Abstract
Background:
This scoping review provides a timely synthesis of the use of continuous glucose monitoring in obesity research with considerations to adherence to continuous glucose monitor devices and metrics most frequently reported.
Methods:
This scoping review was conducted adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist. Eligible studies (n=31) evaluated continuous glucose monitor use in research on participants, of all ages, with overweight or obesity.
Results:
Reviewed studies varied in duration from one to 84 days (mean: 8.74 d, SD 15.2, range 1 to 84 d) with 889 participants total (range: 11-118 participants). Across all studies, the mean percent continuous glucose monitor wear time (actual/intended wear time in days) was 92% (numerator - mean: 266.1 d, SD: 452, range: 9-1596 d/denominator - mean: 271.6 d, SD: 451.5, range: 9-1596 d). Continuous glucose monitoring was utilized to provide biofeedback (n=2, 6%), monitor dietary adherence (n=2, 6%), and assess glycemic variability (n=29, 93%). The most common variability metrics reported were standard deviation (n=19, 62%), area under the curve (n=12, 39%), and glycemic range (n=12, 39%).
Conclusions:
Available evidence suggests that continuous glucose monitoring is a well-tolerated and versatile tool for obesity research in pediatric and adult patients. Future investigation is needed to substantiate the feasibility and utility of continuous glucose monitors in obesity research and maximize comparability across studies.
Keywords: Continuous Glucose Monitor, Obesity, Overweight, Adherence
1. Introduction
Continuous glucose monitors (CGM) are wearable devices that track glucose levels in interstitial fluid by taking measurements at regular and frequent intervals throughout the day and night [1, 2]. These measurements generate dynamic information on the glycemic profile of the patient throughout the day [3]. CGM devices have been validated for measuring blood glucose levels and are well tolerated by children and adults with diabetes in clinical settings. Though CGM devices were initially deployed to help manage type-1 diabetes, their use is now expanding into the care of adults and children with type-2 diabetes as well [4].
More recently, researchers have considered the potential utility of CGM in obesity research with adults and children, both as an outcome measure and to supplement behavioral weight management interventions. This interest emerged in response to studies supporting the effectiveness of other mHealth devices, such as activity monitors, on intervention engagement [5-7]. Available research further suggests that the provision of real-time feedback on biological indicators of health can increase adherence, motivate behavior change and promote weight loss and physical activity in both clinical and research settings [8-12].
CGM real-time feedback and data collection will only be a useful research tool if the participants are able to utilize the devices appropriately and for the prescribed wear time. However, unlike health technology involving smartphone-based applications, CGM is not well known or understood by individuals without diabetes. The majority of CGM acceptability data comes from type-1 diabetes studies [13-16], and those patients with diabetes are usually highly motivated to adhere to CGM use to achieve euglycemia [17, 18]. It is unclear whether the perceived benefits of CGM are substantial enough to motivate adherence to continuous wear among research participants without type-I diabetes. Therefore, demonstrating adherence to prescribed wear times is essential to support the use of CGM in obesity research.
Glycemic variability refers to changes in blood glucose levels that occur throughout the day. Although mostly used in diabetes care and beta cell pathology research, glycemic variability is a physiological process involving a complex array of regulatory hormones, and also depends on variations in glucose tolerance and insulin activity [19]. There are multiple metrics commonly used to calculate glycemic variability, including percent time in range (TIR), standard deviation (SD) of glucose measurements, continuous overlapping net glycemic action (CONGA), mean amplitude of glycemic excursions (MAGE), mean of daily difference (MODD), and area under the curve (AUC) [19, 20]. Glycemic variability is a potentially useful research metric to understand the impact of obesity interventions on adiposity and glucose metabolism. Given inconsistent reports in the obesity literature regarding the utility of glycemic variability, we are cataloging the varying definitions of CGM derived glycemic variability, seeking to better delineate the potential of CGM devices in obesity research.
To date, there has been no comprehensive review of CGM use in obesity research. Furthermore, no previous review has summarized data on CGM adherence in children and adults with obesity, nor cataloged the glycemic variability metrics used. This information is needed to appraise the utility of CGM use among individuals without diabetes and, also, to evaluate the relevance of currently used metrics to obesity research. Since CGM use in obesity research is relatively new, the overarching aim of this scoping review was to synthesize current evidence on the use of CGM in clinical trials and observational studies. The two specific aims of this review were: 1) examine participants’ adherence to CGM, across studies (operationalized as actual wear time relative to prescribed wear time), and 2) catalog the metrics used to evaluate glycemic variability in obesity research.
2. Methods
2.1. Eligibility Criteria
Eligible studies included randomized controlled trials (RCT), non-RCT, and/or quasi-experimental studies (observational) involving children, adolescents, and/or adults with overweight or obesity. Research conducted in community, outpatient, inpatient, and/or primary care settings was included. CGM use was required to be part of the study, either as the main intervention, or as a tool to collect research data. Studies were excluded if they did not use human participants or enrolled participants with type-1 diabetes. Studies of participants that included groups with obesity alone and with type-2 diabetes (n=3, 9%) were included. Studies with lean participants were included, as long as they also included participants with overweight/obesity as well. Studies were excluded if specific data were not collected or reported, including demographic characteristics, Body Mass Index (BMI), intervention setting, intervention duration, or CGM metrics. Intervention duration had to be at least 24 hours. No limitations were placed on length of follow-up, or study date. All studies identified in the search that met the eligibility criteria were included in this scoping review.
2.2. Identification of Relevant Studies
This scoping review was conducted using the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist [21]. A systematic search of published articles up to April 2020 was undertaken using electronic databases: PubMed, Science, Cochrane Library, PsychINFO, CINAHL database, Google Scholar, and clinicaltrials.gov. Keywords were searched using both the National Library of Medicine’s Medical Subject Headings (MeSH) and as independent search terms (e.g., overweight AND continuous glucose monitor OR monitoring, obesity AND continuous glucose monitor OR monitoring). A research librarian was consulted to design the most effective strategies for each database. The search terms were intentionally broad, to capture relevant studies and prevent omissions. Key journals and references cited in all systematic reviews identified during those initial searches were also manually reviewed for additional relevant studies. Initial search of articles was conducted manually by one reviewer (first author EH). The database search resulted in an initial pool of 961 articles. Deduplication was completed using Endnote online software (Clarivate, Philadelphia, PA). All records were then independently reviewed for inclusion by two reviewers (AV and EH) using the defined inclusion criteria, evaluating articles first by title and abstract, followed by full text review. Discrepancies were resolved through discussion between investigators.
