Abstract
Aims
As survival among people living with HIV (PLHIV) improves with universal HIV treatment, new strategies are needed to support management of co‐morbidities like type 2 diabetes (T2D). We assessed prediabetes and T2D prevalence and risk factors using haemoglobin A1c (HbA1c) among PLHIV on antiretroviral therapy (ART) in Central Kenya.
Methods
This cross‐sectional study, conducted at a rural and urban site, enrolled PLHIV aged ≥35 years on ART for at least 5 years. HbA1c was assayed using Cobas b 101®, a point‐of‐care device. HbA1c levels ≥6.5% were considered diagnostic of T2D. For pre‐diabetic HbA1c levels (5.7%–6.4%), participants were requested to return the following day for a fasting blood glucose (FBG) to rule out T2D. Risk factors were assessed using multivariable log‐binomial regression.
Results
Of the 600 completing study procedures, the prevalence of diabetes was 5% (30/600). Ten participants were known to have diabetes; thus, prevalence of newly diagnosed T2D was 3.4% (20/590). Prevalence of prediabetes (HbA1c 5.7%–6.4%) was 14.2% (84/590). Significant predictors of elevated HbA1c were increase in age (Prevalence ratio [PR]: 1.10, CI: 1.02, 1.18, p = .012), hypertension (PR: 1.43, CI: 1.07–2.3, p = .015), central adiposity (PR: 2.11, CI: 1.57–2.84, p < .001) and use of Efavirenz (PR: 2.09, CI: 1.48, 2.96, p < .001).
Conclusion
There is a high prevalence of prediabetes, a significant predictor of T2D, among PLHIV in Central Kenya. Point‐of‐care HbA1c may help identify PLHIV with prediabetes in a single screening visit and provide an opportunity for early intervention.
Keywords: HIV, prediabetes, type 2 diabetes, viral suppression
The article highlights the need for T2D screening especially among African people with HIV who are aviremic using standard diagnostic measures. The results show a high prevalence of prediabetes (14%) with a significant proportion of them expected to develop overt diabetes and ultimately its costly complications.
1. INTRODUCTION
Globally, people living with HIV (PLHIV) have been shown to have a higher prevalence of type 2 diabetes (T2D) and prediabetes relative to those without HIV. 1 Chronic systemic inflammation and toxicity related to antiretroviral therapy (ART) have been linked to pancreatic insufficiency and peripheral insulin resistance. 2 , 3 With increased life expectancy following early initiation of ART, PLHIV are also experiencing age‐associated metabolic conditions, including T2D and cardiovascular disease. 4 We sought to characterize the prevalence of prediabetes, T2D and associated risk factors for hyperglycaemia among ART‐experienced PLHIV in a rural and urban environment in Central Kenya.
Prediabetes is important to identify as it has a better predictive value for developing T2D than individual risk factors including obesity and familial history. 5 , 6 Outside of Africa, the incidence of T2D among PLHIV is high. In South East Asia, it ranges from 3.4 to 11.1 per 100 person years. 7 , 8 However, findings on prevalence of T2D among PLHIV in sub‐Saharan Africa (SSA) are mixed. While earlier studies found up to fourfold increased risk for T2D among PLHIV compared to individuals without HIV, 9 , 10 , 11 a review of recent studies in SSA reported T2D prevalence closer to twofold greater among people with HIV compared to adults without HIV, with prevalence ranging from 0.5%–9.3% among PLHIV and 0.5%–3.6% among individuals without HIV. 12 These conflicting findings could be due to use of different tests and criteria for diagnosis of T2D. Heterogeneity in ART use, for example including both ART‐naïve and ART‐experienced patients could also explain the mixed findings. Earlier studies also suggested that treatment with protease inhibitors (PI), high viral load and low CD4 count were predictors of T2D among PLHIV. 13 With universal ART roll‐out, there is a need to identify persistent risk factors for T2D among PLHIV with immune reconstitution hence a high CD4 count and viral suppression and are using newer classes of ART drugs.
Optimal screening approaches for diabetes suitable for PLHIV in resource‐limited settings are critical first steps in this process. Glycated haemoglobin (HbA1c) captures the glycaemic status over a 3‐month period and does not require fasting, therefore a feasible and acceptable screening tool in this population. 14 While older studies reported that compared to fasting blood glucose (FBG) or oral glucose tolerance test (OGTT), HbA1c underestimated T2D prevalence in PLHIV populations, these studies were conducted among adults with severe HIV‐associated immunosuppression. 15 , 16 Recent studies that include PLHIV on ART have found similar test performance comparing HbA1c to other screening tests. 17 , 18 Availability of consistent and standardized point‐of‐care (POC) HbA1c devices has increased access to HbA1c testing and POC HbA1c could be a feasible screening tool in the Kenyan population. 19 , 20 With this in mind, we conducted a cross‐sectional study using HbA1c screening and a confirmatory blood glucose to assess the burden of prediabetes, T2D and associated risk factors for hyperglycaemia among ART‐experienced PLHIV in Central Kenya.
