Abstract
Background
Genital herpes is incurable, and is caused by the herpes simplex virus (HSV). First‐episode genital herpes is the first clinical presentation of herpes that a person experiences. Current treatment is based around viral suppression in order to decrease the length and severity of the episode.
Objectives
To determine the effectiveness and safety of the different existing treatments for first‐episode genital herpes on the duration of symptoms and time to recurrence.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (from inception to April 2016), MEDLINE (from inception to April 2016), the Specialised Register of the Cochrane Sexually Transmitted Infections Review Group (from inception to April 2016), EMBASE (from inception to April 2016), PsycINFO (from inception to April 2016), CINAHL (from inception to April 2016), LILACS (from inception to April 2016), AMED (from inception to April 2016), and the Alternative Medicines Specialised Register (from inception to April 2016). We handsearched a number of relevant journals, searched reference lists of all included studies, databases of ongoing trials, and other Internet databases.
Selection criteria
We included randomised controlled trials (RCTs) on participants with first‐episode genital herpes. We excluded vaccination trials, and trials in which the primary objective assessed a complication of HSV infection.
Data collection and analysis
All studies written in English were independently assessed by at least two review authors for inclusion, risk of bias for each trial, and to extract data. Studies requiring translation were assessed for inclusion, trial quality, and data extraction by external translators.
Main results
We included 26 trials with 2084 participants analysed. Most of the studies were conducted in the United Kingdom (UK) and United States (US), and involved men and women experiencing their first episode of genital herpes, with the exception of three studies which included only women. We rated the majority of these studies as having an unclear risk of bias; largely due to lack of information supplied in the publications, and due to the age of the trials. This review found low quality evidence from two studies of oral acyclovir, when compared to placebo, reduced the duration of symptoms in individuals undergoing their first episode of genital herpes (mean difference (MD) ‐3.22, 95% confidence interval (CI) ‐5.91 to ‐0.54; I2 = 52%). In two studies (112 participants), intravenous acyclovir decreased the median number of days that patients with first‐episode herpes suffered symptoms. Oral valaciclovir (converted to acyclovir) also showed a similar length of symptom duration when compared to acyclovir in two studies. There is currently no evidence that topical acyclovir reduces symptoms (MD ‐0.61 days, 95% CI ‐2.16 to 0.95; 3 RCTs, 195 participants, I2 statistic = 56%). There is also no current evidence that the topical treatments of cicloxolone cream, carbenoxolone sodium cream, adenosine arabinoside, idoxuridine in dimethyl sulfoxide, when compared to placebo reduced the duration of symptoms in people undergoing their first episode of herpes.
Two studies reported no evidence of a reduction in the number of median days to recurrence following treatment with oral acyclovir versus placebo. Adverse events were generally poorly reported by all of the included studies and we were unable to quantitatively analyse this outcome. For those taking acyclovir, there were no serious adverse events; the most common adverse events reported for oral acyclovir were coryza, dizziness, tiredness, diarrhoea and renal colic. For intravenous acyclovir these were phlebitis, nausea and abnormal liver function tests and for topical acyclovir there was pain with the topical application.Those undergoing interferon treatment had significantly more adverse events compared to those taking placebo.
Authors' conclusions
There is low quality evidence from this review that oral acyclovir reduced the duration of symptoms for genital herpes. However, there is low quality evidence which did not show that topical antivirals reduced symptom duration for patients undergoing their first episode of genital herpes. This review was limited by the inclusion of skewed data, resulting in few trials that we were able to meta‐analyse.
Plain language summary
Treatment for the first time men and women get genital herpes (first‐episode genital herpes)
Review question The aim of this research was to look at the positive and adverse effects of treatments, on the duration of symptoms, in people who have their first episode of genital herpes.
Background Genital herpes is caused by the herpes simplex virus (HSV) that is primarily sexually transmitted (skin‐to‐skin contact). First‐episode genital herpes is the first time a person experiences the symptoms of genital herpes. The main feature of genital herpes are painful skin lesions. Treatment is based around viral suppression in order to decrease the length and severity of the symptoms.
Study characteristics We included 26 randomised controlled trials (RCTs) trials with 2084 participants that looked at treatments for first‐episode genital herpes versus placebo or another treatment. The trials all included people who were having their first episode of genital herpes and were conducted in various countries around the world. Three of the trials included only women, and in all the trials the participants had had symptoms for eight days or less. Fifteen of the 26 trials were funded by a pharmaceutical company. Key results The evidence is current to April 2016. The evidence shows that oral and intravenous acyclovir may be effective in reducing the number of days of symptoms in someone with first‐episode genital herpes. Oral valaciclovir showed a similar length of symptom duration as acyclovir. We did not find enough evidence to support the use of topical treatments. There was also no evidence that any of the treatments reduced the time between episodes for people with genital herpes. The evidence presented here is mostly of low quality. The studies included were mainly conducted in the 1980s and at this time the brief way studies were reported does not allow us to adequately judge the quality of the included studies. Quality of the evidence The evidence provided by this review is of low quality. Although there are 26 included studies, the meta‐analyses created in this review at the most, had three included studies. This was mainly due to the low number of studies that looked at each different type of antiviral. It was also unclear as to how well the included studies were conducted, as the methods for each of the individual studies did not report enough detail to judge each study's quality, inconsistence and this also affected the overall quality of the review.
Summary of findings
Background
Description of the condition
Genital herpes is a sexually transmitted infection caused by herpes simplex virus (HSV) type 1 and 2. HSV‐2 infections usually cause more recurrent and severe symptoms, and initial infections (primary infections) are generally more severe than recurrences. HSV‐2 infection is more common in women, possibly because the rate of male‐to‐female transmission is at least twice that of female‐to‐male transmission (Azwa 2009). The prevalence of genital HSV infection increases with age and numbers of sexual partners, with higher rates in specific ethnic and lower socioeconomic groups (Azwa 2009). The strongest predictor for genital HSV infection is a person's number of lifetime partners (Azwa 2009). HSV infection results in lifelong infection, which can be asymptomatic or present with recurrent lesions. It is estimated that up to 70% of all genital HSV‐2 is transmitted during asymptomatic shedding from an index partner with HSV‐2 (Azwa 2009). The virus enters the body by direct contact of the infected person's secretions or mucus membranes with the skin or mucus membrane of another. The herpes virus multiplies in the basal epithelial layer and then becomes latent in the dorsal root ganglion where it can reactivate spontaneously and travel back to the epithelium. This is known as viral shedding (Whitley 1998).
The initial infection may or may not cause symptoms, and is followed by seroconversion with type‐specific antibodies four to six weeks after infection. There are two types of symptomatic first‐episode occurrences. The first is a first episode of herpes in non‐primary infection which occurs in a person who was non‐symptomatic when initially infected with HSV, the second is a first episode of herpes in primary infection which is when HSV causes a symptomatic episode in a HSV‐seronegative person. First episode of herpes in non‐primary infections which is in an already infected individual are associated with fewer systemic symptoms, a shorter duration of disease, a shorter duration of viral shedding, and fewer lesions than a first episode of herpes with a primary infection(Azwa 2009). A first episode can last up to two weeks if untreated (Cernik 2008). As symptomatic, first episode herpes with primary infection is usually more severe than a first episode of herpes with a non primary infection, it is important to ascertain that interventions are effective for these individuals experiencing a first episode of herpes with a primary infection.
After an incubation period of one to 26 days, classical primary genital herpes begins with prodromal symptoms, characterised by localised pain or tingling lasting up to 24 hours. Clinical manifestations of herpes are diverse (Corey 1983b). However, 'classic' prodromal symptoms are followed by the appearance of randomly distributed vesicles clustered on a red base. Tiny papules develop into vesicles, which subsequently ulcerate and crust. Constitutional symptoms such as fever, chills, fatigue, and muscle aches accompany the disease and last 10 to 14 days. Enlarged inguinal or femoral glands may accompany constitutional symptoms, and dysuria is common in women.
For women, the classic clinical picture is that of painful vaginal and vulva lesions (Corey 1982b). However, infection of the cervix, often subclinical, is common. Men typically develop lesions on the glans, prepuce, or shaft of the penis (Corey 1982b). Male circumcision significantly reduces the incidence of HSV‐2 infection (Tobian 2009), and appears to reduce the number of recurrences and evidently prolongs the disease‐free period between two recurrences (Jerath 2009). Male circumcision does not affect HSV‐2 acquisition among female partners (Tobian 2012).
Extragenital complications occur in a minority of people who present with primary HSV infection. These include central nervous system disease, such as aseptic meningitis, encephalitis, or transverse myelitis; end organ disease including hepatitis or pneumonitis; and disseminated HSV (Corey 1982b).
Description of the intervention
There is no therapy or vaccine to prevent HSV, though the use of condoms offers moderate protection from acquisition (Martin 2009). The aim of treatment is to improve symptoms and time to recovery. Antiviral agents have been shown to reduce the duration and severity of symptoms, reduce healing times, and decrease the duration of viral shedding in first episode genital herpes (Azwa 2009). Which antiviral provides the best treatment and in which form (oral, topical, subcutaneous, intramuscular, or intravenous) needs to be confirmed. Treatment of symptomatic episodes of HSV does not alter the clinical course of the disease and has no effect on the rates of recurrences of genital herpes (Azwa 2009).
Currently, there are three classes of drugs licensed for the treatment of HSV symptomatic episodes, all of which target viral deoxyribonucleic acid (DNA) replication. Guanosine analogues, including acyclovir, valicyclovir, famciclovir, and ganciclovir, are the drugs of choice for the management of first episode HSV. The acyclic nucleotide analogue, cidofovir, and the pyrophosphate analogue, foscarnet, are reserved for use in resistant viruses. Acyclovir, a thymidine nucleoside analogue, was the first drug introduced to treat HSV infection. It has poor bioavailability and a short half‐life and, as a result, requires frequent dosing. Valacyclovir is a prodrug of acyclovir, and famciclovir is a prodrug of the guanosine nucleoside analogue, penciclovir (Azwa 2009).
Acyclovir can be administered topically, orally, or intravenously. When administered within 72 hours of the formation of the lesions, acyclovir shortens the course of the first episode attack, prevents new lesion formation, and helps decrease any accompanying constitutional symptoms (Azwa 2009).
Adverse effects caused by Acyclovir, valacyclovir, and famciclovir are rare and include nausea, vomiting, headache, and diarrhoea (Azwa 2009). Ganciclovir (myelosuppressive), foscarnet (nephrotoxic), and cidofovir (nephrotoxic) are very toxic drugs and are not used as a first‐line treatment (Vajpayee 2000).
Imidazoquinolines such as imiquimod and resiquimod have been found in preclinical studies to be immune response modifiers by inducing cytokines (Stanley 2002). Imiquimod is currently used as a topical treatment for external genital and perianal warts in adults (approved by the US Food and Drug Administration (FDA) in 1997). Application is topical, which appears to have minimal systemic absorption. Adverse reactions are mainly related to the application site with some people reporting systemic symptoms (Gupta 2002).
Interferons are well known for their antiviral effects, and are also potent cell growth regulators, and have immunomodulation properties (Katze 2002). Some randomised double‐blind placebo‐controlled trials have reported positive results with the use of Interferon topically. The treatment was also reported to be well tolerated and only minor local reactions were noted (Chiu 2011).
Natural products include plant extracts, antioxidants, and vitamins. Many small molecules, including phenols, polyphenols, terpenes, flavonoids, and sugar‐containing compounds, have potential anti‐HSV activity (Zhong 2012). Some of the products that have been trialed include Clinacanthus nutans (C. nutans) (Kongkaew 2011), lysine, vitamin C, zinc, vitamin E, and adenosine monophosphate (Gaby 2006). However, most of the studies were for recurrent genital herpes, so treatment of first episodes needs to be studied further.
How the intervention might work
Acyclovir, valacyclovir, and famciclovir are competitive inhibitors of viral DNA polymerase, resulting in inhibition of viral DNA synthesis. The drugs have an excellent margin of safety because they are converted by viral thymidine kinase to the active drug only inside virally infected cells (Cernik 2008).
Imiquimod and its potent analogue (100 times more), resiquimod, are from the family of imidazoquinolines. Both have mechanisms of action that modify the immune response. This is mediated through the induction of various cytokines, including tumour necrosis factor‐alpha (TNF‐α), interferon‐alpha (IFN‐α), and interleukins (IL) such as IL‐1, IL‐6, and IL‐12 (Brown 2002). It is thought that it may stimulate or enhance the innate and adaptive immune system (Gupta 2002).
Interferon works by stimulating the host immune system by increasing activation of natural killer cells, macrophages, and cytotoxic T cells, therefore interfering with the lifecycle of the virus (Chiu 2011). Natural products contain a wide variety of compounds that have been found to have anti‐HSV properties. The majority have a mechanism of action that inhibits attachment and entry of the virus into the host cell. However, the specific mechanisms and targets of most of the active natural products are unknown and still require investigation (Zhong 2012).
Why it is important to do this review
HSV is a major global health problem. It is the leading cause of encephalitis and genital ulcerative disease, and a major cofactor for HIV infection (Azwa 2009). The virus can establish latency, reactivate frequently, and be horizontally and vertically transmitted during periods of unrecognised or asymptomatic shedding. Seroprevalence varies widely between different geographical and population groups and is particularly high in HIV‐infected individuals, reaching levels over 90% in countries where HIV is endemic (Malkin 2004; Weiss 2004).
Genital herpes is a significant risk factor for acquiring HIV for both men and women, which is of serious concern. Many mechanisms have been suggested as to how this takes place. It is thought to be due to the presence of broken skin giving transmission enhancement, or that HSV interacts with HIV leading to increased success of the infection (Huang 2012). One systematic review found an approximately three‐fold increase in risk of HIV acquisition in men and women infected with HSV‐2 (Freeman 2006).
There is significant concern surrounding maternal herpes infection due to the risk of neonatal infection, which has been shown to lead to significant morbidity and mortality (Brown 2005). The most critical determinant of neonatal infection is first episode of primary infection genital HSV infection near delivery. This clinical observation may be related to the absence of maternal anti‐HSV antibodies and to greater viral exposure during first episode of primary infection herpes. Other predictors of neonatal infection include viral shedding during labour, invasive fetal monitoring and premature delivery (Brown 2003). Although cesarean section does not completely eliminate the risk for HSV transmission to the infant, women with genital herpetic lesions at the onset of labour should deliver by cesarean section to prevent neonatal HSV infection (Workowski 2010).
Treatment of the disease in the most effective and beneficial way is imperative. If HSV‐2 is a cofactor for HIV infection then HSV‐2 treatment may have a role as an HIV prevention strategy. This review will help to provide clarity on which is the most effective treatment regime in terms of medication, dose, and application by demonstrating a clear picture of the current evidence that exists within the literature. This will in turn help to clarify the situation for health practitioners regarding the extent of improvement of health outcomes for particular treatments, along with their adverse events.
This review addresses first‐episode disease only. A separate Cochrane review addresses the use of oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients (Le Cleach 2014).
Objectives
To determine the effectiveness and safety of the different existing treatments for first‐episode genital herpes on the duration of symptoms and time to recurrence.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished parallel randomised controlled trials (RCTs) and cluster‐RCTs. We excluded quasi‐RCTs.
Inclusions:
drug dosing trials;
suppressive therapy regimens (long‐term therapy) for first episodes.
Exclusions:
studies of vaccinations;
studies for which the objective was to look at the treatment for complications of herpes simplex virus (HSV), for example, herpes simplex encephalitis or herpes proctitis.
Types of participants
Men and women, inclusive of pregnant women, with their first episode of genital herpes (including immunocompetent and immunodeficient individuals). We included studies if they included participants with first‐episode disease and data were reported separately for this group.
Types of interventions
We looked at antivirals (both topical and systemic), interferon (both topical and systemic), immune modulators such as imiquimod (topical or analogue, e.g. resiquimod) and natural products which were compared with no treatment, placebo, other medication, or differing drug dosages. The timing of the treatments is in relation to the onset of symptoms. The interventions are:
Antivirals
antiviral (such as acyclovir: topical and systemic) versus placebo
antiviral (topical and systemic) versus no treatment
antiviral (topical and systemic) versus other medication
Interferon (immune modulator)
Interferon (topical and systemic) versus placebo
Interferon (topical and systemic) versus no treatment
Interferon (topical and systemic) versus other medication
Imiquimod (immune modulator)
imiquimod (topical or analogue) versus placebo
imiquimod (topical or analogue) versus no treatment
imiquimod (topical or analogue) versus other medication
Natural product
natural product versus placebo
natural product versus no treatment
natural product versus other medication
Antiviral + natural product
antiviral + natural product versus placebo
antiviral + natural product versus no treatment
antiviral + natural product versus other medication
Dosage studies
antiviral versus antiviral (both topical or systemic)
interferon versus interferon (both topical or systemic)
imiquimod versus imiquimod (both topical and analogue)
natural product versus natural product
Types of outcome measures
Primary outcomes
1. Duration of symptoms from onset of treatment: symptoms are defined by the trial authors. When several symptoms are reported, we included the longest duration. 2. Time to first recurrence. 3. Adverse events.
Secondary outcomes
4. Duration of lesions from onset of treatment: we defined this as time to complete lesion healing. 5. Neonatal effects: as defined by the trial authors. 6. Caesarean section delivery.
Search methods for identification of studies
RH and VJ identified as many relevant RCTs as possible of 'antiviral agents, interferon, imiquimod, and biological agents' for 'genital herpes', irrespective of their language of publication, publication date and publication status (published, unpublished, in press, and in progress).
Electronic searches
The Trials Search Co‐ordinator (TSC) of the Cochrane Sexually Transmitted Infections Review Group (STIG) conducted a comprehensive search strategy to capture as many relevant RCTs as possible in electronic databases. We used both electronic searching in bibliographic databases and handsearching, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We downloaded and managed the search results using Endnote bibliographic software. We deleted duplicate records of the same study. See Appendix 1 for the electronic search strings.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) Ovid platform (from inception to April 2016), MEDLINE Ovid platform (from inception to April 2016), EMBASE.com (from inception to April 2016), PsycINFO EBSCOHost platform (from inception to April 2016), CINAHL EBSCOHost platform (from inception to April 2016), LILACS iAHx interface (from inception to April 2016) and AMED (from inception to April 201)
We used the Cochrane highly sensitive search strategy for identifying RCTs (sensitivity‐ and precision‐maximising version; 2008 revision) in Ovid format in MEDLINE (Higgins 2011).
Searching other resources
We attempted to identify additional relevant RCTs by using the following methods.
Searching in Complementary and Alternative Medicines (CAM) Specialised Register (ProCite Database): inception to present.
-
Searching in trial registers:
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP portal) (http://apps.who.int/trialsearch/): inception to present.
ClinicalTrials.gov (http://clinicaltrials.gov/): inception to present.
Searching in Web of Science (http://thomsonreuters.com/web‐of‐science/): inception to present.
Searching in Proquest Dissertations and Theses (http://search.proquest.com): inception to present.
Searching for grey literature in System for Information on Grey Literature in Europe 'OpenGrey' (http://www.opengrey.eu/): inception to present.
Searching by contacting pharmaceutical companies producing 'antiviral agents, interferon, imiquimod, and biological agents' for 'genital herpes'.
-
Handsearching conference proceedings from the following meetings:
International Society for Sexually Transmitted Diseases Research ‐ ISSTDR (http://www.isstdr.org/): 2007, 2009, 2011, 2013, and 2015.
British Association for Sexual Health and HIV ‐ BASHH (http://www.bashh.org/): 2004, 2006, 2007, 2009, 2013, 2014, and 2015.
International Congress on Infectious Diseases ‐ ICID (http://www.isid.org/): 2010 and 2012, and 2014.
International Union against Sexually Transmitted Infections ‐ IUSTI (http://www.iusti.org/): 2011 and 2012.
International Society for Infectious Diseases ‐ ISID (http://www.isid.org/): 2011.
International Meeting on Emerging Diseases and Surveillance ‐ IMED (http://www.isid.org/): 2007, 2009, 2011, 2013, and 2014.
Interscience Conference on Antimicrobial Agents and Chemotherapy ‐ ICAAC (http://www.icaac.org/): 2011, 2012, 2013, 2014, and 2015.
International Federation of Gynecology and Obstetrics ‐ FIGO (http://www.figo.org/ ): 2012 and 2015.
Handsearching within previous systematic reviews and other relevant publications on the same topic.
Handsearching within reference lists of all relevant RCTs identified by others methods.
Contacting drug companies for trials.
Data collection and analysis
Selection of studies
After all searches were conducted, we checked for duplicates using EndNote. Two review authors (RH, VJ) independently assessed trials for inclusion by scanning the titles and abstracts based on the established inclusion criteria. We then compared which trials had been identified and obtained full‐text articles in order to select the final studies for possible inclusion in the review. A third review author (HR) helped resolve any disagreements regarding study inclusion. We sought additional information from the trial authors if there was insufficient information to make a decision about eligibility. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and 'Characteristics of excluded studies' table. We did not impose any language restrictions.
Data extraction and management
Three review authors (RH, DF, VJ) independently extracted data from eligible studies using a data extraction form that was developed by the review authors (Appendix 2). We resolved any differences by discussion or by consultation (or both) with a third review author (HR, VJ) to reach consensus. Extracted data included study characteristics and outcome data (see data extraction form, Appendix 2). Where there were multiple publications of one study, we used the main trial report as the reference and extracted any additional details from secondary papers. We contacted trial authors if further data was required, such as methods or results so as to confirm the suitability of the study for meta‐analysis. We routinely sought information on whether data was recorded that was not reported in the published paper from the corresponding authors for all included trials.
Assessment of risk of bias in included studies
Two individuals (RH, VJ or DF) independently assessed the quality of each of the selected studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We classified studies as 'low risk of bias', 'high risk of bias', or 'unclear risk of bias', reporting on the following seven domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias.
We resolved any disagreements regarding bias by consensus or discussion with a third review author (HR or VJ).
We searched for within‐trial selective reporting, such as reporting of outcomes in insufficient detail or trials failing to report obvious outcomes. We sought protocols and compared the outcomes of the protocol with the outcomes in the final study.
The conclusion of all judgements is presented in the 'Risk of bias' table, which by means of sensitivity analysis, is incorporated into the interpretation of review findings.
Measures of treatment effect
We expressed dichotomous data, such as caesarean section delivery, as a risk ratio (RR) and 95% confidence intervals (CIs). We expressed continuous data, such as duration of symptoms, as a mean difference (MD) between treatment groups, with 95% CIs. If similar outcomes were reported on different scales we planned to use the standardised mean difference (SMD). We utilised the most detailed numerical data available that provided a similar way to analyse the included studies (e.g. P values, test statistics) where data required to calculate RRs or MDs were unavailable. We used hazard ratios (HRs) to express time‐to‐event outcomes, where data permitted (duration of symptoms, duration of lesions, time to recurrence). Many studies presented data as medians as the data were heavily skewed. These medians are presented in additional tables.
Unit of analysis issues
In the case of cross‐over trials, we planned to include only the first phase data. However, we did not include any cross‐over trials in this review.
In the case of cluster‐randomised data, we planned to employ the interclass correlation coefficient (ICC) as discussed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If the ICC was not available then we planned to borrow a suitable factor from other trials as an estimate of relative variability. We did not, however, have any cluster‐randomised trials.
For adverse events where it was not clear if individuals had suffered from more than one adverse event, we did not generate a summary statistic. If we found studies with more than one intervention versus placebo or a third intervention, we planned to ensure double‐counting of the participants would not have occurred by splitting the comparison group between the two interventions (Higgins 2011).
Dealing with missing data
We attempted to contact the primary trial author for further information when there were missing data. However, due to the age of the included trials, we were unable to get additional information for the majority of trials. We noted characteristics of any participants that left the study; this enabled us to determine if the groups remain balanced. We looked at the method used to impute the missing data if intention‐to‐treat (ITT) analyses were supplied by the primary trial authors.
When there was sufficient detail reported to calculate the MDs but no information on the corresponding standard deviation (SD) was given, we assumed the outcome to have a standard SD that is equal to the highest SD, after it has been approximately matched for sample size with the study from where the SD is borrowed. We planned to explore the robustness of this decision separately by sensitivity analysis.
Assessment of heterogeneity
We carried out meta‐analyses when studies were sufficiently homogeneous in terms of their clinical and methodological characteristics. In addition to visual inspection of the forest plots, we used the I2 statistic to quantify any heterogeneity in the meta‐analysis (Higgins 2011). For I2 statistic levels up to 50%, we considered heterogeneity to be mild to moderate. For I2 statistic levels between 50% and 80%, we considered heterogeneity to be moderate and, where possible, we used random‐effects models to allow for heterogeneity. If the I2 statistic exceeded 80%, we considered heterogeneity to be substantial, and did not present pooled results; instead, we planned to report any observations as a narrative (Higgins 2011).
Assessment of reporting biases
We minimised the risk of reporting bias by undertaking a comprehensive search over multiple electronic databases and additional resources for both unpublished and published articles. We did not impose any language restrictions. We were alert for duplication of data. We were unable to construct a funnel plot to assess publication bias, as there were fewer than 10 studies in any analysis (Higgins 2011).
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). If the studies were sufficiently similar, we combined the data using a fixed‐effect model. If we detected moderate heterogeneity, we used a random‐effects model (Higgins 2011).
We meta‐analysed dichotomous data using the Mantel‐Haenszel method to calculate RRs; and for continuous data we used the MD, or SMD, as appropriate. We meta‐analysed time to recurrence data as HRs using the generic inverse variance method.
We planned to use the Peto odds ratio if the obtained data included rare events (as might be the case for adverse events).
We conducted separate analyses according to the route of drug administration (oral, topical, subcutaneous, intramuscular, or intravenous).
Some of the primary studies did not report combined findings for all first‐episode participants. For example, separate data were reported for male and female participants, or for first episode of primary infection and first episode of non primary infection. We stratified our analyses as required, to facilitate maximum pooling of data.
Subgroup analysis and investigation of heterogeneity
Where data were available, we conducted subgroup analyses to determine the separate evidence within the following subgroups.
Gender.
Length of treatment (five days or less, more than five days). The rationale for this subgroup was that the usual recommended length of treatment is five days with no evidence of benefit for longer periods of time (Azwa 2009).
Type of drug within a class
Duration of time between appearance of lesions and initiation of treatment (five days or less, more than five days). The rationale for this subgroup was that it is usually recommended that treatment be initiated as soon as possible once a clinical diagnosis has been made (Azwa 2009).
Immunodeficiency e.g. HIV.
First episode of primary infection versus first episode of non‐primary infection.
If we detected substantial statistical heterogeneity, we explored clinical and methodological differences between the studies that might account for this. We took any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions to arbitrary decisions made regarding the eligibility and analysis were robust. These analyses included consideration of whether the review conclusions would have differed if:
eligibility were restricted to studies without high risk of bias;
a random‐effects model had been adopted; and
imputed data were included by ITT.
Overall quality of the evidence: 'Summary of findings' table
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEproGDT software (GRADEproGDT 2015).We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.We then imported these tables into Review Manager 5 (RevMan 2014).
We included the following outcomes in the 'Summary of findings' tables: Duration of symptoms from onset of treatment, Adverse events, Duration of lesions from onset of treatment and Time to recurrence.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
We conducted the searches in April 2016. The searches were broad and included antiviral medications. After de‐duplication we had 3349 studies. After extensive screening and assessment, we identified 26 studies eligible for inclusion in this review (see Figure 1). Two studies are awaiting classification (see Characteristics of studies awaiting classification table). We have not identified any ongoing studies in this area.
1.
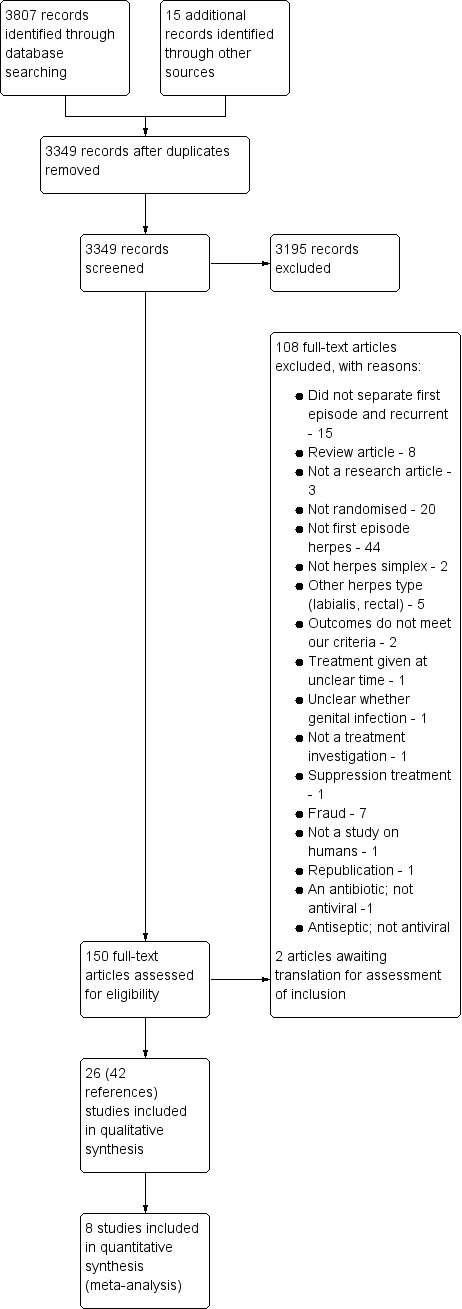
Study flow diagram.
Included studies
Design and setting of the included studies
We included 26 studies (documented by 42 publications) which analysed 2084 participants. Most of the studies were conducted in the US (Adams 1976; Bryson 1983; Corey 1982a; Corey 1982b; Corey 1983; Levin 1989; Mertz 1984; Pazin 1987; Peacock 1988; Silvestri 1982; Wald 1994), and the UK (Csonka 1984; Fiddian 1983; Kinghorn 1986a; Kinghorn 1986b; Mindel 1982; Mindel 1986; Mindel 1987), with additional studies in New Zealand (Batcheler 1986), Canada (Mendelson 1986), China (Lai 2000), Sweden (Nilsen 1982), Mexico (Zavala 1988), and Japan (Niimura 1996); one multicentre study included participants from the US, UK, and Australia (Fife 1997). The largest study included 643 participants (Fife 1997), and the smallest study included 11 (Csonka 1984). Overall, the studies were not recent, with the oldest study published in 1976 (Adams 1976), and the newest study published in 2000 (Lai 2000).
Sixteen studies stated that they received commercial funding (Corey 1982a; Corey 1982b; Corey 1983; Fiddian 1983; Fife 1997; Kinghorn 1986a; Kinghorn 1986b; Levin 1989; Mendelson 1986; Mertz 1984; Mindel 1982; Mindel 1986; Mindel 1987; Nilsen 1982; Peacock 1988; Wald 1994). Four studies apparently received no commercial funding (Adams 1976; Csonka 1984; Pazin 1987; Silvestri 1982). Six studies did not mention their funding source (Altomare 1985; Batcheler 1986; Bryson 1983; Lai 2000; Niimura 1996; Zavala 1988).
Participants in the included studies
Three studies included only women (Batcheler 1986; Mindel 1986; Pazin 1987), and the remaining 23 studies included men and women.
The duration of symptoms required for participant eligibility differed between studies. These criteria are presented in Table 3.
1. Number of days from onset of symptoms that patients were included.
| Time | Less than 24 hrs | Less than 2 days | Less than 3 days | Less than 4 days | Less than 5 days | Less than 6 days | Less than 7 days | Less than 8 days | Did not state |
| Studies of oral antivirals | Fife 1997 | Lai 2000; Mindel 1986; Nilsen 1982; Wald 1994 | Bryson 1983; Kinghorn 1986b; Mertz 1984 | Niimura 1996 included from day 2, 3, 4, 5, 6, 7 or more, but subgrouped data of results was not available | |||||
| Studies of topical antivirals | Fiddian 1983 | Corey 1982a; Corey 1982b; Kinghorn 1986a | |||||||
| Studies of intravenous antivirals |
Mindel 1982 Severe GH |
Corey 1983; Peacock 1988 | |||||||
| Studies of topical interferon | Batcheler 1986 | ||||||||
| Studies of IM/SC interferon | Pazin 1987 (IM) | Levin 1989 (IM) | Mendelson 1986 (SC) | ||||||
| Studies of adenosine arabinoside | Adams 1976 | ||||||||
| Studies of topical carbenoxolone versus topical cicloxone | Csonka 1984 | ||||||||
| Studies of ribavirin | Zavala 1988 | ||||||||
| Studies of topical idoxuridine versus dimethyl sulfoxide | Silvestri 1982 | ||||||||
| Studies of oral inosine pranobex | Mindel 1987 | ||||||||
| Studies of topical tromantadine | Altomare 1985 |
GH: genital herpes IM: intramuscular SC: subcutaneous
Adams 1976 and Altomare 1985 included participants with onset of symptoms of less than two days.
Fife 1997, Pazin 1987, and Zavala 1988 included participants with onset of symptoms of less than three days.
Levin 1989 included participants with onset of symptoms of less than four days.
Csonka 1984, Fiddian 1983, Lai 2000, Mendelson 1986, Mindel 1986, Mindel 1987, Nilsen 1982, and Wald 1994 included participants with onset of symptoms of less than five days.
Bryson 1983, Corey 1982a, Corey 1982b, Kinghorn 1986a, Kinghorn 1986b, Mertz 1984, and Mindel 1982 included participants with onset of symptoms of less than six days.
