The TPX2 protein family comprises WAVE-DAMPENED2-like (WDL) and TPX2-like (TPXL) groups. WDLs govern cell expansion in response to ethylene, light, brassinosteroids, and abiotic stresses. TPXLs function in mitotic spindle assembly and activating Aurora kinase.
Keywords: Brassinosteroids, cell expansion, ethylene, hormonal signalling, microtubules, TPX2, WAVE-DAMPENED
Abstract
TPX2 proteins were first identified in vertebrates as a key mitotic spindle assembly factor. Subsequent studies demonstrated that TPX2 is an intricate protein, with functionally and structurally distinct domains and motifs including Aurora kinase-binding, importin-binding, central microtubule-binding, and C-terminal TPX2 conserved domain, among others. The first plant TPX2-like protein, WAVE-DAMPENED2, was identified in Arabidopsis as a dominant mutation responsible for reducing the waviness of roots grown on slanted agar plates. Each plant genome encodes at least one ‘canonical’ protein with all TPX2 domains and a family of proteins (20 in Arabidopsis) that diversified to contain only some of the domains. Although all plant TPX2-family proteins to date bind microtubules, they function in distinct processes such as cell division, regulation of hypocotyl cell elongation by hormones and light signals, vascular development, or abiotic stress tolerance. Consequently, their expression patterns, regulation, and functions have diverged considerably. Here we summarize the current body of knowledge surrounding plant TPX2-family proteins.
Introduction
Microtubules in plants direct intracellular trafficking and govern spatial distribution of organelles. Organization of microtubules changes to suit any developmental or physiological situation that a plant could encounter throughout its life cycle. One of the most spectacular examples is assembly of structurally and functionally distinct microtubule arrays during mitosis: the pre-prophase band, mitotic spindle, anaphase spindle, the phragmoplast, and cortical microtubule array. The pre-prophase band forms during transition from G2 to prophase and marks the position of the division plane; the metaphase spindle ensures equal inheritance of information between daughter cells; the anaphase spindle separates daughter chromatids; and the phragmoplast constructs partitions between daughter cells (reviewed in Wasteneys, 2002). The cortical microtubule array forms during interphase and amongst many roles determines the direction of cell expansion. Typically, cells expand perpendicular to the orientation of cellulose microfibrils in the cell wall, which in turn mimics orientation of cortical microtubules (Baskin, 2001, 2005; Bringmann et al., 2012b). The ability to direct orientation of cellulose microfibrils makes microtubules a key effector of signalling pathways that regulate plant morphogenesis (Lindeboom et al., 2013; Shaw, 2013).
Organization of microtubules is governed by an ensemble of microtubule-associated proteins (MAPs) that facilitate formation of new microtubules; stabilize, destabilize, or sever microtubules; link microtubules together or to other structures; and facilitate trafficking along microtubules (reviewed in Hamada, 2014). Some MAPs are conserved across all eukaryotes, such as the microtubule-nucleating γ-tubulin ring complex (γ-TuRC; Hashimoto, 2013), the microtubule-severing protein katanin (Burk et al., 2007), or the microtubule polymerization factor MOR1/GEM1 (Whittington et al., 2001; Twell et al., 2002). Other MAPs are plant specific, such as the microtubule-stabilizing protein MAP70 (Korolev et al., 2005; Korolev et al., 2007). Several groups of MAPs are partially conserved. One such group is TPX2-like (TPXL) and WAVE-DAMPENED2 (WVD2)-like proteins that combine evolutionarily conserved and plant-specific domains (Rajangam et al., 2008b; Evrard et al., 2009). Here we overview the current body of knowledge about this family of proteins and highlight the key knowledge gaps.
