Abstract
Background
Extracorporeal membrane oxygenation (ECMO) was frequently used to treat patients with severe coronavirus disease-2019 (COVID-19)-associated acute respiratory distress (ARDS) during the initial outbreak. Care of COVID-19 patients evolved markedly during the second part of 2020. Our objective was to compare the characteristics and outcomes of patients who received ECMO for severe COVID-19 ARDS before or after July 1, 2020.
Methods
We included consecutive adults diagnosed with COVID-19 in Paris–Sorbonne University Hospital Network ICUs, who received ECMO for severe ARDS until January 28, 2021. Characteristics and survival probabilities over time were estimated during the first and second waves. Pre-ECMO risk factors predicting 90-day mortality were assessed using multivariate Cox regression.
Results
Characteristics of the 88 and 71 patients admitted, respectively, before and after July 1, 2020, were comparable except for older age, more frequent use of dexamethasone (18% vs. 82%), high-flow nasal oxygenation (19% vs. 82%) and/or non-invasive ventilation (7% vs. 37%) after July 1. Respective estimated probabilities (95% confidence intervals) of 90-day mortality were 36% (27–47%) and 48% (37–60%) during the first and the second periods. After adjusting for confounders, probability of 90-day mortality was significantly higher for patients treated after July 1 (HR 2.27, 95% CI 1.02–5.07). ECMO-related complications did not differ between study periods.
Conclusions
90-day mortality of ECMO-supported COVID-19–ARDS patients increased significantly after July 1, 2020, and was no longer comparable to that of non-COVID ECMO-treated patients. Failure of prolonged non-invasive oxygenation strategies before intubation and increased lung damage may partly explain this outcome.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-021-03780-6.
Keywords: Extracorporeal membrane oxygenation, Venovenous ECMO, Acute respiratory distress syndrome (ARDS), COVID-19, SARS-CoV-2, Outcomes
Introduction
Extracorporeal membrane oxygenation (ECMO) was frequently used to treat coronavirus disease-2019 (COVID-19) patients with severe acute respiratory distress syndrome (ARDS) during the initial outbreak from January to June 2020 [1–4]. High-volume ECMO centers and large ECMO networks reported similar survival rates for these patients compared to ECMO-supported patients with non-COVID-associated ARDS [5–7].
Management of COVID-19 patients evolved over the following months, as knowledge of the disease improved with the publication of landmark randomized trials. In June 2020, RECOVERY-trial results showed that dexamethasone (6 mg/day for 10 days) significantly reduced mortality compared to usual care for patients receiving either invasive mechanical ventilation or oxygen alone. That dexamethasone regimen was recommended by the World Health Organization and largely prescribed to COVID-19 patients. High-flow oxygen through a nasal cannula (HFNO) or noninvasive ventilation (NIV) was also shown to lower COVID-19 patients’ need for invasive mechanical ventilation and was recommended in national and international guidelines.
Because the care of COVID-19 patients evolved during the second part of 2020, we compared the characteristics and outcomes of patients who received ECMO support before and after July 1, 2020, for laboratory-confirmed severe acute respiratory distress syndrome (ARDS)-associated coronavirus-2 (SARS-CoV-2) infection in Paris–Sorbonne Hospitals.
Methods
Study settings
All consecutive adults, with laboratory-confirmed SARS-Cov-2 infection admitted to Paris–Sorbonne University Hospital Network ICUs, who received venoarterial (VA)- or venovenous (VV)-ECMO for severe ARDS from March 8, 2020, to January 28, 2021, were included, and provided 90-day survival status. The characteristics and outcomes of the first 83 cohort patients were reported previously [2]. We chose to split that cohort into two periods, before and after July 1, 2020, respectively. That date corresponds to the publication of the RECOVERY trial [8], and the transition period between the first and second COVID-19 waves in France. ECMO support was provided in four ICUs at Pitié–Salpêtrière and Tenon Hospitals before July 1, and in one Pitié–Salpetrière Hospital ICU thereafter. The Sorbonne University Ethics Committee approved the study protocol (CER-SU-2020–46).
ECMO indications and organization
ECMO indications for COVID-19 patients are detailed elsewhere [2, 4] and did not change throughout the study period. Briefly, indication(s) for ECMO implantation were centralized and evaluated in staff meetings, including at least two intensivists. Severe ARDS patients eligible for ECMO had to fulfill EOLIA trial respiratory severity criteria [5], with pre-ECMO use of neuromuscular-blocking agents and prone-positioning strongly recommended. Similarly, ECMO contraindications were still age > 70 years, severe comorbidities, cardiac arrest, refractory multiorgan failure or Simplified Acute Physiology Score (SAPS) II [9] > 90, irreversible neurological injury and/or invasive mechanical ventilation for > 10 days. Once the indication was retained, the Pitié–Salpêtrière Mobile ECMO Retrieval Team, comprising a cardiovascular surgeon and a perfusionist, was sent at the patient’s bedside for ECMO cannulation and retrieval to our department, as described previously [10, 11].
Management of ECMO for COVID-19
Throughout the study period, femoral–jugular percutaneous cannulation under ultrasonography guidance with a large drainage femoral cannula (25–29Fr) was strongly recommended for VV-ECMO. Pump speed was adjusted to obtain > 90% arterial oxygen saturation. Optimal cannula positioning was verified by ultrasonography and chest X-ray. Because of frequent thromboembolic events on-ECMO, including massive pulmonary embolism [12], and based on our early experience [2], the targeted activated partial thromboplastin time (aPTT) for VV-ECMO anticoagulation with unfractionated heparin was 60–75 s or anti-Xa activity 0.3–0.5 IU/mL. The hemoglobin threshold for red-cell transfusion was 7–8 g/dL and platelet transfusions were discouraged except for severe thrombocytopenia (< 50 G/L) or thrombocytopenia < 100 G/L with bleeding. To enhance protection against ventilator-induced lung injury, ultra-protective lung ventilation on-ECMO was recommended [6], and early prone-positioning on-ECMO was encouraged in the absence of hemodynamic instability and contraindications [13]. Based on the first descriptions [1, 2, 14] of very long ECMO runs and mechanical ventilation of COVID-19 patients, our team decided to decrease on-ECMO neuromuscular blocking-agent use for these patients. Patients were assessed daily for possible ECMO-weaning using the EOLIA clinical and physiological criteria [5, 15]. On-ECMO tracheostomy was considered after having identified, before the procedure, that the patient would likely tolerate decreased sedation. All tracheostomy decisions were made after discussion within the medical team of the balance between risks and benefits of that procedure on-ECMO [16]. When VA-ECMO was initiated, the ipsilateral limb received percutaneous cannulation via an anterograde perfusion catheter.
