Abstract
Current evidence from randomized controlled trials (RCTs) and systematic reviews on the utility of convalescent plasma (CP) in patients with coronavirus disease 2019 (COVID-19) suggests a lack of benefit. We conducted an updated meta-analysis of RCTs with trial sequential analysis to investigate whether convalescent plasma is futile in reducing mortality in patients hospitalized with COVID-19. We searched 6 databases from December 1, 2019 to August 1, 2021 for RCTs comparing the use of CP with standard of care or transfusion of non-CP standard plasma in patients with COVID-19. The risk of bias was assessed using the Cochrane Risk-of-Bias 2 Tool. Random effects (DerSimonian and Laird) meta-analyses were conducted. The primary outcome was the aggregate risk for in-hospital mortality between both arms. We conducted a trial sequential analysis (TSA) based on the pooled relative risks (RRs) for in-hospital mortality. Secondary outcomes included the pooled RR for receipt of mechanical ventilation and mean difference in hospital length of stay. We included 18 RCTs (8702 CP, 7906 control). CP was not associated with a significant mortality benefit (RR: 0.95, 95%-CI: 0.86-1.04, P = .27, high certainty). Subgroup analysis did not find any significant differences (pinteraction = 0.30) between patients who received CP within 8 days of symptom onset (RR: 0.97, 95%-CI: 0.79-1.19, P = .80), or after 8 days (RR: 0.79, 95%-CI: 0.57-1.10, P = .16). TSA based on a RR reduction of 10% from a baseline mortality of 20% found that CP was not effective, with the pooled effect within the boundary for futility. CP did not significantly reduce the requirement for mechanical ventilation (RR: 1.00, 95%-CI: 0.91-1.10, P = .99, moderate certainty) or hospital length of stay (+1.32, 95%-CI: -1.86 to +4.52, P = .42, low certainty). CP does not improve relevant clinical outcomes in patients with COVID-19, especially in severe disease. The pooled effect of mortality was within the boundary of futility, suggesting the lack of benefit of CP in patients hospitalized with COVID-19.
Keywords: Coronavirus disease 2019, Severe acute respiratory syndrome coronavirus 2, Convalescent plasma, Meta-analysis, Mortality
Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues to progress, and a spike in cases has been noted worldwide since March 2021, with many countries experiencing a second or third wave. Healthcare systems have been burdened globally, and up to 20% of infected patients progress to severe disease and 5% require admission to the intensive care unit (ICU) [1]. It has been reported that mortality rates of patients with severe COVID-19 reach up to 20% [2], and as many as 45% of patients requiring invasive mechanical ventilation do not survive [3]. Several potential therapies have been investigated, but only glucocorticoids, interleukin-6 receptor antagonists, and monoclonal antibodies have shown some survival benefits till date [4], [5], [6].
Convalescent plasma (CP) is a potential therapy that has been previously investigated against other respiratory viruses [7,8]. It supposedly engenders a temporary immune response against the viral particles, before the peak production of endogenous IgM and IgG antibodies by the native immune response is established in the patient [9]. The transient reduction in viral load reduces the stimulus for a hyperinflammatory cytokine storm that mediates further progression. Early administration of convalescent plasma in disease has been proven to be beneficial in some viral illnesses [9,10]. Numerous observational studies, randomized controlled trials (RCTs) and systematic reviews have been published thus far in patients with COVID-19 [11,12], with most recent reviews highlighting a lack of benefit of CP [13].
Multiple RCTs have been terminated early due to the decreasing prevalence of COVID-19, as well as refusal of consent from eligible patients [14], [15], [16], [17], [18]. As a result of this, the sample size of these RCTs is insufficient, and they are unable to provide strong evidence for or against CP in COVID-19. As such, we conducted a systematic review and meta-analysis of CP use in patients hospitalized with COVID-19. In addition to this, we performed a trial sequential analysis (TSA) via cumulative meta-analysis similar to interim analyses in RCTs, in order to assess the conclusiveness of the findings from our meta-analysis.
Methodology
Search Strategy and Selection Criteria
This study was registered with PROSPERO (CRD42021253826), and was conducted in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-analyses Statement [19]. We searched MEDLINE, Embase, Cochrane, Scopus, MedRXiv and COVID-NMA databases from December 1, 2019 to August 1, 2021, using the following keywords and their variations: “COVID-19,” “convalescent plasma,” and “randomised controlled trials” (Supplementary Data 1). We assessed all relevant studies and their citation lists to identify articles for inclusion.
We included all RCTs comparing the use of CP with standard of care or transfusion of non-CP standard plasma in 10 or more adult patients (≥18 years) hospitalized with severe COVID-19 reporting on in-hospital mortality, receipt of mechanical ventilation, or hospital length of stay. We excluded any non-human or pediatric studies (<18 years). In the case of overlapping patient data, we included the largest study and excluded any other overlapping studies. Three reviewers (RRL, JJLS, FLT) were involved in the screening process and any conflicts were resolved by a fourth reviewer (KR).
Assessment of Risk of Bias and Certainty of Evidence
Study quality was assessed using the Cochrane Risk-of-Bias 2 tool for RCTs [20]. We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach to assess the certainty of evidence [21]. The assessment for risk of bias and certainty of evidence was conducted by 3 reviewers (RRL, JJS, FLT) independently, and conflicts were resolved by a fourth reviewer (KR)
Outcomes of Interest and Data Collection
The primary outcome for our meta-analysis is in-hospital mortality, and this is quantified based on the relative risk (RR) between the treatment versus control arms. Secondary outcomes included the receipt of mechanical ventilation, and the hospital length of stay. Other outcomes that were reported descriptively include the ICU length of stay, as well as any other adverse outcomes. Data were collected independently by 4 reviewers (RRL, JJLS, FLT, SM) using a prespecified datasheet, and conflicts were resolved by a fifth reviewer (KR). Data collection covered study characteristics, patient demographics, CP characteristics, mortality, and other relevant clinical outcomes (Supplementary Data 2).
