Abstract
Chromosome 4q21 microdeletion leads to a human syndrome that exhibits restricted growth, facial dysmorphisms, mental retardation, and absent or delayed speech. One of the key genes in the affected region of the chromosome is PRKG2, which encodes cGMP-dependent protein kinase II (cGKII). Mice lacking cGKII exhibit restricted growth and deficits in learning and memory, as seen in the human syndrome. However, vocalization impairments in these mice have not been determined. The molecular pathway underlying vocalization impairment in humans is not fully understood. Here, we employed cGKII knockout (KO) mice as a model for the human microdeletion syndrome to test whether vocalizations are affected by loss of the PRKG2 gene. Mice emit ultrasonic vocalizations (USVs) to communicate in social situations, stress, and isolation. We thus recorded ultrasonic vocalizations as a model for human speech. We isolated postnatal day 5–7 pups from the nest to record and analyze USVs and found significant differences in vocalizations of KO mice relative to wild-type and heterozygous mutant mice. KO mice produced fewer calls that were shorter duration and higher frequency. Because neuronal activation in the arcuate nucleus in the hypothalamus is important for the production of animal USVs following isolation from the nest, we assessed neuronal activity in the arcuate nucleus of KO pups following isolation. We found significant reduction of neuronal activation in cGKII KO pups after isolation. Taken together, our studies indicate that cGKII is important for neuronal activation in the arcuate nucleus, which significantly contributes to the production of USVs in neonatal mice. We further suggest cGKII KO mice can be a valuable animal model to investigate pathophysiology of human microdeletion 4q21 syndrome.
Keywords: cGMP-dependent protein kinase II, ultrasonic vocalization, human microdeletion 4q21 syndrome, speech impairment, and the arcuate nucleus
Introduction
Microdeletions of chromosome 4q21 are associated with a human syndrome that includes restricted growth, mental retardation, facial dysmorphisms, and absent or delayed speech [1, 2]. The affected region contains five genes; Protein Kinase G 2 (PRKG2), RasGEF Domain Family Member 1B (RASGEF1B), Heterogeneous Nuclear Ribonucleoprotein D (HNRNPD), Heterogeneous nuclear ribonucleoprotein D-like (HNRPDL), and Enolase-Phosphatase 1 (ENOPH1) [1, 2]. Studies of human patients with different but overlapping deletions suggest that disruption of the PRKG2 and RASGEF1B genes are important for intellectual disability and speech defects, while defects in HNRNPD and HNRNPDL genes are involved in growth retardation, hypotonia, and some aspects of developmental delay [2]. However, the exact molecular pathway underlying the symptoms in the human microdeletion syndrome has not been fully elucidated.
Among genes located in the affected chromosome region, PRKG2 encodes cGMP-dependent protein kinase II (cGKII), which is a serine/threonine kinase activated by the second messenger cGMP [3, 4]. cGKII is expressed abundantly in several tissues including the intestines, kidney, and brain, whereas it is found at lower levels in the lungs, prostate, growth plates, pancreas, and salivary glands [4, 5]. cGKII phosphorylates various targets that are important for several biological functions. For example, it plays a key role in modulating secretion from cells in the kidney, epithelium, and adrenal gland by phosphorylating the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) chloride channel [3, 5–8]. Importantly, loss-of-function mutations or genetic ablation of cGKII cause dwarfism in humans and rodents as cGKII regulates bone mass [6, 9–11], as seen in human microdeletion 4q21 syndrome [1, 2]. In neurons, cGKII plays important roles in fast synaptic transmission by controlling the AMPA receptor (AMPAR) subunit GluA1 trafficking [12–14]. cGKII phosphorylates serine 845 of GluA1 (pGluA1) that is important for activity-dependent trafficking of GluA1-containing AMPARs and increases the level of extrasynaptic receptors [12, 15–18]. In the hippocampus, cGKII-mediated GluA1 phosphorylation is critical for long-term potentiation (LTP), which is important for learning and memory [12, 13, 19]. In fact, cGKII knockout (KO) mice exhibit severe impairment in hippocampus-dependent learning and memory [20, 21], which is relevant to mental retardation found in human microdeletion 4q21 syndrome [1, 2]. Taken together, loss of the cGKII (PRKG2) gene likely underlies neurological symptoms in microdeletion 4q21 syndrome, which includes intellectual disability and speech defects.
