Abstract
A Lactobacillus-dominated vaginal microbiota (VMB) has been associated with health and considered an important host defense mechanism against urogenital infections. Conversely, depletion of lactobacilli and increased microbial diversity, amplifies the risk of adverse gynecologic and obstetric outcomes. A common clinical condition that exemplifies dysbiosis is bacterial vaginosis (BV). BV is currently treated with antibiotics, but frequently recurs, due in part to persistent dysbiosis and failure of lactobacilli to repopulate the vagina. New treatment options are needed to address BV. The VMB is relatively simple and optimally dominated by one or several species of Lactobacillus. Lactobacillus crispatus is strongly associated with vaginal health and depleted in dysbiosis. Replenishing the dysbiotic VMB with protective L. crispatus CTV-05 is a promising approach to prevent recurrent infections and improve women’s health. Here we discuss confirmation of this approach with the microbiome-based biologic drug, LACTIN-V (L. crispatus CTV-05), focusing on prevention of BV recurrence.
Keywords: LACTIN-V, Lactobacillus crispatus CTV-05, bacterial vaginosis (BV), vaginal microbiota (VMB), live biotherapeutic product (LBP), women’s health
THE VAGINAL MICROBIOTA AND WOMEN’S HEALTH
The role of the vaginal microbiota (VMB) in the female reproductive tract health is well established [1–3]. Lactobacillus acidophilus was once considered the major vaginal species until the 1980s when molecular identification methods showed it to be a complex of multiple species. Subsequently, Lactobacillus crispatus, Lactobacillus gasseri, and Lactobacillus jensenii were identified as major species of the VMB [4, 5], and more recently Lactobacillus iners emerged as another prevalent species [6, 7], although its role in vaginal health is still under debate [8]. Newer culture-independent techniques, using DNA sequencing techniques, revealed the same 4 Lactobacillus species dominating separate bacterial community state types (CSTs), as well as a heterogeneous CST that is not dominated by Lactobacillus [6, 7].
Although every woman harbors a unique bacterial community, the VMB is optimally dominated by H2O2-producing lactobacilli, which create a low-pH [9], noninflammatory environment [3]. Lactic acid produced by lactobacilli acidifies the vagina and suppresses the growth of many opportunistic pathogens [10, 11]. L. crispatus is the most prevalent H2O2-producing Lactobacillus species of the female reproductive tract and L. crispatus-dominated bacterial communities exhibit the lowest vaginal pH, lowest proinflammatory cytokine levels, and lowest risk of gynecologic and obstetric complications [10–14].
VAGINAL DYSBIOSIS
Bacterial vaginosis (BV) is a common ecological disorder of the VMB characterized by increased microbial diversity with expansion of mainly anaerobic bacteria and loss of H2O2-producing lactobacilli [15, 16]. BV affects 15%–50% of reproductive-aged women globally and can recur in 20%–75% within 3 months following standard antibiotic treatment [17, 18]. Dysbiosis can be associated with increased levels of proinflammatory cytokines [19] and increased numbers of activated CD4+ T lymphocytes [20]. Cervicovaginal bacterial communities are major modulators of the host inflammatory response [11]. Several negative sequelae accompany proinflammatory dysbiosis, such as increased risk of sexually transmitted infections (STIs) [21, 22], including human immunodeficiency virus (HIV) [23], pelvic inflammatory disease [24], preterm birth [25], and enhanced progression of cervical cancer human papillomavirus (HPV) [26].
BV is currently treated with antibiotics (ie, metronidazole) [27]. Metronidazole kills the BV-associated anaerobic bacteria while sparing vaginal lactobacilli, which are intrinsically resistant to nitroimidazoles. However, metronidazole treatment alone does not restore a Lactobacillus-dominated microbiome and dysbiosis can persist.
FROM PROBIOTICS TO LIVE BIOTHERAPEUTIC PRODUCTS
Probiotics have rapidly grown into a multibillion-dollar industry that is lightly regulated as food or dietary supplements in the United States [28]. This has led to a call for stricter requirements for scientific substantiation of putative health benefits conferred by microorganisms [29]. Although most probiotics are for gastrointestinal use, several are marketed for vaginal health. However, many of these products contain species that are not naturally present in the VMB. It is not clear whether these products can sustainably colonize or benefit the vaginal ecosystem because vaginal strains differ from those found in food or the gastrointestinal tract. A number of recent meta-analyses have been published on probiotics to treat/prevent BV [30–32], and while the products were generally safe, there was no clear or consistent indication that commercially available probiotics improve outcomes related to women’s health. Because probiotic products are not regulated as drugs in the United States, they cannot make specific health claims.
Fueled by the Human Microbiome Project [33], the roles of the microbiome in health have become better appreciated and spurred the development of microbiome-based products intended to treat or prevent disease. This activity prompted the Center for Biologics Evaluation and Research at the Food and Drug Administration (FDA), to respond with a draft guidance, document in 2012, addressing the early development of live biotherapeutic products (LBPs), thus establishing a new class of biologic drugs [34]. LBP was defined as a biological product that: (1) contains live microorganisms, such as bacteria; (2) is applicable to the prevention, treatment, or cure of a disease or condition of human beings; and (3) is not a vaccine.
LACTIN-V: THE FIRST VMB-BASED LBP
L. crispatus has long been associated with reproductive health and has strong inverse relationships with vaginal dysbiosis and its clinical sequelae [10, 11]. For example, depletion of vaginal Lactobacillus, particularly H2O2-producing strains such as L. crispatus, has been associated with both BV and recurrent urinary tract infections (rUTI) [35, 36]. For these reasons, a strain of L. crispatus was carefully selected as the active ingredient of LACTIN-V, which became the first VMB-based LBP. LACTIN-V is being developed under an Investigational New Drug application with the FDA as an adjuvant therapy to prevent recurrence of BV and rUTI following antimicrobial treatment.
LACTIN-V contains L. crispatus CTV-05 (CTV-05), a specific strain isolated from the vagina of a healthy woman [5, 37]. L. crispatus is found naturally in the vagina of many healthy women and has also been detected in the rectum [38–40]. CTV-05 is a homofermenter of glucose to lactic acid (both d and l isomers), and an H2O2 producer. Unlike most commercially available probiotic Lactobacillus strains, which are not vaginal strains, CTV-05 adheres to vaginal epithelial cells and is capable of colonizing the vagina [37, 41, 42]. CTV-05 has an antibiotic-susceptibility profile similar to other L. crispatus strains and is intrinsically resistant to metronidazole. In addition, CTV-05 antagonizes a number of urogenital pathogens in vitro (Table 1). The strain has an excellent preclinical and clinical safety record. There have been no reports of L. crispatus causing bacteremia or endocarditis, as noted with some probiotic lactobacilli [45].