Data Synthesis
We estimated the summary RRs for in-hospital mortality between the treatment and control arms for each study, and pooled them using random effects (DerSimonian and Laird) meta-analysis based on the Freeman-Tukey double-arcsine transformation [22], [23], [24]. Confidence intervals were computed using the Clopper-Pearson method. We assessed the possibility of publication bias via visual inspection of the funnel plot as well as Egger's regression test. Small-study effects were corrected using the random-effects trim-and-fill (R0 estimator) procedure. A previous meta-analysis suggested the exclusion of the study by Agarwal et al. as majority of their patients had received low antibody titers, following which they found significant survival benefits [12]. To test this hypothesis, we conducted a sensitivity analysis excluding the study from the meta-analysis. Another sensitivity analysis was conducted by excluding any studies with high risks of bias.
To further elicit the effect of CP in COVID-19, we performed TSA using TSA v0.9.5.10 (www.ctu.dk/tsa), which combines an information size calculation for a meta-analysis with the threshold of statistical significance whenever an additional trial is included via cumulative meta-analysis. This is similar to group sequential monitoring boundaries in RCTs during interim analyses. The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial, which recruited patients to detect a RR reduction in mortality of 20%, did not find significant benefits from CP use. As such, in our meta-analysis, we sought to determine if CP had a significant benefit based on a smaller reduction in mortality. Using a type I error of 0.05 and a power of 0.80, we estimated the required information size assuming a RR reduction in mortality of 10% (which is closer to the pooled estimate in the meta-analysis) and a baseline in-hospital mortality rate of 20% as reported in the published literature for patients with severe COVID-19, anticipating low-moderate levels of heterogeneity [2].
Subgroup analysis was conducted based on the timing at which CP was given (within 8 days of symptom onset, and later than 8 days). We then pooled the RRs for receipt of mechanical ventilation, and the mean differences in hospital length of stay. Pooling of RRs was conducted with continuity correction by adding a constant of 0.5 to allow inclusion of studies with zero events. For continuous variables, means and standard deviations were derived from the aggregate data as per Wan et al. [25]. Statistical heterogeneity was measured as part of the assessment of certainty of evidence outlined by the GRADE approach. Statistical analysis was conducted on R3.6.1. Nominal P < .05 was considered statistically significant in our analysis.
Post-Hoc Analyses
We conducted several additional post-hoc analyses after extracting the data. Firstly, we conducted a subgroup analysis by regional variation as well as the risk of bias. Secondly, we conducted an additional trim-and-fill analysis for hospital length of stay in view of the significant publication bias (pegger < .05).
Results
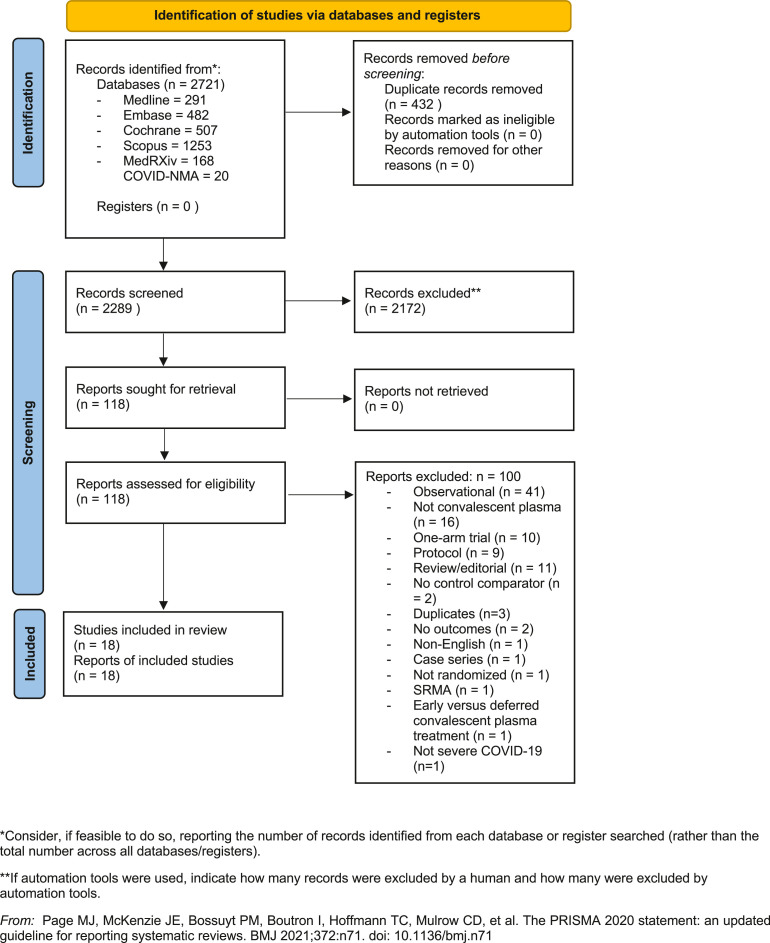
Of 2721 references, we identified 118 potentially relevant studies for full-text evaluation. 20 RCTs were identified and 2 were excluded – one compared the effects of early and deferred CP [26], while another RCT did not report any of the prespecified primary or secondary outcomes [27]. In total, 18 RCTs comprising 16,608 hospitalized patients (CP vs control: 8702 vs 7906 patients) were included (Figure 1 ) [[14], [15], [16], [17], [18],[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. 15 RCTs reported on 30-day mortality, and one RCT reported on 60-day mortality [36], and in-hospital mortality [37]. The REMAP-CAP trial randomized 2084 patients, but only reported mortality outcomes in 2076 patients. Of note, 3 trials reported on patients with nonsevere and severe COVID-19: Bennett-Guerrero reported on 14 patients with nonsevere COVID-19 [15], while Gharbharan reported on one patient with moderate COVID-19 [16]. 897 patients (8%) in the RECOVERY trial did not receive supplemental oxygen therapy [31]. Various adjuvant therapies including corticosteroids, antiviral medications, and hydroxychloroquine were used in each study; the proportion of patients receiving each adjuvant therapy is summarized in Supplementary Data 3. Study details, patient characteristics and details on CP are summarized in Table 1 .