In humans, speech production involves multiple brain regions as well as neuronal control of muscles in the lung, larynx, and pharynx [22–24]. Significantly, speech defects are one of the primary characteristics of the human microdeletion 4q21 syndrome, but the mechanisms underlying speech delay are unknown [1, 2]. Rodents communicate using high frequency, ultrasonic vocalizations (USVs) in male-female interactions, juvenile social interactions, and mother-infant interactions [25–28]. Pups vocalize in the range of 30–90 kHz in response to aversive stimuli such as isolation from the natal nest, and this elicits a retrieval response from the mother [29–34]. Interestingly, isolation from the nest results in a decrease in body temperature of pups which, in turn, triggers emission of USVs [31]. Notably, a recent study demonstrated that dropping body temperature of isolated pups induces strong activation of agouti-related peptide (AgRP) neurons in the arcuate nucleus in the hypothalamus following 90 min isolation from the nest, which is sufficient to produce USVs [35]. Therefore, thermal insulation is an important factor regulating the activation of AgRP neurons in the arcuate nucleus of pups following isolation from the nest and is associated with the emission of USVs [35].
Given that cGKII KO mice exhibit dwarfism and disrupted learning and memory, which resembles traits of human patients with microdeletion 4q21 syndrome, these mice can be a potential animal model to study this disorder. However, whether defects in the cGKII gene are sufficient to produce vocalization changes in addition to the cognitive defects and growth retardation seen in KO animals is unknown. Here, we measure USVs to examine whether loss of the cGKII gene is associated with altered vocalizations in mice, which would indicate cGKII gene mutations likely underlie the speech impairment in the human syndrome. We found significant differences in USVs of KO mice relative to wild-type (WT) mice. We also revealed significant reduction in activation of AgRP neurons in the cGKII KO arcuate nucleus, which contributes to reduced USV production in KO pups. Finally, the current study combined with previous work further suggest that cGKII KO mice can serve as a valuable animal model to investigate pathophysiology of human microdeletion 4q21 syndrome.
Materials and Methods
Animals
cGKII KO animals were maintained in the C57Bl6 background as previously described [20]. Pups, a mixture of males and females, were produced from breeding heterozygous cGKII KO mice. Genomic DNA for genotyping was collected from tail biopsy using Extracta™ DNA Prep for PCR according to the manufacturer’s instructions (QuantaBio). For PCR genotyping, three primers were used - AV3R: 5’-ATTAAGGGCCAGCTCATTC-3’, e2fb-av: 5’-GGTGAAGTTTTAGGTGAAACCAAG-3’, and av9r: 5’-CTGCTTAATGACGTAGCTGCC-3’. The PCR program used was: 94°C for 5 min, followed by 10 cycles consisting of 94°C for 30 s, 66°C for 30 s, and 72°C for 1 min, followed by 27 cycles consisting of 94°C for 30 s, 59°C for 1 min, and 72°C for 1 min, and a final extension at 72°C for 10 min. Litters consisting of a mixture of WT, KO, and heterozygous mutant mice were used for the study. Colorado State University’s Institutional Animal Care and Use Committee reviewed and approved the animal care and protocol (16–6779A).
USV Recording and analysis
A decrease in body temperature of pups upon isolation from the nest triggers emission of USVs. However, after the second week of life, pups are able to self-maintain their body temperature, resulting in fewer USVs [31]. Therefore, the ages of the pups used for experiments ranged from postnatal day 5 to postnatal day 7 (P5-P7) as reported previously [36]. Neonatal P5–7 mice were separated from the home cage and placed in a round transparent container with an open top. The genotype of each pup was not known at the time of recording and was determined after USV recordings were complete. The ultrasound microphone (Avisoft-Bioacoustics CM16/CMPA) was placed above the container in the sound-proof box. Vocalizations were recorded for 5 min using Avisoft Recorder software. After the recording was finished for each pup, tail biopsy was carried out for genotyping, and brain tissues were collected for analyzing cGKII expression. Between animals, the container was cleaned with 70% ethanol and dried to get rid of any odors that could affect pup vocalizations. Spectrograms of USVs were generated and analyzed by using Avisoft SASLab pro (Fig. 1). Recording data were filtered in a range of 30–90kHz, and start/end time, duration, peak frequency, and peak amplitude for each of the calls were collected. With the data extracted, the number of calls, average duration, average frequencies, and average amplitudes were determined for each pup individually and then averaged for each genotype.
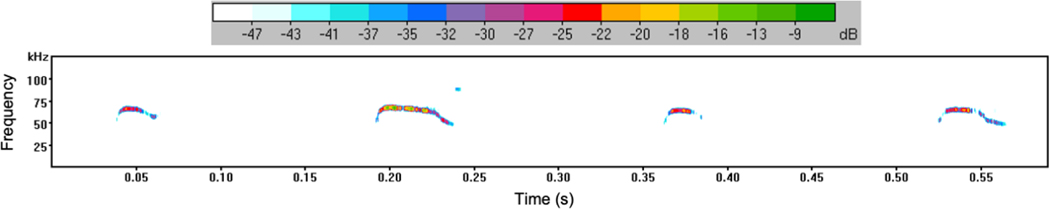
Figure 1. Spectrogram of mouse pup ultrasonic vocalizations.
