Abstract
Background
There is growing evidence suggesting that air pollution may act as an important environmental risk factor in the development and aggravation of childhood atopic dermatitis (AD).
Methods
We collected data from the Taiwan National Health Insurance (NHI) research database and linked the data to the Taiwan Air Quality-Monitoring Database. From January 1, 2000 to December 31, 2012; children aged below 18 years were selected from the database and followed longitudinally until the diagnosis of AD, withdrawal from the NHI, or December 31, 2012. Children with missing data or those diagnosed with AD before enrolment in this study were excluded. We measured the incidence rate and hazard ratios (HRs) for AD and stratified them by quartiles (Q1–Q4) of air pollutant concentration. Multivariable Cox proportional hazards models were also applied by adjusting for age, sex, monthly income, and level of urbanization.
Results
When compared with the concentrations of pollutants in the Q1 quartile, the adjusted HR for AD increased with an increase in the exposure concentrations of total hydrocarbons (THCs), non-methane hydrocarbons (NMHCs), and methane (CH4) from 1.65 (95% confidence interval [CI]: 1.47–1.84) to 10.6 (95% CI: 5.85–7.07), from 1.14 (95% CI: 1.06–1.24) to 2.47 (95% CI: 2.29–2.66), and from 1.70 (95% CI: 1.52–1.89) to 11.9 (95% CI: 10.8–13.1), respectively. Patients exposed to higher levels of THCs, NMHCs, and CH4 exhibited greater incidence rates of childhood AD.
Conclusions
The present study demonstrated that exposure to higher concentrations of THCs, NMHCs, and CH4 were associated with an increased risk of childhood AD.
Keywords: Air pollution, Atopic dermatitis, Children, Cohort study, Hydrocarbons, Non-methane hydrocarbon, Total hydrocarbon, Methane, Environmental pollutants
Background
Atopic dermatitis (AD) is a common chronic relapsing inflammatory skin disease associated with intense itching and recurrent eczematous lesions [1]. Hanifin-Rajka major diagnostic criteria including pruritus, typical morphology, chronic or chronically relapsing dermatitis, and personal or family history of atopy have been used most frequently to diagnose AD in Taiwan [2]. In a process referred to as the “atopic march,” food allergy and AD are usually an early sign of other subsequent allergic disorders [3, 4]. Up to 80% of the children with AD eventually develop allergic rhinitis or asthma later in their childhood [5]. AD begins most commonly in the early childhood. Approximately 15–30% of the children and 10% of the adults are affected worldwide [6, 7]. In approximately 80% of the cases, childhood AD did not persist beyond 8 years of age and in less than 5% of the cases, it persisted into adulthood. Particularly, prolonged persistence of AD (more than 10 years) was associated with greater severity of the disease [8]. AD obviously influences patients’ quality of life and has financial implications. Itching and scratching are the two main symptoms that affect the quality of life in childhood AD. These symptoms affect the quality of sleep, thus requiring a treatment regime, affect the ability to participate in sporting activities, and result in social embarrassment [9]. The 2006 report from the American Academy of Dermatology, the most comprehensive contemporary research on the economic impact of AD, revealed that the total annual burden of AD was $4.228 billion. AD was associated with the fifth-highest overall treatment cost among all skin diseases in the US, placing a tremendous financial burden on the society [10]. Hence, it is critical to identify and control the risk factors in susceptible subjects for successful treatment and prevention of childhood AD.
Over the past 30 years, the worldwide prevalence of AD has increased considerably, particularly in industrialized countries [6]. Although both genetic and environmental factors are involved in the etiology of AD, the recent increase in the prevalence of is mainly attributed to environmental factors [11]. There is growing evidence suggesting that air pollution may act as an important environmental risk factor for the development and aggravation of childhood AD [12–15]. A variety of air pollutants such as particulate matter (PM); nitrogen oxide compounds (NOx); environmental tobacco smoke (ETS); traffic-related air pollution (TRAP) caused by pollutants such as PM, NO, NO2, SO2, CO, CO2, O3; and volatile organic compounds (VOCs) have been mentioned as risk factors for the development or aggravation of AD [11]. Skin barrier dysfunction is considered the initial step in the development of AD. The skin barrier plays pivotal roles in immune surveillance and homeostasis and in preventing the penetration of irritants and allergens [6, 16, 17]. Air pollutants may induce oxidative stress in the skin, leading to skin barrier dysfunction or immune dysregulation [18, 19]. TRAP, especially O3, has been observed to alter the resident skin flora and cause predisposition to S. aureus colonization [18]. Other dust particles and diesel exhaust particulates have also been demonstrated to exert toxicological effects on human skin [19]. Further research is needed to determine the mechanism behind the role of air pollutants in AD.
Although several studies support the development or aggravation of childhood AD due to air pollutants, current evidence regarding the skin aspects of air pollution remains relatively scarce in contrast to that regarding airway diseases such as asthma [20]. Moreover, previous studies have limitations such as inaccurate study design and assessment and the presence of confounding variables such as obesity, genetics, and comorbidities. For example, several studies have considered a mixture of substances including ETS, VOCs, and NOx, representing a combined impact on human health. Selection bias was also observed due to potential misclassification in some cross-sectional studies, since the diagnosis of AD was not confirmed by a physician and was based simply on reports from the patients or their parents [11].