2. SUBJECTS, MATERIALS AND METHODS
2.1. Study subjects and setting
During a routine clinic visit, PLHIV aged ≥35 years were recruited from the HIV Comprehensive Care Centres (CCC) at Kiambu and Kerugoya, urban and rural referral hospitals, respectively, through systematic random sampling from January through July 2018. Eligibility criteria included having been on ART for at least 5 years and having had a clinical review within the previous 6 months. Patients with a history of blood transfusion or anaemia in the preceding 6 months and pregnant women were excluded. Written informed consent was obtained by a dedicated study nurse prior to all study procedures. The study was approved by the Kenyatta National Hospital/University of Nairobi Ethics Review Committee and the University of Washington Human Subjects Division.
At enrolment, a questionnaire adapted from the World Health Organization (WHO) Stepwise Approach to Surveillance (STEPS) instrument (Annex 1) was administered by a trained study nurse to obtain demographics, risk factors such as smoking and alcohol use, family history of diabetes and prior diagnosis or treatment of diabetes and/or hypertension. Questions assessing participants’ history of screening for diabetes, including type of screening test used and the indication for screening, and knowledge of increased risk of hyperglycaemia secondary to HIV were asked, since people with HIV are expected to have annual screening for diabetes as per the national HIV treatment guidelines. 21 Factors influencing haemoglobin levels such as other medications and self‐reported haemoglobinopathies, including sickle cell anaemia, were also obtained.
HIV‐specific data including the date of HIV diagnosis, current and previous ART regimens and blood pressure (BP) readings from the two previous clinic visits were extracted by the study nurse from participants’ clinical charts and confirmed from the electronic medical records. Weight, height, waist and hip circumferences were measured according to WHO international standards. 22 A blood pressure reading was obtained with the participant seated and legs uncrossed, as part of routine clinic visit procedures.
2.2. Glucose screening
The HbA1c assay was done using Cobas b 101 ® by Roche©, a point‐of‐care instrument which measures per cent HbA1c and mmol/mol HbA1c by photometric transmission measurement. Internal quality controls were implemented as per the device manufacturer's instructions and run every 2 weeks. The device's coefficient of variation provided by the manufacturer was 0.19% HbA1c. 23 Venous samples were obtained from 1% of the participants and assayed in an accredited laboratory for comparison with the POC values, showing good collinearity, with a correlation coefficient of 0.97 and a % mean difference of 2%.
For HbA1c levels ≥6.5% (≥48 mmol/mol), a random blood glucose obtained on the same day was used to confirm T2D diagnosis [Figure 1], as per the American Diabetes Association (ADA) guidelines where a diagnosis needs to be confirmed using a different test. For HbA1c levels between 5.7% and 6.4% (39–47 mmol/mol), participants were requested to return while fasting the following day for a fasting blood glucose using Accu‐Check® by Roche©. Some patients who also needed a fasting lipid profile (details not presented here) also got a fasting blood glucose regardless of their HbA1c.
FIGURE 1.
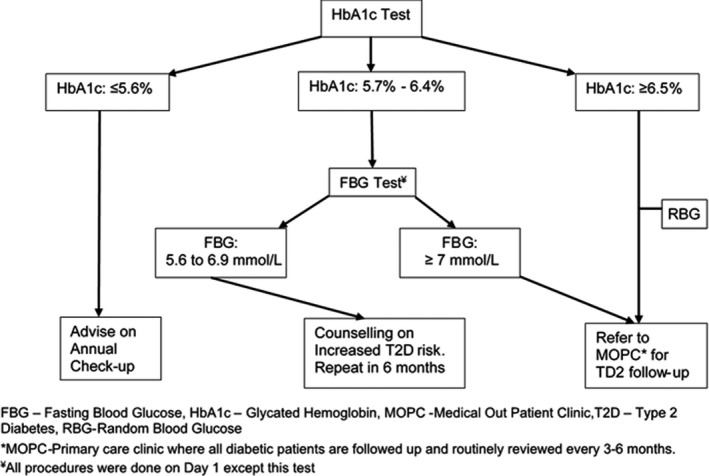
Study procedures flowchart
2.3. Definitions of diabetes and hypertension
Hypertension was defined by two parameters: (1) two readings where systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg, 24 using the enrolment BP measurement and a second BP reading from the clinical chart or (2) reported use of antihypertensive medications. Prediabetes was defined as a HbA1c between 5.7 and 6.4% (39–47 mmol/mol). Diabetes was defined by three parameters: (1) a HbA1c ≥6.5% and random blood glucose ≥11.1 mmol/L or (2) a HbA1c between 5.7 and 6.4% and fasting blood glucose ≥7.0 mmol/L or (3) reported use of insulin or oral hypoglycaemics. 25 , 26
2.4. Statistical analysis
The sample size was based on an a priori estimated prevalence of diabetes among the PLHIV population of 16%–25%. 11 , 27 Using a normal approximation to the binomial distribution, we needed a sample size of 588 to observe an estimate of 25% prevalence of T2D with a precision interval of ±3.5%.