Spectrogram generated from P5-P7 pup isolation calls using Avisoft SASLab Pro software. Four individual vocalizations are shown in the representative spectrogram. The horizontal axis shows time (in seconds) from which duration of each USV could be determined as well as a total duration of vocalization during the 5-minute testing period. The vertical axis shows frequency (kHZ) of vocalizations emitted by mouse pups. The amplitude of calls (dB) can also be determined by the color (scale shown in top bar).
Tissue sample preparation and immunoblots
Whole brain tissue from each mouse was prepared as described previously [20, 37–39]. Brain samples were homogenized and collected by using a RIPA buffer (150mM NaCl, 0.1% TritonX-100, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris, pH 8.0). Equal amounts of protein were loaded on 10% SDS-PAGE gel and transferred to nitrocellulose membranes. Membranes were blotted with anti-cGKII [12, 20] (Covance, 1:1000) and anti-actin (Abcam, 1:2000) antibodies and developed with chemiluminescence.
Immunohistochemistry
Immunohistochemistry was carried out as described previously [37]. 5–7-day old WT and cGKII KO pups (both males and females) were subjected to 90 mins isolation from the nest. Both WT and KO pups in the nest were used as the basal condition. Brains were then extracted and fixed with 4% paraformaldehyde for 3 days, and then 40μm coronal sections were collected using a vibratome. The expression of the activity-regulated gene, c-Fos, was used to determine activation of neurons in the arcuate nucleus in the hypothalamus before or after isolation from the nest. Sections containing the arcuate nucleus in the hypothalamus were collected, permeabilized with diluting buffer (1% BSA, 0.4% Triton-X, 1% normal goat serum in pH 7.6 TBS), and blocked with 3% normal goat serum in TBS. The brain tissues were then incubated with anti-c-Fos antibody (Synaptic Systems) diluted to 1:500 in diluting buffer at 4°C for 18 hours. Afterwards, the sections were incubated for 2 hours with a secondary goat anti-rabbit IgG antibody conjugated with Alexa Fluor 647 (Invitrogen). Nuclei were labeled by 4′,6-diamidino-2-phenylindole (DAPI). The sections were then mounted on microscope slides and coverslipped. Sections were imaged using the Olympus inverted microscope IX73. The Olympus cellSENS Dimensions software was used to first measure c-Fos positive immunoreactivity on DAPI-positive cells in the hypothalamic region by co-localization analysis. Numbers of c-Fos-positive cells were then counted using particle analysis in the NIH ImageJ software.
Statistics
Statistical comparisons were analyzed with GraphPad Prism 9 software. Unpaired two-tailed Student t-tests were used in single comparisons. To determine whether our samples had homogeneity of variance, we first performed the Levene’s test. When Levene’s test showed significance, we used the non-parametric Kruskal-Wallis test followed by Dunn’s post-hoc test. In contrast, when the population variances of our samples were equal, we used a one-way ANOVA followed by Tukey’s post-hoc test to determine statistical significance. Results are represented as mean ± SEM, and p < 0.05 was considered statistically significant.