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses Flow Diagram.
Table 1.
Demographics and outcomes of included studies
| Study | Year | Design | Groups | No. of patients | Patient characteristics | Medication regimen | Mortality | Follow-up duration |
|---|---|---|---|---|---|---|---|---|
| Agarwal | 2020 | Multicenter RCT in India | CP | 235 | 52 (42-60) y 177 males 30 seronegative BMI 26.2 ± 4.3 |
2 × 200 mL CP, 24 h apart | 34 | 28 d |
| Control | 229 | 52 (41-60) y 177 males 40 seronegative BMI 26.1 ± 4.2 |
HCQ, Remdesivir, Lopinavir/Ritonavir, PRED, Dexamethasone, Hydrocortisone, Tocilizumab, Heparin, Azithromycin, Antibiotics | 31 | ||||
| Al Qahtani | 2020 | Multicenter RCT in Bahrain | CP | 20 | 52.6±14.9 y 17 Males |
2 × 200 mL CP, 24 h apart | 1 | 28 d |
| Control | 20 | 50.7 ± 12.5 y 15 Males |
HCQ, Lopinavir/Ritonavir, Ribavirin, Azithromycin, Peginterferon, Tocilizumab, PRED, antibiotics, anticoagulation, PPI, ACE-I/ARB, CCB, B blocker, Aspirin, Diuretics, Insulin, Metformin, Thyroxine, Acetylcysteine | 2 | ||||
| Avendano- Sola |
2020 | Multicenter RCT in Spain | CP | 38 | 61.3 ± 16.3 y 2 immunocompromised 21 seronegative 20 males |
1 × 250-300 mL CP VMNT-ID50 assay titer 1:292 Pseudovirus neutralizing ID50 assay titer 1:327 |
0 | 30 d |
| Control | 43 | 60.3 ± 15.0 y 5 immunocompromised 19 seronegative 24 males |
HCQ, Lopinavir-Ritonavir, Azithromycin, Remdesivir, Glucocorticoid, Tocilizumab, LMWH | 4 | ||||
| Bajpai | 2020 | Single center RCT in Lok Nayak hospital in India | CP | 14 | 48.1±9.1 y 11 males BMI 26.3 ± 2.5 |
2 × 250 mL CP, 24 hours apart | 3 | 29 d |
| Control | 15 | 48.3 ± 10.8 y 11 males BMI 26.1 ± 2.2 |
HCQ, Azithromycin, Oseltamivir, standard medications for diabetes mellitus and hypertension control | 1 | ||||
| Bennett- Guerrero |
2021 | Single center RCT in a New York Hospital | CP | 59 | 67±15.8 y 36 males 4 immunocompromised BMI 28.9 (24.0-33.6) |
2 × 240 mL CP over 1-4 h each Pseudotype assay titer1:334 Plaque neutralization assay titer 1:526 |
14 (28 d) 16 (90 d) |
90 d |
| Control | 15 | 64±17.4 y 8 males 2 immunocompromised BMI 27.8 (23.1-30.2) |
Glucocorticoids, Remdesivir, HCQ, Tocilizumab | 4 (28 d) 5 (90 d) |
||||
| Concor | 2021 | MulticenterRCT in Canada, the United States, and Brazil | CP | 614 | 67.8±16.0 y 362 males BMI 29 (25-33) |
1 × 500 mL CP from 1 - 2 donors Viral neutralizing antibodies at a titer of >1:160 or antibodies against the receptor binding domain (RBD) of the SARS-CoV-2 Spike protein at a titer of >1:100 Azithromycin, Systemic Corticosteroids, Antiviral Medications, Anticoagulants, Other Covid-19 medications, Other Antibiotics |
141 (30 days) 156 (90 d) |
90 d |
| Control | 307 | 67.3 ± 14.8 y 183 males BMI 29 (25,33) |
Azithromycin, Systemic Corticosteroids, Antiviral Medications, Anticoagulants, Other Covid-19 medications, Other Antibiotics | 63 (30 d) 69 (90 d) |
||||
| Estcourt (REMAP- CAP) |
2021 | International multicenter RCT | Early CP | 1120 | 60.3 ± 12.8 y 753 males 67 immunocompromised 271 seronegative BMI 30.8 (26.9-35.6) |
2 × 250-310 mL CP within 48 h of randomization Antibody titer ≥ 1:80 |
406/1117 | 28 d |
| Delayed CP | 31 | 61.1 ± 17.5 y 18 males BMI 32.4 (28.7-39.7) |
9 | |||||
| Control | 933 | 60.2±13.1 y 633 males 60 immunocompromised 149 seronegative BMI 31.1 (26.9-36.5) |
Steroids, Remdesivir, immunomodulators, Tocilizumab, Sarilumab | 354/928 | ||||
| Gharbharan | 2020 | Multicenter RCT in Netherlands | CP | 43 | 61 (56-70) y 29 males 7 seronegative |
1 × 300 mL CP 2nd unit of CP after 5 days in patients without a clinical response and a persistently positive RT-PCR Antibody titer 1:640 |
6 | 60 d *No deaths occurred beyond Day 30. |
