Abstract
The stress of Antenatal Maternal Hypoxia (AMH) can lead to a number of physiological and pathological changes in both mother and fetus, changes which can be linked to alterations in placental morphology and gene regulation. Recently, in the Brown Norway rat “model” of placental insufficiency, we reported alterations in placental renin-angiotensin system (RAS) genes. Moreover, AMH can lead to reduced oxygen availability to the fetus, similar to a state of placental insufficiency. Thus, in pregnant mice dams we tested the hypothesis that antenatal maternal hypoxic stress leads to alterations in the placental RAS. These alterations may, in part, account for the phenotypic changes in both pregnant mice dams as well as fetus and adult offspring.
Methods:
Pregnant FVB/NJ mice dams were either maintained as controls, or exposed to 10.5% O2 for 48 h from 15.5 to 17.5 day post coitum. We then measured placental mRNA and protein expression of several RAS genes (n = 4 to 5; P < 0.05 was considered significant).
Result:
In murine placenta: (1) angiotensinogen (AGT) mRNA was undetectable; however, AGT protein was detectable and increased significantly with AMH. (2) In AMH, although renin mRNA was reduced protein expression increased, in association with decreased microRNA (miRNA) 199b, which can lead to increased renin translation. (3) Also in AMH placenta, angiotensin converting enzyme (ACE) −1 mRNA was unaltered; however, protein expression increased significantly, in association with decreased miRNA 27a, which can result in increased ACE-1 translation. (4) In AMH placenta, ACE-2 mRNA was reduced significantly, whereas protein expression was significantly greater, in association with reduced miRNA 429. (5) In AMH placenta, angiotensin II type (AT) −1a receptor mRNA expression was unaltered while AT-1b receptor mRNA was undetectable in both groups. Moreover, AT-1 receptor protein expression was unchanged in response to AMH. (6) AT-2 receptor mRNA and proteins were undetectable in both groups.
Conclusion:
The normal murine placenta possesses several components of RAS, and in response to AMH several of these elements undergo important changes. In addition, differential expression of RAS mRNA, miRNA and protein, indicate post-transcriptional regulatory mechanisms involved with hypoxic stress, and necessitate further investigation.
Keywords: Developmental programming, Acclimatization, Pre-eclampsia, Angiotensin II, Vasoconstriction
1. Introduction
In several laboratory animal studies, antenatal maternal hypoxia (AMH) and the following placental blood flow dysregulation has been shown to be associated with a number of important problems. These include placental insufficiency [1], intrauterine growth restriction (IUGR) [2], a pre-eclampsia-like syndrome [3], altered fetal heart growth and vascular function [4], and in the adult permanent neurological deficits [5], pulmonary arterial dysfunction [6], and atherosclerosis [7]. Some of these conditions may be a direct consequence of relative tissue hypoxia/ischemia. Another factor playing an important role in the development of these disorders may be significant alterations in the placental gene expression as a consequence of AMH [8,9], which can lead to important changes in placental phenotype [10,11]. Of note, in a recent study in a Brown Norway Rat which has inherently inadequate placentation and insufficiency, we demonstrated significant changes in the expression of a number of genes, including those of the placental renin-angiotensin system (RAS) [12]. In other studies we have demonstrated that as a consequence of maternal stressors such as hypoxia or protein deprivation, both systemic as well as local RAS undergo important epigenetic and genetic alterations [13–15].
Over the past several decades, the renin-angiotensin system has received increased attention, and our understanding of this system has changed dramatically. Originally, RAS was regarded as a systemic cascade, in which the α2-globulin angiotensinogen (AGT) is produced constitutively, chiefly by the liver, and released into the circulation. A substrate for renin (secreted by kidneys), AGT is converted into the decapeptide angiotensin (Ang) I, and subsequently by angiotensin converting enzyme (ACE) −1 to the octapeptide Ang II. By the action of ACE-2, Ang II activity is terminated by its conversion to Ang 1–7. An important regulator of peripheral vascular resistance, Ang II also regulates sodium and water metabolism [16].
Importantly, the RAS exists not only as a systemic pathway, but also in its entirety in various tissues and organ systems which function independently [14,17]. Accumulating evidence from our and other laboratories indicates that the RAS is altered as a consequence of antenatal maternal stressors in number of different organ systems [13,14,18]. Of critical importance, a local RAS exists in the placenta, which interacts with other regulatory systems and modulates various aspects of tissue function. The uteroplacental RAS also has been reported to be important for endometrial regeneration, decidualization, implantation, and placentation [19,20]. In addition, the local placental RAS participates in the regulation of uteroplacental blood flow, prostaglandin synthesis, and estradiol-17-beta secretion [19,21]. Of importance, disturbances of placental RAS may lead to disorders such as IUGR and pre-eclampsia [19]. Moreover, evidence indicates that antenatal maternal hypoxia can lead to pre-eclampsia and IUGR as well as disorders in fetus [1–7], and the placental RAS may be involved in these disorders. It is not known, however, the extent to which maternal hypoxic stress can lead to alterations in the placental RAS. Of note, in human placentae from IUGR and pre-eclamptic pregnancy, alterations in the RAS are well documented [22–26]. Thus, in pregnant mice dams, we tested the hypothesis that antenatal maternal hypoxic stress leads to alterations in the placental RAS. These alterations may, in part, account for the phenotypic changes in both pregnant mice dams, as well as in the fetus and offspring as adults.
2. Methods
2.1. Experimental animal and tissues
All experimental procedures were approved by the Animal Care and Use Committee of Loma Linda University. At 16 weeks of age, we bred FVB/NJ mice by keeping the males and females together for 12 h (overnight). In the morning, we confirmed the mating by examination of vaginal plugs, and considered that 0.5 days post coitum (DPC). Pregnancy was confirmed by increase in the weight at 7 DPC. As previously described in Ref. [9], at 17.5 DPC the dams were sacrificed by cervical dislocation. The uterus was removed rapidly and placed in a Petri dish containing ice cold physiological buffered saline. Entire placentae were isolated under a dissection microscope and maternal decidua and endometrial tissue were removed [27]. The isolated and cleaned placentae were snap frozen in liquid nitrogen, and stored at −80 °C for later analysis. For each study, tissues obtained from the fetuses from one mother were used for either real-time PCR or western immunoblots assay, and considered n = 1. We determined the developmental stages of the embryos by visual inspection according to a modified Theiler staging system[28]. Details of the staging system are available online at http://genex.hgu.mrc.ac.uk/Databases/Anatomy/MAstaging.shtml.