Discovery of plant TPX2 proteins
TPX2 was originally identified in vertebrates as a targeting protein for Xenopus laevis kinesin-like protein 2 (Wittmann et al., 1998; Gruss et al., 2001). Depletion of TPX2 from Xenopus egg total protein extracts using TPX2 antibody abrogated targeting of Xenopus laevis kinesin-like protein 2 (Xklp2) to the spindle poles and resulted in mitotic failure. Subsequent studies revealed that TPX2 is an evolutionarily conserved microtubule-binding protein in vertebrates and invertebrates (Gruss et al., 2002; Kufer et al., 2002; Ozlu et al., 2005; Goshima, 2011). The list of TPX2 functions during mitosis in vertebrates encompasses targeting Aurora A protein kinase to the spindle poles; activating Aurora A (Kufer et al., 2002; Wadsworth, 2015); and promoting branching microtubule nucleation by increasing the efficiency of microtubule nucleation in concert with γ-TuRC (Petry et al., 2013; Alfaro-Aco et al., 2017). TPX2 can also promote microtubule nucleation by stabilizing early nucleation intermediates (Roostalu et al., 2015; Wieczorek et al., 2015; Reid et al., 2016).
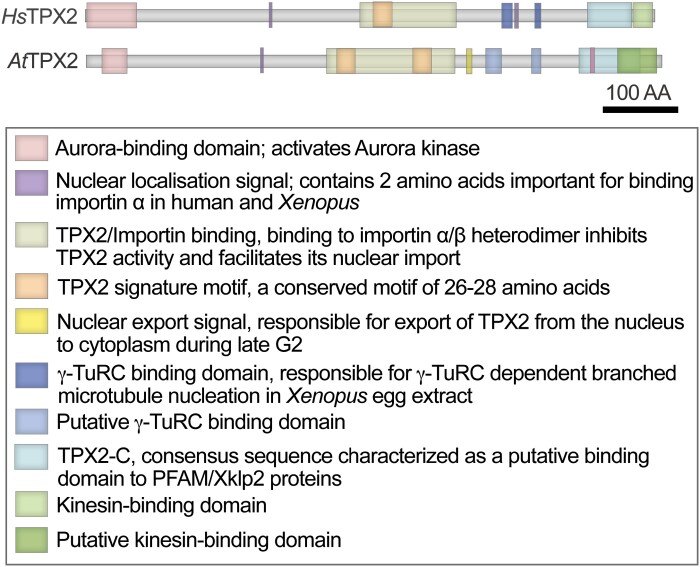
Each of the above TPX2 functions is undertaken by specialized domains (Fig. 1). The N-terminus contains an Aurora-binding and activation domain (Bird and Hyman, 2008). The centrally positioned importin-binding domain, called the TPX2/importin domain, mediates the localization of Xklp2 to the mitotic spindle (Boleti et al., 1996; Brunet et al., 2004; Neumayer et al., 2012). A nuclear localization signal (NLS) keeps TPX2 in the nucleus during interphase (Schatz et al., 2003). During mitosis, the activity of TPX2 is inhibited through association with the α/β importin complex (Wittmann et al., 2000; Gruss et al., 2002). A high concentration of RanGTP, produced by the chromatin component Ran-GEF RCC1, sequesters importin β, causing dissociation of importin α from TPX2. Then TPX2 can contribute to microtubule nucleation, and Aurora A targeting and activation (Carazo-Salas et al., 1999; Gruss et al., 2001). γ-TuRC activation is facilitated by a motif near the C-terminus of the protein (Alfaro-Aco et al., 2017). The kinesin-interacting domain and a highly conserved, but poorly characterized, TPX2-C domain (PF6886) are located on the very C-terminus (Fig. 1).
Fig. 1.
Conserved domains in human (Hs) and Arabidopsis (At) TPX2.
The first TPX2-family protein in plants, WVD2 was identified in an Arabidopsis thaliana activation tag screen for altered root growth on slanted agar plates (Yuen et al., 2003). Overexpression of WVD2 resulted in altered anisotropic cell expansion and, consequently, shorter and thicker roots and etiolated hypocotyls, with shorter and wider leaves (Yuen et al., 2003). Analysis of the A. thaliana reference genome revealed one TPX2-family protein, AtTPX2, showing ~40% amino acid sequence similarity to human TPX2 and containing all conserved domains (Fig. 1) (Vos et al., 2008; Petrovska et al., 2013). In addition, a TPX2 signature motif composed of 26–28 amino acids was identified within the TPX2/importin-binding domain of this protein (PF12214) (Vos et al., 2008).