| Control | 43 | 63 (55-77) y 33 males 6 seronegative |
EMA-approved drugs (chloroquine, azithromycin, lopinavir/ritonavir, tocilizumab, anakinra) | 11 | ||||
| Korper | 2021 | MulticenterRCT in Germany | CP | 53 | 59 (53-65) y 42 males 10 seronegative BMI 29.4 (27.6-33.4) |
3 units of CP on day 1, 3, and 5 with a total median of 846 mL (824-855 mL). PRNT50 neutralisation titer 1:160 |
8 | 35 d |
| Control | 52 | 62 (55-66) y 35 males 10 seronegative BMI 29.1 (25.6-31.5) |
Antivirals, Steroids, Antibiotics, Vasopressors, Anticoagulants, Platelet aggregation inhibitor | 14 | ||||
| Li | 2020 | Multicenter RCT in China | CP | 51 | 70 (62-80) y 27 males |
1 × 4-13 mL/kg of CP – 10 mL for the first 15 mins, 100 mL/h subsequently Antibody titer 1:160 |
8 | 28 d |
| Control | 50 | 69 (63-76) y 33 males |
Antivirals, Interferon, Chinese herbal medicine, Antibacterials, Antifungals, Steroids, Human immunoglobulin | 12 | ||||
| Libster | 2018 | Multicenter RCT in Argentina | CP | 80 | 76.4 ± 8.7 y 26 males |
Antihypertensives, antidiabetics Antibody titer 1:3200 |
2 | 25 d |
| Control | 80 | 77.9 ± 8.4 y 34 males |
Antihypertensives, antidiabetics | 4 | ||||
| O'Donnell | 2021 | Multicenter RCT in New York and Brazil | CP | 150 | 60 (48-71) y 96 males BMI 30.1 (26.6-34.7) |
1 × 200-250 mL CP over 2h Antibody titer 1:160 |
19 | 28 d |
| Control | 73 | 63 (49-72) y 51 males BMI 29.4 (26.2-33.0) |
Corticosteroids, Remdesivir, HCQ, Antibacterials |
18 | ||||
| Pouladzadeh | 2021 | Single center RCT in Iran Ahvaz Jundishapur University of Medical Sciences | CP | 30 | 53.5±10.3 y 16 males |
1 × 500 mL CP, 2nd unit if no improvement seen after 24 h | 3 | 2 mo |
| Control | 30 | 57.2 ± 17.0 y 17 males |
Chloroquine phosphate, Lopinavir/Ritonavir | 5 | ||||
| Rasheed | 2020 | Multicenter RCT in Iraq | CP | 21 | 21 ± 55.7 y 18 seronegative |
1 × 400 mL CP over 2h | 1 | 30 d |
| Control | 28 | 28 ± 47.8 y | HCQ, Azithromycin, PRED | 8 | ||||
| Ray | 2020 | Multicenter RCT in India | CP | 40 | 27 males | 2 × 200 mL CP, 24 h apart | 10 | 30 d |
| Control | 40 |
30 males |
Tocilizumab, Remdesivir, HCQ, AZA, Ivermectin, Doxycycline, Corticosteroids, LMWH / unfractionated heparin, Antibiotics, Antidiabetics, Antihypertensives | 14 | ||||
| RECOVERY (Horby) | 2021 | Multicenter RCT in UK | CP | 5795 | 63.6 ± 14.7 y 1982 seronegative 3643 males |
1 × 275 mL CP, 2nd unit 75 mL at least 12 hrs later the following day (4675 (81%)) Antibody titers ≥1:100 |
1398 | 28 d |
| Control | 5763 | 63.4±14.6 y 1629 seronegative 3787 males |
Dexamethasone, Lopinavir-Ritonavir, HCQ, azithromycin, colchicine, REGN-COV2 (monoclonal neutralising antibody cocktail), aspirin, tocilizumab | 1408 | ||||
| Sekine | 2021 | Single center RCT in Brazil, Hospital de Clinicas de Porto Alegre | CP | 80 | 59.0 (48.0 - 68.5) y 49 seronegative 49 males |
2 × 300 mL, 48 h apart Glucocorticoids and Antibacterials |
18 | 28 d |
| Control | 80 | 62.0 (49.5 - 68.0) y 52 seronegative 44 males |
Glucocorticoids and Antibacterials | 13 | ||||
| Simonovich | 2020 | International multicenter RCT | CP | 228 | 62.5 (53-72.5) y 65 seronegative 161 males |
5-10 mL/kg/h of CP with an inferior limit 400 mL for patients <70 kg and a superior limit of 600 mL for those >70 kg. Median 500 mL (IQR 415-600 mL) Antibody titer 1:3200 |
25 | 30 d |
| Control | 105 | 62 (49-71) y 34 seronegative 64 males |
Steroids, Lopinavir/Ritonavir, Tocilizumab, Ivermectin | 12 |
ACE-I/ARB, Angiotensin converting enzyme inhibitor/angiotensin receptor blocker; B blocker, beta blocker; CCB, calcium channel blocker; CP, convalescent plasma; HCQ, Hydroxychloroquine; LMWH, Low molecular weight heparin; PRED, Methylprednisolone; PPI, Proton pump inhibitor.