2.2. Hypoxic exposure
As described previously in Ref. [9], at 15.5 DPC, the pregnant mice were placed in a custom sealed Plexiglas cage. A mixture of compressed air and nitrogen was infused, and O2 concentration was measured using an in-line oxygen meter (Oxychecq Expedition; Oxychecq, Fort Pierce, FL). Mice were maintained in an environment of 10.5% O2 (one-half atmosphere equivalent) for 48 h. To ensure a consistent hypoxic exposure, the O2 concentration in the cages was checked at hourly intervals during the day, and every 3 h at night.
2.3. mRNA and protein quantification
Western immunoblot assays and real-time PCR were conducted, as described and validated previously by our laboratory [13,29–30]. We isolated and quantified RNA and protein by Allprep DNA/RNA Mini Kit, according to the manufacturer’s instructions (Qiagen Inc, Valencia, CA Cat # 80204). To check for RNA quality and quantity, isolated mRNA was analyzed using a Beckman Spectrophotometer at 260/280 wavelength UV rays, and a 260/280 ratio of 1.8–2 was accepted for quantification with real-time PCR. The mRNA was treated with genomic DNA wipeout buffer, and then reverse transcribed using Quantitect cDNA synthesis kit (Qiagen, Inc). Real-time PCR was performed on Light Cycler 1.5 (Roche Inc., Indianapolis, IN) using hydrolysis Taqman probes and primers (Table 1), designed using the Universal Probe Library, a web based software (Roche Inc.), and the Quantifast real-time PCR kit. Cycles required (based on initial mRNA of a particular gene) to reach a threshold detection limit by the real-time PCR were recorded and normalized to a house-keeping gene (18 S ribosomal RNA). We have observed that these normalized cycle threshold values (ΔCT-values) are reliable and repeatable. Moreover, these values are an accurate representation of the initial mRNA amount of a particular gene, and are reported in results. After performing validation curves, we determined the fold changes with ΔΔCT method [28,31].
Table 1.
Primers used in the study.
| No. | Name | Gene Acc. No. | Primer/Probe | Sequence |
|---|---|---|---|---|
| 1 | Angiotensinogen | NM_007428.3 | Left primer | caacacctacgttcacttccaa |
| Right primer | cagacaccgagatgctgttg | |||
| Probe | ctggagtc | |||
| 2 | Renin | NM_031192.2 | Left primer | acggatcagggagagtcaaa |
| Right primer | cacagtgattccacccacag | |||
| Probe | ctcagcca | |||
| 3 | ACE-1 | NM_207624.4 | Left primer | ccctaggacctgccaatct |
| Right primer | tgctcatgttgcttagcagag | |||
| Probe | ggcagcag | |||
| 4 | ACE-2 | NM_027286.3 | Left primer | tgaaaaagtggtgggagatga |
| Right primer | aacagagatgcagggtcaca | |||
| Probe | tggtggag | |||
| 5 | AT-1a | NM_177322.3 | Left primer | actcacagcaaccctccaag |
| Right primer | ctcagacactgttcaaaatgcac | |||
| Probe | catcacca | |||
| 6 | AT-1b | NM_175086.3 | Left primer | cgccagcagcactgtaga |
| Right primer | ggagggggtgaattcaaaa | |||
| Probe | gggagcag | |||
| 7 | AT-2 | NM_007429.4 | Left primer | ggagctcggaactgaaagc |
| Right primer | ctgcagcaactccaaattctt | |||
| Probe | cttcagcc |
For western immunoblot experiments, frozen samples were homogenized in the 1× cell lysing buffer (Cell Signaling Technology, Beverly, MA) containing 1× phosphatase and protease inhibitors cocktail (Sigma, St. Loius, MO). Nuclei and debris were pelleted by centrifugation at 1000× g for 10 min. The supernatant was collected and stored at −80° C. SDS-gel and western blot were performed by use of appropriate antibodies (Table 2) [13,14,32]. All secondary antibodies were obtained from Abcam Inc. (Cambridge, MA). 20 μg protein from each sample was loaded on a SDS-gel and electrophoresed at 100V for 3h. Proteins were transferred to a nitro-cellulose membrane, and subjected to immunoblotting with antibodies (Table 2). Bands were detected with enhanced chemiluminescence using a ChemiImager (Alpha-Innotech, San Leandro, CA). The results are expressed as fraction of control. We performed control experiments with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), beta-tubulin, alpha-actin, and mitogen activated kinase (MAPK); our results demonstrated MAPK protein expression was unaltered with hypoxia in placental tissue. Moreover, MAPK integrated density (arbitrary unit) on densitometry analysis correlated well with the amount of protein loaded, and was used as an internal control to account for uniform loading.
Table 2.
Antibodies used in the study.
| No. | Name | Company | Cat. No. | Dilution |
|---|---|---|---|---|
| 1 | Angiotensinogen | Swant Inc. Switzerland | 138 | 1:1000 |
| 2 | Renin | Santa Cruz Biotechnologies | sc-27320 | 1:300 |
| 3 | ACE-1 | Abcam Inc. | ab39172 | 1:1000 |
| 4 | ACE-2 | Abcam Inc. | ab59351 | 1:1000 |
| 5 | AT-1 | Abcam Inc. | ab59018 | 1:250 |
| 6 | AT-2 | Abcam Inc. | ab19134 | 1:250 |
| 7 | GAPDH | Abcam Inc. | ab9485 | 1:2000 |
| 8 | Alpha-Actin | Abcam Inc. | Ab1801 | 1:1000 |
| 9 | ERK1/2 | Cell Signaling Technology | #9102 | 1:1000 |
| 10 | Beta-tubulin | Santa Cruz Biotechnology | sc-5274 | 1:1000 |
2.4. MicroRNA studies
MicroRNAs (miRNA) were identified for the 3′UTR of the RAS mRNAs, using the web based bio-informatics software e TargetScan 4.2 (http://www.targetscan.org). Of 170 miRNA suggested by the bio-informatics software, we choose 17 miRNA for the present study, by the use of the Context Score of more than 90th percentile, as described by Grimson et al. [33]. The Context Score was established using computational and experimental approaches and is based on five criteria: (1) AU-rich nucleotide composition near the site, (2) proximity to sites for coexpressed miRNAs (which leads to cooperative action), (3) proximity to residues pairing to miRNA nucleotides 13–16, (4) positioning within the 3′UTR at least 15 nucleotides from the stop codon, and (5) positioning away from the center of long UTRs. The identified miRNA levels were measured by the use of Real-Time Taqman microRNA PCR assays, according to manufacturer’s instructions (Applied Biosystems Inc. Carlsbad, CA).
