Abstract
Purpose of Review
Endocrine disrupting chemicals (EDCs) potentially have a role in causing hypospadias malformation through modifiable in-utero exposure. Considering the emerging literature on the role of potential endocrine disrupting substances on the occurrence of hypospadias and the potential to inform public health efforts to prevent the occurrence of these malformations, we have summarized the current literature, identified areas of consensus, and highlighted areas that warrant further investigation.
Recent Findings
Pharmaceuticals, such as diethylstilbestrol, progestin fertility treatments, corticosteroids, and valproic acid, have all been associated with hypospadias risk. Data on exposure to dichlorodiphenyltrichloroethane and hexachlorobenzene pesticides, as well as non-persistent pollutants, particularly phthalates, is less consistent but still compelling.
Summary
Improving exposure assessment, standardizing sample timing to relevant developmental windows, using clear case identification and classification schemes, and elucidating dose-response relationships with EDCs will help to provide clearer evidence. Promising directions for future research include identification of subgroups with genetic hypospadias risk factors, measurement of intermediate outcomes, and study of EDC mixtures that will more accurately represent the total fetal environment.
Keywords: Hypospadias, Endocrine disrupting chemicals, Pharmaceuticals, Phthalates, Pesticides, In-utero
Introduction
Hypospadias is a congenital abnormality of penile development that manifests as a urethral orifice that opens on the ventral aspect of the penis proximal to the anatomically correct location at the tip of the penile glans. Hypospadias is one of the most common congenital anomalies of the genitourinary system, occurring in approximately 1 of 200–300 live births in North America [1]. Up to 30% of hypospadias cases can be linked to specific gene mutations, leaving 70% of cases with unknown etiology [2]. Xenobiotic chemicals, including certain drugs and environmental contaminants that influence hormonal pathways, may influence risk for this malformation. In particular, endocrine disrupting chemicals (EDCs) influence hormonal signaling pathways involved in fetal development, and those with androgenic or anti-androgenic properties specifically are suspected to contribute to the development of hypospadias. Male sex organs form between 8 and 14 weeks’ gestation, and the mechanism of penile shaft development and urethral folding is a complex developmental cascade in which the androgen receptor plays an essential role. Until the ninth week of gestation, female and male genitalia are grossly identical. Subsequent urethral development includes the differentiation of the embryonic cloaca into the rectum resulting in the formation of the urogenital sinus (embryonic urinary tract), and the genital tubercle, which is the precursor to the phallus. As the phallus forms and becomes solid at the end, the urogenital membrane is absorbed, and folding occurs to leave the hollow urethra within the phallus. Tubularization results in an opening at the urethral meatus, which is normally located at the distal end of the glans. This process concludes at 16 weeks’ gestation, marking the 8–16-week window as the critical exposure window for disruptions in urethral formation [3]. Mutations in the androgen receptor gene have been implicated in congenital hypospadias creating interest in compounds that interact with the androgen receptor as possible environmental causes of hypospadias. Rarely, concurrent hypospadias and cryptorchidism result from a disorder of sexual development (DSD). DSD patients have an underlying genetic abnormality (e.g., an androgen receptor mutation) and constellations of malformations [4]. Isolated hypospadias occurs more commonly with over 95% of hypospadias occurring without cryptorchidism. Thus isolated hypospadias warrants independent study as it likely has distinct pathogenesis from cryptorchidism [5, 6]. Additionally, it is thought that the underlying mechanism behind cryptorchidism is prooncogenic with an observed increase in testicular cancer risk in both testicles in patients with unilateral undescended testes [7]. This risk is not observed in hypospadias, further supporting individual study of isolated hypospadias.
Because they may share common modes of action, we consider pharmaceuticals and hormonal compounds (e.g., estrogenic compounds) along with environmental EDCs, such as non-persistent pollutants (e.g., phthalates) and persistent organic pollutants (POPs), such as pesticides in this review. Pharmaceutical EDCs are a heterogeneous group of compounds with largely unexplored mechanisms of action pertaining to hypospadias. Sex-steroid medications have been examined in several studies due to their inherent androgenic and anti-androgenic properties. Less is known about environmental EDCs. However, an increased risk of hypospadias and of reduced anogenital distance has been related to EDCs, such as phthalates in animal experiments with evidence to support a risk to humans in some observational studies [8, 9]. Mechanistically, these chemicals may interact with the androgen receptor or act through upregulation of other transcription factors such as TFG-beta1 [10]. Some POPs, such as the pesticide dichlorodiphenyltrichloroethane (DDT), also are a suspected cause of hypospadias in rat models and through mechanisms including inhibition of androgen synthesis, anti-androgenic activity, oxidative stress, and epigenetic mechanisms [11]. The impact of these EDC exposures further may be influenced by genetic predisposition.
In light of the emerging literature on the role of potential endocrine disrupting substances on the occurrence of hypospadias and the potential to inform public health efforts to prevent the occurrence of these malformations, we have summarized the current literature and identified areas of consensus, as well as gaps in our current knowledge base, highlighting areas that warrant further investigation.
Methods
A PubMed search of studies published since 2000 was performed, and aMeSH search with hypospadias, environmental, and chemical as major headings was conducted, yielding 298 articles. Case-control and cohort studies were included that met the following criteria: human study, analysis of hypospadias independently from other disorders, and the maternal exposure identified as a specific drug or environmental chemical exposure or class of exposure. Studies with the following characteristics were excluded: combined outcome of hypospadias along with other disorders (e.g., cryptorchidism) or lacking a comparison or control group. Studies using In vitro fertilization (IVF) as a proxy for progestin exposure were excluded. We also excluded studies of occupational exposure. These included studies that estimated occupational EDC exposure through a job exposure matrix or that examined geographical proximity to known EDC sources (e.g., farms and factories). Thus, we restricted our review to investigations that documented exposure directly and with reasonable reliability, e.g., via biomarker concentrations or reported exposure. Finally, we cross-checked the citations of eligible studies to identify relevant studies that were not retrieved in the original database query. A flowchart of our selection process can be found in Fig. 1.
Fig. 1.
