Abstract
Objective:
Cigarette smoke (CS) is a primary risk factor for the development of numerous benign and malignant laryngeal diseases. The epithelium and mucus lining the vocal folds (VF) are the first barriers against CS. The primary objective of this study was to investigate epithelial and mucus barrier changes in the mouse laryngeal mucosa upon exposure to subacute CS. The secondary objective was to compare mucus barrier changes in mice and human smokers and nonsmokers.
Methods:
Mice were exposed to CS for 4-weeks for 4-hours [N=12, high dose, (HD)] or 1-hour [N=12, (low dose (LD)] per day. Air-exposed mice were used as a control group (N=10). Larynges were harvested and VF epithelial barrier integrity was evaluated including cellular proliferation and expression of cell junctions. We also investigated mucus production by examining mucus cell area and mucin expression in mice and human smokers and nonsmokers.
Results:
HD CS increased VF epithelial cellular proliferation but did not alter expression of cell junctions. HD CS also induced hypertrophy of the mucus-producing submucosal glands. However, only LD CS increased MUC5AC gene expression. MUC5AC staining appeared elevated in laryngeal specimens from smokers, but this was not significant as compared to nonsmokers.
Conclusion:
These findings help us identify potential adaptive mechanisms to CS exposure as well as set the foundation for further study of key aspects of epithelial and mucus barrier integrity that may be implicated in laryngeal disease development following prolonged smoking.
Keywords: Cigarette smoke, Mouse model, Vocal fold, Epithelium, Mucus
INTRODUCTION
Multiple laryngeal diseases including chronic laryngitis, Reinke’s edema, laryngeal leukoplakia, and laryngeal cancer are associated with environmental exposure to cigarette smoke (CS).1–4 CS is a mixture of more than 7000 toxicants, including carcinogens and currently, an estimated 40 million adults smoke.5, 6 CS-induced benign and malignant diseases are not trivial and impair critical laryngeal functions including respiration, coughing, swallowing, and in humans, voice production. Specifically, CS may influence voice production directly by causing inflammation and dehydration of the vocal folds.7 The larynx is also a target of CS in terms of the significant discomfort associated with smoking.8 CS may induce a phonotraumatic cough and throat clearing behaviors, which can lead to vocal fold edema and inherent cellular damage.3 (Dworkin, inflammation) It is imperative to elucidate the pathophysiological mechanisms of CS-induced laryngeal disease in order to improve clinical management. However, our understanding remains incomplete, especially as pertains to the outermost portions of the vocal folds (VF), the epithelial and mucus barriers.
The VF epithelium is the first layer of cellular contact upon inhalation of CS and serves a critical barrier function.9 It is stratified squamous type and composed of multiple cell layers joined together by tight and adherens cell junctions.10, 11 The epithelium is derived from a population of basal progenitor cells that express transcription factor TP63 (p63).12 Epithelial turnover, or proliferation, begins in the basal layer and following division, cells replenish the epithelium as they differentiate and migrate towards the surface.13 Earlier studies have evaluated the effects of CS on the epithelium in the supraglottic and subglottic regions of the larynx.14–16 However, these regions are lined by pseudostratified columnar epithelium, a different type than the stratified squamous cells covering the VF. Furthermore, previous work has demonstrated regional heterogeneity among airway epithelial cell types in response to CS.17 It has been shown that CS induces VF epithelial hyperplasia.18 However, the impact of CS on VF epithelial cell junctions and proliferation, both key to barrier integrity, have not been evaluated in detail.
A thin layer of fluid, or mucus, covers the VF epithelial surface.9 Mucus is secreted from goblet cells embedded within the supraglottic and subglottic epithelium and underlying submucosal glands. Mucus binds and traps inhaled irritants for clearance from the larynx through coughing or swallowing and lubricates the VF during vibration.19 Mucin glycoproteins are the most important component of mucus and dictate its defensive and physical properties.20 While there is some evidence CS induces laryngeal subglandular hypertrophy and increases mucus production,8, 21 there is an overall paucity of information related to CS-induced changes in mucus production, particularly mucin expression, in the larynx.
Sustained insults from CS that disrupt VF epithelial integrity or alter mucus production may diminish the protective capacity offered by these barriers and contribute to disease development. Consequently, the primary objective of this study was to investigate epithelial and mucus barrier changes in mouse laryngeal mucosa upon exposure to CS. This model was chosen as mouse laryngeal organization is similar to that of humans22 and used extensively to evaluate CS-induced inhalation injuries in other regions of the airway.23, 24 Mice were exposed to CS for 4-weeks for 4-hours [high dose, (HD)] or 1-hour [(low dose (LD)] per day. Air-exposed mice were utilized as a control group. We evaluated critical aspects of VF epithelial barrier integrity including cellular proliferation and cell junctions. We also examined CS-induced mucus production by examining mucus cell area and mucin expression. While it is established that the mouse VF epithelial barrier is similar to humans in terms of cellular composition and junctions,25 minimal work has been published regarding use of a mouse model for evaluating laryngeal mucus production. Consequently, a secondary objective of this study was to evaluate mucin expression in human laryngeal specimens from smokers and nonsmokers and compare those findings to that observed in the mouse.
MATERIALS AND METHODS
Animals
Animal protocols were performed with prior approval from the Stanford University Institutional Animal Care and Use Committee. Adult male C57BL/6 mice were given standardized food, water ad libitum and housed separately in ventilated cages in a designated temperature-controlled room with 12:12h light–dark cycle in the Stanford School of Medicine Veterinary Service Center. Animal health was monitored daily.
Cigarette Smoke Exposure (CSE)
Fifteen-week-old mice were randomly assigned into three groups: HD CSE (n=12), LD CSE (n=12), and room air-exposed controls (n=10). Mice in the CSE groups were exposed 5 days/week, for 4-weeks (Fig. 1A). HD and LD group mice were exposed to 4-hours and 1-hour of CS per day, respectively (Fig. 1B). Based on Organization for Economic Co-operation and Development (OECD) inhalation exposure guidelines, 4-weeks exposure is considered a subacute inhalation toxicity study (OECD TG 412). In addition, previous studies demonstrate that 1–4 hours of CSE per day induces pathological changes within the airways.18, 26, 27 Age-matched control mice were left in room air in their original housing conditions.
Figure 1.
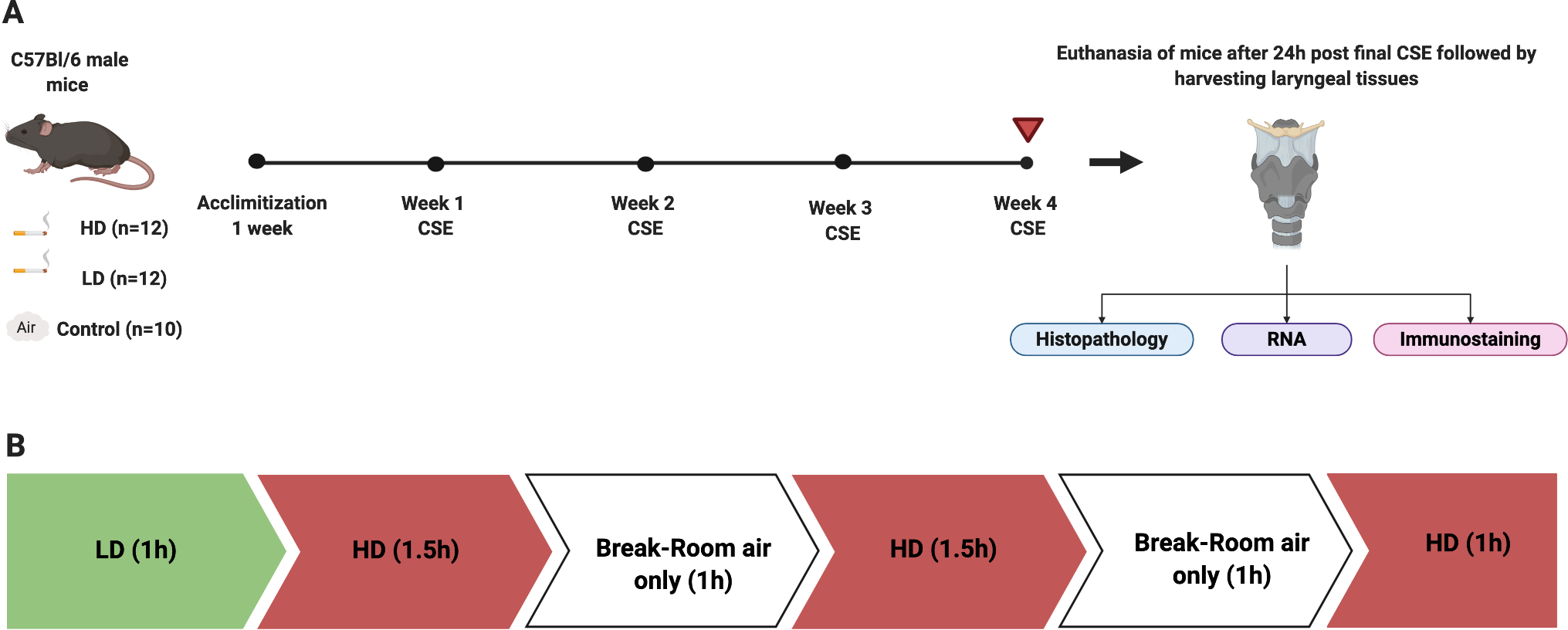
Experimental schematic. Mice were exposed to HD or LD CSE or air control exposures for 4 weeks (A). Prior to the start of 4-week CS exposures, LD and HD group mice were acclimatized to 20min of CS per day, for 1-week (M-F). HD exposures occurred in 1–1.5 hour increments with a 30 minute break in order to minimize Carboxyhemoglobin (CoHB) buildup and exposure-related stress (Fig. 1B). Following euthanasia, larynges were harvested and processed for histopathology, gene expression, or immunostaining. LD exposures included 1 hour of CS per day and HD exposures included 4 hours per day (B).