2.5. Statistical analysis
To determine significant differences between groups, we analyzed the data using unpaired, two-tailed Student’s t-test and Chi-square test, by the use of GraphPad Prism software (GraphPad Software Inc., San Diego, CA). The hypothesis was accepted at P < 0.05. For each study, the fetuses from one mother were considered n = 1. For each experiment n was equal to four or more, as noted.
3. Results
3.1. Antenatal maternal hypoxia and placental angiotensinogen expression
Our results indicate that angiotensinogen protein is present in the murine placenta; however, with quantitative real-time PCR techniques, we were unable to detect AGT mRNA. In response to AMH, we observed a significant increase in angiotensinogen protein levels by western immunoblot (Fig. 1), the relative integrated density values (IDV; arbitrary units) being 0.60 ± 0.08, N = 5 and 0.93 ± 0.12, N = 4, (P < 0.05), for placentae from control and hypoxic dams, respectively. Thus, the experiments suggest that AGT is present in placenta, and AMH was associated with increased placental AGT protein concentration, however, it may be not expressed de novo in placenta.
Fig. 1.
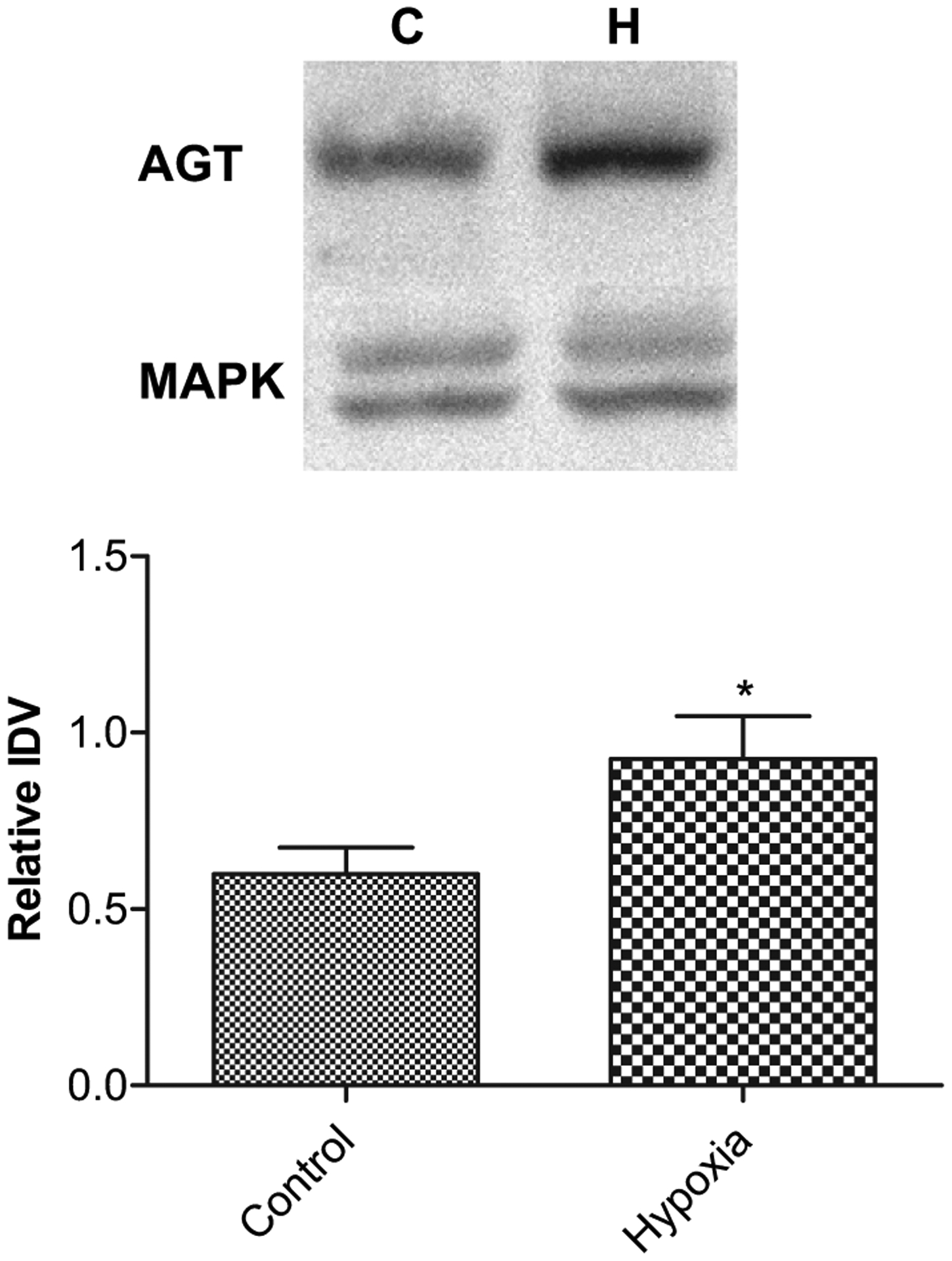
Acute antenatal hypoxia and angiotensinogen (AGT) protein expression in placenta. Top, representative Western immunoblot showing AGT expression with mitogen activated kinase (MAPK) as control. Bottom, bar graph demonstrating normalized relative integrated density value (IDV) of AGT protein expression in control and hypoxic placentae. *Denotes significant difference by an unpaired Student’s t-test (P < 0.05; n = 5).
3.2. Antenatal maternal hypoxia and placental renin expression
Our study also demonstrates that Renin is locally expressed in the placenta. Renin mRNA expression decreased ~4-fold in the fetus as a consequence of AMH (Table 3). In contrast, renin protein expression was similar in placenta from hypoxic dams, as compared to control, relative IDVs being 0.49 ± 0.08, N = 5 and 0.56 0.06, N = 4 for placentae from control and hypoxic dams, respectively (Data not shown). Despite reduced mRNA levels in hypoxic placentae, a normal protein expression may result from reduced expression of specific miRNA. Thus, with the use of miRNA complementary to the 3′UTR of renin mRNA, we observed ~2-fold decrease of miRNA mmu-mir-199b (P < 0.05) in placenta from hypoxic dams, as compared to control (Table 4). We observed no change in expression of miRNA mmu-mir-742 or mmu-mir-199a. These results suggest that renin is expressed locally in placenta, and as a consequence of AMH protein expression is unchanged despite reduced mRNA levels. These studies also suggest that miRNA mmu-mir-199b may be involved in post-transcriptional (translational) regulation of renin in response to AMH.
