Abstract
Streptomyces sp. strain S-9 was studied for its effect in inducing systemic resistance in Pigeon pea against the plant pathogen Fusarium udum causing wilt. The 16S rRNA gene sequencing and phylogenetic analysis indicated that S-9 is closely related to genus Streptomyces for which it was referred to as Streptomyces sp. S-9. Streptomyces sp. S-9 caused 85% inhibition of the pathogen and showed various attributes of plant growth-promoting such as the production of IAA, P-solubilization, and -1, 3-Glucanase activity. Proline and malondialdehyde (MDA) content was significantly higher whereas the chlorophyll content decreased in the pathogen-infected plant when compared to S-9 treated Pigeon pea plants. The anatomical research assisted the biocontrol-mediated stress tolerance findings in the Pigeon pea plant through increased root epidermis and enhanced stress-related xylem tissues. Fungus inoculation elevated the antioxidative enzymatic activities of superoxide dismutase (SOD; 78%) and catalase (CAT; 56%). Marked reductions in antioxidant enzymes were associated with the antagonistic effects of the different treatments. Conclusions showed that S-9 bioinocula applied as a seed coating enhanced soil availability of nitrogen (N), phosphate (P), and potassium (K), indicating their suitability for direct application invigorating plant growth and persuade resistance in the plant Pigeon pea against Fusarium wilt.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02989-0.
Keywords: Fusarium wilt, Actinomycetes, Pigeon Pea, MDA
Introduction
Soil-borne pathogenic fungi, such as Fusarium udum, Fusarium oxysporum, Phytophthora sp., Alternaria sp., etc., can cause serious plant diseases, affect crop yields, and impair product quality (Morrissey et al. 2004). Such fungal pathogens with a huge host variety ensuing into the disease of the various economically agricultural plants. Traditionally, synthetic fungicides such as Bavistin and Thiram are particularly accustomed manipulate the prevalence of Fusarium and different soil pathogenic fungi (Melent’ev et al. 2006). Consequently, alternate approaches are required for his or her eco-friendly and sustainable management. Consequently, alternate approaches are required for their eco-friendly and sustainable management. Plants are quite well bestowed upon with various types of defense mechanisms to shield and protect them from many diseases. In modern times, crop farming is a complex network of interactions among plants, fertilizers, rhizobacteria, and soil. There is an earnest requirement for eco-friendly sustainable activities in the agriculture input. The ability of the rhizospheric microorganisms to give sufficient stock of fundamental supplements for the improvement of agricultural products is unquestionable (Kumar et al. 2018) As plants are known to harbor various beneficial microscopic organisms in all organs as epiphytes and endophytes, and control of these microorganisms have been demonstrated to build the profitability of harvests, we propose to call them aggregately as plant-beneficial microbes (PBR). This is the time of sustainable and evergreen agricultural food crop production; therefore, these tripartite (plant–microbe–soil) interactions in the spermosphere and rhizosphere play a humungous role.
Plant growth-promoting actinomycetes (PGPA) are root colonizing microbes with beneficial effects including plant growth promotion and disease control (Misra et al. 2017). Streptomyces, is the most abundant genus of actinobacteria representing 50% of the total population of soil actinobacteria (Parte et al. 2020). Streptomyces is a filamentous bacterium with a high G + C content that belongs to the Streptomycetaceae family and order Actinomycetales (Gopalakrishnan et al. 2020). It is well-known for producing a large number of bioactive metabolites. About 75% of the reported antibiotics were identified from Streptomyces (Olanrewaju and Babalola 2019). The filaments of Streptomyces promote the maximum absorption of nutrients from the soil and support colonization on substrates, particularly plant roots. As a result, Streptomyces populations are frequently reported in strain isolation, metagenomics, genome mining, and sequencing (Newitt et al. 2019). Streptomyces are known for their biocontrol potential against a variety of phytopathogens in addition to their plant growth-promoting abilities.
Numerous Streptomyces strains are considered biocontrol since they produce a wide scope of antimicrobials, can endure in unforgiving conditions, and proficiently colonize the rhizosphere of various plant species including rice (Qin et al. 2011; Kinkel et al. 2012). Moreover, Streptomyces can inspire initiated obstruction, as it has been depicted previously (Conn et al. 2008; Kurth et al. 2014). Because of these highlights, it is not astounding that assorted Streptomyces strains had been concentrated to control fungal and bacterial diseases of rice such as bacterial leaf blight caused by Xanthomonas oryzae, however, very few Streptomyces are currently being developed as biocontrol products.
Pigeon pea (Cajanus cajan (L. Mill) is one of the most significant pulse crops in the semi-arid tropics. Pigeon pea cultivation has a direct impact on the overall economic, financial and nutritional status of subsistence farmers in South and East Africa, Asia, and the South American subcontinent (Sharma et al. 2016). Pigeon pea [Cajanus cajan (L.) Millsp.], also known as red gram, is the second most important edible legume crop in India with an area of 5.10 hectares and a yield of 3.31 million tonnes (FAOSTAT 2019). Pea seeds are the main source of protein and vitamins, especially for the vegetarian population of the world.
In Pigeon pea cultivation, an important biological factor that causes Fusarium wilt is Pigeon pea wilt, which harms crop growth (30–60% of the incidence occurs in the flowering and mature period) and yield (Sharma et al. 2016). Managing Fusarium infection is often a difficult task, primarily due to its soil-borne nature, the deep interior of host tissues, the synthesis of persistent latent structures, and the ability to survive and sustain longer without host plants (Boukerma et al. 2017). In recent decades, the prevention and control of soil-borne diseases in crops have generally been achieved through the use of disease-resistant varieties, crop rotation, synthetic fungicides, and fumigation (Wang et al. 2018; Tang et al. 2020).
However, Pigeon pea suffers high mortality because of serious seed and soil-borne fungal pathogens such as F. udum and F. oxysporum. Economically important crops such as Pigeon pea need protection from various diseases as the demand for such crops are expected to steadily increase, particularly in the developing regions of the world. Presently, chemical fungicides such as thiram or captan are used to control wilt, but the increasing environmental awareness of pesticide-related hazards has emphasized the need for biological methods. The fungi Fusarium udum is a soil-borne plant pathogen and, therefore, chemical control is impractical in established cases. Moreover, extensive and inordinate use of synthetic and semi-synthetic compounds to improve Pigeon pea productivity and disease control is a growing concern. In light of the above facts, it can be observed the application of PGPA can contribute to sustainable high yield and plant protection. Therefore, the present investigation was undertaken to see the effect of PGPA strains particularly Streptomyces sp. S-9 on antagonistic activity against F. udum in vitro and further using pot, field trials in Pigeon pea.
PGPA alone or in combination can reduce the level of active free radicals through antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT). These enzymes can eliminate free radicals, thus reducing the formation of reactive oxygen species (ROS) (Manoj et al. 2016).
Many Streptomyces strains are considered biocontrol agents, since they produce a wide range of antimicrobials, can persist in harsh environments, and efficiently colonize the rhizosphere of different plant species including rice (Qin et al. 2011; Kinkel et al. 2012). Furthermore, Streptomyces can elicit induced resistance, as it has been described before (Conn et al. 2008; Kurth et al. 2014). Because of these features, it is not surprising that diverse Streptomyces strains had been studied to control fungal and bacterial diseases of rice such as Bacterial Leaf Blight caused by Xanthomonas oryzae, however, very few Streptomyces are currently being developed as biocontrol products.