2.3. Data Extraction
Data was independently extracted from eligible articles by using a data extraction form that mirrored the Joanna Briggs Institute extraction instrument for scoping reviews (Microsoft, Redmond, WA) [22]. The following information was extracted: authors, year of publication, location, study design, sample size, sample characteristics, intervention duration, CGM use duration, research outcomes, glycemic variability metrics (if reported), and CGM-relevant outcomes data. Data were collated, summarized, and reported in Table 1. Diligent efforts were made to reach out to the original authors to collect unreported data. Studies were excluded if the data required for analysis could not be obtained.
Table 1.
Scoping review on the use of continuous glucose monitors in obesity research.
| Author (Date,Country) | Participants | Design | Study Aims | Metrics | Wear Time | Grading |
|---|---|---|---|---|---|---|
| Adult Studies | ||||||
| Glycemic Dysfunction | ||||||
| Farabi, et al. (2015, USA) | N=38 Adults with BMI ≥30 kg/m2 with T2D | Crossover RCT | Assess impact of single bout of exercise on diurnal and nocturnal oxidative stress and glycemic variability | SD CONGA |
3 day-wear x 2 | IB |
| Gay, et al. (2018, USA) | N=9 Adults with BMI ≥25 kg/m2 with prediabetes | Crossover RCT | Determine the effects of 2-min and 4-min bouts of vigorous-intensity stair-climbing on glucose levels | AUC GR |
1 day | IIB |
| Little, et al. (2014, Canada) | N=10 Adults with BMI ≥25 kg/m2 with T2D | Crossover RCT | Examine the effect of acute high-intensity interval training compared with continuous moderate-intensity exercise on postprandial hyperglycemia | SD MAGE |
3-day wear x 2 | IA |
| Parr, et al. (2018, Australia) | N=13 Adults with BMI ≥25 kg/m2 and prediabetes | Crossover RCT | Compare the effect of a high versus low energy intake first meal on glucose and insulin responses during prolonged sitting in individuals with prediabetes | SD CONGA MAGE AUC |
1-day wear x 2 | IIB |
| Post- Gastric Bypass | ||||||
| Christfort ∅hrstrøm, et al. (2019, Denmark) | N=11 Women with BMI ≥30 kg/m2 post Roux-en-Y gastric bypass | Crossover RCT | Investigate the effects of acarbose, sitagliptin, verapamil, liraglutide and pasireotide on post-bariatric hypoglycemia | SD TIR |
6-day wear x 5 | IIA |
| Halperin, et al. (2011, USA) | N=16 Women post-Roux-en-Y gastric bypass | Observational | Investigate if CGM is an effective tool for characterizing glycemic variability after Roux-en-Y Gastric Bypass | GR | 3 days | IIB |
| Ritz, et al. (2012, France) | N=8 Adults s/p Gastric Bypass Surgery | Observational | Evaluate the effect of treatment with dietary counseling plus acarbose administration on the symptoms of dumping syndrome and on the characteristics of the CGM profile | SD MAGE TIR |
3-day wear x 2 | IIIB |
| Wysocki, et al. (2019, Poland) | N=32 Adults with BMI >30kg/m2 with T2D | Observational | Compare effects of laparoscopic sleeve gastrectomy vs. gastric bypass on blood glucose using CGM | SD TIR |
10 days | IIIA |
| Pregnancy | ||||||
| Harmon, et al. (2011, USA) | N=38 Pregnant Women with BMI ≥18 kg/m2 | Observational | Define 24-h glycemia in normal-weight and pregnant women with obesity using CGM | AUC GR |
4 day-wear x 2 | IIA |
| Yogev, et al. (2004, USA) | N=57 Pregnant women with BMI ≥30kg/m2 | Observational | Evaluate the ambulatory daily glycemic profile in the second half of pregnancy in women without diabetes | GR | 3 days | IIA |
| Weight Based | ||||||
| Buscemi, et al. (2013, Italy) | N=40 Adults with BMI ≥30 kg/m2 | RCT | Determine the effects of hypocaloric diets with different glycemic indexes and glycemic loads on endothelial function and glycemic variability | SD GR CV |
2-day wear x 2 | IIA |
| Climie, et al. (2018, Australia) | N=9 Adults with BMI ≥25 kg/m2 | Crossover RCT | Test the effect of brief activity breaks vs. uninterrupted sitting on postprandial glucose and insulin levels | SD CONGA MAGE AUC |
4 days | IIA |
| Crespo, et al. (2016, USA) | N=11 Adults with BMI ≥25 kg/m2 | Crossover RCT | Compare the 24-h and postprandial glucose responses to incremental intervals of standing, walking, and cycling compared to sitting | SD MAGE AUC |
4 days | IIA |
| Jamshed, et al. (2019, USA) | N=11 Adults with BMI ≥25 kg/m2 | Crossover RCT | Investigate the impact of Time Restricted Feeding on cardiovascular disease risk | SD MAGE AUC GR |
4-day wear x 2 | IIB |
| Jiménez-Domínguez, et al. (2015, Mexico) | N=20 Adults with BMI ≥18 kg/m2 | Crossover RCT | Examine the effects of acute ingestion of native banana starch on glycemic profiles | SD MAGE AUC CV |
4 days | IIB |
| Jospe, et al. (2020, New Zealand) | N=40 Adults with BMI >30 kg/m2 | RCT | Assess hunger training using CGM with fingerprick glucose monitoring on weight loss and adherence | NA | 30 days |
IIA |
| Kim, et al. (2019, USA) | N=31 Adults with BMI ≥30 kg/m2 | Observational | Determine the predictive effect of circulating glucose levels on eating in free-living individuals | SD MAGE |
7 days | IIA |
| Liao, et al. (2020, USA) | N=19 Adults with BMI ≥25 kg/m2 | Observational | Determine the acceptability of a physical activity intervention that incorporated the use of CGMs in sedentary adults with overweight and obesity without diabetes and evaluate the changes in exercise motivation | NA | 10 days |
IIA |
| Philippou, et al. (2008, UK) | N=18 Adults with BMI ≥30kg/m2 | RCT | Compare the effects of two energy-restricted healthy diets on heart disease risk factors and weight loss | AUC GR |
1-day wear x 2 | IIIA |
| Rafiei, et al. (2019, Canada) | N=15 Women with BMI ≥25 kg/m2 | RCT | Compare the effects of high-intensity interval training with moderate-intensity continuous training for improvement of CGM-derived markers of glycemic variability | SD MAGE AUC |
2-day wear x 2 | IIA |
| Salkind, et al. (2014, USA) | N=36 Adults with BMI ≥30 kg/m2 | Observational | Investigate glycemic profile in adults with severe obesity | SD MAGE CONGA MODD |
3 days | IIA |
| Wennberg, et al. (2015, Australia) | N=19 Adults with BMI ≥25 kg/m2 | Crossover RCT | Compare the acute effects of uninterrupted sitting with sitting interrupted by brief bouts of light-intensity walking on neuroendocrine biomarkers | SD AUC TIR |