Table 3.
mRNA levels in control and hypoxic murine placentae.
| Gene | Control ΔCT | Hypoxic ΔCT | P-Value | Fold Change |
|---|---|---|---|---|
| Angiotensinogen | Not detectable | Not detectable | ||
| Renin | 16.05 ± 0.48 | 18.03 ± 0.56 | 0.03 | 3.96 (decrease) |
| ACE-1 | 12.84 ± 0.67 | 12.19 ± 0.54 | – | – |
| ACE-2 | 10.44 ± 1.21 | 13.98 ± 0.08 | 0.01 | 11.63 (decrease) |
| AT-1a | 15.44 ± 0.96 | 16.10 ± 0.13 | – | – |
| AT-1b | Not detectable | Not detectable | ||
| AT-2 | Not detectable | Not detectable |
Table 4.
miRNA levels in the control and hypoxic murine placentae.
| Regulating Gene | miRNA | ΔCt | Fold Change | P-Value | |
|---|---|---|---|---|---|
| Control | Hypoxic | ||||
| Renin | 742 | 15.89 ± 0.15, N = 5 | 15.71 ± 0.38, N = 5 | – | – |
| 199a | 14.08 ± 0.27, N = 5 | 14.62 ± 0.28, N = 5 | – | – | |
| 199b | 13.15 ± 0.36, N = 5 | 14.27 ± 0.23, N = 5 | −2.1 | 0.020 | |
| ACE-1 | 27a | 7.30 ± 0.39, N = 5 | 8.47 ± 0.11, N = 5 | −2.2 | 0.010 |
| 27b | 8.51 ± 0.40, N = 5 | 8.63 ± 0.59, N = 5 | |||
| ACE-2 | 200b | 8.22 ± 0.43, N = 5 | 8.60 ± 0.23, N = 5 | ||
| 200c | 9.47 ± 0.21, N = 5 | 9.47 ± 0.35, N = 5 | |||
| 429 | 12.91 ± 0.35, N = 5 | 14.31 ± 0.35, N = 5 | −2.0 | 0.02 | |
3.3. Antenatal maternal hypoxia and placental ACE-1 expression
ACE-1 protein also was expressed locally in the placenta, and in association with AMH it was up-regulated significantly (Fig. 2), despite no change in the mRNA expression (Table 3); the relative IDVs being 0.85 ± 0.15, N = 5 and 1.6 ± 0.28, N = 5, placentae from control and hypoxic dams, respectively. Moreover, with hypoxic stress we also observed ~2-fold decrease in miRNA mmu-mir-27a (P < 0.05) in placentae from hypoxic dams, as compared to control (Table 4). These results suggest miRNA-mediated post-transcriptional up-regulation of ACE-1, as a consequence of AMH.
Fig. 2.
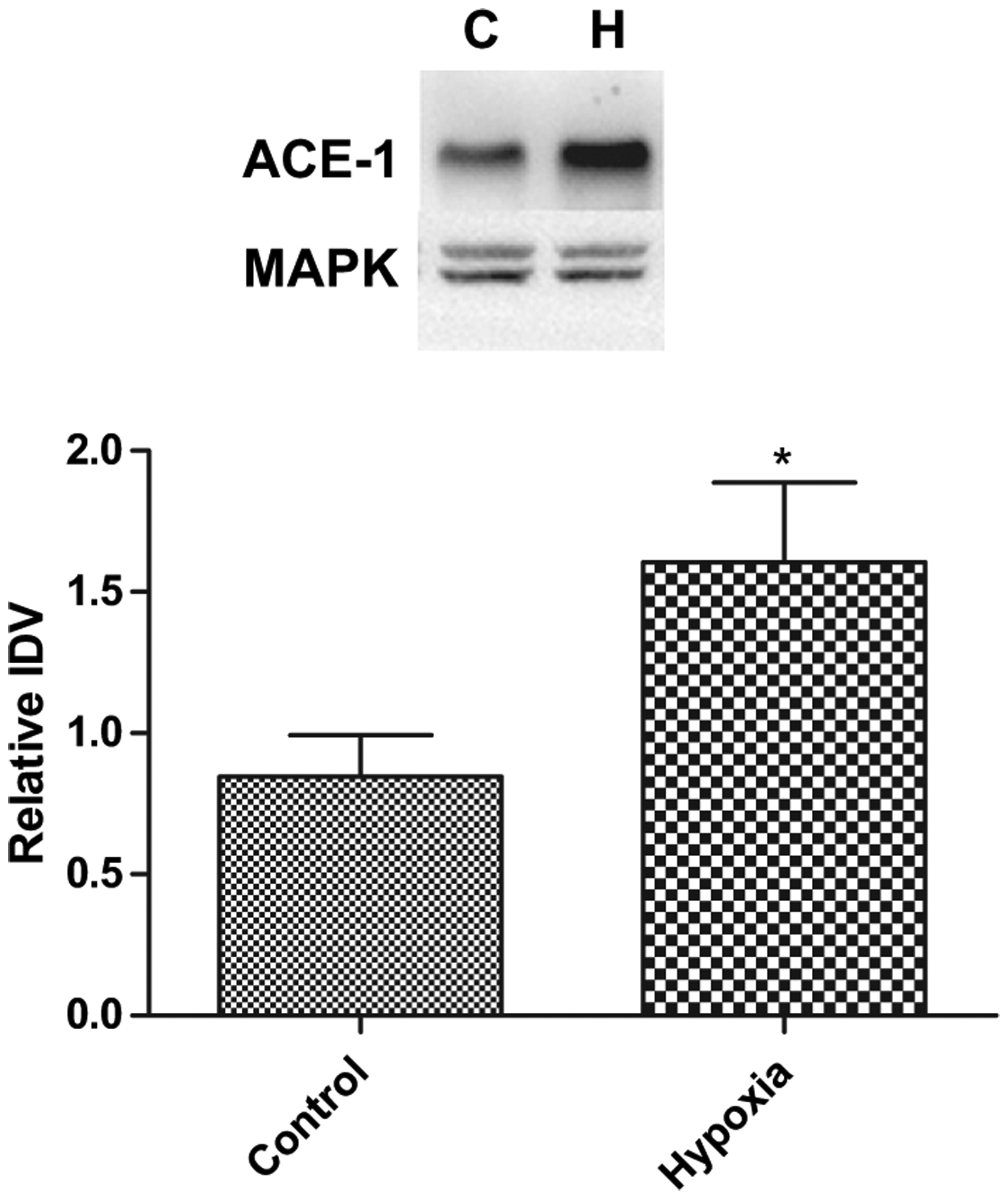
Acute antenatal hypoxia and expression of angiotensin converting enzyme-1 (ACE-1) protein in placenta. Top, representative Western immunoblot showing ACE-1 expression. Bottom, bar graph demonstrating normalized relative IDV for ACE-1 protein in control and hypoxic placenta. (Symbol same as in Fig. 1).