Mice in the HD and LD groups were exposed to whole body mainstream CS (Delivered dose: 5.4882 mg/kg/cigarette; Supplementary data S1) using the “inExpose” smoking system and Flexiware software (SCIREQ, Montreal, Canada). 3R4F reference research cigarettes (University of Kentucky, KY, USA) were smoked according to the following standards (ISO 1991): one 35 ml puff of 2-second duration followed by 58 seconds of fresh air at a rate of 2L/min. The CS was directed in a 5-liter volume whole body exposure chamber in which mice were divided by separators. The average total particulate matter per cubic meter of air (TPM) was 621 mg/m3 for both LD and HD exposures (Supplementary data S2). Mice were euthanized 24-hours following the final exposure. Larynges were harvested for morphometric, gene, and immunofluorescent (IF) assessments (Fig. 1A). Number of animals used in each assessment are provided in figure legends.
Human Sample Acquisition
Six healthy participants (23–42 years) with no history of laryngeal, respiratory, or gastrointestinal disease were recruited. The protocol was approved by the Institutional Review Board of Stanford University and informed consent was obtained from all participants. Three nonsmokers (2 males, 1 female) had not smoked during the previous year. Three smokers (3 males) consumed a minimum of 2 cigarettes/day (range 2–3 cigarettes) for one or more years. All smokers also used electronic nicotine delivery systems (range 1x/month – 3x/day) for at least one year.
False vocal fold (FVF) tissue was sampled from smokers and non-smokers. An Olympus ENF-T3 flexible fiberoptic laryngoscope with biopsy forceps passed through the biopsy channel was used to collect two biopsies, 2 mm each, following topical anesthesia with 4% lidocaine. FVF samples were fixed in 4% paraformaldehyde overnight at 4°C and sent to histology services in the Department of Pathology, for paraffin embedding and microtomy. 5μm sized sections were obtained and processed for IF of mucin.
Mouse Larynx Morphometric Analysis
Mouse larynges were fixed in 4% paraformaldehyde overnight at 4°C and sent to HistoWiz, Inc. (http://www.histowiz.com; Brooklyn, NY, USA) in 70% ethanol for processing, paraffin embedding and sectioning. Larynges were sectioned anterior to posterior and coronal sections (5μm) were stained with Hematoxylin & Eosin (H&E) and Alcian blue/Periodic Acid Schiff (AB/PAS). Whole-stained slides were scanned and digitized (Aperio AT2, Leica Biosystems, Germany) for morphometric analysis of vocal fold epithelial thickness (H&E) and submucosal gland area and composition (AB/PAS). Morphometric analysis was conducted on a single section per larynx. The region of interest for analysis was at an approximate laryngeal depth of 150–200 μm, which corresponds to the mid-membranous VF. For epithelial thickness, the right and left VF epithelium was traced using Aperio ImageScope v.12.3.2.8013 (Leica Biosystems) and thickness was computed as an average between five different points. For submucosal gland area, the exterior border of the entire subglandular region across the right and left subglottis spanning from the lower border of the VF to the first tracheal ring was traced and the average area was computed using Fiji (derivative of ImageJ; NIH, USA). For quantification of mucus composition, individual images of the AB-stained acidic and PAS-stained neutral mucus were obtained using color deconvolution. The exterior borders of AB- and PAS-stained glands were then traced across the left and right subglottis and average area of acidic and neutral mucus, respectively, was obtained. Finally, the percentage of acidic and neutral mucus was calculated by dividing the average area of acidic or neutral mucus by the average total subglandular area and multiplying by 100.
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Mucosa and submucosal tissues from the upper border of the mouse glottis to the lower level of the cricoid cartilage were harvested for qPCR analyses. Mouse-specific primer sequences for cell junction and mucin genes are displayed in Supplementary Table 1. ß-actin was selected as the endogenous reference gene. qPCR was performed using iTaq™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA) in the CFX96 Real-Time PCR Machine (Bio-Rad). Relative quantitative analysis of gene expression was completed using the standard comparative Ct method (2-ΔΔCt).28 All samples were run in duplicate.
Immunofluorescence (IF)
Antibodies against cell junction and mucin proteins are displayed in Supplementary Table 2. Heat-induced antigen retrieval was performed, followed by blocking. Primary antibodies were applied overnight, washed, and then slides were treated for 1-hour with secondary antibodies (Supplementary Table 2). Slides were mounted with ProLong Gold Antifade Mountant formulated with DAPI (Invitrogen™, CA, USA). Images were acquired using an AxioImager M1 microscope (Carl Zeiss, Gottingen, Germany). Ki67 and p63 labeled mouse VF epithelial cells were counted across a length of 450μm (Fiji). Mean fluorescence intensity (MFI) was calculated across a fixed epithelial length of 230μm for mouse cell junctions and 350μm for human MUC5AC (Zen 3.1, Carl Zeiss, Gottingen, Germany). MFI for mouse MUC5AC was computed across six randomly selected circular regions with a 130μm diameter in the right and left subglottis.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism version 9.0. All mouse epithelial and mucus barrier assessments were analyzed by one-way analysis of variance (ANOVA) and post hoc Fisher’s Least Significant Difference (LSD) multiple comparison test. Mann-Whitney U test was used to evaluate mucin expression between human smokers and nonsmokers. Values less than p ≤ 0.05 were considered significant. All data are represented in figures as means ± standard deviations (SD).
RESULTS
CSE and Epithelial Barrier Integrity
VF epithelial thickness was evaluated on H&E-stained slides in control (Fig. 2A), LD (Fig. 2B), and HD (Fig. 2C) groups. Thickness was significantly increased in the HD as compared to control (p = 0.0056, 95% confidence interval [CI]: −9.515 to −2.041) and LD groups (p = 0.0147, 95% confidence interval [CI]: −9.190 to −1.224) (Fig. 2D).
Figure 2.