Results
Loss of cGKII function in mouse pups disrupts USVs
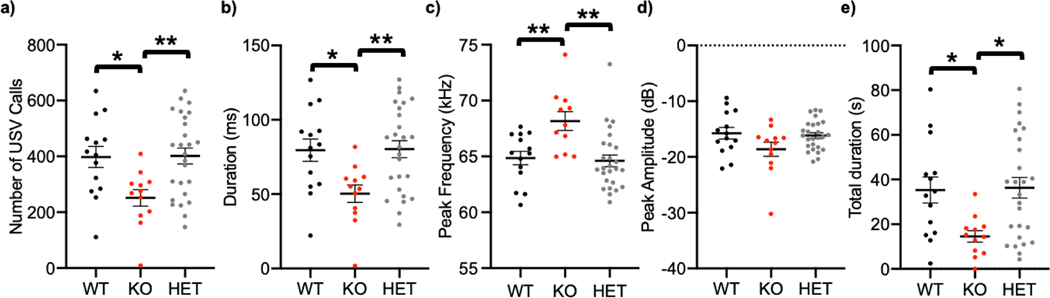
Following isolation from the nest, we recorded USVs from P5–7 pups of WT, cGKII KO, and heterozygous mutant mice. As mouse pup USVs occur in the 40–80 kHz range [40], spectrograms in this range for each recording were created and analyzed (Fig. 1). We found that pups lacking the cGKII gene emitted less vocalizations than both WT and heterozygous mutant (HET) pups during the 5 min testing period (F(2, 49)=1.875, p=0,0067, WT, 397.9 ± 37.55 calls; KO, 251.4 ± 29.51 calls; HET, 401.4 ± 28.52 calls) (Fig. 2a). The duration of each call of KO pups was also significantly reduced compared with WT and HET mice (F(2, 49)=1.875, p=0,0067, WT, 79.57 ± 7.51 ms; KO, 50.25 ± 5.90 ms; HET, 80.28 ± 5.70 ms) (Fig. 2b). We also analyzed the peak frequency of USVs and found that KO pups produced higher frequency USVs than WT and HET pups (F(2, 49)=0.7716, p=0.0133, WT, 64.85 ± 0.60 kHz; KO, 67.51 ± 1.01 kHz; HET, 64.6 ± 0.53 kHz) (Fig. 2c). When comparing the peak amplitude of USVs from each genotype, there was no significant difference between WT and KO pups (F(2, 49)=0.8641, p=0,0781, WT, −15.76 ± 1.03 dB; KO, −18.62 ± 1.27 dB; HET, −16.15 ± 0.52 dB) (Fig. 2d). Given that KO pups generated less and shorter calls (Fig. 2a and 2b), the total time of USVs for 5 min in KO animals was significantly less than WT and HET mice (F(2, 49)=7.732, p=0,016, WT, 35.32 ± 5.88 s; KO, 14.56 ± 2.58 s; HET, 36.29 ± 4.58 s) (Fig. 2e). Interestingly, there were no significant differences between WT and HET pups in USV production when pups were isolated from the nest. Taken together, these results show that the complete loss of the cGKII gene in mice is sufficient to alter USVs.
Figure 2. Abnormal ultrasonic vocalizations in isolated cGKII KO pups relative to WT and HET pups.
a) Total number of USVs emitted, b) duration of individual calls, c) peak frequency, d) peak amplitude (or intensity), and e) total duration of calls in each genotype during 5-minute isolation period. (n=14 WT, 12 KO, and 26 HET animals, *p<0.05 and **p<0.01, one-way ANOVA, Tukey test).
WT and HET mice exhibit normal cGKII expression
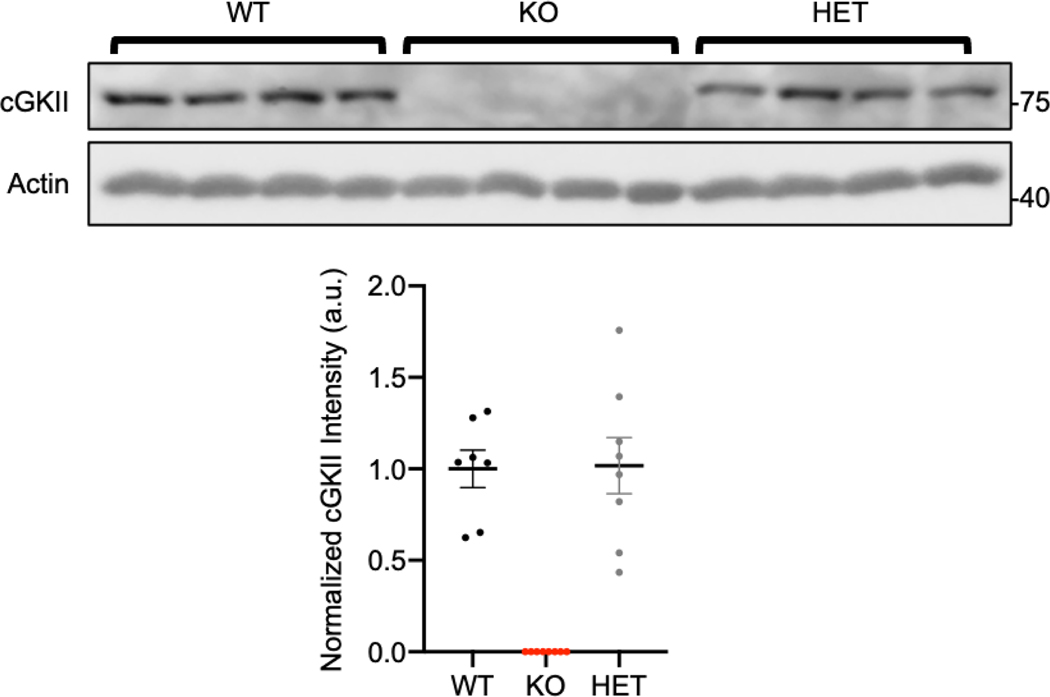
Many genetic disorders are caused by haploinsufficiency, in which having only one copy of the WT allele is not sufficient to produce the WT phenotype when the other allele is a loss-of-function mutation or deleted [41]. Interestingly, for all characteristics of USVs, such as number of calls, duration of each call, peak frequency, and peak amplitude, there were no significant differences between WT and HET pups (Fig. 2). We isolated brain tissues from each genotype and measured cGKII expression levels by immunoblots to determine whether cGKII expression in HET pups is comparable to that in WT pups (Fig. 3). As expected, cGKII KO animals had no cGKII expression, whereas there was no difference in cGKII levels between WT and HET brains (WT, 1 ± 0.10 a.u. and HET, 1.07 ± 0.15 a.u.) (Fig. 3). Therefore, one copy of the cGKII gene in mice is sufficient to produce cGKII protein, ultimately leading to normal USV production in HET pups.