All angiosperm genomes sequenced to date contain at least one TPX2 protein with all conserved domains and a family of proteins lacking certain domains (Rajangam et al., 2008a, b; Evrard et al., 2009; Du et al., 2016; Lei et al., 2019). For example, the A. thaliana genome has 20 TPX2-family genes (Table 1; Fig. 2; Supplementary Table S1) with highly diverse sequences and functions. The functions of TPX2-family proteins extend beyond cell division into facilitating anisotropic cell expansion in response to developmental and environmental cues. Below we summarize the current knowledge on plant TPX2-family proteins.
Table 1.
Accession numbers, properties, and published nomenclature of Arabidopsis TPX2-family proteins.
| Accession Number | Length (no. of amino acids) | Mol. wt | pI | Yuen et al. (2003) | Perrin et al. (2007) | Rajangam et al. (2008a) | Vos et al., (2008) | Wang et al. (2012) | Tomstikova et al. (2015) | Ma et al. (2018) | Unpublished |
|---|---|---|---|---|---|---|---|---|---|---|---|
| At3g04630 | 287 | 32.1 | 10.1 | WDL1 | |||||||
| At5g28646 | 202 | 23.3 | 9.1 | WVD2 | |||||||
| At1g54460 | 338 | 37.4 | 10.3 | WDL2 | |||||||
| At3g23090 | 338 | 37.8 | 9.6 | WDL3 | |||||||
| At2g35880 | 432 | 46.7 | 10.5 | WDL4 | |||||||
| At4g32330 | 437 | 47.5 | 9.0 | WDL5 | |||||||
| At2g25480 | 404 | 44.3 | 9.3 | WDL6 | |||||||
| At1g70950 | 478 | 53.2 | 7.8 | WDL7 | |||||||
| At3g01710 | 391 | 43.9 | 10.3 | WDL8 | |||||||
| At3g26050 | 533 | 58.9 | 10.8 | WDL9 | |||||||
| At5g44270 | 309 | 35.9 | 11.0 | TPXL7 | |||||||
| At1g03780 | 758 | 86.5 | 10.2 | TPX2 | |||||||
| At3g01015 | 488 | 56.2 | 9.9 | TPXL1 | MDP60 | ||||||
| At5g15510 | 497 | 56.5 | 10.0 | TPXL5 | |||||||
| At5g37478 | 178 | 20.4 | 9.92 | MAP20 | TPXL6 | ||||||
| At4g22860 | 509 | 58.1 | 10.1 | TPXL3 | |||||||
| At4g11990 | 501 | 57.2 | 9.5 | TPXL2 | |||||||
| At5g07170 | 397 | 45.1 | 9.7 | TPXL4 | |||||||
| At5g62240 | 377 | 43.2 | 10.2 | TPXL8 | |||||||
| At1g23060 | 361 | 41.0 | 10.6 | MDP40 |
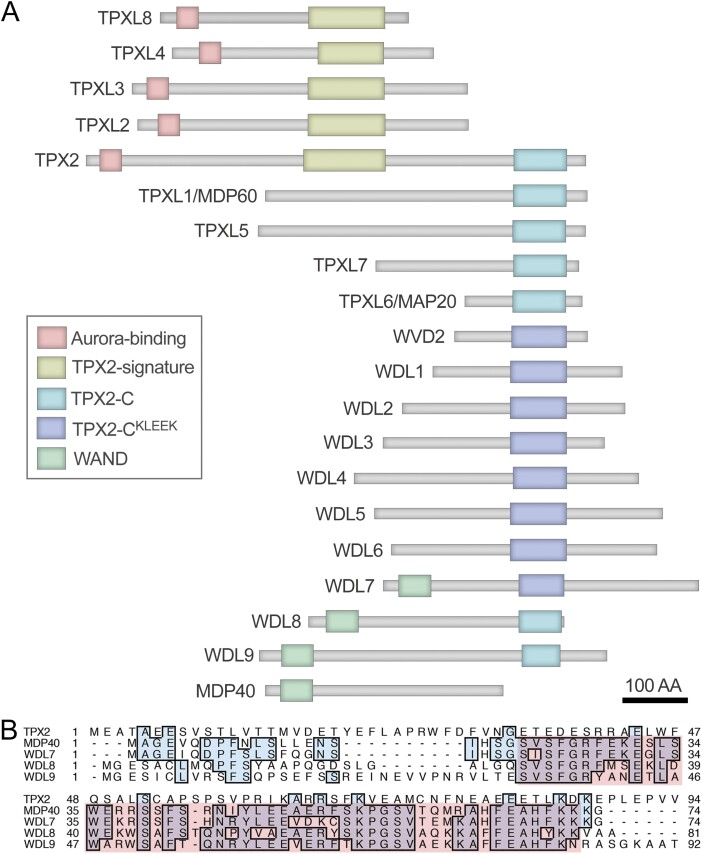
Fig. 2.