Due to lower‐than‐expected numbers of participants with diabetes in the sample, prediabetes and diabetes were combined to create a binary outcome for the regression analysis. Multivariable log‐binomial regression was used to assess factors associated with prediabetes and diabetes. Covariates considered include age, sex, family history of T2D, time since HIV diagnosis, duration on ART, individual ART drugs, alcohol use, hypertension, body mass index (BMI) and waist circumference. Following bivariable analysis, covariates with a p‐value of ≤.05 were included in the multivariable model.
3. RESULTS
3.1. Cohort demographics and prevalence of prediabetes and T2D
Of the 600 participants who completed all study procedures, 383 (63.8%) were female. [Table 1] The median age was 46.8 years (interquartile range [IQR]: 41.6, 53.1).
TABLE 1.
Demographics, anthropometric measurements and HIV‐related characteristics among PLHIV in Central Kenya
| Covariate | All participants | Urban Clinic | Rural Clinic |
|---|---|---|---|
| n = 600 | n = 302 | n = 298 | |
| Median (IQR) or n (%) | Median (IQR) or n (%) | Median (IQR) or n (%) | |
| Age (years) | 46.8 (41.6, 53.1) | 46.6 (41.2, 53.0) | 46.9 (42.0, 53.4) |
| Sex (female) | 383 (63.8%) | 177 (58.6%) | 206 (69.1%) |
| Education Level | |||
| Primary | 334 (55.7%) | 155 (51.3%) | 179 (60.1%) |
| Secondary | 191 (31.7%) | 107 (35.4%) | 84 (28.2%) |
| Post‐Secondary | 56 (9.4%) | 34 (11.3%) | 22 (7.3%) |
| None | 19 (3.2%) | 6 (2.0%) | 13 (4.4%) |
| Time since HIV diagnosis (years) | 9.3 (7.6, 11.1) | 9.1 (7.7, 11.0) | 9.7 (7.6, 11.4) |
| Time on ART (years) | 8.1 (6.5, 10.0) | 8.0 (6.7, 9.6) | 8.3 (6.4, 0.3) |
| Viral load (>0 copies/ml) | 45 (7.5%) | 10 (3.6%) | 35 (12.2%) |
| Current CD4 (cells/ml) | 512 (354, 690) | 482 (324, 660) | 540 (387, 719) |
| Current ART Regimen | |||
| 2NRTI+NNRTI | 560 (93.3%) | 284 (94.0%) | 276 (92.6%) |
| NRTI+NNRTI+PI | 39 (6.5%) | 17 (5.6%) | 22 (7.4%) |
| Other | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) |
| Smoking | |||
| Never | 431 (71.8%) | 206 (68.2%) | 225 (75.5%) |
| Previous | 144 (24.0%) | 82 (27.2%) | 62 (20.8%) |
| Current | 25 (4.2%) | 14 (4.6%) | 11 (3.7%) |
| Alcohol Use¥ | 63 (10.5%) | 42 (13.9%) | 21 (7.0%) |
| Hypertension¥ | 215 (35.8%) | 79 (26.2%) | 136 (45.6%) |
| Waist‐Hip Ratio (≥0.9 for males and ≥0.85 for females) | 329 (54.8%) | 158 (52.3%) | 171 (57.4%) |
| Waist Circumference (>102 for males and >88 cm for females) | 200 (33.3%) | 102 (33.8%) | 98 (32.9%) |
| Previously diagnosed T2D | 10 (1.7%) | 3 (1.0%) | 7 (2.3%) |
| Family history of T2D | 125 (20.8%) | 60 (19.9%) | 65 (21.8%) |
| Previous Blood Sugar Screening¥ | 145 (24.2%) | 56 (18.5%) | 89 (29.9%) |
| Random Blood Glucose | 136 (93.8%) | 54 (96%) | 82 (92%) |
| Fasting Blood Glucose | 8 (5.5%) | 1 (2%) | 7 (8%) |
| Haemoglobin A1c | 1 (0.7%) | 1 (2%) | 0 (0%) |
| Reasons for Blood Sugar Screening¥ | |||
| Free, for example medical camp | 52 (35.9%) | 19 (34%) | 33 (37%) |
| Test annually by myself | 41 (28.3%) | 12 (21%) | 29 (33%) |
| Test annually by physician | 27 (18.6%) | 13 (23%) | 14 (16%) |
| Part of clinical work‐up | 22 (15.1%) | 9 (16%) | 13 (15%) |
| Due to familial history | 1 (0.7%) | 1 (2%) | 0 (0%) |
| Others | 2 (1.4%) | 2 (4%) | 0 (0%) |
Abbreviations: 3TC, lamivudine; ART, antiretroviral therapy; BMI body mass index; IQR, interquartile range; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; T2D, type 2 diabetes.