In the present study, we focused on the association between hydrocarbons and the development of childhood AD. Total hydrocarbons (THCs), which are organic chemical compounds consisting of non-methane hydrocarbons (NMHCs) and methane (CH4), are responsible for approximately 85% of the global energy consumption due to rapid industrialization and urbanization. It is unclear whether air pollutants released during combustion of hydrocarbons, particularly CH4, affect the body’s largest organ, the skin. Hence, we conducted this nationwide retrospective study using real-world data in Taiwan to evaluate the effect of exposure to these air pollutants on the risk of AD in children.
Methods
Data source
We conducted a retrospective cohort study using the Children’s File, a representative database including data from half of all children randomly selected from the registry of beneficiaries of the Taiwan National Health Insurance (NHI) Research Database (NHIRD) for the year 2000. The NHIRD was established in 1995 and covers more than 99% of the total population in Taiwan [21]. It contains all medical records including de-identified demographic information such as sex, birth dates, occupation of the beneficiaries, and place of residence and clinical information such as diagnostic codes based on the International Classification of Disease, 9th Revision, Clinical Modification [ICD-9-CM] [22]; health management; and treatment. Since all the research data were anonymized and encrypted to protect the individuals’ privacy, the requirement for consent was waived for this study. The study was approved by the Institute Review Board of China Medical University Hospital (CRREC-103-048) and the study procedures were in accordance with the principles of the Declaration of Helsinki.
Study population, outcome of interest, endpoints, and confounding factors
From January 1, 2000 to December 31, 2012; We obtained data from children aged below 18 years. Candidates with missing data or those diagnosed with AD before enrolment in this study were excluded. The diagnosis of AD by a physician in Taiwan relies on clinical features listed in the Hanifin and Rajka diagnostic criteria and the American Academy of Dermatology Consensus Criteria [1, 2]. The AD diagnosis guideline established by the Taiwanese Dermatological Association committee (2015 and 2020) is also based on the aforementioned diagnostic criteria [1]. AD has chronic and relapsing characteristics. Hence, AD (the outcome of interest) was defined as at least three records of ICD-9-CM codes 691 (atopic dermatitis and related conditions) or 691.8 (other atopic dermatitis and related conditions) made by dermatologists or pediatricians in any diagnostic field during the inpatient or ambulatory claim process. All participants were followed up from baseline until the diagnosis of AD, withdrawal from the NHI, or December 31, 2012. The mean follow-up duration in AD patients was 6.50 years (standard deviation [SD]: 3.39 years). The confounding factors included age, sex, level of urbanization, and monthly income. The level of urbanization was defined based on the population density and was graded into four levels. Urbanization level was defined according to a National Health Research Institutes report [21]. City districts and townships where the subjects were registered for insurance purposes were grouped into seven urbanization levels based on population density (population/km2). Levels 1 and 7 referred to the most and the least urbanized areas, respectively. However, since very few patients were included in levels 5, 6, and 7; these three levels were combined with level 4. Monthly income was also classified into four groups: < 14,400 New Taiwan dollar (NT$); 14,400–18,300 NT$; 18,301–21,000 NT$; and ≥ 21,000 NT$.
Exposure measurement
The Taiwan Air Quality Monitoring Network was established by the Taiwan Environmental Protection Administration (TEPA) in 1993 [23, 24]. It comprises of 74 monitoring stations around the island. The monitoring stations are fully automated and record daily readings of THCs, NMHCs, and CH4 using ultraviolet fluorescence. Air pollution data were extracted from all monitoring stations and averaged on each day. The databases of these air pollutants were obtained from the Taiwan Air Quality-Monitoring Database (TAQMD), released by the TEPA. We linked the NHIRD and the TAQMD data according to the residential areas of candidates and the location of air quality-monitoring stations. A residential area was defined based on the location of the clinic and the hospital that treated acute nasopharyngitis (common cold) (ICD-9-CM code 460). Since acute nasopharyngitis is a common health problem, patients tend to visit the local clinic or other medical institution nearest to their residential areas. The average daily concentrations of air pollutants were calculated by dividing the cumulative daily air pollutant concentration by the duration from enrolment in this study to the endpoint for each candidate. Air pollutant concentrations were categorized into four groups based on quartiles: Q1, Q2, Q3, and Q4. THC concentrations were categorized into Q1 (< 2.29 ppm), Q2 (2.29–2.40 ppm), Q3 (2.40–2.60 ppm), and Q4 (> 2.60 ppm). NMHC concentrations were categorized into Q1 (< 0.27 ppm), Q2 (0.27–0.35 ppm), Q3 (0.35–0.51 ppm), and Q4 (> 0.51 ppm). CH4 concentrations were categorized into Q1 (< 2.01 ppm), Q2 (2.01–2.06 ppm), Q3 (2.06–2.11 ppm), and Q4 (> 2.11 ppm).
Statistical analysis
The demographic data in our study included age, sex, monthly income, level of residential urbanization, and daily average exposure to air pollutants. The chi-squared test was used to analyze the distributed difference among daily average concentrations for each air pollutant by quartile and urbanization. We used person-years as the denominator for estimating the incidence rate. The incidence rate of AD (per 1000 person-years) was calculated at four different air pollutant concentration levels. Cox proportional hazards regression models were applied to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for AD in Q2–Q4 levels of air pollutant concentrations when compared with the Q1 concentrations. The multivariable model was adjusted for age, sex, monthly income, and urbanization level. We also utilized the Kaplan–Meier method to estimate the cumulative incidence of AD during the follow-up. The log-rank test was used to analyze the difference among air pollutant concentration levels. All data analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and SPSS 15.1 (SPSS Inc., Chicago, IL, USA). The significance level was set at p < 0.05 in all statistical tests.