3.4. Antenatal maternal hypoxia and placental ACE-2 expression
As shown in Table 3 ACE-2 mRNA was reduced significantly (P < 0.05) in placenta from hypoxic dams. In contrast, AMH was associated with a marked increase in ACE-2 protein expression (P < 0.05), as compared to control, the relative IDVs being 0.08 ± 0.03, N = 4 and 0.24 ± 0.04, N = 4, respectively (Fig. 3). With hypoxic stress, we also observed a ~2-fold decrease in miRNA mmu-mir-429 (P < 0.05; Table 4), although no changes were observed in expression of miRNA mmu-mir-200b or mmu-mir-200c (Table 4). These experiments suggest up-regulation of ACE-2 production as a consequence of AMH.
Fig. 3.
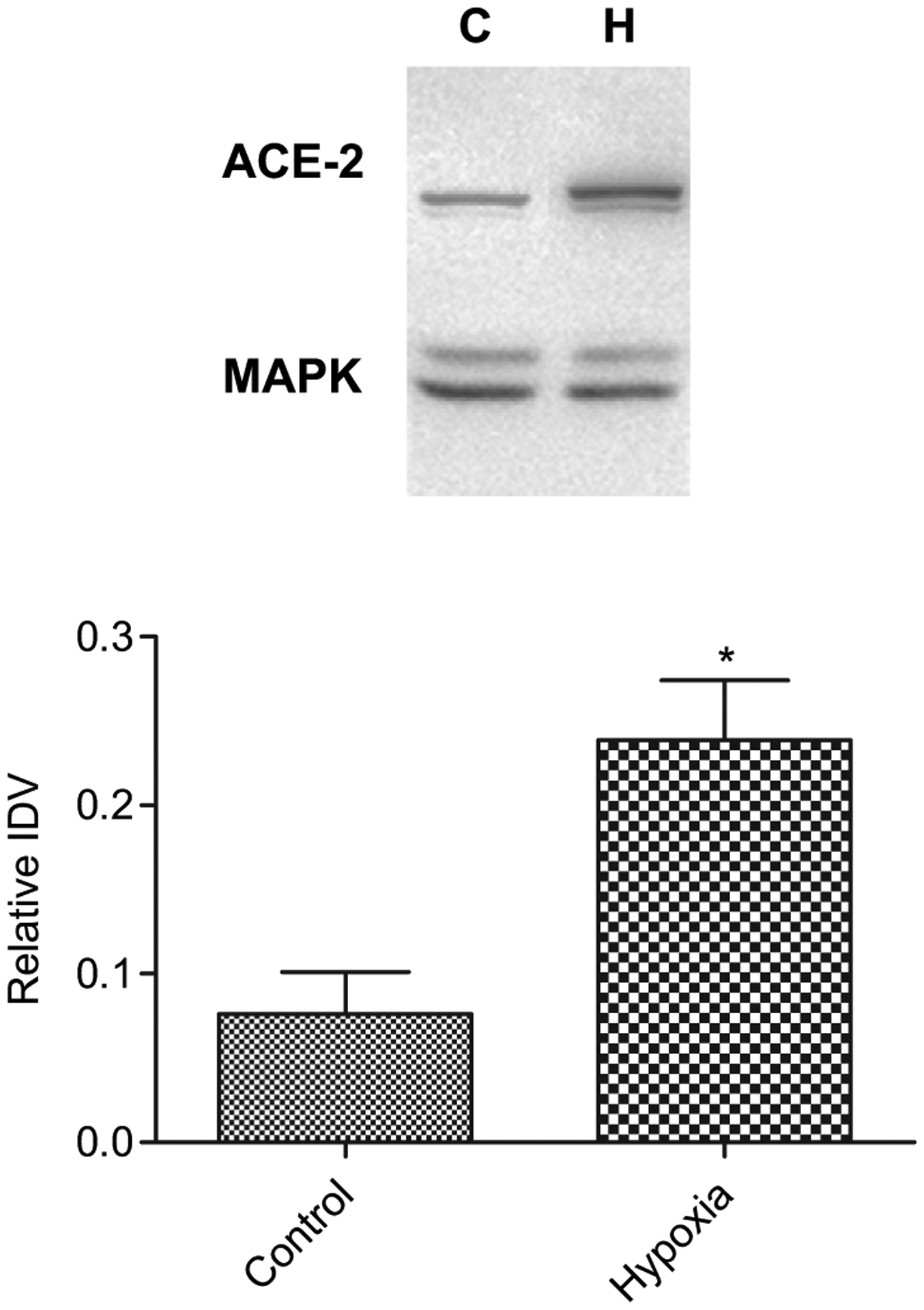
Acute antenatal hypoxia and expression of angiotensin converting enzyme-2 (ACE-2) protein in placenta. Top, representative Western immunoblot showing ACE-2 expression. Bottom, bar graph demonstrating normalized relative IDV for ACE-2 protein in control and hypoxic placenta. (Symbol same as in Fig. 1).
3.5. Antenatal maternal hypoxia and placental angiotensin II receptor expression
Two AT-1 receptors transcripts are known to exist in mice, AT-1a and AT-1b; but our results indicate the presence of only AT-1a transcripts in placenta. Furthermore, we were unable to detect AT-2 receptor mRNA or protein in the placenta from either group. Of note, there was no change in either AT-1a mRNA expression (Table 3) or protein in murine placenta as a consequence of AMH. The relative IDVs for protein expression were 15.44 ± 0.96 N = 4, and 16.10 ± 0.13, N = 4, respectively (Data not shown).
