By now, most are familiar with the graph of the distinctive arching breast cancer mortality curve from about 1975 (the advent of Surveillance, Epidemiology, and End Results reporting) and currently tracking 4 decades forward. The curve, peaking around 1990 and then beginning an impressive decline, marks the turning point in progress attributable to more comprehensive screening and genuinely effective treatment (1). When plotted separately for White and Black women (Figure 1), a strikingly different pattern emerges, with the peak for Black women being higher and occurring later (about 1995, despite lower breast cancer incidence than White women throughout this period), followed by a less pronounced descent resulting in a consistent mortality gap exceeding 9 deaths per 100 000 in 2010, much wider than the pre-1990 disparity. The gap persists to present, at best no longer widening and possibly converging slightly in more recent years, with an average mortality rate difference of 7.8 over 2013-2017 (1,2). The convergence is encouraging but slow and not guaranteed to continue.
Figure 1.
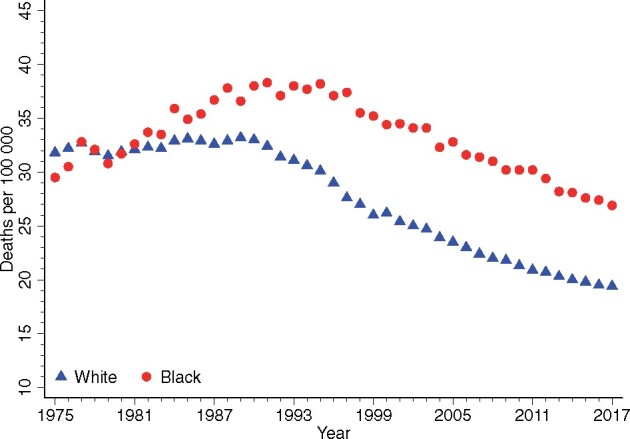
Age-adjusted annual breast cancer mortality for Black women and White women 1975-2017. Source: NCI Surveillance, Epidemiology, and End Results Program (1).
A multitude of investigations across diverse settings have explored potential causal factors from multiple domains, with disparities in clinical, biologic, care access and delivery, and health status all emerging as contributory. A consistent focus has been on greater frequency of unfavorable disease features, including more clinically advanced disease and absence of hormone receptors (later combined with HER2− negative status) among Black patients. In contrast, the study in this issue of the Journal by Albain et al. (3) among participants in the landmark TAILORx trial focuses on the historically better-prognosis patients, who have estrogen receptor (ER)+, HER2− tumors and negative lymph nodes. It is worth noting that despite proportional overrepresentation in higher risk groups by Black women, the ER+, HER− tumor type represents the majority diagnosis in all race groups (1,2), and here we perhaps expect more similar outcomes given the clear treatment rubric. Earlier (clinical trial–based) studies predating widespread HER2 testing found variable results among ER+ patients, with one study showing mostly similar breast cancer outcomes for Black women and White women (mostly lymph node–negative) and another finding a moderate disparity in trials of node-positive patients (4,5). The studies were statistically congruent for overall survival (OS) disparities (6). A subsequent study incorporating HER2 status showed less favorable outcomes for Black women specifically for ER+, HER2− disease (7).
The current investigation, like other contemporary studies among ER+, HER2− trial participants cited therein, does show a deficit in outcome for Black women compared with Whites (3). For the primary trial endpoint (invasive disease-free survival) and OS, at least a small degree of deficit is perhaps unsurprising, because invasive disease-free survival includes second primary cancers, for which incidence and survival disparities are present in Black women for several types, and both endpoints include noncancer deaths. There remains a substantial life expectancy gap that persists throughout life between Black and White women, equaling about 2.1 years among those reaching 50 years of age (8). Although the trial excluded those with profound comorbidities, some important health conditions did differ, including body mass index (likely a greater contributor to OS than breast cancer endpoints). Thus, the trial cohort appropriately reflects health disparities in the population at large, and, consequently, a degree of influence on endpoints such as OS may thus manifest within the trial, particularly over longer follow-up.
The differences in breast cancer–specific outcomes (relapse-free interval and distant disease-free interval) raise concern regarding both unmeasured tumor heterogeneity that influences treatment response potential among ER+, HER2− patients as well as the myriad of other factors, including treatment nonadherence, that could exert sufficient influence to diminish treatment efficacy even in a clinical trial setting. There were moderate differences in potential disease explanatory factors, but interestingly the 21-gene recurrence score was not among these, suggesting that to the extent that the score captures predicted risk based on tumor biology, both broadly (ie, the discrete risk partitions commonly employed) and on a continuous scale, it does so similarly across race and ethnic groups. The difference emerges relatively early for relapse-free interval, and it may be of interest to parse the distribution of anatomic failure sites to explore any difference in local disease control, a prior focus of interest in relation to race (9). As in the case of disease-free survival and OS, it is useful to examine events comprising the composite recurrence endpoint to glean all available information about sources of differential outcomes.