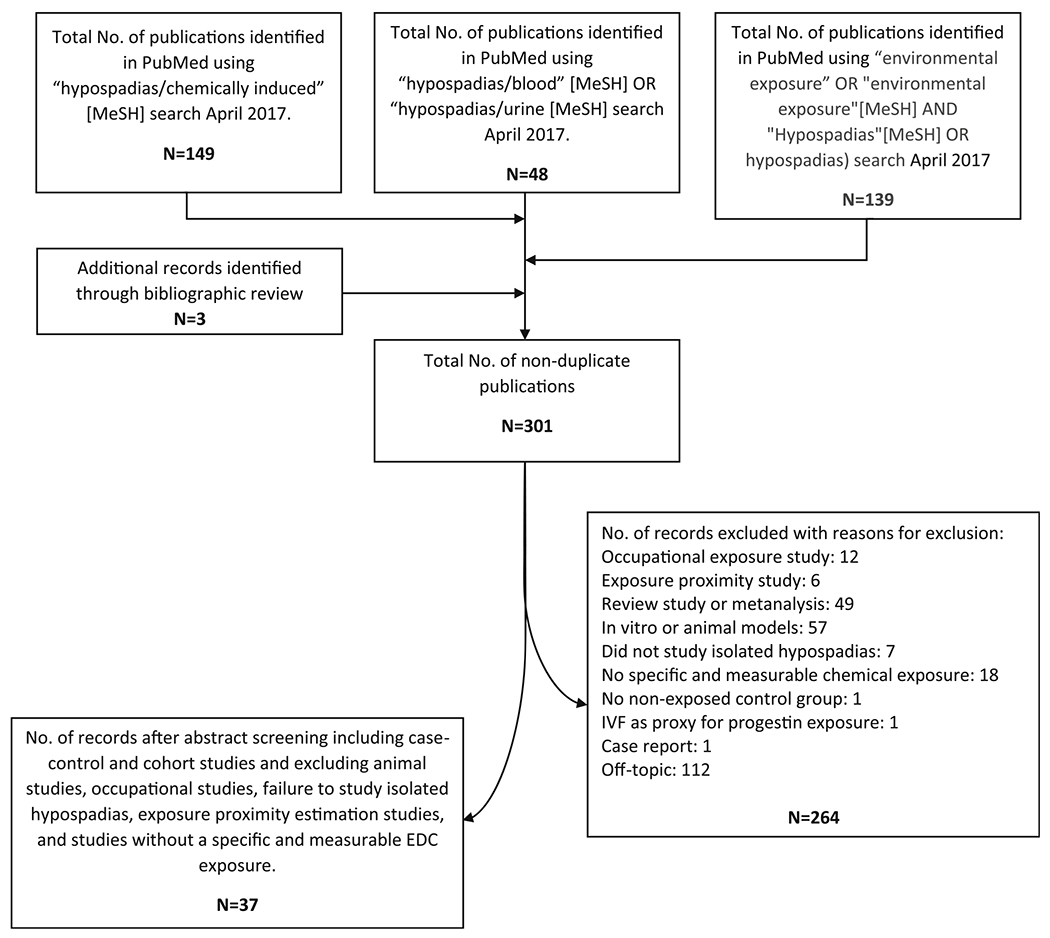
Literature review methodology flowchart
After application of these criteria, 37 studies remained. These studies examined: (1) exogenous sex steroid hormones and other pharmaceutical agents and (2) persistent and non-persistent environmental EDCs studied in relation to hypospadias. The most important case-control studies can be found in Tables 1 and 2.
Table 1.
Case-control studies of hypospadias and pharmaceutical use or sex steroid exposure in pregnancy
| Author, citation number (year) | Country Subjects (cases) |
Agent Data source (relation to pregnancy) |
Covariates | Significant findings |
|---|---|---|---|---|
| Pharmaceuticals | ||||
| Anderka, [12] (2012) | USA 10,383 (4524 cases) |
PPI, steroids, ondansetron Maternal survey (during pregnancy) | Maternal age, race-ethnicity, education, parity, first trimester smoking, plurality, previous miscarriage, infant sex, folic acid intake, BMI, study site, race/ethnicity, nulliparity, preeclampsia, chronic hypertension, family history of diabetes, family history of hypertension, total urinary arsenic, and fish consumption | • PPI use (OR 4.36; 95% CI 1.21–15.81) • Steroid use (OR 2.37; 95% CI 1.03–7.97) • Ondansetron use, no association or effect |
| Carmichael, [13] (2009) | USA 4165 (1165 cases) |
Corticosteroids Maternal survey (4 weeks prior-18 weeks after conception) |
Maternal education, race-ethnicity, maternal age, parity, folic acid intake, smoking, BMI, subfertility, and study site | • Corticosteroid use (OR 1.4; 95% CI 0.9–2.4) |
| Lind, [14] (2013) | USA 5851 (1537 cases) |
Venlafaxine, clomiphene citrate, 62 other medications and 24 herbal supplements Maternal survey (1 month before–4 months after conception) |
Maternal age, race-ethnicity, education, pre-pregnancy BMI, previous live births, maternal subfertility, study site, and year | • Venlafaxine use (OR 2.4; 95% CI 1.0–6.0) • Clomiphene citrate use (OR 1.9; 95% CI 1.2–3.0) |
| Pederson, [15] (2006) | Denmark 9 (1 case) for Loratadine 27 (4 cases) for other anti-histamine use |
Loratadine, other anti-histamines Maternal survey (1 month before–4 months after conception) |
Maternal age, birth order, smoking status, preeclampsia, use of clomiphene (proxy for IVF), diabetes, and epilepsy | • Loratadine use (OR 1.4; 95% CI 0.0–10.5) •Other anti-histamine use (OR 1.9; 95% CI 0.5–5.8) |
| Reis, [16] (2010) | Sweden 15,017 |
Antidepressants (SSRI and TCA) Swedish National Birth Register (during pregnancy) |
Maternal characteristics, maternal delivery diagnoses, infant neonatal diagnoses and the presence of congenital malformations | • TCA use (OR 1.93; 95% CI 0.88–3.67) • Citalopram (SSRI) use (OR 1.30; 95% CI 0.94–1.80) • Paroxetine (SSRI) use (OR 2.45; 95% CI 1.12–4.64) |
| Rodríguez-Pinilla, [17] (2008) | Spain 14,858 (2393 cases) |
Valproic acid Registry data (first trimester treatment for epilepsy) |
Maternal age, education, epilepsy, other chronic diseases, ethnic group, first degree relatives with congenital malformations, first degree relatives with hypospadias, fever during pregnancy, smoking, alcohol consumption, maternal intake of vitamins, sexual hormones and anticonvulsant drugs (other than VA) during first trimester | • Valproic acid use (OR 5.71; 95% CI 1.78–18.36) |
| Hormonal exposure Brouwers, [18] (2006) | Netherlands 834 (251 cases) |
DES Parental survey (parental exposure in utero) |
Parental education level, parents of cases chose matching control of approximately same age as their son | • Maternal in utero DES exposure (OR 4.9; 95% CI 1.1–22.3) • Paternal exposure, no association or effect |
| Carmichael, [19] (2005) | USA 1788 (502 cases of middle and posterior hypospadias) |
Progestin Maternal survey (4 weeks before through 14 weeks after conception) |
Birth weight, presence of concurrent major or minor anomalies (excluding chordee, unspecified hypospadias, epispadias, ambiguous genitalia, recognizable single gene anomaly or chromosomal abnormality), singleton births, and family history | • Progestin intake for fertility (OR 3.7; 95% CI 2.3–6.0). • Progestin use for contraception (OR 0.8; 95% CI 0.5–1.1) |
| Meijer, [20] (2006) | Netherlands 4920 (392 cases) |
Clomiphene Prescription history (90 days prior to conception through first trimester) |
Multiple birth, exposure to DES, peri-conceptional use of folic acid, and maternal age | • Maternal clomiphene exposure and proximal hypospadias (OR 6.08; 95% CI 1.40–26.33) • No association with midshaft or distal hypospadias |
| Norgaard, [21] (2009) | Denmark 17,333 (1683 cases) |
Oral contraceptives National databases (30 days before pregnancy through first trimester) |
Maternal age, birth order, maternal smoking, prescriptions for ovulation-inducing drugs, antiepileptics, antidiabetics, and maternal pre-eclampsia | • No notable effect |
| Sorensen, [22] (2005) | Denmark 3509 (319 cases) |
Clomiphene Prescription history (90 days prior to conception through first trimester) |
Maternal age, birth order, maternal preeclampsia, maternal epilepsy, maternal diabetes | • No association(OR 0.48; 95% CI 0.15–1.54) |
| van Rooij, [23] (2013) | Netherlands 1031 (405 cases) |
Hormonal contraceptives Maternal survey (Use during early pregnancy) |
Maternal and paternal age at childbirth, ethnicity, education, maternal and paternal BMI, family history of hypospadias or cryptorchidism, time-to-pregnancy without fertility treatment, maternal or paternal subfertility, primiparity, twin or triplet pregnancy, preeclampsia, hormone containing contraceptive use, maternal smoking during first 4 months of pregnancy, paternal smoking prior to pregnancy, preterm birth, SGA | • Hormonal contraceptives with all hypospadias(OR 6.6; 95% CI 1.6–27.2) • With middle hypospadias (OR 8.5; 95% CI 1.5–47.9) • With proximal hypospadias (OR 11.5; 95% CI 1.9–72.0) |
| Wogelius, [24] (2006) | Hungary 27,837 (3038 cases) |
Oral contraceptives Maternal survey (Use during early pregnancy) |
Birth order, maternal age, employment status, diabetes, and preeclampsia | • Comparing cases with congenital abnormality-free controls (OR 1.21; 95% CI 0.67–2.17). • Comparing cases with boys with other congenital abnormalities (OR 0.83; 95% CI 0.46–1.50). |
Abbreviations: DES, diethylstilbestrol; PPI, proton pump inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; VA, valproic acid
Table 2.