Six RCTs were rated as having low risk of bias, 11 RCTs with some concerns of bias, and 1 RCT with high risks of bias. Most RCTs had some concerns with deviations from the intended intervention. The risk of bias assessment is summarized in Supplementary Data 4. The GRADE assessment for certainty of evidence for primary outcome was high and is summarized in Supplementary Data 5.
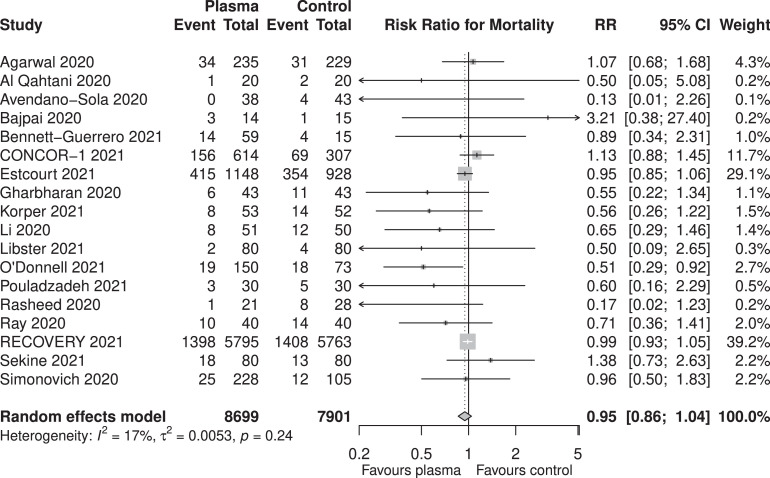
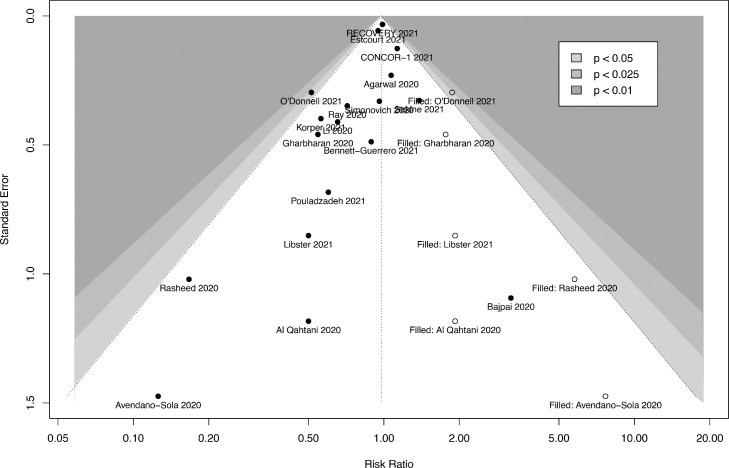
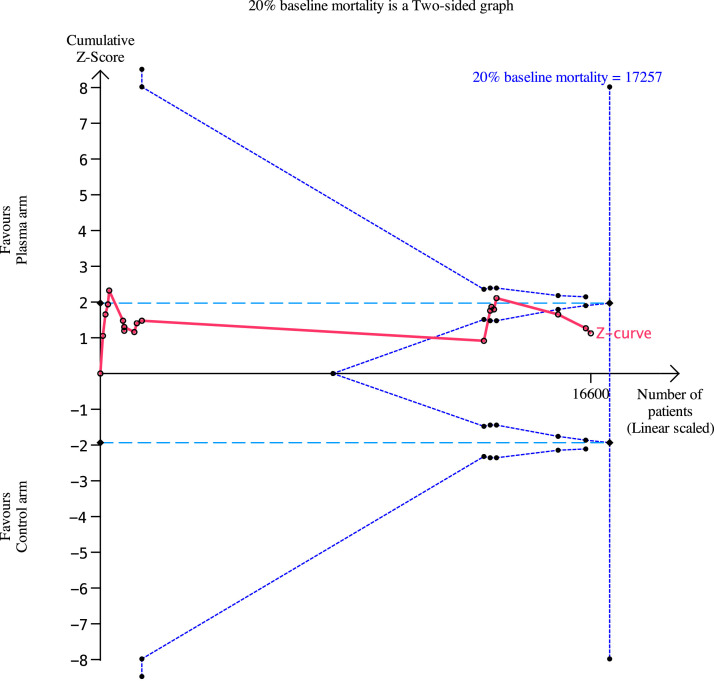
From the 18 RCTs, 2121 (24.4%) of 8699 patients from the CP arm and 1984 (25.1%) of 7901 patients from the control arm did not survive. The pooled RR for in-hospital mortality was 0.95 (95%-CI: 0.86- 1.04, P= .27 Figure 2 , high certainty). While there was possible evidence of publication bias (pegger = .032), the pooled estimate remained relatively similar after correction of small-study effects using the trim-and-fill (R0 estimator) procedure (RR: 0.98, 95%-CI: 0.86-1.11, P = .71, Figure 3 ). A futility analysis was conducted based on in-hospital mortality reported by 18 RCTs, the required information size was 17,257. The cumulative Z-curve did not cross the boundaries for benefit or harm, and was within the boundary for futility (Figure 4 ), suggesting the postulated benefit of CP is unlikely to be achieved with further randomization of patients.
Fig. 2.
Pooled in-hospital mortality for patients receiving convalescent plasma and standard of care for COVID-19.
Fig. 3.
Funnel plot after correcting for small-study effects using the trim-and-fill (R0) estimator.
Fig. 4.