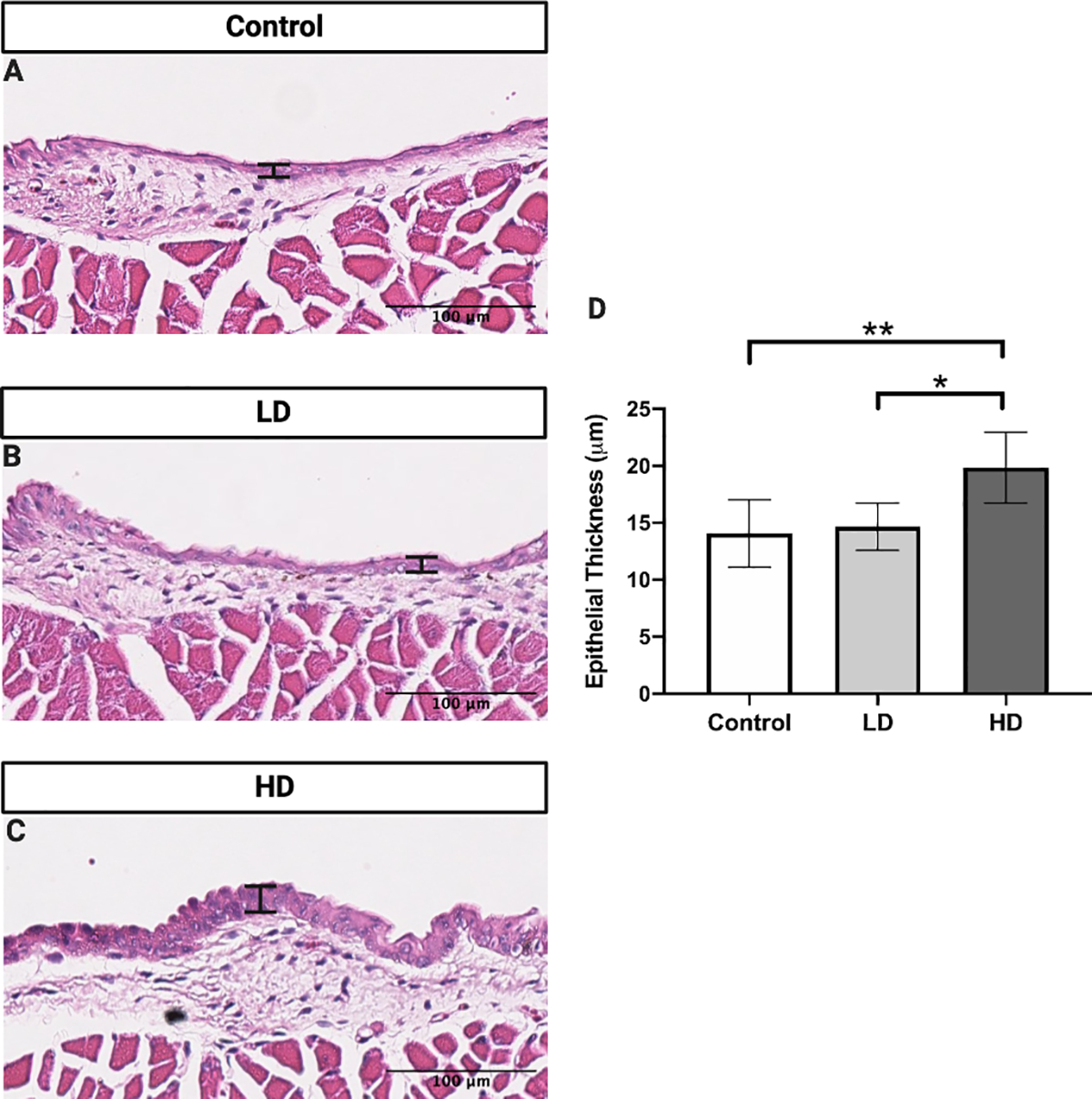
VF epithelial thickness following control (A), LD (B), and HD (C) CSE. VF epithelial thickness was significantly increased following HD CSE when compared to control and LD CSE (D). N=5 in control group. N=4 in LD group. N=6 in HD group. H&E stained images are at a magnification of 200x. Bar graph shows the mean with standard deviation (SD). * indicates p ≤ 0.05. ** indicates p ≤ 0.01.
VF epithelial proliferation was evaluated by staining for Ki67, a marker for proliferation in control (Fig. 3A), LD (Fig. 3B), and HD (Fig. 3C) groups. HD mice had significantly greater Ki67+ cells than the control mice (p = 0.0162, 95% confidence interval [CI]: −12.75 to −1.913) (Fig. 3D). Ki67 labeled proliferative cells remained unaltered in the LD group in comparison to the controls.
Figure 3.
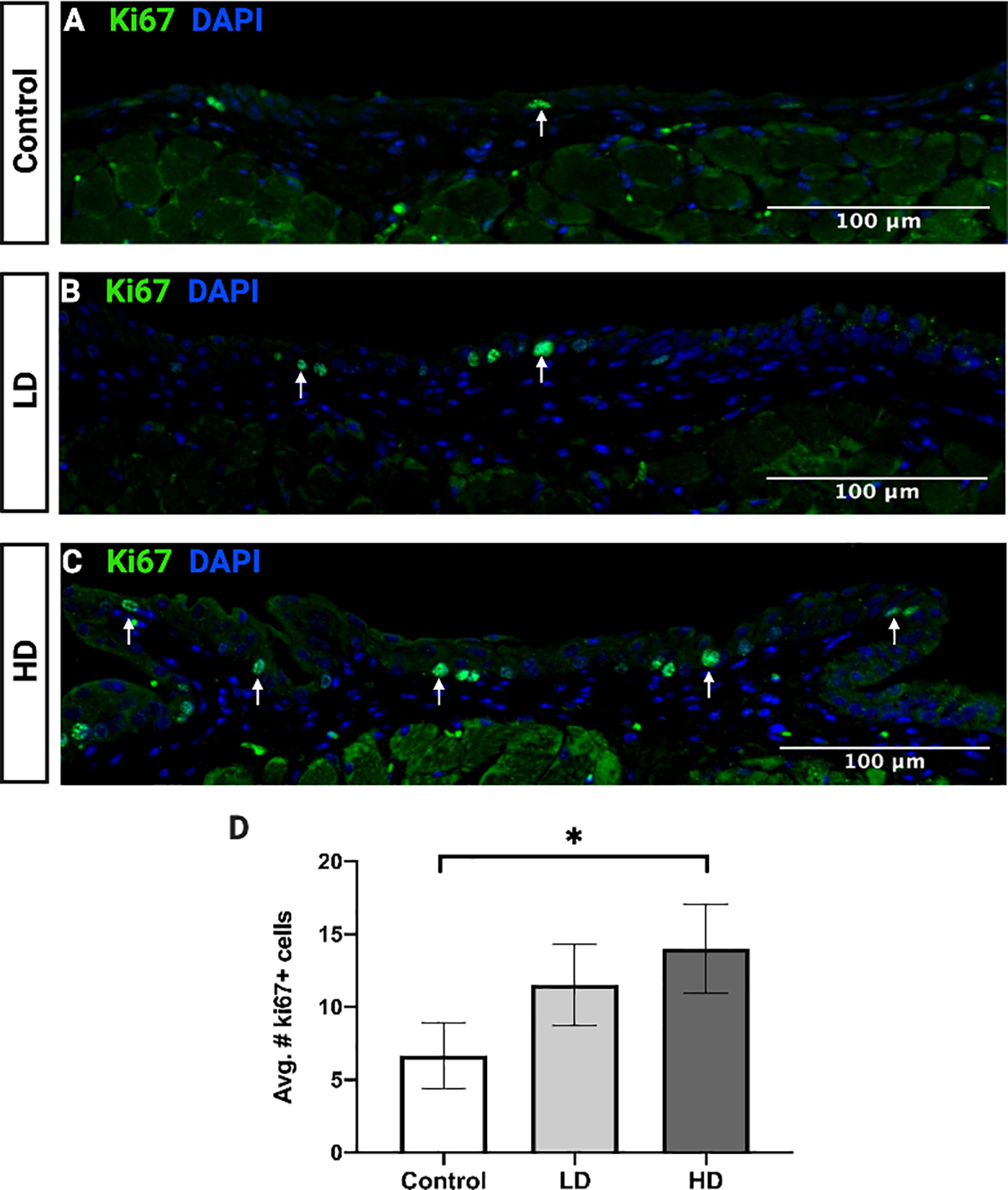
VF epithelial proliferative cells following control (A), LD (B), and HD (C) CSE. The number of Ki67 labeled proliferative cells (green) was significantly increased following HD CSE when compared to control (D). N=3 in all groups. Fluorescent stained images are at a magnification of 200x. Hoechst (blue) was used as a nuclear stain. Bar graph shows the mean with SD. * indicates p ≤ 0.05.
VF epithelial basal cells were evaluated by staining for p63 in control (Fig. 4A), LD (Fig. 4B), and HD (Fig. 4C) groups. CS exposed mice in both LD (p = 0.0067, 95% confidence interval [CI]: −30.47 to −7.528) and HD (p = 0.0039, 95% confidence interval [CI]: −32.81 to −9.861) groups exhibited a significantly greater number of p63+ cells in the VF in comparison to controls (Fig. 4D).
Figure 4.