Case-control studies examining hypospadias and environmental endocrine-disrupting chemicals (EDCs) during pregnancy
| Author, citation number (year) | Country Subjects (cases) | EDCs Data source (relation to pregnancy) | Covariates | Significant findings |
|---|---|---|---|---|
| Phthalates/phthalate metabolites | ||||
| Chevrier, [25] (2012) | France 63 (21 cases) |
Low MWP, ΣDEHP metabolites, high MWP Maternal urine (after birth) |
Cases and controls matched for residence area, gestational age at urine sample collection, and date and day of collection (Sunday–Monday vs. other) | • MEP, MBP, and MiBP at intermediate concentrations (OR 0.15; 95% CI 0.02–0.9) |
| Jensen, [26•] (2015) | Denmark 300 (75 cases) |
Metabolites of DEHP, DiNP Amniotic fluid (second trimester) |
No confounders described. Amniocentesis indication was generally maternal age > 35 but there were some cases with identified congenital abnormalities | • No association with DiNP metabolites (OR 1.69; 95% CI 0.78–3.67) or DEHP metabolites. |
| Persistent organic pollutants | ||||
| Carmichael, [27] (2010) | USA 48 (28 cases) |
5 PBDE and 4 PCB congeners Maternal serum (second trimester) |
Foreign-born Hispanic race-ethnicity, time elapsed between blood collections, and AFP analysis | • No notable association found for PBDE 28, 47, 99, 100, 153 or for PCB 52, 99, 153, 180. |
| Giordano, [28] (2010) | USA 58 (21 cases) |
DDE, HCB, and 4 PCB congeners Maternal serum (third trimester) |
Lipid (%), maternal age, and breastfeeding duration | • Serum HCB concentration above the median of all subjects (OR 5.50; 95% CI 1.24-24.31) • HCB as a continuous variable (OR 1.26; 95% CI 1.04–1.52) • PCB118 (OR 1.40; 95% CI 0.99–1.97). |
| McGlynn, [29] (2009) | USA 794 (201 cases) |
11 PCB congeners Maternal serum (third trimester) |
Serum triglycerides, cholesterol, DDE levels | • No notable associations |
| Rignell-Hydbom, [30] (2012) | Sweden 472 (237 cases) |
PBC-153, p,p′-DDE, HCB Maternal serum (week 14 of gestation) |
Controls matched for maternal age, birth year, parity and maternal smoking habits in early pregnancy | • p,p’-DDE levels above the median (OR 1.69; 95% CI 0.97–2.93) • p,p’-DDE highest quartile (OR 1.65; 95% CI 1.02–2.69) |
| Toft, [31] (2016) | Denmark 375 (75 cases) |
Perfluorooctane Sulfonate Amniotic fluid (after 15 weeks gestation from amniocentesis) |
Gestational age of amniocentesis, year of amniocentesis, maternal age, gestational age at delivery, birth weight, and maternal cotinine (smoking) | • No notable association |
| Pesticides | ||||
| Agopian, [32] (2013) | USA 16,433 (9647 cases) |
Atrazine Estimated concentration in maternal water supply based on US Geological Survey data (Water levels at birth and at delivery) |
Season of conception, birth year, and maternal age, race-ethnicity, education, history of previous live births, birthplace, and smoking | • Medium-low (OR 1.11; 95% CI 1.04–1.18) and medium levels (OR 1.22; 95% CI 1.12–1.33) of atrazine associated with all hypospadias. • Medium-low (OR 1.52; 95% CI 1.25–1.85) and medium levels (OR 1.44; 95% CI 1.11–1.85) of atrazine associated with midshaft and proximal hypospadias. • High levels protective (OR 0.82; 95% CI 0.74–0.91) |
| Bhatia, [33] (2005) | USA 353 (70 cases) |
DDT and DDE Maternal serum (latest possible serum from pregnancy, otherwise earliest postpartum serum) |
Triglyceride level, cholesterol level, maternal age, pre-pregnancy BMI, parity, maternal ethnicity, maternal place of birth, maternal occupation before pregnancy, birth weight, gestational age, date of blood draw, and season of birth | • No notable effect of DDT or DDE |
| Dugas, [34] (2010) | England 961 (471 cases) |
Biocides and insect repellants Maternal questionnaire and tallying of insect repellant exposure and “biocide score” exposure index (first trimester of pregnancy |
Maternal smoking, maternal age, parental income, birth weight, previous stillbirth, folate intake during first 3 months of pregnancy) | • Use of insect repellent (OR 1.81; 95% CI 1.06–3.11). • High biocide scores (score 3; OR 1.73; 95% CI 1.02–2.94; scores 4 and 5 combined: OR 2.98; 95% CI 1.01–8.78) |
| Flores-Luévano, [35] (2003) | Mexico 69 (41 cases) |
DDT and DDE Maternal Serum |
Age of the mother, age of the father, lactation and employment during the pregnancy | • No notable effect |
| Longnecker, [36] (2002) | USA 751 (199 cases) |
DDE Maternal serum (during the first and third trimesters) |
Race, triglyceride levels, cholesterol levels | • No notable effect |
| Trabert, [37] (2012) | USA 754 (197 cases) |
Chlordanes: trans-nonachlor and oxychlordane Maternal serum (third trimester of pregnancy) |
Serum p,p′-DDE (5 categories), total PCBs (four categories), serum triglycerides, serum cholesterol, socioeconomic index | • Trans-nonachlor concentrations (ORs 0.84–1.08; 95% CI all including 1.0). • Oxychlordane concentrations (ORs 1.06–1.24; 95% CI all including 1.0) |
| Winston, [38] (2016) | USA 1765 (343 cases) |
Atrazine Estimated concentration in maternal water supply based on US Geological Survey data (Gestation weeks 6–16) |
Private well use, residential use of water filtration, state of residence, maternal age, maternal race-ethnicity, multiple or singleton birth, previous pregnancies, maternal education, maternal diabetes, maternal hypertension, maternal BMI, choline use, and use of artificial reproductive technology. | • No notable effect |
Abbreviations: DDE, dichlorodiphenyldichloroethylene; DEHP, bis(2-ethylhexyl) phthalate; DiNP, diisononyl phthalate; HCB, hexachlorobenzene; MBP, 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene; MiBP, monoisobutyl phthalate; MWP, molecular weight pthalate; p,p′-DDE, 1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene; PBDE, polybrominated diphenyl ethers; PCB, polychlorinated biphenyl
Exogenous Sex Steroids
Estrogens were the first chemicals to be studied in the context of maternal exposure and hypospadias. The synthetic non-steroidal estrogen, diethylstilbestrol (DES), is a known carcinogen formerly administered to pregnant women to prevent miscarriage prior to evidence of adverse health effects and a lack of efficacy for that indication [39]. An early cohort study conducted in the Netherlands identified four cases of hypospadias among 205 sons of women exposed to DES in utero (~2% prevalence) versus 8 cases out of 8729 sons of mothers without DES exposure (0.09% prevalence). This study observed a strong association (prevalence odds ratio (pOR) 21.3; 95% CI 6.5–70.1) between maternal in utero DES exposure and hypospadiac son [40••]. In a US cohort study, maternal DES exposure was related to a higher but not statistically significant increase in risk of offspring hypospadias with ten cases per 2552 live births from exposed mothers and three cases per 1336 live births from unexposed mothers (pOR 1.7; 95% CI 0.4–6.8) [41]. A case-control study surveying 834 mothers with 251 hypospadiac children observed that women exposed to DES in utero were nearly five times more likely to have infants with hypospadias (OR 4.9; 95% CI 1.1–22.3) [18]. A French, multigenerational cohort study also observed a relation between maternal DES exposure during pregnancy and increased prevalence of hypospadias for the next two generations, suggesting that the underlying biological mechanism may be epigenetic [42]. Thus, studies on DES raise the possibility of an association with hypospadias. While no longer prescribed, DES is similar in chemical structure with other xenobiotic compounds and thus, the epidemiologic findings are still of relevance. It is important to note, however, that the studies of DES exposure all suggest a possible epigenetic effect on the development of hypospadias in later generations. While DES is historically important and chemically relevant to this discussion, its effect may derive from interaction with the maternal oocyte rather than the developing male penis itself.