Figure 3. cGKII protein expression levels in each genotype.
Representative immunoblots of cGKII protein expression in brain tissues from 4 animals in each genotype. Actin protein expression is used as a loading control. Normalized cGKII protein levels are shown in graph below. KO pups have no protein expression, while WT and HET pups have the same amount of protein present.
Reduction of neuronal activation in the cGKII KO arcuate nucleus following isolation
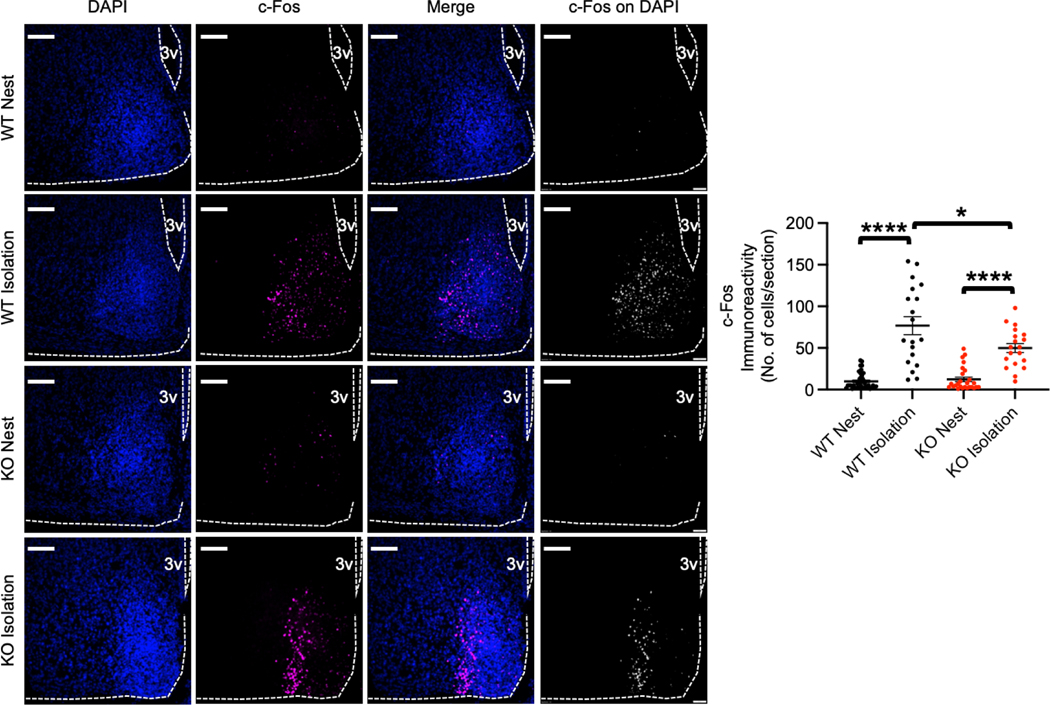
Given that the activity of AgRP neurons in the arcuate nucleus in the hypothalamus is important for USV emission in infant mice [35] and cGKII is able to regulate neuronal activity by controlling AMPAR trafficking [12–14], we hypothesized that loss of cGKII function can alter neuronal activation in the arcuate nucleus following isolation from the nest, which in turn affects USVs. We thus measured expression of the activity-regulated gene, c-Fos, as a neuronal activity marker [42, 43] to determine activation of arcuate nucleus neurons before or after isolation (Fig. 4). To quantify c-Fos immunoreactivity at the level of individual neurons, nuclei were labeled with DAPI, and we measured c-Fos signals on individual DAPI-positive cells in the arcuate nucleus. WT mice after a 90 min isolation exhibited significantly increased neuronal activity in the hypothalamus compared to WT mice in the nest (WT nest, 9.97 ± 1.56 cells and WT isolation, 76.79 ± 10.82 cells, p<0.0001) (Fig. 4), which is consistent with the previous report [35]. There was no difference in c-Fos immunoreactivity of WT and KO mice before the isolation (KO nest, 12.35 ± 2.76 cells) (Fig. 4). Interestingly, a 90 min isolation also significantly elevated c-Fos immunoreactivity in the arcuate nucleus of KO mice compared to KO nest pups (KO isolation, 49.95 ± 5.39 cells, p<0.0001) (Fig. 4). However, this increased neuronal activation in isolated KO animals was significantly lower than neuronal activation in WT pups following isolation (WT isolation, 76.79 ± 10.82 cells and KO isolation, 49.95 ± 5.39 cells, p=0.0352) (Fig. 4). These results suggest that the loss of cGKII reduces neuronal activity in the arcuate nucleus following isolation, and this decrease may underlie altered USVs in KO mice.