Corey 1983 and Peacock 1988 included participants with onset of symptoms of less than seven days.
Silvestri 1982 included participants with onset of symptoms of less than eight days.
Batcheler 1986 did not state how many days of symptoms their participants had before treatment
Niimura 1996 included from day 2, 3, 4, 5, 6, 7 or more, but subgrouped data of results was not available.
Bryson 1983, Corey 1982a, and Corey 1982b subgrouped by antibody status (first episode of primary infection and first episode of non‐primary infection).
It is important to note that Mindel 1982 only included participants with severe first episode genital herpes.
Adams 1976, Bryson 1983, Fiddian 1983, Kinghorn 1986b, and Nilsen 1982 looked at both males and females and did not provide results subgrouped by antibody status.
The remaining studies did not report results by either gender or antibody status.
Interventions in the included studies
Antiviral versus placebo
Oral acyclovir versus placebo (Bryson 1983; Kinghorn 1986b; Mertz 1984; Nilsen 1982). Some participants in both arms of Kinghorn 1986b also received co‐trimoxazole. The oral dose of acyclovir was 1 gm daily in most studies.
Oral ribavirin versus placebo (Zavala 1988).
Intravenous acyclovir versus placebo (Corey 1983; Mindel 1982; Peacock 1988).
Topical acyclovir versus placebo (Corey 1982a; Corey 1982b; Fiddian 1983; Kinghorn 1986a).
Topical cycloxolone versus placebo (Csonka 1984).
Topical carbenoxolone versus placebo (Csonka 1984).
Topical tromantadine versus placebo (Altomare 1985).
Topical adenosine arabinoside versus placebo (Adams 1976).
Topical idoxuridine in dimethyl sulfoxide versus dimethyl sulfoxide alone or saline (Silvestri 1982).
Antiviral versus other antiviral
Topical 2% carbenoxolone cream versus 2% cicloxolone cream (Csonka 1984).
Oral acyclovir versus inosine prabonex (with or without oral acyclovir in control arm) (Mindel 1987).
Antiviral regimen comparisons
Long versus short course oral acyclovir (Mindel 1986).
High (4 gm/day) versus standard dose (1 gm/day) oral acyclovir (Wald 1994).
Famciclovir at doses, 125 mg, 250 mg and 500 mg (Niimura 1996).
Antiviral versus interferon
Topical acyclovir versus intramuscular interferon (Levin 1989).
Interferon versus placebo
Intramuscular interferon versus placebo (Pazin 1987).
Topical interferon versus placebo (Batcheler 1986).
Subcutaneous interferon versus placebo (Mendelson 1986).
We did not find any studies of imiquimod, antiviral + natural product, or natural products that met the inclusion criteria.
Outcomes in the included studies
Primary outcomes
1. Duration of symptoms from onset of treatment
Most studies reported duration of symptoms, but many either reported only median values (Corey 1983; Fiddian 1983; Mertz 1984; Mindel 1982; Mindel 1986; Mindel 1987; Nilsen 1982; Peacock 1988; Wald 1994), or reported dichotomous data (e.g. number healed by six days) (Csonka 1984; Niimura 1996). We could not meta‐analyse these data.
Other studies reported means, but some failed to report standard deviations (SDs) or standard errors (SEs) (Adams 1976; Bryson 1983; Fife 1997; Mendelson 1986; Silvestri 1982), and so we had to impute the SD or report the data in additional tables. Only one study reported hazard ratios for this outcome (Fife 1997).
2. Time to first recurrence
Nine studies reported time to first recurrence (Bryson 1983; Corey 1982a; Corey 1982b; Mendelson 1986; Mertz 1984; Mindel 1987; Pazin 1987; Peacock 1988; Wald 1994), but none reported hazard ratios. Six of these studies reported median time to first recurrence (Bryson 1983; Corey 1982a; Corey 1982b; Mertz 1984; Mindel 1987; Wald 1994). Mean times were given by Peacock 1988, but the remaining two studies just declared there were no differences in time to first recurrence between the two groups and did not provide numerical data (Mendelson 1986; Pazin 1987). The proportion of participants who were adequately followed up varied across studies, but was around 80% in those that declared numbers of patients followed up (Corey 1982a; Corey 1982b; Wald 1994); however, the majority of studies did not declare the number of patients adequately followed up.
3. Adverse events
Nearly all studies reported adverse events. Many failed to report comparative data, but this was often because (as they reported in the text) there were no adverse events reported in either arm. Although, one study reported other outcomes separately for first‐episode participants, for this particular outcome separate data were not provided (Altomare 1985) .
Secondary outcomes
1. Duration of lesions from onset of treatment
Most studies reported duration of lesions, although as noted above, many reported data unsuitable for meta‐analysis.
2. Neonatal effects
No studies reported this outcome.
3. Caesarean section delivery
No studies reported this outcome.
Excluded studies
We excluded 106 studies; the reasons are reported in the Characteristics of excluded studies table. Common reasons for exclusion are that the studies were not looking at first‐episode genital herpes, or the study design did not appear to be randomised.
We excluded seven studies due to suspected fraudulent publication (Syed 1995a; Syed 1995b; Syed 1995c; Syed 1997a; Syed 1997b; Syed 1998a; Syed 1998b). When we attempted to gain more information from T. A. Syed regarding his studies, we discovered that he is currently serving a prison sentence for 64 counts including practicing medicine without a license, grand theft, perjury, and forgery. It appears he was never employed at universities he claimed the research was from. Other authors listed on his studies were unable to be identified. For these reasons we have chosen not to include these studies despite the fulfilment of the inclusion criteria of this review. Our Review Group has contacted all relevant journals where his studies were published to highlight this information.
Risk of bias in included studies
We rated most of the studies at unclear risk of bias in most domains. We attempted to contact all trial authors for more information about randomisation and blinding procedures. For a graphical representation of the results of the risk of bias assessment see Figure 2 and Figure 3 .
2.
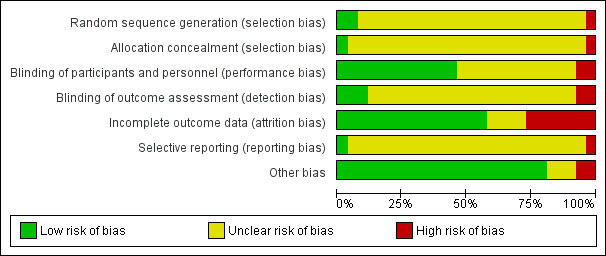
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
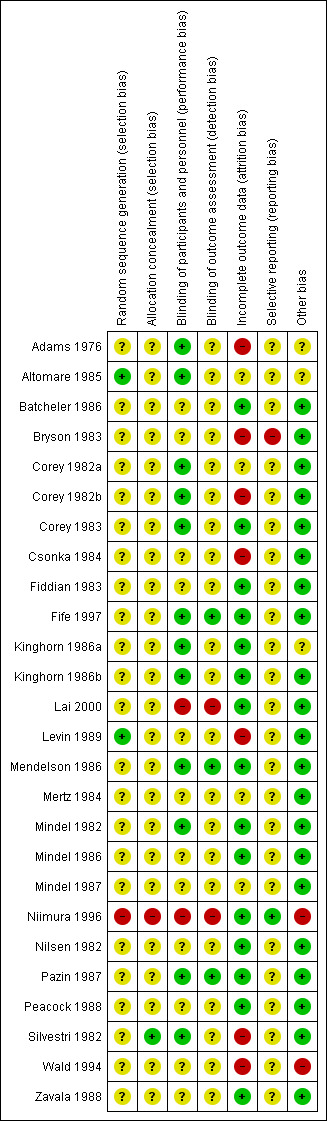
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of random sequence
Only two studies (8%) reported acceptable methods of random sequence generation (Altomare 1985; Levin 1989). We judged one study (4%) to be at high risk of bias (Niimura 1996), and the remaining studies (88%) at unclear risk of bias in this domain.
Allocation concealment
Only one study (4%) reported acceptable methods of allocation concealment (Silvestri 1982). We judged one study (4%) to be at high risk of bias (Niimura 1996), and the remaining studies (92%) at unclear risk of bias in this domain.
Blinding
Twelve studies (46%) described acceptable methods of blinding of participants and study personnel (Adams 1976; Altomare 1985; Corey 1982a; Corey 1982b; Corey 1983; Fife 1997; Kinghorn 1986a; Kinghorn 1986b; Mendelson 1986; Mindel 1982; Pazin 1987; Silvestri 1982). We judged two studies (8%) at high risk of bias in this domain (Lai 2000; Niimura 1996), and the remaining studies (46%) at unclear risk; many of these studies mentioned that they were double‐blinded but failed to provide further details.
Only three studies (12%) described acceptable methods of blinding of outcome assessment (Fife 1997; Mendelson 1986; Pazin 1987). We judged two studies (8%) at high risk of bias in this domain (Lai 2000; Niimura 1996), and the remaining studies (80%) at unclear risk; many of these studies mentioned that they were double‐blinded but failed to provide further details.
Incomplete outcome data
Fifteen studies (58%) analysed all or most randomised participants for at least two of our primary outcomes and we judged these at low risk of attrition bias (Batcheler 1986; Corey 1983; Fiddian 1983; Fife 1997; Kinghorn 1986a; Kinghorn 1986b; Lai 2000; Niimura 1996; Mendelson 1986; Mindel 1982; Mindel 1986; Nilsen 1982; Pazin 1987; Peacock 1988; Zavala 1988). We judged seven studies (27%) at high risk of bias, in most cases because they failed to analyse a high proportion (< 20%) of randomised participants (Adams 1976; Bryson 1983; Corey 1982b; Csonka 1984; Levin 1989; Silvestri 1982; Wald 1994). We judged the remaining studies (15%) at unclear risk of bias in this domain (Altomare 1985; Corey 1982a; Mertz 1984; Mindel 1987).
In the studies reporting time to first recurrence, the proportion of participants who were adequately followed‐up varied across the studies see Description of studies.
Selective reporting
We judged one study (4%) at high risk of bias in this domain as it was unclear why outcomes were not reported for one group of randomised participants (Bryson 1983); we judged all other studies (96%) at unclear risk of selective reporting, as study protocols were not available and there was no statement in the publication stating that all measured outcomes had been reported. Therefore it was unclear whether all prespecified outcomes were reported.
Other potential sources of bias
We judged two studies (8%) at high risk of bias for this domain. One study was due to baseline imbalance (Wald 1994), and one study was due to the data and analyses being undertaken by the funder (Niimura 1996). We rated three studies (12%) at unclear risk of other bias, for example, due to changes in the intervention during the study, or possible co‐intervention (Adams 1976; Altomare 1985; Kinghorn 1986a). We judged all other studies (80%) at low risk of bias in this domain, as no potential source of other bias was identified.
Effects of interventions
Summary of findings for the main comparison. Oral acyclovir versus placebo for men and women with their first episode of genital herpes.
| Oral acyclovir versus placebo for men and women with their first episode of genital herpes | |||||
| Patient or population: men and women with their first episode of genital herpes Setting: STD and family planning clinics Intervention: oral acyclovir Comparison: placebo | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Risk with oral acyclovir | |||||
| Duration of symptoms from onset of treatment | The mean duration of symptoms from onset of treatment in the intervention group was 3.22 days fewer than that with placebo (5.91 fewer to 0.54 fewer) | ‐ | 82 (2 RCTs) | ⊕⊕⊝⊝ Low1, 2 |
|
| Adverse events | Study population | Not pooled | 130 (2 RCTs) | ⊕⊝⊝⊝ Very low2, 3,5 |
There were no severe adverse events. Adverse events were unable to be pooled as they were only reported in two studies and were not reported in a consistent way. Adverse events recorded for those taking this medication included coryza, dizziness, tiredness, diarrhoea and renal colic |
| Not pooled | |||||
| Duration of lesions from onset of treatment | The mean duration of lesions from onset of treatment in the intervention group was 3.51 fewer days than that with placebo (6.19 fewer to 0.82 fewer) | ‐ | 86 (2 RCTs) | ⊕⊕⊝⊝ Low1, 2 |
|
| Time to recurrence | Data were not analysed using the correct method but statistical analysis did not show any difference in median time to recurrence in the two groups | Not pooled | 198 (2 RCTs) |
⊕⊝⊝⊝ Very low2,4 |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 Downgraded by 1 for risk of bias. There was unclear risk in both studies for randomisation and allocation concealment. Only one study used blinding and one study was graded high risk for attrition and reporting biases. 2 Downgraded by 1 for imprecision. There were very low sample numbers in these two studies. 3 Downgraded by 1 for risk of bias. Studies reporting adverse events were rated as unclear for the majority of the risk of bias items.
4 Downgraded by 2 for risk of bias as both studies were unclear for allocation concealment and randomisation and there is the potential for a substantial effect due to dropouts as actual numbers followed up were not reported
5 Downgraded by 1 for imprecision based on very wide confidence intervals
Summary of findings 2. Topical acyclovir versus placebo for men and women with their first episode of genital herpes.
| Topical acyclovir versus placebo for men and women with their first episode of genital herpes | ||||||
| Patient or population: men and women with their first episode of genital herpes Setting: STD and family planning clinics Intervention: topical acyclovir Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with topical acyclovir | |||||
| Duration of symptoms from onset of treatment | The mean duration of symptoms from onset of treatment in the intervention group was 0.61 days fewer than that with placebo (2.16 fewer to 0.95 more) | ‐ | 195 (3 RCTs) | ⊕⊕⊝⊝ Low1, 2 |
One included study had given all subjects oral acyclovir | |
| Duration of lesions from onset of treatment | The mean duration of lesions from onset of treatment in the intervention group was 0.86 days fewer than that with placebo (2.15 fewer to 0.42 more) | ‐ | 195 (3 RCTs) | ⊕⊕⊝⊝ Low1, 3 |
One included study had given all subjects oral acyclovir | |
| Adverse events ‐ pain with topical application | Study population | Study population | RR 0.74 (0.46 to 1.20) | 247 (3 RCTs) | ⊕⊕⊝⊝ Low1,5 | |
| 242 per 1000 | 179 per 1000 (111 to 290) | |||||
| Moderate | Moderate | |||||
| 235 per 1000 | 174 per 1000 (108 to 282) | |||||
| Time to recurrence | Data were not pooled. Time to reccurrence ranged from 70‐116 days | Data were not pooled. Time to recurrence ranged from 70‐116 days | The were no differences reported between the two groups | 129 (3 RCTs) |
⊕⊝⊝⊝ Very low1,4 |
Data were not analysed using the correct method. Medians were presented |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by 1 for risk of bias. All trials had unclear risk of bias for randomisation and allocation concealment. 2 Downgraded by 1 for inconsistency. Heterogeneity was 56%. 3 Downgraded by 1 for inconsistency. Heterogeneity was 72%.
4 Downgraded by 2 for risk of bias with regard to incomplete data not many participants were followed up.
5 Downgraded by 1 for imprecision based on very wide confidence intervals
Antiviral versus placebo
1.1 Oral acyclovir versus placebo
Four studies with 227 participants were included in this comparison (Bryson 1983; Kinghorn 1986b; Mertz 1984; Nilsen 1982).
Primary outcomes
1.1.1 Duration of symptoms from onset of treatment
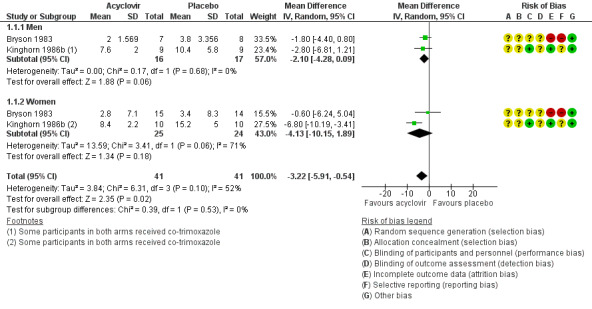
When two studies were pooled, symptom duration was significantly shorter in the acyclovir group (Bryson 1983; Kinghorn 1986b) (mean difference (MD) ‐3.22 days, 95% confidence interval (CI) ‐5.91 to ‐0.54; two RCTs, 82 participants, I2 statistic = 52%) (Analysis 1.1; Figure 4). There was moderate heterogeneity for this outcome for which there was no clear explanation. Use of a random‐effects model did not affect the significance of the findings.
1.1. Analysis.
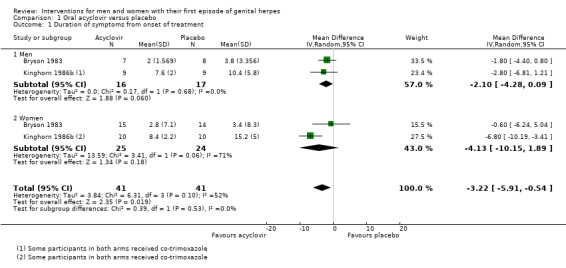
Comparison 1 Oral acyclovir versus placebo, Outcome 1 Duration of symptoms from onset of treatment.
4.
Forest plot of comparison: 1 Oral acyclovir versus placebo, outcome: 1.1 Duration of symptoms from onset of treatment.
Two studies reported medians only (Mertz 1984; Nilsen 1982). One found that the duration of symptoms was significantly shorter in the acyclovir group than in the placebo group among participants (n = 101) with first episode of primary infection (P < 0.5) (Mertz 1984). There was no significant difference between the groups in participants with first episode of non‐primary infection (n = 13). The other study found that the duration of symptoms was significantly shorter in the acyclovir group (Nilsen 1982) (n = 31, P < 0.05). See Table 4.
2. Medians: oral acyclovir versus placebo.
| Acyclovir | Placebo | ||||||
| Outcome | Study |
Median (days) |
No. participants |
Median (days) |
No. participants | P value | Favours intervention |
| Duration of symptoms from onset of treatment | Nilsen 1982 | 4 | 17 | 9 | 14 | < 0.05 | ✓ |
| Duration of symptoms from onset of treatment by antibody status | |||||||
| Primary | Mertz 1984 | 5 | 52 | 7 | 49 | < 0.05 | ✓ |
| Non‐primary | Mertz 1984 | 2 | 9 | 4 | 15 | > 0.1 | ✕ |
| Duration of symptoms from onset of treatment by gender | |||||||
| Females | Nilsen 1982 | 5 | 10 | 8 | 7 | NS | ✕ |
| Males | Nilsen 1982 | 3 | 7 | 9 | 7 | < 0.05 | ✓ |
| Duration of lesions from onset of treatment | Nilsen 1982 | 6 | 17 | 11 | 14 | < 0.01 | ✓ |
| Duration of lesions from onset of treatment by antibody status | |||||||
| Primary | Mertz 1984 | 12 | 61 | 16 | 58 | < 0.01 | ✓ |
| Non‐primary | Mertz 1984 | 9 | 12 | 13 | 19 | > 0.1 | ✕ |
| Duration of lesions from onset of treatment by gender | |||||||
| Females | Nilsen 1982 | 4.5 | 10 | 6 | 7 | < 0.05 | ✓ |
| Males | Nilsen 1982 | 7 | 7 | 11 | 7 | 0.06 | ✕ |
| Time to recurrence | ✕ | ||||||
| Participants with 4‐9 month follow‐up | Bryson 1983 | 94 | Unclear | 101 | Unclear | NS | ✕ |
| HSV‐2 group | Mertz 1984 | 71 | Unclear | 108 | Unclear | NS | ✕ |
Mertz 1984: Duration of symptoms refers specifically to pain HSV‐2: herpes simplex virus type 2 NS: not statistically significant ✓: favours intervention ✕: does not favour intervention
Due to the moderate heterogeneity (52%), we used a random‐effects model for this analysis. The evidence for this outcome was of low quality. We downgraded the quality of evidence due to the risk of bias of associated studies and because this finding is based on very low sample numbers (Table 1).
Subgroup analysis by gender
When we pooled Bryson 1983 and Kinghorn 1986b, and considered men and women separately, for males there is a difference in the duration of symptoms after treatment with acyclovir (MD ‐2.10 days, 95% CI ‐4.28 to 0.09; 2 RCTs, 33 men, I2 statistic = 0%). In females there was high heterogeneity between the two trials included in the meta analysis and it did not show any statistical difference between those taking acyclovir and those taking placebo (MD ‐4.13 days, 95% CI ‐10.15 to 1.89; 2 RCTs, 49 women, I2 statistic = 71%). However overall, we did not observe any statistical difference between men and women (Test for subgroup differences: Chi2 = 0.39, P = 0.53) for the duration of symptoms from onset of treatment.
When median duration of symptoms was subgrouped by gender in Nilsen 1982, findings remained statistically significant among males (n = 14, P < 0.05) but not among females (n = 17). See Table 4.
These subgroup findings should be regarded with caution and are as a result of low to very low quality evidence due to the small sample sizes, heterogeneity in one of the female subgroups, and inconsistency in the findings.
Subgroup analysis by antibody status (first episode of primary infection or first episode of non‐primary infection)
No data were available to allow subgrouping by antibody status. However, one study that had reported medians did report these two groups separately (Mertz 1984). This study showed a significant reduction in duration of symptoms for those undergoing their first episode of primary infection as indicated by their antibody status. In those whose antibody status indicated a previous infection, there was no observed reduction (Table 4).
1.1.2 Time to first recurrence
No studies reported hazard ratios. Two studies reported no significant difference between the two groups in the median days to recurrence among participants with adequate follow‐up (Bryson 1983; Mertz 1984). See Table 4.
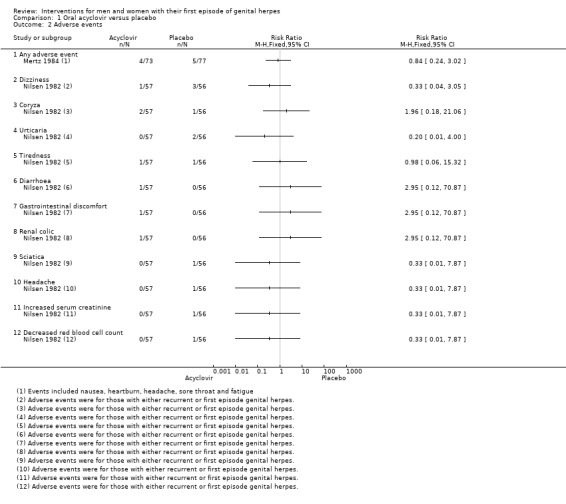
1.1.3 Adverse events
Very few adverse events were reported (Analysis 1.2). Reported events included headache, nausea, heartburn, fatigue, and sore throat. Two studies reported that no adverse events occurred in either group (Bryson 1983; Kinghorn 1986b). This evidence was of low quality as there was a high level of heterogeneity and the risk of bias of the included studies was unclear for most of the domains (Table 1).
1.2. Analysis.
Comparison 1 Oral acyclovir versus placebo, Outcome 2 Adverse events.
Secondary outcomes
1.1.4 Duration of lesions from onset of treatment
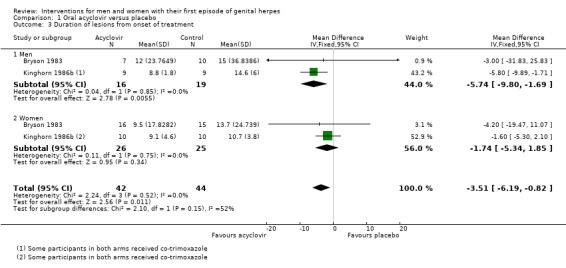
When we pooled two studies (Bryson 1983; Kinghorn 1986b), lesion duration was significantly shorter in the acyclovir group (MD ‐3.51 days, 95% CI ‐6.19 to ‐0.82; two RCTs, 86 participants, I2 statistic = 0%, Analysis 1.3).
1.3. Analysis.
Comparison 1 Oral acyclovir versus placebo, Outcome 3 Duration of lesions from onset of treatment.
Two studies reported medians only (Mertz 1984; Nilsen 1982). One study found that the duration of lesions was significantly shorter in the acyclovir group than in the placebo group among participants (n = 119) with first episode of primary infection (P < 0.01) (Mertz 1984). There was no significant difference between the groups for participants with first episode of non‐primary infections (n = 31). The other study found that the duration of symptoms was significantly shorter in the acyclovir group (n = 31, P < 0.01) (Nilsen 1982). See Table 4. These findings should be regarded with caution due to the low quality evidence shown here. This is a result of small sample sizes and high levels of bias associated with the included studies (Table 1).
Subgroup analysis by gender
When we pooled Bryson 1983 and Kinghorn 1986b, and considered men and women separately, for males there is a difference in the duration of lesions after treatment with acyclovir (MD ‐5.74 days, 95% CI ‐9.80 to ‐1.69; 35 men, I2 statistic = 0%). In females the meta analysis did not show any statistical difference between those taking acyclovir and those taking placebo for duration of lesions (MD ‐1.74 days, 95% CI ‐5.34 to 1.85; 51 women, I2 statistic = 0%). However overall, we did not observe any statistical difference between men and women (Test for subgroup differences: Chi2 = 2.10, P = 0.15) for the duration of lesions from onset of treatment.
However, when findings were subgrouped by gender in Nilsen 1982 (data supplied as medians), among males there was no difference between the acyclovir and placebo groups (n = 14, P = 0.06), but among females there was a significantly shorter lesion duration in the acyclovir group (n = 17, P < 0.05). See Table 4.
These subgroup findings should be regarded with caution due to the low grade of the evidence as a result of small sample sizes and inconsistency in the findings.
Subgroup analysis by antibody status (first episode of primary infection or first episode of primary non‐primary infection)
No data were available to allow subgrouping by antibody status. However, one study that had reported medians did report these two groups separately (Mertz 1984). This study showed a significant reduction in duration of lesions for those undergoing their first episode of primary infection, as indicated by their antibody status. In those whose antibody status indicated they were having a first episode of non primary infection, there was no observed reduction (Table 4).
No other secondary outcomes were reported.
1.2 Oral ribavirin versus placebo
One study made this comparison (Zavala 1988). The study was in Spanish, and the data were provided by a translator.
Primary outcomes
1.2.1 Duration of symptoms from onset of treatment
The mean duration of symptoms from the onset of treatment for the treatment group of 30 patients was 5.7 days, and for the placebo group of 30 patients was 15.5 days. No standard errors were provided, so we have reported the available data in Table 5.
3. Mean: oral ribavirin versus placebo.
| Oral ribavirin | Placebo | |||||
| Outcome | Study |
Mean (days) |
No. participants |
Mean (days) |
No. participants | Favours intervention |
| Duration of symptoms from the onset of treatment | Zavala 1988 | 5.7 | 30 | 15.5 | 30 | ✓ |
✓: favours intervention
Our other primary outcomes were not reported.
Secondary outcomes
Our other secondary outcomes were not reported.
1.3 Intravenous acyclovir versus placebo
Three studies compared this outcome (Corey 1983; Mindel 1982; Peacock 1988). Nearly all outcomes were reported as median values. Peacock 1988 reported mean values for time to first recurrence but did not report standard deviations. Mindel 1982 only included patients with severe first episode of primary infection genital herpes that warranted hospital admission.
Primary outcomes
1.3.1 Duration of symptoms from onset of treatment
Two studies reported a shorter median duration of symptoms in the acyclovir group (Mindel 1982: n = 30, P < 0.05; Peacock 1988: n = 82, P = 0.019). The third study reported no significant difference between the groups (Corey 1983: n = 31, P = 0.17). See Table 6.
4. Medians: intravenous acyclovir versus placebo.
| Acyclovir | Placebo | ||||||
| Outcome | Study |
Median (days) |
No. participants |
Median (days) |
No. participants | P value | Favours intervention |
| Duration of symptoms from onset of treatment ‐ all | |||||||
| Mindel 1982 | 6.5 | 15 | 8.5 | 15 | < 0.05 | ✓ | |
| Peacock 1988 | 4.3 | 42 | 4.8 | 40 | 0.019 | ✓ | |
| Corey 1983 | 4 | 15 | 7 | 16 | 0.17 | ✕ | |
| Duration of symptoms from onset of treatment by antibody status | |||||||
| Primary | Mindel 1982 | 6.3 | 12 | 8.8 | 8 | NS | ✕ |
| Peacock 1988 | 4.2 | 10.6 | 0.009 | ✓ | |||
| Corey 1983 | 3 | 14 | 7 | 13 | 0.17 | ✕ | |
| Non‐primary | Peacock 1988 | 4.4 | 3.8 | 0.55 | ✓ | ||
| Duration of symptoms from onset of treatment by gender | |||||||
| Female | Mindel 1982 | 6.8 | 12 | 7.3 | 12 | NS | ✕ |
| Duration of lesions from onset of treatment | |||||||
| Mindel 1982 | 7.0 | 15 | 14.0 | 15 | < 0.001 | ✓ | |
| Peacock 1988 | 8.4 | 42 | 11.5 | 40 | 0.02 | ✓ | |
| Corey 1983 | 9 | 15 | 21 | 16 | 0.002 | ✓ | |
| Duration of lesions from onset of treatment by antibody status | |||||||
| Primary | Mindel 1982 | 9.0 | 12 | 15.0 | 8 | < 0.05 | ✓ |
| Peacock 1988 | 8.3 | 22 | 14.2 | 22 | 0.015 | ✓ | |
| Corey 1983 | 9 | 14 | 21 | 13 | 0.007 | ✓ | |
| Non‐primary | Peacock 1988 | 8.4 | 20 | 8.2 | 18 | NS | ✕ |
| Duration of lesions from onset of treatment by gender | |||||||
| Female | Mindel 1982 | 7.0 | 12 | 12.5 | 12 | < 0.05 | ✓ |
| Time to first recurrence by HSV type | |||||||
| HSV‐1 | Corey 1983+Mindel 1982 | 279 | 7 | 184 | 7 | 0.4 | ✕ |
| HSV‐2 | Corey 1983+Mindel 1982 | 64 | 23 | 74 | 23 | 0.4 | ✕ |
Duration of symptoms: Peacock 1988 refers specifically to pain; Corey 1983 refers to constitutional symptoms
HSV‐1: herpes simplex virus type 1 HSV‐2: herpes simplex virus type 2 NS: not statistically significant ✓: favours intervention ✕: does not favour intervention
Subgroup analysis by gender
One study reported data for females only (n = 24) and found no significant difference between the acyclovir and the placebo group (Mindel 1982; Table 6).
Subgroup analysis by antibody status (first episode of primary infection or first episode of non‐primary infection)
One study reported data separated into first episode of primary infection and first episode of non‐primary infection based on antibody status (Peacock 1988). Acyclovir reduced the symptoms in the first episode of primary infection group only, and no difference was seen in the first episode of non‐primary infection group (Table 6).
1.3.2 Time to first recurrence
One study reported mean time to first recurrence (Peacock 1988). No measurement of error was provided for this information but this study observed a mean time to first recurrence in the acyclovir group of 89 days and 93 days in the placebo group.
Corey 1983 and Mindel 1982 combined their data in a follow‐up publication, subgrouped into participants with HSV‐1 infection (n = 14) and those with HSV‐2 infection (n = 46). They reported no significant difference (P = 0.04) between the groups in median time to first recurrence. See Table 6.
1.3.3 Adverse events
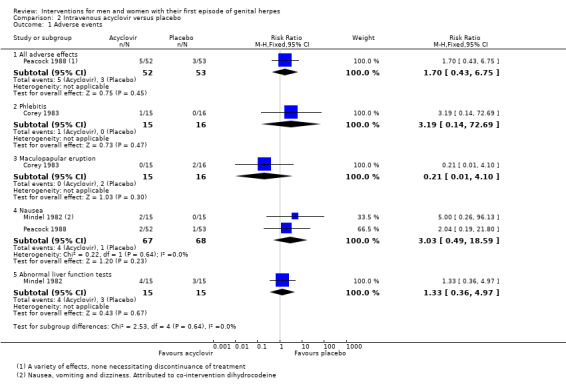
None of the three studies reported a difference between the groups in the rate of adverse events, though sample sizes were small for individual outcomes (Analysis 2.1). Reported events included mild phlebitis or pain at the infusion site, rashes, abnormal liver function tests, nausea, vomiting, and dizziness. Some of these effects were attributed to co‐administration of codeine.
2.1. Analysis.
Comparison 2 Intravenous acyclovir versus placebo, Outcome 1 Adverse events.
Secondary outcomes
1.3.4 Duration of lesions from onset of treatment
All three studies reported a shorter median duration of lesions in the acyclovir group (Mindel 1982: n = 30, P < 0.001; Peacock 1988: n = 82, P = 0.02; Corey 1983: n = 31, P = 0.002). See Table 6.
Subgrouped by gender
One study reported data for females only (n = 24) and found a significantly shorter median duration of lesions in the acyclovir group (P < 0.05) (Mindel 1982; Table 6).
Subgroup analysis by antibody status (first episode of primary infection or first episode of non‐primary infection)
One study reported data separated into first episode of primary infection and first episode of non‐primary infection based on antibody status. Acyclovir reduced the duration of lesions in the first episode of primary infection group (P < 0.015) but not in the first episode of non‐primary infection group (Peacock 1988; Table 6).
No other secondary outcomes were reported.
1.4 Topical acyclovir versus placebo
Four studies made this comparison (Corey 1982a; Corey 1982b; Fiddian 1983; Kinghorn 1986a). It should be noted that in the Kinghorn 1986a study all participants received oral acyclovir in addition to the topical acyclovir or placebo.