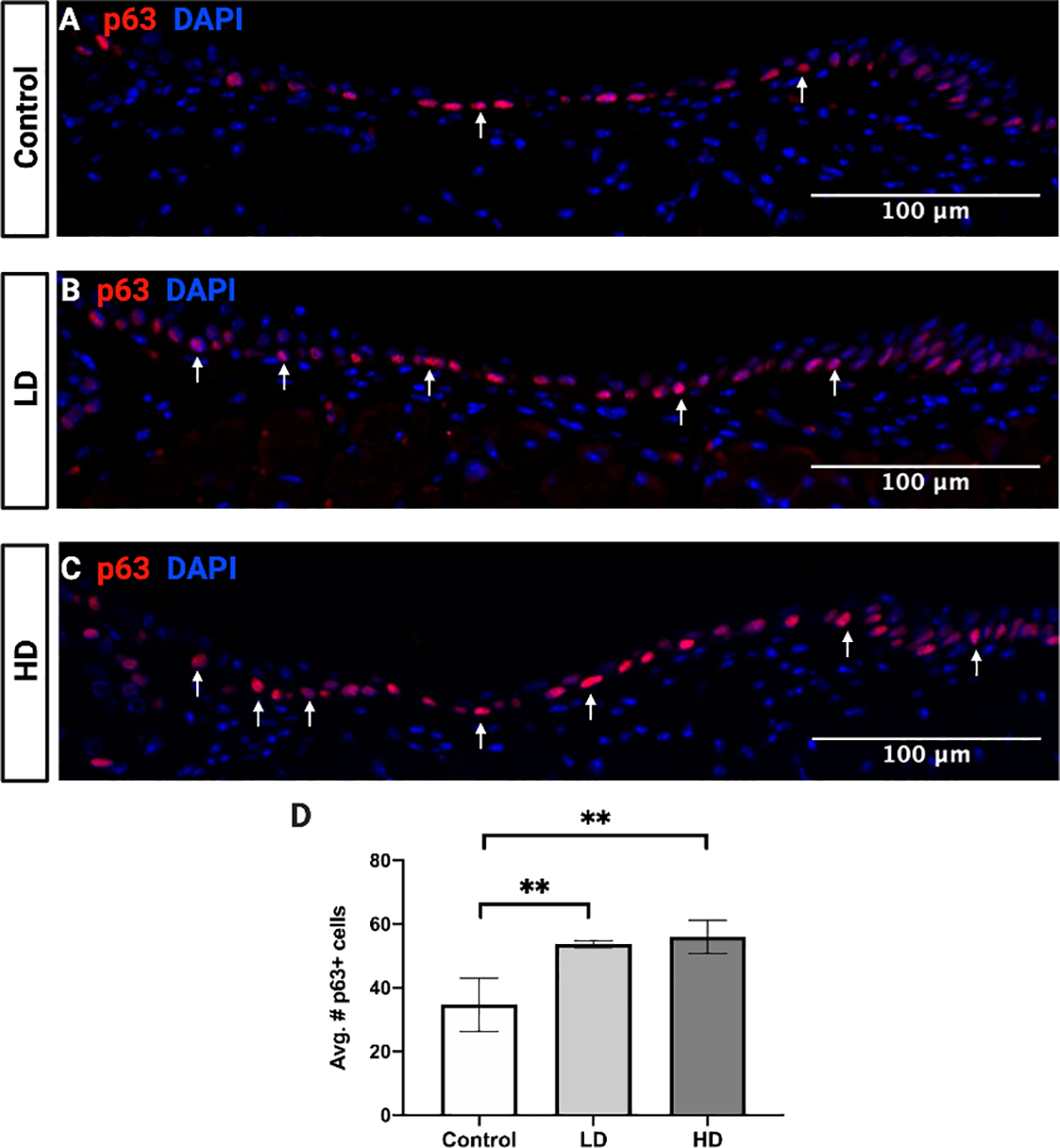
VF epithelial basal cells following control (A), LD (B), and HD (C) CSE. The number of p63 labeled basal cells (red) was significantly increased following LD and HD CSE when compared to control (D). N=3 in all groups. Fluorescent stained images are at a magnification of 200x. DAPI (blue) was used as a nuclear stain. Bar graph shows the mean with SD. ** indicates p ≤ 0.01.
Expression of an epithelial adherens junction proteins (E-cadherin and β-catenin) and tight junction protein (Zona occludens 1, [ZO-1])were quantified by qPCR (Fig. 5) and IF (Fig. 6). Both analyses indicated no significant change in E-cadherin (Fig. 5A, Fig. 6A–D) and β-catenin (Fig. 5B, Fig. 6E–H) expression between groups. LD and HD groups exhibited a 2.5- to 3-fold increase in ZO-1 gene expression compared to controls (LD: p = 0.0025, 95% confidence interval [CI]: −2.669 to −0.7873; HD: p = 0.0019, 95% confidence interval [CI]: −2.749 to −0.8677) (Fig. 5C). However, IF analysis for ZO-1 revealed no significant differences between groups (Fig. 6I–L).
Figure 5.
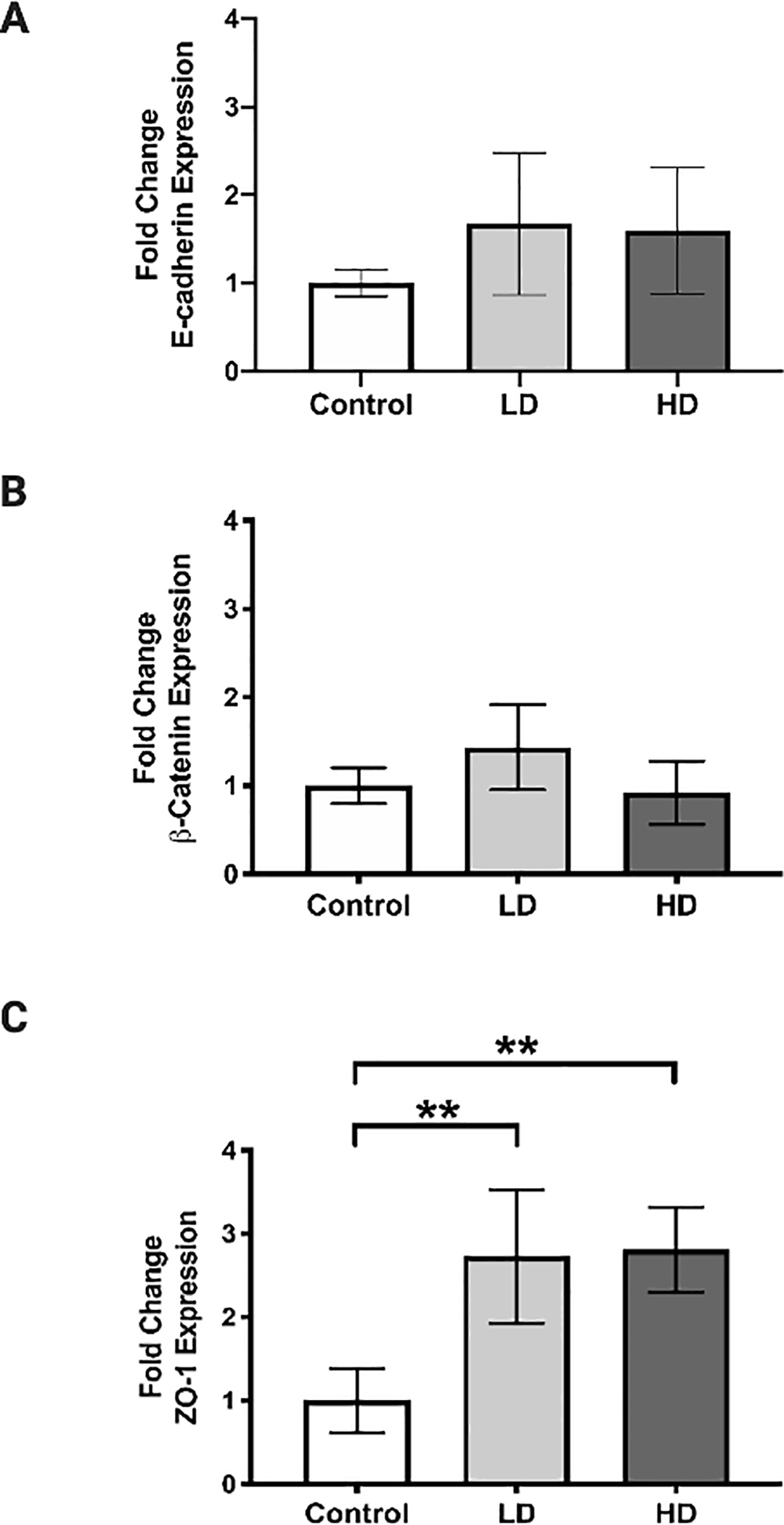
VF epithelial cell junction gene expression following control, LD, and HD CSE. Expression of E-cadherin (A) and ß-catenin (B) was not significantly altered following CSE. Expression of ZO-1 was significantly increased following LD and HD CSE (C). N=3 in all groups. Bar graph shows the mean with SD. ** indicates p ≤ 0.01.
Figure 6.