Table 1.
Inhibition of Vaginal and Urinary Pathogens by CTV-05
| Microorganism | Strain No. | Results: Zone of Inhibition, mma |
|---|---|---|
| Vaginal pathogens | ||
| N. gonorrhoeae | F6b | 61 (S) |
| N. gonorrhoeae | SPD 600b | 62.5 (S) |
| N. gonorrhoeae | 7603389b | 55 (S) |
| N. gonorrhoeae | 87016589b | 55 (S) |
| N. gonorrhoeae | 85044571b | 65 (S) |
| B. fragilis | 25285c | 32.5 (S) |
| B. fragilis | 43860c | 42.5 (S) |
| B. fragilis | 43858c | 70 (S) |
| S. agalactiae group B | 13813c | 57.5 (S) |
| S. pyogenes | Clinical isolated | Complete inhibition |
| G. vaginalis | ATCC 14018 | Complete inhibition |
| G. vaginalis | Clinical isolate 9d | Complete inhibition |
| G. vaginalis | Clinical isolate 10d | Complete inhibition |
| G. vaginalis | Clinical isolate 11d | Complete inhibition |
| Dialister sp. | Clinical isolated | Partial inhibition |
| Urinary tract pathogens | ||
| E. coli | 3052-961 | Complete inhibition |
| E. coli | 3100-961 | 63 (S) |
| E. coli | 3171-961 | 70 (S) |
| E. coli | 3196-961 | Complete inhibition |
| E. coli | 3265-961 | 63 (S) |
| E. coli | 3301-971 | Complete inhibition |
| E. coli | 3077-971 | 63 (S) |
| E. coli | 3058-981 | 67.5 (S) |
| E. coli | 3163-981 | 60 (S) |
| E. coli | 3201-981 | 67.5 (S) |
| E. coli | 49161c | 68 (S) |
| E. coli | 11775c | 71.5 (S) |
| E. coli | 29194c | 62.5 (S) |
| E. coli | 25922c | 60 (S) |
| Staph. aureus | 25923c | 68 (S) |
Abbreviations: B., Bacillus;E., Escherichia; G., Gardnerella; N., Neisseria;S., Streptococcus; Staph., Staphylococcus; S, sensitive.
aZone of inhibition method used an agar bilayer technique to detect inhibition by Lactobacillus crispatus CTV-05 against the test organisms [43, 44]. For organisms requiring special nutrient agars, the process was modified to use commercially prepared agar, which was aseptically removed from a petri dish and placed directly over an MRS agar surface creating a bilayer of equal thickness. All MRS agar plates were inoculated by streaking 0.01 mL of an overnight culture of CTV-05 across the diameter of the plate. All plates were incubated under optimum conditions for 24, 48, or 72 hours prior to overlay.
bStrains provided by the Centers for Disease Control and Prevention.
cStrain obtained from the American Type Culture Collection.
dClinical vaginal isolates obtained from women under IRB 06157-01 with Planned Parenthood Mar Monte.
LACTIN-V DEVELOPMENT
LACTIN-V was originally formulated as a vaginally administered gelatin capsule with a potency of 5 × 108 colony-forming units (CFU)/capsule. The capsule was tested in phase 1 and phase 2 clinical trials of healthy female volunteers and women with rUTI or BV. Following a successful phase 1 safety study in healthy women with a history of rUTI [46], a phase 2 rUTI trial (NCT00305227) was conducted in 100 women who received standard antibiotic treatment for uncomplicated cystitis, followed by LACTIN-V or placebo capsules daily for 5 days, then once weekly for 10 weeks [47]. Although not statistically significant, the rUTI incidence in the LACTIN-V arm (15%) was about half of that in the placebo arm (27%), similar to prophylactic antibiotic treatment. However, high-level vaginal colonization with L. crispatus (>106 16S rRNA gene copies/mL by quantitative polymerase chain reaction [qPCR]) in the LACTIN-V arm was associated with a significant rUTI reduction (risk ratio [RR] = 0.07; 95% confidence interval [CI], .02–.3). Interestingly, women receiving placebo who achieved comparably high levels of endogenous L. crispatus did not appear to have equivalent protection against rUTI (RR = 1.1), suggesting that CTV-05 was superior to the endogenous strains.
LACTIN-V capsules were tested in a phase 2 multisite randomized placebo-controlled trial of 149 women treated for BV with topical metronidazole or clindamycin (unpublished). The product was administered for 5 consecutive days, then once weekly for 10 weeks, with follow-up clinic visits at 4, 10, and 16 weeks. LACTIN-V administration appeared to modestly decrease the rate and incidence of recurrent BV compared to placebo. The time to first BV recurrence was longer in the LACTIN-V arm (118.7 days) versus the placebo arm (98.7 days). Subjects in the LACTIN-V arm who were colonized with CTV-05 were less likely to experience another BV episode than those in the placebo arm. CTV-05 colonization was determined by culture and repetitive element PCR (repPCR) [37]. In the per protocol cohort, the incidence of BV recurrence in CTV-05–colonized subjects was 12.5% compared to 30.3% in placebo, and 16% for the modified intention to treat cohort compared to 33.8% in placebo. Colonization in the most compliant per protocol cohort was 59% compared to 42% in the modified intention to treat cohort. Although not statistically significant, these results suggested for the first time that CTV-05 colonization may be a surrogate marker for efficacy, and that improving efficacy would require achieving higher colonization rates. Because the capsule formulation dissolved poorly in the vagina, likely hindering CTV-05 colonization, a specially designed vaginal applicator was developed to deliver LACTIN-V powder directly to the vaginal mucosa.
LACTIN-V VAGINAL APPLICATOR
The new dosage form was tested in a phase 1 escalating dose trial to assess safety, tolerability, and acceptability of the LACTIN-V applicator at 3 doses ranging from 5 × 108 to 2 × 109 CFU/applicator (NCT00537576) [48]. Twelve healthy women received the study product for 5 consecutive days and returned for follow-up visits on days 7 and 14. The adverse events (AEs) were mild or moderate and predominantly local (genitourinary) and evenly distributed across dose levels and treatment arms. LACTIN-V delivered at doses up to 2 × 109 CFU/applicator appeared to be safe and well tolerated.