Domain organization of Arabidopsis TPX2-family proteins. (A) TPX2 has all conserved domains whereas other members of TPX2-family retain only some domains. In addition, several members have a plant-specific WAND. The positions of the domains is listed in Supplementary Table S1. (B) Alignment of the N-terminal region of TPX2-family proteins containing WAND with the corresponding region of TPX2. The WAND is highlighted in red.
Diversity of TPX2 proteins in plants
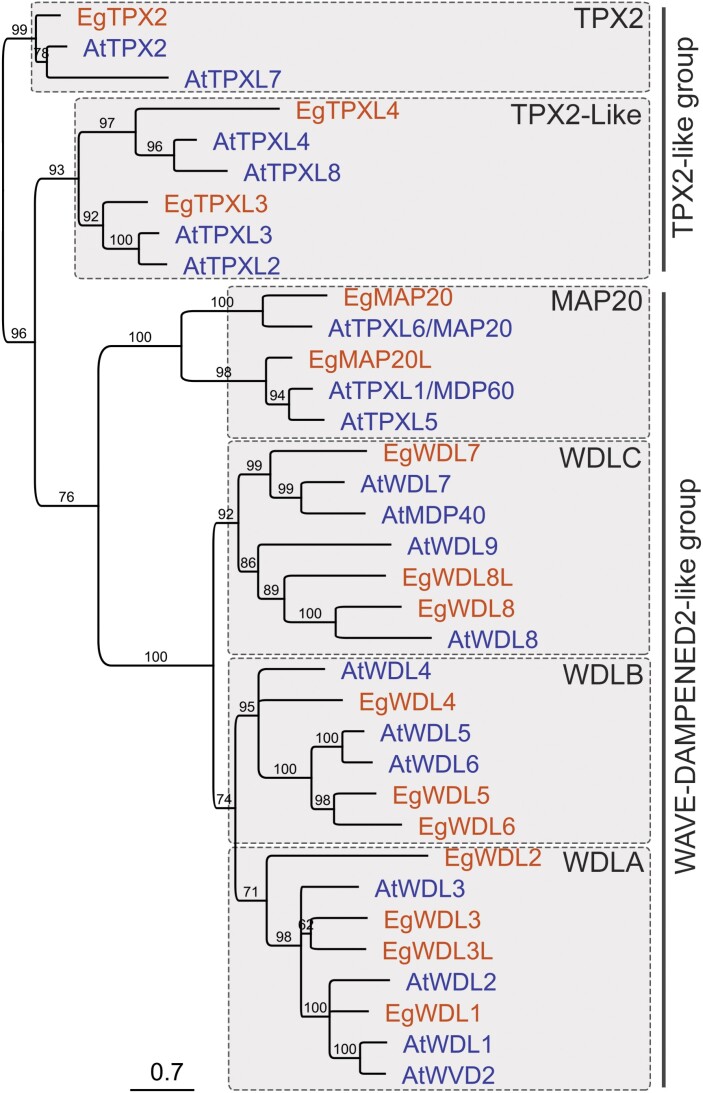
TPX2-family proteins were first classified according to the TPX2-C sequence of WVD2 (Yuen et al., 2003; Perrin et al., 2007; Rajangam et al., 2008a; Du et al., 2016). TPX2-C of A. thaliana WVD2 has been noted to have a second haplotype containing a plant-specific ‘KLEEK’ motif (Yuen et al., 2003), hereinafter referred to as TPX2-CKLEEK. Four major clades of WAVE-DAMPENED2-like (WDL) proteins were identified in Arabidopsis, aspen, Eucalyptus, cotton, moss, and others (Fig. 3) (Rajangam et al., 2008a, b; Evrard et al., 2009; Du et al., 2016; Lei et al., 2019; Kozgunova et al., 2020, Preprint). Proteins with TPX2-CKLEEK form two clades, WDLA and WDLB (Figs 2, 3), whereas proteins lacking TPX2-CKLEEK constitute the MAP20 clade (Du et al., 2016; Lei et al., 2019). Currently, no information is available on what protein sequence features contribute to segregation of proteins into WDLA and WDLB clades.