Clomiphene, structurally similar to DES, is a selective estrogen receptor modulator given to improve fertility. A large population-based case-control study from Denmark that used national registry and pharmacy data observed no association between clomiphene exposure during pregnancy and hypospadias in the offspring of mothers who were surveyed about their exposure [22]. A comparable study in the Netherlands made a distinction between severity of hypospadias, namely severe (posterior) versus moderate (middle) and mild (anterior) hypospadias, determined by where the urethra opens on the penile shaft (Fig. 2) [43, 44]. In this study, an increased risk of posterior, but not anterior and middle hypospadias, was observed with clomiphene exposure, adjusting for DES exposure among other variables (OR 6.1, 95% CI 1.40–26.3) [20]. A US study surveying a sample of 1788 patients with 502 cases of middle or posterior hypospadias also found an increased risk associated with progestins when used for fertility treatments (OR 3.7, 95% CI 2.3–6.0) [19]. Pregnadiene, a metabolic derivative of progesterone and synthetic ovulation stimulants as a class have also been associated with a modestly increased risk of hypospadias (OR for pregnadiene 1.40; 95% CI 1.10–1.76; OR for synthetic ovulation stimulants 1.89; 95% CI 1.28–2.70) [45].
Fig. 2.
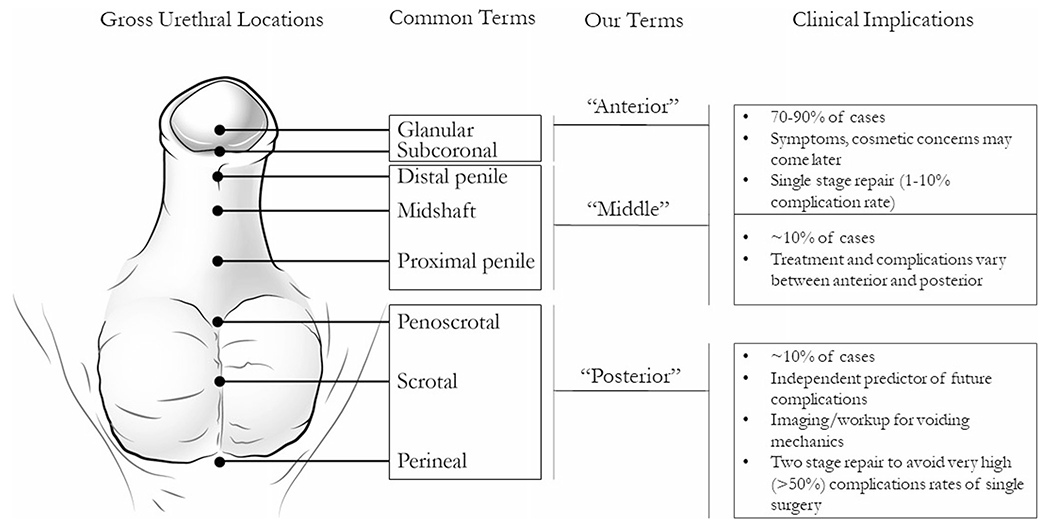
Hypospadias classification schemes and clinical implications. Some common terms found in the literature are described and associated with the terms that we used to consistency in this review. Clinical information regarding complications and epidemiologic data are provided. Incidence of each hypospadias grade is subject to debate due to inconsistent classification schemes [22, 43]
Oral contraceptive (OCP) use in the first trimester of pregnancy has been investigated as a potential cause of hypospadias. A Dutch case-control study (n = 1031 patients with 405 cases) investigated OCP use, among other risk factors, and specifically mild (anterior), moderate (middle), and severe (posterior) hypospadias. A large effect was observed in all three groups (ORs ranging from 3.8 to 11.5), although results were not statistically significant for mild (anterior) hypospadias [23]. A larger study of 3038 cases with recorded early pregnancy OCP use and 24,799 controls observed no association [24]. Another study of 7333 patients and 1683 cases similarly found no relation. This study specifically defined early pregnancy exposure as OCP use between 30 days pre-conception to the end of the first trimester, controlled for maternal ovulation treatment, and distinguished between hypospadias diagnosed before or after 6 months of age [21]. A further study of progestin only OCP use in 1788 patients also observed no association [19]. It is possible that the inter-study heterogeneity in results is due to differences in the effects of OCPs by severity of hypospadias, but this will need to be confirmed by future studies.
Other Pharmaceuticals
Drugs, other than sex steroids, commonly administered to pregnant women may play a role in the pathogenesis of hypospadias via endocrine-disrupting mechanisms. Glucocorticoids, which are structurally related to sex hormones, may be administered for nausea and vomiting of pregnancy or in the treatment of autoimmune disorders. One small study surveyed mothers with (n = 28) and without (n = 20) hypospadias offspring for corticosteroid use during pregnancy. The investigators reported a modestly elevated hypospadias risk (OR 1.4; 95% CI 0.9–2.4) associated with maternal steroid use between 5 and 14 weeks post-conception, which is the period corresponding to genital tubercle development [13]. Another case-control study from the USA with 10,383 patients and 4524 cases surveyed for medications, including steroids, used for nausea and vomiting of pregnancy. There was an elevated risk of hypospadias (OR 2.87; 95% CI 1.03–7.97) after adjusting for similar maternal covariates as the previous study. Use of proton pump inhibitors (PPI) in pregnancy also were associated with elevated risk of hypospadias (OR 4.36; 95% CI 1.21–15.81) in this study. The wide confidence interval is likely attributable to the small sample size, with fewer than ten patients in each case and control group [12]. PPI use was also investigated in a retrospective cohort study of 2962 patients. Analyses were conducted for exposure between 30 days prior to conception until the end of the first trimester as well as for exposure throughout the pregnancy. There was no association for either time period [46]. Additionally, there was no association with antihistamine use during pregnancy. A further study using a national prescription database from Denmark found an elevated risk with antihistamine use during pregnancy, but it was not statistically significant (OR 1.9; 95% CI 0.5–5.8) [15].