A small phase 2a trial followed to assess colonization, safety, tolerability, and acceptability of applicator-delivered LACTIN-V (NCT00635622) [42]. All participants were treated for BV with 0.75% metronidazole vaginal gel (MetroGel), followed by LACTIN-V (2 × 109 CFU/applicator) or matching placebo applicator daily for 5 days, then once weekly for 2 weeks. The participants returned for follow-up on days 10 and 28. Overall, 61% in the LACTIN- V group were colonized with CTV-05 as determined by culture and repPCR. Among LACTIN-V users with complete adherence to the protocol, 78% were colonized [42]. The AEs were mild or moderate in severity and evenly distributed between the LACTIN-V and placebo arms. The applicator product appeared to be safe, well tolerated, and accepted by the participants.
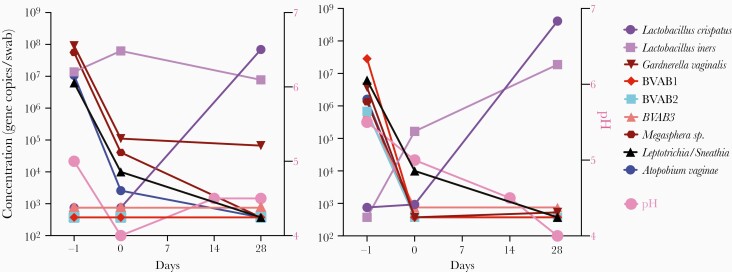
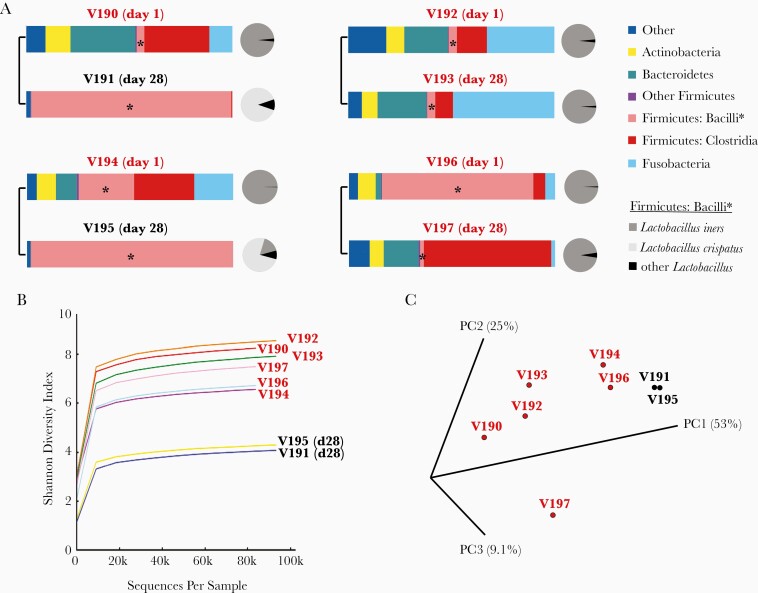
The effects of endogenous vaginal bacteria on L. crispatus colonization during the phase 2a study were examined by qPCR for 7 BV-associated bacteria, (Leptotrichia/Sneathia sp., Gardnerella vaginalis, BVAB-1, BVAB-2, BVAB-3, Megasphera sp., and Atopobium vaginae), L. iners, and L. crispatus [49]. The concentrations of the 7 BV species declined between the screening and enrollment visits with successful Metrogel treatment, and L. crispatus levels generally increased upon application of LACTIN-V (Figure 1). Overall, this study provided additional evidence that vaginal colonization with L. crispatus following LACTIN-V treatment was associated with reduced levels of BV-associated anaerobes, potentially reducing the risk of BV recurrence. A preliminary microbiome analysis of a subset of samples from the phase 2a study showed that women colonized with CTV-05 underwent a shift from a diverse VMB at enrollment, to a L. crispatus-dominated bacterial community at day 28 (Figure 2A). Results from 2 women not colonized with CTV-05 are also shown (Figure 2A). Figure 2B shows the concomitant decrease in the Shannon diversity index for the 2 women colonized with CTV-05. Figure 2C shows a principal component analysis of the 4 women day 1 and day 28 post CTV-05 treatment. For women not colonized, the Shannon diversity index remained high and L. iners was the main Lactobacillus species present at enrollment and day 28.
Figure 1.
Changes in the concentrations of vaginal bacteria species of 2 women successfully colonized with Lactobacillus crispatus-CTV-05 in the LACTIN-V arm of the phase 2a trial. Vaginal swabs were taken at screening (before metronidazole treatment), at enrollment (after metronidazole and before LACTIN-V treatment), and at day 28 (after LACTIN-V treatment). DNA was extracted from vaginal swabs and bacterium-specific qPCR was performed for targeted vaginal bacteria. Bacteria concentrations are expressed as mean log10 16S rRNA gene copies per swab. Vaginal pH was measured at screening (day −1), enrollment (day 0), and days 14 and 28. Abbreviations: BVAB, bacterial vaginosis-associated bacteria; qPCR, quantitative polymerase chain reaction.
Figure 2.
Changes in the microbiota of 4 women during the phase 2a LACTIN-V trial. A, The bacterial communities of 4 women before (day 1) and after (day 28) dosing with Lactobacillus crispatus-CTV-05 in the LACTIN-V arm of the phase 2a trial. Left, 2 women who were successfully colonized; right, 2 women who were not. Note that the bacterial species in the phylum Firmicutes, class Bacilli became L. crispatus-dominant (light grey circle) in the women who were colonized. Lactobacillus iners (medium grey) was the predominant lactobacillus species in women who were not colonize with L. crispatus. B, Shannon diversity indices were calculated for the 2 time points of each woman. Note that the day-28 time points for the women in whom L. crispatus became dominant (V191 and V195) had the lowest Shannon diversity. C, A principal component analysis (PCA) was performed to visualize the differences in microbiota among the 4 women at pre- and posttreatment time points. Note that the posttreatment time points for the 2 women who were successfully colonized with L. crispatus (in response to LACTIN-V treatment) and thus had the highest proportion of lactobacilli, V191 and V195, cluster together by PCA. Microbiome metrics (relative abundance and Shannon diversity indices) were calculated and PCA graphs were plotted using QIIME software (version 1) [50]. Individual vaginal swab samples are denoted with a “V###” abbreviation, while each of the 3 principal component axes are denoted with a “PC#” abbreviation.