¥p‐value <.05 comparing urban to rural clinic.
The median time since HIV diagnosis was 9.3 years (IQR: 7.6, 11.1), while the median duration on ART was 8.1 years (IQR: 6.5, 10.0). Median current CD4 was 512 cells/ml (IQR: 354,690) and though 45 individuals (7.5%) had a detectable viral load, only 22 (3.7%) had more than 1000 copies/ml, the cut‐off for virologic treatment failure. A majority (93%) were on a combination of two nucleoside reverse transcriptase inhibitors (NRTI); tenofovir [TDF] or zidovudine [AZT], lamivudine 28 ), and a non‐nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz 28 or nevirapine. Only 7% were using a protease inhibitor. One hundred and thirty‐six (45.6%) participants from the rural clinic were hypertensive compared to 79 (26.2%) from the urban clinic (p‐value: <.001). Of note, among hypertensive patients on antihypertensives, none of their medication information was recorded in the electronic medical system.
At enrolment, 10 (1.7%) of 600 participants were known to have diabetes. Among those not known to have diabetes, 20 were newly diagnosed to have T2D. The overall prevalence of diabetes was therefore 5% (30/600) while the prevalence of newly diagnosed T2D was 3.4% (20/590), with fourteen cases (2.4%) having diabetes as defined by HbA1c ≥6.5% (≥48 mmol/mol) and random blood glucose >11.1 mmol/L and six cases having diabetes as defined by HbA1c 5.7% ‐ 6.4% and fasting glucose ≥7.0 mmol/L [Figure 2]. Of the participants requested to return for fasting glucose to rule out diabetes, 17% (19/113) failed to return. Excluding participants known to have T2D, the prevalence of newly diagnosed prediabetes (HbA1c 5.7%–6.4%) was therefore 14.2% ([84/590) [Figure 2]. Overall, newly diagnosed hyperglycaemia (T2D and prediabetes) was 21% (62/299) in Kiambu compared to 14% (42/291) in Kerugoya. (p = .045).
FIGURE 2.
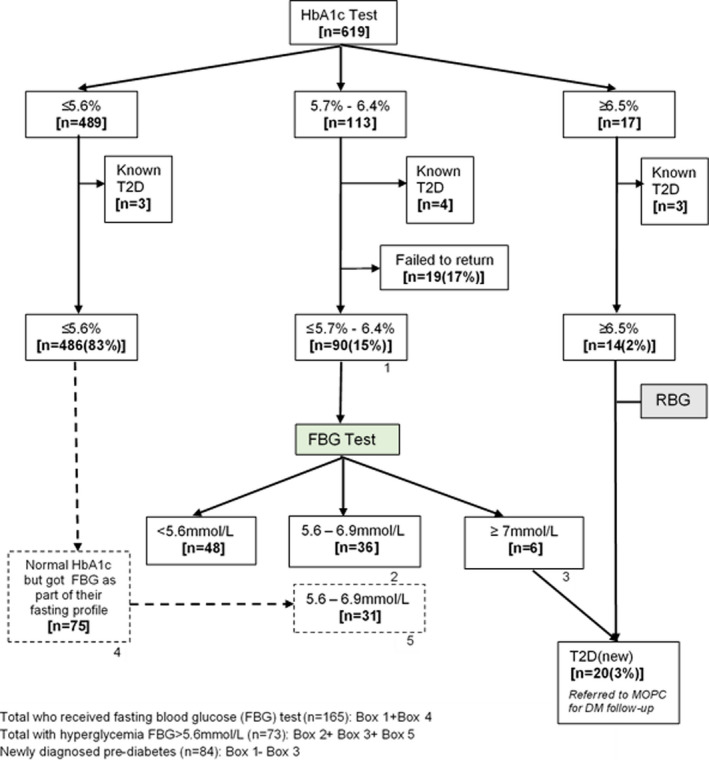
Prediabetes and diabetes testing and results among 619 HIV‐positive study participants
3.2. Factors associated with prediabetes and diabetes.
Factors associated with a diagnosis of prediabetes or diabetes are shown in Table 2 and included age, hypertension, central obesity and an ART regimen containing EFV. In multivariable log‐binomial regression analysis, age was significantly associated with prediabetes and diabetes, with a 13% increase in the prevalence ratio for every 5‐year increase in age (adjusted prevalence ratio [aPR]: 1.13, CI: 1.03, 1.25, p = .004). Hypertensive patients were 1.61 times as likely to have prediabetes and diabetes compared to those without hypertension (aPR: 1.61, CI: 1.15, 2.26, p = .006) [Table 2]. Hyperglycaemic HbA1c values were twice as likely among those with central adiposity (waist circumference>102 cm for males and >88 cm for females) compared to those with normal waist circumference (aPR: 2.09, CI: 1.50, 2.92, p < .001). BMI and waist circumference were not included in the same model as these variables were highly colinear (Pearson's r = 0.83).