1-day wear x 2 | IA |
| Wilkinson, et al. (2020, USA) | N=19 Adults with BMI ≥25 mg/m2 | Single-arm paired-sample trial | Investigate if 10-h time restricted eating intervention in patients with metabolic syndrome would result in a significant improvement in mean blood glucose, fasting insulin, and inflammatory markers | SD | 12 weeks | IIA |
| Winn, et al. (2019, USA) | N=10 Adults with BMI ≥25 kg/m2 | Crossover RCT | Determine whether mild energy restriction preserves glycemic control during physical inactivity and whether this preservation is more effectively achieved with a higher-protein diet | GR | 3-day wear x 2 | IIA |
| Pediatric Studies | ||||||
| Glycemic Dysfunction | ||||||
| Choudhary, et al. (2013, USA) | N=17 Children ages 7-18, with BMI ≥95th%ile and prediabetes | Observational | Examine daily glycemic excursions in children with prediabetes | MAGE GR |
4 days | IIIC |
| Weight Based | ||||||
| Bauer, et al. (2015, USA) | N=28 Adolescents, ages 14-18, with BMI ≤85th %ile | RCT | Examine the effect of high vs. normal protein breakfast consumption on glycemic control in youth | SD | 3-day wear x 2 | IIB |
| Chan, et al. (2015, USA) | N=118 Children ages 10-18, with BMI ≥85th%ile | Observational | Determine if HbA1c or oral glucose tolerance test is a better predictor of free-living glycemia as measured by CGM | SD AUC TIR |
3 days | IIIA |
| Ghane, et al. (2019, USA) | N=33 Children ages 10-11 of all BMI %iles | RCT | Determine the accuracy of Freestyle Pro for estimating plasma glucose during oral glucose tolerance test in healthy children | AUC GR |
1 day | IIB |
| Kaya, et al. (2017, Turkey) | N=50 Children ages 10-18, with BMI ≥95th %ile | Observational | Investigate the relationship between glycemic variability and inflammatory parameters in children with insulin resistance and obesity | SD AUC |
1 day | IIIB |
| Schiaffini (2016, Italy) | N=30 Children ages 7-17, with BMI ≥120th % of the 95th %ile | Observational | Evaluate the glucose profile in children with non-alcoholic fatty liver disease | SD GR |
4-day wear x 2 | IA |
| Zou, et al. (2008, China) | N=84 Children ages 6-15, with BMI ≥95th %ile | Observational | Assess glucose metabolism disorder by CGMs in children with obesity | GR | 1 day | IIA |
Abbreviations: Body Mass Index (BMI), Percentile (%ile), Randomized Controlled Trial (RCT), standard deviation (SD), continuous overlapping net glycemic action (CONGA), mean amplitude of glycemic excursions (MAGE), area under the curve (AUC), absolute means of daily differences (MODD), time in range (TIR), coefficient of variability (CV), and glycemic range (GR).
Grading System: Classification and scores were based on the rigor of study design (level I, II, or II) and the quality of research being reported (high, medium, or low quality). Scores range from IA (RCT with high quality results) to IIIC (non-experimental with low quality results or major flaws) [23].
AV and EH independently appraised the methodological quality of included studies using the Johns Hopkins Nursing Evidence-Based Practice to access evidence level and quality of research [23]. Articles were classified according to their quality of evidence and rigor of study design. Classification and scores were based on the rigor of study design (level I, II, or III) and the quality of research being reported (high, medium, or low quality). Scores range from IA (RCT with high quality results) to IIIC (non-experimental with low quality results or major flaws). At each level of classification, there are specific metrics the study must reach [23]. The appraisal tool provides a systematic and standardized approach to categorizing journal articles. This methodology was selected because it integrates the scientific evidence with the best available experimental (patient and practitioner) evidence.
2.4. Measures of Adherence
Adherence was defined as the actual total CGM wear time by the research participant, divided by the prescribed wear time stated in the study protocols. This data was either calculated from quantitative data reported in the results section of the study or based on summary adherence outcomes reported by authors in their discussion section.
2.5. Categorization of Patient Cohorts
To better understand the current populations that CGM are being deployed in obesity research, the cohort of studies included in this scoping review were categorized by patient cohort. The categories were generated after the relevant studies in this scoping review were demographics. Studies were divided into adult and pediatric categories based of the age of participants, then further subdivided by glycemic dysfunction (cohort of subjects with glycemic dysfunction, for example type-2 diabetes or pre-diabetes), post-gastric bypass (cohort of patients after gastric bypass surgery), pregnancy (cohort of patients who are pregnant), and weight based (cohort of patients with no specific requirements).
2.6. Data Synthesis
Results were synthesized by AV and EH, following data extraction. Given the studies’ extreme heterogeneity, no meta-analysis or other statistical tests were performed on the data set.
3. Results
3.1. Study and Participant Characteristics
The 31 studies that met criteria for data extraction are summarized in Table 1. The majority of studies were conducted in adult populations (n=24) [12, 24-46] and seven studies were conducted in children [47-53]. Most studies took place in the US (n=16) [12, 24, 25, 29, 32, 33, 36, 37, 40, 43, 45-50]. The remaining studies were conducted in Australia (n=3)[27, 35, 44], Canada (n=2) [26, 42], China (n=1) [53], Denmark (n=1) [28], France (n=1) [30], Italy (n=2) [34, 52], Mexico (n=1) [38], Poland (n=1) [31], New Zealand (n=1) [39], Turkey (n=1) [51], and the UK (n=1) [41]. Participant ages ranged from 6 to 78 years of age. The sample size ranged from 12 adult participants in a post-gastric bypass observational study [30] to 118 participants in an observational study evaluating the relationship between CGM use and hemoglobin A1C values in a pediatric population [49]. The majority of studies recruited both male and female participants; four studies recruited females only [28, 29, 32, 33]. All studies, except one, reported the BMI of participants.