Materials and methods
Cultivar and test pathogen used in the experiment
Pigeon pea seeds (var.BDN-2) were procured from the Pulse Research Station, Model Farm, located in Vadodara, Gujarat, India. The seeds were surface sterilized by soaking into 0.1% sodium hypochlorite solution (SDFCL, Mumbai) for 3 min followed by three washings with sterile distilled water (SDW). Fusarium udum (ITCC 3241) was procured from the Indian Type Culture Collection (ITCC), Indian Agriculture Research Institute (IARI), and New Delhi, India. The strain was grown and maintained on the media, viz., potato dextrose agar (PDA), (Hi-media, Mumbai, India) at 27 ± 1 °C for 5 days, until sporulation (200 g potato infusion, 20 g dextrose, 15 g agar) as suggested by ITCC.
Isolation and characterization of actinomycetes
Actinomycetes were isolated from soil samples procured from the rhizosphere of (about 2-month-old growing crop) Pigeon pea from Lasundra, Vadodara (N22°56′16″, E73°22′20″), Gujarat (India) during July 2016. About 1 g of soil sample enriched with calcium carbonate (CaCO3) was dissolved in 10 mL 0.85% sterile normal saline (SNS) and vortexed meticulously. These samples were serially diluted up to 10–3 and spread over sterilized actinomycetes isolation agar media enriched with 50 µgmL−1 each of cycloheximide and nystatin to prevent fungal contamination and incubated at 27 ± 1°Cfor 4 days.
Screening of actinomycetes
Fusarium udum and Streptomyces sp. S-9 were cultured on potato dextrose agar medium at 28 °C for 7 days. An agar block of indicator fungus was prepared using a sterile cork borer with a diameter of 8 mm and placed at the center of the plate. Inoculate an agar block containing a single colony of actinomycetes around the fungus block 10 mm from the edge of the agar block. (Bredholdt et al. 2007). The plates were incubated at 28 °C, and mycelial growth inhibition was measured after 7 days (Bredholdt et al. 2007). Inhibition of mycelial development against the bacterial isolate was indicative of antagonistic behavior. The percentage of inhibition of radial mycelial growth is determined according to Ji et al. (2013) as follows:
Phenotypic characterization of Streptomyces sp.
Scanning electron microscopy (JEOL JSM-6380 LV, Japan) was used to examine morphological characteristics of cultures grown on ISP 3 agar at 28 °C for 2 weeks (Jin et al. 2019). Cutting a block from an agar plate and fixing it in 2.5 percent glutaraldehyde buffer (pH 7.2) at 4 °C for around 1.5 h yielded samples for scanning electron microscopy. Samples were dehydrated through a graded sequence of ethanol, passed through tertiary-butanol, and then critically point dried after being rinsed twice with phosphate buffer. Under vacuum, the dried samples were mounted on a stub-bearing adhesive and spatter-coated with gold (Guan et al. 2015).
Amplification of 16S rDNA genes by polymerase chain reaction (PCR)
Actinomycetes (Streptomyces sp. strain S-9) was inoculated into ISP-1broth and kept overnight for shaking, genomic DNA was extracted using SDS-lysozyme extraction method and PCR targeting the 16S rDNA gene amplified using universal primer gene corresponding to positions 8–27 for the forward primer and 1492–1510 for the reverse primer (Forward primer (27F): 5′-AGAGTTTGATCMTGGCTCAG-3′ Reverse primer (1492R): 5′-TACGGYTACCTTGTTACGACTT-3′. The phylogeny was inferred using the maximum likelihood method based on the Kimura 2-parameter model (Kimura et al. 1980).
Scanning electron microscopy
For SEM, F. udum mycelia samples were fixed in 4% glutaraldehyde at 4 ± 1 °C overnight, then rinsed three times with 0.05 M sodium cacodylate (Sigma-Aldrich, USA) buffer (pH 7.2) for 10 min at 4 ± 1 °C. Samples were subsequently fixed with 1% osmium tetroxide (Sigma–Aldrich, USA) for 2 h at 4 ± 1 °C and washed with distilled water twice briefly. The hyphae were dehydrated in series of ethanol concentrations (50, 70, 80, and 90%) for 10 min each and then in 100% ethanol for 20 min to ensure complete dehydration. The hyphae were then placed in isoamyl acetate. After a critical point in drying, the samples were mounted on stubs and sputter-coated with gold–palladium and examined with the scanning electron microscope (Model-JEOL JSM-6380 LV, Japan) at 20 kV.
Quantification of plant growth-promoting activities
Screening for the plant growth and biocontrol activity performed following standard procedures such as IAA production (Patten and Glick 1996), P-solubilization (Pikovskaya 1948), siderophore production (Schwyn and Neilands 1987), chitinolytic activity (Vyas and Deshpande 1989). The β-1, 3-glucanase activity was assayed using laminarin (from Laminaria digitata) (Sigma-Aldrich) as a substrate (Liang et al. 1995).
Pre-emergence wilt incidence (%)
Pre-emergence (symptoms of the disease such as root rot/brownish lesions on root/poor/no radicle emergence) and gravity indices of post-emergence wilt disease were determined by counting the number of germinating seeds and surviving seedlings (those seedlings that did not display any symptoms of wilt disease such as brownish lesions/premature drooping of leaves/partial or full wilting of part or wholly wilting) among those germinated. A percentage of disease incidences was calculated based on visible wilt symptoms observed on the plant after 15 days up to 35 DAS (Days after sowing). The number of plants infected with F. udum was counted in each plot at 90 DAS and the mortality of the plants was determined.
Effect of bacterial inoculation on biocontrol and mitigation of stress under greenhouse condition
Following the characterization of bacterial strains based on their biocontrol attributes under normal and stress conditions in vitro, the bacterial strains were also tested for their competence of biocontrol under plant test using Pigeon pea (Cajanus cajan) plastic pot conditions (15 cm in diameter). Experiments were conducted in a completely randomized block design with three replicates in pots containing 2.0 mm sieved unsterilized field soils (2.0 kg soil per pot) of Pulse Research Station, Model Farm, located in Vadodara, Gujarat, India (latitude/longitude 73°1771′ N/22°3125′ E). Surface-sterilized seeds were sown in each pot (4 seeds pot−1) and daily observations were taken for germination and wilt incidence. Each treatment had three replications. Seven-day-old Pigeon pea seedlings (Cajanus cajan) var. BDN-2 was used for transplantation in earthen pots filled with field soil. Plants were grown under natural greenhouse conditions, and the treatments for host plants with concern bacterial strains were as follows: Uninoculated, S-9, S-9 + Fusarium udum, and Fusarium udum. The surface-sterilized seeds were soaked into the culture with CMC coating broth of Streptomyces sp. strain S-9 (108 CFU mL−1) for 2 h. A 100 mL spore suspension (106 CFU mL−1) of F. udum was added into the pots having sterile soil, a pot with 20 mL distilled water served as control non-infested control. Pots were watered daily with 20 ml sterile distilled water. The plants were harvested after 30 d and selected parameters such as root length, shoot length, fresh root weight, dry root weight, fresh shoot weight, and dry shoot weight was measured.
T-1—Control (Uninoculated),
T-2—Treated with isolate S-9.
T-3—Treated with isolate S-9 + F. udum.
T-4—F. udum only.
Plant vegetative parameters, biochemical, and antioxidative assays
Lipid peroxidation assay was carried out by thiobarbituric acid (TBA) (Sigma-Aldrich, USA) method, wherein thiobarbituric acid reacting substances (TBARS) act as an indicator of membrane lipid peroxidation that was measured in terms of malondialdehyde (MDA) (Sigma Aldrich, USA) concentration (Fazeli et al. 2007). For this purpose, about 0.2 g leaf samples were homogenized in 4 mL of 0.1% trichloroacetic acid (TCA) solution and centrifuged at 10,000 rpm for 10 min, and the supernatant was collected and 1 ml of 20% TCA containing 0.5% TBA was added to 0.5 ml of supernatant. Samples were shaken thoroughly and placed in a boiling water bath for 30 min. They were stored in a cooled ice bath. These samples were again centrifuged at 10,000 rpm for 15 min and supernatants were collected. Their absorbance was measured at 532 and 600. TBARS content was expressed in nmol per g FM.