TABLE 2.
Predictors of diabetes and prediabetes among 590 persons living with HIV
| Covariate | Unadjusted PR (95% CI) | p‐value for unadjusted PR | Adjusted PR1 (95% CI) | p‐value for adjusted PR |
|---|---|---|---|---|
| Age (5 years) | 1.16 (1.05, 1.27) | .002 | 1.13 (1.03, 1.25) | .004 |
| Sex (male) | 0.71 (0.48, 1.05) | .08 | ||
| Time since HIV diagnosis (years) | 1.05 (0.98, 1.14) | .22 | ||
| Time on ART | 1.06 (0.98, 1.16) | .14 | ||
| Family history of DM | 1.42 (0.96, 2.09) | .08 | ||
| Alcohol Use | 0.70 (0.36, 1.37) | .29 | ||
| Hypertension | 1.69 (1.19, 2.38) | .003 | 1.61 (1.15, 2.26) | .006 |
| Waist Circumference (>102 for males & >88 cm for females) | 2.19 (1.55, 3.09) | <.001 | 2.09 (1.50, 2.92) | <.001 |
| Viral load (detectable) | 0.73 (0.34, 1.58) | .425 | ||
| Current CD4(cells/ml) | 1.00 (0.99, 1.01) | .087 | ||
| ART (Having the drug as part of their current or previous combination) | ||||
| TDF (n = 456) | 1.62 (1.00, 2.63) | .05 | ||
| NVP (n = 459) | 1.08 (0.71, 1.64) | .74 | ||
| EFV (n = 274) | 1.83 (1.28, 2.62) | .001 | 2.09 (1.48, 2.96) | <.001 |
| AZT (n = 215) | 0.77 (0.52, 1.13) | .18 | ||
| ABC (n = 6) | 0.95 (0.16, 5.70) | .95 | ||
| LPV/r (n = 26) | 0.64 (0.22, 1.90) | .43 | ||
| ATV/r (n = 15) | 0.75 (0.19, 2.63) | .67 | ||
Bold values indicates the statistical significance.
Covariates with a p‐value of <.05 in bivariable analysis were included in the multivariable model.
Abbreviation: PR, Prevalence Ratio.
People on current or previous regimens containing EFV were twice as likely to have prediabetes and diabetes (aPR: 2.09, CI: 1.48, 2.96, p < 0.001) [Table 2]. Time since HIV diagnosis, duration on ART, sex, CD4 count, viral load or being on a PI‐containing regimen were not associated with prediabetes and diabetes in bivariable analyses and were excluded from the multivariable model.
Among the 486 participants who had a normal HbA1c, 75 of them returned the following day for a fasting lipid profile and also got a fasting blood glucose; hence, a total of 165 people had fasting blood glucose [Figure 2]. Using FBG as the outcome, the prevalence of hyperglycaemia (>5.6 mmol/L) was 44% (73/165) and the direction of association between the risk factors and elevated FBG remained the same, although statistical significance was lost due to the smaller sample size.
3.3. Diabetes awareness and screening
Overall, 145 participants (24.2%) reported being previously screened for diabetes, with 89 (29.9%) from the rural clinic compared to 56 (18.5%) in the urban clinic, a difference that was statistically significant (p = .001). This screening had been done using a random blood glucose (93.8%) or fasting blood glucose (5.5%). Majority of patients (35.9%) reported prior screening because the blood glucose test had been offered free of charge. While self‐initiated blood sugar screening was higher in the rural (70%) compared to the urban clinic (55%), provider‐driven screening (e.g., as part of clinical work‐up) was higher (39%) in the urban clinic compared to 31% in the rural clinic (p = .039).