Data collection
Our first-wave, ECMO-treated COVID-19 patients’ data were reported previously [2]. Briefly, they included pre-ECMO demographic information; severity scores (i.e., SAPS II [9]; Sequential Organ-Failure Assessment (SOFA) score [17]; Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score [18]); rescue therapies; pre-ECMO ventilatory mechanics, including driving pressure (ΔP), mechanical power [19] and ventilatory ratio [20]; arterial blood-gas parameters, and routine laboratory values. Because we expected that pre-intubation management had changed over the study period, HFNO and NIV use and their durations were also noted.
Mechanical ventilation settings, arterial blood gases, adjuvant therapies on-ECMO and ECMO-related complications were recorded daily from day 1 to 7, then every 7 days until ECMO-day 60, ECMO-weaning or death, whichever occurred first. Major bleeding was defined as the need for ≥ 2 units of packed red blood cells for an obvious hemorrhagic event, necessitating a surgical or interventional procedure, an intracerebral hemorrhage or being fatal, while massive hemolysis was defined as plasma-free hemoglobin > 500 mg/L associated with clinical signs of hemolysis.
Lastly, specific COVID-19 treatments were recorded. We specified whether dexamethasone (6 mg/day) was initiated before or during ECMO, and if the patient received high-dose corticosteroids, defined as > 1 mg/kg/day of prednisone or equivalent since ARDS onset, during the ECMO run [21].
Outcomes
Patient outcomes on days 28, 40, 50, 60, 70, 80 or 90 post-ECMO implantation included the following endpoints: on-ECMO, in-ICU and weaned-off ECMO, alive and out of ICU or died. The time spent in each of those four states until the specified days were also calculated. Other outcomes included ICU- and ECMO-related complications.
Statistical analyses
Patient characteristics are expressed as n (%) for categorical variables or median (interquartile range, IQR) for continuous parameters, as appropriate.
To describe patients’ in-ICU trajectories over time, a multi-state model was used as in our previous study [2, 22]. Briefly, this framework considers that a patient can go through different states during follow-up. Herein, the starting time was the ECMO-initiation day, making on-ECMO the initial state for all patients, potentially followed by two intermediate states: in-ICU & weaned-off ECMO or alive & out of the ICU. Because patients could die at any time during follow-up, either in-ICU or after discharge, the death is the only final absorbing state (the final state that a patient can enter but once entered cannot be left). In this four-state model (Additional file 1), each box represents a state and each arrow represents possible transitions from one state to another.
After assessing patient status, participants who did not reach the final absorbing state were right-censored. A Cox model stratified on each possible transition state was fitted to estimate transition (from one state to another) and state-occupation (for each of the four states) probabilities over time; the percentages of patients occupying each possible state were represented simultaneously over time with a stacked probability plot and reported with their 95% confidence interval (CI) on days 28, 40, 50, 60, 70, 80 and 90 post-ECMO initiation. Another figure (Additional file 2) individually displays all possible transition probabilities from one state to another over time. Mean state-occupation times (i.e., the expected length of stay in each possible state of the multi-state model) were also reported at the same times. Finally, median on-ECMO duration and length of ICU stay were established. These analyses were computed separately for the first and second waves.
Pre-ECMO risk factors for 90-day mortality were assessed for the entire cohort using univariate and multivariate Cox regression models. The variables included in the multivariate model were defined a priori, without any variable selection. Multiple imputations were used to replace missing values when appropriate. Briefly, 10 copies of the dataset were created with the missing values replaced by imputed values, based on observed data including participants’ outcomes and pre-ECMO characteristics. Each dataset was then analyzed and the results from each dataset were pooled into a final result applying Rubin’s rule [23]. Hazard ratios and their 95% CIs were estimated.
Finally, adjusted Kaplan–Meier probabilities of survival were estimated from the multivariate Cox regression model. Each subject’s survival probability over time was estimated from the model, first considering all subjects included during the first wave, and second considering all subjects included during the second wave. Then, the survival probabilities were averaged across all individuals. Finally, unadjusted and adjusted Kaplan–Meier curves were plotted on the same figure.
All the analyses were computed at a two-sided α level of 5% with R software, version 4.0.3.
Results
Pre-ECMO patient characteristics
Eighty-eight patients were admitted before July 1, 2020, and 71 thereafter (Table 1). Briefly, patients admitted after July 1 were significantly older, while the numbers and types of failing organs at ECMO cannulation were comparable. The first COVID-19-symptoms-to-intubation interval was longer during the second period. Respective before and after HFNO (19% vs. 82%) and/or NIV (7% vs. 37%) use and their durations before intubation were significantly higher after July 1, while the intubation-to-ECMO interval was comparable for the two periods. At ECMO cannulation, ventilation parameters, respiratory mechanics and blood gases did not differ between groups. More than 90% of the patients had received neuromuscular blocking agents and undergone prone-positioning before ECMO during both periods, while inhaled nitric oxide or prostacyclin (35% vs. 52%, p = 0.032) and dexamethasone (18% vs. 82%, p < 0.001) were used significantly more frequently after July 1. Notably, d-dimer concentrations were significantly lower in patients admitted during the second period.
Table 1.