Primary outcomes
1.4.1 Duration of symptoms from onset of treatment
When three studies were pooled, there was no difference between the groups in symptom duration (Corey 1982a; Corey 1982b; Kinghorn 1986a) (MD ‐0.61 days, 95% CI ‐2.16 to 0.95; 3 RCTs, 195 participants, I2 statistic = 56%; Analysis 3.1; Figure 5). As there was moderate heterogeneity for this analysis for which there was no obvious explanation, we used a random‐effects model.
3.1. Analysis.
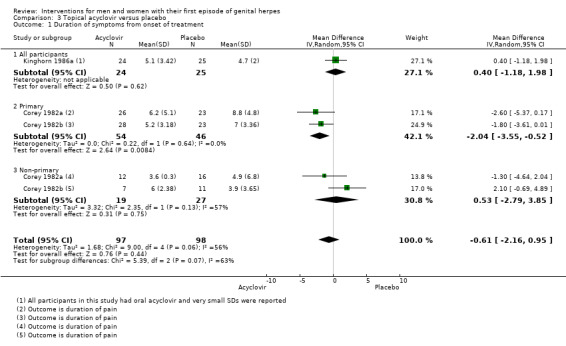
Comparison 3 Topical acyclovir versus placebo, Outcome 1 Duration of symptoms from onset of treatment.
5.
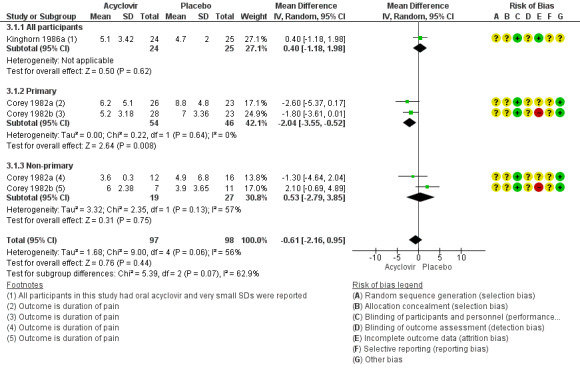
Forest plot of comparison: 4 Topical acyclovir versus placebo, outcome: 4.1 Duration of symptoms from onset of treatment.
One study reported medians only (Fiddian 1983). The duration of symptoms was significantly shorter in the acyclovir group than in the placebo group (n = 101, P = 0.01; see Table 7). We graded this evidence as low quality due to the high risk of bias associated with the included studies and the reasonably high heterogeneity (Table 2).
5. Medians: topical acyclovir versus placebo.
| Topical acyclovir | Topical placebo | ||||||
| Outcome | Study |
Median (days) |
No. participants |
Median (days) |
No. participants | P value | Favours intervention |
| Duration of symptoms from onset of treatment ‐ all | |||||||
| Fiddian 1983 | 5 | 54 | 8 | 47 | 0.01 | ✓ | |
| Duration of symptoms from onset of treatment by gender | |||||||
| Females | Fiddian 1983 | 6 | 35 | 9 | 31 | < 0.05 | ✓ |
| Males | Fiddian 1983 | 3.5 | 19 | 6 | 16 | > 0.1 | ✕ |
| Duration of lesions from onset of treatment ‐ all | |||||||
| Fiddian 1983 | 8 | 54 | 13 | 47 | 0.01 | ✓ | |
| Duration of lesions from onset of treatment by gender | |||||||
| Females | Fiddian 1983 | 8 | 35 | 13 | 31 | < 0.001 | ✓ |
| Males | Fiddian 1983 | 8 | 19 | 11 | 16 | < 0.01 | ✓ |
| Time to first recurrence | |||||||
| Corey 1982a | 116 | 116 | ✕ | ||||
| Corey 1982b | 79 | 79 | ✕ | ||||
✓: favours intervention ✕: does not favour intervention
Subgrouped analysis gender
Kinghorn 1986a analysed females separately and found no difference between the acyclovir and placebo groups (MD 0.50 days, 95% CI ‐1.35 to 2.35; 35 women). (See Analysis 3.2; Figure 6). In the study that reported median values only, there was no statistically significant difference between the groups among men (Fiddian 1983, n = 35), but symptom duration was significantly shorter in the acyclovir group among women (Fiddian 1983, 64 women, P < 0.05). See Table 7.
3.2. Analysis.
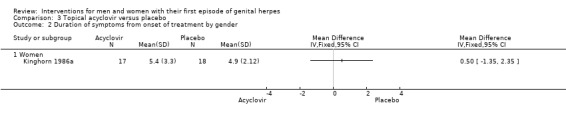
Comparison 3 Topical acyclovir versus placebo, Outcome 2 Duration of symptoms from onset of treatment by gender.
6.
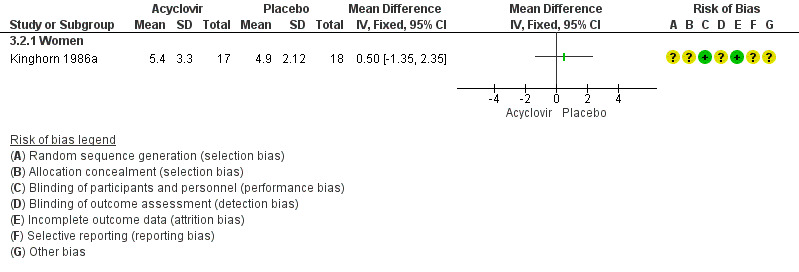
Forest plot of comparison: 4 Topical acyclovir versus placebo, outcome: 4.2 Duration of symptoms from onset of treatment by gender.
1.4.2 Time to first recurrence
Corey 1982b reported no difference in the median time to recurrence among 58 participants (84% of total) who had adequate follow‐up; the median was 79 days in both groups, but it was unclear how many in each group were followed up. No further information about time to recurrence was available.
Corey 1982a reported no difference in the median time to recurrence among 25 participants (78% of total) with HSV‐2 infection who had adequate follow up; the median was 116 days in both groups, but it was unclear how many in each group were followed up. No further information about time to recurrence was available.
Kinghorn 1986a also failed to show a difference between those on topical acyclovir and those receiving placebo in those who had had a recurrence in the six months following treatment (risk ratio (RR) 1.2, 95% CI 0.6 to 2.3; P = 0.6). Of the 46 patients who completed follow‐up, 11 (50%) out of 22 who received acyclovir compared with 10 (42%) out of 24 treated with placebo had a recurrence within six months of their first episode.
1.4.3 Adverse events
None of the studies reported a difference between the groups in the rate of adverse events, though sample sizes were small for individual outcomes (Analysis 3.5, Figure 7). Reported events included pain with topical application, rashes, and itching. This evidence is of moderate quality with the only concern being in regard to the risk of bias associated with the included studies (Table 2).
3.5. Analysis.
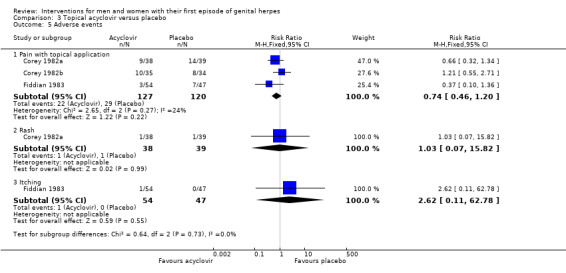
Comparison 3 Topical acyclovir versus placebo, Outcome 5 Adverse events.
7.
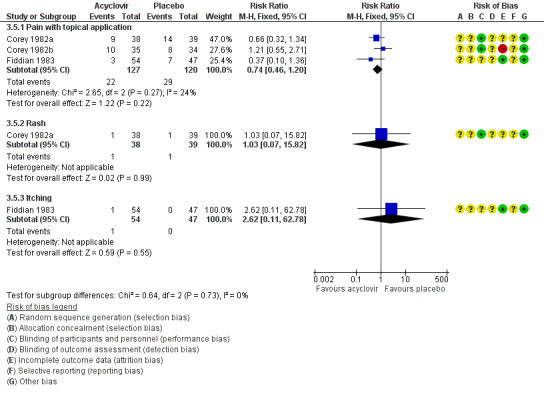
Forest plot of comparison: 4 Topical acyclovir versus placebo, outcome: 4.3 Adverse events.
Secondary outcomes
1.4.4 Duration of lesions from onset of treatment by antibody status
When Corey 1982a, Corey 1982b, and Kinghorn 1986a were pooled there was no difference between the groups in lesion duration (MD ‐0.86 days, 95% CI ‐2.15 to 0.42; 195 participants, I2 statistic = 51%, Analysis 3.3; Figure 8). As there was moderate heterogeneity for this outcome for which there was no clear explanation we used a random‐effects model.
3.3. Analysis.
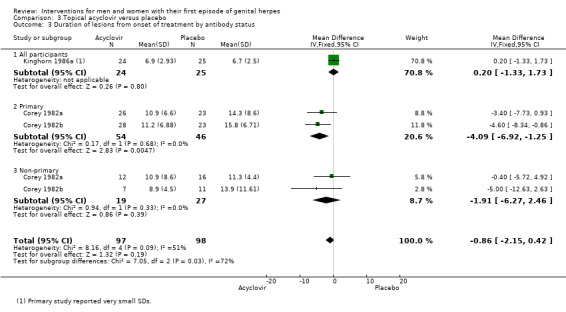
Comparison 3 Topical acyclovir versus placebo, Outcome 3 Duration of lesions from onset of treatment by antibody status.
8.
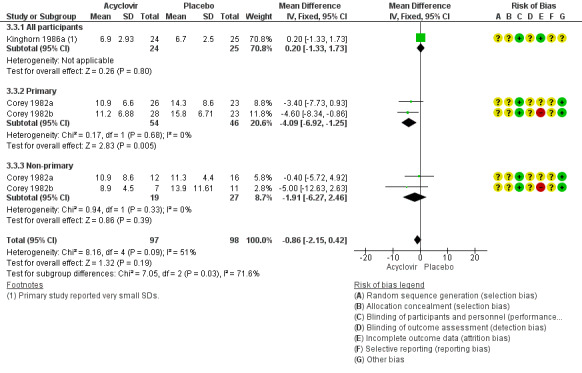
Forest plot of comparison: 4 Topical acyclovir versus placebo, outcome: 4.4 Duration of lesions from onset of treatment by antibody status.
The study which reported medians only found that the duration of symptoms was significantly shorter in the acyclovir group than in the placebo group (Fiddian 1983, n = 101, P = 0.01; see Table 7). As a result of the substantial heterogeneity and the risk of bias of included studies we have judged this evidence as low quality (Table 2).
Subgroup analysis by gender
Kinghorn 1986a analysed females as a separate subgroup and found no difference between the acyclovir and placebo groups (MD ‐0.10 days, 95% CI 1.78 to 1.58; 35 women). See Analysis 3.4.
3.4. Analysis.
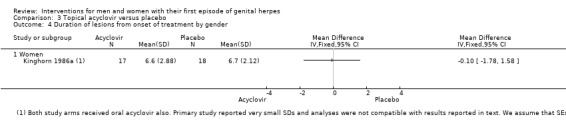
Comparison 3 Topical acyclovir versus placebo, Outcome 4 Duration of lesions from onset of treatment by gender.
The study which reported medians only found a significantly shorter lesion duration in the acyclovir group among men (Fiddian 1983) (n = 35, P < 0.01) and also among women (64 women, P < 0.001). See Table 7.
No other secondary outcomes were reported.
1.5 Topical 2% cicloxolone cream versus placebo
One small three‐arm study made this comparison (Csonka 1984). It included a total of only 19 participants with first‐episode disease, of whom only 11 (57%) were included in the analysis.
Primary outcomes
1.5.1 Duration of symptoms from onset of treatment
There was no difference between the groups in the duration of symptoms at five or seven days. However, no conclusions can be drawn, as analysis included only five participants in the cicloxolone group and four in the placebo group. See Analysis 4.1 and Analysis 4.2.
4.1. Analysis.
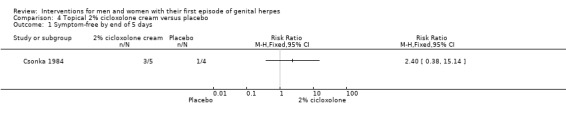
Comparison 4 Topical 2% cicloxolone cream versus placebo, Outcome 1 Symptom‐free by end of 5 days.
4.2. Analysis.
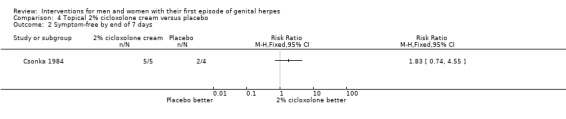
Comparison 4 Topical 2% cicloxolone cream versus placebo, Outcome 2 Symptom‐free by end of 7 days.
1.5.2 Time to first recurrence
This outcome was not reported.
5.3 Adverse events
No comparative data were reported among participants with first‐episode disease. Among all participants with either first‐episode or recurrent genital herpes, one in each group had slight irritation after application of the cream. The reaction was not sufficiently severe to discontinue treatment.
Secondary outcomes
1.5.4 Duration of lesions from onset of treatment
There was no difference between the groups in the duration of lesions at five or seven days. However, no conclusions can be drawn as analysis included only five participants in the cicloxolone group and four in the placebo group. See Analysis 4.3 and Analysis 4.4.
4.3. Analysis.
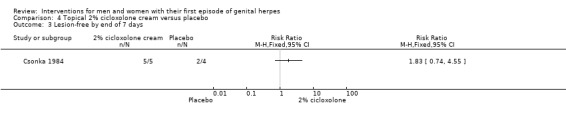
Comparison 4 Topical 2% cicloxolone cream versus placebo, Outcome 3 Lesion‐free by end of 7 days.
4.4. Analysis.
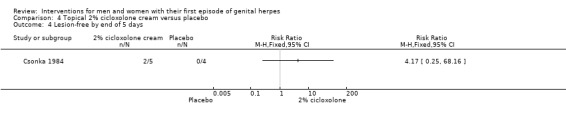
Comparison 4 Topical 2% cicloxolone cream versus placebo, Outcome 4 Lesion‐free by end of 5 days.
No other secondary outcomes were reported.
1.6 Topical carbenoxolone sodium cream versus placebo
One small three‐arm study made this comparison (Csonka 1984). It included a total of only 19 participants with first‐episode disease, of whom only 11 (57%) were included in analysis.
Primary outcomes
1.6.1 Duration of symptoms from onset of treatment
There was no difference between the groups in the duration of symptoms at five or seven days. However, no conclusions can be drawn as there were only two participants in the carbenoxolone group and four in the placebo group. See Analysis 5.1 and Analysis 5.2.
5.1. Analysis.
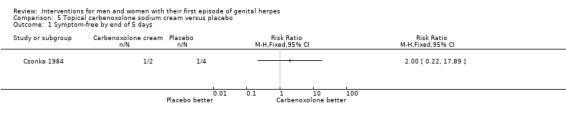
Comparison 5 Topical carbenoxolone sodium cream versus placebo, Outcome 1 Symptom‐free by end of 5 days.
5.2. Analysis.
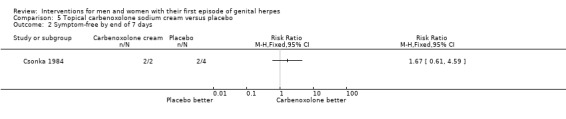
Comparison 5 Topical carbenoxolone sodium cream versus placebo, Outcome 2 Symptom‐free by end of 7 days.
1.6.2 Time to first recurrence
This outcome was not reported.
1.6.3 Adverse events
No comparative data were reported among participants with first‐episode disease. Among all participants with either first‐episode or recurrent genital herpes, one in each group had slight irritation after application of the cream; the reaction was not severe enough to discontinue treatment.
Secondary outcomes
1.6.4 Duration of lesions from onset of treatment
There was no difference between the groups in the duration of lesions at five or seven days. However, no conclusions can be drawn as there were only two participants in the carbenoxolone group and four in the placebo group. See Analysis 5.3 and Analysis 5.4.
5.3. Analysis.
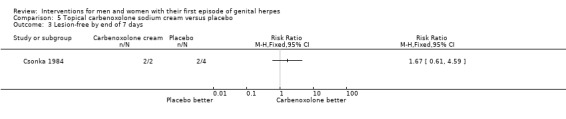
Comparison 5 Topical carbenoxolone sodium cream versus placebo, Outcome 3 Lesion‐free by end of 7 days.
5.4. Analysis.
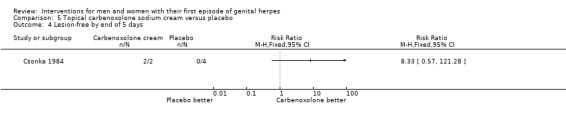
Comparison 5 Topical carbenoxolone sodium cream versus placebo, Outcome 4 Lesion‐free by end of 5 days.
Our other secondary outcomes were not reported.
1.7 Topical adenosine arabinoside versus placebo or no treatment
One study made this comparison (Adams 1976). Partway through this study the intervention was changed from adenosine arabinoside topical ointment to gel that was applied topically and intravaginally. Due to poor reporting of study methods, we could not extract data for analysis. The study authors concluded that the intervention was ineffective in both men and women. Available data are presented in Table 8.
6. Mean: adenosine arabinoside versus placebo.
| Adenine arabinoside | Placebo | No treatment | Untreated (no contraception) | |||||||
| Study |
Mean (days) |
No. participants |
Mean (days) |
No. participants |
Mean (days) |
No. participants |
Mean (days) |
No. participants | Favours intervention | |
| Duration of symptoms from onset of treatment by gender | ||||||||||
| Females | Adams 1976 | 10.4 | 8 | 6.8 | 10 | 8.8 | 4 | 7.0 | 5 | ✕ |
| Males | Adams 1976 | 7.8 | 9 | 6.3 | 9 | 6.5 | 4 | ✕ | ||
| Duration of lesions from onset of treatment by gender | ||||||||||
| Females | Adams 1976 | 16.1 | 8 | 11 | 10 | 10 | 4 | 13.6 | 5 | ✕ |
| Males | Adams 1976 | 11.9 | 9 | 13.1 | 9 | 11.5 | 4 | ✕ | ||
✕: does not favour intervention
1.8 Topical 30% idoxuridine in dimethyl sulfoxide versus dimethyl sulfoxide alone or saline
One study made this comparison but data were not in a format that we could use in a meta‐analysis (Silvestri 1982).
Primary outcomes
1.8.1 Duration of symptoms from onset of treatment
There was no difference between the groups in the mean duration of symptoms. See Table 9.
7. Mean: topical 30% idoxuridine in dimethyl sulfoxide versus control.
| Topical 30% idoxuridine | Control* | |||||
| Outcome | Study | Mean (days) | No. participants | Mean (days) | No. participants | Favours intervention |
| Duration of symptoms from the onset of treatment ‐ all | ||||||
| Silvestri 1982 | 10.7 | 9 | 12.9 | 23 | ✕ | |
| Duration of lesions from the onset of treatment ‐ all | ||||||
| Silvestri 1982 | 19.6 | 9 | 17.9 | 23 | ✕ | |
| Adverse effects | Events | No. Participants | Events | No. participants | ||
| Burning on application | Silvestri 1982 | 5 | 9 | 6 | 23 | ✕ |
*Control: either dimethyl sulfoxide alone or saline alone ✕: does not favour intervention
Time to first recurrence
This outcome was not reported.
1.8.2 Adverse events
There was no difference between the groups in the rate of adverse events. Burning on application was reported in both study groups. See Table 9.
Secondary outcomes
1.8.3 Duration of lesions from onset of treatment
There was no difference between the groups in the duration of symptoms. See Table 9.
Our other secondary outcomes were not reported.
1.9 Topical tromantadine versus placebo
One small study made this comparison (Altomare 1985). It included 14 males and seven female participants.
Primary outcomes
1.9.1 Duration of symptoms from onset of treatment
Duration of symptoms and duration of lesions were reported as a combined outcome in this study and data were dichotomous. There was no difference between the groups at three days (Analysis 6.1), six days (Analysis 6.2), or nine days (Analysis 6.3) from onset of treatment, but at 12 days the intervention group were significantly more likely to have healed (RR 2.27, 95% CI 1.04 to 4.97; 1 RCT, 21 participants) (Analysis 6.4).
6.1. Analysis.
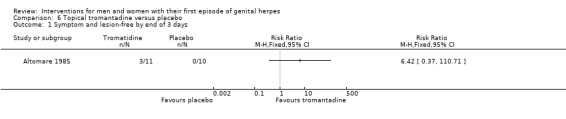
Comparison 6 Topical tromantadine versus placebo, Outcome 1 Symptom and lesion‐free by end of 3 days.
6.2. Analysis.
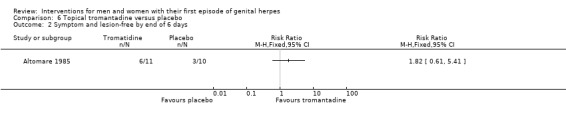
Comparison 6 Topical tromantadine versus placebo, Outcome 2 Symptom and lesion‐free by end of 6 days.
6.3. Analysis.
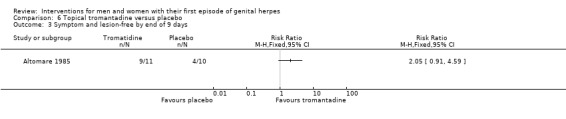
Comparison 6 Topical tromantadine versus placebo, Outcome 3 Symptom and lesion‐free by end of 9 days.
6.4. Analysis.
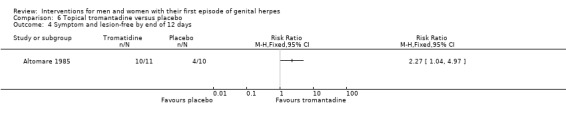
Comparison 6 Topical tromantadine versus placebo, Outcome 4 Symptom and lesion‐free by end of 12 days.
1.9.2 Time to first recurrence
This outcome was not reported.
1.9.3 Adverse events
Adverse events were poorly reported in this study and no reliable comparative data were available for first‐episode participants.
Secondary outcomes
Other secondary outcomes were not reported.
2. Antiviral versus other antiviral
2.1 Oral valaciclovir versus acyclovir
Two studies made this comparison (Fife 1997; Lai 2000). One was a large study which used survival analysis and reported hazard ratios (Fife 1997), and the other small study which reported mean values (Lai 2000).
Primary outcomes
2.1.1 Duration of symptoms from onset of treatment
Both studies reported that there was no difference between the oral valaciclovir group and the oral acyclovir group in the duration of symptoms (hazard ratio (HR) 1.02, 95% CI 0.85 to 1.22; 1 RCT, 643 participants, Analysis 7.1; MD 0.30 days, 95% CI ‐0.81 to 1.41; 1 RCT, 28 participants, Analysis 7.2).
7.1. Analysis.
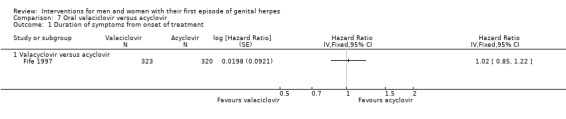
Comparison 7 Oral valaciclovir versus acyclovir, Outcome 1 Duration of symptoms from onset of treatment.
7.2. Analysis.
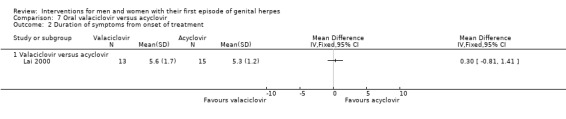
Comparison 7 Oral valaciclovir versus acyclovir, Outcome 2 Duration of symptoms from onset of treatment.
2.1.2 Time to first recurrence
This outcome was not reported.
2.1.3 Adverse events
There was no difference between the groups in the rate of adverse events. Reported events included nausea and headache, however they were uncommon in both arms of the included studies. See Analysis 7.3.
7.3. Analysis.
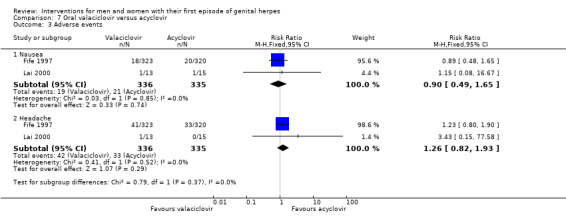
Comparison 7 Oral valaciclovir versus acyclovir, Outcome 3 Adverse events.
Secondary outcomes
2.1.4 Duration of lesions from onset of treatment
One study reported this outcome (Fife 1997). There was no significant difference between the groups in the duration of lesions (HR 1.08, 95% CI 0.92 to 1.27; 1 RCT, 643 participants; Analysis 7.4)
7.4. Analysis.
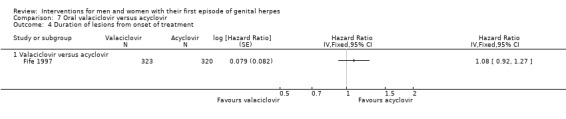
Comparison 7 Oral valaciclovir versus acyclovir, Outcome 4 Duration of lesions from onset of treatment.
Our other secondary outcomes were not reported.
2.2 Topical carbenoxolone sodium versus topical cicloxolone
One small three‐arm study made this comparison (Csonka 1984). It included a total of only 19 participants with first‐episode disease, of whom only 11 (57%) were included in the analysis.
Primary outcomes
2.2.1 Duration of symptoms from onset of treatment
There was no difference between the groups in the duration of symptoms at five or seven days. However, no conclusions can be drawn as analysis included only two participants in the carbenoxolone group and five participants in the cicloxolone group. See Analysis 8.1.
8.1. Analysis.
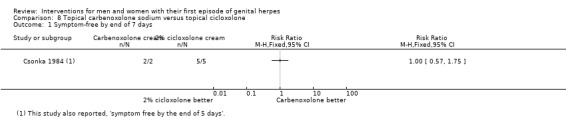
Comparison 8 Topical carbenoxolone sodium versus topical cicloxolone, Outcome 1 Symptom‐free by end of 7 days.
2.2.2 Time to first recurrence
This outcome was not reported.
2.2.3 Adverse events
No comparative data were reported among participants with first‐episode disease. Among all participants with either first‐episode or recurrent genital herpes, one in each group had slight irritation after application of the cream. The reaction was not sufficiently severe to discontinue treatment.
Secondary outcomes
2.2.4 Duration of lesions from onset of treatment
There was no difference between the groups in the duration of lesions at five or seven days. However, no conclusions can be drawn as analysis included only two participants in the carbenoxolone group and five participants in the cicloxolone group. See Analysis Analysis 8.2.
8.2. Analysis.
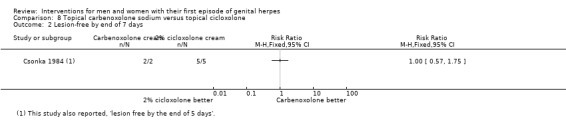
Comparison 8 Topical carbenoxolone sodium versus topical cicloxolone, Outcome 2 Lesion‐free by end of 7 days.
Our other secondary outcomes were not reported.
2.3 Oral acyclovir alone versus inosine prabonex with or without acyclovir
One study with 52 participants compared acyclovir alone versus inosine prabonex with or without acyclovir (Mindel 1987). It reported median values.
Primary outcomes
2.3.1 Duration of symptoms from onset of treatment
There was no significant difference between any of the groups in duration of symptoms. See Table 10.
8. Medians: oral acyclovir versus inosine pranobex versus both.
| Acyclovir | Inosine pranobex | Both | |||||||
| Study |
Median (days) |
No. participants |
Median (days) |
No. participants |
Median (days) |
No. participants | P value | Favours Acyclovir | |
| Duration of symptoms from onset of treatment ‐ all | |||||||||
| Mindel 1987 | 7 (range 3 to 19) | 24 | 8 (range 4 to 23) | 28 | 7 (range 3 to 19) | 25 | Acyclovir versus inosine: NS Acyclovir versus both: NS |
✕ | |
| Duration of symptoms from onset of treatment by gender | |||||||||
| Females | Mindel 1987 | 7 (range 3 to 19) | 9.5 (range 4 to 23) | 7 (range 3 to 19) | Acyclovir versus inosine: P < 0.05 Acyclovir versus both: NS |
✓ | |||
| Duration of lesions from onset of treatment ‐ all | |||||||||
| Mindel 1987 | 9 (range 4 to 24) | 24 | 13 (range 1 to 26) | 28 | 9 (range 5 to 18) | 25 | Acyclovir versus inosine: P < 0.05 Acyclovir versus both: NS |
✓ | |
| Duration of lesions from onset of treatment by gender | |||||||||
| Females | Mindel 1987 | 9.5 (range 4 to 24) | 13 (range 1 to 26) | 9 (range 5 to 18) | Acyclovir versus inosine: NS Acyclovir versus both: NS |
✕ | |||
| Time to recurrence | |||||||||
| Mindel 1987 | 187.4 | 142.5 | 132.7 | NS | ✕ | ||||
NS: not statistically significant ✓: favours acyclovir ✕: does not favour acyclovir
Subgroup analysis by gender
When analysis was restricted to women only, duration of symptoms was significantly shorter in the acyclovir‐only group (n = 21) than in the inosine prabonex‐only group (n = 24) (P < 0.05), but did not differ from the group receiving both interventions (n = 21). See Table 10.
2.3.2 Time to first recurrence
The authors reported that there was no significant difference between the groups in time to first recurrence. It was not stated what proportion of participants were followed up for this outcome. See Table 10.
2.3.3 Adverse events
The study authors stated that no adverse effects were noted.
Secondary outcomes
2.3.4 Duration of lesions from onset of treatment
Duration of lesions was significantly shorter in the group receiving acyclovir only (n = 24) than in those receiving inosine prabonex only (n = 28). There was no difference between the groups when the group receiving acyclovir only (n = 24) was compared with those receiving acyclovir plus inosine prabonex (n = 49). See Table 10.
Subgroup analysis by gender
When analysis was restricted to women only, duration of lesions did not significantly differ between any of the groups. See Table 10.
Our other secondary outcomes were not reported.
3. Antiviral regimen comparisons
3.1 Long course versus short course acyclovir
One study made this comparison (Mindel 1986; n = 60). It reported median values. Sixty women were treated with either oral acyclovir for 42 days or oral acyclovir for five days followed by placebo for 37 days.
Primary outcomes
3.1.1 Duration of symptoms from onset of treatment
There was no significant difference between the groups in duration of symptoms (Table 11).
9. Medians: oral acyclovir long course versus standard course.
| Long course acyclovir | Short course acyclovir | ||||||
| Outcome | Study | Median (days) | No. participants | Median (days) | No. participants | P value | Favours long course acyclovir |
| Duration of symptoms from onset of treatment by gender | |||||||
| Female | Mindel 1986 | 11 (range 1 to 31) | 30 | 11 (range 2 to 28) | 30 | NS | ✕ |
| Duration of lesions from onset of treatment by gender | |||||||
| Female | Mindel 1986 | 11 (range 5 to 34) | 30 | 11 (range 5 to 32) | 30 | NS | ✕ |
NS: not statistically significant ✕: does not favour long course acyclovir
3.1.2 Time to first recurrence
The median time to first recurrence in the long course group was 66.5 days, and 24 days in the short course group (P < 0.0001). However the study authors reported that the difference between the groups was only significant during the treatment period (42 days) and not for longer follow‐up.
3.1.3 Adverse events
There was no difference between the groups in the rate of adverse events, which were uncommon in both groups. Reported events included constipation, diarrhoea, and bilirubin elevation. See Analysis 9.1.
9.1. Analysis.
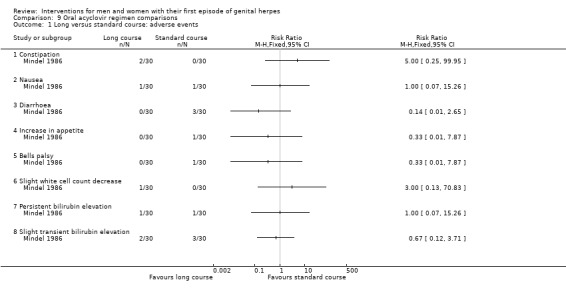
Comparison 9 Oral acyclovir regimen comparisons, Outcome 1 Long versus standard course: adverse events.
Secondary outcomes
3.1.4 Duration of lesions from onset of treatment
There was no significant difference between the groups in duration of lesions (Table 11).
Our other secondary outcomes were not reported.
3.2 High dose versus standard dose acyclovir
One study made this comparison (Wald 1994; n = 56). It reported median values. Participants were treated with either oral acyclovir 1 gm daily or 4 gm daily for five days.
Primary outcomes
3.2.1 Duration of symptoms from onset of treatment
There was no significant difference between the groups in duration of symptoms. See Table 12.
10. Medians: oral acyclovir high dose versus standard dose.
| High dose acyclovir | Low dose acyclovir | ||||||
| Outcome | Study |
Median (days) |
No. participants |
Median (days) |
No. participants | P value | Favours high dose acyclovir |
| Duration of symptoms from onset of treatment ‐ all | |||||||
| Wald 1994 | 7 (range 5 to 10) | 59 | 9 (range 7 to 12) | 28 | NS | ✕ | |
| Duration of lesions from onset of treatment ‐ all | |||||||
| Wald 1994 | 11 (range 8 to 14) | 59 | 10 (range 7 to 11) | 28 | NS | ✕ | |
| Time to recurrence ‐ all | |||||||
| Wald 1994 | 45 (range 20 to 128) | See footnote | 53 (range 11 to 196) | See footnote | NS | ✕ | |
Recurrence occurred in 80% of participants NS: not statistically significant ✕: does not favour high dose acyclovir
3.2.2 Time to first recurrence
There was no significant difference between the groups in time to first recurrence. See Table 12.
3.2.3 Adverse events
There was no statistically significant difference between the groups in the rate of adverse events (RR 7.08, 95% CI 0.41 to 121.05), but all reported events occurred in the intervention group (gastric disturbance in seven participants and headache in two participants). See Analysis 9.2.