Trial sequential analysis for a baseline mortality rate of 20%. As the RECOVERY trial had found no significant benefit at a relative risk reduction (RRR) in mortality of 20%, modelled our TSA based on a 10% RRR in mortality to further elicit the effect of convalescent plasma. The required information size is 17,257, and this is not achieved. The cumulative Z-curve (red line) does not cross the boundary for conventional (light-blue dotted lines) or TSA-adjusted (upper and lower-most curves) boundaries for benefit or harm. The Z-curve is within the boundary of futility (triangular lines beginning from the middle of the graph).
We then conducted a subgroup analysis based on the timing of CP (pinteraction = .30). Patients who were treated with CP within 8 days of symptom onset (8 studies, 2,204 patients, RR: 0.97, 95%-CI: 0.79-1.19, P = .80) did not have a significantly different risk for mortality compared to those who were treated beyond 8 days (7 studies, 12,251 patients, RR: 0.79, 95%-CI: 0.57-1.10, P = .16).
Administration of CP did not impact the proportion of patients requiring mechanical ventilation between both groups (8 studies, 6511 CP, 6363 control, RR: 1.00, 95%-CI: 0.91-1.10, P = .99, pegger = .73, moderate certainty), nor did it reduce the hospital length of stay (5 studies, 549 CP, 467 control, +1.32 days, 95%-CI: -1.86 to +4.51, P = .42, pegger = .033, low certainty). Given the significant Egger's test, trim-and-fill analysis was conducted for hospital length of stay; after correcting for small-study effects, CP increased the hospital length of stay by 5.08 days (95%-CI: 2.22-7.94, P= .0005). Ten studies reported on adverse outcomes related to COVID-19 therapy while 3 studies reported on the ICU length of stay (ranging from 5 to 39 days). The details of the adverse outcomes as well as other clinical outcomes are summarized in Supplementary Data 6.
Post-hoc subgroup analyses by geographical region (Asia, Europe, The Americas, pinteraction = .84) and by risk of bias (low risk vs some concerns and high risk, pinteraction = .75, Supplementary Data 7) found no difference in the reduction in mortality across the individual subgroups.
Discussion
Our analysis of the current literature suggests that CP does not appear to have any significant mortality benefits for patients hospitalized with COVID-19 (RR: 0.95, 95%-CI: 0.86-1.04, P = .27), majority of whom had severe disease. Sensitivity analyses excluding Agarwal et al. [12,28], and studies with high risks of bias [33], did not significantly change the pooled estimate. Previously published meta-analyses suggested no survival benefits [11]. However, Klassen et al. found significant survival benefits after excluding one study where patients had received “low-levels” of antibodies during transfusion [12,28]. While our meta-analysis had 5 newer RCTs included, our sensitivity analysis after excluding the study did not find any significant survival benefits for patients receiving CP.
Apart from its direct antiviral neutralizing action [9], CP therapy is postulated to provide additional benefit via immunomodulation of the infected host [41]. However, its effectiveness is confounded by the emergence of the newer alpha (B.1.1.7), gamma (P.1) and delta (B.1.617.2) variants, some of which may evade immune responses. Secondly, the lowest effective dose and neutralizing antibody titer required for viral clearance remain uncertain. The mortality rates in patients hospitalized due to COVID-19 have been variable globally. While the RECOVERY trial, which enrolled the largest number of patients receiving CP, reported a 24% mortality in the control and intervention arms of predominantly severe COVID-19 patients [31], other observational studies have shown lower mortality rates in hospitalized patients with COVID-19 [2,42]. Our TSA analysis found that assuming a baseline mortality rate of 20% in this cohort and a RR reduction of 10%, the cumulative Z-curve was within the boundary for futility. This implies that CP in its current state and timing of administration is unlikely to confer survival benefit amongst patients hospitalized with COVID-19, and recruitment of further patients for CP would be futile.
Two interrelated factors could explain the lack of demonstrable efficacy of CP in COVID-19: the serostatus of the recipient and the timing of plasma administration. SARS-CoV-2 viremia peaks at the first week of illness and viral clearance which follows the primary immune response occurs by 10-14 days. Beyond 8 days after symptom onset, nearly all patients with COVID-19 had neutralizing antibody responses [43]. In our analysis, antibody titers across both arms were reported to be similar [28], and as many as 80% of patients receiving CP were seropositive at baseline [14,16,31,40], which could also potentially account for the lack of survival benefit from CP.
Several studies have found that receipt of CP within 72 hours in milder forms of COVID-19 provided significant benefits to survival and disease progression [18,44,45]. Of note, 2 RCTs randomized their patients within 3 days of severe symptoms, and both did not find any survival benefits [30,37]. Patients even in the early stages of severe COVID-19 are likely to have been seroconverted, and as such, CP confers no additional benefit. It is interesting to note that the recent COVID-19 Convalescent Plasma in Outpatients (C3PO) trial, which randomized patients to receive relatively high-titer (1:160) CP within 7 days of symptoms, found no significant mortality benefits of CP, which argues against the possibility of better efficacy of CP in patients who are seronegative. A recent Bayesian re-analysis of the RECOVERY trial data suggested some mortality benefit in seronegative patients receiving CP [46], concordant with a propensity-score matched study investigating CP in patients with immunodeficiency [47]. This may suggest the need to select for recipients who are unable to mount an adequate immune response for transfer of passive immunity and to maximize the effects of CP.