Psychiatric medications, particularly antidepressants, are commonly administered in pregnancy and have potential endocrine disrupting effects. A case-control study through the Swedish National Birth Register of 14,821 women investigated rates of birth defects and complications associated with the use of either tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs) during pregnancy. Elevated risks of borderline significance were observed with TCAs and citalopram, an SSRI (OR for TCA 1.93; 95% CI 0.88–3.67; OR for citalopram 1.30; 95% CI 0.94–1.80). A significant SSRI association also was found with paroxetine (OR 2.45; 95% CI 1.12–4.64), with only nine exposed cases. [16] A US case-control study of 5851 patients investigating 64 drugs and 24 supplements for association with hypospadias observed a previously unidentified association with maternal venlafaxine use during pregnancy (OR 2.4; 95% CI 1.0–6.0) [14]. This and other SSRIs will need to be investigated further. Moreover, it will be important to disentangle whether these are independent effects since the rates of a variety of pathologies and complications may be increased in the offspring of users of these medications.
Valproic acid is a known teratogen that is uncommonly used during pregnancy for the treatment of seizure disorders. Data from the Spanish Collaborative Study of Congenital Malformations investigated the effect of valproic acid use in the first trimester for treatment of epilepsy in 14,858 patients (2393 cases) adjusting for common confounders, including several unspecified diseases in the mother. The study reported an increased risk of hypospadias with maternal valproate use in pregnancy (OR 5.71; 95% CI 1.78–18.36) [17]. Further studies of pharmaceuticals administered during pregnancy are warranted. Particularly, studies to better elucidate dose-response relationships and to consider confounding by indication, a special type of confounding that is of concern when the clinical indication for use of a specific pharmaceutical agent or treatment is an independent risk factor for the outcome of interest [47]. Above, we have described preliminary evidence to support potential associations between maternal use of certain antidepressant medications during pregnancy and hypospadias risk. However, it is presently unclear whether maternal depression during (or before) pregnancy is an independent risk factor for hypospadias. Therefore, the ability to adjust for the clinical indication (i.e., major depressive disorder) or severity of maternal depression is an important component of understanding any possible associations between maternal antidepressant use in pregnancy and hypospadias risk in future studies.
Persistent Organic Pollutants
POPs are compounds that have a long residence time in the environment, long half-lives in the human body, and have potential endocrine disrupting properties [48]. Industrial pesticides are some of the most well-studied POPs, and there have been several occupational and geographic proximity studies indicating a potential link with hypospadias [49]. Four studies in geographically distinct populations measured maternal serum levels of DDT and its metabolite dichlorodiphenyldichloroe thylene (DDE) during pregnancy and found no association of DDT or DDE with hypospadias although they had small sample sizes [35–39]. A larger study (237 cases, 472 controls) with the ability to adjust for multiple potentially confounding factors found an increased risk of hypospadias among women with serum DDE concentrations at the highest quartile (OR 1.65; 95% CI 1.02–2.69) compared to women in the first quartile during the 14th week gestation of pregnancy [30].
Two studies have investigated parental exposure to the pesticide atrazine estimated through public drinking water composition records. The first found an association with all hypospadias and estimated atrazine exposure in the second (OR 1.11; 95% CI 1.04–1.18) and third quartiles (ORs 1.52; 95% CI 1.25–1.85), with a stronger effect for middle and posterior hypospadias. The trend was inconsistent, however, making the results difficult to interpret [32]. A subsequent study observed no association between drinking water atrazine exposure and hypospadias [38]. In both studies, there was significant geographic variation by state in water atrazine concentration. Exposure to self-applied biocides and insect repellants during pregnancy has been investigated in one English case-control study of 961 patients that found an increased risk of hypospadias with first trimester maternal use of insect repellant (OR 1.81; 95% CI 1.06–3.11) and with the highest biocide exposure categories (OR 2.98; CI 1.01–8.78). This study did not specify the specific chemical used or frequency of use [34]. Chlordanes have been investigated in one US study of 754 patients, but there were no associations found between hypospadias incidence and maternal third-trimester serum concentrations of trans-nonachlor or oxychlordane[37].
Non-pesticide organohalogenated POPs also have been investigated. Polychlorinated biphenyls (PCBs) are an important POP subgroup of synthetic compounds undetectable by odor, color, and taste that can be found in electrical products and coolants made before 1977, at which point their manufacture was banned in the US due to possible carcinogenicity [50]. Maternal serum concentrations from pregnancy are often used as a proxy for fetal exposure to PCBs. A case-control study from the US of 794 patients studied 11 PCB congeners and found no significant association of hypospadias with third trimester maternal serum levels of any individual PCB. An analysis of the sum of all 11 PCB congeners adjusted for serum DDE concentration, triglycerides, and cholesterol indicated weak associations found at the highest and lowest concentrations, but not at intermediate concentrations. The authors noted that the levels of PCBs in the population studied (children born from 1959 to 1965) were much higher than would be found in populations today [29]. Another study collected serum after birth of the child for 58 patients with 21 cases in the USA and measured four PCB congeners and the fungicide, hexachlorobenzene (HCB). No association was observed with PCBs, but a positive association was found between maternal serum HCB concentrations and hypospadias (OR 5.50; 95% CI 1.24–24.31). This study adjusted for breastfeeding duration, as increased duration of breastfeeding decreases maternal serum levels of certain POPs, specifically PCBs [28]. An additional study of 472 patients with 237 cases in Sweden looked at PCB-153 and HCB and did not find any associations [30]. A small study (28 cases) using maternal serum samples in the USA investigated several PCB congeners as well as polybrominated diphenyl ether flame retardants (PBDEs). Adjusting for time elapsed between blood collection and analysis among other factors, no associations were detected [27]. Perfluorooctane sulfonate is another POP that was measured in routinely collected amniotic fluid samples in one 375 patient study in Denmark. Analyses of the relation with hypospadias, adjusted notably for gestational age at time of sampling, found no association [31].
Trihalomethanes (THMs) are a group of organohalogenated water disinfection by-products. Two case-control studies, one in the USA (n = 468 cases, 465 controls) and one in England (n = 320 cases, 614 controls) with both using Geographical Information Systems data to assign exposures, estimated maternal exposure through geographical and water utilities data on drinking water concentrations of THM. Neither found associations with hypospadias [51, 52].
Non-persistent Pollutants
Non-persistent pollutants, such as phthalates have characteristically short residence times in the environment, short half-lives (hours to days) in the human body, and are ubiquitous in synthetic products, particularly plastics [53, 54]. Pregnant mothers also are often exposed through use of personal care products (e.g., lotions). Studies of phthalates typically use urinary concentrations of phthalate metabolites to quantify the exposure [54].
In a case-control study of 80 cases and 80 controls from South Korea, concentrations of eight different phthalate were analyzed in spot urine samples from children with hypospadias and their mothers. Associations were found with hypospadias and elevated levels of bis(2-ethylhexyl) phthalate (DEHP) (p = 0.006) and of nonylphenol (n-NP) (p = 7.26e–6) in children’s urine samples. However, it is important to note that phthalate exposure during early childhood does not directly reflect exposure during the etiologically relevant window of early pregnancy and that substantial within-person variability in urinary phthalate concentrations over time has been observed among children and pregnant women [55, 56]. In contrast, no association was found between hypospadias in the offspring and phthalates in the maternal urine [57]. This study also looked at maternal and infant serum phthalate levels; however, serum phthalate concentrations are not currently considered an accurate proxy of exposure [58].