9.2. Analysis.
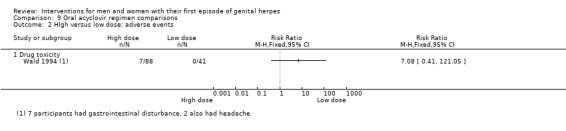
Comparison 9 Oral acyclovir regimen comparisons, Outcome 2 HIgh versus low dose: adverse events.
Secondary outcomes
3.2.4 Duration of lesions from onset of treatment
There was no significant difference between the groups in duration of lesions. See Table 12.
Our other secondary outcomes were not reported.
3.3 High dose versus standard dose famciclovir
One study looked at different dosing regimens of famciclovir (Niimura 1996).
Primary outcomes
3.3.1 Duration of symptoms from onset of treatment
There was no significant difference between the groups in duration of symptoms. See Analysis 13.1.
13.1. Analysis.
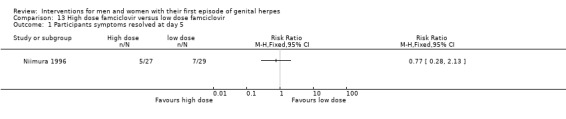
Comparison 13 High dose famciclovir versus low dose famciclovir, Outcome 1 Participants symptoms resolved at day 5.
3.3.2 Time to first recurrence
There were no studies that looked at this outcome.
3.3.3 Adverse events
There were no studies that looked at this outcome.
Secondary outcomes
3.3.4 Duration of lesions from onset of treatment
There was no significant difference between the groups in visibility of lesions at day 5. See Analysis 13.2.
13.2. Analysis.
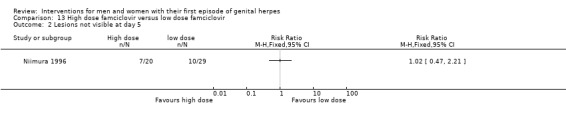
Comparison 13 High dose famciclovir versus low dose famciclovir, Outcome 2 Lesions not visible at day 5.
No other secondary outcomes were reported.
4 Antiviral versus interferon
4.1 Topical acyclovir versus intramuscular interferon
One study with 105 participants made this comparison (Levin 1989).
Primary outcomes
4.1.1 Duration of symptoms from onset of treatment
There was no difference between the groups in symptom duration (MD 1.03 days, 95% CI ‐0.85 to 2.91; 1 RCT, 105 participants; Analysis 10.1).
10.1. Analysis.
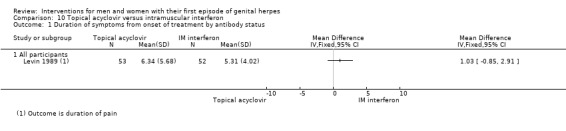
Comparison 10 Topical acyclovir versus intramuscular interferon, Outcome 1 Duration of symptoms from onset of treatment by antibody status.
4.1.2 Time to first recurrence
This outcome was not reported.
4.1.3 Adverse events
Compared to the topical acyclovir group, the intramuscular interferon group reported higher rates of visual disturbances (RR 2.76, 95% CI 1.23 to 6.19), dizziness (RR 6.83, 95% CI 1.59 to 29.25), nausea (RR 1.81, 95% 1.10 to 2.96), anorexia (RR 1.74, 95% CI 1.10 to 2.75), sweating (RR 19.31, 95% CI 1.14 to 327.34), fever (RR 3.82, 95% CI 2.36 to 6.18), fatigue (RR 1.44, 95% CI 1.09 to 1.90), chills (RR 3.13, 95% CI 1.99 to 4.91), headache (RR 1.57, 95% CI 1.14 to 2.17), myalgia (RR 1.74, 95% CI 1.30 to 2.34), and neutropenia (RR 5.69, 95% CI 2.33 to 13.90). There was no difference between the groups in rates of diarrhoea or vomiting (Analysis 10.2).
10.2. Analysis.
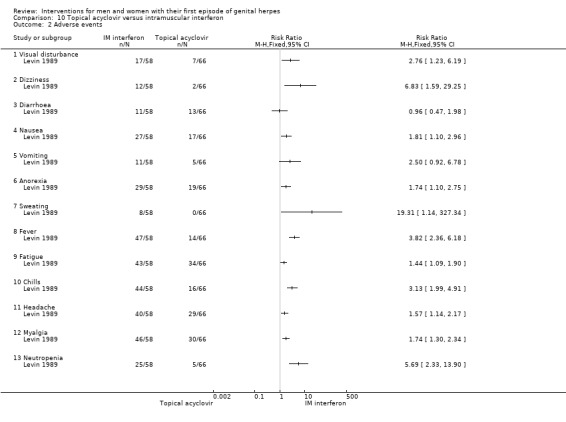
Comparison 10 Topical acyclovir versus intramuscular interferon, Outcome 2 Adverse events.
Secondary outcomes
4.1.4 Duration of lesions from onset of treatment
There was no difference between the groups in lesion duration (MD 1.58, 95% CI ‐0.38 to 3.54; 1 RCT, 105 participants; Analysis 10.3).
10.3. Analysis.
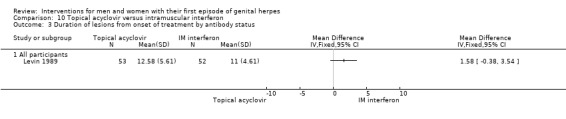
Comparison 10 Topical acyclovir versus intramuscular interferon, Outcome 3 Duration of lesions from onset of treatment by antibody status.
Our other secondary outcomes were not reported.
5 Interferon versus placebo
5.1 Topical interferon cream versus placebo
Batcheler 1986 reported this comparison. Only females (n = 36) were included in this study. Data were unsuitable for analysis as no standard errors were reported.
Primary outcomes
5.1.1 Duration of symptoms from onset of treatment
This outcome was reported for only 30 (83%) of the participants. The authors stated that there was no significant difference between the groups. See Table 13.
11. Mean: topical interferon versus placebo.
| Topical interferon | Placebo | |||||
| Outcome | Study | Mean (days) | No. participants | Mean (days) | No. participants | Favours intervention |
| Duration of symptoms from the onset of treatment | ||||||
| Batcheler 1986 | 7.25 | 12 | 6.33 | 18 | X | |
| Duration of lesions from the onset of treatment | ||||||
| Batcheler 1986 | 8.06 | 16 | 6.52 | 19 | X | |
✓: favours intervention ✕: does not favour intervention
5.1.2 Time to first recurrence
This outcome was not reported.
5.1.3 Adverse events
The authors reported that there were no adverse events in either study arm.
Secondary outcomes
5.1.4 Duration of lesions from onset of treatment
The authors stated that there was no significant difference between the groups (see Table 13).
Our other secondary outcomes were not reported.
5.2 Subcutaneous interferon versus placebo
One study reported this comparison (Mendelson 1986). It included 31 participants.
Primary outcomes
5.2.1 Duration of symptoms from onset of treatment
The authors stated that there was no significant difference between the groups (Analysis 11.1).
11.1. Analysis.
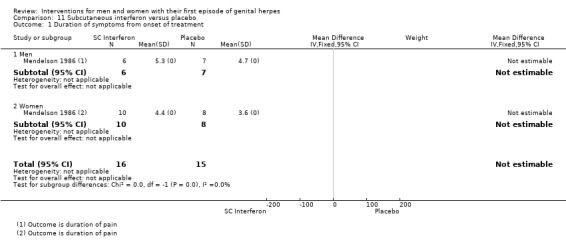
Comparison 11 Subcutaneous interferon versus placebo, Outcome 1 Duration of symptoms from onset of treatment.
5.2.2 Time to first recurrence
The authors stated that there was no difference between the groups in time to first recurrence, but no numerical data were presented for this outcome.
5.2.3 Adverse effects
Compared to the placebo group, the subcutaneous interferon group reported higher rates of fever (RR 12.86, 95% CI 1.91 to 86.44), headache (RR 3.57, 95% CI 1.23 to 10.36), chills (RR 5.89, 95% CI 1.58 to 22.03), anorexia (RR 8.57. 95% CI 1.22 to 60.07), and neutropenia (RR 29.00, 95% CI 1.90 to 443.25). There was no difference between the groups in rates of myalgia, nausea, vomiting, fatigue, and diarrhoea (see Analysis 11.2).
11.2. Analysis.
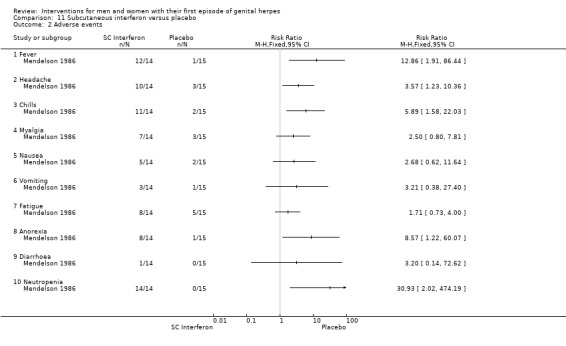
Comparison 11 Subcutaneous interferon versus placebo, Outcome 2 Adverse events.
Secondary outcomes
5.2.4 Duration of lesions from onset of treatment
The authors stated that there was no significant difference between the groups (Analysis 11.3).
Our other secondary outcomes were not reported.
5.3 Intramuscular interferon versus placebo
One study reported this comparison (Pazin 1987). The study included 69 participants, all women.
Primary outcomes
5.3.1 Duration of symptoms from onset of treatment
Data were reported in graphical form. The study authors reported that although the mean duration of pain in the intervention group was consistently two days shorter than in the placebo group, the difference was not significant.
5.3.2 Time to first recurrence
The study authors stated that life table analysis showed no difference between the groups in time to first recurrence. Eighty per cent of participants were followed for at least 230 days.
5.3.3 Adverse events
Compared to the placebo group, the interferon group had increased rates of transient neutropenia (RR 23.91, 95% CI 1.48 to 385.85; 1 RCT, n = 64) and thrombocytopenia (RR 11.51, 95% CI 0.68 to 196.20). See Analysis 12.1.
12.1. Analysis.
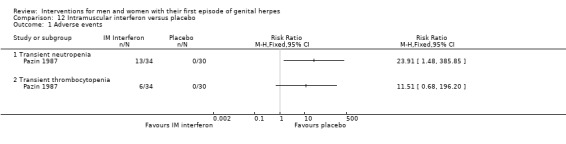
Comparison 12 Intramuscular interferon versus placebo, Outcome 1 Adverse events.
Secondary outcomes
5.3.4 Duration of lesions from onset of treatment
The study authors stated that there was no significant difference between the groups in duration of lesions. See Table 14.
12. Medians: intramuscular interferon versus placebo.
| Intramuscular interferon | Placebo | ||||||
| Outcome | Study |
Median (days) |
No. participants |
Median (days) |
No. participants | P value | Favours intervention |
| Duration of lesions from onset of treatment ‐ women | |||||||
| Pazin 1987 | 16 | 34 | 22 | 30 | P < 0.05 for days 18 to 20 only | ✕ | |
✕: does not favour intervention
Our other secondary outcomes were not reported.
6 Natural products
There were no included studies that looked at this comparison.
Sensitivity Analysis
It was not possible to conduct a sensitivity analysis by excluding studies at high risk of bias, as the included studies did not differ substantially with respect to risk of bias. Nearly all studies were at unclear risk of bias in most domains. Use of a random‐effects model did not change the statistical significance of any of the findings, with the exception of the female subgroup in Analysis 1.1; Figure 4.
Discussion
Summary of main results
There were four randomised controlled studies that compared oral acyclovir with placebo for first‐episode genital herpes. The dose of acyclovir was 200 mg given five times daily for either 5, 7, or 10 days. The pooled data from two studies showed that symptom duration, including lesion duration, was significantly shorter in the acyclovir group (mean difference (MD) ‐3.3 days, 95% CI ‐4.94 to ‐1.46). There was no difference in time to first recurrence. Few adverse effects were reported. One study that compared short duration (5 days) with longer duration (42 days) acyclovir found no difference in symptom duration. Similarly, high dose acyclovir (4 gm x 4 days) did not show any difference in symptom duration compared to standard dose (1 gm x 4 days). The two studies that compared oral acyclovir (200 mg x 5 daily) with oral valaciclovir (300 mg or 500 mg x 2 daily) found no difference in symptom duration. One study of varying regimes of famciclovir (125 mg, 250 mg, or 500 mg X 3 daily) also found no difference in symptom duration. Of the three studies comparing intravenous acyclovir with placebo, two reported shorter median symptom duration and all three a shorter median duration of lesions. Four studies compared topical acyclovir with placebo. Pooled data from three studies showed no difference in symptom duration.
Placebo controlled studies of other antivirals such as cicloxolone, carbenoxolone, adenosine arabinoside, idoxuridine, and tromantadine were either too small or had such poor reporting of study methods to enable any meaningful conclusions. The one study comparing oral acyclovir with inosine prabonex found no overall difference in symptom duration or time to first recurrence, however the duration of lesions was shorter for the acyclovir group. When topical acycylovir, not shown to be beneficial in the previous randomised placebo‐controlled studies, was compared to interferon, in one study, there was no difference in symptom duration. The authors of a study comparing topical interferon cream with placebo reported no difference in symptom duration. Similarly, two studies comparing subcutaneous interferon or intramuscular interferon with placebo also found no benefit. One study of topical proflavine with light exposure and another of the antibiotic, co‐trimoxazole also showed no benefit when compared with placebo.
Overall completeness and applicability of evidence
Many of the included studies in this review were old with data reporting median values that precluded us from being able to pool the data. In addition, the age of the studies also meant that when data were missing, we were unable to obtain additional information. There were no studies comparing either valaciclovir or famciclovir with placebo and no studies comparing famciclovir with acyclovir. We were also lacking studies comparing imiquimod to placebo, no treatment or other medications and studies looking at the use of natural products.
None of the included studies contained pregnant women as part of the participant group. For this reason we were unable to assess the effects of these medications on outcomes looking at neonatal effects and rates of caesarean section delivery. In addition, for the primary outcome of time to first recurrence the available data was limited and not presented in the appropriate form to allow meta‐analysis.
Few studies with acyclovir had antibody levels and so we were unable to confirm that this was a first episode of primary infection. In the one study where this information was available, symptom duration appeared to be reduced only for those with a first episode of primary infection. Whether gender affects the efficacy of acyclovir for symptom duration is not clear due to small sample sizes.
Our intention was to perform subgroup analyses on the following variables gender, length of treatment, type of drug within a class, duration of time between appearance of lesions and initiation of treatment, immunodeficiency and first episode of primary infection versus first episode of non‐primary infection. The was insufficient data to explore the majority of these subgroups and those subgroups we were able to present (gender and first episode of primary infection vs first episode of non primary infection) only contained a limited number of small studies.
Quality of the evidence
We judged most of the studies at unclear risk of bias in most domains. This is in large due to the age of the included studies and the brief reporting of the methods in the included studies. This does not necessarily imply that the studies were of poor quality, but rather that we did not have the information that we required to be able to classify these studies as either low or high risk. In addition to the unclear risk of bias information, there were very few studies that we were able to include in the meta‐analysis. Because of the low number of studies that were able to inform the meta‐analysis the confidence intervals were relatively wide and so for this reason we downgraded the level of evidence for reasons of imprecision. This, in combination with some apparent heterogeneity, led us to grade most of the evidence as low.
Potential biases in the review process
As some of these trials were carried out before there was an international requirement for trial registration, it is possible that some studies may have been missed. However, we were unable to construct a funnel plot to assess publication bias, as there were fewer than 10 studies in any analysis.
Agreements and disagreements with other studies or reviews
Other reviews state that antiviral therapy with oral acyclovir, its prodrug valacyclovir, or with famciclovir, is effective in treating first‐episode genital herpes. All three drugs were found to be equipotent; however, acyclovir is less well absorbed and requires a more frequent dosing schedule. There is agreement that topical antivirals have limited effectiveness (Leung 2000; Patel 2002). The only indication for the use of intravenous therapy is when the patient is unable to swallow or tolerate oral medication. None of these treatments influence subsequent recurrence. All of the studies in this review used acyclovir 200 mg five times daily for either 5, 7, or 10 days. However, current guidelines also recommend 400 mg three times a day for seven days (CDC 2015) or five days (IUSTI 2010). These guidelines recommend that treatment should be commenced within five days of the start of the episode or while new lesions are still forming.
Authors' conclusions
Implications for practice.
There is support in this review for the current recommended treatment of symptomatic first episode genital herpes with oral acyclovir. The evidence presented here is graded as low quality but this is in part due to the poor reporting of the included studies. Most of these studies are from the 1980s and at this time the brief way studies were reported does not allow us to adequately judge the quality of the included studies.
Low quality evidence did not support the use of topical acyclovir as an effective treatment for genital herpes.
We did not find sufficient evidence for many of the possible treatments of first episode herpes nor were we able to assess which was the most advantageous dosage for the treatments looked at within this review.
Implications for research.
There were no studies which looked at immunocompetent individuals or pregnant women. We would like to see research done in this area to determine the most advantageous treatment and regimen for these particularly vulnerable groups to reduce the significant morbidity and mortality associated with them. Asymptomatic disease probably leads to most cases of transmission, so, the role for antivirals in reducing transmission needs ongoing research.
History
Protocol first published: Issue 7, 2013 Review first published: Issue 8, 2016
| Date | Event | Description |
|---|---|---|
| 13 March 2009 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank the Cochrane Sexually Transmitted Infections Group (STIG) and the Cochrane Menstrual Disorders and Subfertility Group (MDSG). In particular Miguel Ortega, former Trials Search Co‐ordinator (TSC) of the Cochrane STIG; John Feliciano, TSC of the Cochrane STIG; and Marian Showell, TSC of MDSG, for constructing and running the electronic searches, Jane Marjoribanks for checking drafts of the review, and Cindy Farquhar (MDSG Co‐ordinating Editor) for support throughout and for contacting drug companies for trials. Thank you to Jonathan Ross for the supply of conference abstracts and Emma Coutts and Sarah Correa for helping in selection of studies. Thank you kindly to Thomas Allersma who assisted with data extraction and risk of bias appraisal. We also wish to thank the translators of non‐English studies, including Ray Zhang, Kensuke Takaoka, Kerstin Mueller, and Luisa Fajardo. Lastly we would like to thank Marialena Trivella and Vasileios Papastamopoulos who were involved in the protocol for this review.
Appendices
Appendix 1. Electronic search strategies
Medline (Ovid)
(02/04/2016)
1 exp Herpes Genitalis/ (4275)
2 herpe$.tw. (76899)
3 exp Herpes Simplex/ (22486)
4 HHV.tw. (4409)
5 HSV.tw. (21157)
6 exp Simplexvirus/ (28145)
7 simplexviru$.tw. (30)
8 (marmoset adj5 virus$).tw. (94)
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (86193)
10 anal.tw. (29839)
11 anogenital.tw. (3386)
12 anorectal.tw. (8969)
13 genital$.tw. (56334)
14 penile.tw. (17150)
15 penis.tw. (12429)
16 perianal.tw. (5491)
17 rectal.tw. (69420)
18 vaginal.tw. (72767)
19 venereal.tw. (5408)
20 vulva$.tw. (13904)
21 vulvovaginal.tw. (1712)
22 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (257408)
23 9 and 22 (6144)
24 exp Herpesvirus 2, Human/ (3711)
25 herpesvirus 2.tw. (413)
26 herpesvirus II.tw. (9)
27 (herpes adj5 herpe$).tw. (52909)
28 (herpes adj5 II).tw. (373)
29 HHV 2.tw. (22)
30 HHV2.tw. (5)
31 HSV 2.tw. (5319)
32 HSV2.tw. (323)
33 1 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 (55351)
34 exp Antiviral Agents/ (303715)
35 antivir$.tw. (63908)
36 anti vir$.tw. (5577)
37 viral inhibitor.tw. (100)
38 virus repressor.tw. (2)
39 virucid$.tw. (1215)
40 vir?static.tw. (313)
41 exp Acyclovir/ (12863)
42 ac?clovir.tw. (7599)
43 valac?clovir.tw. (972)
44 famciclovir.tw. (591)
45 exp Ganciclovir/ (5623)
46 ganc?clovir.tw. (6177)
47 cidof?vir.tw. (1471)
48 exp Foscarnet/ (1484)
49 foscarnet.tw. (1547)
50 exp Interferons/ (119762)
51 interferon$.tw. (127762)
52 IFN.tw. (96393)
53 imiquimod.tw. (2111)
54 resiquimod.tw. (159)
55 exp Biological Factors/ (2841008)
56 (biologic$ adj5 agent$).tw. (12274)
57 (biologic$ adj5 product$).tw. (8626)
58 (natural adj5 product$).tw. (26380)
59 natural compound$.tw. (4368)
60 Clinacanthus nutans.tw. (25)
61 exp Lysine/ (35397)
62 lysine.tw. (55146)
63 exp Ascorbic Acid/ (38528)
64 ascorb$.tw. (38553)
65 xyloascorbic acid.tw. (3)
66 (vitam$ adj5 C).tw. (21237)
67 antiscorbutic vitamin.tw. (3)
68 exp Vitamin E/ (29617)
69 vitamin E.tw. (24099)
70 alpha tocopher$.tw. (14687)
71 alphatocopher$.tw. (39)
72 exp Zinc/ (52222)
73 zinc$.tw. (90668)
74 exp Lithium/ (20694)
75 lithium.tw. (34282)
76 exp Adenosine Monophosphate/ (9325)
77 adenosine.tw. (93521)
78 adenine.tw. (37098)
79 AMP.tw. (57857)
80 adenylic acid.tw. (531)
81 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 (3475012)
82 randomized controlled trial.pt. (411144)
83 controlled clinical trial.pt. (90371)
84 randomized.ab. (340883)
85 placebo.ab. (168016)
86 clinical trials as topic.sh. (175597)
87 randomly.ab. (245501)
88 trial.ti. (148072)
89 82 or 83 or 84 or 85 or 86 or 87 or 88 (1005320)
90 exp animals/ not humans.sh. (4210486)
91 89 not 90 (925788)
92 33 and 81 and 91 (1408)
CENTRAL (Ovid platform)
(02/04/2016)
1 exp Herpes Genitalis/ (333)
2 herpe$.tw. (2144)
3 exp Herpes Simplex/ (772)
4 HHV.tw. (41)
5 HSV.tw. (439)
6 exp Simplexvirus/ (310)
7 simplexviru$.tw. (0)
8 (marmoset adj5 virus$).tw. (0)
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (2293)
10 anal.tw. (1756)
11 anogenital.tw. (110)
12 anorectal.tw. (504)
13 genital$.tw. (1804)
14 penile.tw. (749)
15 penis.tw. (213)
16 perianal.tw. (324)
17 rectal.tw. (5243)
18 vaginal.tw. (7394)
19 venereal.tw. (35)
20 vulva$.tw. (359)
21 vulvovaginal.tw. (238)
22 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 (16670)
23 9 and 22 (504)
24 exp Herpesvirus 2, Human/ (145)
25 herpesvirus 2.tw. (0)
26 herpesvirus II.tw. (0)
27 (herpes adj5 herpe$).tw. (1866)
28 (herpes adj5 II).tw. (12)
29 HHV 2.tw. (1)
30 HHV2.tw. (0)
31 HSV 2.tw. (220)
32 HSV2.tw. (12)
33 1 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 (1937)
34 exp Antiviral Agents/ (12340)
35 antivir$.tw. (2477)
36 anti vir$.tw. (169)
37 viral inhibitor.tw. (0)
38 virus repressor.tw. (0)
39 virucid$.tw. (21)
40 vir?static.tw. (20)
41 exp Acyclovir/ (943)
42 ac?clovir.tw. (942)
43 valac?clovir.tw. (259)
44 famciclovir.tw. (136)
45 exp Ganciclovir/ (284)
46 ganc?clovir.tw. (386)
47 cidof?vir.tw. (38)
48 exp Foscarnet/ (69)
49 foscarnet.tw. (88)
50 exp Interferons/ (4444)
51 interferon$.tw. (9130)
52 IFN.tw. (4533)
53 imiquimod.tw. (232)
54 resiquimod.tw. (12)
55 exp Biological Factors/ (50893)
56 (biologic$ adj5 agent$).tw. (297)
57 (biologic$ adj5 product$).tw. (99)
58 (natural adj5 product$).tw. (202)
59 natural compound$.tw. (35)
60 Clinacanthus nutans.tw. (3)
61 exp Lysine/ (334)
62 lysine.tw. (686)
63 exp Ascorbic Acid/ (1607)
64 ascorb$.tw. (1250)
65 xyloascorbic acid.tw. (0)
66 (vitam$ adj5 C).tw. (2365)
67 antiscorbutic vitamin.tw. (0)
68 exp Vitamin E/ (1983)
69 vitamin E.tw. (2454)
70 alpha tocopher$.tw. (1131)
71 alphatocopher$.tw. (12)
72 exp Zinc/ (1208)
73 zinc$.tw. (2957)
74 exp Lithium/ (626)
75 lithium.tw. (1756)
76 exp Adenosine Monophosphate/ (141)
77 adenosine.tw. (2254)
78 adenine.tw. (243)
79 AMP.tw. (650)
80 adenylic acid.tw. (0)
81 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 (77359)
82 33 and 81 (1156)
EMBASE.com
(02/04/2016)
#1. 'genital herpes'/exp
#2. herpe*:ab,ti
#3. 'herpes simplex'/exp
#4. 'herpes simplex virus'/exp
#5. 'herpes virus'/exp
#6. hhv:ab,ti
#7. hsv:ab,ti
#8. 'simplexvirus'/exp
#9. simplexviru*:ab,ti
#10. (marmoset NEAR/5 virus*):ab,ti
#11. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#12. anal:ab,ti
#13. anogenital:ab,ti
#14. anorectal:ab,ti
#15. genital*:ab,ti
#16. penile:ab,ti
#17. penis:ab,ti
#18. perianal:ab,ti
#19. rectal:ab,ti
#20. vaginal:ab,ti
#21. venereal:ab,ti
#22. vulva*:ab,ti
#23. vulvovaginal:ab,ti
#24. #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23
#25. #11 AND #24 8,221
#26. 'herpes simplex virus 2'/exp
#27. 'herpesvirus 2':ab,ti
#28. 'herpesvirus ii':ab,ti
#29. (herpes NEAR/5 2):ab,ti
#30. (herpes NEAR/5 ii):ab,ti
#31. 'hhv 2':ab,ti
#32. hhv2:ab,ti
#33. 'hsv 2':ab,ti
#34. hsv2:ab,ti
#35. #1 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34
#36. 'antivirus agent'/exp
#37. 'antiviral therapy'/exp
#38. antivir*:ab,ti
#39. (anti NEAR/5 vir*):ab,ti
#40. 'viral inhibitor':ab,ti
#41. 'virus repressor':ab,ti
#42. virucid*:ab,ti
#43. vir?static
#44. 'aciclovir'/exp
#45. ac?clovir
#46. 'valaciclovir'/exp
#47. valac?clovir
#48. 'famciclovir'/exp
#49. famciclovir:ab,ti
#50. 'ganciclovir'/exp
#51. ganc?clovir
#52. 'cidofovir'/exp
#53. cidof?vir
#54. 'foscarnet'/exp
#55. foscarnet:ab,ti
#56. 'interferon'/exp
#57. interferon*:ab,ti
#58. ifn:ab,ti
#59. 'imiquimod'/exp
#60. imiquimod:ab,ti
#61. resiquimod:ab,ti
#62. 'biological product'/exp
#63. (biologic* NEAR/5 agent*):ab,ti
#64. (biologic* NEAR/5 product*):ab,ti
#65. 'natural product'/exp
#66. (natural NEAR/5 product*):ab,ti
#67. (natural NEAR/5 compound*):ab,ti
#68. 'clinacanthus nutans':ab,ti
#69. 'lysine'/exp
#70. lysine:ab,ti
#71. 'ascorbic acid'/exp
#72. ascorb*:ab,ti
#73. 'xyloascorbic acid':ab,ti
#74. (vitam* NEAR/5 c):ab,ti
#75. 'antiscorbutic vitamin':ab,ti
#76. 'alpha tocopherol'/exp
#77. 'vitamin e':ab,ti
#78. (alpha NEAR/5 tocopher*):ab,ti
#79. alphatocopher*:ab,ti
#80. 'zinc'/exp
#81. zinc*:ab,ti
#82. 'lithium'/exp
#83. lithium:ab,ti
#84. 'adenosine phosphate'/exp
#85. adenosine:ab,ti
#86. adenine:ab,ti
#87. amp:ab,ti
#88. 'adenylic acid':ab,ti
#89. #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88
#90. 'randomized controlled trial'/exp
#91. 'single blind procedure'/exp
#92. 'double blind procedure'/exp
#93. 'crossover procedure'/exp
#94. . #90 OR #91 OR #92 OR #93
#95. random*:ab,ti
#96. placebo*:ab,ti
#97. allocat*:ab,ti
#98. crossover*:ab,ti
#99. 'cross over':ab,ti
#100.trial:ti
#101.(doubl* NEXT/1 blind*):ab,ti
#102. #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101
#103. #94 OR #102
#104.'animal'/de
#105.'animal experiment'/de
#106.'nonhuman'/de
#107. #104 OR #105 OR #106
#108.'human'/de
#109. #107 AND #108
#110. #107 NOT #109
#111. #103 NOT #110
#112. #35 AND #89 AND #111
PsycINFO and CINAHL (EBSCOHost platform)
(11/04/2015)
S1 (MH "Herpes Genitalis") 1,074
S2 TX Herpes Genital* 1,512
S3 TX herpesvirus 1,569
S5 (S1 OR S2 OR S3 OR S4) 8,923
S4 TX herpes 7,925
S6 Antiviral agent 127
S7 antiviral 16,089
S8 (MH "Antiviral Agents+") OR (MH "Antiretroviral Therapy, Highly Active") OR (MH "Acyclovir") 27,872
S9 TX imiquimod 249
S10 TX interferron 1
S11 TX interferon 8,169
S12 TX famciclovir 163
S13 TX valaciclovir 49
S14 TX ac?clovir 1,385
S15 TX anti viral 283
S16 TX virucid 0
S17 TX virucide 5
S18 S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S1736,571
S19 S5 AND S18 1,981
S20 TX first time 32,862
S21 TX initial 150,983
S22 TX first episode* 8,304
S23 TX first occurrence* 879
S24 TX primary episode* 390
S25 TX primary occurrence* 405
S26 TX index 300,549
S27 TX primary presentation 740
S28 S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 479,355
S29 S19 AND S28 125
LILACS (iAHx interface)
(02/04/2016)
(mh:(herpes genital)) OR (ti:(herpes)) OR (ab:(herpes)) AND db:("LILACS") AND type_of_study:("clinical_trials") RCTs filter: ((PT:"ensayo clinico controlado aleatorio" OR PT:"ensayo clinico controlado" OR PT:"estudio multicéntrico" OR MH:"ensayos clinicos controlados aleatorios como asunto" OR MH:"ensayos clinicos controlados como asunto" OR MH:"estudios multicéntricos como asunto" OR MH:"distribución aleatoria" OR MH:"método doble ciego" OR MH:"metodo simple‐ciego") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animales OR MH:conejos OR MH:ratones OR MH:ratas OR MH:primates OR MH:perros OR MH:gatos OR MH:porcinos OR PT:"in vitro")
Specialised Register of the Cochrane Sexually Transmitted Infections Review Group
(11/04/2016)
# 1 (herpes:AB) AND (INREGISTER) 57
# 2 (herpes:TI) AND (INREGISTER) 127
#3 #1 OR #2 132
AMED (Allied and Complementary Medicine)
(02/04/2016)
1 exp Herpes Genitalis/ (8)
2 herpes.tw. (327)
3 exp Herpes Simplex/ (86)
4 simplexviru$.tw. (0)
5 simplex viru$.tw. (110)
6 marmoset virus$.tw. (0)
7 or/2‐6 (327)
8 genital$.tw. (672)
9 venereal.tw. (12)
10 anogenital.tw. (2)
11 rectal.tw. (265)
12 anal.tw. (99)
13 anorectal.tw. (22)
14 perianal.tw. (8)
15 penile.tw. (80)
16 penis.tw. (39)
17 vaginal.tw. (200)
18 vulva$.tw. (20)
19 vulvovaginal.tw. (8)
20 or/8‐19 (1276)
21 7 and 20 (23)
22 hsv 2.tw. (31)
23 hsv2.tw. (0)
24 or/1,21‐23 (52)
25 antivir$.tw. (589)
26 ac?clovir.tw. (27)
27 valac?clovir.tw. (7)
28 famciclovir.tw. (2)
29 exp Interferons/ (32)
30 interferon$.tw. (255)
31 imiquimod.tw. (1)
32 exp Plants medicinal/ (19460)
33 exp Plant extracts/ (22462)
34 (biological adj5 agent$).tw. (65)
35 (biologic$ adj5 product$).tw. (818)
36 (natural adj5 product$).tw. (911)
37 exp Antiviral agents/ (521)
38 Plant extract$.tw. (15650)
39 or/25‐38 (27988)
40 24 and 39 (33)
Alternative Medicines Specialised Register
(26/06/2015)
herpe*
Appendix 2. Data extraction form
Data collection form
Notes on using a data extraction form:
Be consistent in the order and style you use to describe the information for each report.
Record any missing information as unclear or not described, to make it clear that the information was not found in the study report(s), not that you forgot to extract it.