Leaf chlorophyll was determined using a chlorophyll meter (SPAD-502, Minolta, Japan). Three measurements at random locations in the middle of the leaf were made for each plant and the average used for the analysis. Twenty leaves with incremental chlorophyll levels (determined by SPAD-502 readings) were then harvested to construct a standard curve for quantification of chlorophyll content using the method for chlorophyll analysis described by Arnon (1949).
About 0.5 g of fully expanded 'sun' leaves from field-grown Pigeon pea plants were sampled was homogenized in 10 ml of 3% aqueous sulfosalicylic acid. The homogenate was filtered through Whatman # 2 filter paper. To the 2 ml of filtrate, 2 ml acidic ninhydrin, and 2 ml of glacial acetic acid (Merck, Mumbai) were and incubated at 100 °C for 1 h the reaction was terminated in an ice bath. The reaction mixture was extracted with 4 ml toluene, mixed vigorously for 15–20 s. The chromophore containing toluene was aspirated from the aqueous phase, warmed to 28 °C ± + 10 °C and the absorbance was read at 520 nm using toluene for a blank. The proline concentration was determined from a standard curve prepared with pure proline (100 µg/ml) (Bates 1973) and calculated on a fresh weight basis as follows:
Estimation of defense enzymes of leaf samples was done using established standard protocols. The NBT reduction was measured for evaluating the SOD (EC 1.15.1.1) activity as per the protocol of Beauchamp and Fridovich (1971). CAT (EC 1.11.1.6) activity was estimated by the reduction in absorbance by induced decomposition of H2O2 in the presence of the enzyme (Aebi 1984).
Detection of H2O2 in Pigeon pea leaves
Qualitative assessment of 3, 3′-diaminobenzidine (DAB) staining was performed to capture the H2O2 in Pigeon pea leaf tissues. In short, the leaves harvested from all treatments (Control) were vacuum infiltrated for 5 min into DAB solution (PALL Life Sciences, India) with a concentration of 1 mg ml−1 (Rangani et al. 2016). Following this, infiltrated leaves in DAB solution were incubated for 24 h to get stained. After incubation, leaves were washed in distilled water and then, boiled in 95% ethanol for 10 min for the purpose to remove the excess stain (Thordal-Christensen et al. 1997). H2O2 in DAB-stained leaves were visualized as reddish-brown coloration.
Histology of Pigeon pea roots under greenhouse conditions
Microscopic examination of changes in root tissue anatomical features was observed following the protocol of O'Brien et al. (1964). Here, hand-cut sections (ca. 10–50 μ) of fresh root materials corresponding to different treatments (Control, S-9, S-9 + F. udum, and F. udum) allowed soaking for at least 2–3 min. These sections were immersed in a staining solution for 1 min. The staining solution was referred to 0.05% toluidine blue in 0.1 M phosphate buffer of pH 6.8. Following staining, sections were washed with tap water and examined at both × 40 and × 100 under a microscope (Olympus CX1, Leica Microsystems, GmbH, Germany). The root samples were analyzed for the thickness of endodermis; size and number of xylem cells.
Amount of estimation of the contents of N and P in soil
Soil samples were randomly taken from each pot 0–20 cm depth before planting, bulked, air-dried, and sieved using a 2 mm sieve for analysis. The particle size analysis was done by pipette method Gee et al. Soil pH in water was determined using soil: water ratio of 1:2 with a glass electrode pH meter. Organic carbon was determined using Walkley and Black method (Nelson and Sommers 1996). Total nitrogen (N) in the soil was determined by Kjeldahl digestion Exchangeable bases in the samples were extracted in 1 M NH4 OAC (Sigma–Aldrich, Bangalore) at pH 7.0. Potassium (K) was analyzed by flame photometry. Available phosphorus (P) was determined by Bray-1 extraction and determined colorimetrically by the molybdenum blue procedure Soil samples were air-dried and ground to powder and analyzed with wet digestion method using 5:1:1 ml of HNO3:H2SO4:HClO4 acid. Total N was determined by micro–Kjeldahl method. For P, K, samples (0.5 g) were ashed, dissolve in 10% hydrogen chloride (HCl), and diluted to 50 ml. Phosphorous was determined using vandal molybdate colorimetric. The Physico-chemical properties of soil used were analyzed at the Department of Agricultural Chemistry and soil science, Anand Agriculture University, Anand, Gujarat, India.
Statistical analysis
The data obtained were statistically analyzed using one-way ANOVA and Duncan’s Multiple Range Test using SPSS (Version 20). Differences were considered statistically significant at P ≤ 0.05. Data were presented as mean ± standard error of the mean (SEM) of three replicates except otherwise stated. The graph was prepared using Graphpad Prism (Version 8).
Results
Phenotypic characteristics of Streptomyces sp.
The morphology of the strain S-9 culture grown in ISP 3 medium for 4 weeks indicated that it was consistent with the Streptomyces genus. The S-9 strain was identified as an aerobic gram-positive actinomycete, which produced a well-developed, branched, and non-fragmented mycelial matrix, but no aerial mycelium. On the substrate mycelium, nonmotile and oval spores (2 µm) are formed individually (Supplementary Fig. 1).
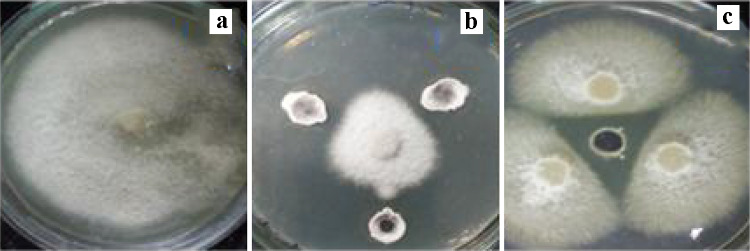
Fig. 1.
Suppression of F. udum mycelial growth formation by selected bacterial isolates; a Control of F. udum (b), The mycelial growth pattern of F. udum in the presence of bacteria. The experiment was performed with three replicates. Images were taken 15 days after inoculation (DAI) (c) After 7 days of bacterial treatment and incubated at 28 °C, and a clear halo zone (antagonism) was observed
Isolation and molecular characterization of actinomycetes
Results indicated that the inhibition zones between bacterial isolates and fungal isolates are generally confirmed by calculating the percent inhibition of radial mycelial development against bacterial isolates was observed (Fig. 1). Streptomyces sp. strain S-9 showed 85% mycelial inhibition of F. udum (Fig. 1). The 16S rRNA gene sequence of strain S-9 showed a close phylogenetic relationship with Streptomyces spinoverrucosus NIIST A67 and Streptomyces pseudogriseolus strain 99 (Supplementary Fig. 2). The sequence has been submitted to GenBank under the accession number MK 158,952.
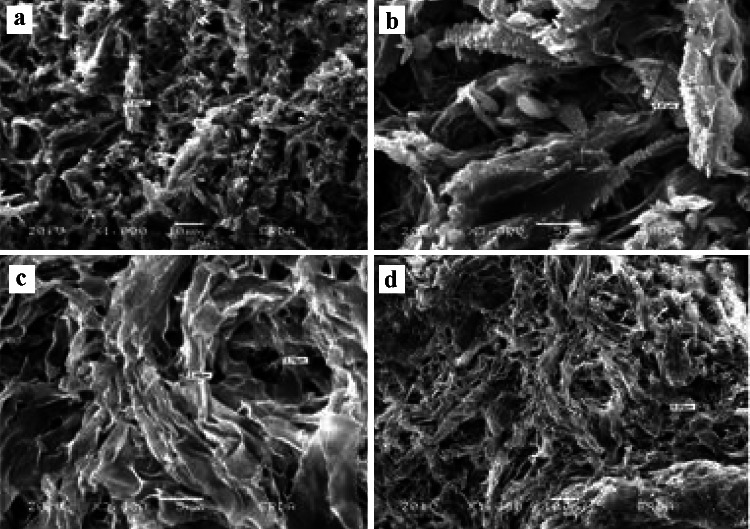
Fig. 2.