Maternal urinary concentrations of 11 phthalate metabolites and nine phenols in mothers of hypospadias offspring were compared to those of normal offspring in a case-control study from France (n = 21 cases, 42 controls). Phthalates were grouped as low molecular weight, DEHP metabolites, and high molecular weight compounds and categorized by low, medium, or high concentration within groups. The low molecular weight group, comprised of three phthalate metabolites, was inversely related to hypospadias (OR 0.15; 95% CI 0.02–0.9), but the study had only three cases and 25 controls [25]. This study also used a single maternal urine sample collected at birth to indicate fetal exposure, which makes exposure misclassification likely [59•].
A case-control study of 300 total maternal-infant dyads with 75 cases from Denmark assessed DEHP and di-isononyl phthalate (DiNP) metabolites measured in amniotic fluid samples from the second trimester. Concentrations of DEHP metabolites in amniotic fluid were not strongly associated with hypospadias, and DiNP metabolites were associated with a statistically non-significant increased likelihood of hypospadias (OR 1.69; 95% CI 0.78–3.67). Notably, the indication for amniocentesis in these patients was advanced maternal age (> 35), and there were other developmental abnormalities in some of the cases, and thus, the possibility of residual confounding [26•].
Conclusion
The current literature on hypospadias provides some indication of the role of exogenous endocrine disruptors in the pathogenesis of hypospadias. Among the pharmaceutical agents, sex steroids, DES, and progestin fertility treatments have all been associated with hypospadias risk. However, results are not entirely consistent, and in some studies, associations were confined to subgroups, i.e., among posterior hypospadias. Likewise, steroid pharmaceuticals and valproic acid also may be related to the risk of hypospadias, but results vary among a limited number of studies. Studies of other pharmaceutical agents remain incomplete, as some common medications administered to pregnant women have not yet been evaluated, such as beta blockers, antibiotics, and diabetes medications. For the drugs that have been studied, further confirmatory studies are warranted. Evidence for associations of hypospadias with gestational exposure to DDT/DDE and HCB pesticides, as well as non-persistent pollutants, particularly phthalates, is less consistent but still compelling. Several challenges exist in our ability to investigate the impact of environmental factors on hypospadias occurrence.
Inter-individual variability in absorption, metabolism, or distribution of EDCs may limit the ability to observe associations, particularly when sample sizes are small. For example, with respect to use of sex steroids, fetal exposure may depend on transplacental permeability and maternal-fetal metabolism [60]. Further, aspects of absorption and metabolism may be influenced by genetics or behaviors. Genetic polymorphisms related to metabolizing enzymes have been demonstrated to influence individual susceptibility to chemical exposures and may be relevant for gene-environment interactions [61]. Women’s exposure to phthalates and certain phenols will also be influenced by their frequency of use and choice of certain personal care products [53, 62–64].
As with many other areas of environmental epidemiology, assessing exposure to EDCs and identifying windows of vulnerability remain among the greatest challenges [65]. Much of the reviewed literature determined exposure through medical records or patient surveys. This type of data is usually insufficient to characterize dose-response relationships. Use of biomarkers to assess exposure presents some advantages, but includes challenges as well, especially for non-persistent chemicals with short half-lives in the body. Studies on organohalogenated chemicals and pesticides differed in their methods of exposure measurement. For non-persistent pollutants, differences in study findings may be influenced by varying biomarkers of exposure (e.g., urine versus amniotic fluid). Even given well developed exposure assessment methodologies, applying sampling methods that capture the etiologically relevant timing of exposure is another critically important factor. Because male sex organs form between 8 and 14 weeks’ gestation, maternal exposures at or prior to that time are likely to be most important when considering hypospadias risk. Many of the reviewed studies assessed exposure during late pregnancy or occasionally after pregnancy. In the case of long half-life compounds such as POPs, this may be a reasonable approach, but for many EDCs, variability in exposures over time may result in exposure misclassification [59, 65]. Specifically capturing exposure data during the critical genital developmental period may help to elucidate exposure-disease relationships in future studies. Further, because there appears to be a rather short critical window during which it is likely that exposure could influence male genital development (i.e., prior to 14 weeks’ gestation), it may be possible to leverage exposures captured outside of the critical window as a negative control exposure [66].
EDC exposures often occur in mixtures but are analyzed individually in most of the hypospadias literature, thus presenting another methodologic challenge. In future studies that collect data on multiple exposures, analysis should account for mixtures to better characterize the effect of the individual studied exposures and their combinations [67, 68]. However, such studies often require large sample sizes, which can be difficult to achieve with rare outcomes such as hypospadias. Some studies of EDCs adjusted for related EDCs, while others did not. Further, in studies that consider multiple correlated exposures, it is difficult to tease out the potential etiologic agent or mixtures of compounds that may act synergistically or through a common mechanistic pathway.
A final challenge to consider is that of outcome heterogeneity and outcome misclassification. Hypospadiases vary in their presentation, diagnostic accuracy, and classification scheme. Mild cases of hypospadias may not be obvious at birth but are noticed as the child grows and develops symptoms or cosmetic concerns. Gradations of severity have significant implications on quality of life for the child as well as the clinical and surgical management and potential etiology [69]. Development of a standardized hypospadias grading scheme in the future would help to ensure comparability and potential pooling of studies to increase statistical power. It would be helpful for future studies to consider isolated hypospadias, as it may have different pathogenesis and distinct etiology from hypospadias secondary to DSD-causing mutations that are also related to cryptorchidism. Additionally, the EDCs investigated in the future could cause hypospadias by varying mechanisms that may not primarily act on the androgen receptor at all.
In conclusion, despite methodological challenges, further investigation is warranted to identify exposures that increase risk of hypospadias and ultimately to inform efforts to reduce the incidence of this disorder. Hypospadias is very likely to be hormonally-mediated given its pathophysiology. Thus, understanding the role of modifiable exposures to EDCs may represent a largely untapped opportunity for prevention. Focusing on improving exposure assessment, standardizing sample timing to relevant developmental windows of exposure, using clear case identification and classification schemes, and elucidating dose-response relationships with EDCs will help to provide clearer evidence. This is particularly important for suspected EDCs for which there is limited epidemiologic data (e.g., non-persistent pollutants). Potentially fruitful areas of further research include identification of subgroups with genetic hypospadias risk factors, measurement of intermediate outcomes (e.g., fetal testosterone, insulin-like growth factor, or anogenital distance), and study of EDC mixtures that will more accurately represent the total fetal environment.