PHASE 2 PROOF OF CONCEPT
Recently, a larger phase 2b multisite randomized, placebo-controlled trial of LACTIN-V to prevent BV recurrence was completed (NCT02766023) [51]. In this study, 228 women with BV were treated with Metrogel for 5 days, then randomized 2:1 to receive LACTIN-V (2 × 109 CFU/applicator) or a matching placebo applicator for 5 consecutive days, followed by twice-weekly doses for 10 additional weeks. Follow-up clinic visits occurred at weeks 4, 8, 12, and 24. The primary end points were the proportion of women with recurrent BV by week 12 and safety by week 24. Secondary end points included detectable CTV-05 colonization at each study visit and the proportion of women with recurrent BV by week 24.
In the intention-to-treat analysis, BV recurrence through the 12-week visit was significantly less common in the LACTIN-V arm than in the placebo arm (30% vs 45%, respectively; RR = 0.66; 95% CI, .44–.87; P = .01). In addition, BV recurrence remained significantly less common through the 24-week visit, 13 weeks after the last dose of LACTIN-V (39% vs 54%, respectively; RR = 0.73; 95% CI, .54–.92). The local solicited genitourinary AEs were mostly mild or moderate in severity, and their frequency and severity were similar between arms (Table 2). Additional information of solicited, unsolicited, and systemic AEs can be found in Cohen et al supplemental material [51].
Table 2.
Frequency and Severity of Local Genitourinary Adverse Events
| LACTIN-V | Placebo | |||
|---|---|---|---|---|
| Symptoms | % Mild | % Moderate | % Mild | % Moderate |
| Abnormal vaginal discharge | 41.8 | 25.5 | 34.8 | 34.8 |
| Abnormal vaginal odor | 35.4 | 21.2 | 21.1 | 30.8 |
| External genital irritation | 24.1 | 19.8 | 18.1 | 12.1 |
| External genital swelling | 10.6 | 10.6 | 7.5 | 7.5 |
| Genital burning | 18.4 | 15.6 | 10.6 | 18.1 |
| Genital itching | 29.7 | 31.9 | 28.7 | 22.7 |
| Genital rash | 9.2 | 5.6 | 6.0 | 4.5 |
| Vaginal bleeding | 23.4 | 3.5 | 28.7 | 4.5 |
CONNECTING THE DOTS
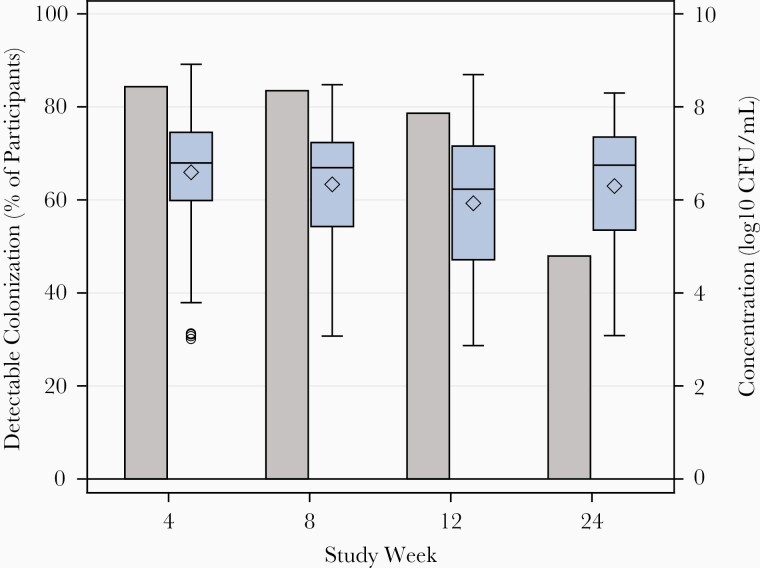
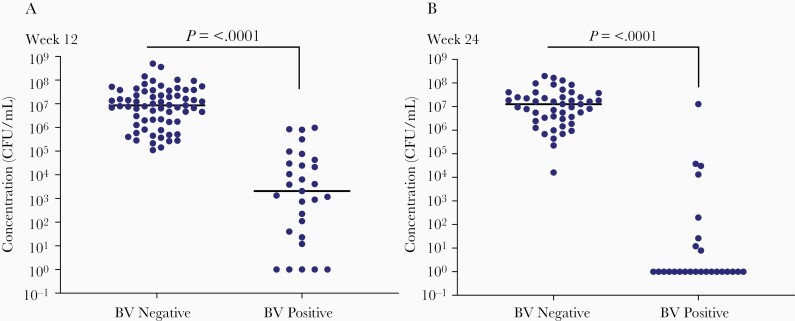
Overall, CTV-05 was detected by qPCR in 79% of women in the LACTIN-V arm at the 12-week visit and 48% at 24 weeks (Figure 3). Detectable colonization was defined as CTV-05 levels above the lower limit of detection at the 95% detection threshold (6.6 × 102 CFU/mL). Among subjects with detectable CTV-05, the median concentration (expressed as CFU/mL) in the LACTIN-V intervention arm ranged from 1.7 × 106 to 6.2 × 106 at different clinic visits during the dosing regimen through week 12, and 5.6 × 106 at week 24, approximately 13 weeks after the last dose of LACTIN-V. Although the proportion of participants with detectable colonization decreased after the last dose of LACTIN-V, the smaller number of women who remained colonized at week 24 still had median levels of CTV-05 comparable to those on treatment up to week 12 (Figure 3). This finding suggested that some women remained durably colonized for at least 13 weeks following the last dose of LACTIN-V. When the concentrations of CTV-05 at 12 and 24 weeks were further analyzed in participants with or without BV recurrence, it was evident that women who did not experience BV recurrence had significantly higher CTV-05 concentrations than those with BV recurrence (Figure 4). Women harboring ≥106 CFU/mL CTV-05 appeared to be protected from recurrent BV.
Figure 3.
Detectable colonization and median concentrations of Lactobacillus crispatus CTV-05 in the LACTIN-V arm of the phase 2b trial at weeks 4, 8, 12, and 24. The grey bars indicate the overall percentage of women colonized with L. crispatus CTV-05 above the lower limit of detection in the LACTIN-V arm. The box and whiskers plots indicate maximum and minimum values of L. crispatus CTV-05, the horizontal lines inside the box and whiskers indicate median values and the diamonds indicate mean values. The circles below the box and whiskers bar at week 4 represent outliers. The size of each box and whiskers represents the interquartile range. Abbreviation: CFU, colony-forming units. Reproduced with permission from the New England Journal of Medicine [52].
Figure 4.