Pre-ECMO characteristics according to ICU admission before or after July 1, 2020
| Characteristic | All (N = 159) | ICU admission | p | |
|---|---|---|---|---|
| Before July 1 (N = 88) | After July 1 (N = 71) | |||
| Age, years | 51 (43–58) | 49 (41–56) | 54 (49–60) | 0.002 |
| Male | 114 (72) | 64 (73) | 50 (70) | 0.784 |
| Body mass index, kg/cm2 | 30.8 (27.7–35.1) | 30.3 (27.7–34.0) | 31.0 (27.2–37.0) | 0.513 |
| SAPS II | 55 (39–64) | 46 (30–57) | 61 (53–68) | < 0.001 |
| RESP score | 4 (2–5) | 4 (2–5) | 3 (2–4) | 0.020 |
| Total SOFA score | 11 (9–13) | 11 (9–13) | 11 (8–13) | 0.599 |
| Renal component ≥ 3 | 24 (15) | 15 (17) | 9 (13) | 0.795 |
| Cardiovascular component ≥ 3 | 79 (50) | 44 (50) | 35 (49) | 0.752 |
| Hematological component ≥ 3 | 4 (3) | 2 (2) | 2 (3) | 0.556 |
| Comorbidities | ||||
| Hypertension | 64 (40) | 34 (39) | 30 (42) | 0.644 |
| Diabetes | 54 (34) | 38 (43) | 26 (37) | 0.525 |
| Chronic respiratory diseasea | 24 (15) | 10 (11) | 14 (20) | 0.143 |
| Immunocompromisedb | 9 (6) | 3 (3) | 6 (8) | 0.189 |
| Time from | ||||
| First symptoms to ICU admission, days | 7 (5–10) | 7 (5–10) | 8 (5–11) | 0.452 |
| First symptoms to intubation, days | 10 (7–13) | 8 (6–11) | 11 (8–17) | < 0.001 |
| ICU admission to intubation, days | 1 (0–3) | 0 (0–1) | 3 (1–8) | < 0.001 |
| ICU admission to ECMO, days | 7 (3–9) | 6 (3–7) | 9 (4–12) | < 0.001 |
| Intubation to ECMO, days | 4 (1–7) | 4 (3–6) | 3 (1–7) | 0.115 |
| MERT retrieval on-ECMO from another hospital | 135 (85) | 66 (75) | 69 (97) | < 0.001 |
| High-flow oxygen before intubation | 75 (47) | 17 (19) | 58 (82) | < 0.001 |
| Duration, days | 4 (1–7) | 2 (0–4) | 5 (1–8) | 0.009 |
| Non-invasive ventilation before intubation | 32 (20) | 6 (7) | 26 (37) | < 0.001 |
| Duration, days | 3 (1–6) | 2 (3–4) | 3 (1–8) | 0.575 |
| Volume-assist control ventilation | 156 (98) | 88 (100) | 68 (96) | 0.094 |
| Ventilation parameters | ||||
| FiO2 | 100 (100–100) | 100 (100–100) | 100 (100–100) | 0.359 |
| PEEP, cmH2O | 12 (10–14) | 13 (12–14) | 12 (10–14) | 0.019 |
| Tidal volume, mL/kg PBW | 6.0 (5.6–6.4) | 6.0 (5.7–6.4) | 6.1 (5.5–6.3) | 0.661 |
| Respiratory rate, breaths/min | 30 (26–30) | 30 (28–30) | 30 (26–30) | 0.519 |
| Plateau pressure, cmH2O | 31 (29–32) | 31 (29–33) | 30 (30–32) | 0.717 |
| Driving pressure, cmH2O c | 19 (16–21) | 18 (16–20) | 19 (16–22) | 0.289 |
| Static compliance, mL/cmH2O | 21.5 (17.5–26.4) | 22.1 (18.1–26.5) | 20.8 (17.0–23.9) | 0.148 |
| Mechanical power, J/min d | 23.8 (20.2–27.1) | 24.9 (21.9–27.2) | 21.4 (19.2–26.4) | 0.055 |
| Ventilatory ratio | 2.7 (2.2–3.1) | 2.8 (2.3–3.3) | 2.5 (2.0–2.9) | 0.046 |
| Last blood-gas values pre-ECMO | ||||
| pH | 7.32 (7.25–7.39) | 7.32 (7.24–7.38) | 7.33 (7.26–7.41) | 0.143 |
| PaO2/FiO2 | 60 (54–69) | 60 (54–68) | 60 (54–74) | 0.601 |
| PaCO2, mmHg | 56 (49–65) | 57 (50–67) | 53 (48–63) | 0.156 |
| PaO2, mmHg | 63 (54–70) | 64 (54–71) | 63 (55–70) | 0.857 |
| Plasma bicarbonate, mmol/L | 27 (23–32) | 27 (24–32) | 27 (23–33) | 0.705 |
| Arterial lactate, mmol/L | 1.6 (1.3–2.1) | 1.6 (1.4–2.0) | 1.6 (1.3–2.4) | 0.858 |
| Laboratory values | ||||
| White-cell count, G/L | 13.0 (9.5–18.0) | 13.1 (10.0–17.1) | 12.9 (8.6–18.9) | 0.872 |
| Lymphocytes, G/L | 0.85 (0.50–1.33) | 0.96 (0.55–1.46) | 0.80 (0.50–1.14) | 0.328 |
| Dexamethasone (6 mg/day) started pre-ECMO | 74 (47) | 16 (18) | 58 (82) | < 0.001 |
| Rescue therapy pre-ECMO | ||||
| Any | 157 (99) | 86 (98) | 71 (100) | 0.503 |
| Neuromuscular blockade | 150 (94) | 83 (94) | 67 (94) | 1.000 |
| Prone positioning | 146 (92) | 82 (93) | 64 (90) | 0.487 |
| Inhaled nitric oxide or prostacyclin | 68 (43) | 31 (35) | 37 (52) | 0.032 |
| High-dose corticosteroids | 10 (6) | 6 (7) | 4 (6) | 1.000 |
| Almitrine | 1 (0.6) | 1 (1) | 0 (0) | 1.000 |