The meta-analysis and TSA are particularly apt as several trials were terminated early, and results were inconclusive [14], [15], [16], [17], [18]. The use of trial sequential analysis allowed us to assess the pooled effect in relation to the required information size, as well as assess futility. As such, the added value of our study lies in the finding that further recruitment of patients is futile in eliciting the effect of CP in patients with severe COVID-19, which previous meta-analyses have not investigated. More importantly, the finding of futility holds massive public health implications, particularly when the delta variant becomes increasingly prevalent. Immense amounts of resources are diverted away from other potentially beneficial therapies, as well as vaccine production. More importantly, patients who received CP therapy have been advised to defer vaccination for at least 90 days which puts a dent in the goal to vaccinate everyone as quickly as possible [48].
We recognize several limitations of our study. A large proportion of patients had severe to critical COVID-19, and received CP approximately a week after the onset of symptoms. The results of our meta-analysis are therefore not completely generalizable to patients with mild and moderate COVID-19 where CP is used within a week of symptom onset, or in patients who are immunocompromised or seronegative at the time of transfusion [47]. This is further compounded by the fact that volumes of CP and antibody neutralizing titers were heterogenous across studies. While some studies have suggested a dose-response relationship in CP for COVID-19 [40], we were unable to account for this as we were limited by study-level data, and the fact that various instruments and scales were used to determine antibody titers across the studies. There is also likely some residual uncertainty for these highly select groups of patients. Most studies included in the analysis had some concerns of bias, with one study being rated as high risk of bias. As such, the results of these trials should be interpreted with caution, and the certainty of the evidence is impacted. This is further compounded by inclusion of data from preprint servers, which can in itself introduce bias. Nevertheless, the concerns of bias mostly lay in deviations from the intended intervention, where studies had patients crossover from the control arm to the CP arm. Such deviations from interventions may be unavoidable in the context of the current pandemic, where withholding a potentially lifesaving treatment may be considered unethical. In addition, the assessment of evidence found high certainty for the primary outcome, and the pooled results did not significantly change based on the trim-and-fill procedure. Most of the patients receiving CP have also been subjected to a range of other therapies, which might confound the findings of the RCTs.
In this meta-analysis of over 16,000 patients hospitalized with COVID-19, CP did not significantly reduce mortality, nor did it provide any significant benefit for any other clinical outcomes. The pooled effect was within the boundary of futility suggesting further recruitment of patients is unlikely to demonstrate mortality benefit of CP as postulated in patients with severe COVID-19. Nonetheless, its therapeutic use in patients unable to mount an adequate immune response remains undetermined. Rather than determining its effectiveness in COVID-19 in general, further research on CP should be directed in highly select patient groups, and determining which subgroup of patients is most likely to benefit.
Data Sharing Statement
All data generated or analyzed during this study are included in the published studies and their supplementary information files.
Acknowledgments
Acknowledgments
The authors acknowledge Ms. Suei Nee Wong from the National University of Singapore for her assistance with the search strategy.
Declaration of interest
All authors declare no competing interests.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions Statement
Study design: KR, RRL
Search strategy and screening of articles: RRL, JJLS, FLT, KR
Risk of bias assessment: RRL, JJLS, FLT
Data collection: RRL, JJLS, FLT, SM
Data analysis and interpretation: RRL, JJLS, FLT, BCT, NLS, KR,
Tables and figures: RRL, JJLS, FLT
Drafting of manuscript: RRL
Critical revision of manuscript for intellectually important content: KR, SM, RRL, BCT, SSM, BEF
All authors provided critical conceptual input, interpreted the data analysis, read, and approved the final draft.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tmrv.2021.09.001.
Appendix. Supplementary materials
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira E., Parikh A., Lopez-Ruiz A., Carrilo M., Goldberg J., Cearras M., et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim Z.J., Subramaniam A., Ponnapa Reddy M., Blecher G., Kadam U., Afroz A., et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A Meta-analysis, Am J Respir Crit Care Med. 2021;203:54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling R.R., Ramanathan K., Tan W.Q., Yeo L.H.Y., Poon W.H., Syn N.L., et al. Interleukin-6 receptor antagonists for severe coronavirus disease 2019: a meta-analysis of reconstructed individual patient data from randomised controlled trials. SSRN. 2021 doi: 10.2139/ssrn.3844782. [DOI] [Google Scholar]
- 5.Rochwerg B., Siemieniuk R.A., Agoritsas T., Lamontagne F., Askie L., Lytvyn L., et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 6.Mahase E. Covid-19: regeneron's antibody combination cuts deaths in seronegative patients, trial finds. BMJ. 2021;373:n1570. doi: 10.1136/bmj.n1570. [DOI] [PubMed] [Google Scholar]
- 7.Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S., et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV Infection, Saudi Arabia. Emerg Infect Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 10.Arciuolo R.J., Jablonski R.R., Zucker J.R., Rosen J.B. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City. Clin Infect Dis 2017. 2013;65:1843–1847. doi: 10.1093/cid/cix639. [DOI] [PubMed] [Google Scholar]