Figure 4. Reduction of neuronal activation in the arcuate nucleus in isolated cGKII KO pups.
Left panels: Representative c-Fos immunoreactivity (Magenta), DAPI (Blue), merge images, and c-Fos signals colocalized with DAPI images in each condition. The graph on the right shows that neuronal activation is significantly reduced in the KO arcuate nucleus following isolation from the nest (n= WT Nest; 39 sections from 4 animals, WT Isolation; 19 sections from 4 animals, KO Nest; 26 sections from 4 animal, and KO Isolation; 19 sections from 4 animals, *p<0.05 and ****p<0.0001, Kruskal-Walls test, Dunn test). A scale bar indicates 100μm. 3V, the third ventricle.
Discussion
4q21 microdeletion syndrome is a human genomic disorder, which is characterized by neonatal hypotonia, intellectual disability, absent or delayed speech, growth retardation, brain malformation and facial dysmorphism [1, 2]. Given that mice carrying a null mutation of the cGKII gene exhibit postnatal dwarfism and learning disability similar to that observed in human patients [6, 20, 21], it has been proposed that the PRKG2 gene is one of the key genes for the 4q21 microdeletion syndrome [1, 2]. Additionally, cGKII is abundantly expressed in the brain and regulates neuronal activity and cognitive function [12, 13, 20, 21]. However, a role of cGKII in speech has not been investigated in humans. Given that USVs in mice are used for communication and thus may be used as a model for speech [26], we employed cGKII KO mice and recorded USVs in neonatal mice and revealed abnormal vocalization patterns in mice lacking the cGKII gene, suggesting cGKII plays a critical role in the production of USVs. Together with the dwarfism and cognitive deficits exhibited by cGKII KO mice, this indicates that cGKII KO mice can serve as a valuable animal model to investigate pathophysiology of the 4q21 microdeletion syndrome.
A lack of cGKII expression in a specific area of the brain responsible to produce vocalization in fetal life could be a possible reason for USV changes in KO mice. In fact, isolation from the nest decreases body temperature in neonatal mice, which in turn activates neurons that express AgRP in the arcuate nucleus in the hypothalamus [35]. This activation is sufficient to produce USVs, so that the mother can retrieve isolated pups to the nest [29–34]. Consistently silencing these neurons sufficiently decreases USV production [35]. Thus, AgRP neuron activity is crucial for USV production in mice. Given that cGKII is important for neuronal activity, loss of cGKII function would be expected to lower the activity of AgRP neurons in the arcuate nucleus, which may underlie abnormal USVs in KO animals. Notably, the neuronal activity measured by c-Fos immunoreactivity in the arcuate nucleus of KO mice was not significantly different from WT pups in the nest. (Fig. 4). This suggests cGKII may not be important for neuronal activation under the basal condition. In contrast, we found c-Fos immunoreactivity in individual arcuate nucleus cells was significantly lower in KO pups compared with WT mice following isolation (Fig. 4), indicating decreased neuronal activation, which underlies reduced USV production in KO pups following isolation from the nest. Although USV production in mice can serve as a simple model for human speech [26], rodents lack complex language [26], therefore making it to study mechanisms underlying human oral language in animal models. Furthermore, there are significant differences in the neural circuits that control the production of innate vocalizations versus learned vocalizations such as human speech [44–46]. It is thus important to understand how the behavioral and neural circuits underlying mouse USVs can inform our understanding of speech and speech disorders in humans. However, the investigation of altered vocalizations in cGKII KO mice will likely provide information about the speech alterations in human 4q21 microdeletion syndrome since reports indicate that at least two individuals with the 4q21 microdeletion syndrome had decreased crying and a “weak cry” as infants [47], which is consistent with our findings in the mouse model. This suggests disruptions in both innate and learned vocalizations in the 4q21 microdeletion syndrome. Therefore, our findings in which loss of cGKII functions in mice contributes to alterations in USV production following isolation from the nest likely represent impairments in innate vocalization production. This further suggests that cGKII KO mice can be a valuable animal model to investigate the pathophysiology involved in human microdeletion 4q21 syndrome.
Highlights.
Chromosome 4q21 microdeletion leads to a human syndrome that exhibits restricted growth, mental retardation, and absent or delayed speech.