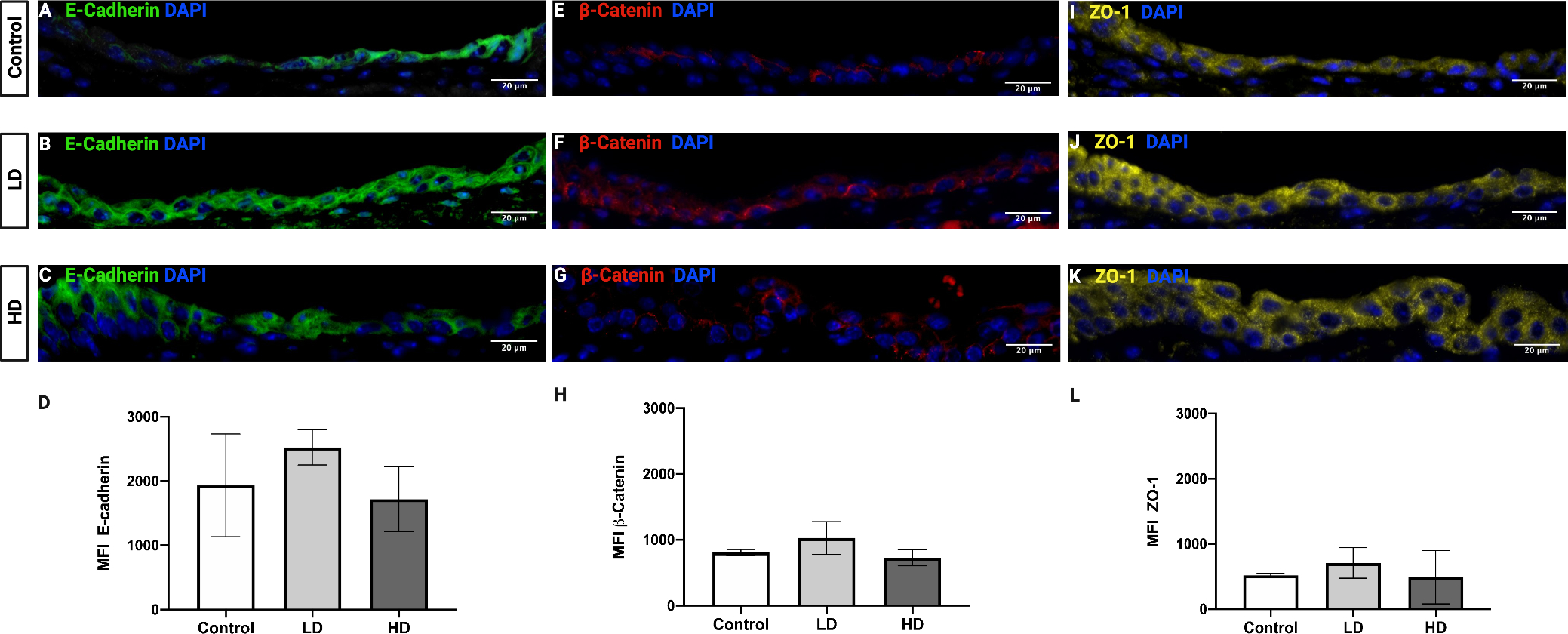
VF epithelial cell junction protein expression following control, LD, and HD CSE. E-cadherin is visualized in green (A-C), ß-catenin in red (E-G), and ZO-1 in yellow (I-K). Intensity of E-cadherin (D), ß-catenin (H), and ZO-1 (L) expression was not significantly altered following CSE. Fluorescent stained images are at a magnification of 200x. DAPI (blue) was used as a nuclear stain. N=3 in all groups. Bar graph shows the mean with SD.
CSE and Laryngeal Mucus Production
Submucosal gland area was evaluated on AB/PAS-stained slides in control (Fig. 7A), LD (Fig. 7B), and HD (Fig. 7C) groups. HD mice showed significant submucosal gland hypertrophy in comparison to the controls (p = 0.0023, 95% confidence interval [CI]: −0.1250 to −0.03410) and LD mice (p = 0.0235, 95% confidence interval [CI]: −0.09946 to −0.008517) (Fig. 7D). Acidic mucins significantly increased in both the LD (p = 0.0287, 95% confidence interval [CI]: −27.42 to −1.778) and HD groups (p = 0.0069, 95% confidence interval [CI]: −30.51 to −5.957) compared to controls (Fig. 7E). Neutral mucin levels remained unaltered.
Figure 7.
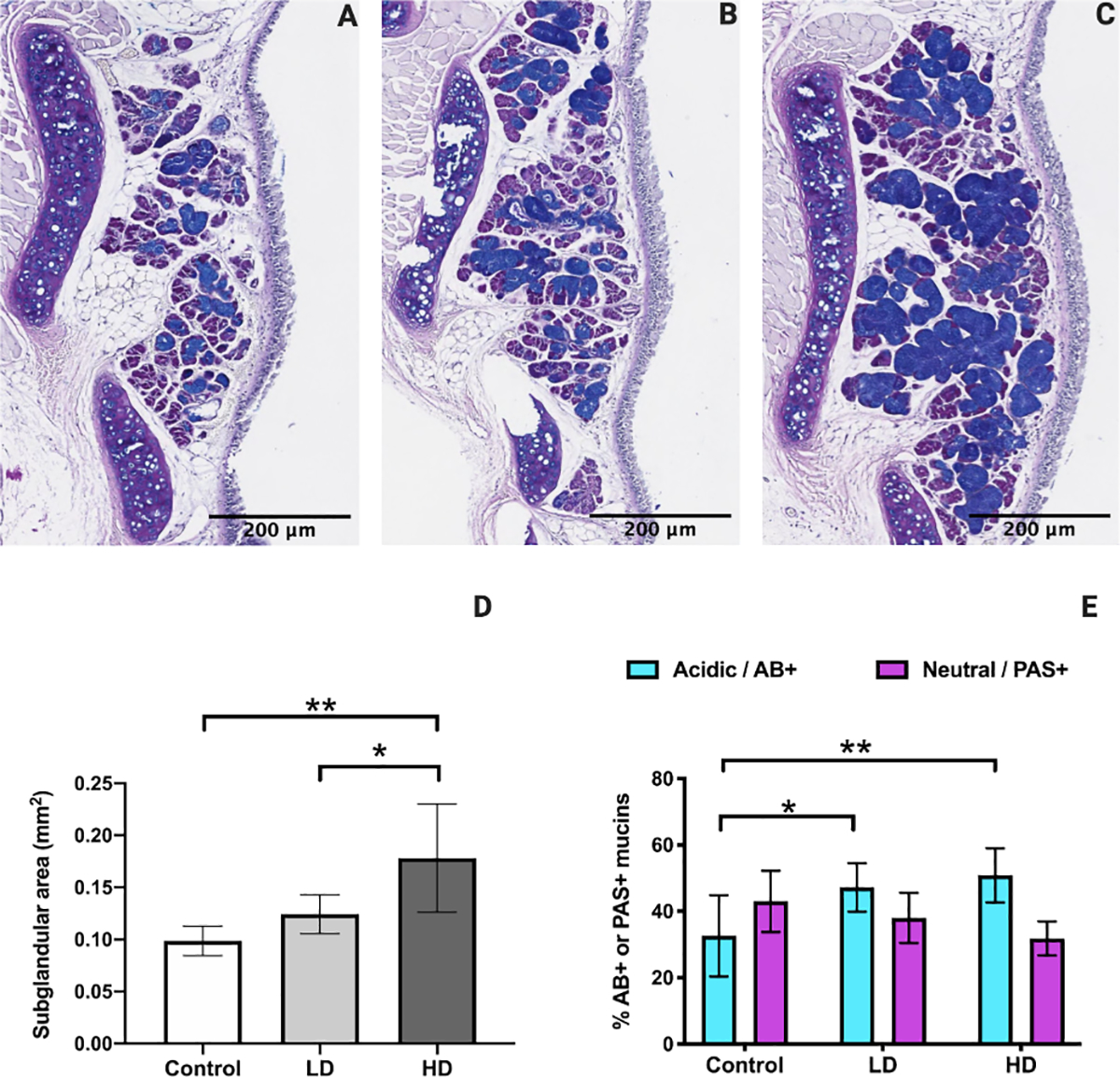
Laryngeal subglandular area and mucin histochemical activity following control (A), LD (B), and HD (C) CSE. Subglandular area was significantly increased following HD CSE when compared to control and LD CSE (D). Percent acidic mucins were significantly increased following LD and HD CSE when compared to controls (E). AB/PAS stained images are at a magnification of 200x. N=5 in control group. N=4 in LD group. N=6 in HD group. Bar graph shows the mean with standard deviation (SD). * indicates p ≤ 0.05. ** indicates p ≤ 0.01.