Results
Altogether, 7304 children (2.96%) were diagnosed with AD within the cohort of 246,844 children (between January 1, 2001 and December 31, 2012). The demographic data of the patients are presented in Table 1. The mean age of the patients was 6.50 years (SD: 3.39 years). The proportion of boys and girls was similar (51.6% vs. 48.4%). Most of the participants were from families belonging to the lowest monthly income category (83.4%) and resided in the most urbanized areas (33.2%).
Table 1.
The demographic information of study population
| N = 246,844 | n | % | |
|---|---|---|---|
| Gender | Boys | 126,256 | 51.6 |
| Age, years | mean, SD | 6.50 | 3.39 |
| ≦6 | 126,967 | 51.4 | |
| 7–12 | 101,653 | 41.2 | |
| > 12 | 18,224 | 7.38 | |
| Monthly income (NTD)a | < 15,000 | 205,871 | 83.4 |
| 15,000 − 19,999 | 30,871 | 12.5 | |
| ≥ 20,000 | 10,102 | 4.09 | |
| Urbanization levelb | 1 (highest) | 81,827 | 33.2 |
| 2 | 79,185 | 32.1 | |
| 3 | 47,013 | 19.1 | |
| 4 (lowest) | 38,819 | 15.7 | |
| Exposure | |||
| THC level (daily average) | mean, SD | 2.43 | 0.23 |
| NMHC level (daily average) | mean, SD | 0.40 | 0.17 |
| CH4 level (daily average) | mean, SD | 2.03 | 0.13 |
| Follow years | mean, SD | 10.6 | 3.02 |
| Outcome | |||
| Atopic dermatitis | 7304 | 2.96 | |
SD standard deviation
aMonthly income, New Taiwan Dollar (NTD), 1 NTD is equal to 0.03 USD
bUrbanization level: The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as the least urbanized. THC total hydrocarbons, NMHCs non-methane hydrocarbons, CH4 methane
We collected the data of participants under conditions of THC, NMHC, and CH4 exposure based on the location of the Taiwan Air Quality Monitoring station. Concentrations of each air pollutant were categorized by quartiles, ranging from Q1 (the lowest concentration) to Q4 (the highest concentration). Tables 2, 3 and 4 show the baseline characteristics of children exposed to four levels of THC, NMHC, and CH4 concentrations. Children with the highest THC, NMHC, and CH4 exposure concentrations lived in areas with higher urbanization.
Table 2.
Baseline characteristics of participants exposed to quartile (Q1-Q4) daily average concentrations of total hydrocarbons (THC)
| Variables | THC N = 246,844 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 N = 63,003 |
Q2 N = 60,660 |
Q3 N = 70,328 |
Q4 N = 52,853 |
p-value | ||||||
| n | % | n | % | N | % | n | % | |||
| Age | mean, SDa | 5.58 | 2.61 | 5.66 | 2.76 | 7.15 | 3.71 | 7.71 | 3.83 | < 0.001 |
| Boys | 32,841 | 52.1 | 31,400 | 51.8 | 36,293 | 51.6 | 26,722 | 50.6 | < 0.001 | |
| Monthly income (NTD)b | < 0.001 | |||||||||
| < 15,000 | 55,885 | 88.7 | 53,572 | 88.3 | 56,140 | 79.8 | 40,274 | 76.2 | ||
| 15,000 − 19,999 | 5794 | 9.20 | 5428 | 8.95 | 10,531 | 15.0 | 9118 | 17.3 | ||
| ≥ 20,000 | 1324 | 2.10 | 1660 | 2.74 | 3657 | 5.20 | 3461 | 6.55 | ||
| Urbanization levelc | < 0.001 | |||||||||
| 1 (highest) | 17,524 | 27.8 | 13,907 | 22.9 | 24,184 | 34.4 | 26,212 | 49.6 | ||
| 2 | 15,355 | 24.4 | 24,605 | 40.6 | 23,393 | 33.3 | 15,832 | 30.0 | ||
| 3 | 14,606 | 23.2 | 10,230 | 16.9 | 14,777 | 21.0 | 7400 | 14.0 | ||
| 4 (lowest) | 15,518 | 24.6 | 11,918 | 19.7 | 7974 | 11.3 | 3409 | 6.45 | ||
| Outcome | ||||||||||
| Atopic dermatitis | 504 | 0.80 | 788 | 1.30 | 2863 | 4.07 | 3149 | 5.96 | < 0.001 | |
Chi-square test; aOne-way ANOVA; SD standard deviation
bMonthly income: New Taiwan Dollar (NTD), 1 NTD is equal to 0.03 USD
cUrbanization level: The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as the least urbanized
Table 3.