| Renal replacement therapy | 5 (3) | 4 (5) | 1 (1) | 0.381 |
| Cardiac arrest | 5 (3) | 3 (3) | 2 (3) | 1.000 |
Values are expressed as median (interquartile range) or number (%)
ECMO extracorporeal membrane oxygenation, ICU intensive care unit, MERT Mobile ECMO Retrieval Team, PaO2/FiO2 ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen, PEEP positive end-expiratory pressure, PBW predicted body weight, PaCO2 partial pressure of arterial carbon dioxide, RESP Respiratory Extracorporeal Membrane Oxygenation Survival Prediction, SaO2 arterial oxygen saturation, SAPS Simplified Acute Physiology Score, SOFA Sequential Organ-Function Assessment
aDefined as chronic obstructive pulmonary disease or asthma
bDefined as hematological malignancies, active solid tumor or having received specific anti-tumor treatment within 1 year, solid-organ transplant or human immunodeficiency virus-infected, long-term corticosteroids or immunosuppressants
cDefined as plateau pressure minus PEEP
dMechanical power (J/min) = 0·098 × tidal volume × respiratory rate × peak pressure – 1/2 × driving pressure. If not specified, peak pressure was considered equal to plateau pressure
On-ECMO patient management
ECMO management during the two periods is described in Table 2 and Additional file 3. During the second period, more patients received airway pressure-release ventilation/bilevel-mode ventilation and two remained non-intubated, awake and on HFNO. Ventilation parameters were comparable during the two periods, with major decreases of driving pressure, static compliance, mechanical power and ventilatory ratio on ECMO-day 1. More than 80% of the patients were prone-positioned during ECMO, with comparable numbers of sessions during both periods, while a significantly lower percentage of patients received continuous neuromuscular blockade during the second period (94% vs. 27%, p < 0.001). After July 1, 60 (85%) patients received dexamethasone during the first 15 days on-ECMO and a significantly higher percentage of patients (15% vs. 37%, p = 0.001) received high-dose corticosteroids for non-resolving ARDS [21].
Table 2.
Characteristics and complications during ECMO according to ICU admission before or after July 1, 2020
| Characteristic | All (N = 159) | ICU admission | p | |
|---|---|---|---|---|
| Before July 1 (n = 88) | After July 1 (n = 71) | |||
| ECMO-day 1 | ||||
| Type of ECMO support | 0.846 | |||
| Femoral–jugular VV | 150 (94) | 81 (92) | 69 (97) | |
| Femoral–femoral VV | 4 (3) | 3 (3) | 1 (1) | |
| Ventilation mode | < 0.001 | |||
| APRV/bilevel | 144 (91) | 75 (85) | 69 (97) | |
| Volume-assist control ventilation | 13 (8) | 13 (15) | 0 (0) | |
| High-flow oxygen | 2 (1) | 0 (0) | 2 (3) | |
| Ventilation parameters | ||||
| Minute ventilation, L/min | 3.8 (2.4–6.2) | 3.5 (2.4–6.1) | 4.6 (2.6–6.2) | 0.455 |
| Driving pressure, cmH2O | 12 (12–14) | 12 (12–14) | 12 (12–14) | 0.623 |
| Compliance, mL/cmH2O | 13.5 (9.5–22.1) | 13.2 (9.0–20.1) | 13.9 (10.0–23.3) | 0.120 |
| Mechanical power, J/min | 7.2 (4.3–12.1) | 6.4 (4.1–11.3) | 8.1 (5.1–12.3) | 0.220 |
| Ventilatory ratio | 0.76 (0.51–1.14) | 0.72 (0.44–1.09) | 0.77 (0.56–1.17) | 0.183 |
| Laboratory values | ||||
| Platelet, × 103/mm3 | 247 (177–317) | 237 (177–310) | 266 (179–333) | 0.320 |
| Hemoglobin, g/dL | 9.4 (8.1–10.7) | 9.1 (8.0–10.3) | 10.0 (8.5–11.0) | 0.023 |
| Fibrinogen, mg/L | 7.1 (5.7–8.2) | 6.7 (5.7–8.1) | 7.1 (5.6–8.2) | 0.711 |
| d–Dimers, ng/L | 4905 (2020–17,340) | 5935 (2320–18,710) | 2485 (1727–3655) | 0.020 |
| aPTT ratio | 1.5 (1.2–1.7) | 1.35 (1.16–1.6) | 1.4 (1.2–1.6) | 0.011 |
| During the ECMO run | ||||
| aPTT ratio ECMO-day 2 | 1.5 (1.2–1.7) | 1.5 (1.3–1.9) | 1.4 (1.2–1.6) | 0.011 |
| aPTT ratio ECMO-day 3 | 1.7 (1.3–2.2) | 1.8 (1.4–2.5) | 1.5 (1.2–2.0) | 0.012 |