Concentrations of Lactobacillus crispatus CTV-05 in participants diagnosed with or without bacterial vaginosis (BV) at (A) week 12 and (B) week 24 of the phase 2b trial. QPCR values were plotted separately for women with and without a positive BV diagnosis at weeks 12 and 24. A positive BV diagnosis was defined as both a positive Amsel test (≥3 Amsel criteria) and Nugent score >3. Values above 106 colony-forming units (CFU)/mL appeared to protect women from BV recurrence. Significance of difference between groups was determined by the Mann-Whitney 2-tailed test in Prism Version 8.4.2.
Furthermore, the colonization of L. crispatus was considerably higher in the LACTIN-V arm than in the placebo arm at both week 12 (82% vs 35%, respectively) and week 24 (64% vs 22%, respectively). Thus, without LACTIN-V treatment, only about one-third of women in the study population would spontaneously recolonize with an endogenous L. crispatus following Metrogel treatment.
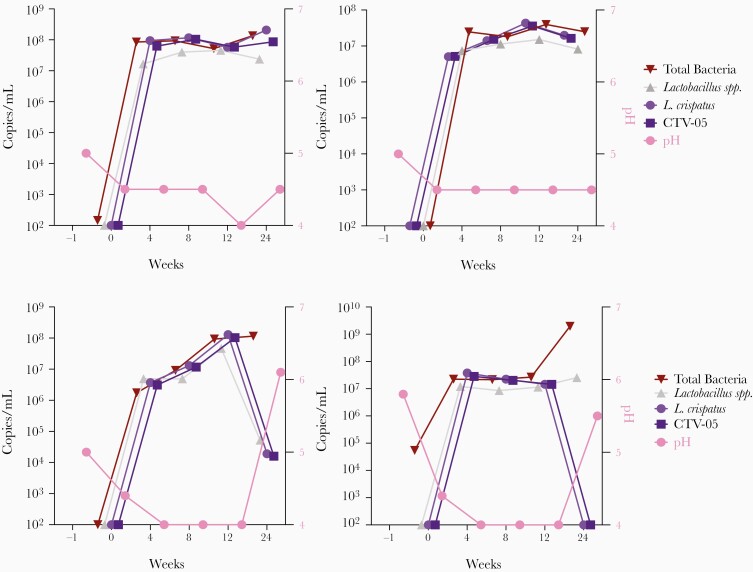
When CTV-05 dominates the VMB, it generally tracks with qPCR measurements of L. crispatus sp., Lactobacillus spp., total bacteria, and low vaginal pH. Figure 5A shows results from 2 women who were successfully colonized throughout the duration of the study to week 24, while Figure 5B shows results from 2 women who were successfully colonized during dosing through week 12, but then lost CTV-05 after the last dose of LACTIN-V. When CTV-05 levels declined in Figure 5B there was a corresponding increase in vaginal pH. An important mechanism for protection from BV recurrence is vaginal acidity, which is largely a function of lactic acid production by lactobacilli. In some instances, L. crispatus sp. levels were below detection while total Lactobacillus spp. levels were high, and vaginal pH was more variable (eg, subject 594). L. iners was suspected as the probable species in many of these cases, as observed in the phase 2a study. Metagenomic next-generation sequencing is being conducted to further identify the bacterial species present and their potential role in BV recurrence.
Figure 5.
Examples of 2 patterns of Lactobacillus crispatus CTV-05 colonization following LACTIN-V administration in the phase 2b trial. A, Two women successfully colonized with L. crispatus CTV-05 throughout the 24-week duration of the study and (B) 2 women successfully colonized with L. crispatus CTV-05 only during LACTIN-V dosing through week 12. Vaginal pH was measured and vaginal swabs were collected at screening, prior to metronidazole treatment (day −1), at enrollment, after metronidazole and before LACTIN-V treatment (day 0), and then at weeks 4, 8, 12 (during treatment) and at 24 (13 weeks post treatment). DNA was extracted from vaginal swabs. Primers were designed for quantitative polymerase chain reaction to amplify pan 16S rRNA for total bacteria, and specific primers for all Lactobacillus species, L. crispatus species, and L. crispatus CTV-05 strain.
CURRENT STATUS, NEXT STEPS, AND FUTURE PROSPECTS
In the phase 2b trial, LACTIN-V significantly reduced BV recurrence by one-third compared to placebo. This result was particularly significant in the context of the study population because most of the women were at high risk of BV recurrence. About half the participants had experienced ≥5 prior BV episodes, and nearly 70% had ≥3 BV episodes. In addition, over half the women self-identified as African American or Hispanic/Latina, 2 populations where BV is particularly prevalent [52].
CTV-05 colonization was closely correlated with prevention of BV recurrence. It appeared that colonization levels ≥106 CFU/mL may be protective, possibly because sufficient lactic acid can be produced to drive vaginal pH down and antagonize the growth of BV-associated bacteria. Nonetheless, some women who were not well colonized with CTV-05 experienced BV recurrence. An important ongoing therapeutic goal is to determine the factors that contribute to persistent colonization of CTV-05 so that BV recurrence can be prevented in a greater proportion of women over longer time periods.
Recent studies have identified multiple factors and temporal dynamics that impact the composition of the VMB and its ability to maintain a Lactobacillus-dominant state and prevent BV. Host genetic factors may lead to a higher risk for disturbances of the VMB in women of African, African American, and Latina ethnicity [7, 53–56]. Genetic associations in Kenyan women suggest a role for the innate immune system and cell signaling in vaginal microbiome composition and susceptibility to nonoptimal vaginal microbiome [57]. In addition, hormonal fluctuations impact the VMB [58–62]; estrogen and vaginal glycogen levels are lowest during menses, and the presence of menstrual blood may increase pH and BV risk [63–66]. However, in the phase 2b trial neither menses nor unprotected sex decreased CTV-05 colonization, possibly due to the twice-weekly dosing regimen. In addition, evidence points toward certain hormonal contraceptives as being potentially protective against BV [61, 67–72].
Sexual behavior affects BV risk. While condoms are protective [73, 74], early sexual debut [75, 76], oral sex [77], untreated sexual partners [67, 78–83], multiple partners [76], and exposure to semen [73, 74, 78, 84] all increase BV risk. BV recurrence may be caused by reinfection from a partner [67]. A polymicrobial biofilm has been identified on desquamated epithelial cells in male urine and semen samples, suggesting a potential reinfection mechanism [85]. Concurrent partner treatment is a promising option [86]. Vaginal cleaning practices, douching [87–91], and smoking [92] are also linked to BV risk. Many of these factors influence vaginal pH, which may alter the growth of lactobacilli and BV-associated bacteria. A pH of >5 is permissive to the growth of many nonacidophilic bacteria. One hypothesis is that the production of biogenic amines by certain taxa may allow them to competitively colonize the vagina by mitigating the protective effects of low pH, thus increasing the risk of BV development [93]. Similarly, the failure of metronidazole to suppress BV bacteria (possibly due to biofilms or resistant strains) following treatment appears to have a negative impact on CTV-05 colonization.