3.2. Adherence
Adherence was defined as percent (%) of actual CGM wear time relative to the study protocol prescribed wear time in days. A large majority, 92% (numerator - mean: 266.1 d, SD: 452, range: 9-1596 d/denominator - mean: 271.6d, SD: 451.5, range: 9-1596 d) of participants wore their CGM for the entire prescribed duration of the research study. Common reasons for premature discontinuation or non-use included skin irritation due to the CGM adhesive, technical difficulty with the device, other concerns regarding wearing a device, and non-CGM related adherence issues. Three studies specifically surveyed patients’ satisfaction regarding CGM as a tool for weight management treatment [54, 55]. In those three studies, self-report patient satisfaction with CGM, was high, further suggesting CGM use is feasible in the weight management context [54, 55].
3.3. CGM Utilization
Continuous glucose monitoring was utilized to provide biofeedback (n=2, 6%), monitor dietary adherence (n=2, 6%), and assess glycemic variability (n=29, 93%). CGM was most often deployed to characterize the glycemic variability of either a patient cohort or during the research intervention (n=29, 93%). Other applications identified for the CGM device were to provide behavioral cues (n=1,3%) [12], hunger cues (n=1, 3%) [39], and monitor adherence (n=2, 6%) [37, 45].
3.4. Glycemic Variability
Glycemic variability was reported in 29 of the 31 included studies. Eight different glycemic variability metrics were identified across all studies Figure 2. The majority of studies (n=23) used multiple metrics to capture glycemic variability. The glycemic variability metrics identified were standard deviation of the glucose measurements (SD) (n=19, 62%), area under the curve (AUC) (n=12, 39%), glycemic range (GR) (n=12, 39%), mean amplitude of glycemic excursions (MAGE) (n=11, 35%), time in range (TIR) (n=5,16%), continuous overlapping net glycemic action (CONGA) (n=4,13%), coefficient of variability (CV) (n=2, 6%), and absolute means of daily differences (MODD) (n=1, 3%).
Figure 2.
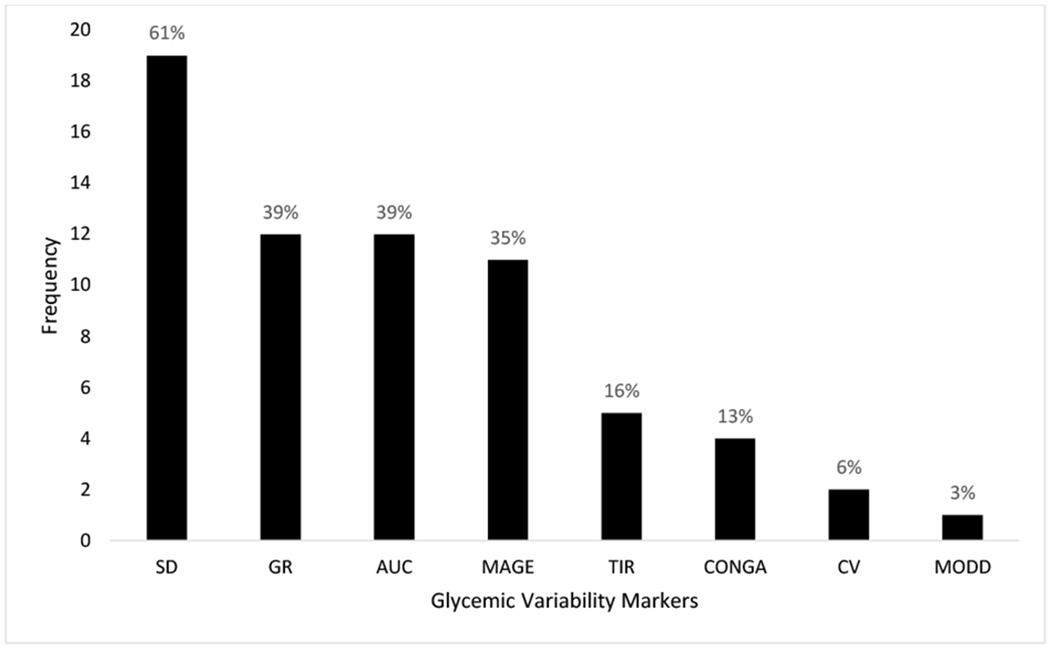
Frequency of glycemic variability metrics reported across included studies. Percentage reflects metric usage across included studies (n=31).
Abbreviations: Standard deviation (SD), continuous overlapping net glycemic action (CONGA), mean amplitude of glycemic excursions (MAGE), area under the curve (AUC), absolute means of daily differences (MODD), time in range (TIR), coefficient of variability (CV), and glycemic range (GR)
3.5. Categorization of Patient Cohorts
Of the adult studies, the following cohorts of patients were identified: glycemic dysfunction (n=4), post-gastric bypass (n=4), pregnancy (n=2), and weight (n=14). Of the pediatric studies, the following cohorts of patients were identified: glycemic dysfunction (n=1) and weight (n=7). These categorizations can be found in Table 1.
4. Discussion
The overarching aim of this scoping review was to synthesize the currently available evidence on uses of CGM in obesity research. We were specifically interested in examining parameters relevant to research implementation, namely participants’ adherence to CGM wear time and the glycemic variability metrics most often used in obesity research. The results suggest that the feasibility of using CGM for obesity research is high, and the utility of those CGM measurements is less well established.
Regarding the feasibility of CGM use in obesity research, participants across studies seemed to tolerate the device well, as demonstrated by the high adherence across varying study protocols and research subject populations. The protocols spanned diverse research settings (community, outpatient, and/or inpatient) and both pediatric and adult populations. Many of the relatively infrequent adherence issues are well recognized and addressed in diabetes related research, including adhesive sensitivity [56, 57]. However, adherence to obesity study protocols may not perfectly capture subjective parameters of tolerability. We acknowledge that some participants may have varying degrees of intrinsic motivation to adhere to CGM protocols based on their underlying health state. For example, participants with obesity and glycemic dysfunction may be highly motivated to adhere to study protocols to prevent disease progression. Future research is necessary to investigate the feasibility across larger subgroups of patients with obesity with no glycemic dysregulation and to uncover the best ways to implement CGM use in obesity research. Additionally, future research should consider explicitly measuring participant satisfaction with CGM wear, while also identifying possible barriers or discomforts that may impede continuous wear.