The effect of CSE on MUC5AC, a primary secreted mucin in the airway, was examined in control (Fig. 8A), LD (Fig. 8B), and HD (Fig. 8C) groups by IF analysis. MUC5AC expression was primarily localized to submucosal glands. There were no significant changes between the groups (Fig. 8D). However, qPCR analysis demonstrated that LD mice exhibited a significant 2.5-fold increase in MUC5AC gene expression in comparison to controls (p = 0.0078, 95% confidence interval [CI]: −4.273 to −0.7702) and HD mice (p = 0.0213, 95% confidence interval [CI]: 0.3618 to 3.864) (Fig. 8E). MUC5AC was also evaluated in FVF specimens from human nonsmokers (Fig. 8F) and smokers (Fig. 8G). IF analysis revealed that FVF specimens demonstrated epithelial goblet cell staining and that smokers exhibited a non-significant increase in MUC5AC expression in comparison to nonsmokers (Fig. 8H).
Figure 8.
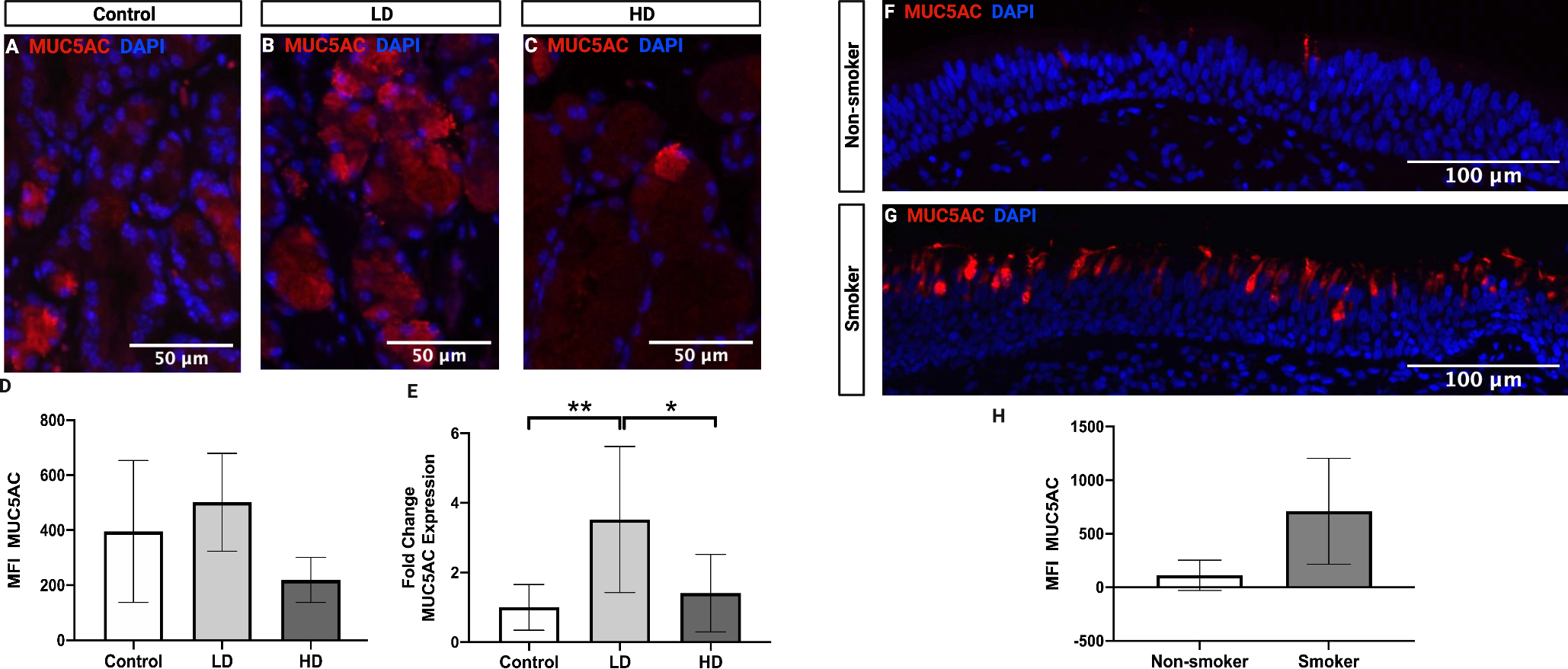
MUC5AC expression following control, LD, and HD CSE in mice (A-E) and in human FVF specimens from nonsmokers and smokers (F-H). Intensity of MUC5AC protein expression in mouse submucosal glands was not significantly altered following CSE (D). MUC5AC gene expression was significantly increased following LD CSE as compared to control and HD (E). Intensity of MUC5AC protein expression in human FVF epithelium was not significantly different in smokers compared to nonsmokers (H). Fluorescent stained images are at a magnification of 200x. DAPI (blue) was used as a nuclear stain. For MFI in mice, N=4 in all groups. For gene expression in mice, N=6 in all groups. N=3 for human smokers and nonsmokers. Bar graphs show the mean with SD. * indicates p ≤ 0.05. ** indicates p ≤ 0.01.
DISCUSSION
In this investigation, mice were exposed to HD or LD CS for 4-weeks and we examined VF epithelial barrier integrity and laryngeal mucus production. HD CS increased epithelial cellular proliferation but did not consistently alter expression of cell junctions. HD CS also induced hypertrophy of the mucus-producing submucosal glands. However, only LD CS increased MUC5AC gene expression. MUC5AC staining appeared elevated in FVF specimens from smokers, but this was not significant as compared to nonsmokers.
In healthy VF epithelium, cell proliferation is tightly regulated and necessary for normal epithelial turnover.9 Following HD CSE, cell proliferation was increased. Specifically, VF epithelial thickness increased, which indicates hyperplasia. This was accompanied by elevated Ki67+ proliferative cells and p63+ basal cells. Ki67+ cells were localized to the basal cell layer. Together, these findings suggest that the cells of the basal layer continually proliferate and differentiate under continuous CS, eventually leading to hyperplasia. In response to CS, basal cells of the lower airways proliferate and can regenerate normally differentiated epithelium or altered histologic phenotypes.29 It is possible that the VF increased proliferation as an adaptive response to CS in order to turnover injured surface cells and regenerate normal epithelium.13, 30, 31 Increased epithelial proliferation and hyperplasia is seen during tissue regeneration following iatrogenic VF injury.32, 33 Even if adaptive, hyperplasia may still have adverse consequences for VF health including reduced epithelial transport efficiency and increased VF mass, which might affect vibration.34 Sustained cell proliferation in toxicant-exposed tissue may also be a site-specific indicator of injury and identify the location and types of cells at risk for carcinogenesis.35 Basal cells in the lower airways undergo transcriptomic changes upon chronic CSE resulting in altered differentiation and a disorganized epithelium.36, 37 In addition, epithelial hyperplasia has been shown to be a precursor to laryngeal dysplasia and carcinoma.38 In order to establish a timeline of events leading up to disease development, it will be necessary to characterize when and how CS impacts the VF basal cell transcriptome.
We investigated the effect of CS on the expression of epithelial tight and adherens junctions. HD and LD CSE increased ZO-1 gene expression. No other changes in cell junction gene or protein expression were observed. In epithelial cells of the lower airways, exposure to CS reduces levels of tight and adherens junctions indicating CS-induced loss of structural barrier integrity.11, 39, 40 Our data suggest that the VF epithelial barrier is structurally intact following a 4-week CSE. The stratified squamous epithelium of the VF may be more resistant to injury than other airway epithelial cell types, at least in the short-term. However, whether a 4-week CSE increases VF epithelial permeability, an indicator of functional barrier integrity, is unknown. Previous work has demonstrated that the presence of cell junctions cannot be equated with intact functional barrier integrity.41
Clinically, thick mucus is observed in the larynx of smokers.3 Consistent with previous studies,8, 21 we observed significant submucosal gland hypertrophy in the HD group, which suggests increased mucus production. Histochemically, mucus is classified as neutral or acidic. HD and LD CSE increased acidic mucus in the laryngeal submucosal glands. Increased acidic mucus has also been observed following CSE in the lower airways.42 While the functional significance of this is unknown, it is possible that as CS-induced laryngeal disease progresses, there may be corresponding changes in mucus content. With further studies, histochemical laryngeal mucus content may be used as a marker of CS-induced disease development.