4. DISCUSSION
After excluding those with known T2D, overall prevalence of newly diagnosed diabetes using HbA1c was low (3.4%) in this virally suppressed PLHIV cohort. However, prevalence of prediabetes was high at 14.2%. With up to 10% of people with prediabetes progressing to develop diabetes each year, identifying and addressing prediabetes through diet, lifestyle modification or metformin could reduce future risk of diseases associated with T2D such as stroke, myocardial infarction and peripheral arterial disease. 29 Risk factors associated with prediabetes and diabetes included age, presence of hypertension, abnormal waist circumference and current or prior use of efavirenz. Hypertension was prevalent in over a third (36%) of the population, with higher proportions observed in the rural compared to the urban clinic (46% vs 26%). These results support previously published findings that PLHIV share similar risk factors for diabetes with the general population. Many studies have described an association between diabetes and age, hypertension and central adiposity, in both general populations and PLHIV sub‐populations. 12 , 30
Our findings are also consistent with several recent studies which have reported a wide range of values for the prevalence of diabetes and its risk factors among individuals on ART in SSA: diabetes (0.5%–8%), 31 prediabetes (12%–21%) 11 , 13 , 32 , 33 and hypertension (12%–39%). 34 , 35 Prevalence of diabetes (3.4%) in our study population was similar to these studies and to other recent studies in Kenya among PLHIV which found 2.1%–4.8%. 36 , 37 It was also consistent with the overall range of T2D prevalence estimates in Kenya among all adults, regardless of HIV status, at 3.5% to 5%. 38 Our estimates of T2D among PLHIV in Kenya were lower than estimates in low‐income countries in South East Asia, where estimates of up to 9% have been reported. 7 These differences could be due to lower prevalence of other risk factors, like alcohol and smoking, in our study population relative to PLHIV in these regions or to other environmental or genetic differences. 30 , 39
The association of hyperglycaemia with Efavirenz is also consistent with what has previously been reported in the literature. 9 , 11 This is particularly significant in Kenya, where a greater proportion of patients are on TDF and EFV as their first‐line regimen, yet diabetes screening is emphasized only among the small proportion on PI‐containing regimens. Dolutegravir, an integrase inhibitor which has been associated with weight gain and hyperglycaemia, 40 , 41 is being rolled out nationally to replace EFV as part of the first‐line regimen. Should the hyperglycaemic effect of both drugs be cumulative, a case can be made for diabetes and prediabetes screening to be offered to all PLHIV as part of comprehensive health services.
Despite being in the ART treatment guidelines, awareness on the need for screening was generally low (24.2%). With the move towards holistic patient care, PLHIV need to be sensitized on the increased risk of prediabetes and diabetes and therefore the need for routine screening. Since self‐initiated blood sugar screening occurred largely when the test was free, cost and affordability should be a key factor when considering feasible diabetes screening strategies.
This study is the first to our knowledge that reports on prevalence of diabetes using the standard ADA HbA1c definition in East Africa. Many previous studies have relied on a single test. A strength of this study is use of a confirmatory test as per the ADA guidelines. Nearly one in five of those asked to return the following day while fasting failed to come back, highlighting the difficulties posed by screening approaches that require multiple clinic visits. Of note, approximately half of those individuals identified as having prediabetes defined as Hgb A1c 5.7–6.4 had normal fasting blood glucose. Ideally, such individuals would undergo OGTT to determine their risk status. However, given the preponderance of other risk factors for cardiovascular disease in this population, their participation in targeted risk reduction strategies such as diet and lifestyle modification or metformin is warranted. With similar prevalence findings to other studies that used fasting glucose, our findings add to the body of literature supporting use of point‐of‐care HbA1c screening, reducing the requirement for a return visit while fasting. 14 , 20 This is important because despite a low prevalence of overt diabetes, the marked prevalence of prediabetes and other risk factors among a population who attend clinic regularly highlights an opportunity to screen for these modifiable risk factors.
Our study limitations include the failure to use 75g‐OGTT test, the gold standard, as part of our algorithm for T2D diagnosis. We did not assess the mean corpuscular volume (MCV) in this population, who are likely to have macrocytosis, a factor that could interfere with HbA1c values. We relied on the last haemoglobin (Hb) assessed in the preceding 6 months and did not assess a current Hb to rule out anaemia. Both macrocytosis and current low haemoglobin would likely be associated with falsely low HbA1c levels. Our findings may therefore underestimate the prevalence of elevated HbA1c in this population.
5. CONCLUSION
In summary, we believe our findings add support to calls for universal HbA1c screening using POC screening methods as part of comprehensive HIV care services. Our cohort reflects the growing population of older PLHIV who have been on ART for long, have high CD4 counts and have achieved viral suppression. With time and without intervention, it is expected that a significant proportion of those with prediabetes will develop overt diabetes and ultimately its costly complications. In addition to counselling regarding positive lifestyle changes for these PLHIV, longitudinal follow‐up of PLHIV with prediabetes will be important in understanding the risk of progression to T2D and to better inform screening and intervention strategies for diabetes among PLHIV in sub‐Saharan Africa.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHORS CONTRIBUTIONS
AN, SP and CF designed the study. AN, CK and MO were significantly involved in implementation of the study and made significant content contributions. AN and CF took lead in interpretation of the results and drafting of the manuscript. OA and NP provided substantial contributions to statistical analysis, interpretation of the results and drafting of the manuscript. All authors critically reviewed and approved the final version.
ACKNOWLEDGEMENTS
We would like to acknowledge all the patients and healthcare workers from Kiambu and Kerugoya County hospitals who participated in the study. Special thanks to Dr. Jared Mecha (University of Nairobi Central Kenya Response Integration, Strengthening and Sustainability Project), Dr. Ndiritu Ndirangu (Kiambu County Health Management Team) and Dr. E. Gakuo (Kirinyaga County Health Management Team), who allowed and supported us to implement the study.
