PubMed] [Google Scholar]