4. Discussion
Hemochorial placentation, which ensures intimate contact between maternal and embryonic blood compartments, is a feature of many mammalian species, including rodents and primates [34–36]. Moreover, we have shown that in response to in utero environmental changes, such as antenatal maternal hypoxia, placental blood flow and its distribution are altered significantly [37] with a more uniform blood flow during hypoxia, as compared to a relatively uneven distribution of cotyledonary maternal and fetal flow during normoxia [37]. In a related study, it was shown that pre-eclampsia is often associated with irregular placental blood supply with hypoperfusion and reperfusion events [38]. Because the renin-angiotensin system plays an important role in placental blood flow regulation, and alterations in the RAS are well documented in human placentae from IUGR and pre-eclamptic pregnancy [22–26], we speculate that the RAS may play an important role in this regard. For instance, several critical elements of the RAS, renin, ACE, and the AT1-receptor were up-regulated in the decidua of patients with pre-eclampsia, as compared to normal controls [24]. Other studies have demonstrated that maternal hypoxia can lead to significant placental vasoconstriction and insufficiency [1]. The molecular mechanisms, however, of hypoxia-induced placental insufficiency are unknown. In a previous study in the Brown Norway rat, we have demonstrated that placental insufficiency is associated with significant alterations of the placental RAS [12]. In addition, we also have shown significant alterations in the systemic and local RAS in association with maternal protein deprivation [13–15].
The present study is an extension of our previous work on maternal stress and its effect on placental gene regulation [12–14,29,39]. In the present report, we demonstrate that maternal hypoxia, which may result in a state similar to that of placental insufficiency [1], also leads to significant changes in the placental RAS. Among the novel findings, we demonstrate that AGT apparently is not produced locally in the murine placenta (mRNA is not detectable); however, AGT protein is present and increases in response to AMH. Previous studies support our finding that AGT can be sequestered into the placenta from the maternal circulation [40]. Both similar to, and in contrast to, our findings in the murine placenta, AGT mRNA has been shown to be both detectable [41] and undetectable [42] in other studies on human placenta. AMH -induced increased placental AGT protein levels, in turn, can lead to increased Ang II production and vasoconstriction [1,43–45]. AGT and renin overexpression have been shown to be associated with pre-eclampsia [46]. AMH also can lead to the development of pre-eclampsia, however, the molecular mechanisms are unknown [47].
As summarized in Fig. 4, we observed several changes that can produce vasoconstriction as a consequence of AMH. These may play an important role in the pathophysiology of pre-eclampsia. Nonetheless, we also observed compensatory changes in the placental RAS expression, which may tip the system towards reducing vasoconstriction. For instance, we observed increased ACE-2 protein expression indicating activation of a compensatory pathway to regulate expression. Another observation of the present study was differential expression of mRNA and protein of several components of the placental RAS as a consequence of AMH. This phenomenon is similar to that of other studies which report mRNA and protein expression being differentially regulated with antenatal maternal stressors [13,14], and other physiological/pathological conditions [48,49].
Fig. 4.
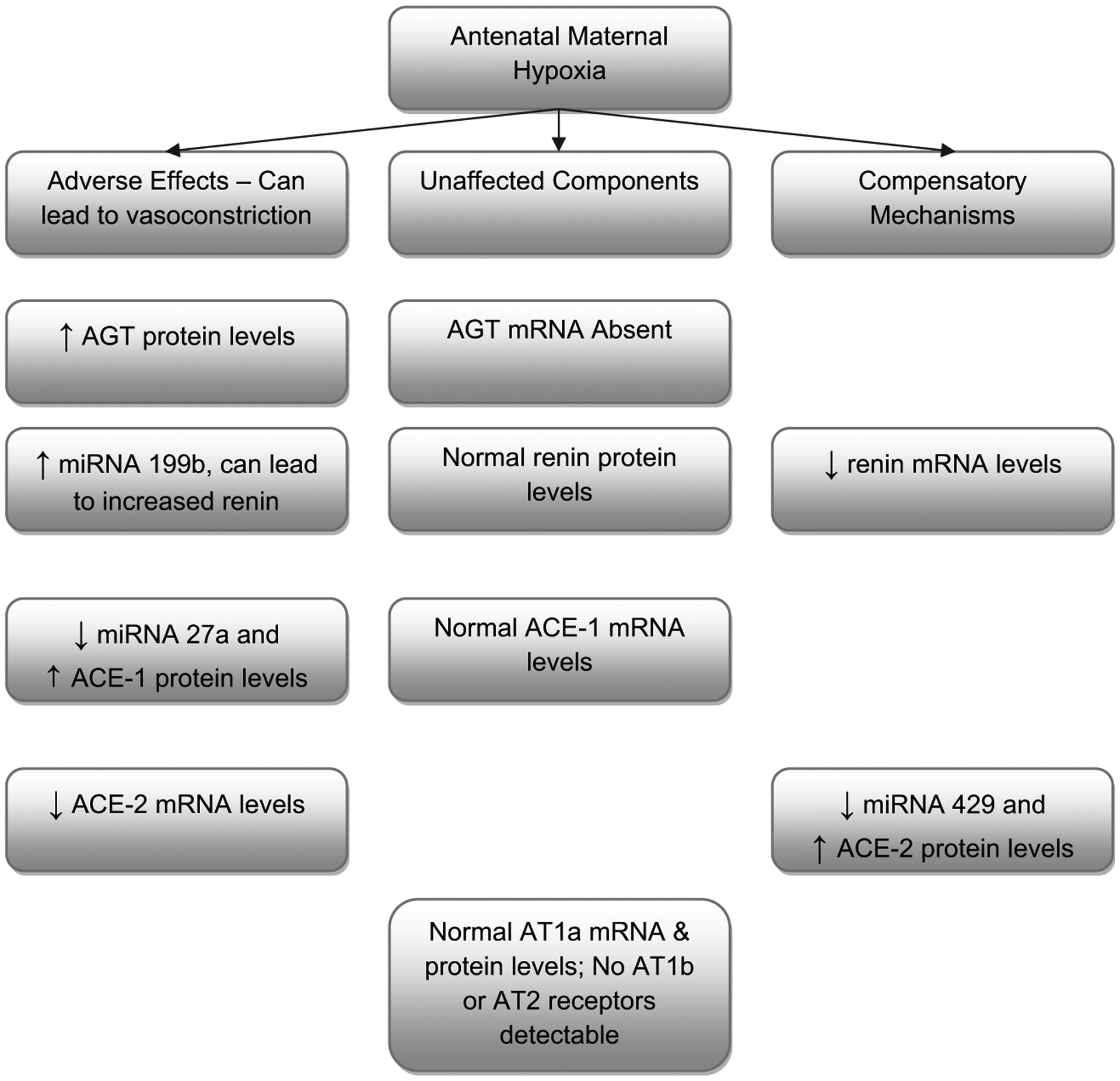
Summarized findings of the present study for mRNA, miRNA, and proteins in placenta in response to antenatal maternal hypoxic stress.