Baseline characteristics of participants exposed to quartile (Q1-Q4) daily average concentrations of non-methane hydrocarbons (NMHC)
| Variables | NMHC N = 246,844 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 N = 55,312 |
Q2 N = 75,581 |
Q3 N = 54,687 |
Q4 N = 61,264 |
p-value | ||||||
| n | % | n | % | N | % | n | % | |||
| Age | mean, SDa | 6.12 | 3.18 | 6.04 | 3.09 | 7.02 | 3.52 | 6.97 | 3.67 | < 0.001 |
| Boys | 28,693 | 51.9 | 39,338 | 52.1 | 27,917 | 51.1 | 31,308 | 51.1 | < 0.001 | |
| Monthly income (NTD)b | < 0.001 | |||||||||
| < 15,000 | 47,529 | 85.9 | 64,814 | 85.8 | 44,185 | 80.8 | 49,343 | 80.5 | ||
| 15,000 − 19,999 | 5766 | 10.4 | 8467 | 11.2 | 7931 | 14.5 | 8707 | 14.2 | ||
| ≥ 20,000 | 2017 | 3.65 | 2300 | 3.04 | 2571 | 4.70 | 3214 | 5.25 | ||
| Urbanization levelc | < 0.001 | |||||||||
| 1 (highest) | 10,156 | 18.4 | 19,922 | 26.4 | 25,416 | 46.5 | 26,333 | 43.0 | ||
| 2 | 16,372 | 29.6 | 26,062 | 34.5 | 15,707 | 28.7 | 21,044 | 34.4 | ||
| 3 | 8878 | 16.1 | 19,178 | 25.4 | 9417 | 17.2 | 9540 | 15.6 | ||
| 4 (lowest) | 19,906 | 36.0 | 10,419 | 13.8 | 4147 | 7.58 | 4347 | 7.10 | ||
| Outcome | ||||||||||
| Atopic dermatitis | 1046 | 1.89 | 1692 | 2.24 | 1878 | 3.43 | 2688 | 4.39 | < 0.001 | |
Chi-square test; aOne-way ANOVA; SD standard deviation
bMonthly income: New Taiwan Dollar (NTD), 1 NTD is equal to 0.03 USD
cUrbanization level: The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as the least urbanized
Table 4.
Baseline characteristics of participants exposed to quartile (Q1-Q4) daily average concentrations of methane (CH4)
| Variables | CH4 N = 246,844 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 N = 57,832 |
Q2 N = 62,400 |
Q3 N = 64,035 |
Q4 N = 62,577 |
p-value | ||||||
| n | % | n | % | N | % | n | % | |||
| Age | mean, SDa | 5.72 | 2.61 | 5.68 | 2.84 | 6.30 | 3.19 | 8.25 | 4.03 | < 0.001 |
| Boys | 30,067 | 52.0 | 32,576 | 52.2 | 32,959 | 51.5 | 31,654 | 50.6 | < 0.001 | |
| Monthly income (NTD)b | < 0.001 | |||||||||
| < 15,000 | 50,495 | 87.3 | 55,406 | 88.8 | 54,061 | 84.4 | 45,909 | 73.4 | ||
| 15,000 − 19,999 | 6026 | 10.4 | 5210 | 8.35 | 7732 | 12.1 | 11,903 | 19.0 | ||
| ≥ 20,000 | 1311 | 2.27 | 1784 | 2.86 | 2242 | 3.50 | 4765 | 7.61 | ||
| Urbanization levelc | < 0.001 | |||||||||
| 1 (highest) | 17,455 | 30.2 | 19,681 | 31.5 | 23,868 | 37.3 | 20,823 | 33.3 | ||
| 2 | 14,939 | 25.8 | 22,318 | 35.8 | 22,219 | 34.7 | 19,709 | 31.5 | ||
| 3 | 14,376 | 24.9 | 11,388 | 18.3 | 10,443 | 16.3 | 10,806 | 17.3 | ||
| 4 (lowest) | 11,062 | 19.1 | 9013 | 14.4 | 7505 | 11.7 | 11,239 | 18.0 | ||
| Outcome | ||||||||||
| Atopic dermatitis | 482 | 0.83 | 921 | 1.48 | 1713 | 2.68 | 4188 | 6.69 | < 0.001 | |
Chi-square test; aOne-way ANOVA; SD standard deviation
bMonthly income: New Taiwan Dollar (NTD), 1 NTD is equal to 0.03 USD
cUrbanization level: The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as the least urbanized
Table 5 shows the increase in incidence rate of AD from 0.69 to 6.45, from 1.72 to 4.37, and from 0.73 to 7.74 per 1000 person-years with an increase in the THC, NMHC, and CH4 exposure concentrations, respectively. In the multivariable Cox proportional hazard regression, the adjusted HR for AD increased with an increase in the THC, NMHC, and CH4 exposure concentrations from 1.65 (95% CI: 1.47–1.84) to 10.6 (95% CI: 5.85–7.07), from 1.14 (95% CI: 1.06–1.24) to 2.47 (95% CI: 2.29–2.66), and from 1.70 (95% CI: 1.52–1.89) to 11.9 (95% CI: 10.8–13.1), respectively when compared with the corresponding exposure concentrations in the Q1 quartile (1.00) (Table 5).
Table 5.