Scanning electron micrographs (SEM) of pathogen-bacterial interaction during antagonism assay against F. udum in dual culture. a, b Images of F. udum from control plate (c, d) in dual culture with Streptomyces sp. S-9 (MK158952). Mycelial abnormality is observed along with a coagulation of cytoplasm, b mycelial shredding and shrinking, c leakage of cytoplasm and mycelial breakage, and d Perforation, breakage, and shrinking, as compared to growth in the absence of antagonistic agent Streptomyces sp. S-9 (MK158952)
Effect of strain S-9 on Fusarium morphology
Results showed that the morphological alterations of Fusarium pathogens affected by strain Streptomyces sp. strain S-9, report of SEM images analysis of hyphae of F. udum (Fig. 2a–d). Minor developed dense hyphae revealed typically long, cylindrical cells with a smooth surface. Test strain had fewer hyphae and degenerated mycelia as shown in Fig. 2. In the degenerated hyphae, the wall intruded and formed small depressions at many sites along the hyphae. F. udum hyphae were also degenerated, progressively shrunken (Fig. 2b, d). Streptomyces sp. strain S-9 had great potential as a biocontrol agent for wilt diseases of Pigeon pea crop. A summary of SEM of pathogen-bacterial interaction during the antagonism assay is presented in Fig. 2
Plant growth-promoting attributes of actinomycetes
Results observed that the antifungal attributes and root hair formation study according to the standard curve the strain Streptomyces sp. strain S-9 had plant growth-promoting traits. Results showed Streptomyces sp. strain S-9 able to produce IAA, β-1, 3 glucanase, and P-solubilization. The quantitative estimation of the IAA production in culture broth with tryptophan in the presence of ranged from 16.8 ± 0.45 μgmL−1. In the case of P-solubilization by Streptomyces sp. strain S-9 observed that it was produced a varying zone of solubilization on rock phosphate around the selected strain colonies. Data showed that the P-solubilization of PGPA varied from 1.5 to 10.5 mm. Nevertheless, isolate Streptomyces sp. strain S-9 produced a larger (10.5 mm) zone of P-solubilization. In bacterized Pigeon pea plants, β-1, 3 glucanases activity (32 ± 0.20 ng glucose/min/mg protein), thereafter declined gradually. In an assessable assessment of P-solubilization of Streptomyces sp. strain, S-9 was 25.50 ± 0.20 mgL−1, indicating potential P-solubilization degradation by isolating Streptomyces sp. strain S-9 (Supplementary Table 1).
Table 1.
In vitro seed germination and Effect of Streptomyces sp. Strain S-9 inoculation on seed germination under F. udum challenged condition
| Treatments | Germination (%) | Seedling length (cm) | Vigor index | Disease severity (%) |
|---|---|---|---|---|
| T-1 | 83.33 | 6.86 | 571.64 | 10.23 |
| T-2 | 100.00 | 5.99 | 599.00 | 12.67 |
| T-3 | 83.33 | 6.19 | 515.81 | 16.89 |
| T-4 | 33.33 | 1.86 | 61.99 | 57.50 |
T-1—Control; T-2—S-9; T-3—S-9 + F. udum; T-4—F. udum only
DAS Days after sowing, MAS Months after sowing data are presented as mean ± SD, n = 3 according to Duncan multiple range test (DMRT) (P < 0.05)
Root hair formation study and wilt incidence
S-9 treated seeds showed higher germination percentage, vigor index, and a significant increase in shoot and root lengths. Results observed that the application of Streptomyces sp. S-9 alone and in a combination with different treatments significantly influenced germination. Significantly highest (100%) percentage of germination detected T-2 followed by T-1 ≥ T-3 and T-4. The same trend was also observed for the seedling length and vigor index (Supplementary Fig. 3; Table 1). Inoculation with bacteria onto seeds promoted a positive effect. Strain S-9 significantly promoted root hair formation in treated seedling roots of Pigeon pea vis-à-vis controls (without bacteria). Seeds treated with Streptomyces sp. S-9 showed abundant production of long root hairs. This isolate also encouraged the development of seedlings, including increased root (5.9 cm) and shoot lengths (8.5 cm) and the formation of root hair.
Fig. 3.
Disease severity of F. udum on Pigeon pea with different treatments. a Control, b T-2 S-9, c T-3 S-9 + F. udum, d T-4 Fusarium udum
Under pathogen-challenged conditions, the lowest (12.67%) pre-emergence disease incidence was observed in T-2 treatment (Streptomyces sp. S-9) followed by T-3 treatment (Streptomyces sp. S-9 + F. udum) (16.89%), and T-1 (Control) (10.23%). The significantly highest pre-emergence disease incidence (57.50%, respectively) was observed in T-4 treatment having only F. udum pre-inoculation. The incidence of wilt in Pigeon pea cultivar BDN-2 was monitored at 30, 60, and 90 DAS, respectively, in pot conditions (Table 2). S-9 treated seedlings challenged with pathogens also exhibited higher germination percentage, vigor index, and a significant increase in root and shoot length over untreated seedlings challenged with pathogens (Table 1; Fig. 3).
Table 2.
Different growth parameters of Pigeon pea plant (BDN2) inoculation (3 MAS)
| Treatment | Length (cm) | Fresh weight (g) | Dry weight (g) | |||
|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | |
| T-1 | 12.10 ± 0.72 | 26.67 ± 1.53 | 1.18 ± 0.08 | 0.93 ± 0.06 | 0.22 ± 0.02 | 0.23 ± 0.02 |
| T-2 | 13.77 ± 0.44 | 31.33 ± 1.00 | 1.55 ± 0.05 | 1.40 ± 0.12 | 0.42 ± 0.02 | 0.29 ± 0.01 |
| T-3 | 12.50 ± 0.61 | 31.00 ± 1.53 | 1.46 ± 0.06 | 0.93 ± 0.10 | 0.39 ± 0.03 | 0.25 ± 0.02 |
| T-4 | 10.90 ± 0.36 | 24.67 ± 0.58 | 0.82 ± 0.07 | 0.80 ± 0.10 | 0.18 ± 0.01 | 0.15 ± 0.01 |
Data are presented as mean ± SD, n = 3 according to Duncan multiple range test (DMRT) (P < 0.05). T-1—Control; T-2—S-9; T-3—S-9 + F. udum; T-4—F. udum
MAS Months after sowing
Effect of bacterial inoculation on biocontrol and stress mitigation under greenhouse condition
Data showed that the highest root (13.77, 12.50, 10.90, and 12.10 cm) and shoot (31.33, 31.00, 26.67, and 24.67 cm) length was observed with T-2 followed by T-3, T-4, and T-1, respectively (Table 2; Fig. 4). Pots with T-3 treatment combination significantly highest fresh root (1.55 g) and shoot (1.40 g) were recorded. In the case of the dry weight of the root and shoot results were observed in the following order T-3 > T-2 > T-1 and T-4 (Table 2). Streptomyces sp. strain S-9 treated Pigeon pea plants revealed a significant increase in the root (13.70 cm) and shoot lengths (31.00 cm), fresh root (1.46 g) and shoot (0.93 g), and dry shoot weights (0.25 g) over the control, it was also observed that the Streptomyces sp. strain S-9 treated plants under- challenged inoculation condition resulted in better plant growth and vigor vis-à-vis plants challenge with pathogens in the absence of seed bacterization.
Fig. 4.