Include any instructions and decision rules on the data collection form, or in an accompanying document. It is important to practice using the form and give training to any other authors using the form.
| Review title or ID |
| Interventions for men and women with their first episode of genital herpes RH124 |
| Study ID(surname of first author and year first full report of study was published e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
Notes: |
1. General Information
| Date form completed(dd/mm/yyyy) |
| Name/ID of person extracting data |
|
Report title (title of paper/ abstract/ report that data are extracted from) |
|
Report ID (ID for this paper/ abstract/ report) |
|
Reference details |
| Report author contact details |
|
Publication type (e.g. full report, abstract, letter) |
|
Study funding sources (including role of funders) |
|
Possible conflicts of interest (for study authors) |
|
Notes: |
2. Study Eligibility
| Study Characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes | No | Unclear |
Location in text (pg & ¶/fig/table) |
|
| Type of study | Published and unpublished randomised controlled trials will be included with the exception of those that are quasi‐randomised Inclusions: ‐ Drug dosing trials ‐ Suppressive therapy regimes (long‐term therapy) for first episodes Exclusions: ‐ Studies of vaccinations. ‐ Studies of complications of HSV |
|||||
|
Participants |
Men and women with their first episode of genital herpes Inclusions: ‐ Immunodeficient individuals Exclusions: ‐ Animal models |
|||||
| Types of intervention |
All to be compared with no treatment or placebo or other medication |
|||||
| Types of outcome measures | Primary outcomes 1. Duration of symptoms from onset of treatment (e.g. pain, itching) 2. Duration of lesions from onset of treatment 3. Time to first recurrence Secondary outcomes 4. Adverse events 5. Neonatal effects 6. Caesarean section delivery 7. Viral shedding |
|||||
| INCLUDE | EXCLUDE | |||||
|
Reason for exclusion |
||||||
|
Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg & ¶/fig/table) |
|||
|
Population description (from which study participants are drawn) |
||||
|
Setting (including location and social context) |
||||
| Inclusion criteria | |
|||
| Exclusion criteria | |
|||
| Method/s of recruitment of participants | ||||
| What day after onset of symptoms was intervention given | Is the day of treatment reported? | Yes No Unclear |
||
| If reported, is it ≤ 5 or > 5 days? | ≤ 5 > 5 days (more than 5) |
|||
|
Informed consent obtained |
Yes No Unclear |
|||
|
Notes: | ||||
4. Methods
|
Descriptions as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Aim of study |
|
||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, cluster/ groups or body parts) |
|||
|
Start date |
|
||
|
End date |
|
||
|
Total study duration |
|||
| Ethical approval needed/obtained for study | Yes No Unclear |
||
|
Notes: | |||
5. 'Risk of bias' assessment
See Chapter 8 of the Cochrane Handbook
| Domain |
Risk of bias |
Support for judgement |
Location in text (pg & ¶/fig/table) |
||
| Low risk | High risk | Unclear | |||
|
Random sequence generation: Was the allocation sequence adequately generated? (selection bias) |
|||||
|
Allocation concealment: Was allocation adequately concealed? (selection bias) |
|||||
|
Blinding of participants: Was knowledge of the allocated intervention adequately prevented during the study? (performance bias) |
|||||
| Blinding of personnel (performance bias) | |
||||
|
Blinding of outcome assessment (detection bias) |
|
||||
|
Incomplete outcome data: Were incomplete outcome data adequately addressed? (attrition bias) |
|||||
|
Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting? (reporting bias) |
|||||
|
Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||||
|
Total no. randomised |
|||||||
|
Clusters (if applicable, no., type, no. people per cluster) |
|||||||
| Baseline imbalances: Were the intervention and control groups comparable at baseline? | |||||||
|
Withdrawals and exclusions (if not provided below by outcome) |
|
||||||
| Age | |
|
|||||
| Sex | |||||||
| Race/ethnicity | |||||||
|
Co‐morbidities |
|
||||||
| Other treatment received(additional to study intervention) | |
||||||
|
Other relevant sociodemographics |
|
||||||
|
Subgroups measured |
|
||||||
|
Subgroups reported |
|
||||||
| Have they detailed by antibodies the type of first episodes? | Primary only Both primary and non‐primary Not stated |
||||||
| If both primary and non‐primary, have they analysed separately? | Yes No |
||||||
|
Notes: | |||||||
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention Group 1 ‐
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
||
|
No. randomised to group (specify whether no. people or clusters) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
|
Co‐interventions |
||
|
Notes: | ||
Intervention Group 2 ‐
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
||
|
No. randomised to group (specify whether no. people or clusters) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
|
Co‐interventions |
||
|
Notes: | ||
Intervention Group 3 ‐
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
||
|
No. randomised to group (specify whether no. people or clusters) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
|
Co‐interventions |
||
|
Notes: | ||
Intervention Group 4 ‐
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Group name |
||
|
No. randomised to group (specify whether no. people or clusters) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
|
Co‐interventions |
||
|
Notes: | ||
8. Outcomes & results
Outcome: Duration of symptoms from onset of treatment
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Outcome name |
||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
|
Notes: | ||
Results: Continuous outcome ‐ duration of symptoms from onset of treatment
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||||
| Results | (Insert name of group) | (Insert name of group) | ||||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||||
| (Insert name of group) | (Insert name of group) | |||||||||||
| Mean | SD (or other variance) | No. Participants | Mean | SD (or other variance) | No. participants | |||||||
| No. missing participants and reasons | |
|||||||||||
| |
||||||||||||
| No. participants moved from other group and reasons | |
|||||||||||
| |
||||||||||||
|
Any other results reported |
||||||||||||
|
Unit of analysis (individuals, cluster/groups or body parts) |
||||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||||
| Reanalysed results | |
|||||||||||
|
Notes: |
||||||||||||
Outcome: Duration of lesions from onset of treatment
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Outcome name |
||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
|
Notes: | ||
Results: Continuous outcome ‐ duration of lesions from onset of treatment
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||||
| Results | (Insert name of group) | (Insert name of group) | ||||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||||
| (Insert name of group) | (Insert name of group) | |||||||||||
| Mean | SD (or other variance) | No. Participants | Mean | SD (or other variance) | No. participants | |||||||
| No. missing participants and reasons | |
|||||||||||
| |
||||||||||||
| No. participants moved from other group and reasons | |
|||||||||||
| |
||||||||||||
|
Any other results reported |
||||||||||||
|
Unit of analysis (individuals, cluster/groups or body parts) |
||||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||||
| Reanalysed results | |
|||||||||||
|
Notes: |
||||||||||||
Outcome: Time to first recurrence
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Outcome name |
||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
|
Notes: | ||
Results: Continuous outcome – time to first recurrence
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||||||||
| Timepoint (specify whether from start or end of intervention) | ||||||||||||
| Results | Intervention | Comparison | ||||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||||
| Comparison 2 | Comparison 3 | |||||||||||
| Mean | SD (or other variance) | No. Participants | Mean | SD (or other variance) | No. participants | |||||||
| No. missing participants and reasons | |
|||||||||||
| |
||||||||||||
| No. participants moved from other group and reasons | |
|||||||||||
| |
||||||||||||
|
Any other results reported |
||||||||||||
|
Unit of analysis (individuals, cluster/groups or body parts) |
||||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||||
| Reanalysed results | ||||||||||||
|
Notes: |
||||||||||||
Outcome: Caesarean section delivery
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
|
Notes: | |||
Results: Dichotomous outcome ‐ caesarean section delivery
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||||
| Results | (Insert name of group) | (Insert name of group) | |||||
| No. events | No. participants | No. events | No. participants | ||||
| (Insert name of group) | (Insert name of group) | ||||||
| No. Events | No. Participants | No. Events | No. participants | ||||
| No. missing participants and reasons | |
|
|||||
| |
|
||||||
|
No. participants moved from other group and reasons |
|
|
|||||
| Any other results reported | |||||||
|
Unit of analysis(by individuals, cluster/groups or body parts) |
|||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | |||||||
| Reanalysis required?(specify) | Yes No Unclear |
||||||
| Reanalysis possible? | Yes No Unclear |
||||||
| Reanalysed results | |||||||
|
Notes: | |||||||
Outcome: Neonatal effects
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|
||
| Outcome definition(with diagnostic criteria if relevant) | |
||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
|
Notes: | |||
Results: Dichotomous outcome ‐ neonatal effects
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||||||
| Results | (Insert name of group) | (Insert name of group) | |||||
| No. events | No. participants | No. events | No. participants | ||||
| (Insert name of group) | (Insert name of group) | ||||||
| No. Events | No. Participants | No. Events | No. participants | ||||
| No. missing participants and reasons | |
|
|||||
| |
|
||||||
|
No. participants moved from other group and reasons |
|
|
|||||
| Any other results reported | |||||||
|
Unit of analysis(by individuals, cluster/groups or body parts) |
|||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | |||||||
| Reanalysis required?(specify) | Yes No Unclear |
||||||
| Reanalysis possible? | Yes No Unclear |
||||||
| Reanalysed results | |||||||
|
Notes: | |||||||
Outcome: Adverse events
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
||
|
Outcome name |
|
||
| Outcome definition(with diagnostic criteria if relevant) | |
||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
|
Notes: | |||
Results: Dichotomous outcome ‐ adverse events
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|||||
| Results | (Insert name of group) | (Insert name of group) | ||||
| No. events | No. participants | No. events | No. participants | |||
| (Insert name of group) | (Insert name of group) | |||||
| No. Events | No. Participants | No. Events | No. participants | |||
| No. missing participants and reasons | |
|
||||
| |
|
|||||
|
No. participants moved from other group and reasons |
|
|
||||
| Any other results reported | ||||||
|
Unit of analysis(by individuals, cluster/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
9. Other information
|
Description as stated in report/paper |
Location in text (pg & ¶/fig/table) |
|
|
Key conclusions of study authors |
|
|
|
References to other relevant studies |
|
|
| Correspondence required for further study information(from whom, what and when) | |
|
|
Notes: | ||
Data and analyses
Comparison 1. Oral acyclovir versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of symptoms from onset of treatment | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐3.22 [‐5.91, ‐0.54] |
| 1.1 Men | 2 | 33 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐4.28, 0.09] |
| 1.2 Women | 2 | 49 | Mean Difference (IV, Random, 95% CI) | ‐4.13 [‐10.15, 1.89] |
| 2 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Any adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Coryza | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Urticaria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Tiredness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Gastrointestinal discomfort | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Renal colic | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Sciatica | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Increased serum creatinine | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Decreased red blood cell count | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Duration of lesions from onset of treatment | 2 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐3.51 [‐6.19, ‐0.82] |
| 3.1 Men | 2 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐5.74 [‐9.80, ‐1.69] |
| 3.2 Women | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐1.74 [‐5.34, 1.85] |
Comparison 2. Intravenous acyclovir versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All adverse effects | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.43, 6.75] |
| 1.2 Phlebitis | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.19 [0.14, 72.69] |
| 1.3 Maculopapular eruption | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.10] |
| 1.4 Nausea | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.49, 18.59] |
| 1.5 Abnormal liver function tests | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.36, 4.97] |
Comparison 3. Topical acyclovir versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of symptoms from onset of treatment | 3 | 195 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐2.16, 0.95] |
| 1.1 All participants | 1 | 49 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐1.18, 1.98] |
| 1.2 Primary | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐2.04 [‐3.55, ‐0.52] |
| 1.3 Non‐primary | 2 | 46 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐2.79, 3.85] |
| 2 Duration of symptoms from onset of treatment by gender | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Women | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Duration of lesions from onset of treatment by antibody status | 3 | 195 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐2.15, 0.42] |
| 3.1 All participants | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐1.33, 1.73] |
| 3.2 Primary | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐4.09 [‐6.92, ‐1.25] |
| 3.3 Non‐primary | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐6.27, 2.46] |
| 4 Duration of lesions from onset of treatment by gender | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Women | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Pain with topical application | 3 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.46, 1.20] |
| 5.2 Rash | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.82] |
| 5.3 Itching | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.62 [0.11, 62.78] |
Comparison 4. Topical 2% cicloxolone cream versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom‐free by end of 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Symptom‐free by end of 7 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Lesion‐free by end of 7 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Lesion‐free by end of 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Topical carbenoxolone sodium cream versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom‐free by end of 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Symptom‐free by end of 7 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Lesion‐free by end of 7 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Lesion‐free by end of 5 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Topical tromantadine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom and lesion‐free by end of 3 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Symptom and lesion‐free by end of 6 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Symptom and lesion‐free by end of 9 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Symptom and lesion‐free by end of 12 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Oral valaciclovir versus acyclovir.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of symptoms from onset of treatment | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 1.1 Valacyclovir versus acyclovir | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Duration of symptoms from onset of treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Valaciclovir versus acyclovir | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 2 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.65] |
| 3.2 Headache | 2 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.82, 1.93] |
| 4 Duration of lesions from onset of treatment | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 4.1 Valaciclovir versus acyclovir | 1 | Hazard Ratio (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Topical carbenoxolone sodium versus topical cicloxolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptom‐free by end of 7 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Lesion‐free by end of 7 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Oral acyclovir regimen comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long versus standard course: adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Constipation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Increase in appetite | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Bells palsy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Slight white cell count decrease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Persistent bilirubin elevation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Slight transient bilirubin elevation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 HIgh versus low dose: adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Drug toxicity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Topical acyclovir versus intramuscular interferon.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of symptoms from onset of treatment by antibody status | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 All participants | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Visual disturbance | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Sweating | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Chills | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.11 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.12 Myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.13 Neutropenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Duration of lesions from onset of treatment by antibody status | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 All participants | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Subcutaneous interferon versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of symptoms from onset of treatment | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Men | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Women | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Chills | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Myalgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.10 Neutropenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Intramuscular interferon versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Transient neutropenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Transient thrombocytopenia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. High dose famciclovir versus low dose famciclovir.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants symptoms resolved at day 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Lesions not visible at day 5 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adams 1976.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of patients randomised: unknown Number of patients analysed: 49 (27 women, 22 men) Number of withdrawals/exclusions: unclear as done on the basis of episodes (of the 74 episodes of genital herpetic infection in men, 63 episodes in 55 men were evaluable according to the criteria outlined above. 3 patients were excluded because Herpesvirus hominis was not recovered from the genital lesions, and 8 were excluded because they did not return after the 3‐day visit. Of the 77 episodes in women, 17 were excluded because the woman was not using reliable contraception. 10 of these 17 women had cultures positive for Herpesvirus hominis and were followed without therapy as described above. Of the remaining 60 episodes in women, 13 were excluded because Herpesvirus hominis was not recovered, and follow‐up study was not possible in 2 other cases) Sources of funding: this research was supported by research grant no. Al‐12192 and institutional research fellowship award no. AI‐00191 from the National Institute of Allergy and Infectious Diseases, and by a grant from Parke, Davis and Company |
|
| Participants | Setting: Harborview Venereal Disease Clinic, Seattle Inclusion criteria: new lesions formed within 2 days of entry into study, women using reliable contraception (however, if not using reliable contraception, still followed with no treatment) Exclusion criteria: pregnant, herpes virus not recovered from lesions, failure to complete protocol Type of first‐episode herpes: unknown |
|
| Interventions | (1) 3% ara‐A (adenosine arabinoside) in petroleum ointment base: topical application to each lesion 4 x daily for 7 days (then after 45% of women had entered the study, 6 women were assigned to treatment with 3% ara‐A in water‐soluble gel to be applied 4 times daily to external lesions together with 5 ml to be instilled twice daily intravaginally) (2) Indistinguishable petrolatum ointment placebo: topical application to each lesion 4 x daily for 7 days (then after 45% of women had entered the study, 3 women were assigned to treatment with placebo water‐soluble gel to be applied 4 times daily to external lesions together with 5 ml to be instilled twice daily intravaginally) (3) No treatment |
|
| Outcomes | Mean total number of lesions (before treatment and at end of treatment), mean duration (days) of lesions (after treatment and total), mean duration (days) of pain (after treatment and total), mean duration (days) of viral shedding (after treatment and total), recurrence during follow‐up | |
| Possible conflicts of interest | ||
| Notes | Data is available in raw form. Application for women was changed during study from topical ointment to gel that was topically and intravaginally applied. Brief results on women with topical versus intravaginal and topical application. More information would be needed for analysis however, as paper is from 1976; we are not expecting to be able to receive this. Have emailed: no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “…to treatment with an indistinguishable placebo gel administered according to the same schedule, or to no therapy. Thus ara‐A or placebo ointment or gel were given in a double‐blind fashion” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear how many first‐episode participants randomised. Overall 15% of male and 40% of female episodes not included in analysis: unclear how many participants this involved and whether they were first‐episode or not, as study also included participants with recurrent disease |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | Potential bias due to change of intervention during study |
Altomare 1985.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of patients randomised: unknown Number of patients analysed: 21 first‐episode (40 altogether) Sex: 14 male, 7 female Age: mean age overall 37.5 +/‐ 12.3 years Number of withdrawals/exclusions: 3 people from the placebo group were considered as dropouts because they did not report to the outcome assessor Sources of funding: not reported |
|
| Participants | Setting: Milano Italy, Institute of Clinical Dermatology Inclusion criteria: patients with clinical symptoms of GH not more than 2 days (first‐episode and recurrent episode included) Exclusion criteria: patients less than 16 years old, pregnant or lactating women, people using topical corticosteroids or general cytostatics, immunosuppressants or other drugs that would interfere with the activity of the treatment. Also excluded patients who could not tolerate the treatment, patients with edematous lesions, and patients who were on a systemic treatment for other diseases Type of first‐episode herpes: unknown |
|
| Interventions | Group 1: tromantadine applied with a light massage 5 times per day for men and 2 times for women who reapplied after washing. All patients had to disinfect their hands after application Group 2: placebo Duration of treatment: 12 days In the case of intravaginal manifestations, investigators provided 3 cc of an 'ointment' applied with a special applicator 2 times a day in the first 4 days, and 1 time for the following days |
|
| Outcomes | Proportion healed at 3, 6, 9, and 12 days: this included the disappearance of objective and subjective symptoms: lesions, oedema, burning, pain, itching, 'sense of tension on lesion', dysuria; and complete re‐epithelisation and no new lesion Side effects |
|
| Possible conflicts of interest | Unknown | |
| Notes | Translated by Nancy Santoressi | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a randomised list |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The treatment and the placebo were absolutely indistinguishable in appearance, consistency, colour |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind, and notes that one doctor performed outcome assessment, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 people from the placebo group were considered as dropouts because they did not report to the outcome assessor ‐ unclear whether these people had first‐episode or recurrent disease (thus up to 15% of relevant data could be missing) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes seem to be reported but data for first‐episode only reported separately for one outcome and only reported for those who healed within 12 days |
| Other bias | Unclear risk | Insufficient details about which participants received ointment prescribed for intravaginal manifestations |
Batcheler 1986.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of patients randomised: 111 (first episode of primary infection and first episode of non‐primary) Number of patients analysed: 77 (first episode of primary infection and first episode of non‐primary), 36 (primary) (1 excluded from final analysis as patient required hospitalisation as attack was so severe) Number of withdrawals/exclusions: 34 (9 due to no follow‐up, 1 due to pregnancy, 16 due to non‐herpetic condition, 8 with uncertain diagnosis) Sources of funding: not specified |
|
| Participants | Setting: patients were informed female volunteers referred to National Women’s Hospital, Auckland, by local doctors and Family Planning Association clinics Inclusion criteria: women with diagnosis of GH Exclusion criteria: pregnancy, serious coexisting medical problems or drug allergies Age: (1) Treatment ‐ 22.4 years (mean) (first‐episode GH) (2) Placebo ‐ 24.1 years (mean) Sex: all female Ethnicity: (1) Treatment ‐ 15 Caucasion, 1 Māori (2) Placebo ‐ 18 Caucasion, 1 Māori Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Treatment: refrigerated cream containing beta‐interferon (20,000 iu/g) applied topically to lesions 5 to 6 x daily until lesion healing (2) Placebo: refrigerated placebo cream applied topically to lesions 5 to 6 x daily until lesion healing |
|
| Outcomes | Duration of symptoms before treatment, duration of pain, duration of discharge, duration of itch, duration of dysuria, time until crusting, time until healing, length of viral shedding | |
| Possible conflicts of interest | ||
| Notes | Have emailed for details regarding randomisation, allocation, blinding, and outcome assessment: no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Cream allocation was done in a random double‐blind way" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Patients were then assigned either cream containing beta‐Interferon (20,000 iu/g) or placebo cream. Cream allocation was done in a random double‐blind way." No details as to appearance of placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient had a particularly severe attack, requiring hospitalisation for a month. As she was so much worse than all other patients she was excluded from the final analysis" 35/36 (> 97%) included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Table seems to have reported all possible outcomes, but no protocol available |
| Other bias | Low risk | No other potential bias identified |
Bryson 1983.
| Methods | Randomised, placebo‐controlled, parallel trial Unit of allocation: individuals Number of patients randomised: 68 (52 with HSV‐1 infection) Number of patients analysed: 48 Number of withdrawals/exclusions: 6 (3 participants dropped out of the study, and 3 participants with vesicular and ulcerative lesions did not have initially positive cultures for HSV) Sources of funding: not specified |
|
| Participants | Setting: University Centre for Infectious Diseases clinic at the University of California in Los Angeles, United States of America Inclusion criteria: first episode of GH Exclusion criteria: prior history of vesicular or ulcerative genital lesions, lesions present for more than 6 days, pregnant or nursing women, underlying diseases, inadequate contraception, patients who had tried to obtain some form of antiviral treatment within one week before entry, initial negative cultures to HSV Age: (1) Acyclovir: 25 (mean) (2) Placebo: 25 (mean) Sex: (1) Male: 7, female: 16 (2) Male: 10, female: 15 Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Acyclovir: 200 mg acyclovir capsule taken orally for 10 days, 5 x a day (2) Placebo: placebo capsule taken orally for 10 days, 5 x a day |
|
| Outcomes | Time to healing, duration of shedding, time to crusting, duration of symptoms, severity of symptoms | |
| Possible conflicts of interest | An author is from Burroughs Wellcome Company. Dr Anthony Segretti from Burroughs Wellcome Company was used for statistical consultation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were randomised". Emailed regarding details of randomisation: no response |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "double‐blind" in title, no other mention elsewhere. Emailed regarding details of blinding: no response |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Three subjects dropped out of the study, and three subjects with vesicular and ulcerative lesions did not have initially positive cultures for herpes simplex virus. Data from these six patients are not included in the analysis.” “Of the 68 patients who were entered into the original study (40 females, 28 males)…. 63 were available for follow‐up. There were 52 subjects with HSV 2 infections”. Not stated which groups the 11 missing subjects were in, and not stated why the non‐HSV‐2 participants were excluded |
| Selective reporting (reporting bias) | High risk | As above – no explanation why HSV‐1 appears to have been excluded from follow‐up |
| Other bias | Low risk | No other potential bias identified |
Corey 1982a.
| Methods | Randomised, parallel multicentre trial Unit of allocation: individuals Number of patients randomised: 94 (patients with first‐episode GH) Number of patients analysed: 77 Number of withdrawals/exclusions: 17 (14 were excluded from the analysis because HSV was not isolated from their external genital lesions before therapy, 3 were excluded because they did not attend follow‐up visits frequently enough to permit evaluation of the effect of therapy of the duration of viral shedding or lesion healing) Sources of funding: Burroughs Wellcome Company and National Institutes of Health |
|
| Participants | Setting: University of Washington Genital Herpes Simplex Virus Clinic located at the Harborview Medical Centre, Seattle, Washington, and at the Emory University and the De‐Kalb County Health Department in Atlanta, Georgia, United Stated of America Inclusion criteria: initial or recurrent GH, presented within 6 days of onset of lesions if first‐episode, and within 48 hours if recurrent, patients in good health Exclusion criteria: HSV not isolated from their external genital lesions before therapy, pregnancy, and if receiving any form of immunosuppressive therapy Age: 27 years (mean) ‐ of participants with first‐episode GH Sex: (1) first episode primary acyclovir ‐ 8 males, 18 females first episode non‐primary acyclovir ‐ 4 males, 8 females (2) First episode of primary infection placebo ‐ 6 males, 17 females First episode of Non‐primary placebo ‐ 7 males, 9 females Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Acyclovir: 5% acyclovir in polyethylene glycol ointment for 7 days, 4 x a day (every 4 hours while awake) (2) Placebo: polyethylene glycol ointment alone for 7 days, 4 x a day (every 4 hours while awake) |
|
| Outcomes | Pain, viral shedding, time to healing, time to crusting, recurrence | |
| Possible conflicts of interest | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Separate randomisation codes were generated for each study centre, for patients with first episodes and those with recurrent episodes, and for male and female patients”. Emailed regarding details of allocation process: received response, however, was not specific enough to be considered low risk |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Packaged in identically coded 15g tubes” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “Ninety four patients with first episodes of infection were enrolled. Fourteen were excluded from analysis because HSV was not isolated from their external genital lesions before therapy and three were excluded because they did not attend follow‐up visits frequently enough to permit evaluation of the effect of therapy of the duration of viral shedding or lesion healing” |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Corey 1982b.
| Methods | Randomised, parallel trial Unit of allocation: individuals Number of patients randomised: 89 Number of patients analysed: 69 Number of withdrawals/exclusions: 13 (7 drug, 6 placebo) did not comply with the study protocol, 13 (7 drug, 6 placebo) did not comply with the study protocol Sources of funding: Burroughs Wellcome Company and National Institutes of Health |
|
| Participants | Setting: Between April 1979 and August 1980, patients referred to the University of Washington Genital Herpes Simplex Virus clinic located Harbourview Medical Center, Seattle, Washington, United States of America Inclusion criteria: episode of GH, initial or recurrent Exclusion criteria: presentation with symptoms that have been present for more than 6 days (for first‐episode) or over 48 hrs (recurrent) Age: 25.7 years (mean) ‐ of participants with first‐episode GH Sex: 64% female ‐ of participants with first‐episode GH Ethnicity: 97% Caucasian ‐ of participants with first‐episode GH Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Acyclovir: 5% acyclovir in polyethylene glycol ointment for 7 days, 4 x a day or 6 x daily if after Jan 1980 (2) Placebo: polyethylene glycol ointment for 7 days, 4 x a day or 6x daily if after Jan 1980 |
|
| Outcomes | Mean duration of time from the onset of lesions until initiation of therapy, mean number of lesions, mean lesion area, itching, pain, dysuria, vaginal discharge, viral shedding from lesions, time to crusting of lesions, duration of lesions | |
| Possible conflicts of interest | Ronald E Keeney and L Gray Davis are from Burroughs Wellcome Company | |
| Notes | Ointment was applied 6 x a day after Jan 1980 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were then randomly assigned". Emailed regarding details of allocation process: received response however was not specific enough to be considered low risk "block randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "In a double‐blind fashion". Emailed response: "placebo was identical" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT. No information on withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Corey 1983.
| Methods | Randomised, placebo‐controlled, parallel trial Unit of allocation: individuals Number of patients randomised: 32 Number of patients analysed: 31 Number of withdrawals/exclusions: 1 (HSV was never isolated during the study period, and because acute and convalescent sera showed unchanging high titres of HSV‐2 neutralising antibody, suggesting past asymptomatic HSV‐2 infection) Sources of funding: Burroughs Wellcome Company and National Institutes of Health |
|
| Participants | Setting: Harborview Medical Center Genital Herpes Clinic and at the Clinical Research Centre, University Hospital, Seattle, Washington, United States of America Inclusion criteria: no previous history of either oral‐labial or GH simplex infection, presented within 7 days of onset of symptoms, extensive genital lesions or systemic symptoms, not pregnant, not receiving any form of immunosuppressive therapy, in general good health Sex: (1) Acyclovir ‐ 1 male, 14 females (all first‐episode GH) (2) Placebo ‐ 3 males, 13 females (1) Acyclovir ‐ 1 male, 13 females (primary first‐episode GH) (2) Placebo ‐ 2 males, 11 females Age: Mean age of all patients was 26 years (nil else stated) Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Acyclovir: 5 mg/kg intravenous for 5 days (15 doses), every 8 hours over 1 hour (2) Placebo: normal saline intravenous for 5 days (15 doses), every 8 hours over 1 hour |
|
| Outcomes | Complete healing of genital lesions, subsequent recurrence rates, viral shedding, complete crusting of genital lesions, pain, itching, vaginal discharge, dysuria (women only), sore throat, constitutional symptoms, toxicity, time to first recurrence | |
| Possible conflicts of interest | People from Burroughs Wellcome Company helped develop the protocol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomly assigned”. Emailed regarding details of allocation process: received response, however, was not specific enough to be considered low risk |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Emailed for information: "IV solutions were administered by a study nurse; all the solutions were made up in a research pharmacy and the bags were identical" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One patient was excluded from all analyses because herpes simplex virus was never isolated during the study period and serology showed high titres of HSV 2 neutralising antibody suggesting past asymptomatic HSV 2 infection" |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Csonka 1984.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of patients randomised: 79 (19 with initial GH) Number of patients analysed: 11 (initial GH) Number of withdrawals/exclusions: 22 (10 participants were excluded from the analysis due to late entry or inadequate viral confirmation, one patient was excluded because of misdiagnosis, and 11 participants defaulted) Sources of funding: Dr FR House of Guy's Hospital Medical School for the statistical analyses, Mrs Ruth Parry for technical assistance, Biorex Laboratories for the supply of materials for the trial and for the in vitro study, and Dr P Thornton for his help with the preparation of the paper |
|
| Participants | Setting: attended Praed Street Clinic of St Mary’s Hospital, London Inclusion criteria: presented within 48 hours of the onset of a recurrent attack or within 5 days of an initial attack, over 16 years, no other clinically obvious genital infection, had not received antiviral treatment within the previous 14 days Exclusion criteria: late entry or inadequate viral confirmation, misdiagnosis, patient defaulting Sex: (1) Carbenoxolone: male – 3, female – 3 (initial GH before exclusions) (2) Cicloxolone: male – 4, female – 3 (3) Placebo: male – 2, female ‐ 4 Type of first‐episode herpes: not specified |
|
| Interventions | (1) Carbenoxolone: 2% carbenoxolone sodium cream applied sparingly to the lesions 5 x daily for 7 days or for the duration of the lesions and for 24 hours after healing, whichever was the shorter period (2) Cicloxolone: 2% cicloxolone sodium cream applied sparingly to the lesions 5 x daily for 7 days or for the duration of the lesions and for 24 hours after healing, whichever was the shorter period (3) Placebo: control cream of the same formula (but containing neither test medication) applied sparingly to the lesions 5 x daily for 7 days or for the duration of the lesions and for 24 hours after healing, whichever was the shorter period |
|
| Outcomes | Free of symptoms at the end of 5 days, free of symptoms at the end of treatment, free of lesions at the end of 5 days, free of lesions at the end of treatment, side effects | |
| Possible conflicts of interest | Unknown | |
| Notes | Data are in dichotomous form. Have emailed for raw data and allocation/randomisation/blinding details: no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomised” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The creams were dispensed in numbered 15 g tubes" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Ten patients were excluded from the analysis due to late entry or inadequate viral confirmation, one patient was excluded because of misdiagnosis, and 11 patients defaulted." Only 11/19 (57%) participants included in analysis and text suggests that there are other "defaulters" not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Fiddian 1983.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 107 (first‐episode) Number of participants analysed: 101 Number of withdrawals/exclusions: 6 (4 in the placebo group and 2 in the acyclovir group failed to return to the clinic for follow‐up visits) Sources of funding: not mentioned |
|
| Participants | Setting: various S.T.D. clinics of the participating centres, United Kingdom Inclusion criteria: presented within 5 days of onset of lesions if initial, and within 24 hours if recurrent, male participants and females adequately protected from pregnancy, aged 16 years or more, who had not received other specific antiviral therapy in the preceding 14 days, not having other infections that might interfere with the assessments Exclusion criteria: none stated Age: (1) Acyclovir: 25.5 years (mean) (2) Placebo: 24.5 years (mean) (participants with first‐episode GH) Sex: (1) Acyclovir: male ‐ 19, female ‐ 35 (2) Placebo: male ‐ 16, female ‐ 31 Type of first‐episode herpes: unknown |
|
| Interventions | (1) Acyclovir: 30 g tube of 5% acyclovir in an aqueous cream base containing propylene glycol applied liberally for 10 days or until healing has occurred, 5 x a day (2) Placebo: 30 g tube of aqueous cream base alone applied liberally for 10 days or until healing has occurred, 5 x a day |
|
| Outcomes | Viral shedding, pain, itching, dysuria, discharge, combined symptoms, healing time of all sites, healing time of original external lesions, adverse events | |
| Possible conflicts of interest | Author AP Fiddian is from Wellcome Research Laboratories, which may be the company producing the drug of interest | |
| Notes | Data is in median form. Emailed to try and obtain data in raw form: our message was forwarded to Wellcome and GSK and they had no information to provide It appears that subgroups of participants in this study are reported in separate publications by some of the co‐authors (see Kinghorn 1983 and Thin 1983, referenced as co‐publications of Fiddian 1983), though this is not clearly reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Male and female patients were randomly allocated separately to the treatment groups." Emailed regarding details of randomisation: no information able to be provided |
| Allocation concealment (selection bias) | Unclear risk | "Male and female patients were randomly allocated separately to the treatment groups under double‐blind conditions" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Male and female patients were randomly allocated separately to the treatment groups under double‐blind conditions." Emailed regarding details of blinding: no information able to be provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Four patients in the placebo group and two in the acyclovir group failed to return to the clinic for any follow‐up visits and so have had to be excluded from the analysis of the results" |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Fife 1997.