Comparisons of differences in atopic dermatitis incidences and associated HRs in participants exposed to quartile (Q1-Q4) daily average concentrations of air pollutants
| Pollutant levels | Event | PY | IR | cHR | 95%CI | aHR | 95%CI | |
|---|---|---|---|---|---|---|---|---|
| THC | ||||||||
| Q1 | 63,003 | 504 | 729,958 | 0.69 | Ref. | Ref. | ||
| Q2 | 60,660 | 788 | 694,338 | 1.13 | 1.64 | (1.47, 1.83) | 1.65 | (1.47, 1.84) |
| Q3 | 70,328 | 2863 | 695,742 | 4.12 | 5.72 | (5.21, 6.29) | 6.43 | (5.85, 7.07) |
| Q4 | 52,853 | 3149 | 487,850 | 6.45 | 8.82 | (8.03, 9.69) | 10.6 | (9.60, 11.6) |
| NMHC | ||||||||
| Q1 | 55,312 | 1046 | 606,958 | 1.72 | Ref. | Ref. | ||
| Q2 | 75,581 | 1692 | 834,767 | 2.03 | 1.18 | (1.09, 1.27) | 1.14 | (1.06, 1.24) |
| Q3 | 54,687 | 1878 | 551,734 | 3.40 | 1.92 | (1.78, 2.07) | 1.93 | (1.79, 2.09) |
| Q4 | 61,264 | 2688 | 614,430 | 4.37 | 2.48 | (2.31, 2.66) | 2.47 | (2.29, 2.66) |
| CH4 | ||||||||
| Q1 | 57,832 | 482 | 664,004 | 0.73 | Ref. | Ref. | ||
| Q2 | 62,400 | 921 | 713,125 | 1.29 | 1.79 | (1.60, 1.99) | 1.70 | (1.52, 1.89) |
| Q3 | 64,035 | 1713 | 689,674 | 2.48 | 3.38 | (3.05, 3.73) | 3.32 | (3.00, 3.67) |
| Q4 | 62,577 | 4188 | 541,086 | 7.74 | 9.99 | (9.09, 11.0) | 11.9 | (10.8, 13.1) |
PY person-years
IR Incidence rate, (per 1000 person-years)
cHR crude hazard ratio
aHR adjusted hazard ratio of a multivariate analysis, after adjustment for age, sex, monthly income, and urbanization level
CI vconfidence interval
Ref. reference group
THC total hydrocarbons, NMHCs non-methane hydrocarbons, CH4 methane
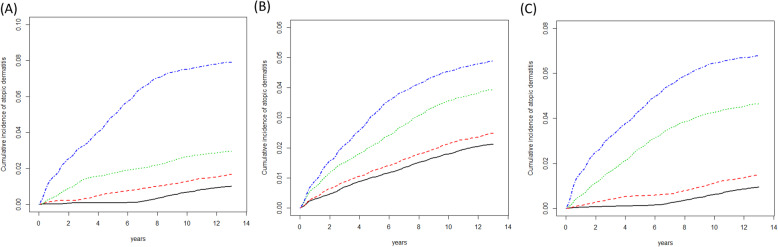
Figure 1 shows the Kaplan–Meier curves for the cumulative incidence separated by pollutant concentrations in each quartile (Q1, Q2, Q3, and Q4). During a follow-up of 12 years, the cumulative incidence rates of AD were lower among children living in areas with lower quartile concentrations of THCs, NMHCs, and CH4 than among those living in areas with higher quartile concentrations.
Fig. 1.
The cumulative incidence of atopic dermatitis in participants exposed to quartile daily average quartile concentrations of (A) total hydrocarbon (THC), (B) non-methane hydrocarbons (NMHC), and (C) methane (CH4). First quartile (solid line), first quartile to second quartile (dashed line), second quartile to third quartile (dotted line), ≧ third quartile concentration (dot dash line)
Discussion
In the present population-based longitudinal study, we demonstrated that Taiwanese children exposed to higher concentrations of THCs, NMHCs, and CH4 were at an increased risk of developing AD regardless of adjustment for potential confounding factors such as age, sex, monthly income, and urbanization level. Our cohort study also revealed a clear dose-response relationship between air pollution and AD. The present study is distinctive in several respects. We assessed the real-world data from the Children’s File. Children are more susceptible than adults to the effects of air pollution, as their lungs and immune systems are still developing, they breathe faster than adults, and their respiratory tracts are more permeable [25]. AD diagnosis in our study was confirmed by a physician, minimizing the potential for selection bias. Our study might be one of the first to investigate the relationship between AD and CH4, an active greenhouse gas, to identify the dermatological effect of a single component.
Taiwan is located in East Asia, the most polluted region in the world. Currently, it is facing severe air pollution, especially in major urban areas, due to rapid increase in population and industrial development as well as transportation demands. While the number of children with AD continues to increase in both developed and developing countries, the prevalence of AD in Taiwan appears to have grown dramatically over recent decades [26]. According to the Taiwan National Study 2000 to 2007, the overall 8-year prevalence of AD is approximately 6.7% [27]. Due to such rapid growth in the number of AD cases with increased urbanization and industrialization, the role of environmental factors, especially airborne pollutants, has garnered increasing attention. Over the past 10 years, a number of studies have shown that TRAP and air pollutants such as PM, VOCs, and ETS are associated with the development and exacerbation of AD. Multiple comprehensive studies have been conducted in pediatric age group with a large dataset. In a French study that enrolled 4907 children who had resided at their current addresses for 3 years or longer, lifetime AD was significantly associated with 3-year averaged concentrations of PM10, NO2, NOx, and CO (adjusted odds ratios [ORs]: 1.13, 1.23, 1.06, and 1.08, respectively) [28]. In a prospective birth cohort study from Munich that included 2860 children aged 4 years, NO2 exposure (per 6.4 mg/m3) was associated with both physician-diagnosed AD and parental reports of AD symptoms (ORs: 1.18 and 1.11, respectively) [29]. In a cross-sectional study from Shanghai during 2011–2012 that enrolled 3358 preschool children, a positive correlation was observed between increased gestational and lifetime exposure to a mixture of SO2, NO2, and PM10 and childhood AD (ORs: 1.78 and 1.87, respectively) [30]. In 91,642 children from the US National Survey of Children’s Health, moderate to severe eczema was associated with elevated levels of NO3 and PM2.5 (ORs: 1.249 and 1.070, respectively) [31]. A few studies also revealed that prenatal exposure to VOCs and ETS are likely to induce a Th2-dominant immune status or the development of AD after birth [32–34]. In the present study, we observed that the adjusted HRs for AD increased with an increase in the exposure concentration of THCs (from 1.65 to 10.6), NMHCs (from 1.14 to 2.47), and CH4 (from 1.70 to 11.9) when compared with exposure to the corresponding concentrations in the Q1 quartile.