This review identified multiple different applications for CGM both as an intervention tool and as an outcome measure. Across all the studies examined, CGM data was used for a variety of purposes including: 1) as a behavioral intervention by providing real-time biofeedback connected to a specific process such as identifying hunger cues, 2) as a method to monitor adherence to dietary interventions, or 3) as a method to assess glycemic variability.
In one study, as a behavioral intervention, CGM was shown to be as reliable as manual finger-checks for teaching hunger training and thus has the potential to be used alongside a behavioral intervention to increase motivation, provide real-time feedback and augment treatment effectiveness in obesity research. Verification of dietary intervention adherence in obesity research is an important factor to consider in any obesity trial. Self-report and interviewled dietary recalls remain the gold standard strategies to evaluate dietary intake in obesity research; however, these tools have been criticized due to potential error in reporting, omission bias and suboptimal compliance. CGM data may provide an alternative to monitoring adherence in dietary interventions and improve the validity of the data collected in ensuring that intervention dosage is implemented as intended (e.g., monitoring actual fast in intermittent fasting studies). The diversity of CGM use across these studies highlights the promising potential of CGM as tool in obesity research.
This review highlights the multiplicity of glucose variability metrics reported from CGM devices. We found that measuring glycemic variability was the most frequent application of the CGM. The most commonly used glycemic variability metric was the standard deviation of the glucose measurements either in isolation or reported with AUC and MAGE. There is no current consensus regarding the most useful metric(s), possibly due to the multiplicity of intended uses. For example, the metric selected will be different if the goal is to motivate users, rather than directly assess an intervention’s impact on weight loss or insulin resistance. This variability is reflected in the studies examined here. In order to advance our understanding of glycemic variability future studies should compare metrics side-by-side to standardize protocols and optimize comparability across studies.
4.4. Strengths and Limitations
Naturally, this review is not without limitations. First, by including CGM as one of our search terms, it is possible that we could have missed studies where data from CGM was reported as a secondary outcome and therefore not pick up by MESH terms. Second, the significant differences in methodology and reported data complicated any quantitative comparison across studies. In addition, few studies provided information on completers versus non-completers, or other potential confounding factors, so it is unclear to what degree selection bias affects high reported adherence. The studies reviewed also differed markedly in their terminology (e.g., ‘tolerability’) and how they operationalized adherence. Third, we included all eligible papers regardless of the quality and rigor of the studies featured, thus potentially introducing biases in our conclusions if study findings were misrepresented. Finally, while we strove to identify all relevant studies, unpublished null-effect studies and manuscripts published in a language other than English were omitted from this review. These omissions limit the generalizability of our conclusions regarding the use of CGM in obesity research.
5. Conclusion
The available evidence to date suggests that CGM is a well-tolerated and versatile tool for obesity research in both pediatric and adult patients. A diversity of metrics was used to report glycemic variability from studies’ CGM data. In order to advance our understanding of glycemic variability as a useful outcome measure in obesity research, future studies should carefully evaluate the validity and reliability of these metrics to support standardization of protocols and comparability across studies. CGM use may also be a useful tool not only to collect glycemic data, but also to augment behavioral interventions and monitor adherence. Realizing this potential requires further investigation to better harness the utility of CGM and strengthen both obesity research and treatment outcomes.
Figure 1.
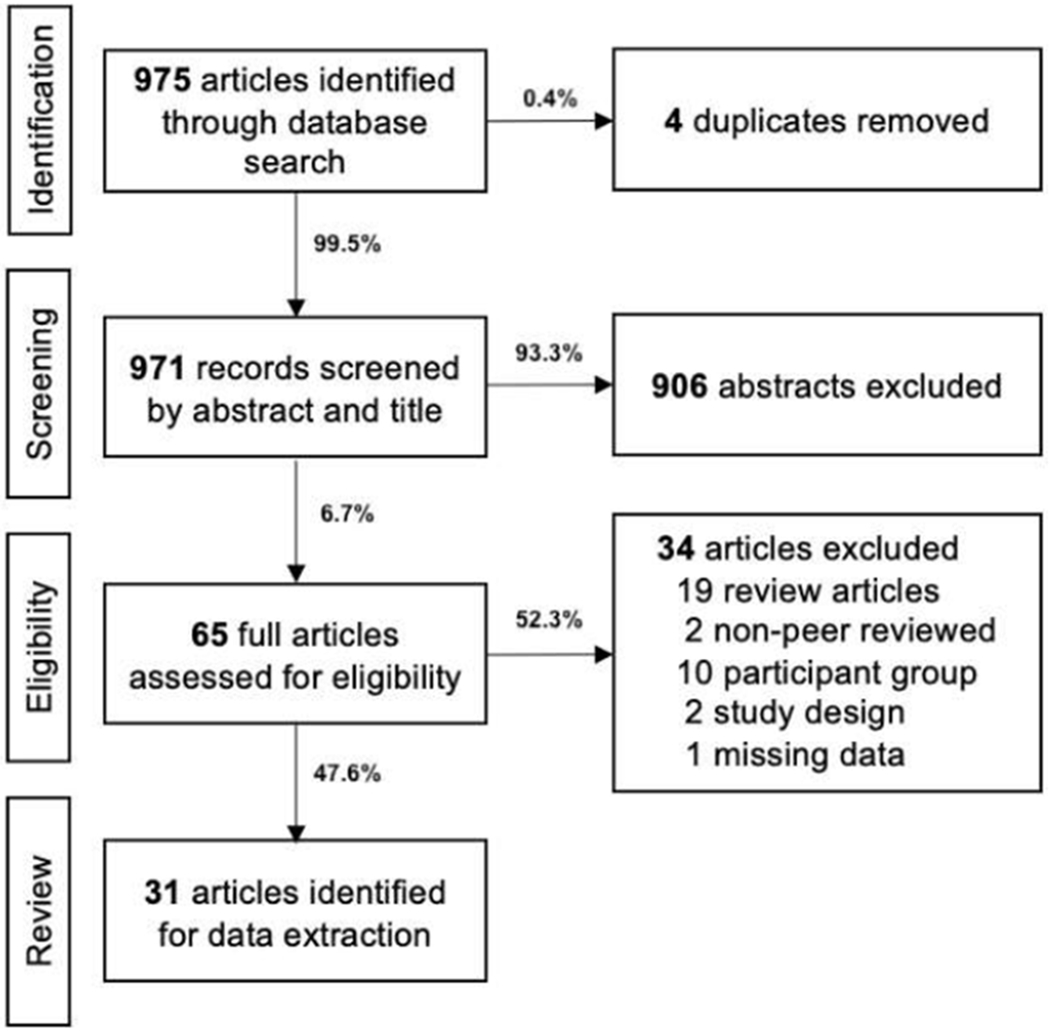
Flow diagram of screening and review process.