The cGMP-dependent protein kinase II (cGKII) gene is one of the genes located in the affected region of the chromosome.
cGKII knockout mice show restricted growth and deficits in learning and memory.
Altered ultrasonic vocalizations and decreased activation in arcuate nuclues neurons are found when infant cGKII knockout pups are isolated from the nest.
cGKII knockout mice can be a valuable animal model for human microdeletion 4q21 syndrome.
Acknowledgements
We thank members of the Kim laboratory for their generous support and Dr. Lon Kendall for use of the USV recording system. We also thank Dr. Ioana Carcea at Rutgers University for helping USV data analysis.
Funding
This work is supported by Student Experiential Learning Grants (LMS and SK), COVID-19 Teaching & Research Student Employment Initiative (JKS and MJD), and College Research Council Shared Research Program from Colorado State University (SK). This research was also supported by funds from NIH/NCATS Colorado CTSA Grant (UL1 TR002535) and the Boettcher Foundation’s Webb-Waring Biomedical Research Program (SK). MJD is a 2019 Boettcher Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonnet C, et al. , Microdeletion at chromosome 4q21 defines a new emerging syndrome with marked growth restriction, mental retardation and absent or severely delayed speech. J Med Genet, 2010. 47(6): p. 377–84. [DOI] [PubMed] [Google Scholar]
- 2.Hu X, et al. , Further defining the critical genes for the 4q21 microdeletion disorder. Am J Med Genet A, 2017. 173(1): p. 120–125. [DOI] [PubMed] [Google Scholar]
- 3.Scott JD, Cyclic nucleotide-dependent protein kinases. Pharmacol Ther, 1991. 50(1): p. 123–45. [DOI] [PubMed] [Google Scholar]
- 4.Schlossmann J, Feil R, and Hofmann F, Insights into cGMP signalling derived from cGMP kinase knockout mice. Front Biosci, 2005. 10: p. 1279–89. [DOI] [PubMed] [Google Scholar]
- 5.Vaandrager AB, Hogema BM, and de Jonge HR, Molecular properties and biological functions of cGMP-dependent protein kinase II. Front Biosci, 2005. 10: p. 2150–64. [DOI] [PubMed] [Google Scholar]
- 6.Pfeifer A, et al. , Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science, 1996. 274(5295): p. 2082–6. [DOI] [PubMed] [Google Scholar]
- 7.Vaandrager AB, et al. , cGMP stimulation of cystic fibrosis transmembrane conductance regulator Cl- channels co-expressed with cGMP-dependent protein kinase type II but not type Ibeta. J Biol Chem, 1997. 272(7): p. 4195–200. [DOI] [PubMed] [Google Scholar]
- 8.Vaandrager AB, et al. , Differential role of cyclic GMP-dependent protein kinase II in ion transport in murine small intestine and colon. Gastroenterology, 2000. 118(1): p. 108–14. [DOI] [PubMed] [Google Scholar]
- 9.Koltes JE, et al. , A nonsense mutation in cGMP-dependent type II protein kinase (PRKG2) causes dwarfism in American Angus cattle. Proc Natl Acad Sci U S A, 2009. 106(46): p. 19250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikuda H, et al. , Cyclic GMP-dependent protein kinase II is a molecular switch from proliferation to hypertrophic differentiation of chondrocytes. Genes Dev, 2004. 18(19): p. 2418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuchida A, et al. , Phenotypic characterization of the Komeda miniature rat Ishikawa, an animal model of dwarfism caused by a mutation in Prkg2. Comp Med, 2008. 58(6): p. 560–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Serulle Y, et al. , A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron, 2007. 56(4): p. 670–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, et al. , Network compensation of cyclic GMP-dependent protein kinase II knockout in the hippocampus by Ca2+-permeable AMPA receptors. Proc Natl Acad Sci U S A, 2015. 112(10): p. 3122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diering GH and Huganir RL, The AMPA Receptor Code of Synaptic Plasticity. Neuron, 2018. 100(2): p. 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche KW, et al. , Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron, 1996. 16(6): p. 1179–88. [DOI] [PubMed] [Google Scholar]
- 16.Derkach VA, et al. , Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nature reviews. Neuroscience, 2007. 8(2): p. 101–13. [DOI] [PubMed] [Google Scholar]
- 17.Malinow R. and Malenka RC, AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci, 2002. 25: p. 103–26. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd JD and Huganir RL, The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol, 2007. 23: p. 613–43. [DOI] [PubMed] [Google Scholar]