| Adjuvant therapies on ECMO | ||||
| Continuous neuromuscular blockers | 102 (64) | 83 (94) | 19 (27) | < 0.001 |
| Prone positioning | 131 (82) | 71 (81) | 60 (85) | 0.529 |
| No. of sessions on-ECMO | 2 (1–3) | 2 (1–3) | 2 (1–4) | 0.121 |
| Nitric oxide or prostacyclin | 5 (3) | 5 (6) | 0 (0) | 0.066 |
| High-dose corticosteroids | 39 (25) | 13 (15) | 26 (37) | 0.001 |
| Renal replacement therapy | 63 (40) | 39 (44) | 24 (34) | 0.178 |
| Tracheostomy | 47 (30) | 26 (30) | 21 (30) | 0.996 |
| Received COVID-19 specific treatment | ||||
| Remdesivir | 17 (11) | 8 (9) | 9 (13) | 0.467 |
| Lopinavir/ritonavir | 20 (13) | 20 (23) | 0 (0) | < 0.001 |
| Tocilizumab | 9 (6) | 8 (9) | 1 (1) | 0.042 |
| Dexamethasone, ≤ 6 mg/day 1st 15 days | 73 (46) | 13 (15) | 60 (85) | < 0.001 |
| ECMO-related complications | ||||
| Intravascular hemolysis | 29 (18) | 9 (10) | 20 (28) | 0.004 |
| Clogged circuit requiring change | 17 (11) | 3 (3) | 14 (20) | < 0.001 |
| Severe thrombocytopenia (< 50 G/L) a | 10 (6) | 5 (6) | 5 (7) | 0.754 |
| ECMO setting/insertion change(s) b | 8 (5) | 4 (5) | 4 (6) | 0.051 |
| Massive hemorrhage | 70 (44) | 34 (39) | 36 (51) | 0.127 |
| Stroke | 1.000 | |||
| Ischemic | 2 (1) | 1 (1) | 1 (1) | |
| Hemorrhagic | 8 (5) | 4 (5) | 4 (6) | |
| Pulmonary embolism | 23 (14) | 16 (18) | 7 (10) | 0.138 |
| Cardiac arrest | 17 (11) | 9 (10) | 8 (11) | 0.833 |
| Pneumothorax | 17 (11) | 5 (6) | 12 (17) | 0.03 |
| Antibiotic-treated VAP | 159 (100) | 75 (85) | 63 (89) | 0.516 |
| ≥ 1 treated bacteremia episode(s) | 75 (47) | 44 (51) | 31 (44) | 0.388 |
Values are expressed as median (interquartile range) or number (%)
APRV airway pressure release ventilation, aPTT activated partial thromboplastin time, ECMO extracorporeal membrane oxygenation, ICU intensive care unit, Fr French, FiO2 the fraction of inspired oxygen, PEEP positive end-expiratory pressure, PaO2 partial pressure of arterial oxygen, PaCO2 partial pressure of arterial carbon dioxide, PAPV positive airway-pressure ventilation, PBW predicted body weight, and SOFA Sequential Organ-Function Assessment, VA venoarterial, VV venovenous, VAP ventilator-associated pneumonia
aDuring the first 3 days
bIncluded ECMO-cannulation switches from VA to VV; VA to venous–arteriovenous (V-AV); and VV to V-AV
ICU and ECMO-related complications
Despite significantly lower aPTT ratios during the first days on-ECMO after July 1, massive hemorrhage, hemorrhagic stroke and pulmonary embolism rates did not differ between periods. However, intravascular hemolysis and clogged circuits were more frequent after July 1. Pneumothorax occurred significantly more frequently during the second period (6% vs. 17%, p = 0.03). The frequencies of antibiotic-treated ventilator-associated pneumonia and bacteremia episodes remained very high and comparable between study periods. More than a third of our patients required renal replacement therapy while on-ECMO.
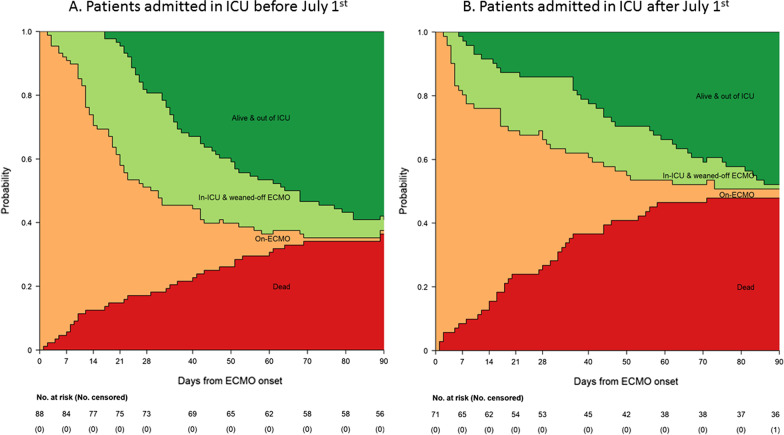
Patient outcomes
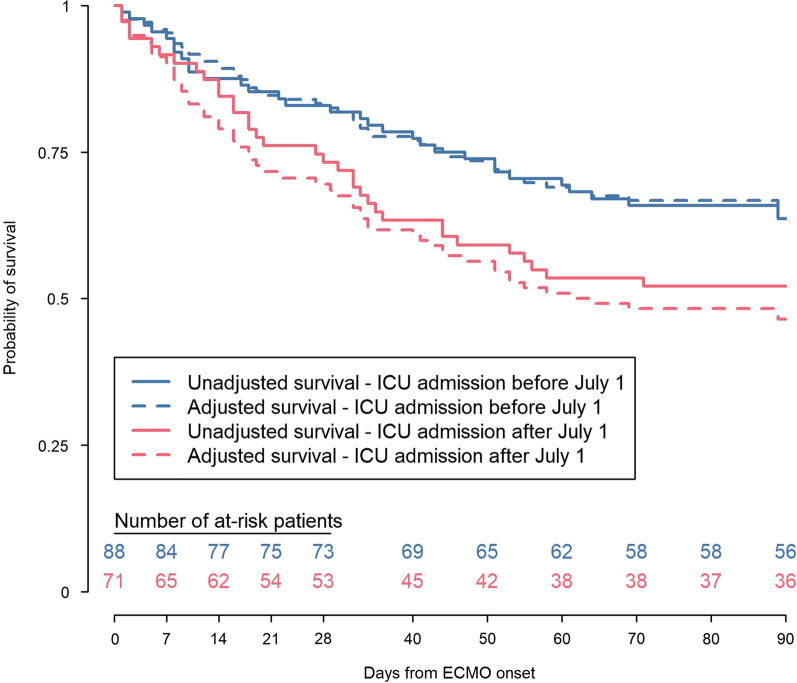
On April 28, 2021, complete 90-day follow-up was obtained for all patients. The estimated state-occupation probabilities (95% CI) of being on-ECMO, in-ICU & weaned-off ECMO, alive and out of ICU or dead 90 days post-ECMO initiation, respectively, were: 1% (0.2–8%), 3% (1–10%), 59% (49–69%) and 36% (27–47%) during the first period, and 3% (0.7–11%), 1% (0.2–10%), 48% (37–60%) and 48% (37–60%) for patients admitted after July 1 (Fig. 1 and Additional file 4). Kaplan–Meier estimates of 90-day survival were 64% and 52%, respectively, for the first and the second periods (log-rank test p = 0.108) (Fig. 2). After adjusting for confounders, patients treated after July 1 had a significantly higher probability of death by day 90 (HR 2.27, 95% CI 1.02–5.07).