Rapid industrialization coupled with urbanization has led to accumulated global waste production due to the continuously increasing demand for energy. Hydrocarbons, which are organic chemical compounds consisting of hydrogen and carbon, form the basis of the majority of global energy production via fossil fuel combustion and evaporation of gasoline. Both NMHCs and CH4 are composed of THCs. Most of the hydrocarbons on earth are naturally derived from decomposition of organic matter in petroleum and are generated by human activity. NMHCs, often referred to as VOCs, are unstable forms of substances such as benzene and their derivatives.
A great number of animal and epidemiological studies have reported negative effects of VOCs on skin barrier function. A prospective study in Korea revealed that an increase of 1 ppb in outdoor benzene and total VOC concentrations was associated with a 27.38 and 25.86% increase in AD symptoms, respectively [14]. Kim et al. observed that exposure to airborne formaldehyde leads to an increase in transepidermal water loss and stratum corneum pH in healthy subjects as well as in AD patients [15]. In a rat AD model, Han et al. showed that formaldehyde exposure aggravated pruritus and skin inflammation. These results suggest that formaldehyde penetrated the injured skin barrier and exacerbated Th1 responses and serum IgE levels in AD rats [35]. In several previous studies, certain VOCs and polycyclic aromatic hydrocarbons have been proposed to activate the ligand-activated transcription factor AhR, leading to downstream activation of inflammation and itch mediators such as artemin [36, 37]. Adverse health effects of direct exposure to CH4, a nontoxic greenhouse gas, have been scarcely reported except suffocation due to high concentrations. The present study is the first one to suggest that CH4 exposure contributes to an increased risk of AD development. Rapid industrialization and urbanization contribute to increased CH4 production. Increasing urbanization has been accompanied by a rise in larger cities with increasing population densities. Densely populated areas aggravate the spread of contagious infectious diseases. Emerging infectious diseases may worse skin inflammation caused by AD [38]. Further studies are needed to confirm this hypothesis.
Although our study was a large-scale population-based cohort study, it has several limitations. Although AD is a complex and multifactorial disorder, we did not consider other environmental factors such as temperature, humidity, and ultraviolet light that might interact with airborne pollutants [39]. Other potential risk factors for AD such as atopic family history, dietary factors, pet and prenatal exposure, and even the severity of AD could not be estimated in the present study due to the lack of information in the Children’s File. The reported prevalence of AD was 7.2% in a previous study wherein 11,874 students from 14 schools in central Taiwan completed the International Study of Asthma and Allergies in Childhood questionnaire [40]. However, the present study revealed a prevalence of 2.96% during the study period. This finding implies that AD might have been underdiagnosed in the present study, particularly in those with mild or infrequent symptoms. This disparity in findings might be explained by the following factors. We defined AD as at least three medical records of ICD-9-CM codes 691 or 691.8 due to the chronic and relapsing nature of AD and to avoid overdiagnosis. Thus, although this approach reduced the risk of false positives, it might have led to a low prevalence of AD diagnosis during the study period. Patients with mild AD may not seek medical services or be coded for the clinical diagnosis of AD by a physician. Moreover, the selection of another ICD-9-CM code for the diagnosis of AD by a physician may also lead to underestimation of AD prevalence. Furthermore, due to the inclusion characteristics of our database, children enrolled during the study period who became older than 18 years of age in 2012 were also taken into account. Thus, the exposure measurement might have been calculated partially after the age of 18. Exposure to indoor air pollution was not investigated in our study. Hence, our results might not represent the overall effect of air pollution on AD [41].
Conclusion
Our findings indicated that exposure to higher concentrations of THCs, NMHCs, and CH4 might lead to an increased risk of AD development. Further studies are needed to gain a better understanding of the role of air pollutants in the pathogenesis of AD.
Acknowledgments
Financial disclosure
The authors have indicated they have no financial relationships relevant to this article to disclose.
Data sharing statement
No additional data.
Abbreviations
- AD
Atopic dermatitis
- CH4
Methane
- CI
Confidence interval
- ETS
Environmental tobacco smoke
- HR
Hazard ratio
- ICD-9-CM
International Classification of Disease, 9th Revision, Clinical Modification
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
- NMHCs
Non-methane hydrocarbons
- NOx
Nitrogen oxide compounds
- NT$
New Taiwan dollar
- OR
Odds ratio
- PM
Particulate matter
- SD
Standard deviation
- TAQMD
Taiwan Air Quality-Monitoring Database
- TEPA
Taiwan Environmental Protection Administration
- THCs
Total hydrocarbons
- TRAP
Traffic-related air pollution
- VOC
Volatile organic compound
Authors’ contributions
Chang-Ching Wei conceptualized and designed the study. Chieh Wang and Jeng-Dau Tsai drafted the initial manuscript. Cheng-Li Lin carried out the acquisition of data and analysis and interpretation of data. Lei Wan and critically reviewed and revised the manuscript. Chang-Ching Wei and Jeng-Dau Tsai coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted.