In an effort to understand more completely differential expression of mRNA and proteins, we examined several miRNA, as these have emerged as important players in post-transcriptional gene regulation. These miRNA which are subtypes of small, noncoding RNA, 21–25 nucleotides in length, are capable of base pairing with mRNA, and fine-tuning gene expression during development and differentiation, by suppressing their expression in sequence specific manner. Following the discovery of first miRNA “lin4” as a small temporal RNA [50], the number of members of this family has grown enormously, as have identification of their targets. Although miRNAs are similar to small interfering RNA (siRNA) in their generation pathway and molecular characteristics, unlike siRNA, miRNA does not degrade the target mRNA. Rather, they target the 3′untranslated regions of mRNAs, with which they share partial sequence complementarity, thereby silencing post-transcriptional gene translation. By this mechanism, the biological system may increase or decrease miRNA production, which up- or down-regulates gene expression according to the developmental need, thus, producing desired morphologic and physiological changes. Moreover, several placental miRNA (miR-141, miR-149, miR-229–5p, and miR135b) have been shown to be secreted in maternal plasma, the concentration of which decreases significantly after parturition [51]. This suggests that placental miRNA, in addition to regulating local gene expression, may be playing an important role in maternal conditions with obscure etiology, such as pre-eclampsia or related hypertensive disorders. Along this line, studies reveal differential expression of miRNA (miR-210 and miR-182) in placenta from patients with pre-eclampsia, and with small for gestational age newborn infants [52]. In the present study, we observed down regulation of miRNA 199b which can lead to increased renin translation, and down regulation of miRNA 27a and 429 which can lead to increased ACE-1 and ACE-2 protein levels. In summary, out of the multiple components or the RAS examined (AGT, Renin, ACE-1, ACE-2, AT-1 receptor, and AT-2 receptor), protein expression of AGT and ACE-1 were increased, which can lead to increased Ang II and vasoconstriction. In contrast, we also observed increased levels of ACE-2 protein which can lead to degradation of Ang II. Previous studies from our and other laboratories also document complex changes in transcriptional and post-transcriptional regulators in the RAS as a consequence of antenatal maternal stressors [14,18].
4.1. Perspective
During the past decade, the renin-angiotensin system has emerged as a major regulator of blood flow. Apart from the systemic renin-angiotensin system, the importance of a local (tissue) RAS in specific organs is being elucidated. In the present study, not only do we demonstrate the presence of several components of the renin-angiotensin system in murine placenta, but also show that this system changes significantly in response to maternal hypoxic stress. Of importance, we demonstrate that stressors such as hypoxia may not directly affect the gene expression, but may mediate its effect through post-transcriptional regulators such as miRNA. The present findings, in the mouse, of differential changes in placental mRNA and protein, with important changes in miRNA, suggest the need for further investigation in the human placenta, examining the role of miRNA in development of hypoxia-induced placental insufficiency and other pathologies.
Acknowledgement
We thank Brenda Kreutzer for assistance in the preparation of this manuscript.
References
- [1].Howard RB. Control of human placental blood flow. Med Hypotheses 1987;23:51–8. [DOI] [PubMed] [Google Scholar]
- [2].Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta 2007;28:714–23. [DOI] [PubMed] [Google Scholar]
- [3].Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 1996;97:540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol 2005;288: R360–7. [DOI] [PubMed] [Google Scholar]
- [5].Golan H, Kashtutsky I, Hallak M, Sorokin Y, Huleihel M. Maternal hypoxia during pregnancy delays the development of motor reflexes in newborn mice. Dev Neurosci 2004;26:24–9. [DOI] [PubMed] [Google Scholar]
- [6].Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Mitchell AA. Risk factors for persistent pulmonary hypertension of the newborn. Pediatrics 2007;120:e272–82. [DOI] [PubMed] [Google Scholar]
- [7].Wang Z, Huang Z, Lu G, Lin L, Ferrari M. Hypoxia during pregnancy in rats leads to early morphological changes of atherosclerosis in adult offspring. Am J Physiol Heart Circ Physiol 2009;296:H1321–8. [DOI] [PubMed] [Google Scholar]
- [8].Gheorghe C, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol 2010;54:507–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gheorghe C, Mohan S, Oberg KC, Longo LD. Gene expression patterns in the hypoxic murine placenta: a role in epigenesis? Reprod Sci 2007;14:223–33. [DOI] [PubMed] [Google Scholar]
- [10].Penninga L, Longo LD. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta 1998;19:187–93. [DOI] [PubMed] [Google Scholar]
- [11].Bacon BJ, Gilbert RD, Kaufmann P, Smith AD, Trevino FT, Longo LD. Placental anatomy and diffusing capacity in guinea pigs following long-term maternal hypoxia. Placenta 1984;5:475–87. [DOI] [PubMed] [Google Scholar]
- [12].Goyal R, Yellon SM, Longo LD, Mata-Greenwood E. Placental gene expression in a rat “model” of placental insufficiency. Placenta 2010;31:568–75. [DOI] [PubMed] [Google Scholar]
- [13].Goyal R, Galffy A, Field SA, Gheorghe CP, Mittal A, Longo LD. Maternal protein deprivation: changes in systemic renin-angiotensin system of the mouse fetus. Reprod Sci 2009;16:894–904. [DOI] [PubMed] [Google Scholar]
- [14].Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD. Brain renin-angiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod Sci 2010;17:227–38. [DOI] [PubMed] [Google Scholar]
- [15].Goyal R, Leitzke A, Goyal D, Gheorghe CP, Longo LD. Antenatal maternal hypoxic stress: epigenetic adaptations in fetal lung renin-angiotensin system. FASEB J 2010;24. 1023.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Burns KD, Li N. The role of angiotensin II-stimulated renal tubular transport in hypertension. Curr Hypertens Rep 2003;5:165–71. [DOI] [PubMed] [Google Scholar]