Effect of inoculation with different treatment on the disease incidence and severity of Fusarium wilt and growth on Pigeon pea (90 DAI (days after inoculation) sown in autoclaved soil. Treatments: a T-1—Control, b T-2—S-9, c T-3—S-9 + F. udum, d T-4—Fusarium udum
Physiological and biochemical evaluation of plant
The degree of MDA, the last disintegration result of lipid peroxidation inside the leaves test plant treated with F. udum was altogether unique to control in all treatments. MDA aggregation in leaves was critical after 8 h of treatment and expanded bit by bit and topped at 48 h, at that point declined subsequently. It was observed in the following order T-3 (16.66μ mol g−1) > T-2 (12.33μ mol g−1) > T-1 (10.66μ mol g−1) and T-4 (7.66μ mol g−1). In case of total Proline concentration it was significantly varied in following order T-4 (14.83 mg g−1) > T-2 (13.16 mg g−1) ≥ T-3 (12.00 mg g−1) ≥ T-4 (11.00 mg g−1). However, maximum chlorophyll content was found in treatment (T-4) which consisted of S-9 (Fig. 5a-c).
Fig. 5.
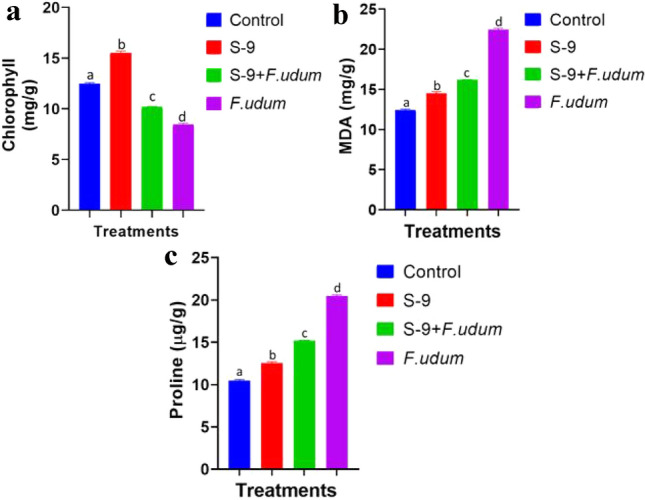
a–c Chlorophyll, proline, and MDA content in Pigeon pea leaves. The data obtained were statistically analysed using one-way ANOVA and Duncan’s Multiple Range Test using SPSS (Version 20). Differences were considered statistically significant at P ≤ 0.05. Data were presented as mean ± standard error of the mean (SEM) of five replicates except otherwise stated. The graph were prepared using Graphpad Prism (Version 8)
Effect of bacterial and fungal inoculation on H2O2 accumulation of Pigeon pea plant
Biocontrol mediated response of Pigeon pea plant towards H2O2 accumulation and modulation of defense enzymes under normal and stress conditions (Fig. 6a–c). In the present study, we observed more accumulation of H2O2 in leaves of fungus-treated Pigeon pea plant than in other treatments as indicated through high retention of DAB stain (Fig. 6a). Moreover, bacteria S-9 inoculation had reduced the formation of H2O2 in Pigeon pea leaves with fewer reddish-brown spots.
Fig. 6.

Representative photograph of in vivo DAB staining for visualization of H2O2 formed in Pigeon pea leaf at the end of the experiment. The reddish-brown colored spots in the leaves attested the take-up and polymerization of DAB to capture H2O2
Effect of fungal inoculation on H2O2 accumulation and defense enzymes of Pigeon pea
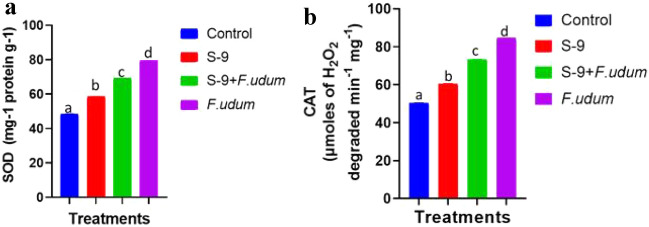
In the present study, we found that in leaves of fungus-treated Pigeon pea plants, DAB stain captured more H2O2 than in other treatments (Fig. 6b) compare to control. Besides, Pigeon pea leaves inoculated by bacteria substantially decreased the formation of H2O2 as conferred by the close non-availability of reddish-brown spots (Fig. 6b). At the same time, to analyze the quenching of accumulated H2O2 and other oxidative stress in Pigeon pea plants, we conducted defense enzyme assays such as superoxide dismutase (SOD) and catalase (CAT). Fungus-treated plants had observed significantly maximum activity for all antioxidant enzymes considered. However, all four biocontrol treatment has significantly lowered the activity for all the antioxidant enzymes under stress (S-9, S-9 + F. udum) when compared with only fungus treatment (Fig. 7). Under stress, F. udum challenged plants have exhibited maximum elevation in the defense enzymatic activity by 79.77% and 70.77% for SOD and CAT, respectively, in Pigeon pea plants (Fig. 7). Moreover, S-9 treatment has exhibited the least decrement by 25.24% and 30.61% for CAT and SOD, respectively, in Pigeon pea plants (Fig. 7).
Fig. 7.
Defense enzyme activities in Pigeon pea leaves. The data obtained were statistically analysed using one-way ANOVA and Duncan’s Multiple Range Test using SPSS (Version 20). Differences were considered statistically significant at P ≤ 0.05. Data were presented as mean ± standard error of the mean (SEM) of five replicates except otherwise stated. The graph were prepared using Graphpad Prism (Version 8)
Effect of fungus inoculation on histology of Pigeon pea root
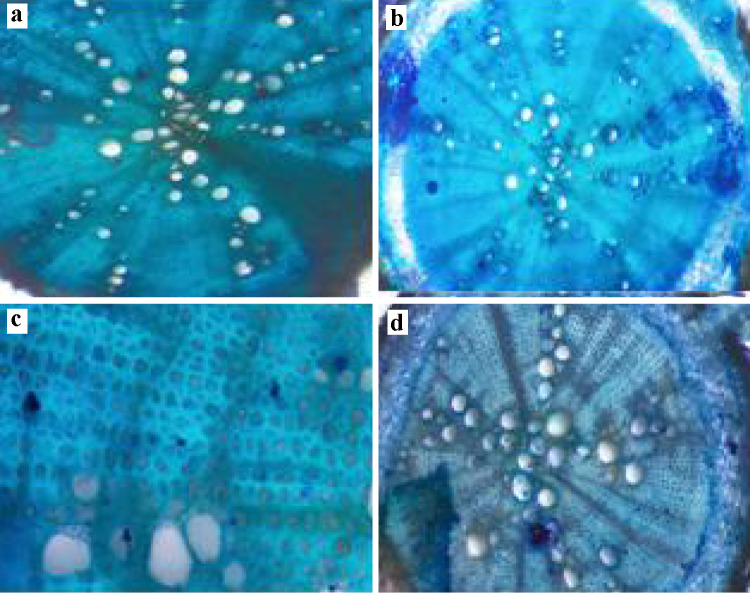
To research the adaptation acquired by the plant in stress environments, the anatomical changes in the root are unique and of utmost importance. We observed some anatomical changes in Pigeon pea root in the current microscopic analysis through various treatments under normal as well as stress condition conditions (Fig. 8), to protect against water loss, the root epidermis is essential and gas exchange also helps in the absorption of water and nutrients. In the current analysis, plants treated with bacterial strains showed an improvement in root epidermis thickness under usual and stressful conditions relative to the respective controls. (Fig. 8a, b). In normal conditions, S-9 treated plant roots showed the maximum thickness of endodermis while Fusarium inoculated plants showed maximum thickness in stress conditions (Fig. 8c, d). Xylem is an essential vascular tissue from root to stem and leaves to provide water and nutrients. It is fascinating that bacterial treatment has not only increased the size of xylem cells but also increased the number of xylem cells as opposed to regulation (Fig. 8a, b). However, it showed increased xylem size under stress, only Fusarium inoculated plant root significantly higher xylem number.