We examined the effect of CS on MUC5AC, one of the most abundant secreted mucins in the airway.43 As there is a paucity of research regarding laryngeal mucus production in both animal models and humans, we investigated MUC5AC expression in both the mouse larynx and in FVF tissue from smokers and nonsmokers. Staining patterns of MUC5AC differed in mice and humans, suggesting that there may be some differences in the mechanisms underlying laryngeal mucus production. In control and CS-exposed mice, MUC5AC was localized to submucosal glands with minimal epithelial goblet cell expression. On the other hand, in FVF specimens from nonsmokers and smokers, we observed epithelial goblet cell MUC5AC expression. In mice, the structure and cellular composition of submucosal glands are similar to those seen in humans; however, airway localization differs.44 In the human larynx submucosal glands are located in the FVF45 and subglottis46 and in the mouse larynx are isolated to the subglottis.44 We did not assess for MUC5AC in human larynx submucosal glands. The limited depth of FVF biopsies precluded reliable submucosal gland MUC5AC analysis and we are unable to obtain subglottic human biopsies in-office. However, previous work has shown that healthy submucosal glands of the larynx46 and lungs43 do not express MUC5AC. In terms of the influence of smoking on laryngeal MUC5AC, in mice, we only observed increased MUC5AC gene expression following LD exposure. This was surprising as CS is well known to induce MUC5AC gene and protein expression in other parts of the airway.47–49 Increased MUC5AC content is also associated with disease severity.50 It is possible that the 4-weeks was not long enough to induce sustained increases in MUC5AC in the larynx. In addition, MUC5AC expression is closely related to inflammation.51 Consequently, future studies are needed to elucidate the influence of CS dose on inflammation and mucin expression in the larynx. Finally, we also did not observe a significant increase in MUC5AC in the FVF of smokers. Admittedly, our smokers were overall healthy and considered to have low CS consumption.52 However, given the trend observed in our data, we suspect that a significant increase in MUC5AC would be observed in human smokers with a greater sample size or longer smoking history.
Although we evaluated two doses of CS, only a 4-week, subacute exposure was performed. Further studies would benefit from acute and chronic timepoints in order to create a detailed timeline of cellular and molecular changes that eventually culminate in CS-induced laryngeal disease. In addition, we quantified cell junction and mucin protein expression using IF. Other techniques such as western blotting may be more sensitive to assessing protein changes following CS. In addition, our protein levels did not always correlate with gene expression findings, demonstrating that transcript levels by themselves may not be sufficient to predict protein levels.53 There are many different types and causes of chronic laryngeal inflammation in addition to CS and these causes often may occur simultaneously.3 Individuals with habitually excessive vocal behaviors (i.e. phonotrauma) are also highly susceptible to laryngeal inflammation and it has been shown previously that the mechanical stresses of vocal fold vibration compromise vocal fold epithelial barrier integrity.10 It is likely that phonotrauma may exacerbate or accelerate CS-induces changes in the VF epithelial and mucus barrier. Consequently, further studies should work to systematically combine different causes of chronic laryngeal inflammation to elucidate synergetic effects that may ultimately present in laryngeal disease. Finally, at least 20 mucins have been identified. We investigated the localization and expression of one predominant secreted mucin, MUC5AC; however, future studies are needed to systematically evaluate all mucins in the mouse and human larynx under normal / healthy conditions and following CS exposure. This is particularly relevant as we observed differences in MUC5AC localization in the mouse and human larynx in the current study. Although we recognize that our human FVF sample size was relatively small and did not include submucosal gland analysis, these preliminary findings suggest possible differences in mechanisms underlying laryngeal mucus production. Future investigations will be useful for understanding the distinct roles of mucins in laryngeal protection and disease development, and also help validate the mouse model for studying human laryngeal mucus production.
CONCLUSION
We investigated the effect of subacute CSE on the VF epithelial and mucus barriers, which have not been investigated in detail. Greater changes in the epithelial and mucus barriers were observed with a higher dose of CS. We speculate that the VF increased epithelial proliferation as an adaptive response to CS; thus, maintaining structural barrier integrity as demonstrated by intact cell junctions. While the mucus-secreted cells were enlarged, we did not see consistent increases in MUC5AC expression. In summary, these findings help identify potential adaptive mechanisms to CSE as well as set the foundation for continued study of key aspects of epithelial and mucus barrier integrity that may be implicated in laryngeal disease development following prolonged smoking.
Supplementary Material
Supplementary Data. Calculation of CS exposure metrics, respiratory minute volume (RMV), and delivered dose (Supplementary Data S1). Calculation of total particulate matter (TPM) in mainstream CS (Supplementary Data S2).
ACKNOWLEDGEMENTS
We thank Phillip A. Gall, Rene Chen, Dr. Tsuguhisa Nakayama, Dr. David Zarabanda and Dr. Xu Ji for their contributions to conducting CS exposures, sample collection, and data analysis. This study was supported by the National Institutes of Health, National Institute of Deafness and Other Communication Disorders [R21 DC016126]. All figures were created with BioRender.com.
Footnotes
Conflict of Interest: None to report
Level of Evidence: N/A
REFERENCES
- 1.Branski RC, Saltman B, Sulica L, et al. Cigarette smoke and reactive oxygen species metabolism: implications for the pathophysiology of Reinke’s edema. Laryngoscope. October 2009;119(10):2014–8. doi: 10.1002/lary.20592 [DOI] [PubMed] [Google Scholar]
- 2.Dworkin JP. Laryngitis: types, causes, and treatments. Otolaryngol Clin North Am April 2008;41(2):419–36, ix. doi: 10.1016/j.otc.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 3.Dworkin JP, Sugihara E, Stern N, Nauman I, Bathula S, Amjad E. Larynageal inflammation. Annals of Otolaryngology and Rhinology. 2015;2(9):1058–10–65. [Google Scholar]
- 4.Hah JH, Sim S, An SY, Sung MW, Choi HG. Evaluation of the prevalence of and factors associated with laryngeal diseases among the general population. Laryngoscope. November 2015;125(11):2536–42. doi: 10.1002/lary.25424 [DOI] [PubMed] [Google Scholar]
- 5.(US) CfDCaP, (US) NCfCDPaHP, (US) OoSaH. The health consequences of smoking: 50 years of progress: A report of the Surgeon General. 2014.
- 6.Current Cigarette Smoking Among Adults in the United States (Centers for Disease Control) (2016).
- 7.Byeon H, Cha S. Evaluating the effects of smoking on the voice and subjective voice problems using a meta-analysis approach. Sci Rep March 2020;10(1):4720. doi: 10.1038/s41598-020-61565-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueha R, Ueha S, Kondo K, et al. Laryngeal mucus hypersecretion is exacerbated after smoking cessation and ameliorated by glucocorticoid administration. Toxicol Lett January 4 2017;265:140–146. doi: 10.1016/j.toxlet.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 9.Levendoski EE, Leydon C, Thibeault SL. Vocal fold epithelial barrier in health and injury: a research review. J Speech Lang Hear Res October 2014;57(5):1679–91. doi: 10.1044/2014_JSLHR-S-13-0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousseau B, Suehiro A, Echemendia N, Sivasankar M. Raised intensity phonation compromises vocal fold epithelial barrier integrity. Laryngoscope. February 2011;121(2):346–51. doi: 10.1002/lary.21364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am J Respir Cell Mol Biol February 2018;58(2):157–169. doi: 10.1165/rcmb.2017-0200TR [DOI] [PubMed] [Google Scholar]
- 12.Lungova V, Verheyden JM, Herriges J, Sun X, Thibeault SL. Ontogeny of the mouse vocal fold epithelium. Dev Biol March 2015;399(2):263–82. doi: 10.1016/j.ydbio.2014.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leydon C, Bartlett RS, Roenneburg DA, Thibeault SL. Localization of label-retaining cells in murine vocal fold epithelium. J Speech Lang Hear Res August 2011;54(4):1060–6. doi: 10.1044/1092-4388(2010/10-0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaquia Leão H, Galleano Zettler C, Cambruzzi E, et al. The Effects of Passive Smoking on Laryngeal and Tracheal Mucosa in Male Wistar Rats During Growth: An Experimental Study. J Voice. January 2017;31(1):126.e19–126.e24. doi: 10.1016/j.jvoice.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 15.Osimitz TG, Droege W, Finch JM. Toxicologic significance of histologic change in the larynx of the rat following inhalation exposure: a critical review. Toxicol Appl Pharmacol December 2007;225(3):229–37. doi: 10.1016/j.taap.2007.08.027 [DOI] [PubMed] [Google Scholar]