Fig. 1.
Multistate-model stacked probability plots for ICU patients admitted a before or b after July 1. The plots illustrate the probability of being in each endpoint state—On-ECMO, In-ICU and weaned-off ECMO, Alive and out of ICU or dead—over the 90 days post-ECMO implantation. The respective probabilities and mean lengths of stay (with 95% confidence intervals) for each of these four states are reported in Additional file 2. ECMO extracorporeal membrane oxygenation, ICU intensive care unit
Fig. 2.
90-day Kaplan–Meier survival estimates post-ICU admission according to admission period. Adjusted (HR 2.27, 95% CI 1.02–5.07; p = 0.05) and unadjusted (log-rank test p = 0.108) survival-model values
Median (IQR) ECMO durations during the first and second periods, respectively, were 19 (10–35) and 18 (5–35) days (p = 0.949), and 22 (12–48) and 33 (8–62) days among 90-day survivors (p = 0.466). Similarly, ICU and hospital lengths of stay did not differ between periods (Additional file 5).
Predictors of 90-day mortality
The multivariable Cox regression model identified the following patient characteristics as being significantly associated with higher 90-day mortality: being admitted to the ICU after July 1, older age and pre-ECMO SOFA cardiovascular component score ≥ 3 (Table 3). In addition, a higher pre-ECMO PCO2 and longer ICU-admission-to-ECMO interval tended to be associated with higher 90-day mortality. Conversely, having received dexamethasone before ECMO implantation was a protective factor, whereas the SOFA renal component score, pre-ECMO driving pressure and PaO2/FiO2 ratio were not independently associated with higher risk of mortality. Multiple imputations of missing data yielded similar results but ICU admission after July 1 and dexamethasone treatment no longer reached statistical significance (Additional file 6).
Table 3.
Factors at ECMO onset associated with 90-day mortality of severe COVID-19–ARDS patients on ECMO
| Factor | Univariate HR (95% CI) | p | Multivariate HR (95% CI) a | p |
|---|---|---|---|---|
| Date of ICU admission | 0.110 | 0.050 | ||
| Before July 1 | – | – | ||
| After July 1 | 1.47 (0.92–2.37) | 2.27 (1.02–5.07) | ||
| Age, years | < 0.001 | 0.006 | ||
| ˂45 | – | – | ||
| 45–55 | 2.81 (1.26–6.29) | 1.86 (0.74–4.65) | ||
| ˃55 | 5.13 (2.39–11.00) | 3.55 (1.48–8.50) | ||
| Days from ICU admission to ECMO | 0.017 | 0.066 | ||
| < 4 days | – | – | ||
| 4–7 days | 1.37 (0.69–2.74) | 2.51 (1.04–6.10) | ||
| 8–10 days | 1.50 (0.68–3.29) | 2.83 (1.04–7.70) | ||
| > 10 days | 2.82 (1.43–5.57) | 3.02 (1.15–7.92) | ||
| Driving pressure pre-ECMO, cmH2O | 0.150 | 0.264 | ||
| < 17 | – | – | ||
| 17–20 | 0.95 (0.45–1.99) | 0.84 (0.37–1.94) | ||
| 20–22 | 1.18 (0.57–2.42) | 1.04 (0.47–2.29) | ||
| ˃22 | 1.98 (1.03–3.81) | 1.85 (0.86–3.98) | ||
| PaO2/FiO2 pre-ECMO | 0.621 | 0.815 | ||
| < 53 | – | – | ||
| 53–61 | 0.80 (0.42–1.53) | 1.08 (0.49–2.39) | ||
| 61–69 | 0.82 (0.42–1.61) | 0.77 (0.33–1.81) | ||
| ˃69 | 1.19 (0.62–2.27) | 1.12 (0.48–2.62) | ||
| PaCO2 pre-ECMO, mmHg | 0.007 | 0.066 | ||
| < 0 | – | – | ||
| 50–57 | 0.72 (0.31–1.67) | 0.79 (0.30–2.13) | ||
| 57–66 | 1.64 (0.82–3.30) | 2.10 (0.91–4.86) | ||
| ˃66 | 2.35 (1.20–4.59) | 2.11 (0.98–4.53) | ||
| Dexamethasone started pre-ECMO | 1.07 (0.67–1.71) | 0.783 | 0.37 (0.17–0.79) | 0.012 |
| SOFA score CV component ≥ 3 | 1.82 (1.13–2.94) | 0.013 | 2.44 (1.31–4.55) | 0.004 |
| SOFA score renal component ≥ 3 | 1.58 (0.87–2.89) | 0.156 | 1.50 (0.72–3.13) | 0.296 |
CV cardiovascular, SOFA sequential organ failure assessment, ICU intensive care unit, HR hazard ratio, CI confidence interval
aComplete analysis of 55 patients
Discussion
Patient mortality 90 days after starting ECMO support in our experienced center for the most severe forms of COVID-19 ARDS increased from 36% before to 48% after July 1, 2020. Patients admitted after that date were older, had longer ICU-admission-to-intubation intervals, with more frequent HFNO or NIV use, and most of them had received dexamethasone at ECMO onset. Independent pre-ECMO predictors of 90-day mortality for the entire cohort were older age, longer ICU-admission-to-ECMO interval, cardiovascular dysfunction, not having received dexamethasone and being admitted after July 1, 2020.