Although transitions between L. crispatus and L. iners-dominated communities occur, temporal studies of the VMB have shown that they tend to be mutually exclusive, suggesting competition for the same niche [94]. The L. iners-dominated community is relatively unstable and more likely to transition to BV. L. iners may suppress CTV-05 colonization in some women or possibly facilitate BV-associated bacteria. In these cases, selective inhibition of L. iners may potentially improve L. crispatus colonization and clinical benefit [95].
As part of our ongoing effort to understand factors that influence BV recurrence, DNA and RNA sequencing are being conducted to analyze microbial content and gene expression profiles are investigating changes in the VMB during LACTIN-V treatment. Ultimately, these data will be combined with vaginal cytokine and chemokine data and clinical metadata to understand why some women are colonized well with CTV-05, while others are not. This multiomic approach is expected to provide insights into biomarkers to potentially customize the dosing regimen or inform on patient stratification. These approaches may identify molecular signatures of strains of L. crispatus (or other species) that might complement CTV-05 and be incorporated into a second-generation product. New insights into the VMB indicate that thousands of L. crispatus strains may exist, and it is possible that some may colonize better or have additional beneficial features [96].
While most women are readily colonized with CTV-05 after successful metronidazole treatment and respond favorably to intermittent LACTIN-V administration, others may need more aggressive BV treatment, or a change in the frequency or duration of LACTIN-V dosing. In the absence of an approved LBP to optimize the VMB and prevent recurrent BV, clinicians may potentially consider recommending hormonal contraception, boric acid, condom-protected sex, partner treatment, and abstaining from vaginal douches and smoking to reduce risk of recurrence.
LACTIN-V represents the first microbiome-based LBP to exhibit significant efficacy in preventing BV recurrence in a rigorous FDA-regulated clinical trial. BV is associated with a number of important clinical sequelae, preterm birth, low birth weight in newborns, STI and HIV susceptibility, and oncogenic HPV progression. Successful prevention of BV and optimization of the VMB is expected to have a positive impact on these and other indications, and to usher in a new approach to improve women’s health.
Notes
Financial support. The phase 2b randomized double-blind placebo-controlled trial of Lactobacillus crispatus-CTV-05 (LACTIN-V) to prevent the recurrence of bacterial vaginosis (NCT02766023) was support by the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (contract numbers HHSN2722013000141 and HHSN27200007). A. H. and C. R. C. are supported by the Division of Microbiology and Infectious Diseases (contract number HHSN272201 3000141).
Supplement sponsorship. This work is part of a supplement sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH) and the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. L. A. L. and T. P. P. are employees of Osel Inc. L. A. L., T. P. P., and P. P. L. are shareholders of Osel. P. P. L. is a cofounder of Osel and Chairman of the Board at Osel. C. R. C. was paid speaking honoraria by Lupin and Miyarisan Pharmaceuticals and is a member of the scientific advisory board of Osel Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Smith SB, Ravel J. The vaginal microbiota, host defence and reproductive physiology. J Physiol 2017; 595:451–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kroon SJ, Ravel J, Huston WM. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil Steril 2018; 110:327–36. [DOI] [PubMed] [Google Scholar]
- 3. Anahtar MN, Gootenberg DB, Mitchell CM, Kwon DS. Cervicovaginal microbiota and reproductive health: the virtue of simplicity. Cell Host Microbe 2018; 23:159–68. [DOI] [PubMed] [Google Scholar]
- 4. Giorgi A, Torriani S, Dellaglio F, Bo G, Stola E, Bernuzzi L. Identification of vaginal lactobacilli from asymptomatic women. Microbiologica 1987; 10:377–84. [PubMed] [Google Scholar]
- 5. Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis 1999; 180:1950–6. [DOI] [PubMed] [Google Scholar]
- 6. Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology (Reading) 2004; 150:2565–73. [DOI] [PubMed] [Google Scholar]
- 7. Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011; 108(suppl 1):4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petrova MI, Reid G, Vaneechoutte M, Lebeer S. Lactobacillus iners: friend or foe? Trends Microbiol 2017; 25:182–91. [DOI] [PubMed] [Google Scholar]
- 9. O’Hanlon DE, Come RA, Moench TR. Vaginal pH measured in vivo: lactobacilli determine pH and lactic acid concentration. BMC Microbiol 2019; 19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lepargneur JP. Lactobacillus crispatus as biomarker of the healthy vaginal tract. Ann Biol Clin 2016; 74:421–7. [DOI] [PubMed] [Google Scholar]
- 11. Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42:965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anton L, Sierra LJ, DeVine A, et al. Common cervicovaginal microbial supernatants alter cervical epithelial function: mechanisms by which Lactobacillus crispatus contributes to cervical health. Front Microbiol 2018; 9:2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pavlova SI, Kilic AO, Kilic SS, et al. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. J Appl Microbiol 2002; 92:451–9. [DOI] [PubMed] [Google Scholar]
- 14. Klebanoff SJ, Hillier SL, Eschenbach DA, Waltersdorph AM. Control of the microbial flora of the vagina by H2O2-generating lactobacilli. J Infect Dis 1991; 164:94–100. [DOI] [PubMed] [Google Scholar]
- 15. Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988; 158:819–28. [DOI] [PubMed] [Google Scholar]
- 16. Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 2012; 7:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faught BM, Reyes S. Characterization and treatment of recurrent bacterial vaginosis. J Womens Health (Larchmt) 2019; 28:1218–26. [DOI] [PubMed] [Google Scholar]
- 18. Sobel JD, Kaur N, Woznicki NA, et al. Prognostic indicators of recurrence of bacterial vaginosis. J Clin Microbiol 2019; 57:e00227-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell CM, Balkus J, Agnew KJ, et al. Bacterial vaginosis, not HIV, is primarily responsible for increased vaginal concentrations of proinflammatory cytokines. AIDS Res Hum Retroviruses 2008; 24:667–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gosmann C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017; 46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin Infect Dis 2003; 36:663–8. [DOI] [PubMed] [Google Scholar]
- 22. Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis 2003; 37:319–25. [DOI] [PubMed] [Google Scholar]