Highlights.
Continuous glucose monitoring is tolerated in obesity research
Standard deviation is the most common glycemic variability metric
Continuous glucose monitoring can provide biofeedback and monitor dietary adherence
Funding Source:
This project was supported by eHealth International, Inc; by National Institutes of Health [grant number UL1 TR000130]; by Obesity Health Disparities Research Center [grant number U54MD000502]; and by National Institute of Child Health and Human Development [grant number R01HD092483].
Abbreviations:
- BMI
Body mass index
- zBMI
Body mass index Z-score
- CI
Confidence Interval
- CGM
Continuous glucose monitor
- GV
Glycemic Variability
- MeSH
Medical Subject Headings
- %BMIp95
Percent over the 95th percentile
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Agreement Statement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We understand that the Corresponding Author is the sole contact for the Editorial process. He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs
Ethical Statement for Solid State Ionics
Hereby, I /Alaina Vidmar/ consciously assure that for the manuscript / Use of Continuous Glucose Monitoring in Obesity Research: A Scoping Review / the following is fulfilled:
- This material is the authors’ own original work, which has not been previously published elsewhere.
- The paper is not currently being considered for publication elsewhere.
- The paper reflects the authors’ own research and analysis in a truthful and complete manner.
- The paper properly credits the meaningful contributions of co-authors and co-researchers.
- The results are appropriately placed in the context of prior and existing research.
- All sources used are properly disclosed (correct citation). Literally copying of text must be indicated as such by using quotation marks and giving proper reference.
- All authors have been personally and actively involved in substantial work leading to the paper, and will take public responsibility for its content.
The violation of the Ethical Statement rules may result in severe consequences.
To verify originality, your article may be checked by the originality detection software iThenticate. See also http://www.elsevier.com/editors/plagdetect.
I agree with the above statements and declare that this submission follows the policies of Solid State Ionics as outlined in the Guide for Authors and in the Ethical Statement.
Conflict of Interest:
The authors have no financial relationships or conflict of interest relevant to this article to disclose.
Consent for Publication:
Not applicable.
Contributor Information
Elizabeth Hegedus, Children’s Hospital Los Angeles and Keck School of Medicine of USC, Center for Endocrinology, Diabetes and Metabolism
Sarah-Jeanne Salvy, Cancer Research Center on Health Equity, Cedars-Sinai Medical Center, West Hollywood, CA
Choo Phei Wee, Southern California Clinical and Translational Science Institute (SC-CTSI), Department of Preventive Medicine, Keck School of Medicine, Los Angeles
Monica Naguib, Children’s Hospital Los Angeles and Keck School of Medicine of USC, Center for Endocrinology, Diabetes and Metabolism
Jennifer K. Raymond, Children’s Hospital Los Angeles and Keck School of Medicine of USC, Center for Endocrinology, Diabetes and Metabolism
D. Steven Fox, Department of Pharmaceutical and Health Economics, School of Pharmacy of the University of Southern California
Alaina P. Vidmar, Children’s Hospital Los Angeles and Keck School of Medicine of USC, Center for Endocrinology, Diabetes and Metabolism
Availability of Data and Material
The datasets from this study will be available from the corresponding author on written request.
References:
- [1].Continuous glucose monitoring for patients with diabetes: an evidence-based analysis, Ont Health Technol Assess Ser 11(4) (2011) 1–29. [PMC free article] [PubMed] [Google Scholar]
- [2].Joubert M, Reznik Y, Personal continuous glucose monitoring (CGM) in diabetes management: review of the literature and implementation for practical use, Diabetes research and clinical practice 96(3) (2012) 294–305. [DOI] [PubMed] [Google Scholar]
- [3].Gross TM, Mastrototaro JJ, Efficacy and reliability of the continuous glucose monitoring system, Diabetes Technol Ther 2 Suppl 1 (2000) S19–26. [DOI] [PubMed] [Google Scholar]
- [4].Welsh JB, Derdzinski M, Parker AS, Puhr S, Jimenez A, Walker T, Real-Time Sharing and Following of Continuous Glucose Monitoring Data in Youth, Diabetes Ther (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mendoza JA, Baker KS, Moreno MA, Whitlock K, Abbey-Lambertz M, Waite A, Colburn T, Chow EJ, A Fitbit and Facebook mHealth intervention for promoting physical activity among adolescent and young adult childhood cancer survivors: A pilot study, Pediatr Blood Cancer 64(12) (2017). [DOI] [PubMed] [Google Scholar]
- [6].Cheatham SW, Stull KR, Fantigrassi M, Motel I, The efficacy of wearable activity tracking technology as part of a weight loss program: a systematic review, J Sports Med Phys Fitness 58(4) (2018) 534–548. [DOI] [PubMed] [Google Scholar]
- [7].Lee AM, Chavez S, Bian J, Thompson LA, Gurka MJ, Williamson VG, Modave F, Efficacy and Effectiveness of Mobile Health Technologies for Facilitating Physical Activity in Adolescents: Scoping Review, JMIR Mhealth Uhealth 7(2) (2019) e11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Darling KE, Sato AF, Systematic Review and Meta-Analysis Examining the Effectiveness of Mobile Health Technologies in Using Self-Monitoring for Pediatric Weight Management, Child Obes (2017). [DOI] [PubMed] [Google Scholar]
- [9].Chaplais E, Naughton G, Thivel D, Courteix D, Greene D, Smartphone Interventions for Weight Treatment and Behavioral Change in Pediatric Obesity: A Systematic Review, Telemed J E Health 21(10) (2015) 822–30. [DOI] [PubMed] [Google Scholar]