- 11.Janiaud P., Axfors C., Schmitt A.M., Gloy V., Ebrahimi F., Hepprich M., et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325:1185–1195. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klassen S.A., Senefeld J.W., Johnson P.W., Carter R.E., Wiggins C.C., Shoham S., et al. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clinic Proceedings. 2021;96:1262–1275. doi: 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piechotta V., Iannizzi C., Chai K.L., Valk S.J., Kimber C., Dorando E., et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;5 doi: 10.1002/14651858.CD013600.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avendaño-Solà C., Ramos-Martínez A., Muñez-Rubio E., Ruiz-Antorán B., de Molina R.M., Torres F., et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.08.26.20182444. 2020.2008.2026. [DOI] [Google Scholar]
- 15.Bennett-Guerrero E., Romeiser J.L., Talbot L.R., Ahmed T., Mamone L.J., Singh S.M., et al. Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in New York: a double-blind randomized trial. Crit Care Med. 2021 doi: 10.1097/ccm.0000000000005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharbharan A., Jordans C.C.E., Geurtsvankessel C., den Hollander J.G., Karim F., Mollema F.P.N., et al. Convalescent plasma for COVID-19. A randomized clin trial, medRxiv. 2020 doi: 10.1101/2020.07.01.20139857. 2020.2007.2001. [DOI] [Google Scholar]
- 17.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Clopper C.J., Pearson E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika. 1934;26:404–413. doi: 10.2307/2331986. [DOI] [Google Scholar]
- 23.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Miller J.J. The inverse of the freeman – Tukey double arcsine transformation. The American Statistician. 1978;32:138. doi: 10.1080/00031305.1978.10479283. [DOI] [Google Scholar]
- 25.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balcells M.E., Rojas L., Le Corre N., Martínez-Valdebenito C., Ceballos M.E., Ferrés M., et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamdy Salman O., Ail Mohamed H.S. Efficacy and safety of transfusing plasma from COVID-19 survivors to COVID-19 victims with severe illness. A double-blinded controlled preliminary study. Egypt J Anaesth. 2020;36:264–272. doi: 10.1080/11101849.2020.1842087. [DOI] [Google Scholar]
- 28.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AlQahtani M., Abdulrahman A., Almadani A., Alali S.Y., Al Zamrooni A.M., Hejab A.H., et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci Rep. 2021;11:9927. doi: 10.1038/s41598-021-89444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajpai M., Kumar S., Maheshwari A., Chhabra K., kale P., Gupta A., et al. Efficacy of Convalescent Plasma Therapy compared to Fresh Frozen Plasma in Severely ill COVID-19 Patients: A Pilot Randomized Controlled Trial. medRxiv. 2020 doi: 10.1101/2020.10.25.20219337. 2020.2010.2025. [DOI] [Google Scholar]
- 31.Horby P.W., Estcourt L., Peto L., Emberson J.R., Staplin N., Spata E., et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021 doi: 10.1101/2021.03.09.21252736. 2021.2003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell M.R., Grinsztejn B., Cummings M.J., Justman J., Lamb M.R., Eckhardt C.M., et al. A randomized, double-blind, controlled trial of convalescent plasma in adults with severe COVID-19. medRxiv. 2021 doi: 10.1101/2021.03.12.21253373. 2021.2003.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasheed A.M., Fatak D.F., Hashim H.A., Maulood M.F., Kabah K.K., Almusawi Y.A., et al. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 2020;28:357–366. [PubMed] [Google Scholar]
- 34.Ray Y., Paul S.R., Bandopadhyay P., D'Rozario R., Sarif J., Lahiri A., et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. 2020 doi: 10.1101/2020.11.25.20237883. 2020.2011.2025. [DOI] [Google Scholar]
- 35.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pouladzadeh M., Safdarian M., Eshghi P., Abolghasemi H., bavani A.G., Sheibani B., et al. A randomized clinical trial evaluating the immunomodulatory effect of convalescent plasma on COVID-19-related cytokine storm. Intern Emerg Med. 2021 Apr 10:1–11. doi: 10.1007/s11739-021-02734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estcourt L.J. The REMAP-CAP Investigators. convalescent plasma in critically ill patients with Covid-19. medRxiv. 2021 doi: 10.1101/2021.06.11.21258760. 2021.2006.2011. [DOI] [Google Scholar]
- 38.Sekine L., Arns B., Fabro B.R., Cipolatt M.M., Machado R.R.G., Durigon E.L., et al. Convalescent plasma for COVID-19 in hospitalised patients: an open-label, randomised clinical trial. Eur Respir J. 2021 doi: 10.1183/13993003.01471-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The C.-S.G., committee C.-w., Bégin P., Callum J., Jamula E., Cook R., et al. C.-S.G. for The. Convalescent plasma for hospitalized patients with COVID-19 and the effect of plasma antibodies: a randomized controlled, open-label trial. medRxiv. 2021 doi: 10.1101/2021.06.29.21259427. 2021.2006.2029. [DOI] [Google Scholar]
- 40.Körper S., Weiss M., Zickler D., Wiesmann T., Zacharowski K., Corman V.M., et al. Results of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients. J Clin Invest. 2021 doi: 10.1172/jci152264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lünemann J.D., Nimmerjahn F., Dalakas M.C. Intravenous immunoglobulin in neurology–mode of action and clinical efficacy. Nat Rev Neurol. 2015;11:80–89. doi: 10.1038/nrneurol.2014.253. [DOI] [PubMed] [Google Scholar]
- 42.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Vol. 395. Lancet; 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan; pp. 1054–1062. (China: a retrospective cohort study). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joyner M.J., Carter R.E., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med. 2021;384:1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salazar E., Christensen P.A., Graviss E.A., Nguyen D.T., Castillo B., Chen J., et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Spike Protein IgG. Am J Pathol. 2021;191:90–107. doi: 10.1016/j.ajpath.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamilton F.W., Lee T.C., Arnold D.T., Lilford R., Hemming K. Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomised controlled trial. Int J Infect Dis. 2021;109:114–117. doi: 10.1016/j.ijid.2021.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson M.A., Henderson J.P., Shah P.K., Rubinstein S.M., Joyner M.J., Choueiri T.K., et al. COVID-19, C. Consortium. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol. 2021;7:1167–1175. doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention Summary Document for interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized or Approved in the United States. 2021 https://www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf (Accessed: 16 August 2021) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.