Funding
This study is supported in part by Clinical Trial Center and Department of Chinese Medicine and Pharmacy, Ministry of Health and Welfare (MOHW109-TDU-B-212-114004), China Medical University Hospital (CRS-108-015, DMR-HHC-109-9, and DMR-HHC-110-7).
Availability of data and materials
Data available on request due to privacy/ethical restrictions.
Declarations
Ethics approval and consent to participate
The data were analyzed anonymously and informed consent is not applicable. This study has been approved by the Institute Review Board of China Medical University Hospital (CRREC-103-048) and complies with the principles outlined in the Helsinki Declaration.
Consent for publication
This manuscript is an original article that has not been previously published and will not be submitted to any other journal. All the authors have read this manuscript and agree that the work is ready for submission, and accept responsibility for the manuscript’s contents.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chieh Wang and Chang-Ching Wei contributed equally to this work.
References
- 1.Chan TC, Wu NL, Wong LS, Cho YT, Yang CY, Yu Y, Lai PJ, Chang YT, Shih IH, Lee CH. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120(1 Pt 2):429–442. doi: 10.1016/j.jfma.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Rudzki E, Samochocki Z, Rebandel P, Saciuk E, Gałecki W, Rączka A, Szmurło A. Frequency and significance of the major and minor features of Hanifin and Rajka among patients with atopic dermatitis. Dermatology. 1994;189(1):41–46. doi: 10.1159/000246781. [DOI] [PubMed] [Google Scholar]
- 3.Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(6 Suppl):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Mastrorilli C, Caffarelli C, Hoffmann-Sommergruber K. Food allergy and atopic dermatitis: prediction, progression, and prevention. Pediatr Allergy Immunol. 2017;28(8):831–840. doi: 10.1111/pai.12831. [DOI] [PubMed] [Google Scholar]
- 5.Eichenfield LF, Hanifin JM, Beck LA, Lemanske RF, Jr, Sampson HA, Weiss ST, Leung DY. Atopic dermatitis and asthma: parallels in the evolution of treatment. Pediatrics. 2003;111(3):608–616. doi: 10.1542/peds.111.3.608. [DOI] [PubMed] [Google Scholar]
- 6.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 7.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatology. 2014;150(6):593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JP, Chao LX, Simpson EL, Silverberg JI. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75(4):681–687. doi: 10.1016/j.jaad.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, Gould C, Gemmen E, Dall T. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134(5):993–999. doi: 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Lu C, Deng L, Ou C, Yuan H, Chen X, Deng Q. Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children. J Dermatol Sci. 2017;85(2):85–95. doi: 10.1016/j.jdermsci.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Deng Q, Lu C, Li Y, Sundell J, Dan N. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res. 2016;150:119–127. doi: 10.1016/j.envres.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Kim EH, Oh I, Jung K, Han Y, Cheong HK, Ahn K. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132(2):495–498. doi: 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Han Y, Ahn JH, Kim SW, Lee SI, Lee KH, Ahn K. Airborne formaldehyde causes skin barrier dysfunction in atopic dermatitis. Br J Dermatol. 2016;175(2):357–363. doi: 10.1111/bjd.14357. [DOI] [PubMed] [Google Scholar]
- 16.Hendricks AJ, Eichenfield LF, Shi VY. The impact of airborne pollution on atopic dermatitis: a literature review. Br J Dermatol. 2020;183(1):16–23. doi: 10.1111/bjd.18781. [DOI] [PubMed] [Google Scholar]
- 17.Hassoun Y, James C, Bernstein DI. The effects of air pollution on the development of atopic disease. Clin Rev Allergy Immunol. 2019;57(3):403–414. doi: 10.1007/s12016-019-08730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He QC, Tavakkol A, Wietecha K, Begum-Gafur R, Ansari SA, Polefka T. Effects of environmentally realistic levels of ozone on stratum corneum function. Int J Cosmet Sci. 2006;28(5):349–357. doi: 10.1111/j.1467-2494.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 19.Choi H, Shin DW, Kim W, Doh SJ, Lee SH, Noh M. Asian dust storm particles induce a broad toxicological transcriptional program in human epidermal keratinocytes. Toxicol Lett. 2011;200(1–2):92–99. doi: 10.1016/j.toxlet.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Araviiskaia E, Berardesca E, Bieber T, Gontijo G, Sanchez Viera M, Marrot L, Chuberre B, Dreno B. The impact of airborne pollution on skin. J Eur Acad Dermatol Venereol. 2019;33(8):1496–1505. doi: 10.1111/jdv.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Health Insurance Administration Ministry of Health and Welfare. http://www.nhi.gov.tw/English/. Accessed 31 Mar 2021.