The higher mortality rate of our ECMO-treated COVID-19 patients admitted after July 1, 2020, was unexpected. Differences in patient characteristics, management and SARS-CoV-2 pathogenicity might explain that observation. First, the responsibility of pathogenicity seems unlikely, since the original European SARS-CoV-2 represented > 90% of strains circulating in France until mi-January 2021, after which the B.1.1.7 (Alpha) variant progressively became dominant. Second, most patient characteristics were comparable for the two periods, except for a 5-year higher median age during the second period. However, pre-ECMO patient management differed markedly after July 1, 2020. After RECOVERY trial results (published in June 2020) demonstrated lower mortality of patients randomized to receive 10 days of dexamethasone (6 mg/day) compared to usual care of mechanically ventilated COVID-19 patients (29% vs. 41%, respectively), most patients received corticosteroids. Pertinently, having started dexamethasone pre-ECMO was significantly associated with lower 90-day mortality in our predictive model. Also, more frequent use of dexamethasone and high-dose corticosteroids for persistent ARDS after July 1 was not associated with a higher rate of infectious complications.
The other notable patient-management differences were more frequent HFNO or NIV use and for longer durations before intubation during the second period. Although noninvasive respiratory support was shown to lower the need for intubation and invasive mechanical ventilation, COVID-19 patients for whom this strategy failed may have been at increased risk of morbidity and mortality [24, 25]. Indeed, strong and dysregulated spontaneous respiratory efforts, associated with wide transpulmonary pressure swings might heighten the risk of harmful “self-inflicted lung injury” on HFNO or NIV, with more frequent fibrotic evolution of COVID-19 pneumonia [26, 27]. That phenomenon could even be more difficult to detect in COVID-19 patients, who frequently experience dissociation between profound hypoxemic respiratory failure, and only moderate feelings and signs of respiratory distress and dyspnea [28]. Pertinently, the higher pneumothorax rate after July 1, despite similar ventilatory management during both periods, supports that hypothesis, even though pre-ECMO respiratory mechanic parameters did not differ significantly between periods. It should also be noted that although on-ECMO prone-positioning remained frequent (> 80%) after July 1, fewer patients received continuous neuromuscular blockade. Lastly, pulmonary embolism, stroke and hemorrhage rates did not change, while intravascular hemolysis and clogged circuits requiring change were more frequent after July 1. Those observations might reflect the significantly lower anticoagulation delivered to our second-period patients.
We acknowledge several limitations of this study. Our patients were treated in a high-volume, experienced ECMO center. Because better post-ECMO outcomes have been reported in such centers [4, 29], caution is required when extrapolating these results to less-experienced ECMO centers. Second, our study took place before SARS-CoV-2 variants associated with more severe ARDS forms became more prevalent (UK 20I/501Y.V1, South African 20H/501Y.V2, BR-P1 Brazilian, and Delta variants). ECMO outcomes of patients infected with those more virulent strains should be evaluated urgently. Third, COVID-19 management evolved throughout the study period, with widespread use of corticosteroids and interleukin-6 inhibitors. Therefore, patients refractory to several COVID-19 treatments who received ECMO during the second period might be considered sicker than those managed before July 1, 2020. Moreover, we cannot exclude that some residual confounding factors may not have been taken into account in our logistic model. Lastly, our cohort’s mortality rate could evolve further, as some patients were still hospitalized 90 days after ICU admission.
Conclusion
In conclusion, survival of ECMO-rescued patients with severe COVID-19 ARDS in our experienced center has declined over time and is no longer comparable to that of non-COVID ECMO patients. Failure of prolonged non-invasive oxygenation strategies before intubation and increased lung damage, as well as selection of patients already refractory to specific COVID-19 treatments might partially explain this outcome. Although other residual confounding factors may not have been considered herein, the duration of noninvasive respiratory support, e.g., continuous positive airway pressure, HFNO or NIV, should perhaps be integrated in the decision to initiate ECMO for severe COVID-19 patients.
Supplementary Information
Additional file 1. All possible transition probabilities from one state to another over time.
Additional file 2. The estimated probabilities (95% CI) of all possible transitions from one state to another for (A) 88 patients admitted to the ICU before July 1, 2020, and (B) 71 patients admitted to the ICU after July 1, 2020.
Additional file 3. Additional information concerning patients’ characteristics and complications on-ECMO according to ICU-admission period.
Additional file 4. Probabilities of being in each of the four endpoint states and the mean time spent in each one on days 28, 40, 50, 60, and 90 post-ECMO onset according to the ICU-admission date.
Additional file 5. ECMO duration, and ICU and hospital lengths of stay according to the ICU-admission period.
Additional file 6. Predictive factors associated with 90-day mortality of 159 adults with COVID-19 severe ARDS with multiple imputations.
Acknowledgements
GRC-RESPIRE Sorbonne Université:
Groupe de Recherche Clinique en REanimation et Soins intensifs du Patient en Insuffisance Respiratoire aiguE.
Abbreviations
- aPTT
Activated partial thromboplastin time
- ARDS
Acute respiratory distress syndrome
- ECMO
Extracorporeal membrane oxygenation
- VA/VV-ECMO
Venoarterial/venovenous-extracorporeal membrane oxygenation
- ICU
Intensive care unit
- COVID-19
Coronavirus disease 2019
- HFNO
High-flow oxygen through a nasal cannula
- NIV
Noninvasive ventilation
- SAPS
Simplified Acute Physiology Score
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus-2
- RESP
Respiratory extracorporeal membrane oxygenation survival prediction
- SOFA
Sequential organ-failure assessment
Authors' contributions
MS, EL, SAJ, JC, NB, PB, LL, MP, GE, CEL, MD, JMC, MF, PL, GL, and AC were involved in data generation. MS, DH, and AC were involved in the analysis of the data. MS, DH, and AC wrote the manuscript. All authors contributed to critical reading of the text and its revision. All authors read and approved the final manuscript.