Fig. 8.
Effect of Fungus inoculation on Pigeon pea root anatomy (vascular bundle), root anatomy (epidermis) under normal and stress condition. a Root anatomy of Pigeon pea plant under control conditions. b Pigeon pea root anatomy (vascular bundle) under control condition. c Root anatomy of Pigeon pea plant under stress conditions. d Pigeon pea root anatomy (vascular bundle) under stress condition
Analysis of soil sample before and after treatment
The soil was slit loamy and slightly alkaline. Supplementary Table 1 shows the values of the soil physicochemical properties of the pot sample soil. There was a decrease in NPK and organic carbon content in the soil treated with F. udum. NPK content was higher in S-9 treated soil. Since many of the actinomycete isolates had a phosphate solubilizing activity, the available phosphorous content in the soil increases significantly due to the inoculation of Streptomyces sp. S-9. N and P content in the soil as compared to control by the inoculation of Streptomyces sp S-9 consistent. Under pot conditions, inoculation with isolate produced significant improvement in N and P content in the soil. Consistently there were seasonal variations among treatments over the year. This might be attributed to variations in bacterial population due to rapid wetting and drying of the soil. As the soil was deficient in available phosphorous and soil pH was very conductive for phosphate solubilization, microbial phosphate solubilization would have played a role in better plant growth and nutrient uptake.
Discussion
In this study, the actinomycete strain S-9 was identified as Streptomyces sp. This has shown antifungal activity against plant pathogenic fungal strain and particularly strong inhibition of the pathogen F. udum.
Streptomyces sp. isolates S-9 exhibited multiple PGPR traits such as IAA production, phosphate solubilization. This isolate possessing an IAA-producing trait enhanced the growth and nutrient uptake of Pigeon pea, cultivar BDN-2, under potted conditions. The plant rhizosphere is a flexible and dynamic biological condition of intense microbe plant interactions for outfitting fundamental micro–macro nutrients from a limited supplement pool (Jeffries et al. 2003). The plant advantageous qualities of these microorganisms are associated with various lytic enzymes and metabolites Either these molecules are liable for the concealment of pathogens using of Production of lytic enzymes and antimicrobial compounds or through ISR mediated plant boosting resistance just as the advancement of plant development through controllers creation (Lutgenberg and Kamilova 2009; Toumatia et al. 2015; Barka et al. 2016; Bubici, 2018). As soil conditions were conducive for IAA production and P-solubilization, this trait could have been involved in inhibiting pathogens in the rhizosphere and thus, suppressing the incidence of diseases (Zhao et al. 2013). If it is believed that IAA affects plant height due to hormonal impact and if increased plant height has a strong positive association with biomass, IAA may be presumed to be involved in boosting growth. The IAA yields of Streptomyces sp. strain S-9 were maximum (60.5 μgmL−1). Similar results were also reported by Sousa and Olivares (2016). Additionally, our outcomes confirmed that the Streptomyces sp. S-9 additionally produces an enzyme of β- 1, 3 glucanase that is responsible for indirect growth promotion of the test plant via inhibition of phytopathogenic fungi consisting of F. udum (Anupama et al. 2015).
Streptomyces sp S-9 decreased the infection in plants and it demonstrated the best in diminishing infection rate because of F. udum (Kumar et al. 2010). It has been observed in our experiments that Streptomyces sp. isolate S-9 which consistently enhanced growth, and the use of nutrients Pigeon pea under potted conditions had multiple plant growth-promoting traits. For a certain point in time, all the PGPR features could not be expressed. Thus the continuous supply of available nutrients was required for sustaining plant growth and development (Verma et al. 2015).
The potential of Streptomyces sp. S-9 was further verified by conducting potted plant growth experiments. Improved plant biological characteristics, such as shoot length, root length, and biomass, establish the PGP potential of Streptomyces sp. S-9.
Besides conferring protection against Fusarium infection, Streptomyces sp S-9 increased growth of Cajanus cajan seedlings as evidenced by an increase in plumule and radicle lengths and overall weight. Treatment of seeds with cultures especially, Streptomyces sp. S-9 resulted in a considerable increase in radical length. The effect was enhanced when the seed was treated with Streptomyces sp. S-9. An increase in radical length is important for seedlings as the increase in the radicle length increase the root surface area that results in increased water and mineral absorption from soil hence faster and enhanced growth of the plants (Fassler et al. 2010).
Streptomyces sp. S-9 effectively reduced the wilt rate on the Cajanus cajan plant. In this study, Streptomyces sp. S-9 has been shown to significantly reduce Fusarium head blight and populations in the rhizosphere. Numerous microorganisms colonize the rhizosphere improve the hurtful impacts of biotic stress, and upgrade plant development and advancement.
Plants infected with F. udum in the absence of Streptomyces strain as biocontrol, agents reduced the defense-related antioxidant enzyme activity (CAT and SOD). In response to pathogen infection, numerous studies have revealed that PGPA can activate a variety of defense responses in host plant structure, including the activation of antioxidant defense enzymes (Senthilraja et al. 2013; Bano and Muqarab 2017).
In the present study, we found that inoculating plants with Streptomyces sp S-9 significantly increased nutritional and biochemical indices such as chlorophyll content (total). Furthermore, under stressful conditions, Proline accumulation was significantly reduced. Previous studies have shown that inoculating PGPA from other host plants under stress circumstances has similar effects (Ullah and Bano 2015; Vurukonda et al. 2016; Li and Jiang 2017). The bacterial strain S-9 (Streptomyces sp.) was more effective in improving plant vegetative qualities, which could play a role in stress reduction and growth promotion.
Proline accumulation in plants is an important process that plays an important role in maintaining the osmotic balance between the intracellular and extracellular spaces while minimizing damage from stress (Lei et al. 2016).
Antioxidant enzymes regulate ROS production and play an important role in stressed plants. The results of this study showed that the number of antioxidant enzymes was significantly reduced in the Pigeon pea plants that were inoculated with bacteria under stress. In addition, in this study, bacterially treated Pigeon pea plants under upper and stress conditions had thicker root epidermis and increased xylem tissue as an anatomical result compared to their respective control groups. Bacterial-mediated increases in the thickness of the epidermis of pigeon roots and the number of xylem tissues have been demonstrated by previous studies, but only under normal conditions (Rêgo et al. 2014). Some studies have shown that increasing the number and diameter of all vessels promotes water-stress tolerance in legumes (Choate et al. 2008; Purushothaman et al. 2013).
Soil microbes play a very important role in agricultural productivity, mainly by improving soil quality (Barea et al. 2013; Lugtenberg 2015). This study sees that PGPA increases soil fertility, which positively affects that indicator. This is consistent with several previous studies that showed that the total organic carbon content of the soil was directly proportional to PGPA (Valarini et al. 2003; Wu et al. 2005). pH has been reported as an important factor affecting phosphate availability and mineralization from soil (Wu et al. 2005). This discovery was made by Srivastava et al. 2014. PGPA improved soil organic matter content and soil properties, such as total nitrogen, and texture (Wu et al. 2005). Soil pH and EC are commonly used as soil health indicators and have been reported to alter the biological composition of the soil (XiuMei et al. 2008). In addition, based on the results of pot experiments, the possibility of the strain Streptomyces sp. S-9 for improving the growth of pigeon bean plants was evaluated. The results of the greenhouse experiment showed that Streptomyces sp. S-9 inoculation resulted in better plant growth compared to the control group and the 100% recommended NPK.