- 22.Chang LC, Huang N, Chou YJ, Lee CH, Kao FY, Huang YT. Utilization patterns of Chinese medicine and Western medicine under the National Health Insurance Program in Taiwan, a population-based study from 1997 to 2003. BMC Health Serv Res. 2008;8(1):170. doi: 10.1186/1472-6963-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taiwan Air Quality Monitoring Network. Environmental Protection Administration Executive Yuan, R.O.C. (Taiwan). https://airtw.epa.gov.tw/ENG/default.aspx. Accessed 31 Mar 2021.
- 24.Environmental Resource Database. Environmental Protection Administration Executive Yuan, R.O.C. (Taiwan). https://www.epa.gov.tw/ENG/. Accessed 31 Mar 2021.
- 25.Salvi S. Health effects of ambient air pollution in children. Paediatr Respir Rev. 2007;8(4):275–280. doi: 10.1016/j.prrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Lee YL, Li CW, Sung FC, Yu HS, Sheu HM, Guo YL. Environmental factors, parental atopy and atopic eczema in primary-school children: a cross-sectional study in Taiwan. Br J Dermatol. 2007;157(6):1217–1224. doi: 10.1111/j.1365-2133.2007.08215.x. [DOI] [PubMed] [Google Scholar]
- 27.Hwang CY, Chen YJ, Lin MW, Chen TJ, Chu SY, Chen CC, Lee DD, Chang YT, Wang WJ, Liu HN. Prevalence of atopic dermatitis, allergic rhinitis and asthma in Taiwan: a national study 2000 to 2007. Acta Derm Venereol. 2010;90(6):589–594. doi: 10.2340/00015555-0963. [DOI] [PubMed] [Google Scholar]
- 28.Pénard-Morand C, Raherison C, Charpin D, Kopferschmitt C, Lavaud F, Caillaud D, Annesi-Maesano I. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur Respir J. 2010;36(1):33–40. doi: 10.1183/09031936.00116109. [DOI] [PubMed] [Google Scholar]
- 29.Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krämer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, Wichmann HE, Heinrich J, GINI Study Group. LISA Study Group Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177(12):1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- 30.Liu W, Cai J, Huang C, Hu Y, Fu Q, Zou Z, Sun C, Shen L, Wang X, Pan J, Huang Y, Chang J, Zhao Z, Sun Y, Sundell J. Associations of gestational and early life exposures to ambient air pollution with childhood atopic eczema in Shanghai, China. Sci Total Environ. 2016;572:34–42. doi: 10.1016/j.scitotenv.2016.07.197. [DOI] [PubMed] [Google Scholar]
- 31.Kathuria P, Silverberg JI. Association of pollution and climate with atopic eczema in US children. Pediatr Allergy Immunol. 2016;27(5):478–485. doi: 10.1111/pai.12543. [DOI] [PubMed] [Google Scholar]
- 32.Herberth G, Bauer M, Gasch M, Hinz D, Röder S, Olek S, Kohajda T, Rolle-Kampczyk U, von Bergen M, Sack U, Borte M, Lehmann I. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clin Immunol. 2014;133(2):543–550. doi: 10.1016/j.jaci.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 33.Hinz D, Bauer M, Röder S, Olek S, Huehn J, Sack U, Borte M, Simon JC, Lehmann I, Herberth G. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67(3):380–389. doi: 10.1111/j.1398-9995.2011.02767.x. [DOI] [PubMed] [Google Scholar]
- 34.Lehmann I, Thoelke A, Rehwagen M, Rolle-Kampczyk U, Schlink U, Schulz R, Borte M, Diez U, Herbarth O. The influence of maternal exposure to volatile organic compounds on the cytokine secretion profile of neonatal T cells. Environ Toxicol. 2002;17(3):203–210. doi: 10.1002/tox.10055. [DOI] [PubMed] [Google Scholar]
- 35.Han RT, Back SK, Lee H, Lee J, Kim HY, Kim HJ, Na HS. Formaldehyde-induced aggravation of pruritus and dermatitis is associated with the elevated expression of Th1 cytokines in a rat model of atopic dermatitis. PLoS One. 2016;11(12):e0168466. doi: 10.1371/journal.pone.0168466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancebo SE, Wang SQ. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol. 2015;29(12):2326–2332. doi: 10.1111/jdv.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidaka T, Ogawa E, Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Fujimura T, Aiba S, Nakayama K, Okuyama R, Yamamoto M. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017;18(1):64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- 38.Neiderud CJ. How urbanization affects the epidemiology of emerging infectious diseases. Infect Ecol Epidemiol. 2015;5(1):27060. doi: 10.3402/iee.v5.27060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patella V, Florio G, Palmieri M, Bousquet J, Tonacci A, Giuliano A, Gangemi S. Atopic dermatitis severity during exposure to air pollutants and weather changes with an artificial neural network (ANN) analysis. Pediatr Allergy Immunol. 2020;31(8):938–945. doi: 10.1111/pai.13314. [DOI] [PubMed] [Google Scholar]
- 40.Liao MF, Huang JL, Chiang LC, Wang FY, Chen CY. Prevalence of asthma, rhinitis, and eczema from ISAAC survey of schoolchildren in Central Taiwan. J Asthma. 2005;42(10):833–837. doi: 10.1080/02770900500369892. [DOI] [PubMed] [Google Scholar]
- 41.Breysse PN, Diette GB, Matsui EC, Butz AM, Hansel NN, McCormack MC. Indoor air pollution and asthma in children. Proc Am Thorac Soc. 2010;7(2):102–106. doi: 10.1513/pats.200908-083RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.