Acknowledgements
We wish to thank Dr. Lucas Salas for expert assistance in interpreting Spanish language literature relevant to this review and Leah Hofgesang for her professional illustrative contributions to Fig. 2.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Springer A, van den Heijkant M, Baumann S. Worldwide prevalence of hypospadias. J Pediatr Urol. 2016;12(3):152 e1–7. 10.1016/j.jpurol.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Sagodi L, Kiss A, Kiss-Toth E, Barkai L. Prevalence and possible causes of hypospadias. Orv Hetil. 2014;155(25):978–85. 10.1556/OH.2014.29858. [DOI] [PubMed] [Google Scholar]
- 3.Baskin LS. Hypospadias and urethral development. J Urol. 2000;163(3):951–6. [PubMed] [Google Scholar]
- 4.Aschim EL, Nordenskjold A, Giwercman A, Lundin KB, Ruhayel Y, Haugen TB, et al. Linkage between cryptorchidism, hypospadias, and GGN repeat length in the androgen receptor gene. J Clin Endocrinol Metab. 2004;89(10):5105–9. 10.1210/jc.2004-0293. [DOI] [PubMed] [Google Scholar]
- 5.Ratan SK, Aggarwal S, Mishra TK, Saxena A, Yadav S, Pandey R, et al. Children with isolated hypospadias have different hormonal profile compared to those with associated anomalies. J Pediatr Endocrinol Metab. 2012;25(1–2): 111–9. doi: 10.1515/jpem.2011.421. [DOI] [PubMed] [Google Scholar]
- 6.Thorup J, McLachlan R, Cortes D, Nation TR, Balic A, Southwell BR, et al. What is new in cryptorchidism and hypospadias—a critical review on the testicular dysgenesis hypothesis. J Pediatr Surg. 2010;45(10):2074–86. 10.1016/j.jpedsurg.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Akre O, Pettersson A, Richiardi L. Risk of contralateral testicular cancer among men with unilaterally undescended testis: a meta analysis. Int J Cancer. 2009;124(3):687–9. 10.1002/ijc.23936. [DOI] [PubMed] [Google Scholar]
- 8.Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113(8):1056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boberg J, Christiansen S, Axelstad M, Kledal TS, Vinggaard AM, Dalgaard M, et al. Reproductive and behavioral effects of diisononyl phthalate (DINP) in perinatally exposed rats. Reprod Toxicol. 2011;31(2):200–9. 10.1016/j.reprotox.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Chen X, Zhou X, Zhang W, Yuan J, Feng J. The mechanism underlying dibutyl phthalate induced shortened anogenital distance and hypospadias in rats. J Pediatr Surg. 2015;50(12):2078–83. 10.1016/j.jpedsurg.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 11.Jeng HA. Exposure to endocrine disrupting chemicals and male reproductive health. Front Public Health. 2014;2:55. 10.3389/fpubh.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderka M, Mitchell AA, Louik C, Werler MM, Hernandez-Diaz S, Rasmussen SA, et al. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012;94(1):22–30. 10.1002/bdra.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmichael SL, Ma C, Werler MM, Olney RS, Shaw GM, National Birth Defects Prevention S. Maternal corticosteroid use and hypospadias. J Pediatr. 2009;155(1):39–44, e1. 10.1016/j.jpeds.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind JN, Tinker SC, Broussard CS, Reefhuis J, Carmichael SL, Honein MA, et al. Maternal medication and herbal use and risk for hypospadias: data from the National Birth Defects Prevention Study, 1997–2007. Pharmacoepidemiol Drug Saf. 2013;22(7):783–93. 10.1002/pds.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen L, Norgaard M, Skriver MV, Olsen J, Sorensen HT. Prenatal exposure to loratadine in children with hypospadias: a nested case-control study within the Danish National Birth Cohort. Am J Ther. 2006;13(4):320–4. [DOI] [PubMed] [Google Scholar]
- 16.Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010;40(10):1723–33. 10.1017/S0033291709992194. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Pinilla E, Mejias C, Prieto-Merino D, Fernandez P, Martinez-Frias ML, Group EW. Risk of hypospadias in newborn infants exposed to valproic acid during the first trimester of pregnancy: a case-control study in Spain. Drug Saf.2008;31(6):537–43. [DOI] [PubMed] [Google Scholar]
- 18.Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Hypospadias: a transgenerational effect of diethylstilbestrol? Hum Reprod. 2006;21(3):666–9. 10.1093/humrep/dei398. [DOI] [PubMed] [Google Scholar]
- 19.Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Arch Pediatr Adolesc Med. 2005;159(10):957–62. 10.1001/archpedi.159.10.957. [DOI] [PubMed] [Google Scholar]
- 20.Meijer WM, de Jong-Van den Berg LT, van den Berg MD, Verheij JB, de Walle HE. Clomiphene and hypospadias on a detailed level: signal or chance? Birth Defects Res A Clin Mol Teratol. 2006;76(4):249–52. 10.1002/bdra.20243. [DOI] [PubMed] [Google Scholar]
- 21.Norgaard M, Wogelius P, Pedersen L, Rothman KJ, Sorensen HT. Maternal use of oral contraceptives during early pregnancy and risk of hypospadias in male offspring. Urology. 2009;74(3):583–7. 10.1016/j.urology.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen HT, Pedersen L, Skriver MV, Norgaard M, Norgard B, Hatch EE. Use of clomifene during early pregnancy and risk of hypospadias: population based case-control study. BMJ. 2005;330(7483): 126–7. 10.1136/bmj.38326.606979.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rooij IA, van der Zanden LF, Brouwers MM, Knoers NV, Feitz WF, Roeleveld N. Risk factors for different phenotypes of hypospadias: results from a Dutch case-control study. BJU Int. 2013;112(1): 121–8. 10.1111/j.1464-410X.2012.11745.x. [DOI] [PubMed] [Google Scholar]
- 24.Wogelius P, Horvath-Puho E, Pedersen L, Norgaard M, Czeizel AE, Sorensen HT. Maternal use of oral contraceptives and risk of hypospadias - a population-based case-control study. Eur J Epidemiol. 2006;21(10):777–81. 10.1007/s10654-006-9067-0. [DOI] [PubMed] [Google Scholar]
- 25.Chevrier C, Petit C, Philippat C, Mortamais M, Slama R, Rouget F, et al. Maternal urinary phthalates and phenols and male genital anomalies. Epidemiology. 2012;23(2):353–6. 10.1097/EDE.0b013e318246073e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.•.Jensen MS, Anand-Ivell R, Norgaard-Pedersen B, Jonsson BA, Bonde JP, Hougaard DM, et al. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology. 2015;26(1):91–9. 10.1097/EDE.0000000000000198. [DOI] [PubMed] [Google Scholar]; One of the few studies that examines EDC levels in the direct fetal environment.
- 27.Carmichael SL, Herring AH, Sjodin A, Jones R, Needham L, Ma C, et al. Hypospadias and halogenated organic pollutant levels in maternal mid-pregnancy serum samples. Chemosphere. 2010;80(6): 641–6. 10.1016/j.chemosphere.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giordano F, Abballe A, De Felip E, di Domenico A, Ferro F, Grammatico P, et al. Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res A Clin Mol Teratol. 2010;88(4):241–50. 10.1002/bdra.20657. [DOI] [PubMed] [Google Scholar]
- 29.McGlynn KA, Guo X, Graubard BI, Brock JW, Klebanoff MA, Longnecker MP. Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environ Health Perspect. 2009;117(9):1472–6. 10.1289/ehp.0800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rignell-Hydbom A, Lindh CH, Dillner J, Jonsson BA, Rylander L. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants and the risk of hypospadias. PLoS One. 2012;7(9):e44767. 10.1371/journal.pone.0044767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toft G, Jonsson BA, Bonde JP, Norgaard-Pedersen B, Hougaard DM, Cohen A, et al. Perfluorooctane sulfonate concentrations in amniotic fluid, biomarkers of fetal leydig cell function, and cryptorchidism and hypospadias in Danish boys (1980–1996). Environ Health Perspect. 2016;124(1):151–6. 10.1289/ehp.1409288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agopian AJ, Lupo PJ, Canfield MA, Langlois PH. Case-control study of maternal residential atrazine exposure and male genital malformations. Am J Med Genet A. 2013;161A(5):977–82. 10.1002/ajmg.a35815. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia R, Shiau R, Petreas M, Weintraub JM, Farhang L, Eskenazi B. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ Health Perspect. 2005;113(2):220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dugas J, Nieuwenhuijsen MJ, Martinez D, Iszatt N, Nelson P, Elliott P. Use of biocides and insect repellents and risk of hypospadias. Occup Environ Med. 2010;67(3):196–200. 10.1136/oem.2009.047373. [DOI] [PubMed] [Google Scholar]
- 35.Flores-Luevano S, Farias P, Hernandez M, Romano-Riquer P, Weber JP, Dewailly E, et al. DDT/DDE concentrations and risk of hypospadias. Pilot case-control study. Salud Publica Mex. 2003;45(6):431–8. [PubMed] [Google Scholar]
- 36.Longnecker MP, Klebanoff MA, Brock JW, Zhou H, Gray KA, Needham LL, et al. Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am J Epidemiol. 2002;155(4):313–22. [DOI] [PubMed] [Google Scholar]
- 37.Trabert B, Longnecker MP, Brock JW, Klebanoff MA, McGlynn KA. Maternal pregnancy levels of trans-nonachlor and oxychlordane and prevalence of cryptorchidism and hypospadias in boys. Environ Health Perspect. 2012;120(3):478–82. 10.1289/ehp.1103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winston JJ, Emch M, Meyer RE, Langlois P, Weyer P, Mosley B, et al. Hypospadias and maternal exposure to atrazine via drinking water in the National Birth Defects Prevention Study. Environ Health. 2016;15(1):76. 10.1186/s12940-016-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bamigboye AA, Morris J. Oestrogen supplementation, mainly diethylstilbestrol, for preventing miscarriages and other adverse pregnancy outcomes. Cochrane Database Syst Rev. 2003;3:CD004353. 10.1002/14651858.CD004353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.••.Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero: a cohort study. Lancet. 2002;359(9312):1102–7. 10.1016/S0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]; This study is important as it is the landmark study that launched interest in EDC exposure and its effect on hypospadias.