Conclusions
Application of Streptomyces sp. strain S-9 on a soil–plant system under greenhouse/field conditions can be a valuable tool for increased Pigeon pea growth and development; therefore, in this study, an examination of Streptomyces sp. strain S-9 with different treatment combinations to plant growth-promoting traits was carried out under in vitro and pot conditions. Influence of Streptomyces sp. strain S-9 production of plant growth traits such as antagonistic activity against the fungal pathogen F. udum and in controlling the wilt disease in Pigeon pea. We also reported that plant development advancing traits IAA and P-solubilization stimulated the vegetative and reproductive growth of Pigeon pea. Strains were able to efficiently plant growth promotion, therefore, showed great potential for use as novel biofertilizers. Overall, the efficient application of PGPA can be an alternative and promising technology to Pigeon pea crop sustainability under sustainable agriculture. In short, these strains of actinomycetes may be potential biological control candidates for the ecological management of Fusarium wilt and, therefore, may limit the excessive use of synthetic fungicides and their associated harmful effects on humans and the environment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported in part by University Grants Commission (UGC) for providing Basic Science Research (BSR) fellowship. We are also grateful to Department of Microbiology and Biotechnology Centre, The Maharaja Sayajirao University of Baroda for providing basic infrastructure to support this research.
Author contributions
SI conceived and co-ordinate the research. AD conducted experiments and analyzed the data. AD and SI wrote the manuscript. Both authors read and approved the manuscript.
Funding
This work was supported in part by University Grants Commission (UGC) for providing Basic Science Research (BSR) fellowship (Grant No. F. 25-1/2014-15(BSR)/7-128/2007(BSR)). We are also grateful to Department of Microbiology and Biotechnology Centre, The Maharaja Sayajirao University of Baroda for providing basic infrastructure to support this Research.
Data availability
All data generated or analysed during this study are included in this published article and additional supplementary file.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Contributor Information
Anand Dave, Email: ananddave16@gmail.com.
Sanjay Ingle, Email: ingle05@yahoo.co.in.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Anupama NB, Jogaiah S, Ito S, Nagraj KA, Tran LS. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci. 2015;231:62–73. doi: 10.1016/j.plantsci.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano A, Muqarab R. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.) Plant Boil (stuttgart, Germany) 2017;19:406–412. doi: 10.1111/plb.12535. [DOI] [PubMed] [Google Scholar]
- Barea JM, Pozo MJ, Azcón R, Azcón Aguilar C. Microbial interactions in the rhizosphere. In: de Bruijn FJ, editor. Molecular microbial ecology of the rhizosphere. Hoboken: Wiley; 2013. pp. 29–44. [Google Scholar]
- Barka EA, Vatsa P, Sanchez L. Taxonomy, physiology, and natural products of Actinobacteria. Microbiol Mol Biol Rev. 2016;80:1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Boukerma L, Benchabane M, Charif A, Khelifi L. Activity of plant growth promoting rhizobacteria (PGPRs) in the biocontrol of tomato Fusarium wilt. Plant Protect Sci. 2017;53:78–84. doi: 10.17221/178/2015-PPS. [DOI] [Google Scholar]
- Bredholdt H, Galatenko OA, Engelhardt K, Fjærvik E, TErekhova LP, Zotchev SB. Rare actinomycete bacteria from the shallow water sediments of the Trondheim fjord, Norway: isolation, diversity and biological activity. Environ Microbiol. 2007;9:2756–2764. doi: 10.1111/j.1462-2920.2007.01387.x. [DOI] [PubMed] [Google Scholar]
- Bubici G. Streptomyces spp. as biocontrol agents against Fusarium species. Cab Reviews (050) 2018 doi: 10.1079/PAVSNNR201813050. [DOI] [Google Scholar]
- Choate B, Cobb AR, Jansen S. Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytol. 2008;177:608–626. doi: 10.1111/j.1469-8137.2007.02317.x. [DOI] [PubMed] [Google Scholar]
- Conn VM, Walker AR, Franco CM. Endophytic actinobacteria induce defense pathways in Arabidopsis thaliana. Mol Plant Microbe Interact. 2008;21:208–218. doi: 10.1094/MPMI-21-2-0208. [DOI] [PubMed] [Google Scholar]
- FAOSTAT (2019) http://www.fao.org/faostat/en/#data/QC
- Fassler E, Evangelou MW, Robinson BH, Schulin R. Effects of indole-3- acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS) Chemosphere. 2010;80:901–907. doi: 10.1016/j.chemosphere.2010.04.077. [DOI] [PubMed] [Google Scholar]
- Fazeli F, Ghorbanli M, Niknam V. Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant. 2007;51(1):98–103. doi: 10.1007/s10535-007-0020-1. [DOI] [Google Scholar]
- Gopalakrishnan S, Srinivas V, Prasanna SL. Streptomyces. In: Amaresan N, Kumar MS, Annapurna K, Kumar AK, Sankaranarayanan A, editors. Beneficial microbials in ago-ecology. Paperback: Elsevier; 2020. pp. 55–71. [Google Scholar]
- Guan XJ, Liu CX, Zhao JW, Fang BZ, Zhang YJ, Li LJ, Jin PJ, Wang XJ, Xiang WS. Streptomyces maoxianensis sp. nov., a novel actinomycete isolated from soil in Maoxian, China. Antonie Van Leeuwenhoek. 2015;107:1119–1126. doi: 10.1007/s10482-015-0403-9. [DOI] [PubMed] [Google Scholar]
- Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils. 2003;37:1–16. doi: 10.1007/s00374-002-0546-5. [DOI] [Google Scholar]
- Ji SH, Paul NC, Deng JX, Kim YS, Yun BS, Yu SH. Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology. 2013;41(4):234–242. doi: 10.5941/MYCO.2013.41.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LY, Zhao Y, Song W, Duan LP, Jiang SW, Wang XJ, Zhao JW, Xiang WS. Streptomyces inhibens sp. nov., a novel actinomycete isolated from rhizosphere soil of wheat (Triticum aestivum L.) Int J Syst Evol Microbiol. 2019;69:688–695. doi: 10.1099/ijsem.0.003204. [DOI] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:112–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kinkel LL, Schlatter DC, Bakker MG, Arenz BE. Streptomyces competition and co-evolution in relation to plant disease suppression. Res Microbiol. 2012;163:490–499. doi: 10.1016/j.resmic.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Kumar H, Bajpai VK, Dubey RC, Maheshwari DK, Kang SC. Wilt disease management and enhancement of growth and yield of Cajanus cajan (L) var. Manak by bacterial combinations amended with chemical fertilizer. Crop Prot. 2010;29(6):591–598. doi: 10.1016/j.cropro.2010.01.002. [DOI] [Google Scholar]
- Kumar P, Thakur S, Dhingra GK, Singh A, Pal MK, Harshvardhan K, Maheshwari DK. Inoculation of siderophore producing rhizobacteria and their consortium for growth enhancement of wheat plant. Biocatal Agric Biotechnol. 2018;15:264–269. doi: 10.1016/j.bcab.2018.06.019. [DOI] [Google Scholar]
- Kurth F, Mailander S, Bonn M, Feldhahn L, Herrmann S, Grosse I. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol Plant Microbe Interact. 2014;27:891–900. doi: 10.1094/MPMI-10-13-0296-R. [DOI] [PubMed] [Google Scholar]
- Lei P, Xu Z, Liang J, Luo X, Zhang Y, Feng X. Poly (γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. Plant Growth Regul. 2016;78:233. doi: 10.1007/s10725-015-0088-0. [DOI] [Google Scholar]