Fig. 3.
The grouping of TPX2-family proteins. Phylodendrogram of Arabidopsis (blue font) and Eucalyptus (red font) TPX2-family proteins. The bootstrap values were calculated from 1000 repeats. Branches with the bootstrap values <60% were collapsed. TPX2, TPX2-like, MAP20, WDLA, WDLB, and WDLC clades constitute TPX2-like and WDL2-like groups.
Although analysis of 574 TPX2-like plant protein sequences in GenBank identified TPX2-CKLEEK in representatives of the WDLC clade, Arabidopsis members of this clade, with the exception of AtWDL7, lack the KLEEK motif (Fig. 2). Most probably, these sequences group for the conserved N-terminal WAVE-DAMPENED2-like New Domain (WAND) with as yet unknown functions (Figs 2, 3). One member of this clade, MDP40, lacks any TPX2 domains, meaning that MDP40 de facto is not a TPX2-family protein.
Several TPX2-family proteins contain the N-terminal Aurora-binding domain and the central TPX2/Importin-binding domain, but lack TPX2-C (Tomastikova et al., 2015, 2020; Boruc et al., 2019). These proteins were named TPX2-like proteins (TPXLs). The latest phylogenetic study in A. thaliana with the sequences of the N-terminal Aurora-binding domain and central TPX2 signature domain identifies a novel clade consisting of TPXL proteins TPXL2/3/4/8 (Figs 2, 3; Supplementary Table S1) (Tomastikova et al., 2020).
TPX2-family genes have a complex non-overlapping transcription pattern. The most ubiquitous is the WDLB clade followed by WDLA and TPX2 (Du et al., 2016; Lei et al., 2019). The TPX2 clade exhibits the highest expression in roots of Eucalyptus (Du et al., 2016), whereas expression of WDLA and WDLB is the highest in developing cotton fibres (Lei et al., 2019). Members of the WDLA clade in cotton are strongly up-regulated 20 d post-anthesis (Lei et al., 2019). MAP20 is expressed mostly during the late differentiation stages of phloem and xylem in poplar and Brachypodium distachyon (Rajangam et al., 2008a; Smertenko et al., 2020).
In summary, the hodgepodge of domain architecture within members of TPX2-family proteins taken together with the transcriptomics data indicates that the functions of these proteins were evolved to fine-tune microtubule dynamics in the context of distinct, but highly specialized cellular processes (Supplemental Table 2).
Functions of TPX2-family proteins during cell division
Activation of Aurora kinase and targeting of Aurora kinase to microtubules is an essential activity of animal TPX2. Animal Aurora kinase regulates mitotic spindle assembly and karyokinesis (Kufer et al., 2002; Wadsworth, 2015). Arabidopsis thaliana Aurora kinase, in addition to the above functions, also contributes to establishing new tissues by orienting formative cell divisions (Van Damme et al., 2011). Animal Aurora kinase requires activation, and one mechanism is autophosphorylation of the activation loop (reviewed in Willems et al., 2018). Using ancestor sequence reconstruction, it was shown that TPX2 is the second mechanism to evolve for allosteric activation of Aurora A in humans (Hadzipasic et al., 2020).