- 19.Zhuo M, et al. , Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature, 1994. 368(6472): p. 635–9. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, et al. , Brain region-specific effects of cGMP-dependent kinase II knockout on AMPA receptor trafficking and animal behavior. Learn Mem, 2016. 23(8): p. 435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wincott CM, et al. , Spatial memory deficits and motor coordination facilitation in cGMP-dependent protein kinase type II-deficient mice. Neurobiol Learn Mem, 2013. 99: p. 32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffy J, Motor speech disorders: substrates, differential diagnosis, and management. Fourth. ed. 2019, Maryland Heights: Elsevier. pages cm. [Google Scholar]
- 23.Hodgson JC and Hudson JM, Speech lateralization and motor control. Prog Brain Res, 2018. 238: p. 145–178. [DOI] [PubMed] [Google Scholar]
- 24.Moser D, et al. , Brain damage associated with apraxia of speech: evidence from case studies. Neurocase, 2016. 22(4): p. 346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portfors CV, Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci, 2007. 46(1): p. 28–34. [PubMed] [Google Scholar]
- 26.Fischer J. and Hammerschmidt K, Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav, 2011. 10(1): p. 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn ME and Lavooy MJ, A review of the methods of studies on infant ultrasound production and maternal retrieval in small rodents. Behav Genet, 2005. 35(1): p. 31–52. [DOI] [PubMed] [Google Scholar]
- 28.Branchi I, Santucci D, and Alleva E, Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res, 2001. 125(1–2): p. 49–56. [DOI] [PubMed] [Google Scholar]
- 29.Ferhat AT, et al. , Recording Mouse Ultrasonic Vocalizations to Evaluate Social Communication. J Vis Exp, 2016(112). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scattoni ML, Crawley J, and Ricceri L, Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev, 2009. 33(4): p. 508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noirot E, Ultra-sounds in young rodents. I. Changes with age in albino mice. Anim Behav, 1966. 14(4): p. 459–62. [DOI] [PubMed] [Google Scholar]
- 32.Sewell GD, Ultrasonic communication in rodents. Nature, 1970. 227(5256): p. 410. [DOI] [PubMed] [Google Scholar]
- 33.Zimprich A, et al. , Assessing Sociability, Social Memory, and Pup Retrieval in Mice. Curr Protoc Mouse Biol, 2017. 7(7): p. 287–305. [DOI] [PubMed] [Google Scholar]
- 34.Hofer MA, Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology, 1996. 21(2): p. 203–17. [DOI] [PubMed] [Google Scholar]
- 35.Zimmer MR, et al. , Functional Ontogeny of Hypothalamic Agrp Neurons in Neonatal Mouse Behaviors. Cell, 2019. 178(1): p. 44–59 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofer MA, Shair HN, and Brunelli SA, Ultrasonic vocalizations in rat and mouse pups. Curr Protoc Neurosci, 2002. Chapter 8: p. Unit 8 14. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, et al. , Sucrose withdrawal induces depression and anxiety-like behavior by Kir2.1 upregulation in the nucleus accumbens. Neuropharmacology, 2018. 130: p. 10–17. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Violette CJ, and Ziff EB, Reduction of increased calcineurin activity rescues impaired homeostatic synaptic plasticity in presenilin 1 M146V mutant. Neurobiol Aging, 2015. 36(12): p. 3239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shou J, et al. , Distinct Roles of GluA2-lacking AMPA Receptor Expression in Dopamine D1 or D2 Receptor Neurons in Animal Behavior. Neuroscience, 2018. 398: p. 102–112. [DOI] [PubMed] [Google Scholar]
- 40.Ehret G. and Haack B, Categorical perception of mouse pup ultrasound by lactating females. Naturwissenschaften, 1981. 68(4): p. 208–9. [DOI] [PubMed] [Google Scholar]
- 41.Johnson AF, Nguyen HT, and Veitia RA, Causes and effects of haploinsufficiency. Biol Rev Camb Philos Soc, 2019. 94(5): p. 1774–1785. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg ME and Ziff EB, Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature, 1984. 311(5985): p. 433–8. [DOI] [PubMed] [Google Scholar]
- 43.Bullitt E, Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol, 1990. 296(4): p. 517–30. [DOI] [PubMed] [Google Scholar]
- 44.Nieder A. and Mooney R, The neurobiology of innate, volitional and learned vocalizations in mammals and birds. Philos Trans R Soc Lond B Biol Sci, 2020. 375(1789): p. 20190054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tschida K, et al. , A Specialized Neural Circuit Gates Social Vocalizations in the Mouse. Neuron, 2019. 103(3): p. 459–472 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michael V, et al. , Circuit and synaptic organization of forebrain-to-midbrain pathways that promote and suppress vocalization. Elife, 2020. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dukes-Rimsky L, et al. , Microdeletion at 4q21.3 is associated with intellectual disability, dysmorphic facies, hypotonia, and short stature. Am J Med Genet A, 2011. 155A(9): p. 2146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]