- Li HQ, Jiang XW. Inoculation with Plant Growth-Promoting Bacteria (PGPB) improves salt tolerance of maize seedling. Russ J Plant Physiol. 2017;64:235–241. doi: 10.1134/S1021443717020078. [DOI] [Google Scholar]
- Liang ZC, Hseu RS, Wang HH. Partial purification and characterization of a 1, 3-β-D-glucanase from Ganoderma tsugae. J Ind Microbiol. 1995;14:5–9. doi: 10.1007/BF01570058. [DOI] [Google Scholar]
- Lugtenberg B. Life of microbes in the rhizosphere. In: Lugtenberg B, editor. Principles of plant-microbe interactions. Heidelberg: Springer; 2015. pp. 7–15. [Google Scholar]
- Lutgenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 2009;63:541–556. doi: 10.1146/annurev.micro.62.081307.162918. [DOI] [PubMed] [Google Scholar]
- Manoj KP, Kumar V, Kumar M, Shrivastava N, Varma A. Potassium solubilizing microorganisms for sustainable agriculture. New York: Springer; 2016. Rhizosphere microbes: potassium solubilization and crop productivity present and future aspects; pp. 315–325. [Google Scholar]
- Melent’ev AI, Helisto P, Kuzmina LY, Galimzyanova NF, Aktuganov GE, Korpela T. Use of antagonistic bacilli for biocontrol of fungi degrading fresh wood. Appl Biochem Microbiol. 2006;42:62–66. doi: 10.1134/S000368380601009. [DOI] [Google Scholar]
- Misra S, Dixit VK, Khan MH, Kumar MS, Dviwedi G, Yadav S, et al. Exploitation of agro-climatic environment for selection of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing salt tolerant indigenous plant growth promoting rhizobacteria. Microbiol Res. 2017;205:25–34. doi: 10.1016/j.micres.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Morrissey J, Dow J, Mark G, O'Gara F. Are microbes at the root of a solution to world food production? EMBO rept. 2004;5(10):922–926. doi: 10.1038/sj.embor.7400263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. Methods Soil Anal Part 3 Chem Methods. 1996;5:961–1010. [Google Scholar]
- Newitt JT, Prudence SMM, Hutchings MI, Worsley SF. Biocontrol of cereal crop diseases using Streptomycetes. Pathogens. 2019;8:1–25. doi: 10.3390/pathogens8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien TP, Feder N, McCully ME. Polychromatic staining of plant cell walls by toluidine blue. Protoplasma. 1964;59:368–373. doi: 10.1007/BF01248568. [DOI] [Google Scholar]
- Olanrewaju OS, Babalola OO. Streptomyces: implications and interactions in plant growth-promotion. Appl Microbiol Biotechnol. 2019;103:1179–1188. doi: 10.1007/s00253-018-09577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parte AC, Sarda CJ, Meier-Kolthoff JP, Reimer LC, Goker M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol. 2020;70:5607–5612. doi: 10.1099/ijsem.0.004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CL, Glick BR. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol. 1996;42(3):207–220. doi: 10.1139/m96-032. [DOI] [PubMed] [Google Scholar]
- Pikovskaya RI. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiology. 1948;17:362–370. [Google Scholar]
- Purushothaman R, Zaman-Allah M, Mallikarjuna N, Pannirselvam R, Krishnamurthy L, Gowda CLL. Root anatomical traits and their possible contribution to drought tolerance in grain legumes. Plant Prod Sci. 2013;16:1–8. doi: 10.1626/pps.16.1. [DOI] [Google Scholar]
- Qin S, Xing K, Jiang JH, Xu LH, Li WJ. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl Microbiol Biotechnol. 2011;89:457–473. doi: 10.1007/s00253-010-2923-2926. [DOI] [PubMed] [Google Scholar]
- Rangani J, Parida AK, Panda A, Kumari A. Coordinated changes in antioxidative enzymes protect the photosynthetic machinery from salinity induced oxidative damage and confer salt tolerance in an extreme halophyte Salvadora persica L. Front Plant Sci. 2016;7:50. doi: 10.3389/fpls.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rêgo MCF, Ilkiu-Borges F, de Filippi MCC, Gonçalves LA, da Silva GB (2014) Morphoanatomical and biochemical changes in the roots of Rice plants induced by plant growth-promoting microorganisms J Bot: 818797
- Schwyn B, Neilands JB. Universal chemica lassay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Senthilraja G, Anand T, Kennedy J, Raguchander T, Samiyappan R. Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leaf miner insect and collar rot pathogen. Physiol Mol Plant Pathol. 2013;82:10–19. doi: 10.1016/j.pmpp.2012.12.002. [DOI] [Google Scholar]
- Sharma M, Ghosh R, Telangre R, Rathore A, Saifulla M, Mahalinga DM, Saxena DR, Jain YK. Environmental influences on Pigeon pea-Fusarium udum interactions and stability of genotypes to Fusarium wilt. Front Plant Sci. 2016;7:253. doi: 10.3389/fpls.2016.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa JADJ, Olivares FL. Plant growth promotion by Streptomycetes: ecophysiology, mechanisms and applications. Chem Biol Technol Agric. 2016;3(1):24. doi: 10.1186/s40538-016-0073-5. [DOI] [Google Scholar]
- Tang L, Xia Y, Fan C, Kou J, Wu F, Li W, Pan K. Control of Fusarium wilt by wheat straw is associated with microbial network changes in watermelon rhizosphere. Sci Rep. 2020;10:12736. doi: 10.1038/s41598-020-69623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- Toumatia O, Yekkour A, Goudjal Y. Antifungal properties of an actinomycin D-producing strain, Streptomyces sp. IA1, isolated from a Saharan soil. J Basic Microbiol. 2015;55:221–228. doi: 10.1002/jobm.201400202. [DOI] [PubMed] [Google Scholar]
- Ullah S, Bano A. Isolation of plant-growth-promoting rhizobacteria from rhizospheric soil of halophytes and their impact on maize (Zea mays L.) under induced soil salinity. Can J Microbiol. 2015;61:307–313. doi: 10.1139/cjm-2014-0668. [DOI] [PubMed] [Google Scholar]
- Valarini PJ, Diaz alvarez MC, Gasco JM, Guerrero F, Tokeshi H. Assessment of soil properties by organic matter and EM microorganism incorporation. R Bras Ci Solo. 2003;27:519–525. doi: 10.1590/S0100-06832003000300013. [DOI] [Google Scholar]
- Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, Saxena AK, Suman A. Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol. 2015;65(4):1885–1899. doi: 10.1007/s13213-014-1027-4. [DOI] [Google Scholar]
- Vurukonda SSKP, Vardharajula S, Shrivastava M, Ali SKZ. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Vyas P, Deshpande MV. Chitinase production by Myrothecium verrucaria and its significance for fungal mycelia degradation. J Gen Appl Microbiol. 1989;35(5):343–350. doi: 10.2323/jgam.35.343. [DOI] [Google Scholar]
- Wang X, Wang C, Li Q, et al. Isolation and characterization of antagonistic bacteria with the potential for biocontrol of soil-borne wheat diseases. J Appl Microbiol. 2018;125(6):1868–1880. doi: 10.1111/jam.14099. [DOI] [PubMed] [Google Scholar]
- Wu SC, Cao ZH, Li ZG, Cheung KC, Wong MH. Effects of biofertilizers containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial. Geoderma. 2005;125:155–166. doi: 10.1016/j.geoderma.2004.07.003. [DOI] [Google Scholar]
- Xiu-Mei L, Qi L, Wen-Ju L, Yong J. Distribution of soil enzyme activities and microbial biomass along a latitudinal gradient in farmlands of Songliao plain, Northeast China. Pedosphere. 2008;18:431–440. doi: 10.1016/S1002-0160(08)60034-X. [DOI] [Google Scholar]
- Zhao J, Xue QH, Niu GG, Xue L, Shen GH, Du JZM. Extracellular enzyme production and fungal mycelia degradation of antagonistic Streptomyces induced by fungal mycelia preparation of cucurbit plant pathogens. Ann Microbiol. 2013;63:809–812. doi: 10.1007/s13213-012-0507-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and additional supplementary file.