- [10].Spruijt-Metz D, Wen CK, O’Reilly G, Li M, Lee S, Emken BA, Mitra U, Annavaram M, Ragusa G, Narayanan S, Innovations in the Use of Interactive Technology to Support Weight Management, Curr Obes Rep 4(4) (2015) 510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Svetkey LP, Batch BC, Lin PH, Intille SS, Corsino L, Tyson CC, Bosworth HB, Grambow SC, Voils C, Loria C, Gallis JA, Schwager J, Bennett GB, Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology, Obesity (Silver Spring) 23(11) (2015) 2133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liao Y, Basen-Engquist KM, Urbauer DL, Bevers TB, Hawk E, Schembre SM, Using continuous glucose monitoring to motivate physical activity in overweight and obese adults: a pilot study, AACR, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Taylor PJ, Thompson CH, Brinkworth GD, Effectiveness and acceptability of continuous glucose monitoring for type 2 diabetes management: A narrative review, J Diabetes Investig 9(4) (2018) 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liao Y, Schembre S, Acceptability of Continuous Glucose Monitoring in Free-Living Healthy Individuals: Implications for the Use of Wearable Biosensors in Diet and Physical Activity Research, JMIR Mhealth Uhealth 6(10) (2018) e11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barnard KD, Kropff J, Choudhary P, Neupane S, Bain SC, Kapitza C, Forst T, Link M, Mdingi C, DeVries JH, Acceptability of Implantable Continuous Glucose Monitoring Sensor, J Diabetes Sci Technol 12(3) (2018) 634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moser O, Mader JK, Tschakert G, Mueller A, Groeschl W, Pieber TR, Koehler G, Messerschmidt J, Hofmann P, Accuracy of Continuous Glucose Monitoring (CGM) during Continuous and High-Intensity Interval Exercise in Patients with Type 1 Diabetes Mellitus, Nutrients 8(8) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Taylor PJ, Thompson CH, Luscombe-Marsh ND, Wycherley TP, Wittert G, Brinkworth GD, Efficacy of Real-Time Continuous Glucose Monitoring to Improve Effects of a Prescriptive Lifestyle Intervention in Type 2 Diabetes: A Pilot Study, Diabetes Ther (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hilliard ME, Levy W, Anderson BJ, Whitehouse AL, Commissariat PV, Harrington KR, Laffel LM, Miller KM, Van Name M, Tamborlane WV, DeSalvo DJ, DiMeglio LA, Benefits and Barriers of Continuous Glucose Monitoring in Young Children with Type 1 Diabetes, Diabetes Technol Ther 21(9) (2019) 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Suh S, Kim JH, Glycemic Variability: How Do We Measure It and Why Is It Important?, Diabetes Metab J 39(4) (2015) 273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Umpierrez GE, B PK, Glycemic Variability: How to Measure and Its Clinical Implication for Type 2 Diabetes, Am J Med Sci 356(6) (2018) 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp ӧ., Straus SE, PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation, Ann Intern Med 169(7) (2018) 467–473. [DOI] [PubMed] [Google Scholar]
- [22].Peters M, Godfrey C, McInerney P, Munn Z, Tricco A, Khalil H, Chapter 11: Scoping reviews, 2020. 2020). [Google Scholar]
- [23].Dang D, Dearholt S, Johns Hopkins nursing evidence-based practice: model and guidelines, 2017. [Google Scholar]
- [24].Farabi SS, Carley DW, Smith D, Quinn L, Impact of exercise on diurnal and nocturnal markers of glycaemic variability and oxidative stress in obese individuals with type 2 diabetes or impaired glucose tolerance. [DOI] [PubMed] [Google Scholar]
- [25].Gay JL, Buchner DM, Erickson ML, Lauture A, Effect of short bouts of high intensity activity on glucose among adults with prediabetes: A pilot randomized crossover study, Diabetes Res Clin Pract 141 (2018) 168–174. [DOI] [PubMed] [Google Scholar]
- [26].Little JP, Jung ME, Wright AE, Wright W, Manders RJ, Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults, Applied physiology, nutrition, and metabolism 39(7) (2014) 835–841. [DOI] [PubMed] [Google Scholar]
- [27].Parr EB, Devlin BL, Lim KHC, Moresi LNZ, Geils C, Brennan L, Hawley JA, Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study, Nutrients 12(11) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].∅hrstrøm CC, Worm D, Højager A, Andersen D, Holst JJ, Kielgast UL, Hansen DL, Postprandial hypoglycaemia after Roux-en-Y gastric bypass and the effects of acarbose, sitagliptin, verapamil, liraglutide and pasireotide, Diabetes Obes Metab 21(9) (2019) 2142–2151. [DOI] [PubMed] [Google Scholar]
- [29].Halperin F, Patti ME, Skow M, Bajwa M, Goldfine AB, Continuous glucose monitoring for evaluation of glycemic excursions after gastric bypass, Journal of obesity 2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ritz P, Vaurs C, Bertrand M, Anduze Y, Guillaume E, Hanaire H, Usefulness of acarbose and dietary modifications to limit glycemic variability following Roux-en-Y gastric bypass as assessed by continuous glucose monitoring, Diabetes Technol Ther 14(8) (2012) 736–40. [DOI] [PubMed] [Google Scholar]
- [31].Wysocki M, Szopa M, Stefura T, Dudek A, Torbicz G, Gajewska N, Pedziwiatr M, Malczak P, Pisarska M, Budzynski A, Major P, Continuous Glucose Monitoring in Bariatric Patients Undergoing Laparoscopic Sleeve Gastrectomy and Laparoscopic Roux-En-Y Gastric Bypass, Obes Surg 29(4) (2019) 1317–1326. [DOI] [PubMed] [Google Scholar]
- [32].Harmon KA, Gerard L, Jensen DR, Kealey EH, Hernandez TL, Reece MS, Barbour LA, Bessesen DH, Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth, Diabetes Care 34(10) (2011) 2198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yogev Y, Chen R, Ben-Haroush A, Hod M, Diurnal Glycemic profile characterization in non-diabetic non-obese subjects during the first trimester, Journal of Maternal-Fetal & Neonatal Medicine 19 (2006) 44. [Google Scholar]
- [34].Buscemi S, Verga S, Cottone S, Azzolina V, Buscemi B, Gioia D, Cerasola G, Glycaemic variability and inflammation in subjects with metabolic syndrome, Acta Diabetol 46(1) (2009) 55–61. [DOI] [PubMed] [Google Scholar]
- [35].Climie R, Grace MS, Larsen R, Dempsey PC, Oberoi J, Cohen N, Owen N, Kingwell BA, Dunstan D, Regular brief interruptions to sitting after a high-energy evening meal attenuate glycemic excursions in overweight/obese adults, Nutrition, Metabolism and Cardiovascular Diseases 28(9) (2018) 909–916. [DOI] [PubMed] [Google Scholar]