| Methods | Randomised, double‐blind, parallel trial Unit of allocation: individuals Number of participants randomised: 643 Number of participants analysed: 643 (114 had primary disease) Number of withdrawals/exclusions: 70 (13 valaciclovir and 11 acyclovir had antibodies to both HSV‐1 and HSV‐2 at enrolment, they may have unrecognised first episodes ‐ they were included in the analysis as non‐primary cases, exclusion of these participants did not affect the results, 25 valaciclovir and 21 acyclovir failed to take at least 80% of study medication ‐ these participants were included in the ITT and safe analysis) Sources of funding: Glaxo Wellcome Inc |
|
| Participants | Setting: Recruited from 54 sites in the United States, UK, and Australia. Most were student health clinics, STD clinics or family planning clinics Inclusion criteria: no prior history of GH, diagnosis with first‐episode genital HSV infection, > 18 years old, otherwise healthy, presented for enrolment within 72 hours of lesion onset Exclusion criteria: pregnant or nursing, HIV positive Age: (1) Acyclovir group: 23 years (median) (2) Valaciclovir group: 23 years (median) Sex: (1) Acyclovir group: female ‐ 208, male ‐ 112 (2) Valaciclovir group: female ‐ 207, male 116 Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | 1) Acyclovir group: oral 5 capsules with 200 mg acyclovir and 4 tablets of placebo daily with for 10 days, 5 x daily (200 mg) (2) Valaciclovir group: Oral 5 capsules of placebo and 4 tablets daily with 500 mg valaciclovir for 10 days, twice daily (1000 mg) |
|
| Outcomes | Duration of viral shedding, duration of pain, time to resolution of all symptoms, time to healing ‐ ITT, time to healing ‐ HSV proven | |
| Possible conflicts of interest | Some authors from the Valaciclovir International Herpes Simplex Virus Study Group are from Glaxo Wellcome Inc | |
| Notes | Says participants were assigned "drug or matching placebo" however, cannot see information on a placebo group. Emailed for more information: "All subjects received one of the active treatments. All participants received a total of five capsules and 4 tablets each day. The tablets contained either 500 mg of valaciclovir or were matching placebos, while the capsules contained 200 mg of acyclovir or were matching placebos" No totals were provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Subjects were then randomised (1:1)” |
| Allocation concealment (selection bias) | Unclear risk | Email response: “Both the patients and the clinical evaluation team were masked to treatment assignment” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Email response: "Both the patients and the clinical evaluation team were masked to treatment assignment” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both the participants and the clinical evaluation team were masked to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All 643 patients were included in the intent‐to‐treat analysis of primary efficacy endpoints and in the evaluation of safety” |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Kinghorn 1986a.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 50 Number of participants analysed: 49 Number of withdrawals/exclusions: 1 (patient defaulted before completing the protocol and was excluded from the analysis) Sources of funding: not mentioned |
|
| Participants | Setting: patients presenting to the department of genitourinary medicine, Royal Hallamshire Hospital, Sheffield, England, United Kingdom Inclusion criteria: men and women aged 16 years or more who presented within 6 days of the onset of symptoms of first‐episode GH Exclusion criteria: patients were excluded if they had used other antiviral or immune stimulation treatment within the preceding seven days, if they had underlying immune deficiency, hepatic or renal disease, or were women who were not using a valid form of contraception Age: (1) Acyclovir cream: 21.5 years (mean) (2) Placebo cream: 20.7 years (mean) Sex: (1) Acyclovir cream: male ‐ 7, female ‐ 17 (2) Placebo cream: male ‐ 7, female ‐ 18 Type of first‐episode herpes: primary and non‐primary (have not analysed separately) |
|
| Interventions | (1) Acyclovir cream: 5% acyclovir cream applied topically 5 x a day for 7 days (2) Placebo cream: matching placebo cream applied topically 5 x a day for 7 days All participants were treated with oral acyclovir 200 mg 4 x daily for 7 days |
|
| Outcomes | Mean (SD) duration of symptoms (days): itching, pain, dysuria, discharge, all symptoms; mean (SD) duration (days) of viral shedding: external lesions, urethra or cervix; mean (SD) time (days) to healing: original external lesions, all lesions; recurrence; adverse events | |
| Possible conflicts of interest | D Jones and E Hickmott are from Clinical and Applied Research Division, Wellcome Research Laboratories, Beckenham, Kent – the company that may have funded and supplied intervention | |
| Notes | Treatment within 6 days of presentation of symptoms. Have emailed the author for more information on the allocation process and blinding, and also for more time to first recurrence data: no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “...they were given topical treatment with either 5% acyclovir cream or a matching placebo cream. Patients in both treatment groups were given identical advice regarding additional symptomatic treatment.” “The treatment was dispensed in a double‐blind fashion" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Of 50 patients who entered the study one defaulted before completing the protocol and was excluded from the analysis” |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | Unclear whether data are a subset of Fiddian 1983 |
Kinghorn 1986b.
| Methods | Randomised, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 40 Number of participants analysed: 38 Number of withdrawals/exclusions: 2 (were withdrawn from analysis because they failed to comply with the protocol) Sources of funding: Wellcome Foundation |
|
| Participants | Setting: patients presenting to the department of genitourinary medicine, Sheffield, England, United Kingdom Inclusion criteria: presented within 6 days of onset of symptoms Exclusion criteria: used other antiviral or immune stimulation treatment within the preceding 7 days, underlying immune deficiency, hepatic or renal disease, inadequate contraception Age: (1) Acyclovir: 23.2 years (mean), 1.18 SEM (2) Placebo: 23.3 years (mean), 1.15 SEM (3) Co‐trimoxazole: 23.4 years (mean), 1.31 SEM (4) No co‐trimoxazole: 23.4 (mean), 1.02 SEM Sex: (1) Acyclovir: male ‐ 9, female ‐ 10 (2) Placebo: male ‐ 9, female ‐ 10 (3) Co‐trimoxazole: male ‐ 10, female ‐ 8 (4) No co‐trimoxazole: male ‐ 8, female ‐ 12 Type of first‐episode herpes: primary and non‐primary (have not analysed separately) |
|
| Interventions | (1) Acyclovir: 200 mg acyclovir tablets taken orally 5 x daily for 7 days (2) Placebo: placebo tablets identical to acyclovir tablets taken orally 5 x daily for 7 days (3) Acyclovir tablets + co‐trimoxazole tablets: 200 mg acyclovir tablets + 160 mg trimethoprim and 800 mg sulphamethoxazole taken orally (acyclovir 5 x a day, co‐trimoxazole 2 x daily) for 7 days (4) Placebo acyclovir and co‐trimoxazole tablets: placebo identical to acyclovir tablets and then co‐trimoxazole tablets taken orally (placebo identical to acyclovir tab 5 x a day and co‐trimoxazole tablet 2 x daily) for 7 days |
|
| Outcomes | Mean (SD) duration (days) of: viral shedding, pain, all symptoms; mean (SD) time to healing (days) of: original external lesions, all lesions; recurrences; adverse events | |
| Possible conflicts of interest | Al‐Hasani G, CW Potter and E Hickmott are all from Wellcome Research Labs | |
| Notes | Have emailed the author for more information on the allocation process and blinding, and also for more time to first recurrence data: no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated (with separate stratification for men and women) to one of four treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "...acyclovir tablets (Zovirax, Wellcome) 200 mg 5 times daily for seven days; placebo tablets identical to acyclovir taken five times daily for seven days; acyclovir tablets in the above dosage plus co‐trimoxazole tablets (Septrin, Wellcome; 160 mg trimethoprim and 800 mg sulphamethoxazole) twice daily for seven days; or placebo tablets identical to acyclovir." "Patients in all four treatment groups were given identical advice regarding additional symptomatic treatment" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two were withdrawn from analysis for failure to comply with study protocol" |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Lai 2000.
| Methods | Randomised parallel trial Unit of allocation: individuals Number of participants randomised: 28 Number of participants analysed: 28 Number of withdrawals/exclusions: None Source of funding: not reported |
|
| Participants | Setting: outpatient, Peking Union Medical College of Dermatology Inclusion criteria: first‐episode GH; duration no longer than 3 days; no antiviral drug usage in the past week, no syphilis or other disease that can result in genital ulcers Exclusion criteria: pregnant or breastfeeding women, abnormal renal or liver functions, immunodeficiency, immunosuppression, or failure of multiple organs, neurological or psychiatric participants; allergic or intolerant to the tested drugs Age: acyclovir group ‐ mean age 33.8 (range 23 to 54); valaciclovir group ‐ mean age 33.5 years (range 22 to 42) Sex: acyclovir group ‐ male ‐ 9, female ‐ 6 valaciclovir group ‐ male ‐ 10, female ‐ 3 Ethnicity: Chinese Type of first‐episode herpes: not stated |
|
| Interventions | (1) Acyclovir: 1 gm/day, 5 doses of 200 mg daily for 7 to 10 days (2) Valaciclovir: 600 mg day/ two doses of 300 mg daily for 7 to 10 days |
|
| Outcomes | Duration of symptoms from onset of treatment Duration of lesions from onset of treatment |
|
| Possible conflicts of interest | ||
| Notes | Translated by Ray Zhang | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “randomization according to stratification” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding (described as open) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding (described as open) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Outcome definitions vague |
| Other bias | Low risk | No other potential bias identified. Participants comparable at baseline |
Levin 1989.
| Methods | Randomised, parallel trial Unit of allocation: individuals Number of participants randomised: 131 Number of participants analysed: 105 Number of withdrawals/exclusions: 26 (only 3 participants withdrew because of adverse effects ‐ 2 in the high dose rIFN‐2A group, the remainder of the participants were excluded because they failed to complete at least 6 months of follow‐up) “Twenty‐six patients did not complete the study; only three patients withdrew because of adverse effects (two in the high‐dose rIFN‐2A group). The remainder of the patients were excluded because they failed to complete at least 6 months of follow‐up” "65 randomised in rIFN‐2A group, 12 did not complete the study with adverse effect data collected from 58 patients. 66 randomised in acyclovir group, 14 did not complete the study but adverse effect data collected from 66 patients" Sources of funding: Hoffmann‐La Roche Inc., Nutley, N.J., and the Louis and Sidell Bruckner Memorial Fund |
|
| Participants | Setting: participants were enrolled from July 1983 to January 1985 at 4 centres: Denver Disease Control Service, Denver, Colorado; The Fairfax Hospital, Falls Church, Va.; University of California at Los Angeles Herpes Research Clinic, Los Angeles; and the University of Washington Herpes Research Clinic at Harborview Medical Center, Seattle. United States of America Inclusion criteria: otherwise healthy individuals, 18 years or older, were eligible for the study if they were entered within 96 hrs of the onset of lesions characteristic of a primary or non‐primary initial episode of herpes genitalis. Participants were retained for analysis only if HSV‐1 or HSV‐2 was isolated from skin or mucus membrane lesions Exclusion criteria: participants were excluded if they had underlying medical conditions, were pregnant, used inadequate contraception, had abnormal results in pre therapy laboratory tests, had other dermatologic disease in the genital region, or were unable to complete a 1‐year follow‐up period Age: (1) Intramuscular injection of rIFN‐2A: 25.4 (mean) (2) Topical acyclovir: 26.4 years (mean) Sex: (1) Intramuscular injection of rIFN‐2A: male ‐ 17, female ‐ 36 (2) Topical acyclovir: male ‐ 20, female ‐ 32 Type of first‐episode herpes: primary and non‐primary (not analysed separately) |
|
| Interventions | (1) Intramuscular injection of rIFN‐2A + topical placebo: rIFN‐2A was 18 million IU per injection but then was changed to 9 million IU per injection as the adverse reaction rate for the first 7 participants was unacceptable, given for 9 days on day of entry (day 1), 2, 3, 4 or 5, 7 or 8 and 9. Topical placebo was applied every 4 hours while awake for 7 days (2) Intramuscular placebo + topical acyclovir: Intramuscular placebo was given for 9 days on day of entry (day 1), 2, 3, 4 or 5, 7 or 8 and 9. 5% acyclovir ointment was applied every 4 hours while awake for 7 days All participants received: acetaminophen (650 mg) orally at the time of each injection and every 4 hrs thereafter while participants were awake for 24 hrs |
|
| Outcomes | Cessation of pain, fully crusted, skin half‐healing, skin fully healed, negative culture, adverse events, mean number of recurrences: per month, first 6 months, second 6 months | |
| Possible conflicts of interest | Author Robert R Scheer is from Hoffmann‐La Roche Inc. – the company that funded the trial | |
| Notes | 7 participants in the Intramuscular injection of rIFN‐2A + topical placebo group received 18 million IU injection and these data are included in the results. Emailed the first author for more details regarding allocation, blinding and outcome assessment and he responded, confirming published information. Due to the age of the study, full details are not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were assigned from a computer‐generated list of random numbers to receive…” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blinded and placebo controlled. Email from first author reported that investigator and subject were both blinded. No further details available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% of participants (26/131) not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Mendelson 1986.
| Methods | Randomised, double‐blind parallel trial Unit of allocation: individuals Number of participants randomised: 31 Number of participants analysed: 31 (17 with initial disease, 14 with primary initial disease) Number of withdrawals/exclusions: 2 women were excluded during the maintenance phase of the study because of non‐compliance Sources of funding: the work was supported by a grant from Schering Corporation. Kenilworth, New Jersey, United States of America |
|
| Participants | Setting: we studied participants referred to the infectious diseases clinic at this hospital: Division of Infectious Diseases, Sir Mortimer B Davis Jewish General Hospital, McGill University, Montreal, Canada Inclusion criteria: first episodes of GH of less than five days' duration who had no history of vesicular or ulcerative genital lesions. Patients included had no other clinically important disease, were aged 18 or older, and were men or women who were not pregnant and had been taking oral contraceptives or using intrauterine contraceptive devices for at least 3 months before the start of the study Exclusion criteria: patients were excluded from the study if they had appreciable secondary genitourinary infections; had any cardiac, hepatic, gastrointestinal, renal, or neurological disease or clotting abnormality; had been exposed to any investigational drug within one month before the start of the study; had been exposed to any other interferon preparations within one month before the start of the study; had received any other systemic antiviral treatment within 30 days before entry to the study; had received any topical antiviral agents within seven days of entry into the study; had any immunosuppressive disease or were receiving immunosuppressive treatment; required concomitant prostaglandin synthetase inhibiting compounds; or yielded negative viral cultures for HSV at the time of enrolment Age: (1) Men: 31 years , women: 27 years (mean) (2) Men: 33 years, women: 25 years Sex: (1) Interferon: male ‐ 6, female ‐ 10 (2) Placebo: male ‐ 7, female ‐ 8 Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Interferon: subcutaneous interferon 5 X 106 IU injections twice daily for 5 days (treatment phase), then subcutaneous interferon 1 X 106 IU injections 3 times weekly for 12 weeks (2) Placebo: subcutaneous placebo injections twice daily for 5 days (treatment phase), then subcutaneous placebo injections 3 times weekly for 12 weeks |
|
| Outcomes | Mean duration of pain, mean duration of healing, mean duration of itching, mean duration of dysuria, mean duration of inguinal adenopathy, mean duration of vaginal discharge, mean duration of viral shedding, adverse events | |
| Possible conflicts of interest | Unknown | |
| Notes | Cannot find contact email | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “randomised” |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Control patients received placebo injections according to the same schedule" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Differentiation between the side effects of the interferon and the symptoms of the disease was not possible while the study was blind, but the differences became evident when the code was broken” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two women were excluded during the maintenance phase of the study because of non‐compliance. The remaining 29 patients completed their entire course of interferon treatment" |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Mertz 1984.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 180 Number of participants analysed: 150 Number of withdrawals/exclusions: 30 (10 were excluded from analysis because of failure to complete the study protocol, 12 were excluded because the antibody specificity of their acute phase serum suggested past HSV‐2 infection, and 8 were excluded because HSV was not isolated and a fourfold rise in HSV‐2 antibody did not occur) Sources of funding: National Institutes of Health grant AI‐20381, Venereal Disease Research Foundation support (for Dr Mertz), and a grant from the Burroughs‐Wellcome Co |
|
| Participants | Setting: University of Washington, Seattle; University of Vermont, Burlington; University of California‐San Diego, La Jolla; University of Alberta, Edmonton; and Sir Mortimer B. Davis‐Jewish General Hospital, Montreal. Of the 150 participants who remained for evaluation, 72 were enrolled at the University of Washington, 27 at the University of Vermont, 22 at the University of California‐San Diego, 16 at the University of Alberta, and 13 at Jewish General Hospital Inclusion criteria: patients with first episodes of GH who were seen within 6 days of the onset of lesions Exclusion criteria: pregnant women and women without adequate contraception, menopausal and postmenopausal women, participants younger than 18 and older than 50 years, participants who had used any form of antiviral or immunostimulant therapy, participants who were immunosuppressed, and participants with significant renal or hepatic disease Age: (1) Primary ‐ Oral acyclovir: 26 years (mean) (2) Primary ‐ Placebo: 25.6 years (mean) (3) Non‐primary ‐ Oral acyclovir: 27.3 years (mean) (4) Non‐primary ‐ Placebo: 27.7 years (mean) Sex: (1) Primary ‐ Oral acyclovir: male ‐ 23, female ‐ 38 (2) Primary ‐ Placebo: male ‐ 21, female ‐ 37 (3) Non‐primary ‐ Oral acyclovir: male ‐ 3, female ‐ 9 (4) Non‐primary ‐ Placebo: male ‐ 8, female ‐ 11 Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Oral acyclovir: 200 mg acyclovir in capsule taken orally 5 x daily for 10 days (2) Placebo: placebo capsule taken orally 5 x daily for 10 days |
|
| Outcomes | Duration of pain, duration of dysuria, duration of any constitutional symptoms, time to crusting, time to healing, % forming new lesions after 48 hr of therapy, viral shedding: all genital lesions, cervix, time to first recurrence, adverse events | |
| Possible conflicts of interest | Author Dr. Keeney is from Burroughs‐Wellcome which helped fund this study | |
| Notes | Have email regarding randomisation, allocation, blinding, and outcome assessment: no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “patients were randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | "double‐blind, placebo‐controlled trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "patients were randomly assigned capsules containing 200 mg of acyclovir or placebo in a coded container" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data missing for 17% (30/180) of participants |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Mindel 1982.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 30 Number of participants analysed: 30 Number of withdrawals/exclusions: not mentioned Sources of funding: unknown |
|
| Participants | Setting: the Department of Genito‐urinary medicine, Middlesex Hospital, London, United Kingdom. Inclusion criteria: within 6 days of the first appearance of genital sore Exclusion criteria: history of previous GH, age less than 16 years, renal impairment, or specific antiviral therapy in the previous 14 days. Females were excluded if they were pregnant or were not using adequate contraceptive measures (oral contraception or intrauterine device) Age: (1) Acyclovir: 22 (median), 18 to 43 (range) (2) Placebo: 21 (median), 16 to 31 (range) Sex: (1) Acyclovir: female ‐ 12, male ‐ 3 (2) Placebo: female ‐ 12, male ‐ 3 Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Acyclovir: acyclovir 5 mg/kg 45 to 60 min intravenous infusions through an indwelling intravenous cannula every 8 hours (except for the first 4 participants who had a bolus injection) for 15 doses (2) Placebo: mannitol 45 to 60 min intravenous infusions through an indwelling intravenous cannula every 8 hours (except for the first 4 participants who had a bolus injection) for 15 doses |
|
| Outcomes | Viral shedding time (all lesions), duration of new lesion formation, duration of vesicles, duration pain, duration all symptoms, healing time (all lesions), time to first recurrence, adverse events | |
| Possible conflicts of interest | A Paul Fiddian is an author of "Wellcome Research Laboratories", "We thank Mrs C A Burke, Clinical Research Division, Wellcome Research Laboratories, for statistical analysis" | |
| Notes | Data in median form. Included if presented within 6 days. Emailed asking for raw data, and details on randomisation, blinding, allocation and outcome assessment and received a response: due to the age of the studies, the raw data and details are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “We conducted a randomised, double‐blind, placebo‐controlled trial” |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “We conducted a randomised, double‐blind, placebo‐controlled trial.” “The drug and placebo were packaged in indistinguishable vials with individual code numbers, so that the trial was double‐blind” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data appear to be complete |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Mindel 1986.
| Methods | Randomised, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 60 Number of participants analysed: 60 Number of withdrawals/exclusions: 1 (all but one of the participants were followed up for at least six months; the exception was a patient receiving a short course of treatment, who was lost to follow‐up after 37 days) Sources of funding: not stated |
|
| Participants | Setting: Women participants attending the genitourinary clinic of Middlesex Hospital Medical School, London, England, United Kingdom Inclusion criteria: women participants attending the genitourinary clinic of this hospital within five days of a first attack of GH were offered the opportunity of participating in the study. We limited the study to women participants as they usually have more severe infections Exclusion criteria: a history of previous GH, age less than 16 years, renal impairment, or specific antiviral therapy in the previous 14 days. Females were excluded if they were pregnant or were not using adequate contraceptive measures (oral contraception or intrauterine device) Age: (1) Prolonged course of acyclovir: 24.3 (mean), 5.7 SD (2) Short course of acyclovir: 25.2 (mean), 7 SD Sex: All women Type of first‐episode herpes: unknown |
|
| Interventions | (1) Prolonged course of acyclovir: 200 mg acyclovir taken orally 5 x daily for 5 days, then 4 x daily for 37 days (2) Short course of acyclovir: 200 mg acyclovir taken orally 5 x daily for 5 days, then placebo 4 x daily for 37 days |
|
| Outcomes | Local symptoms, systemic symptoms, viral shedding, healing, frequency of recurrence a month, adverse events | |
| Possible conflicts of interest | IV Weller is a Wellcome Trust Senior Lecturer in Infectious Diseases | |
| Notes | Data is in median form. Emailed asking for raw data, and details on randomisation, blinding, allocation, and outcome assessment and received a response: due to the age of the studies, the raw data and details are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “We randomised patients into two treatment groups” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was a placebo group, but no further details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was a placebo group, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “One patient lost to follow‐up after 37 days” |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Mindel 1987.
| Methods | Randomised, double‐blind, parallel trial Unit of allocation: individuals Number of participants randomised: 88 Number of participants analysed: 77 Number of withdrawals/exclusions: 11 (8 who were lost to follow‐up after the initial visit, 1 who lost her tablets, 1 who proved to have varicella zoster and not HSV, and 1 who was virus‐negative with no antibody response) Sources of funding: not stated |
|
| Participants | Setting: Departments of Genitourinary Medicine at the Middlesex Hospital, London, or the Royal Hallamshire Hospital, Sheffield. United Kingdom Inclusion criteria: first attack of GH presenting within 5 days of onset Exclusion criteria: patients under 16 years; females not using adequate contraception; participants unable to attend at the required intervals, and those who had used any antiviral drugs in the preceding 2 weeks; participants with a history of gout, hyperuricaemia, or immunodepression. Since a high proportion of men attending the Middlesex Hospital clinic were homosexual, with a high attendant prevalence of HIV infection, all men from this centre were excluded Age: (1) Acyclovir: 25.5 (mean), 7.02 SD (2) Inosine pranobex: 23.3 (mean), 4.9 SD (3) Both: 24.3 (mean), 7.9 SD Sex: (1) Acyclovir: women ‐ 21, men ‐ 3 (2) Inosine pranobex: women ‐ 24, men ‐ 4 (3) Both: women ‐ 21, men ‐ 4 Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Acyclovir: 200 mg acyclovir taken orally 4 x daily for 7 days, "dummy" inosine pranobex taken orally 4 x daily for 7 days (2) Inosine pranobex: 1 g inosine pranobex taken orally 4 x daily for 7 days, "dummy" acyclovir taken orally 4 x daily for 7 days (3) Both: 200 mg acyclovir taken orally 4 x daily for 7 days, 1 g inosine pranobex taken orally 4 x daily for 7 days |
|
| Outcomes | All participants: viral shedding, dysuria, all symptoms, healing; women: viral shedding, dysuria, all symptoms, healing; adverse events | |
| Possible conflicts of interest | "We thank the Wellcome Research Laboratories in Beckenham, Kent, for help and support" | |
| Notes | Data is in median form. Emailed asking for raw data, and details on randomisation, blinding, allocation, and outcome assessment and received a response: due to the age of the studies, the raw data and details are no longer available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomly allocated” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12.5 % of data missing (11/88) |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Niimura 1996.
| Methods | Randomised parallel trial Unit of allocation: individuals Number of participants randomised: 161 (made up of first‐episode GH and kaposi's varicelliform eruption) Number of participants analysed: 85 (first‐episode GH) Number of withdrawals/exclusions: 34 (14 ‐ mild disease, 1 ‐ too severe, 5 ‐ lost after first visit, 4 ‐ patient withdrawal, 1 ‐ diagnosis changed, 9 ‐ unclear) Sources of funding: Not reported. |
|
| Participants | Setting: both outpatient and inpatient setting in 60 Hospitals all over Japan. Inclusion criteria: patients (male and female), 16 years and older, with their first episode of GH HSV infection. Included as soon as possible Exclusion criteria: Patients with renal dysfunction, patients with severe liver dysfunction, or severe cardiovascular dysfunction, patients with severe underlying medical problem (especially patients with severely weakened immune system), patients who were administered other antiviral drugs (acyclovir, vidarabine, or interferon etc), or gamma globulin preparations within two weeks prior to the trial, patients with gestation, possibility of gestation or breast feeding, patients whom a primary physician assesses unsuitable Patient characteristics: patients with first‐episode GH were not subgrouped Type of first‐episode herpes: unclear |
|
| Interventions | (1) Famciclovir 125 mg tablets orally 3 x daily for 5 days (2) Famciclovir 250 mg tablets orally 3 x daily for 5 days (3) Famciclovir 500 mg tablets orally 3 x daily for 5 days |
|
| Outcomes | Overall improvement, residual lesion ratio | |
| Possible conflicts of interest | Analyses of study were completed by SmithKline Beecham | |
| Notes | Japanese study translated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A rule for allocating interventions to participants is not specified. No explanation of the choice of 60 hospitals |
| Allocation concealment (selection bias) | High risk | No mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All incomplete outcome data was addressed in detail |
| Selective reporting (reporting bias) | Low risk | All outcomes seemed to be reported |
| Other bias | High risk | Famciclovir in this study was made in SmithKline Beecham. Data analyses in this study were done by SmithKline Beecham |
Nilsen 1982.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 116 (31 initial, 85 recurrent) Number of participants analysed: 31 (initial) Number of withdrawals/exclusions: not mentioned Sources of funding: Grant from Wellcome Foundation Ltd (Detailed in preliminary study) |
|
| Participants | Setting: Special clinics of the University Hospital, Bergen, the Municipal Health Centre, Oslo, and the Karnsjukhuset, Skovde Inclusion criteria: presented within 5 days of onset of lesions if initial, and within 48 hours if recurrent Exclusion criteria: under 16 years, pregnant women, not adequately protected against pregnancy, concomitant other infections, treatment with other antivirals, participants not giving informed consent Age: (1) Acyclovir: 26.5 years (mean) (2) Placebo: 24.8 years (mean) (participants with first‐episode GH) Sex: (1) Acyclovir: male ‐ 7, female ‐ 10 (2) Placebo: male ‐ 7, female ‐ 7 Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Acyclovir: 2 100 mg oral capsules, for 5 days, 5 x a day (2) Placebo: 2 placebo oral capsules, for 5 days, 5 x a day |
|
| Outcomes | Duration of pain, duration of all symptoms, duration of viral shedding, duration of itching, new lesion formation, assessed healing time, averaged healing time, crusting time, cessation of new lesions, adverse events | |
| Possible conflicts of interest | Dr. Fiddian is from Wellcome Research Lab | |
| Notes | Data is in median form. Emailed to try and obtain data in raw form: no response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Random allocation according to a predetermined code” |
| Allocation concealment (selection bias) | Unclear risk | “Patients were randomly allocated under double‐blind conditions” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “The trial was double‐blind and placebo‐controlled”. Emailed regarding details of blinding: no response |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “2 patients in the acyclovir group and 1 patient in the placebo group were lost to follow‐up before completion of therapy and have been excluded from the safety analysis" |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Pazin 1987.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 69 Number of participants analysed: 64 Number of withdrawals/exclusions: 5 (due to lack of virological confirmation of diagnosis) Sources of funding: this work was supported by contract AI‐02661 from the National Institute of Allergy and Infectious Diseases and grant RR‐00056 from the Division of Research Resources, National Institutes of Health |
|
| Participants | Setting: 62 of the qualified participants were enrolled in Pittsburg and the remaining two in Rochester, New York Inclusion criteria: eligible participants had lesions for less than 72 hrs; no prior history of GH; a negative pregnancy test (urine chorionic gonadotropin test and confirmatory serum assay); no major cardiac, renal, or pulmonary disease; no personal, emotional, or professional factors that could be expected to interfere with the course of treatment or follow‐up; no psychiatric or addictive disorders that could preclude informed consent; a leukocyte count of ≥5,000/mm³, a platelet count of ≥ 100,000/mm³, and a haemoglobin level of ≥ 12g /dl Exclusion criteria: lack of virological confirmation of diagnosis Age: (1) Interferon: 25.8 years (mean), 18 to 40 years (range) (2) Placebo: 24.9 years, 17‐38 years Sex: all women Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Interferon: on the day of enrolment, the patient received 2 doses of interferon (5 x 10⁴ U/kg) by intramuscular injection. On the 2nd day, 3rd, 4th, 5th, 6th, 7th, 8th, and 10th, 12th, and 14th, single doses were given. The total amount of interferon received over 14 days was 6 x 10⁵ U/kg (2) Placebo: on the day of enrolment, the patient received 2 doses of an equivalent volume of 4.5 mg of human serum albumin/ml by intramuscular injection. On the 2nd day, 3rd, 4th, 5th, 6th, 7th, 8th, and 10th, 12th, and 14th, single doses were given |
|
| Outcomes | Pain, time to healing, time to first recurrence, duration of viral shedding, frequency of recurrences, adverse effects | |
| Possible conflicts of interest | Unknown | |
| Notes | More details provided on interferon preparation. Full results not provided, and more information required on allocation and blinding ‐ have emailed: no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to receive interferon or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither the clinical personnel nor the patient knew which group she was in" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Neither the clinical personnel nor the patient knew which group she was in" ‐ probably blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64/69 (93%) participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Peacock 1988.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 105 Number of participants analysed: 82 Number of withdrawals/exclusions: 23 (7 because of misdiagnoses or protocol violations and 16 because of failure to obtain a positive pre‐therapy culture result for HSV) Sources of funding: this work was supported in part by a grant from the Burroughs Wellcome Company and in part by Training Grant AI‐07001 from the National Institute of Allergy and Infectious Diseases (University of North Carolina‐Chapel Hill) and General Clinical Research Center Grant RR‐032 (University of Alabama‐Birmingham) |
|
| Participants | Setting: participating centres included the University of North Carolina at Chapel Hill, the University of Alabama at Birmingham, New York University, the University of California, Los Angeles, and Duke University. United States of America Inclusion criteria: males and females older than 15 years of age with no prior history of genital HSV infection, a clinical diagnosis of GH with extensive genital lesions present for less than 7 days, and systemic symptoms or signs such as fever, tender lymphadenopathy, headaches, and so forth. All participants were otherwise in good general health Exclusion criteria: pregnancy or ineffective contraceptive methods in females, significant pre‐existing hepatic or renal dysfunction, and antiviral or immunomodulating therapies given within the previous 10 days or 30 days, respectively Age: (1) Acyclovir: 24.5 years (mean) (2) Placebo: 23.5 years (mean) Sex: (1) Acyclovir: male ‐ 11, female ‐ 31 (2) Placebo: male ‐ 13, female ‐ 27 Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Acyclovir: acyclovir 5 mg/kg of body weight given intravenously every 8 hours over 60 minutes for 5 days (i.e. 15 doses) (2) Placebo: normal saline given intravenously every 8 hours over 60 minutes for 5 days (i.e. 15 doses) |
|
| Outcomes | Viral shedding: all lesions, group 1 lesions; median duration of pain after onset of therapy; crusting ‐ all lesions; healing ‐ all lesions, group 1 lesions; incidence of recurrence; time to first recurrence; adverse effects of therapy | |
| Possible conflicts of interest | Gail M Knowlton and L Gray Davis are from the company which funded the research | |
| Notes | Data is in median form. Wanted to contact for details of randomisation and blinding, for the total participants followed up and also for the raw data so we can use the data in the meta‐analysis however could not find contact details. There are more participants in the adverse event groups than are analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were then randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22% participants (23/105) not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Silvestri 1982.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial Unit of allocation: individuals Number of participants randomised: 58 Number of participants analysed: 32 (primary GH) Number of withdrawals/exclusions: 26 (21 had HSV antibody present in the first serum obtained, providing serological evidence of previous HSV infection. Of the remaining 37 participants with primary infection, five were excluded from analysis because of negative culture results or inadequate follow‐up) Sources of funding: National Institutes of Health Grant AI‐14495 and program project grant AI‐12191, Public Health Service project grant SEA‐78‐06‐72, and grants from Research Industries, Salt Lake City, and the Bureau of Medical Services |
|
| Participants | Setting: patients with genital HSV infection who were referred to the University of Washington Herpes Clinics at either the Harborview Medical Center or the University of Washington Student Health Center, Seattle, were enrolled in the study Inclusion criteria: all were at least 18 years of age and otherwise in good health. Patients were enrolled only if initial lesions had been present no more than 8 days Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) Idoxuridine: 8 ml treatment vial of topical 30% weight per volume idoxuridine in 100% dimethyl sulfoxide applied topically 4 x daily with a cotton tipped applicator for 7 days (2) Dimethyl sulfoxide alone: 8 ml treatment vial of 100% dimethyl sulfoxide applied topically 4 x daily with a cotton tipped applicator for 7 days (3) Normal saline: 8 ml treatment vial of normal saline applied topically 4 x daily with a cotton tipped applicator for 7 days |
|
| Outcomes | Symptoms, mean no. of days after initiation of treatment: tender lymphadenopathy, pain, dysuria, vaginal discharge; viral shedding: mean no. of days from initiation of treatment to last positive HSV culture result, participants shedding virus during treatment, %, mean duration of viral shedding, days; mean no. of days from initiation of treatment to healing; mean no. of days from initiation of treatment to crusting; mean no. of days from onset of lesions to healing, participants in whom new lesions developed during treatment, %; time to first recurrence; adverse events | |
| Possible conflicts of interest | Unknown | |
| Notes | Have emailed for more information required on demographics and allocation: no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “patients were assigned randomly” |
| Allocation concealment (selection bias) | Low risk | “All treatment solutions were prepared and dispensed into identical, coded vials by a pharmacist and technician who were not otherwise involved in the study” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “All treatment solutions were prepared and dispensed into identical, coded vials by a pharmacist and technician who were not otherwise involved in the study” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blinded, but no further details given about outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 55% (32/58) participants analysed. Unclear why (or whether) 21 participants with non‐primary infection were randomised then excluded, as they appear to meet study inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Low risk | No other potential bias identified |
Wald 1994.