As the authors note, a reassuring finding is a lack of evidence of any differential treatment efficacy by race in the randomized cohort, consistent with the overall trial conclusion. In trials, the size of the non-White cohorts dictates that only large interaction effects would be reliably detected. However, demonstration of effects nominally in the same direction still provides important supporting evidence. Extrapolation of observed outcome disparities to incorrectly infer that modern appropriately targeted treatments fail to deliver any benefit would only serve to magnify disparities.
Although the findings represent material deficits on outcomes for Black women, it is nonetheless worthwhile to contrast these with patients at large, and more current population-based data allow us to do so, as data on tumor subtypes have become more widely available. A recent summary from combined Surveillance, Epidemiology, and End Results and National Program of Cancer Registries data of ER+, HER2– (all stages) cases diagnosed over years 2010-2015 showed 5-year breast cancer–specific survival of 93% for White women and 86% for Black women, not directly comparable but likely representing a considerably larger disparity relative to the current study (2). Earlier national (National Comprehensive Cancer Network Breast Cancer Outcomes Database, 2000-2007) or large regional population-based (Carolina Breast Cancer Study, 1993-2001; California Cancer Registry, 2005-2012) studies have similarly found the disparity seemingly greatest in this patient class after accounting for other factors (10–12). These studies focused on breast cancer–specific survival and even after adjustment for possible confounders from multiple domains tended to show larger disparities than this endpoint would yield in TAILORx. However, results specifically for lymph node–negative patients were not reported. That the largest disparity outside of trials may reside among those considered the best-prognosis patients may not be paradoxical from the perspective of systemic issues in thorough delivery of established and highly effective treatments. We might reasonably surmise that within trials, disparities would be lessened relative to those in the population, because many key confounding disease and treatment access factors are implicitly accounted for, but, as repeatedly seen, meaningful differences persist.
As the current pandemic experience once again illuminates how disparities in multiple aspects of health can magnify societal disease burden and impact, we find ourselves revisiting breast cancer survival disparities. To explain this unfortunately reproducible observation and find intervention points, 3 strategies may shed light, 2 of which have been called for by the authors. First, foster more diverse participation to permit detection of response variation reliably, and we might consider enriching enrollment of nonmajority populations to provide for more robust detection of any differential treatment response. Second, intensively study the tumor sample data in this cohort and others to discover novel biologic bases for variation in prognosis and treatment response among ER+ patients. For example, emerging concepts such as the role of androgen receptors may prove informative (13). Third, for future trials among these patients, incorporate the thoughtful collection of key personal and system-level determinants of health. As we strive to make trials less burdensome, little-used data are still collected while we lament the absence of key potentially explanatory factors from social domains. If we want to address the complex constellation of factors that contribute to outcome disparities, we need to prospectively measure these additional dimensions of the problem. All of these data together may inform how to further narrow and finally close the gap.
Funding
This work was supported in part by the National Cancer Institute, U.S. National Institutes of Health award U10 CA180822.
Notes
Role of the funder: The funder had no role in the writing of this editorial or decision to submit it for publication.
Disclosures: The author has no conflicts of interest to disclose.
References
- 1. DeSantis CE, Ma J, Gaudet MM, . et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. [DOI] [PubMed] [Google Scholar]
- 2. Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site. Accessed April, 2020.
- 3. Albain KS, Gray RJ, Makowar DF, et al. Race, ethnicity and clinical outcomes in hormone-receptor positive, HER2-negative node negative breast cancer in the randomized TAILORx Trial. J Natl Cancer Inst. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001;2001(30):36–43. [DOI] [PubMed] [Google Scholar]
- 5. Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dignam JJ. Re: Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2010;102(4):279–280. [DOI] [PubMed] [Google Scholar]
- 7. Sparano JA, Wang M, Zhao F, et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J Natl Cancer Inst. 2012;104(5):406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics, Centers for Disease Control and Prevention. National Vital Statistics System. Revised United States Life Tables, 2001-2011. https://www.cdc.gov/nchs/nvss/mortality/lewk3.htm. Published December 16, 2016. Accessed August 1, 2020. [PubMed]
- 9. Srokowski TP, Fang S, Duan Z, et al. Completion of adjuvant radiation therapy among women with breast cancer. Cancer. 2008;113(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warner ET, Tamimi RM, Hughes ME, et al. Racial and ethnic differences in breast cancer survival: mediating effect of tumor characteristics and sociodemographic and treatment factors. J Clin Oncol. 2015;33(20):2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carey L, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. [DOI] [PubMed] [Google Scholar]
- 12. Tao L, Gomez SL, Keegan THM, Kurian AW, Clarke CA. Breast cancer mortality in African-American and Non-Hispanic White women by molecular subtype and stage at diagnosis: a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cochrane DR, Bernales S, Jacobsen BM, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16(1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]