Njoroge A, Augusto O, Page ST, et al. Increased risk of prediabetes among virally suppressed adults with HIV in Central Kenya detected using glycated haemoglobin and fasting blood glucose. Endocrinol Diab Metab. 2021;4:e292. 10.1002/edm2.292
Funding information
National Institutes of Health Grant D43W009580‐03S1.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Hernandez‐Romieu AC, Garg S, Rosenberg ES, Thompson‐Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV‐infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borato DCK, Parabocz GC, Ribas SRW, et al. Changes of metabolic and inflammatory markers in HIV infection: glucose, lipids, serum Hs‐CRP and myeloperoxidase. Metabolism. 2012;61(10):1353‐1360. [DOI] [PubMed] [Google Scholar]
- 3. Chehter EZ, Bacci MR, Alessi R, et al. Pancreatic insufficiency in HIV: is it possible? AIDS Res Hum Retroviruses. 2013;29(3):423‐428. [DOI] [PubMed] [Google Scholar]
- 4. Negin J, Wariero J, Cumming RG, Mutuo P, Pronyk PM. High rates of AIDS‐related mortality among older adults in rural Kenya. J Acquir Immune Defic Syndr. 2010;55(2):239‐244. [DOI] [PubMed] [Google Scholar]
- 5. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high‐risk state for diabetes development. Lancet. 2012;379(9833):2279‐2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Numata Y, Ohya T, Nitta Y, Yoshinaka Y, Shogakiuchi A, Toyota A. Repetition of prediabetes enhances the risk of developing diabetes. J Diabetes Res. 2019;2019:4916546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chhoun P, Tuot S, Harries AD, et al. High prevalence of non‐communicable diseases and associated risk factors amongst adults living with HIV in Cambodia. PLoS One. 2017;12(11):e0187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paengsai N, Jourdain G, Chaiwarith R, et al. Incidence and clinical outcomes of diabetes mellitus in HIV‐infected adults in Thailand: a retrospective cohort study. BMC Public Health. 2018;18(1):1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub‐Saharan Africa: a systematic review and meta‐analysis. Int J Epidemiol. 2013;42(6):1754‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayega RW, Guwatudde D, Makumbi F, et al. Diabetes and pre‐diabetes among persons aged 35 to 60 years in eastern Uganda: prevalence and associated factors. PLoS One. 2013;8(8):e72554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dave JA, Lambert EV, Badri M, West S, Maartens G, Levitt NS. Effect of nonnucleoside reverse transcriptase inhibitor‐based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV‐infected patients. J Acquir Immune Defic Syndr. 2011;57(4):284‐289. [DOI] [PubMed] [Google Scholar]
- 12. Prioreschi A, Munthali RJ, Soepnel L, et al. Incidence and prevalence of type 2 diabetes mellitus with HIV infection in Africa: a systematic review and meta‐analysis. BMJ Open. 2017;7(3):e013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levitt NS, Peer N, Steyn K, et al. Increased risk of dysglycaemia in South Africans with HIV; especially those on protease inhibitors. Diabetes Res Clin Pract. 2016;119:41‐47. [DOI] [PubMed] [Google Scholar]
- 14. Mayega RW, Guwatudde D, Makumbi FE, et al. Comparison of fasting plasma glucose and haemoglobin A1c point‐of‐care tests in screening for diabetes and abnormal glucose regulation in a rural low income setting. Diabetes Res Clin Pract. 2014;104(1):112‐120. [DOI] [PubMed] [Google Scholar]
- 15. Kim PS, Woods C, Georgoff P, et al. A1C underestimates glycemia in HIV infection. Diabetes Care. 2009;32(9):1591‐1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slama L, Palella FJ, Abraham AG, et al. Inaccuracy of haemoglobin A1c among HIV‐infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. J Antimicrob Chemother. 2014;69(12):3360‐3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tien PC, Schneider MF, Cox C, et al. Association of HIV infection with incident diabetes mellitus: impact of using hemoglobin A1C as a criterion for diabetes. J Acquir Immune Defic Syndr. 2012;61(3):334‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eckhardt BJ, Holzman RS, Kwan CK, Baghdadi J, Aberg JA. Glycated Hemoglobin A(1c) as screening for diabetes mellitus in HIV‐infected individuals. AIDS Patient Care STDS. 2012;26(4):197‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirst JA, McLellan JH, Price CP, et al. Performance of point‐of‐care HbA1c test devices: implications for use in clinical practice ‐ a systematic review and meta‐analysis. Clin Chem Lab Med. 2017;55(2):167‐180. [DOI] [PubMed] [Google Scholar]