- 41.Palmer JR, Wise LA, Robboy SJ, Titus-Ernstoff L, Noller KL, Herbst AL, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero. Epidemiology. 2005;16(4):583–6. [DOI] [PubMed] [Google Scholar]
- 42.Kalfa N, Paris F, Soyer-Gobillard MO, Daures JP, Sultan C. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: a multigenerational national cohort study. Fertil Steril. 2011;95(8):2574–7. 10.1016/j.fertnstert.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 43.Subramaniam R, Spinoit AF, Hoebeke P. Hypospadias repair: an overview of the actual techniques. Semin Plast Surg. 2011;25(3):206–12. 10.1055/s-0031-1281490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinoit AF, Poelaert F, Van Praet C, Groen LA, Van Laecke E, Hoebeke P. Grade of hypospadias is the only factor predicting for re-intervention after primary hypospadias repair: a multivariate analysis from a cohort of 474 patients. J Pediatr Urol. 2015;11(2): 70 e1–6. 10.1016/j.jpurol.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Given JE, Loane M, Luteijn JM, Morris JK, de Jong van den Berg LT, Garne E, et al. EUROmediCAT signal detection: an evaluation of selected congenital anomaly-medication associations. Br J Clin Pharmacol. 2016;82(4):1094–109. 10.1111/bcp.12947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erichsen R, Mikkelsen E, Pedersen L, Sorensen HT. Maternal use of proton pump inhibitors during early pregnancy and the prevalence of hypospadias in male offspring. Am J Ther. 2014;21(4):254–9. 10.1097/MJT.0b013e3182456a8f. [DOI] [PubMed] [Google Scholar]
- 47.Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. JAMA. 2016;316(17):1818–9. 10.1001/jama.2016.16435. [DOI] [PubMed] [Google Scholar]
- 48.Gregoraszczuk EL, Ptak A. Endocrine-disrupting chemicals: some actions of pops on female reproduction. Int J Endocrinol. 2013;2013:828532. 10.1155/2013/828532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rocheleau CM, Romitti PA, Dennis LK. Pesticides and hypospadias: a meta-analysis. J Pediatr Urol. 2009;5(1):17–24. 10.1016/j.jpurol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossberg M, Wilhelm L, Pfleiderer G, Tögel A, Dreher E-L, Langer E, et al. Ullmann’s encyclopedia of industrial chemistry—chlorinated hydrocarbons. 2006. doi: 10.1002/14356007.a06_233.pub2. [DOI] [Google Scholar]
- 51.Iszatt N, Nieuwenhuijsen MJ, Nelson P, Elliott P, Toledano MB. Water consumption and use, trihalomethane exposure, and the risk of hypospadias. Pediatrics. 2011;127(2):e389–97. 10.1542/peds.2009-3356. [DOI] [PubMed] [Google Scholar]
- 52.Luben TJ, Nuckols JR, Mosley BS, Hobbs C, Reif JS. Maternal exposure to water disinfection by-products during gestation and risk of hypospadias. Occup Environ Med. 2008;65(6):420–9. 10.1136/oem.2007.034256. [DOI] [PubMed] [Google Scholar]
- 53.Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(e 5):459–66. 10.1038/jes.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva MJ, Samandar E, Preau JL Jr, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;860(1):106–12. 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, et al. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol. 2014;48(15):8881–90. 10.1021/es501744v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119(1): 131–7. 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi H, Kim J, Im Y, Lee S, Kim Y. The association between some endocrine disruptors and hypospadias in biological samples. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012;47(13):2173–9. 10.1080/10934529.2012.680387. [DOI] [PubMed] [Google Scholar]
- 58.Calafat AM, Koch HM, Swan SH, Hauser R, Goldman LR, Lanphear BP, et al. Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res. 2013;15(5):403. 10.1186/bcr3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.•.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environ Int. 2015;85:27–39. 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study speaks the exposure assessment issues that are widespread in the literature on this topic.
- 60.Li LX, Chen L, Meng XZ, Chen BH, Chen SQ, Zhao Y, et al. Exposure levels of environmental endocrine disruptors in mother-newborn pairs in China and their placental transfer characteristics. PloS One. 2013;8(5):e62526. 10.1371/journal.pone.0062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thier R, Bruning T, Roos PH, Rihs HP, Golka K, Ko Y, et al. Markers of genetic susceptibility in human environmental hygiene and toxicology: the role of selected CYP, NAT and GST genes. Int J Hyg Environ Health. 2003;206(3):149–71. 10.1078/1438-4639-00209. [DOI] [PubMed] [Google Scholar]
- 62.Calafat AM, Wong L-Y, Ye X, Reidy JA, Needham LL. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ Health Perspect. 2008;116(7):893–7. 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duty SM, Ackerman RM, Calafat AM, Hauser R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect. 2005;113(11):1530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo Y, Kannan K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol. 2013;47(24):14442–9. 10.1021/es4042034. [DOI] [PubMed] [Google Scholar]
- 65.Calafat AM. Contemporary issues in exposure assessment using biomonitoring. Curr Epidemiol Rep. 2016;3(2):145–53. 10.1007/s40471-016-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weisskopf MG, Tchetgen Tchetgen EJ, Raz R. Commentary: on the use of imperfect negative control exposures in epidemiologic studies. Epidemiology. 2016;27(3):365–7. 10.1097/EDE.0000000000000454. [DOI] [PubMed] [Google Scholar]
- 67.Taylor KW, Joubert BR, Braun JM, Dilworth C, Gennings C, Hauser R, et al. Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environ Health Perspect. 2016;124(12):A227–A9. 10.1289/EHP547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braun JM, Gennings C, Hauser R, Webster TF. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect. 2016;124(1):A6–9. 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manzoni GA, Reali L. Management of hypospadias. J Pediatr Surg Subspecialties. 2017;11(1). [Google Scholar]