Funding
No funding was received for this study. The corresponding author had full access to all data and the final responsibility to submit for publication.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Sorbonne University Ethics Committee (CER-SU-2020-46) approved the study protocol. Moreover, the dataset used for the analysis was anonymized.
Consent for publication
Not applicable.
Competing interests
Matthieu Schmidt reports lecture fees from Getinge, Drager, and Xenios outside the submitted work. Martin Dres reports personal fees from Lungpacer, outside the submitted work. Muriel Fartoukh reports non-financial support from bioMérieux, and personal fees from Pfizer, bioMérieux, Fisher & Paykel, and grants from French Ministry of Health, outside the submitted work. Jean-Michel Constantin reports personal fees and non-financial support from Drager, GE Healthcare, Sedana Medical, Baxter, and Amomed; personal fees from Fisher, Paykel Healthcare, Orion, Philips Medical, and Fresenius Medical Care; and non-financial support from LFB, and Bird Corporation, outside of the submitted work. Alain Combes reports grants from Getinge, personal fees from Getinge, Baxter, and Xenios outside the submitted work. The other authors declare that they have no conflicts of interest related to this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Matthieu Schmidt and Elise Langouet jointly first authors
References
- 1.Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121–1131. doi: 10.1016/S2213-2600(20)30328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz RA, Graf J, Zambrano JM, Ruiz C, Espinoza JA, Bravo SI, et al. ECMO for COVID-19-associated severe ARDS in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021;204:34–43. doi: 10.1164/rccm.202011-4166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebreton G, Schmidt M, Ponnaiah M, Folliguet T, Para M, Guihaire J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt M, Pham T, Arcadipane A, Agerstrand C, Ohshimo S, Pellegrino V, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome. An international multicenter prospective cohort. Am J Respir Crit Care Med. 2019;200:1002–1012. doi: 10.1164/rccm.201806-1094OC. [DOI] [PubMed] [Google Scholar]
- 7.Shaefi S, Brenner SK, Gupta S, O’Gara BP, Krajewski ML, Charytan DM, et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021;47:208–221. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 10.Bréchot N, Mastroianni C, Schmidt M, Santi F, Lebreton G, Hoareau A-M, et al. Retrieval of severe acute respiratory failure patients on extracorporeal membrane oxygenation: any impact on their outcomes? J Thorac Cardiovasc Surg. 2018;155:1621–9.e2. doi: 10.1016/j.jtcvs.2017.10.084. [DOI] [PubMed] [Google Scholar]
- 11.Beurtheret S, Mordant P, Paoletti X, Marijon E, Celermajer DS, Léger P, et al. Emergency circulatory support in refractory cardiogenic shock patients in remote institutions: a pilot study (the cardiac-RESCUE program) Eur Heart J. 2013;34:112–120. doi: 10.1093/eurheartj/ehs081. [DOI] [PubMed] [Google Scholar]
- 12.Hékimian G, Lebreton G, Bréchot N, Luyt C-E, Schmidt M, Combes A. Severe pulmonary embolism in COVID-19 patients: a call for increased awareness. Crit Care. 2020;24:274. doi: 10.1186/s13054-020-02931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guervilly C, Prud’homme E, Pauly V, Bourenne J, Hraiech S, Daviet F, et al. Prone positioning and extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: time for a randomized trial? Intensive Care Med. 2019;45:1040–1042. doi: 10.1007/s00134-019-05570-9. [DOI] [PubMed] [Google Scholar]
- 14.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combes A, Schmidt M, Hodgson CL, Fan E, Ferguson ND, Fraser JF, et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020;46:2464–2476. doi: 10.1007/s00134-020-06290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M, Fisser C, Martucci G, Abrams D, Frapard T, Popugaev K, et al. Tracheostomy management in patients with severe acute respiratory distress syndrome receiving extracorporeal membrane oxygenation: an international multicenter retrospective study. Crit Care. 2021;25:238. doi: 10.1186/s13054-021-03649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 19.Gattinoni L, Tonetti T, Cressoni M, Cadringher P, Herrmann P, Moerer O, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42:1567–1575. doi: 10.1007/s00134-016-4505-2. [DOI] [PubMed] [Google Scholar]
- 20.Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiological analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;199:333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 22.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valley TS, Walkey AJ, Lindenauer PK, Wiener RS, Cooke CR. Association between noninvasive ventilation and mortality among older patients with pneumonia. Crit Care Med. 2017;45:e246–e254. doi: 10.1097/CCM.0000000000002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang BJ, Koh Y, Lim C-M, Huh JW, Baek S, Han M, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41:623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 26.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 27.Brochard L. Ventilation-induced lung injury exists in spontaneously breathing patients with acute respiratory failure: yes. Intensive Care Med. 2017;43:250–252. doi: 10.1007/s00134-016-4645-4. [DOI] [PubMed] [Google Scholar]
- 28.Wilkerson RG, Adler JD, Shah NG, Brown R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020;38(2243):e5–6. doi: 10.1016/j.ajem.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbaro RP, Odetola FO, Kidwell KM, Paden ML, Bartlett RH, Davis MM, et al. Association of hospital-level volume of extracorporeal membrane oxygenation cases and mortality. Analysis of the extracorporeal life support organization registry. Am J Respir Crit Care Med. 2015;191:894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. All possible transition probabilities from one state to another over time.
Additional file 2. The estimated probabilities (95% CI) of all possible transitions from one state to another for (A) 88 patients admitted to the ICU before July 1, 2020, and (B) 71 patients admitted to the ICU after July 1, 2020.
Additional file 3. Additional information concerning patients’ characteristics and complications on-ECMO according to ICU-admission period.
Additional file 4. Probabilities of being in each of the four endpoint states and the mean time spent in each one on days 28, 40, 50, 60, and 90 post-ECMO onset according to the ICU-admission date.
Additional file 5. ECMO duration, and ICU and hospital lengths of stay according to the ICU-admission period.
Additional file 6. Predictive factors associated with 90-day mortality of 159 adults with COVID-19 severe ARDS with multiple imputations.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.