- 16.de Oliveira Semenzati G, de Souza Salgado B, Rocha NS, Michelin Matheus SM, de Carvalho LR, Garcia Martins RH. Histological and immunohistochemical study of the expression of p53 and ki-67 proteins in the mucosa of the tongue, pharynx and larynx of rats exposed to cigarette smoke. Inhal Toxicol September 2012;24(11):723–31. doi: 10.3109/08958378.2012.715317 [DOI] [PubMed] [Google Scholar]
- 17.Baskoro H, Sato T, Karasutani K, et al. Regional heterogeneity in response of airway epithelial cells to cigarette smoke. BMC Pulm Med September 2018;18(1):148. doi: 10.1186/s12890-018-0715-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson-DiRenzo E, Singh SP, Martinez JD, Sanchez SE, Easwaran M, Valdez TA. Cigarette smoke-induced changes in the murine vocal folds: a Raman spectroscopic observation. Analyst November 2020;145(23):7709–7717. doi: 10.1039/d0an01570a [DOI] [PubMed] [Google Scholar]
- 19.Roy N, Tanner K, Gray SD, Blomgren M, Fisher KV. An evaluation of the effects of three laryngeal lubricants on phonation threshold pressure (PTP). J Voice. September 2003;17(3):331–42. [DOI] [PubMed] [Google Scholar]
- 20.Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther March 2009;121(3):332–48. doi: 10.1016/j.pharmthera.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mouadeb DA, Belafsky PC, Birchall M, Hood C, Konia T, Pinkerton KE. The effects of allergens and tobacco smoke on the laryngeal mucosa of guinea pigs. Otolaryngol Head Neck Surg April 2009;140(4):493–7. doi: 10.1016/j.otohns.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 22.Thomas LB, Stemple JC, Andreatta RD, Andrade FH. Establishing a new animal model for the study of laryngeal biology and disease: an anatomic study of the mouse larynx. J Speech Lang Hear Res June 2009;52(3):802–11. doi: 10.1044/1092-4388(2008/08-0087) [DOI] [PubMed] [Google Scholar]
- 23.Coggins CR. A review of chronic inhalation studies with mainstream cigarette smoke in rats and mice. Toxicol Pathol 1998. May-Jun 1998;26(3):307–14; discussion 315. doi: 10.1177/019262339802600301 [DOI] [PubMed] [Google Scholar]
- 24.Coggins CR. A minireview of chronic animal inhalation studies with mainstream cigarette smoke. Inhal Toxicol October 2002;14(10):991–1002. doi: 10.1080/08958370290084746 [DOI] [PubMed] [Google Scholar]
- 25.Gill GA, Buda A, Moorghen M, Dettmar PW, Pignatelli M. Characterisation of adherens and tight junctional molecules in normal animal larynx; determining a suitable model for studying molecular abnormalities in human laryngopharyngeal reflux. J Clin Pathol December 2005;58(12):1265–70. doi: 10.1136/jcp.2004.016972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebel S, Diehl S, Pype J, et al. The transcriptome of Nrf2−/− mice provides evidence for impaired cell cycle progression in the development of cigarette smoke-induced emphysematous changes. Toxicol Sci May 2010;115(1):238–52. doi: 10.1093/toxsci/kfq039 [DOI] [PubMed] [Google Scholar]
- 27.Smith JC, Sausville EL, Girish V, et al. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell. June 2020;53(5):514–529.e3. doi: 10.1016/j.devcel.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. December 2001;25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 29.Crystal RG. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med December 2014;190(12):1355–62. doi: 10.1164/rccm.201408-1492PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesfaigzi Y. Roles of apoptosis in airway epithelia. Am J Respir Cell Mol Biol May 2006;34(5):537–47. doi: 10.1165/rcmb.2006-0014OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renne RA, Gideon KM. Types and patterns of response in the larynx following inhalation. Toxicol Pathol 2006;34(3):281–5. doi: 10.1080/01926230600695631 [DOI] [PubMed] [Google Scholar]
- 32.Branski RC, Rosen CA, Verdolini K, Hebda PA. Acute vocal fold wound healing in a rabbit model. Ann Otol Rhinol Laryngol January 2005;114(1 Pt 1):19–24. [DOI] [PubMed] [Google Scholar]
- 33.Leydon C, Imaizumi M, Bartlett RS, Wang SF, Thibeault SL. Epithelial cells are active participants in vocal fold wound healing: an in vivo animal model of injury. PLoS One. 2014;9(12):e115389. doi: 10.1371/journal.pone.0115389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ling C, Raasch JL, Welham NV. E-cadherin and transglutaminase-1 epithelial barrier restoration precedes type IV collagen basement membrane reconstruction following vocal fold mucosal injury. Cells Tissues Organs 2011;193(3):158–69. doi: 10.1159/000318605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.March TH, Kolar LM, Barr EB, Finch GL, Ménache MG, Nikula KJ. Enhanced pulmonary epithelial replication and axial airway mucosubstance changes in F344 rats exposed short-term to mainstream cigarette smoke. Toxicol Appl Pharmacol December 1999;161(2):171–9. doi: 10.1006/taap.1999.8798 [DOI] [PubMed] [Google Scholar]
- 36.Ryan DM, Vincent TL, Salit J, et al. Smoking dysregulates the human airway basal cell transcriptome at COPD risk locus 19q13.2. PLoS One. 2014;9(2):e88051. doi: 10.1371/journal.pone.0088051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staudt MR, Buro-Auriemma LJ, Walters MS, et al. Airway Basal stem/progenitor cells have diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am J Respir Crit Care Med October 2014;190(8):955–8. doi: 10.1164/rccm.201406-1167LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hellquist H, Lundgren J, Olofsson J. Hyperplasia, keratosis, dysplasia and carcinoma in situ of the vocal cords--a follow-up study. Clin Otolaryngol Allied Sci February 1982;7(1):11–27. doi: 10.1111/j.1365-2273.1982.tb01557.x [DOI] [PubMed] [Google Scholar]
- 39.Tatsuta M, Kan-O K, Ishii Y, et al. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37. Respir Res November 2019;20(1):251. doi: 10.1186/s12931-019-1226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J February 2012;39(2):419–28. doi: 10.1183/09031936.00193810 [DOI] [PubMed] [Google Scholar]
- 41.Leydon C, Imaizumi M, Yang D, Thibeault SL, Fried MP. Structural and functional vocal fold epithelial integrity following injury. Laryngoscope. December 2014;124(12):2764–9. doi: 10.1002/lary.24818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones R, Reid L. Secretory cells and their glycoproteins in health and disease. Br Med Bull. January 1978;34(1):9–16. doi: 10.1093/oxfordjournals.bmb.a071466 [DOI] [PubMed] [Google Scholar]
- 43.Okuda K, Chen G, Subramani DB, et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am J Respir Crit Care Med March 2019;199(6):715–727. doi: 10.1164/rccm.201804-0734OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Innes BA, Dorin JR. Submucosal gland distribution in the mouse has a genetic determination localized on chromosome 9. Mamm Genome. February 2001;12(2):124–8. doi: 10.1007/s003350010244 [DOI] [PubMed] [Google Scholar]
- 45.Kutta H, Steven P, Kohla G, Tillmann B, Paulsen F. The human false vocal folds -- an analysis of antimicrobial defense mechanisms. Anat Embryol (Berl). July 2002;205(4):315–23. doi: 10.1007/s00429-002-0255-8 [DOI] [PubMed] [Google Scholar]
- 46.Kutta H, Willer A, Steven P, Brauer L, Tsokos M, Paulsen F. Distribution of mucins and antimicrobial substances lysozyme and lactoferrin in the laryngeal subglottic region. J Anat October 2008;213(4):473–81. doi: 10.1111/j.1469-7580.2008.00960.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang G, Xu Z, Wang R, et al. Genes associated with MUC5AC expression in small airway epithelium of human smokers and non-smokers. BMC Med Genomics. 2012;5:21. doi: 10.1186/1755-8794-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Innes AL, Woodruff PG, Ferrando RE, et al. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. October 2006;130(4):1102–8. doi: 10.1378/chest.130.4.1102 [DOI] [PubMed] [Google Scholar]
- 49.Wu Q, Jiang D, Chu HW. Cigarette smoke induces growth differentiation factor 15 production in human lung epithelial cells: implication in mucin over-expression. Innate Immun August 2012;18(4):617–26. doi: 10.1177/1753425911429837 [DOI] [PubMed] [Google Scholar]
- 50.Kesimer M, Ford AA, Ceppe A, et al. Airway Mucin Concentration as a Marker of Chronic Bronchitis. N Engl J Med September 2017;377(10):911–922. doi: 10.1056/NEJMoa1701632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samsuzzaman M, Uddin MS, Shah MA, Mathew B. Natural inhibitors on airway mucin: Molecular insight into the therapeutic potential targeting MUC5AC expression and production. Life Sci August 2019;231:116485. doi: 10.1016/j.lfs.2019.05.041 [DOI] [PubMed] [Google Scholar]
- 52.Bjartveit K, Tverdal A. Health consequences of smoking 1–4 cigarettes per day. Tob Control October 2005;14(5):315–20. doi: 10.1136/tc.2005.011932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Beyer A, Aebersold R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell. April 2016;165(3):535–50. doi: 10.1016/j.cell.2016.03.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data. Calculation of CS exposure metrics, respiratory minute volume (RMV), and delivered dose (Supplementary Data S1). Calculation of total particulate matter (TPM) in mainstream CS (Supplementary Data S2).