- [36].Crespo NC, Mullane SL, Zeigler ZS, Buman MP, Gaesser GA, Effects of Standing and Light-Intensity Walking and Cycling on 24-h Glucose, Med Sci Sports Exerc 48(12) (2016) 2503–2511. [DOI] [PubMed] [Google Scholar]
- [37].Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM, Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans, Nutrients 11(6) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jimenez-Dominguez G, Ble-Castillo JL, Aparicio-Trapala MA, Juarez-Rojop IE, Tovilla-Zarate CA, Ble-Castillo DJ, Garcia-Vazquez C, Olvera-Hemandez V, Perez-Pimienta B, Diaz-Zagoya JC, Mendez JD, Effects of Acute Ingestion of Native Banana Starch on Glycemic Response Evaluated by Continuous Glucose Monitoring in Obese and Lean Subjects, Int J Environ Res Public Health 12(7) (2015) 7491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jospe MR, de Bruin WE, Haszard JJ, Mann JI, Brunton M, Taylor RW, Teaching people to eat according to appetite—Does the method of glucose measurement matter?, Appetite (2020)104691. [DOI] [PubMed] [Google Scholar]
- [40].Kim J, Lam W, Wang Q, Parikh L, Elshafie A, Sanchez-Rangel E, Schmidt C, Li F, Hwang J, Belfort-DeAguiar R, In a Free-Living Setting, Obesity Is Associated With Greater Food Intake in Response to a Similar Premeal Glucose Nadir, The Journal of Clinical Endocrinology & Metabolism 104(9) (2019) 3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Philippou E, McGowan BM, Brynes AE, Dornhorst A, Leeds AR, Frost GS, The effect of a 12-week low glycaemic index diet on heart disease risk factors and 24 h glycaemic response in healthy middle-aged volunteers at risk of heart disease: a pilot study, Eur J Clin Nutr 62(1) (2008) 145–9. [DOI] [PubMed] [Google Scholar]
- [42].Rafiei H, Robinson E, Barry J, Jung ME, Little JP, Short-term exercise training reduces glycaemic variability and lowers circulating endothelial microparticles in overweight and obese women at elevated risk of type 2 diabetes, Eur J Sport Sci 19(8) (2019) 1140–1149. [DOI] [PubMed] [Google Scholar]
- [43].Salkind SJ, Huizenga R, Fonda SJ, Walker MS, Vigersky RA, Glycemic variability in nondiabetic morbidly obese persons: results of an observational study and review of the literature, Journal of diabetes science and technology 8(5) (2014) 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wennberg P, Boraxbekk CJ, Wheeler M, Howard B, Dempsey PC, Lambert G, Eikelis N, Larsen R, Sethi P, Occleston J, Hernestål-Boman J, Ellis KA, Owen N, Dunstan DW, Acute effects of breaking up prolonged sitting on fatigue and cognition: a pilot study, BMJ Open 6(2) (2016) e009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wilkinson MJ, Manoogian EN, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda S, Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome, Cell Metabolism 31(1) (2020) 92–104. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Winn NC, Pettit-Mee R, Walsh LK, Restaino RM, Ready ST, Padilla J, Kanaley JA, Metabolic implications of diet and energy intake during physical inactivity, Medicine & Science in Sports & Exercise 51(5) (2019) 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Choudhary A, Antal Z, Assessment of impaired fasting glucose in obese and overweight insulin-resistant children by continuous glucose monitoring, J Diabetes Sci Technol 7(6) (2013) 1646–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bauer LB, Reynolds LJ, Douglas SM, Kearney ML, Hoertel HA, Shafer RS, Thyfault JP, Leidy HJ, A pilot study examining the effects of consuming a high-protein vs normal-protein breakfast on free-living glycemic control in overweight/obese ‘breakfast skipping’ adolescents, International Journal of Obesity 39(9) (2015) 1421–1424. [DOI] [PubMed] [Google Scholar]
- [49].Chan CL, Pyle L, Newnes L, Nadeau KJ, Zeitler PS, Kelsey MM, Continuous glucose monitoring and its relationship to hemoglobin A1c and oral glucose tolerance testing in obese and prediabetic youth, J Clin Endocrinol Metab 100(3) (2015) 902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ghane N, Broadney MM, Davis EK, Trenschel RW, Collins SM, Brady SM, Yanovski JA, Estimating plasma glucose with the FreeStyle Libre Pro continuous glucose monitor during oral glucose tolerance tests in youth without diabetes, Pediatr Diabetes 20(8) (2019) 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kaya A, Koçyiǧit C, Çath G, ӧzkan EB, Dündar BN, The Relationship Between Glycemic Variability and Inflammatory Markers in Obese Children with Insulin Resistance and Metabolic Syndrome, Journal of Clinical Research in Pediatric Endocrinology 9(3) (2017) 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schiaffini R, Liccardo D, Alisi A, Benevento D, Cappa M, Cianfarani S, Nobili V, Early Glucose Derangement Detected by Continuous Glucose Monitoring and Progression of Liver Fibrosis in Nonalcoholic Fatty Liver Disease: An Independent Predictive Factor?, Horm Res Paediatr 85(1) (2016) 29–34. [DOI] [PubMed] [Google Scholar]
- [53].Zou CC, Liang L, Hong F, Zhao ZY, Glucose metabolism disorder in obese children assessed by continuous glucose monitoring system, World J Pediatr 4(1) (2008) 26–30. [DOI] [PubMed] [Google Scholar]
- [54].Sawyer BJ, Effects of eight weeks of high-intensity interval training on blood glucose regulation, endothelial function, and visceral fat in obese adults [thesis], Ariz, USA: Arizona State University; (2013). [Google Scholar]
- [55].de Bruin WE, Ward AL, Taylor RW, Jospe MR, ‘Am I really hungry?’ A qualitative exploration of patients’ experience, adherence and behaviour change during hunger training: a pilot study, BMJ open 9(12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pleus S, Ulbrich S, Zschornack E, Kamann S, Haug C, Freckmann G, Documentation of Skin-Related Issues Associated with Continuous Glucose Monitoring Use in the Scientific Literature, Diabetes Technol Ther 21(10) (2019) 538–545. [DOI] [PubMed] [Google Scholar]
- [57].Faulds ER, Militello LK, Tubbs-Cooley H, Happ MB, Evaluating Feasibility of Personal Diabetes Device Data Collection for Research, Nurs Res 69(6) (2020) 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets from this study will be available from the corresponding author on written request.