| Methods | Randomised, parallel, double‐blind, comparative trial Unit of allocation: individuals Number of participants randomised: 139 Number of participants analysed: 87 Number of withdrawals/exclusions: 52 (4 did not have culture proven herpes, 9 did not start taking the drug within 5 days, 29 had evidence of recurrence rather than first‐episode disease, 10 failed to return to a follow up appointment) Sources of funding: Burroughs Wellcome and National Institutes of Health |
|
| Participants | Setting: University of Washington Viral Disease Research Clinic at Harbour View Medical Centre, Seattle, Washington, United States of America Inclusion criteria: healthy women and men with first episode of GH, no prior antiviral therapy Exclusion criteria: did not have culture proven herpes, did not start taking the drug within 5 days, evidence of recurrent disease, pregnancy Age: (1) 22 (median), 17 to 39 (range) (2) 22 (median), 18 to 33 (range) Sex: (1) male: 21 (36%), female: 38 (64%) (2) male: 4 (14%), female: 24 (86%) Type of first‐episode herpes: primary and non‐primary |
|
| Interventions | (1) High dose acyclovir: oral 800 mg for 10 days, 5 x a day (2) Low dose acyclovir: oral 200 mg for 10 days, 5 x a day |
|
| Outcomes | Duration of symptoms from onset of treatment, duration of lesions from onset of treatment, time to first recurrence | |
| Possible conflicts of interest | Gray Davis is from the company producing the drug | |
| Notes | Emailed to try and obtain raw data so results can be used in the meta‐analysis: it is not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Subjects were randomised to one of the following regimes”. Emailed author and they responded saying they no longer have record of the process |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Subjects and investigators were blinded to the group assignment”. Emailed author and they responded saying they no longer have record of the process |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants and investigators were blinded to the group assignment: no further details about outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 82/139 (59%) of participants analysed |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | Significant differences between groups at baseline in gender balance and symptom severity |
Zavala 1988.
| Methods | Randomised, parallel, double‐blind, parallel trial Unit of allocation: individuals Number of participants randomised: 60 Number of participants analysed: 60 Number of withdrawals/exclusions: 0 Sources of funding: No information provided |
|
| Participants | Setting: outpatient clinic of Hospital Angel Leaño. Guadalajara, Jalisco Inclusion criteria: patients from both sexes aged 18 to 48 years, diagnosis of primary GH, confirmatory ELISA test Exclusion criteria: duration of disease more than 3 days, co‐morbidities (any additional disease), treatment with antivirals prior to the beginning of the study Demographic information: "No se encontraron diferencias significativas en cuanto a sexo, edad, peso, días de evolución del padecimiento, temperatura, presencia de vesículas, intensidad del dolor y malestar general antes del tratamiento." "We found no significant differences with respect to sex, age, weight, days of evolution, temperature, presence of vesicles, pain intensity and general discomfort before treatment" Type of first‐episode herpes: not specified |
|
| Interventions | (1) Ribavirin: 400 mg ribavirin every 8 hours taken orally for 10 days (2) Placebo: placebo every 8 hours taken orally for 10 days |
|
| Outcomes | Average duration of pain, number of vesicles (average), number of recurrences in 30 days after onset of treatment, adverse events | |
| Possible conflicts of interest | No information available | |
| Notes | Data was extracted by Luis Carlos Salazar Díaz. Article in Spanish. Only one person extracted data. Have not made contact for more information at this stage on the basis of language | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Se realizó un estudio prospectivo, doble ciego al azar, en 60 pacientes (…)” "We performed a prospective, randomised double‐blind study, in 60 patients (...)" The randomisation method is not stated |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment method is not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “…el tratamiento consistió en la administración oral de ribavirina (400 mg/8 horas) o placebo, durante 10 días, ambos presentados en idéntica forma farmacéutica" “... the treatment consisted of oral administration of ribavirin (400 mg/8 hours) or placebo for 10 days, both delivered in the same pharmaceutical form" It does not state how personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | “Se realizó un estudio prospectivo, doble ciego al azar, en 60 pacientes (…)” "We performed a prospective, randomised double‐blind study, in 60 patients (...)" It was a double‐blind placebo controlled trial. The blinding procedure was not explained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The report showed all the outcomes specified in the methods section of the report, as well as, all the expected outcomes for the year of the study (1988) There is not data concerning pregnant women. Protocol not available |
| Other bias | Low risk | No other potential bias identified |
ELISA: enzyme‐linked immunosorbent assay GH: genital herpes HSV: herpes simplex virus HSV‐1: herpes simplex virus type 1 HSV‐2: herpes simplex virus type 2 ITT: intention‐to‐treat IU: international units IV: intravenous rIFN‐2A: intramuscular recombinant alpha interferon SD: standard deviation SEM: standard error of the mean STD: sexually transmitted diseases
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Hasani 1986 | Did not separate out first episode and recurrent genital herpes |
| Anonymous 1985 | Review |
| Anonymous 1997 | Only reference is news article cited. Attempts to obtain more details from manufacturers (Glaxo) were unsuccessful |
| Anonymous 2004 | Not randomised |
| Armstrong 1983 | Not a RCT and not looking at first episode genital herpes |
| Ashley 1984 | Not randomised |
| Ashley 1988 | Not randomised |
| Baeten 2008 | Not first episode genital herpes |
| Balfour 1994 | An HIV trial not HSV |
| Beeson 2002 | Facial infection |
| Belec 2006 | Not first‐episode genital herpes |
| Bernstein 1984 | Not randomised |
| Bierman 1981 | Recurrent genital herpes |
| Blough 1979 | No mention of randomisation |
| Bollen 2008 | Not first episode genital herpes |
| Brocklehurst 1990 | Letter not RCT |
| Cattamanchi 2011 | HIV trial rather than first‐episode genital herpes |
| Celum 2008 | Not first‐episode genital herpes |
| Celum 2010 | Not first‐episode genital herpes |
| Chen 2000 | Unclear what proportion of participants had first‐episode infection. Repeated attempts to contact study authors unsuccessful |
| Clewell 2012 | Results not separated into initial and recurrent infection. Also studied other forms of herpes that was not analysed separately. Emailed for more information: Dynamiclear responded and said there was no division between first‐episode and recurrent genital herpes, or between genital and facial herpes |
| Conant 2002 | Recurrent genital herpes |
| Cowan 2008 | Not separated into initial and recurrent infection |
| Crespi 1988 | Not separated into initial and recurrent infection |
| Dannenmaier 1985 | Not solely genital or first‐episode |
| Delany 2009 | Not separated into initial and recurrent infection |
| Diaz‐Mitoma 1998 | Recurrent genital herpes |
| Drake 2010 | Not first‐episode genital herpes |
| Drake 2012 | Not first‐episode genital herpes |
| Emoedi 1983 | Not first‐episode genital herpes |
| Fife 2008 | Randomisation takes place following lesion healing |
| Garcia 2001 | Not randomised |
| Glezerman 1988 | Not first‐episode genital herpes |
| Goodman 1975 | Viral shedding was primary outcome, which is not relevant to our review |
| Guillaume 2002 | Not randomised |
| Gunby 1983 | Not randomised |
| Guo 2001 | Does not mention "first‐episode" herpes |
| Guo 2002 | Does not mention "first‐episode" herpes |
| Handsfield 2007 | Randomisation takes place following lesion healing |
| Harris 1995 | Review |
| Haverkos 1980 | Recurrent herpes labialis |
| Hellgren 1983 | Not first‐episode genital herpes |
| Hilton 1978 | Mostly recurrent genital herpes. Does not appear to be randomised. Emailed authors: no information able to be provided |
| Hjorth 1982 | Study of recurrent herpes labialis |
| Holzgreve 2005 | Not randomised. Not first‐episode genital herpes |
| Hu 2001 | Participants with first‐episode disease not reported separately from those with recurrence |
| Hudson 2004 | Review |
| Johnston 2011 | Emailed authors and more information was provided: was not first‐episode genital herpes |
| Johnston 2012 | Emailed authors and more information was provided: was not first‐episode genital herpes |
| Jones 1979 | Not first‐episode genital herpes |
| Kalinin 1990 | Review |
| Kaufman 1978 | Uses an antiseptic intervention |
| Koytchev 1999 | Not first‐episode genital herpes |
| Kuang 2008 | Does not mention "first‐episode" herpes |
| LeGoff 2007 | Not first‐episode genital herpes |
| Leone 2007 | Does not report on outcomes of interest |
| Levien 1995 | Review |
| Li 1998 | Insufficient information as to which participants had first‐episode genital herpes: 16 of 22 patients (73%) in the treatment group were “first‐episode“; unclear in the control group |
| Loveless 1997 | Re‐publication of three already included studies: Bryson 1983; Mertz 1984; Nilsen 1982 |
| Macotela 1984 | Looking at recurrent genital herpes |
| Mark 2007 | Study of recurrent genital herpes |
| Martens 2009 | Emailed to try to find out whether treatment was given while the patients were still experiencing their first‐episode genital herpes: no response |
| Mayaud 2009 | Emailed authors and more information was provided: was not first‐episode genital herpes |
| Meyers 1982 | Emailed Dr. Balfour asking whether the participants had first‐episode or recurrent genital herpes. He replied "None of the Minnesota subjects had genital herpes. I have no data from other centres" |
| Nagot 2007 | Not first‐episode genital herpes |
| Niimura 1987 | First‐episode genital herpes was not separated from recurrent episodes |
| Nunes 2008 | Not first‐episode genital herpes |
| Pang 2003 | Does not mention "first‐episode" herpes |
| Paz‐Bailey 2009 | Not first‐episode genital herpes. Emailed for more information: no response |
| Petersen 1993 | Results for first‐episode and recurrent episodes are not provided separately |
| Phiri 2010 | Results of first‐episode genital herpes not reported |
| Posevaia 1991 | Not looking at treatment |
| Qadripur 1976 | Does not mention first‐episode. Only 3 of 41 have genital herpes |
| Rompalo 1988 | First‐episode rectal herpes |
| Roy 1982 | Not an antiviral |
| Ruhnek‐Forsbeck 1985 | Review |
| Safrin 1991 | Not first‐episode genital herpes |
| Safrom 1995 | Not first‐episode genital herpes |
| Saltzman 1994 | Review |
| Schacker 1998 | Not first‐episode genital herpes |
| Schneider 1985 | Herpes labialis only |
| Scott 1996 | There were no data for first‐episode |
| Scott 2001 | Not randomised. No comparison group |
| Skinner 1983 | Recurrent herpes |
| Sperling 2008 | Treatment is suppression |
| Strachan 2011 | Not first‐episode genital herpes |
| Strand 2004 | Recurrent genital herpes |
| Syed 1995a | Fraud |
| Syed 1995b | Fraud |
| Syed 1995c | Fraud |
| Syed 1997a | Fraud |
| Syed 1997b | Fraud |
| Syed 1998a | Fraud |
| Syed 1998b | Fraud |
| Tardivo 2012 | Did not mention first‐episode genital herpes |
| Taylor 1975 | Not randomised |
| Twiss 2011 | Not first‐episode genital herpes |
| Vazquez 1998 | Not randomised |
| Vennemann 1985 | Not first‐episode genital herpes. Does not appear to be randomised |
| Wald 1995 | Not randomised |
| Wald 1996 | Not first‐episode genital herpes |
| Wald 2008 | Unclear whether first‐episode and not analysed separately |
| Walker 1985 | Review |
| Wenner 2005 | Not randomised |
| Wenz 1981 | Advertisement for study participation |
| Whitley 1984 | Not first‐episode genital herpes |
| Yarnell 2009 | Not randomised |
| Zu 2010 | Laboratory‐based study |
HSV: herpes simplex virus RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Grebeniuk 1981.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Waiting for full‐text translation for assessment for inclusion. In Russian |
Skerk 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Set for inclusion. Waiting for full‐text translation. In Croatian |
Differences between protocol and review
'Severity' was removed from the objectives in consultation with clinical experts, as this was determined to be not as clinically relevant and would not be reported by the primary studies as it is too difficult to measure.
In this review we have added the words "inclusive of pregnant women" to the participant inclusion criteria. This resulted from a suggestion made during the editorial process of this review in which we were asked to clarify this aspect of the inclusion criteria.
In the protocol we initially planned to subgroup by mode of delivery and dose however with further clinical input with regard to the structure of this review we subsequently decided to analyse the different modes of delivery separately and it was concluded that dose would not be substantially different in any of the studies. In addition a new subgroup was introduced, 'first episode of primary infection versus first episode of non‐primary infection.' As symptomatic first episode of primary infection herpes is usually more severe than a first episode of non primary infection in an already infected individual, it is important to ascertain that interventions are effective for these individuals experiencing a true first episode of primary infection. Please refer to Background.
The summary of findings table outcomes were not prespecified in the original protocol for this review and so these have been chosen based on perceived clinical interest.
Contributions of authors
Rachel Heslop ‐ Took the lead in writing the protocol, co‐ordinating and performing the original search and subsequent searches, performed independent data extraction and quality assessment of the included trials, entered study details and data into Review Manager 5 (RevMan 2014), primarily constructed additional tables and figures, took the lead in writing the description of studies and 'Characteristics of included studies' tables, contributed to 'Effects of interventions' and provided comments on the 'Discussion' and 'Authors' conclusions'.
Helen Roberts ‐ Wrote the first draft of the 'Background', 'Discussion' and 'Authors' conclusions'. Helped with editing of the final draft.
Deralie Flower ‐ Helped with assessing trials for inclusion, as well as data extraction of the included studies. Deralie read and commented on the text of this review.
Vanessa Jordan ‐ Participated in screening of studies and extracting data, either as a second party or as a third party reviewer. Vanessa also created the 'Summary of findings' tables and wrote and edited some of the text of this review. Vanessa took the lead in the co‐ordination of the conclusion of this project.
Sources of support
Internal sources
University of Auckland, New Zealand.
External sources
No sources of support supplied
Declarations of interest
Rachel Heslop: None known. Helen Roberts: None known. Deralie Flower: None known. Vanessa Jordan: None known.
New
References
References to studies included in this review
Adams 1976 {published data only}
- Adams HG, Benson EA, Alexander ER, Vontver LA, Remington MA, Holmes KK. Genital herpetic infection in men and women: clinical course and effect of topical application of adenine arabinoside. Journal of Infectious Diseases 1976;133(Suppl):A151‐9. [DOI] [PubMed] [Google Scholar]
- Adams HG, Benson EA, Alexander ER, Vontver LA, Remington MA, Holmes KK. Genital herpetic infection in men and women: clinical course and effect of topical application of adenine arabinoside [abstract]. British Journal of Venereal Diseases 1977;53(2):152. [DOI] [PubMed] [Google Scholar]
Altomare 1985 {published data only}
- Altomare GF, Polenghi MM, Pigatto PD, Oberhauser V. Tromantadine hydrochlorate in the treatment of genital herpes. A double blind controlled clinical trial. Giornale Italiano di Dermatologia e Venereologia 1985;120(4):XLI‐VI. [PubMed] [Google Scholar]
Batcheler 1986 {published data only}
- Batcheler LM, Bonham DG, Collins R, Liddell HS. Topical interferon cream for the treatment of herpes genitalis: a double‐blind controlled trial. Australian & New Zealand Journal of Obstetrics & Gynaecology 1986;26(3):239‐41. [DOI] [PubMed] [Google Scholar]
Bryson 1983 {published data only (unpublished sought but not used)}
- Bryson Y, Dillon M, Lovett M, Bernstein D, Garratty E, Sayre J. Treatment of first episode genital HSV with oral acyclovir: long term follow‐up of recurrences. A preliminary report. Scandinavian Journal of Infectious Diseases Supplement 1985;47:70‐5. [PubMed] [Google Scholar]
- Bryson YJ, Dillon M, Lovett M. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir: a randomised double‐blind control study trial in normal patients [abstract]. British Journal of Venereal Diseases 1983;59(5):346. [DOI] [PubMed] [Google Scholar]
- Bryson YJ, Dillon M, Lovett M, Acuna G, Taylor S, Cherry JD, et al. Successful treatment of initial genital herpes simplex virus infections with oral acyclovir [abstract]. Clinical Research 1982;30:128A. [Google Scholar]
- Bryson YJ, Dillon M, Lovett M, Acuna G, Taylor S, Cherry JD, et al. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double‐blind controlled trial in normal subjects. New England Journal of Medicine 1983;308(16):916‐21. [DOI] [PubMed] [Google Scholar]
Corey 1982a {published data only}
- Corey L, Nahmias AJ, Guinan ME, Benedetti JK, Critchlow CW, Holmes KK. A trial of topical acyclovir in genital herpes simplex virus infections. New England Journal of Medicine 1982;306(22):1313‐9. [DOI] [PubMed] [Google Scholar]
- Lafferty WE, Brewer LA, Corey L. Alteration of lymphocyte transformation response to herpes simplex virus infection by acyclovir therapy. Antimicrobial Agents and Chemotherapy 1984;26(6):887‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corey 1982b {published data only (unpublished sought but not used)}
- Corey L, Benedetti JK, Critchlow CW, Remington MR, Winter CA, Fahnlander AL. Double‐blind controlled trial of topical acyclovir in genital herpes simplex virus infections. American Journal of Medicine 1982;73(1A):326‐34. [DOI] [PubMed] [Google Scholar]
- Lafferty WE, Brewer LA, Corey L. Alteration of lymphocyte transformation response to herpes simplex virus infection by acyclovir therapy. Antimicrobial Agents and Chemotherapy 1984;26(6):887‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corey 1983 {published data only (unpublished sought but not used)}
- Corey L, Fife KH, Benedetti JK, Winter CA, Fahnlander A, Connor JD. Intravenous acyclovir for the treatment of primary genital herpes. Annals of Internal Medicine 1983;98(6):914‐21. [DOI] [PubMed] [Google Scholar]
- Corey L, Mindel A, Fife KH, Sutherland S, Benedetti J, Adler MW. Risk of recurrence after treatment of first‐episode genital herpes with intravenous acyclovir. Sexually Transmitted Diseases 1985;12(4):215‐8. [DOI] [PubMed] [Google Scholar]
- Lafferty WE, Brewer LA, Corey L. Alteration of lymphocyte transformation response to herpes simplex virus infection by acyclovir therapy. Antimicrobial Agents and Chemotherapy 1984;26(6):887‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Csonka 1984 {published data only}
- Csonka GW, Tyrrell DA. Treatment of herpes genitalis with carbenoxolone and cicloxolone creams: a double blind placebo controlled clinical trial. British Journal of Venereal Diseases 1984 Jun;60(3):178‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiddian 1983 {published data only (unpublished sought but not used)}
- Barton IG, Kinghorn GR, Rowland M, Jeavons M, Al‐Omer LS, Potter CW. Recurrences after first episodes of genital herpes in patients treated with topical acyclovir cream. Antiviral Research 1984;4(5):293‐300. [DOI] [PubMed] [Google Scholar]
- Fiddian AP, Kinghorn GR, Goldmeier D, Rees E, Rodin P, Thin RNT, et al. Topical acyclovir in the treatment of genital herpes ‐ a comparison with systemic therapy. Journal of Antimicrobial Chemotherapy 1983;12:67‐77. [DOI] [PubMed] [Google Scholar]
- Kinghorn GR, Turner EB, Barton IG, Potter CW, Burke CA, Fiddian AP. Efficacy of topical acyclovir cream in first and recurrent episodes of genital herpes. Antiviral Research 1983;3(5‐6):291‐301. [DOI] [PubMed] [Google Scholar]
- Thin RN, Nabarro JM, Parker JD, Fiddian AP. Topical acyclovir in the treatment of initial genital herpes. British Journal of Venereal Diseases 1983;59(2):116‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fife 1997 {published data only (unpublished sought but not used)}
- Fife KH, Barbarash RA, Rudolph T, Degregorio B, Roth R, The Valaciclovir International Herpes Simplex Virus Study Group. Valaciclovir versus acyclovir in the treatment of first‐episode genital herpes infection: results of an international, multicenter, double‐blind, randomized clinical trial. Sexually Transmitted Diseases 1997;24(8):481‐6. [DOI] [PubMed] [Google Scholar]
Kinghorn 1986a {published data only (unpublished sought but not used)}
- Kinghorn GR, Abeywickreme I, Jeavons M, Barton I, Potter CW, Jones D, et al. Efficacy of combined treatment with oral and topical acyclovir in first episode genital herpes. Genitourinary Medicine 1986;62(3):186‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinghorn 1986b {published data only (unpublished sought but not used)}
- Kinghorn GR, Abeywickreme I, Jeavons M, Rowland M, Barton I, Al‐Hasani G, et al. Efficacy of oral treatment with acyclovir and co‐trimoxazole in first episode genital herpes. Genitourinary Medicine 1986;62(1):33‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2000 {published data only}
- Lai WH. Valaciclovir hydrochloride versus aciclovir in the treatment of first‐episode genital herpes: a controlled, randomized open trial [Chinese]. Chinese Journal of Dermatovenereology 2000;14(1):34‐6. [Google Scholar]
Levin 1989 {published data only (unpublished sought but not used)}
- Levin MJ, Judson FN, Eron L, Bryson YJ, Corey L, Murray M, et al. Comparison of intramuscular recombinant alpha interferon (rIFN‐2A) with topical acyclovir for the treatment of first‐episode herpes genitalis and prevention of recurrences. Antimicrobial Agents and Chemotherapy 1989;33(5):649‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendelson 1986 {published data only}
- Mendelson J, Clecner B, Eiley S. Effect of recombinant interferon alpha 2 on clinical course of first episode genital herpes infection and subsequent recurrences. Genitourinary Medicine 1986;62(2):97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mertz 1984 {published data only (unpublished sought but not used)}
- Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first‐episode genital herpes. Infectious Diseases in Clinical Practice 1997;6(Suppl 1):12‐6. [Google Scholar]
- Mertz GJ. Clinical study of a new antiherpes drug: long‐term follow‐up of patients with primary genital herpes infection treated by oral acyclovir therapy: the frequency of recurrence of genital herpes. Therapeutic Research 1985;2(4):652‐4. [Google Scholar]
- Mertz GJ, Critchlow CW, Benedetti J. Double‐blind placebo‐controlled trial or oral acyclovir in first‐episode genital herpes simplex virus infection. JAMA 1984;252(9):1147‐51. [PubMed] [Google Scholar]
Mindel 1982 {published data only}
- Corey L, Mindel A, Fife KH, Sutherland S, Benedetti J, Adler MW. Risk of recurrence after treatment of first‐episode genital herpes with intravenous acyclovir. Sexually Transmitted Diseases 1985;12(4):215‐8. [DOI] [PubMed] [Google Scholar]
- Mindel A, Adler MW, Sutherland S, Fiddian AP. Intravenous acyclovir in genital herpes. An interim report. American Journal of Medicine 1982;73(1A):347‐50. [DOI] [PubMed] [Google Scholar]
- Mindel A, Adler MW, Sutherland S, Fiddian AP. Intravenous acyclovir treatment for primary genital herpes. Lancet 1982;1(8274):697‐700. [DOI] [PubMed] [Google Scholar]
Mindel 1986 {published data only (unpublished sought but not used)}
- Mindel A, Weller IV, Faherty A, Sutherland S, Fiddian AP, Adler MW. Acyclovir in first attacks of genital herpes and prevention of recurrences. Genitourinary Medicine 1986;62(1):28‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mindel 1987 {published data only (unpublished sought but not used)}
- Mindel A. Comparative studies of inosine pranobex and acyclovir. American Journal of Medicine 1988;85(2A):7‐9. [PubMed] [Google Scholar]
- Mindel A, Kinghorn G, Allason‐Jones E, Woolley P, Barton I, Faherty A, et al. Treatment of first‐attack genital herpes‐‐acyclovir versus inosine pranobex. Lancet 1987;1(8543):1171‐3. [DOI] [PubMed] [Google Scholar]
Niimura 1996 {published data only}
- Niimura M, Kobayashi T, Kumamoto Y, Kumazawa J, Kawashima M, Honda M, et al. Clinical efficacy of famciclovir on Herpes Simplex virus infection: double‐blind, dose ranging study. Rinsho Iyaku [Journal of Clinical Therapeutics and Medicines] 1996;12(16):3567‐95. [Google Scholar]
Nilsen 1982 {published data only (unpublished sought but not used)}
- Fiddian AP, Halsos AM, Kinge BR. Oral acyclovir in the treatment of genital herpes: preliminary report of a multicenter trial [abstract]. British Journal of Venereal Diseases 1983;59(2):142. [Google Scholar]
- Fiddian AP, Halsos AM, Kinge BR, Nilsen AE, Wikstrom K. Oral acyclovir in the treatment of genital herpes. Preliminary report of a multicenter trial. American Journal of Medicine 1982;73(1A):335‐7. [DOI] [PubMed] [Google Scholar]
- Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first‐episode genital herpes. Infectious Diseases in Clinical Practice 1997;6(Suppl 1):12‐6. [Google Scholar]
- Nilsen AE, Aasen T, Halsos AM, Kinge BR, Tjotta EA, Wikstrom K, et al. Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet 1982;2(8298):571‐3. [DOI] [PubMed] [Google Scholar]
Pazin 1987 {published data only}
- Pazin GJ, Harger JH, Armstrong JA, Breinig MK, Caplan RJ, Cantell K, et al. Leukocyte interferon for treating first episodes of genital herpes in women. Journal of Infectious Diseases 1987;156(6):891‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peacock 1988 {published data only (unpublished sought but not used)}
- Peacock JE. Intravenous acyclovir therapy of first episodes of genital herpes ‐ a multicenter double‐blind, placebo‐controlled trial [abstract]. Genitourinary Medicine 1989;65(2):137. [DOI] [PubMed] [Google Scholar]
- Peacock JE, Kaplowitz Jr LG, Sparling PF, Durack DT, Gnann Jr JW, Whitley RJ, et al. Intravenous acyclovir therapy of first episodes of genital herpes: a multicenter double‐blind, placebo‐controlled trial. American Journal of Medicine 1988;85(3):301‐6. [DOI] [PubMed] [Google Scholar]
Silvestri 1982 {published data only}
- Silvestri DL, Corey L, Holmes KK. Ineffectiveness of topical idoxuridine in dimethyl sulfoxide for therapy for genital herpes. JAMA 1982;248(8):953‐9. [PubMed] [Google Scholar]
Wald 1994 {published data only (unpublished sought but not used)}
- Wald A, Benedetti J, Davis G, Remington M, Winter C, Corey L. A randomized, double‐blind, comparative trial comparing high‐ and standard‐dose oral acyclovir for first‐episode genital herpes infections. Antimicrobial Agents and Chemotherapy 1994;38(2):174‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zavala 1988 {published data only}
- Zavala Trujillo I, Garibay Valencia M, Fortuno Cordova V. Treatment of genital herpes with ribavirin. Investigacion Medica Internacional 1988;15(4):202‐9. [Google Scholar]
References to studies excluded from this review
Al‐Hasani 1986 {published data only}
- Al‐Hasani AM, Barton IG, Al‐Omer LS, Kinghorn GR, Potter CW. Susceptibility of HSV strains from patients with genital herpes treated with various formulations of acyclovir. Journal of Antimicrobial Chemotherapy 1986;18(Suppl B):113‐9. [DOI] [PubMed] [Google Scholar]
Anonymous 1985 {published data only}
- Anonymous. Oral acyclovir for genital herpes simplex infection. Medical Letter on Drugs and Therapeutics 1985;27(687):41‐3. [PubMed] [Google Scholar]
Anonymous 1997 {published data only}
- Anonymous. Trial will determine how treating patient's first genital herpes outbreak with famciclovir will eliminate future outbreaks. AIDS Patient Care and STDs 1997;11(4):294‐5. [Google Scholar]
Anonymous 2004 {published data only}
- Anonymous. Antiviral drug reduces herpes transmission. Pharmaceutical Journal 2004;272(7282):44. [Google Scholar]
Armstrong 1983 {published data only}
- Armstrong JA, Skicki‐Mullen MB, Breinig MK, Ho M. Interferon susceptibility of herpes simplex virus strains isolated from patients enrolled in clinical trials. Antimicrobial Agents and Chemotherapy 1983;24(1):137‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashley 1984 {published data only}
- Ashley RL, Corey L. Effect of acyclovir treatment of primary genital herpes on the antibody response to herpes simplex virus. Journal of Clinical Investigation 1984;73(3):681‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashley 1988 {published data only}
- Ashley R, Mack K, Critchlow C, Shurtleff M, Corey L. Differential effect of systemic acyclovir treatment of genital HSV‐2 infections on antibody responses to individual HSV‐2 proteins. Journal of Medical Virology 1988;24(3):309‐19. [DOI] [PubMed] [Google Scholar]
Baeten 2008 {published data only}
- Baeten JM, Strick LB, Lucchetti A, Whittington WLH, Sanchez J, Coombs RW, et al. Herpes simplex virus (HSV)‐suppressive therapy decreases plasma and genital HIV‐1 levels in HSV‐2/HIV‐1 coinfected women: a randomized, placebo‐controlled, cross‐over trial. Journal of Infectious Diseases 2008;198(12):1804‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balfour 1994 {published data only}
- Balfour Jr HH, Benson C, Braun J, Cassens B, Erice A, Friedman‐Kien A, et al. Management of acyclovir‐resistant herpes simplex and varicella‐zoster virus infections. Journal of Acquired Immune Deficiency Syndromes 1994;7(3):254‐60. [PubMed] [Google Scholar]
Beeson 2002 {published data only}
- Beeson WH, Rachel JD. Valacyclovir prophylaxis for herpes simplex virus infection or infection recurrence following laser skin resurfacing. Dermatologic Surgery 2002;28(4):331‐6. [DOI] [PubMed] [Google Scholar]
Belec 2006 {published data only}
- Belec L, Gresenguet G, Khonde N, Weiss H, LeGoff J, Frost E, et al. Impact of herpes simplex virus (HSV)‐2 episodic therapy on HIV‐1 and HSV‐2 genital shedding and ulcer healing among women in Ghana and the Central African Republic: randomized double‐blind placebo‐controlled trial (ANRS 1212 study). International Journal of STD & AIDS 2006;17(Suppl 1):26. [Google Scholar]
Bernstein 1984 {published data only}
- Bernstein DI, Lovett MA, Bryson YJ. The effects of acyclovir on antibody response to herpes simplex virus in primary genital herpetic infections. Journal of Infectious Diseases 1984;150(1):7‐13. [DOI] [PubMed] [Google Scholar]
Bierman 1981 {published data only}
- Bierman SM, Kirkpatrick W, Fernandez H. Clinical efficacy of ribavirin in the treatment of genital herpes simplex virus infection. Chemotherapy 1981;27(2):139‐45. [DOI] [PubMed] [Google Scholar]
Blough 1979 {published data only}
- Blough HA, Giuntoli RL. Successful treatment of human genital herpes infection with 2‐deoxy‐D‐glucose. JAMA 1979;241:2798‐801. [PubMed] [Google Scholar]
Bollen 2008 {published data only}
- Bollen LJM, Whitehead SJ, Mock PA, Leelawiwat W, Asavapiriyanont S, Chalermchockchareonkit A, et al. Maternal herpes simplex virus type 2 coinfection increases the risk of perinatal HIV transmission: possibility to further decrease transmission?. AIDS 2008;22(10):1169‐76. [DOI] [PubMed] [Google Scholar]
Brocklehurst 1990 {published data only}
- Brocklehurst P, Carney O, Helson K, Kinghorn G, Mercey D, Mindel A. Acyclovir, herpes, and pregnancy. Lancet 1990;336(8730):1594‐5. [DOI] [PubMed] [Google Scholar]
Cattamanchi 2011 {published data only}
- Cattamanchi A, Saracino M, Selke S, Huang ML, Magaret A, Celum C, et al. Treatment with valacyclovir, famciclovir, or antiretrovirals reduces human herpesvirus‐8 replication in HIV‐1 seropositive men. Journal of Medical Virology 2011;83(10):1696‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celum 2008 {published data only}
- Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany‐Moretlwe S, et al. Effect of aciclovir on HIV‐1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: A randomised, double‐blind, placebo‐controlled trial. Lancet 2008;371(9630):2109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Celum 2010 {published data only}
- Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, et al. Acyclovir and transmission of HIV‐1 from persons infected with HIV‐1 and HSV‐2. New England Journal of Medicine 2010;362(5):427‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2000 {published data only}
- Chen XS, Han GZ, Guo ZP, Lu NZ, Chen J, Wang JB, et al. A comparison of topical application of penciclovir 1% cream with acyclovir 3% cream for treatment of genital herpes: a randomized, double‐blind, multicentre trial. International Journal of STD & AIDS 2000;11(9):568‐73. [DOI] [PubMed] [Google Scholar]
Clewell 2012 {published data only}
- Clewell A, Barnes M, Endres JR, Ahmed M, Ghambeer DKS. Efficacy and tolerability assessment of a topical formulation containing copper sulfate and Hypericum perforatum on patients with herpes skin lesions: A comparative, randomized controlled trial. Journal of Drugs in Dermatology 2012;11(2):209‐15. [PubMed] [Google Scholar]
Conant 2002 {published data only}
- Conant, MA, Schacker TW, Murphy RL, Gold J, Crutchfield LT, Crooks RJ, et al. Valaciclovir versus aciclovir for herpes simplex virus infection in HIV‐infected individuals: two randomized trials. International Journal of STD & AIDS 2002;13(1):12‐21. [DOI] [PubMed] [Google Scholar]
Cowan 2008 {published data only}
- Cowan FMPS, Barlow KL, Langhaug LF, Jaffar S, Hargrove JW, Robinson NJ, et al. A randomised placebo‐controlled trial to explore the effect of suppressive therapy with acyclovir on genital shedding of HIV‐1 and herpes simplex virus type 2 among Zimbabwean sex workers. Sexually Transmitted Infections 2008;84(7):548‐53. [DOI] [PubMed] [Google Scholar]
Crespi 1988 {published data only}
- Crespi H, Mora E, Pueyo S, Jaimovich L, Stringa S, Raimondo E, et al. Therapeutic use of human leukocyte interferon in dermatologic disorders caused by herpes simplex virus: Multicenter study. Medicina Cutanea Ibero‐Latino‐Americana 1988;16(6):459‐65. [PubMed] [Google Scholar]
Dannenmaier 1985 {published data only}
- Dannenmaier B, Hempel B. Chemotherapy of herpes simplex. Therapiewoche 1985;35(27):3276‐9. [Google Scholar]
Delany 2009 {published data only}
- Delany SMN, Clayton T, Akpomiemie G, Capovilla A, Legoff J, Belec L, et al. Impact of aciclovir on genital and plasma HIV‐1 RNA in HSV‐2/HIV‐1 co‐infected women: a randomized placebo‐controlled trial in South Africa. AIDS 2009;23(4):461‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz‐Mitoma 1998 {published data only}
- Diaz‐Mitoma F, Sibbald RG, Shafran SD, Boon R, Saltzman RL. Oral famciclovir for the suppression of recurrent genital herpes: a randomized controlled trial. JAMA 1998;280(10):887‐92. [DOI] [PubMed] [Google Scholar]
- Ritzmann P. Expensive long‐term care with virustatic drugs frequently reduces herpes genitalis episodes. Praxis 1999;88(24):1100. [PubMed] [Google Scholar]
Drake 2010 {unpublished data only}
- Drake AL. HSV‐2 suppression among HIV‐1 infected pregnant and postpartum Kenyan women. Unpublished thesis from University of Washington.