- [17].Valencia JC, Pacheco-Rodriguez G, Carmona AK, Xavier J, Bruneval P, Riemenschneider WK, et al. Tissue-specific renin-angiotensin system in pulmonary lymphangioleiomyomatosis. Am J Respir Cell Mol Biol 2006;35:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ Res 2007;100:520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nielsen AH, Schauser KH, Poulsen K. Current topic: the uteroplacental renin-angiotensin system. Placenta 2000;21:468–77. [DOI] [PubMed] [Google Scholar]
- [20].Squires PM, Kennedy TG. Evidence for a role for a uterine renin-angiotensin system in decidualization in rats. J Reprod Fertil 1992;95:791–802. [DOI] [PubMed] [Google Scholar]
- [21].Kalenga MK, De Gasparo M, Thomas K, De Hertogh R. Angiotensin-II stimulates estradiol secretion from human placental explants through AT1 receptor activation. J Clin Endocrinol Metab 1995;80:1233–7. [DOI] [PubMed] [Google Scholar]
- [22].Ito M, Itakura A, Ohno Y, Nomura M, Senga T, Nagasaka T, et al. Possible activation of the renin-angiotensin system in the feto-placental unit in preeclampsia. J Clin Endocrinol Metab 2002;87:1871–8. [DOI] [PubMed] [Google Scholar]
- [23].Laskowska M, Leszczynska-Gorzelak B, Laskowska K, Oleszczuk J. Evaluation of the renin-angiotensin-aldosterone system in pregnancy complicated by preeclampsia with and without intrauterine growth retardation. Ann Univ Mariae Curie Sklodowska Med 2004;59:451–6. [PubMed] [Google Scholar]
- [24].Herse F, Dechend R, Harsem NK, Wallukat G, Janke J, Qadri F, et al. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension 2007;49:604–11. [DOI] [PubMed] [Google Scholar]
- [25].Herse F, Staff AC, Hering L, Müller DN, Luft FC, Dechend R. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med 2008;86:697–703. [DOI] [PubMed] [Google Scholar]
- [26].Irani RA, Xia Y. The funcitonal role of the reinin-angiotensin system in pregnancy and preeclampsia. Placenta; 2008:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gheorghe C, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta 2009;30: 411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development 1993;118: 1255–66. [DOI] [PubMed] [Google Scholar]
- [29].Gheorghe CP, Mohan S, Oberg KC, Longo LD. Gene expression patterns in the hypoxic murine placenta: a role in epigenesis? Reprod Sci 2007;14:223–33. [DOI] [PubMed] [Google Scholar]
- [30].Gheorghe CP, Goyal R, Holweger JD, Longo LD. Placental gene expression responses to maternal protein restriction in the mouse. Placenta 2009;30:411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhao Y, Long W, Zhang L, Longo LD. Extracellular signal-regulated kinases and contractile responses in ovine adult and fetal cerebral arteries. J Physiol 2003;551:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 2007;27:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Davies J, Glasser SR. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel) 1968;69:542–608. [DOI] [PubMed] [Google Scholar]
- [35].Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta 2002;23:3–19. [DOI] [PubMed] [Google Scholar]
- [36].Enders AC, Welsh AO. Structural interactions of trophoblast and uterus during hemochorial placenta formation. J Exp Zool 1993;266:578–87. [DOI] [PubMed] [Google Scholar]
- [37].Power GG, Longo LD, Wagner N Jr, Kuhl DE, Forster II RE. Uneven distribution of maternal and fetal placental blood flow, as demonstrated using macroaggregates, and its response to hypoxia. J Clin Invest 1967;46:2053–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009;30:473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol 2010;54:507–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Skinner SL, Lumbers ER, Symonds EM. Analysis of changes in the renin-angiotensin system during pregnancy. Clin Sci 1972;42:479–88. [DOI] [PubMed] [Google Scholar]
- [41].Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension 1986;8:544–8. [DOI] [PubMed] [Google Scholar]
- [42].Ihara Y, Taii S, Mori T. Expression of renin and angiotensinogen genes in the human placental tissues. Endocrinol Jpn 1987;34:887–96. [DOI] [PubMed] [Google Scholar]
- [43].Hubloue I, Rondelet B, Kerbaul F, Biarent D, Milani G, Staroukine M, et al. Endogenous angiotensin II in the regulation of hypoxic pulmonary vasoconstriction in anaesthetized dogs. Crit Care 2004;8:R163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zakheim RM, Molteni A, Mattioli L, Park M. Plasma angiotensin II levels in hypoxic and hypovolemic stress in unanesthetized rabbits. J Appl Physiol 1976;41:462–5. [DOI] [PubMed] [Google Scholar]
- [45].Hoeldtke NJ, Napolitano PG, Moore KH, Calhoun BC, Hume JRF. Fetoplacental vascular tone during fetal circuit acidosis and acidosis with hypoxia in the ex vivo perfused human placental cotyledon. Am J Obstet Gynecol 1997;177: 1088–92. [DOI] [PubMed] [Google Scholar]
- [46].Falcao S, Stoyanova E, Cloutier G, Maurice RL, Gutkowska J, Lavoie JL. Mice overexpressing both human angiotensinogen and human renin as a model of superimposed preeclampsia on chronic hypertension. Hypertension 2009;54:1401–7. [DOI] [PubMed] [Google Scholar]
- [47].Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, et al. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 2005;90:4299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].de Waard V, Hansen HR, Spronk HH, Timmerman JJ, Pannekoek H, Florquin S, et al. Differential expression of tissue factor mRNA and protein expression in murine sepsis. The role of the granulocyte revisited. Thromb Haemost 2006; 95:348–53. [DOI] [PubMed] [Google Scholar]
- [49].Eizema K, van den Burg M, Kiri A, Dingboom EG, van Oudheusden H, Goldspink G, et al. Differential Expression of equine myosin heavy-chain mRNA and protein isoforms in a limb muscle. J Histochem Cytochem 2003;51: 1207–16. [DOI] [PubMed] [Google Scholar]
- [50].Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75: 843–54. [DOI] [PubMed] [Google Scholar]
- [51].Chim SS, Shing TK, Hung EC, Leung TY, Lau TK, Chiu RW, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem 2008;54:482–90. [DOI] [PubMed] [Google Scholar]
- [52].Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 2007;196:261–6. [DOI] [PubMed] [Google Scholar]