- 20. Whitley HP, Hanson C, Parton JM. Systematic diabetes screening using point‐of‐care HbA1c testing facilitates identification of prediabetes. Ann Fam Med. 2017;15(2):162‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ministry of Health, N.A.S.C.P. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya. Nairobi: NASCOP; 2018. [Google Scholar]
- 22. WHO . Waist Circumference and Waist–Hip Ratio: Report of a WHO Expert Consultation. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 23. Toro‐Crespo M, Sánchez‐Mora C, Fernández‐Riejos P, Maesa‐Márquez JM, González‐Rodríguez C. Evaluation of 3 hemoglobin A1c point of care instruments. Point of care testing for HbA1c: evaluation of cobas b101, B‐analyst and afinion. Clin Lab. 2017;63(7):1107‐1112. [DOI] [PubMed] [Google Scholar]
- 24. Whelton PK, Carey RM, Wilbert S, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13‐e115. [DOI] [PubMed] [Google Scholar]
- 25. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: The Task Force for diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD). Eur Heart J. 2019;41(2):255‐323. [DOI] [PubMed] [Google Scholar]
- 26. Kenya, M.o.H. Kenya National Clinical Guidelines for the Management of Diabetes Mellitus, T.N.D.P.a.C. Program, Editor. Nairobi, Kenya: Division of Non‐communicable Diseases, Ministry of Health, Kenya; 2018. [Google Scholar]
- 27. Omech B, Sempa J, Castelnuovo B, et al. Prevalence of HIV‐associated metabolic abnormalities among patients taking first‐line antiretroviral therapy in Uganda. ISRN Aids. 2012;2012:960178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McComsey GA, Daar ES, O'Riordan MaryAnn, et al. Changes in fat mitochondrial DNA and function in subjects randomized to abacavir‐lamivudine or tenofovir DF‐emtricitabine with atazanavir‐ritonavir or efavirenz: AIDS Clinical Trials Group study A5224s, substudy of A5202. J Infect Dis. 2013;207(4):604‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Effect of regression from prediabetes to normal glucose regulation on long‐term reduction in diabetes risk: results from the diabetes prevention program outcomes study. Lancet. 2012;379(9833):2243‐2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang C, Chow FC, Han Y, et al. Multicenter cohort study of diabetes mellitus and impaired fasting glucose in HIV‐infected patients in China. J Acquir Immune Defic Syndr. 2015;68(3):298‐303. [DOI] [PubMed] [Google Scholar]
- 31. Njuguna B, Kiplagat J, Bloomfield GS, et al. Prevalence, risk factors, and pathophysiology of Dysglycemia among people living with HIV in Sub‐Saharan Africa. J Diabetes Res. 2018;2018:6916497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manuthu EM, Joshi MD, Lule GN, Karari E. Prevalence of dyslipidemia and dysglycaemia in HIV infected patients. East Afr Med J. 2008;85(1):10‐17. [DOI] [PubMed] [Google Scholar]
- 33. Rhee JY, Bahtila TD, Palmer D, et al. Prediabetes and diabetes among HIV‐infected adults in Cameroon. Diabetes Metab Res Rev. 2016;32(6):544‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kavishe B, Biraro S, Baisley K, et al. High prevalence of hypertension and of risk factors for non‐communicable diseases (NCDs): a population based cross‐sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med. 2015;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peck RN, Shedafa R, Kalluvya S, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross‐sectional study. BMC Med. 2014;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Edwards JK, Bygrave H, Van den Bergh R, et al. HIV with non‐communicable diseases in primary care in Kibera, Nairobi, Kenya: characteristics and outcomes 2010–2013. Trans R Soc Trop Med Hyg. 2015;109(7):440‐446. [DOI] [PubMed] [Google Scholar]
- 37. Achwoka D, Waruru A, Chen T‐H, et al. Noncommunicable disease burden among HIV patients in care: a national retrospective longitudinal analysis of HIV‐treatment outcomes in Kenya, 2003–2013. BMC Public Health. 2019;19(1):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mohamed SF, Mwangi M, Mutua MK, et al. Prevalence and factors associated with pre‐diabetes and diabetes mellitus in Kenya: results from a national survey. BMC Public Health. 2018;18(Suppl 3):1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Putcharoen O, Wattanachanya L, Sophonphan J, et al. New‐onset diabetes in HIV‐treated adults: predictors, long‐term renal and cardiovascular outcomes. Aids. 2017;31(11):1535‐1543. [DOI] [PubMed] [Google Scholar]
- 40. McCann K, Shah S, Hindley L, et al. Implications of weight gain with newer antiretrovirals. Aids. 2021. 10.1097/qad.0000000000002930. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41. Hsu R, Brunet L, Fusco JS, et al. Incident type 2 diabetes mellitus after initiation of common HIV antiretroviral drugs. Aids. 2021;35(1):81‐90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