- 23. Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998; 12:1699–706. [DOI] [PubMed] [Google Scholar]
- 24. Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol 2004; 104:761–9. [DOI] [PubMed] [Google Scholar]
- 25. Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995; 333:1737–42. [DOI] [PubMed] [Google Scholar]
- 26. Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG 2020; 127:171–80. [DOI] [PubMed] [Google Scholar]
- 27. Sobel R, Sobel JD. Metronidazole for the treatment of vaginal infections. Expert Opin Pharmacother 2015; 16:1109–15. [DOI] [PubMed] [Google Scholar]
- 28. Degnan FH. The US Food and Drug Administration and probiotics: regulatory categorization. Clin Infect Dis 2008; 46(suppl 2):S133–6. [DOI] [PubMed] [Google Scholar]
- 29. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11:506–14. [DOI] [PubMed] [Google Scholar]
- 30. van de Wijgert J, Verwijs MC, Agaba SK, et al. Intermittent lactobacilli-containing vaginal probiotic or metronidazole use to prevent bacterial vaginosis recurrence: a pilot study incorporating microscopy and sequencing. Sci Rep 2020; 10:3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van de Wijgert J, Verwijs MC. Lactobacilli-containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG 2020; 127:287–99. [DOI] [PubMed] [Google Scholar]
- 32. Yefet E, Colodner R, Strauss M, Gam Ze Letova Y, Nachum Z. A randomized controlled open label crossover trial to study vaginal colonization of orally administered Lactobacillus reuteri RC-14 and rhamnosus GR-1 in pregnant women at high risk for preterm labor. Nutrients 2020; 12:1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Proctor LM. The Human Microbiome Project in 2011 and beyond. Cell Host Microbe 2011; 10:287–91. [DOI] [PubMed] [Google Scholar]
- 34. Food and Drug Administration. Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information. Guidance for industry. Washington, DC: US Department of Health and Human Services, Food and Drug Administration Center for Biologics Evaluation and Research, 2012. [Google Scholar]
- 35. Eschenbach DA, Davick PR, Williams BL, et al. Prevalence of hydrogen peroxide-producing Lactobacillus species in normal women and women with bacterial vaginosis. J Clin Microbiol 1989; 27:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gupta K, Stapleton AE, Hooton TM, Roberts PL, Fennell CL, Stamm WE. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J Infect Dis 1998; 178:446–50. [DOI] [PubMed] [Google Scholar]
- 37. Antonio MA, Hillier SL. DNA fingerprinting of Lactobacillus crispatus strain CTV-05 by repetitive element sequence-based PCR analysis in a pilot study of vaginal colonization. J Clin Microbiol 2003; 41:1881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Antonio MA, Rabe LK, Hillier SL. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J Infect Dis 2005; 192:394–8. [DOI] [PubMed] [Google Scholar]
- 39. El Aila NA, Tency I, Claeys G, et al. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC Infect Dis 2009; 9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marrazzo JM, Fiedler TL, Srinivasan S, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis 2012; 205:1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kwok L, Stapleton AE, Stamm WE, Hillier SL, Wobbe CL, Gupta K. Adherence of Lactobacillus crispatus to vaginal epithelial cells from women with or without a history of recurrent urinary tract infection. J Urol 2006; 176:2050–4 [DOI] [PubMed] [Google Scholar]
- 42. Hemmerling A, Harrison W, Schroeder A, et al. Phase 2a study assessing colonization efficiency, safety, and acceptability of Lactobacillus crispatus CTV-05 in women with bacterial vaginosis. Sex Transm Dis 2010; 37:745–50. [DOI] [PubMed] [Google Scholar]
- 43. Fitzsimmons N, Berry DR. Inhibition of Candida albicans by Lactobacillus acidophilus: evidence for the involvement of a peroxidase system. Microbios 1994; 80:125–33. [PubMed] [Google Scholar]
- 44. Jack M, Wood BJ, Berry DR. Evidence for the involvement of thiocyanate in the inhibition of Candida albicans by Lactobacillus acidophilus. Microbios 1990; 62:37–46. [PubMed] [Google Scholar]
- 45. Martinez RM, Hulten KG, Bui U, Clarridge JE 3rd. Molecular analysis and clinical significance of Lactobacillus spp. recovered from clinical specimens presumptively associated with disease. J Clin Microbiol 2014; 52:30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Czaja CA, Stapleton AE, Yarova-Yarovaya Y, Stamm WE. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol 2007; 2007:35387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 2011; 52:1212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hemmerling A, Harrison W, Schroeder A, et al. Phase 1 dose-ranging safety trial of Lactobacillus crispatus CTV-05 for the prevention of bacterial vaginosis. Sex Transm Dis 2009; 36:564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ngugi BM, Hemmerling A, Bukusi EA, et al. Effects of bacterial vaginosis-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex Transm Dis 2011; 38:1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010; 7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van de Wijgert JHHM, Verwijs MC. Randomized trial of LACTIN-V to prevent recurrence of bacterial vaginosis. N Engl J Med 2020; 383:790–2. [DOI] [PubMed] [Google Scholar]
- 52. Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of LACTIN-V to prevent recurrence of bacterial vaginosis. N Engl J Med 2020; 382:1906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ness RB, Hillier S, Richter HE, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc 2003; 95:201–12. [PMC free article] [PubMed] [Google Scholar]
- 54. Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis 2008; 35:78–83. [DOI] [PubMed] [Google Scholar]
- 55. Zhou X, Brown CJ, Abdo Z, et al. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 2007; 1:121–33. [DOI] [PubMed] [Google Scholar]
- 56. Si J, You HJ, Yu J, Sung J, Ko G. Prevotella as a hub for vaginal microbiota under the influence of host genetics and their association with obesity. Cell Host Microbe 2017; 21:97–105. [DOI] [PubMed] [Google Scholar]
- 57. Mehta SD, Nannini DR, Otieno F, et al. Host genetic factors associated with vaginal microbiome composition in Kenyan women. mSystems 2020; 5:e00502-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chan RC, Bruce AW, Reid G. Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of gram-negative uropathogens by competitive exclusion. J Urol 1984; 131:596–601. [DOI] [PubMed] [Google Scholar]
- 59. Devillard E, Burton JP, Hammond JA, Lam D, Reid G. Novel insight into the vaginal microflora in postmenopausal women under hormone replacement therapy as analyzed by PCR-denaturing gradient gel electrophoresis. Eur J Obstet Gynecol Reprod Biol 2004; 117:76–81. [DOI] [PubMed] [Google Scholar]