Drake 2012 {published data only}
- Drake AL, Roxby AC, Ongecha‐Owuor F, Kiarie J, John‐Stewart G, Wald A, et al. Valacyclovir suppressive therapy reduces plasma and breast milk HIV‐1 RNA levels during pregnancy and postpartum: a randomized trial. Journal of Infectious Diseases 2012;205(3):366‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emoedi 1983 {published data only}
- Emoedi G, Askenazy J, Suemeghy Z. Preliminary results on tolerance and toxicity tests using human fibroblast interferon (beta‐type) in patients with herpes genitalis infections. International Journal of Clinical Pharmacology Research 1983;3(4):275‐8. [PubMed] [Google Scholar]
Fife 2008 {published data only}
- Fife KH, Warren TJ, Justus SE, Heitman CK, Hs2100275 Study Team. An international, randomized, double‐blind, placebo‐controlled, study of valacyclovir for the suppression of herpes simplex virus type 2 genital herpes in newly diagnosed patients. Sexually Transmitted Diseases 2008;35(7):668‐73. [DOI] [PubMed] [Google Scholar]
Garcia 2001 {published data only (unpublished sought but not used)}
- Garcia A, Garcia S, Sanchez JA, Garcia I, Lanchares JL. Valaciclovir in the treatment of initial infection by genital herpes virus: comparative study. Enfermedades Infecciosas y Microbiologia Clinica 2001;19(1):15‐8. [DOI] [PubMed] [Google Scholar]
Glezerman 1988 {published data only}
- Glezerman M, Lunenfeld E, Cohen V, Sarov I, Movshovitz M, Doerner T, et al. Placebo‐controlled trial of topical interferon in labial and genital herpes. Lancet 1988;1(8578):150‐2. [DOI] [PubMed] [Google Scholar]
Goodman 1975 {published data only}
- Goodman EL, Luby JP, Johnson MT. Prospective double blind evaluation of topical adenine arabinoside in male herpes progenitalis. Antimicrobial Agents and Chemotherapy 1975;8(6):693‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman EL, Luby JP, Johnson MT. Prospective double‐blind evaluation of topical adenine arabinoside in male herpes progenitalis [abstract]. British Journal of Venereal Diseases 1976;52(5):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guillaume 2002 {published data only}
- Guillaume JC. Antiviral and non‐antiviral general treatments for oro‐facial and genital herpes (pregnancy and neonates excluded). Annales de Dermatologie et de Venereologie 2002;129(4):625‐34. [PubMed] [Google Scholar]
Gunby 1983 {published data only}
- Gunby P. Genital herpes research: many aim to tame maverick virus. JAMA 1983;250(18):2417‐9, 23‐4, 27. [DOI] [PubMed] [Google Scholar]
Guo 2001 {published data only}
- Guo SQ, Yang WG. Clinical observation of famciclovir synthesis therapy in treating genital herpes [Chinese]. Chinese Journal of Leprosy and Skin Disease 2001;17(2):116‐7. [Google Scholar]
Guo 2002 {published data only}
- Guo ZP, Liu HJ, Zhang YZ, Zhou GP, Jiang YJ. Clinical observation of recombinant human interferon (alpha)‐2b ointment for the treatment of genital herpes simplex and condyloma acuminatum. Journal of Clinical Dermatology 2002;31(3):169‐70. [Google Scholar]
Handsfield 2007 {published data only}
- Handsfield HH, Warren T, Werner M, Phillips JA. Suppressive therapy with valacyclovir in early genital herpes: a pilot study of clinical efficacy and herpes‐related quality of life. Sexually Transmitted Diseases 2007;34(6):339‐43. [DOI] [PubMed] [Google Scholar]
Harris 1995 {published data only (unpublished sought but not used)}
- Harris JRW. Treatment of the first episode genital herpes with famciclovir. Journal of the European Academy of Dermatology & Venereology 1995;5(Suppl 1):74. [Google Scholar]
Haverkos 1980 {published data only}
- Haverkos, HW, Pazin GJ, Armstrong JA, Ho M. Follow‐up of interferon treatment of herpes simplex. New England Journal of Medicine 1980;303(12):699‐700. [DOI] [PubMed] [Google Scholar]
Hellgren 1983 {published data only}
- Hellgren L, Hermann LS. Tromantadine hydrochloride in the treatment of herpes simplex. Results of a double‐blind therapeutic trial and an open prophylactic investigation. Dermatologica 1983;167(5):267‐72. [PubMed] [Google Scholar]
Hilton 1978 {published data only}
- Hilton AL, Bushell TE, Waller D, Bright J. A trial of adenine arabinoside in genital herpes. British Journal of Venereal Diseases 1978;54(1):50‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hjorth 1982 {published data only}
- Hjorth N, Osmundsen PE, Schmidt H, Thomsen K. Tromantadin HCl (Virumerz) in the treatment of herpes simplex. Ugeskrift for Laeger 1982;144(38):2786‐7. [PubMed] [Google Scholar]
Holzgreve 2005 {published data only}
- Holzgreve H. Honey is better than aciclovir in herpes. MMW Fortschritte der Medizin 2005;147(3):18. [PubMed] [Google Scholar]
Hu 2001 {published data only}
- Hu P, Cheng YJ, Yu KM. Clinical research on comparison of valaciclovir and aciclovir in treating genital herpes [Chinese]. Chinese Journal of Leprosy and Skin Disease 2001;17(2):107‐8. [Google Scholar]
Hudson 2004 {published data only}
- Hudson T. Propolis ointment for genital herpes. Alternative and Complementary Therapies 2004;10(5):287‐8. [Google Scholar]
Johnston 2011 {published data only}
- Johnston C, Saracino M, Kuntz S, Magaret A, Schiffer JT, Selke S, et al. Frequent breakthrough genital HSV‐2 shedding on standard and high dose valacyclovir. Sexually Transmitted Infections 2011;87:A79. [Google Scholar]
Johnston 2012 {published data only}
- Johnston C, Saracino M, Kuntz S, Magaret A, Selke S, Huang M‐l, et al. Standard‐dose and high‐dose daily antiviral therapy for short episodes of genital HSV‐2 reactivation: three randomised, open‐label, cross‐over trials. Lancet 2012;379(9816):641‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1979 {published data only}
- Jones R. Genital herpes and zinc. Medical Journal of Australia 1979;1(7):286. [DOI] [PubMed] [Google Scholar]
Kalinin 1990 {published data only}
- Kalinin Iu T, Vorob'ev AA, Bumialis VV, Denisov LA, Parfenov VV, Klenova AV, et al. The results of clinical trials and the prospects of using the Soviet preparation of human recombinant interferon alpha‐2 (reaferon) in medical practice. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 1990;9:61‐7. [PubMed] [Google Scholar]
Kaufman 1978 {published data only}
- Kaufman RH, Adam E, Mirkovic RR, Melnick JL, Young RL. Treatment of genital herpes simplex virus infection with photodynamic inactivation. American Journal of Obstetrics and Gynecology 1978;132(8):861‐9. [DOI] [PubMed] [Google Scholar]
Koytchev 1999 {published data only}
- Koytchev R, Alken RG, Dundarov S. Hypericum‐extract LI 160 for the therapy of herpes simplex genitalis and labialis: results of two placebo‐controlled, randomised, double‐blind clinical trials. Zeitschrift fur Phytotherapie 1999;20(2):92. [Google Scholar]
Kuang 2008 {published data only}
- Kuang L, Hang YG, Liu X. Curative effects of Longdan Xiegan granule on treatment of damp‐heat genital herpes. Journal of Traditional Chinese Medicine University of Hunan [Hu Nan Zhong Yi Yao Da Xue Xue Bao] 2008;28:68‐70. [Google Scholar]
LeGoff 2007 {published data only}
- LeGoff J, Weiss HA, Gresenguet G, Nzambi K, Frost E, Hayes RJ, et al. Cervicovaginal HIV‐1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS 2007;21(12):1569‐78. [DOI] [PubMed] [Google Scholar]
Leone 2007 {published data only (unpublished sought but not used)}
- Leone P, Warren T, Hamed K, Fife K, Wald A. Famciclovir reduces viral mucosal shedding in HSV‐seropositive persons. Sexually Transmitted Diseases 2007;34(11):900‐7. [DOI] [PubMed] [Google Scholar]
Levien 1995 {published data only}
- Levien TL, Baker DE. Famciclovir and ipratropium nasal spray. Hospital Pharmacy 1995;30(2):153‐4; 60‐2. [Google Scholar]
Li 1998 {published data only}
- Li WW, Zhang LR, Wu JJ. A randomised controlled trial of valaciclovir hydrochloride versus acylovir in the treatment of genital herpes [Chinese]. Journal of Clinical Dermatology 1998;27(3):175. [Google Scholar]
Loveless 1997 {published data only}
- Loveless M, Sacks SL, Harris JRW. Famciclovir in the management of first‐episode genital herpes. Infectious Diseases in Clinical Practice 1997;6(Suppl 1):12‐6. [Google Scholar]
Macotela 1984 {published data only}
- Macotela E, Suarez‐de la Torre RS, Hernandez‐Flores MF, Campos‐Macias P. Topical treatment of genital herpes simplex with a pregnene derivative. Gaceta Medica de Mexico 1984;120(2):59‐60. [PubMed] [Google Scholar]
Mark 2007 {published data only}
- Mark KE, Corey L, Meng TC, Magaret AS, Huang ML, Selke S, et al. Topical resiquimod 0.01% gel decreases herpes simplex virus type 2 genital shedding: a randomized, controlled trial. Journal of Infectious Diseases 2007;195(9):1324‐31. [DOI] [PubMed] [Google Scholar]
Martens 2009 {published data only (unpublished sought but not used)}
- Martens MG, Fife KH, Leone PA, Dix LP, Brennan CA. Once daily valacyclovir for reducing viral shedding in subjects newly diagnosed with genital herpes. Infectious Diseases in Obstetrics and Gynecology 2009;2009:105376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayaud 2009 {published data only}
- Mayaud P, Legoff J, Weiss HA, Gresenguet G, Nzambi K, Bouhlal H, et al. Impact of acyclovir on genital and plasma HIV‐1 RNA, genital herpes simplex virus type 2 DNA, and ulcer healing among HIV‐1‐infected African women with herpes ulcers: a randomized placebo‐controlled trial. Journal of Infectious Diseases 2009;200(2):216‐26. [DOI] [PubMed] [Google Scholar]
Meyers 1982 {published data only}
- Meyers JD, Wade JC, Mitchell CD, Saral R, Lietman PS, Durack DT, et al. Multicenter collaborative trial of intravenous acyclovir for treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. American Journal of Medicine 1982;73(1A):229‐35. [DOI] [PubMed] [Google Scholar]
Nagot 2007 {published data only}
- Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, et al. Reduction of HIV‐1 RNA levels with therapy to suppress herpes simplex virus. New England Journal of Medicine 2007;356(8):790‐9. [DOI] [PubMed] [Google Scholar]
Niimura 1987 {published data only}
- Niimura MOA, Fukaya T, Tagami H, Tomita Y, Honda M, Ishibashi Y, et al. Dose finding study of aciclovir tablet for Herpes Simplex virus infection. Rinsho Iyaku [Journal of Clinical Therapeutics and Medicines] 1987;3(4):337‐50. [Google Scholar]
Nunes 2008 {published data only}
- Nunes Oda S, Pereira Rde S. Regression of herpes viral infection symptoms using melatonin and SB‐73: comparison with acyclovir. Journal of Pineal Research 2008;44(4):373‐8. [DOI] [PubMed] [Google Scholar]
Pang 2003 {published data only}
- Pang CC, Jiang LX, Bai L, Cui SS. Recombinant human alpha‐2b interferon in cream for treatment of genital herpes and herpes zoster. Journal of Dalian Medical University 2003;25(3):185‐7. [Google Scholar]
Paz‐Bailey 2009 {published data only}
- Paz‐Bailey G, Sternberg M, Puren AJ, Markowitz LE, Ballard R, Delany S, et al. Improvement in healing and reduction in HIV shedding with episodic acyclovir therapy as part of syndromic management among men: a randomized, controlled trial. Journal of Infectious Diseases 2009;200(7):1039‐49. [DOI] [PubMed] [Google Scholar]
Petersen 1993 {published data only}
- Petersen CS, Weismann K, Avnstorp C, Rasmussen LP, Fogh H, Tikjob G. Topical tromantadine in the treatment of genital herpes ‐ a double‐blind placebo‐controlled study. Danish Medical Bulletin 1993;40(4):506‐7. [PubMed] [Google Scholar]
Phiri 2010 {published data only}
- Phiri S, Hoffman IF, Weiss HA, Martinson F, Nyirenda N, Kamwendo D, et al. Impact of aciclovir on ulcer healing, lesional, genital and plasma HIV‐1 RNA among patients with genital ulcer disease in Malawi. Sexually Transmitted Infections 2010;86(5):345‐52. [DOI] [PubMed] [Google Scholar]
Posevaia 1991 {published data only}
- Posevaia TA, Tsukerman VG, Shuvaeva NI, Busuek IN, Barinskii IF. The role of herpetic infection in epithelial dysplasias of the cervix uteri and a trial of their treatment with antiherpetic preparations. Voprosy Virusologii 1991;36(1):78‐80. [PubMed] [Google Scholar]
Qadripur 1976 {published data only}
- Qadripur SA. Controlled clinical trial of the herpes antigen 'lupidon' against placebo. Aktuelle Dermatologie 1976;2(3):131‐6. [Google Scholar]
Rompalo 1988 {published data only}
- Rompalo AM, Mertz GJ, Davis LG, Benedetti JB, Critchlow C, Stamm WE, et al. Oral acyclovir for treatment of first‐episode herpes simplex virus proctitis. JAMA 1988;259(19):2879‐81. [PubMed] [Google Scholar]
Roy 1982 {published data only}
- Roy RB. A double‐blind placebo‐controlled study of the use of co‐trimoxazole in the treatment of primary herpes genitalis. British Journal of Sexual Medicine 1982;9(86):22‐3; 41. [Google Scholar]
Ruhnek‐Forsbeck 1985 {published data only}
- Ruhnek‐Forsbeck M. Genital herpes: oral treatment with acyclovir. Scandinavian Journal of Infectious Diseases Supplement 1985;47:63‐6. [PubMed] [Google Scholar]
Safrin 1991 {published data only}
- Safrin S, Crumpacker C, Chatis P, Davis R, Hafner R, Rush J, et al. A controlled trial comparing foscarnet with vidarabine for acyclovir‐resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. New England Journal of Medicine 1991;325(8):551‐5. [DOI] [PubMed] [Google Scholar]
Safrom 1995 {published data only}
- Safrin S, McKinley G, McKeough M, Robinson D, Spruance SL. Treatment of acyclovir‐unresponsive cutaneous herpes simplex virus infection with topically applied SP‐303 [abstract]. Genitourinary Medicine 1995;71(2):138. [DOI] [PubMed] [Google Scholar]
Saltzman 1994 {published data only}
- Saltzman R, Jurewicz R, Boon R. Safety of famciclovir in patients with herpes‐zoster and genital herpes. Antimicrobial Agents and Chemotherapy 1994;38(10):2454‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schacker 1998 {published data only}
- Schacker T, Hu HL, Koelle DM, Zeh J, Saltzman R, Boon R, et al. Famciclovir for the suppression of symptomatic and asymptomatic herpes simplex virus reactivation in HIV‐infected persons: A double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1998;128(1):21‐8. [DOI] [PubMed] [Google Scholar]
Schneider 1985 {published data only}
- Schneider W, Schneider B. Therapeutic effects of tromantadin in different stages of herpes simplex infections. Therapiewoche 1985;35(37):4219‐25. [Google Scholar]
Scott 1996 {published data only (unpublished sought but not used)}
- Scott LL, Sanchez PJ, Jackson GL, Zeray F, Wendel Jr GD. Acyclovir suppression to prevent cesarean delivery after first‐episode genital herpes. Obstetrics and Gynecology 1996;87(1):69‐73. [DOI] [PubMed] [Google Scholar]
Scott 2001 {published data only}
- Scott LL, Hollier LM, McIntire D, Sanchez PJ, Jackson GL, Wendel Jr GD. Acyclovir suppression to prevent clinical recurrences at delivery after first episode genital herpes in pregnancy: an open‐label trial. Infectious Diseases in Obstetrics and Gynecology 2001;9(2):75‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 1983 {published data only}
- Skinner GRB. Lithium ointment for genital herpes. Lancet 1983;2(8344):288. [DOI] [PubMed] [Google Scholar]
Sperling 2008 {published data only}
- Sperling RS, Fife KH, Warren TJ, Dix LP, Brennan CA. The effect of daily valacyclovir suppression on herpes simplex virus type 2 viral shedding in HSV‐2 seropositive subjects without a history of genital herpes. Sexually Transmitted Diseases 2008;35(3):286‐90. [DOI] [PubMed] [Google Scholar]
Strachan 2011 {published data only}
- Strachan E, Saracino M, Selke S, Magaret A, Buchwald D, Wald A. The effects of daily distress and personality on genital HSV shedding and lesions in a randomized, double‐blind, placebo‐controlled, crossover trial of acyclovir in HSV‐2 seropositive women. Brain, Behavior, and Immunity 2011;25(7):1475‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strand 2004 {published data only}
- Strand A. Once daily valaciclovir reduces transmission of genital herpes. Abstract PS06. 2004 Forum for Nordic Dermato‐venereology;9(Suppl 7):16. [Google Scholar]
Syed 1995a {published data only}
- Syed TA. Management of genital herpes in males with human leukocyte interferon‐a in a hydrophilic cream: A placebo‐controlled double blind study. Journal of the European Academy of Dermatology and Venereology 1995;5(Suppl 1):170. [Google Scholar]
Syed 1995b {published data only}
- Syed TA, Lundin S, Cheema KM, Kahlon RC, Khayyami M, Ahmad SA, et al. Human leukocyte interferon‐alpha in cream for the management of genital herpes in Asian women: a placebo‐controlled, double‐blind study. Journal of Molecular Medicine 1995;73(3):141‐4. [DOI] [PubMed] [Google Scholar]
Syed 1995c {published data only}
- Syed TA, Cheema KM, Kahlon BM, Kahlon RC, Khayyami M, Kahlon AM, et al. Human leukocyte interferon‐alpha in cream for the treatment of genital herpes in Asian males: a placebo‐controlled, double‐blind study. Dermatology 1995;191(1):32‐5. [DOI] [PubMed] [Google Scholar]
Syed 1997a {published data only}
- Syed TA, Afzal M, Ashfaq Ahmad S, Holt AH, Ali Ahmad S, Ahmad SH. Management of genital herpes in men with 0.5% Aloe vera extract in a hydrophilic cream: A placebo‐controlled double‐blind study. Journal of Dermatological Treatment 1997;8(2):99‐102. [Google Scholar]
Syed 1997b {published data only}
- Syed TA, Ahmadpour OA, Ahmad SA, Ahmad SH. Human leukocyte interferon‐alpha in a hydrophilic cream versus in a gel for the treatment of genital herpes in males: a placebo‐controlled, double‐blind, comparative study. Journal of Dermatology 1997;24(9):564‐8. [DOI] [PubMed] [Google Scholar]
Syed 1998a {published data only}
- Syed TA, Ahmadpour OA, Ahmad SA, Shamsi S. Treatment of genital herpes in males with imiquimod 1% cream: a randomised, double‐blind, placebo‐controlled study. Clinical Drug Investigation 1998;16(3):187‐91. [DOI] [PubMed] [Google Scholar]
Syed 1998b {published data only}
- Syed T. Management of genital herpes in males with imiquimod analog (5%) in cream: a placebo‐controlled, double‐blind study. Abstract P472; The 7th Congress of the European Academy of Dermatology and Venereology, Nice, 7‐11 October. Journal of the European Academy of Dermatology and Venereology 1998;11(Suppl 2):296. [Google Scholar]
Tardivo 2012 {published data only}
- Tardivo JP, Wainwright M, Baptista MS. Local clinical phototreatment of herpes infection in São Paulo. Photodiagnosis and Photodynamic Therapy 2012;9(2):118‐21. [DOI] [PubMed] [Google Scholar]
Taylor 1975 {published data only}
- Taylor PK, Doherty NR. Comparison of the treatment of herpes genitalis in men with proflavine photoinactivation, idoxuridine ointment, and normal saline. British Journal of Venereal Diseases 1975;51(2):125‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Twiss 2011 {published data only}
- Twiss J, McKenna S, Bloch M, Bonney MA. Patient and clinician perceived benefit of early consumption of famciclovir for the treatment of herpes outbreaks. Value in Health 2011;14(7):A266. [Google Scholar]
Vazquez 1998 {published data only}
- Vazquez E. Gel for resistant herpes. Positively Aware: the Monthly Journal of the Test Positive Aware Network 1998;9(1):16. [PubMed] [Google Scholar]
Vennemann 1985 {published data only}
- Vennemann F, Tholen S, Wolters M. Human alpha‐leukocyte interferon. Preliminary results from a pilot study for the treatment of viral dermatoses. Fortschritte der Medizin 1985;103(42):977‐80. [PubMed] [Google Scholar]
Wald 1995 {published data only}
- Wald A. Acyclovir suppresses subclinical shedding of HSV‐2 in the genital tract. Journal of the European Academy of Dermatology and Venereology 1995;5(Suppl 1):169. [Google Scholar]
Wald 1996 {published data only}
- Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Annals of Internal Medicine 1996;124(1 Pt 1):8‐15. [DOI] [PubMed] [Google Scholar]
Wald 2008 {published data only}
- Wald A, Hughes J, Sanchez J, Reid S, Delany‐Moretlwe S, Cowan F, et al. Effect of aciclovir on HIV‐1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double‐blind, placebo‐controlled trial. Lancet 2008;371(9630):2109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1985 {published data only}
- Walker E. The current status of acyclovir treatment of first episode and recurrent genital herpes simplex infection. Przeglad Dermatologiczny 1985;72(2):214‐6. [PubMed] [Google Scholar]
Wenner 2005 {published data only}
- Wenner C, Nashelsky J. Antiviral agents for pregnant women with genital herpes. American Family Physician 2005;72(9):1807‐8. [PubMed] [Google Scholar]
Wenz 1981 {published data only}
- Wenz C. Herpes on trial. Nature 1981;290(5809):727‐8. [DOI] [PubMed] [Google Scholar]
Whitley 1984 {published data only}
- Whitley RJ, Levin M, Barton N, Hershey BJ, Davis G, Keeney RE, et al. Infections caused by herpes simplex virus in the immunocompromised host: natural history and topical acyclovir therapy. Journal of Infectious Diseases 1984;150(3):323‐9. [DOI] [PubMed] [Google Scholar]
Yarnell 2009 {published data only}
- Yarnell E, Abascal K, Rountree R. Herbs for herpes simplex infections. Alternative and Complementary Therapies 2009;15(2):69‐74. [Google Scholar]
Zu 2010 {published data only}
- Zu Y, Fu Y, Wang W, Wu N, Liu W, Kong Y, et al. Comparative study on the antiherpetic activity of aqueous and ethanolic extracts derived from Cajanus cajan (L.) Millsp. Forschende Komplementarmedizin und Klassische Naturheilkunde 2010;17(1):15‐20. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Grebeniuk 1981 {published data only}
- Grebeniuk VN, Marchenko VI, Parfenov VV, Pozhidaev II, Zubanova NA. Trial of the safety, reactivity and therapeutic effectiveness of an ointment containing swine leukocytic interferon. Antibiotiki 1981;26(3):145‐9. [PubMed] [Google Scholar]
Skerk 2004 {published data only}
- Skerk V, Barsic B, Begovac J, Mahovlic V, Dostal M, Stojkovic A, et al. Randomized, open, comparative, multicentric, pilot study on the efficacy and tolerability of peroral valacyclovir and acyclovir in patients with genital herpes and varicella zoster virus infections. Infektoloski Glasnik 2004;24(4):189‐92. [Google Scholar]
Additional references
Azwa 2009
- Azwa A, Barton SE. Aspects of herpes simplex virus: a clinical review. Journal of Family Planning and Reproductive Health Care 2009;35(4):237‐42. [DOI] [PubMed] [Google Scholar]
Brown 2002
- Brown TJ, McCrary M, Tyring SK. Antiviral agents: Non‐antiretroviral [correction of Nonantiviral] drugs. Journal of the American Academy of Dermatology 2002;47(4):581‐99. [DOI] [PubMed] [Google Scholar]
Brown 2003
- Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003;289(2):203‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Brown 2005
- Brown ZA, Gardella C, Wald A, Morrow RA, Corey L. Genital herpes complicating pregnancy. Obstetrics and Gynecology 2005;106(4):845‐56. [PUBMED: 16199646] [DOI] [PubMed] [Google Scholar]
CDC 2015
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guideline. www.cdc.gov/std/tg2015/.
Cernik 2008
- Cernik C, Gallina K, Brodell RT. The treatment of Herpes Simplex infections: an evidence‐based review. Annals of Internal Medicine 2008;168:1137‐44. [DOI] [PubMed] [Google Scholar]
Chiu 2011
- Chiu HY, Tsai TF. Topical use of systemic drugs in dermatology: a comprehensive review. Journal of the American Academy of Dermatology 2011;65(5):1048. [DOI] [PubMed] [Google Scholar]
Corey 1983b
- Corey L, Adams HG, Brown ZA, Holmes KK. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Annals of Internal Medicine 1983;98(6):958‐72. [DOI] [PubMed] [Google Scholar]
Freeman 2006
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta‐analysis of longitudinal studies. AIDS 2006;20(1):73‐83. [DOI] [PubMed] [Google Scholar]
Gaby 2006
- Gaby AR. Natural remedies for Herpes simplex. Alternative Medicine Review 2006;11(2):93‐101. [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Gupta 2002
- Gupta AK, Browne M, Bluhm R. Imiquimod: a review. Journal of Cutaneous Medicine and Surgery 2002;6(6):554‐60. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Available from www.cochrane‐handbook.org..
Huang 2012
- Huang W, Hu K, Luo S, Zhang M, Li C, Jin W, et al. Herpes simplex virus type 2 infection of human epithelial cells induces CXCL9 expression and CD4+ T cell migration via activation of p38‐CCAAT/enhancer‐binding protein‐beta pathway. Journal of Immunology 2012;188(12):6247‐57. [DOI] [PubMed] [Google Scholar]
IUSTI 2010
- IUSTI/WHO European STD guidelines Editorial Board. 2010 European guideline for the management of genital herpes. www.iusti.org/regions/europe/pdf/2010/Euro_Guideline_2010_herpes.pdf.
Jerath 2009
- Jerath VP, Mahajan VK. Does circumcision influence recurrences in herpes genitalis?. Indian Journal of Dermatology, Venereology and Leprology 2009;75(6):575‐8. [PUBMED: 19915236] [DOI] [PubMed] [Google Scholar]
Katze 2002
- Katze MG, He Y, Gale M Jr. Viruses and interferon: a fight for supremacy. Nature Reviews. Immunology 2002;2(9):675‐87. [DOI] [PubMed] [Google Scholar]
Kongkaew 2011
- Kongkaew C, Chaiyakunapruk N. Efficacy of Clinacanthus nutans extracts in patients with herpes infection: systematic review and meta‐analysis of randomised clinical trials. Complementary Therapies in Medicine 2011;19(1):47‐53. [DOI] [PubMed] [Google Scholar]
Le Cleach 2014
- Cleach L, Trinquart L, Do G, Maruani A, Lebrun‐Vignes B, Ravaud P, et al. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD009036.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2000
Malkin 2004
- Malkin JE. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes 2004;11(Suppl 1):2e23A. [PubMed] [Google Scholar]
Martin 2009
- Martin ET, Krantz E, Gottlieb SL, Magaret AS, Langenberg A, Stanberry L, et al. A pooled analysis of the effect of condoms in preventing HSV‐2 acquisition. Archives of Internal Medicine 2009;169(13):1233‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Patel 2002
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stanley 2002
- Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clinical and Experimental Dermatology 2002;27(7):571‐7. [DOI] [PubMed] [Google Scholar]
Tobian 2009
- Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O. Male circumcision for the prevention of HSV‐2 and HPV infections and syphilis. New England Journal of Medicine 2009;360(13):1298‐309. [PUBMED: 19321868] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tobian 2012
- Tobian AA, Kigozi G, Redd AD, Serwadda D, Kong X, Oliver A. Male circumcision and herpes simplex virus type 2 infection in female partners: a randomized trial in Rakai, Uganda. Journal of Infectious Diseases 2012;205(3):486‐90. [PUBMED: 22147796] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vajpayee 2000
- Vajpayee M, Malhotra N. Antiviral drugs against herpes infections. Indian Journal of Pharmacology 2000;32:330‐8. [Google Scholar]
Weiss 2004
- Weiss H. Epidemiology of herpes simplex virus type 2 infection in developing countries. Herpes 2004;11(Suppl 1):24e35A. [PubMed] [Google Scholar]
Whitley 1998
- Whitley RJ, Kimberlin DW, Roizman B. Herpes Simplex viruses. Clinical Infectious Diseases 1998;26(3):541‐55. [DOI] [PubMed] [Google Scholar]
Workowski 2010
- Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. Morbidity and Mortality Weekly Report 2010;59(RR‐12):1‐116. [PubMed] [Google Scholar]
Zhong 2012
- Zhong M‐G, Xiang Y‐F, Qiu X‐X, Liu Z, Kitazato K, Wang Y‐F. Natural products as a source of anti‐herpes simplex virus agents. RSC Advances 2012 Oct 31 [Epub ahead of print]. [DOI: 10.1039/C2RA21464D] [DOI]
References to other published versions of this review
Heslop 2013
- Heslop R, Jordan V, Trivella M, Papastamopoulos V, Roberts H. Interventions for men and women with their first episode of genital herpes. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010684] [DOI] [PMC free article] [PubMed] [Google Scholar]