- 60. Burton JP, Cadieux PA, Reid G. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl Environ Microbiol 2003; 69:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vodstrcil LA, Hocking JS, Law M, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PLoS One 2013; 8:e73055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Achilles SL, Hillier SL. The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS 2013; 27(suppl 1):S5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Santiago GL, Tency I, Verstraelen H, et al. Longitudinal qPCR study of the dynamics of L. crispatus, L. iners, A. vaginae, (sialidase positive) G. vaginalis, and P. bivia in the vagina. PLoS One 2012; 7:e45281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012; 4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 2010; 5:e10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Eschenbach DA, Thwin SS, Patton DL, et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis 2000; 30:901–7. [DOI] [PubMed] [Google Scholar]
- 67. Bradshaw CS, Vodstrcil LA, Hocking JS, et al. Recurrence of bacterial vaginosis is significantly associated with posttreatment sexual activities and hormonal contraceptive use. Clin Infect Dis 2013; 56:777–86. [DOI] [PubMed] [Google Scholar]
- 68. Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis 2007; 34:954–9. [PubMed] [Google Scholar]
- 69. Shoubnikova M, Hellberg D, Nilsson S, Mårdh PA. Contraceptive use in women with bacterial vaginosis. Contraception 1997; 55:355–8. [DOI] [PubMed] [Google Scholar]
- 70. Rifkin SB, Smith MR, Brotman RM, Gindi RM, Erbelding EJ. Hormonal contraception and risk of bacterial vaginosis diagnosis in an observational study of women attending STD clinics in Baltimore, MD. Contraception 2009; 80:63–7. [DOI] [PubMed] [Google Scholar]
- 71. Baeten JM, Nyange PM, Richardson BA, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol 2001; 185:380–5. [DOI] [PubMed] [Google Scholar]
- 72. Crucitti T, Hardy L, van de Wijgert J, et al. ; Ring Plus Study Group . Contraceptive rings promote vaginal lactobacilli in a high bacterial vaginosis prevalence population: a randomised, open-label longitudinal study in Rwandan women. PLoS One 2018; 13:e0201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sanchez S, Garcia PJ, Thomas KK, Catlin M, Holmes KK. Intravaginal metronidazole gel versus metronidazole plus nystatin ovules for bacterial vaginosis: a randomized controlled trial. Am J Obstet Gynecol 2004; 191:1898–906. [DOI] [PubMed] [Google Scholar]
- 74. Schwebke JR, Desmond R. Risk factors for bacterial vaginosis in women at high risk for sexually transmitted diseases. Sex Transm Dis 2005; 32:654–8. [DOI] [PubMed] [Google Scholar]
- 75. Gallo MF, Macaluso M, Warner L, et al. Bacterial vaginosis, gonorrhea, and chlamydial infection among women attending a sexually transmitted disease clinic: a longitudinal analysis of possible causal links. Ann Epidemiol 2012; 22:213–20. [DOI] [PubMed] [Google Scholar]
- 76. Mitchell CM, Fredricks DN, Winer RL, Koutsky L. Effect of sexual debut on vaginal microbiota in a cohort of young women. Obstet Gynecol 2012; 120:1306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schwebke JR, Richey CM, Weiss2 HL. Correlation of behaviors with microbiological changes in vaginal flora. J Infect Dis 1999; 180:1632–6. [DOI] [PubMed] [Google Scholar]
- 78. Marrazzo JM. Interpreting the epidemiology and natural history of bacterial vaginosis: are we still confused? Anaerobe 2011; 17:186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zozaya M, Ferris MJ, Siren JD, et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome 2016; 4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193:1478–86. [DOI] [PubMed] [Google Scholar]
- 81. Bradshaw CS, Tabrizi SN, Read TR, et al. Etiologies of nongonococcal urethritis: bacteria, viruses, and the association with orogenital exposure. J Infect Dis 2006; 193:336–45. [DOI] [PubMed] [Google Scholar]
- 82. Piot P. Distribution of eight serotypes of Ureaplasma urealyticum in cases of non-gonococcal urethritis and of gonorrhoea, and in healthy persons. Br J Vener Dis 1976; 52:266–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schwebke JR, Rivers C, Lee J. Prevalence of Gardnerella vaginalis in male sexual partners of women with and without bacterial vaginosis. Sex Transm Dis 2009; 36:92–4. [DOI] [PubMed] [Google Scholar]
- 84. Baud D, Pattaroni C, Vulliemoz N, Castella V, Marsland BJ, Stojanov M. Sperm microbiota and its impact on semen parameters. Front Microbiol 2019; 10:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Swidsinski A, Doerffel Y, Loening-Baucke V, et al. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol Obstet Invest 2010; 70:256–63. [DOI] [PubMed] [Google Scholar]
- 86. Amaya-Guio J, Viveros-Carreno DA, Sierra-Barrios EM, Martinez-Velasquez MY, Grillo-Ardila CF. Antibiotic treatment for the sexual partners of women with bacterial vaginosis. Cochrane Database Syst Rev 2016; ( 10):CD011701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by gram stain analysis. Sex Transm Infect 2010; 86:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ness RB, Hillier SL, Richter HE, et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet Gynecol 2002; 100:765. [DOI] [PubMed] [Google Scholar]
- 89. Schwebke JR, Desmond RA, Oh MK. Predictors of bacterial vaginosis in adolescent women who douche. Sex Transm Dis 2004; 31:433–6. [DOI] [PubMed] [Google Scholar]
- 90. Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011; 8:e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Birse KD, Romas LM, Guthrie BL, et al. Genital injury signatures and microbiome alterations associated with depot medroxyprogesterone acetate usage and intravaginal drying practices. J Infect Dis 2017; 215:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Smart S, Singal A, Mindel A. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect 2004; 80:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nelson TM, Borgogna JL, Brotman RM, Ravel J, Walk ST, Yeoman CJ. Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front Physiol 2015; 6:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. France MT, Mendes-Soares H, Forney LJ. Genomic comparisons of Lactobacillus crispatus and Lactobacillus iners reveal potential ecological drivers of community composition in the vagina. Appl Environ Microbiol 2016; 82:7063–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nilsen T, Swedek I, Lagenaur LA, Parks TP. Novel selective inhibition of Lactobacillus iners by Lactobacillus-derived bacteriocins. Appl Environ Microbiol 2020; 86:e01594-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ma B, France MT, Crabtree J, et al. A comprehensive non-redundant gene catalog reveals extensive within-community intraspecies diversity in the human vagina. Nat Commun 2020; 11:940. [DOI] [PMC free article] [PubMed] [Google Scholar]