Activation of Aurora kinase by TPX2 family proteins in plants is supported by several observations in A. thaliana. First, TPXL2 and TPXL3 interact with Aurora 1 and 2 under high-stringency tandem affinity tag purification and yeast two-hybrid analyses, and TPX2 interacts with both kinases only under the least stringent wash conditions (Boruc et al., 2019). Fluorescent lifetime microscopy confirms stronger interaction of Aurora 1–red fluorescent protein (RFP) with green fluorescent protein (GFP) fusions of TPXL2 or TPXL3 than with TPX2–GFP (Boruc et al., 2019). Secondly, either full-length A. thaliana TPX2 or its first 100 amino acids which include the Aurora-binding domain enhance both Aurora 1 autophosphorylation activity and the downstream histone 3 phosphorylation activity (Tomastikova et al., 2015). N-terminal domains of TPXL2 and TPXL3 also activate Aurora 1 kinase (Boruc et al., 2019).
Notably, Aurora 1 phosphorylates both full-length TPX2 and TPX2 lacking the Aurora-binding domain (Tomastikova et al., 2015). Candidate Aurora phosphorylation sites were also identified in TPXL2 and TPXL3 (Boruc et al., 2019). Currently, the role of TPXL phosphorylation by Aurora remains unknown. Thus, plant TPXL can bind and activate Aurora 1 through the conserved Aurora-binding domain on the N-terminus and Aurora 1 can phosphorylate TPX2 independently of the binding domain.
TPXLs exhibit unique co-localization pattern with microtubule arrays. Antibody against human TPX2 labelled nuclei prior to nuclear envelope breakdown (NEB), mitotic spindle and spindle poles, but not the phragmoplast in Nicotiana tabacum BY-2 cells (Vos et al., 2008). Similar localization during mitosis was reported in Arabidopsis root cells stably expressing GFP–TPX2 (Petrovska et al., 2012, 2013; Boruc et al., 2019). TPXL2 and TPXL3 localize to the nuclear envelope during prophase, associate with mitotic spindle microtubules, and concentrate around daughter nuclei after anaphase. In addition, TPXL3 localizes to the phragmoplast distal zone (Boruc et al., 2019).
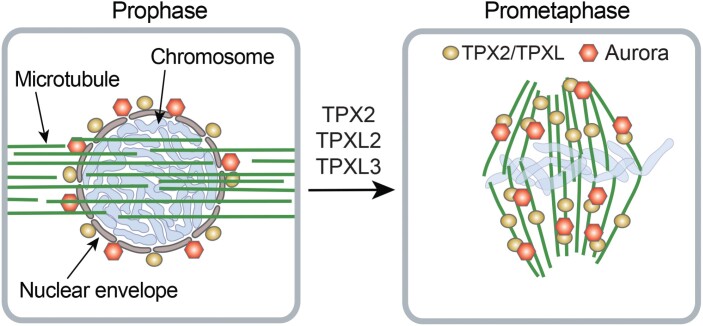
TPXL proteins play an essential role in mitosis. Microinjecting the anti-human TPX2 into Tradescantia stamen hair cells prevented the onset of mitosis and NEB (Vos et al., 2008). Knockout of TPXL3 in Arabidopsis resulted in embryo lethality (Boruc et al., 2019). In contrast, the TPXL2 loss-of-function allele tpxl2 and two TPX2 loss-of-function alleles tpx2-3 and tpx2-4 exhibited no discernible phenotype (Boruc et al., 2019). These observations suggest functional redundancy between TPX2 and TPXL proteins, but a unique role for TPXL3 (Fig. 4).
Fig. 4.
Role of TPXL proteins in cell division. TPX2, TPXL2, and TPXL3 localize to the nuclear envelope, but not to the microtubules of the pre-prophase band during prophase. Aurora kinase also localizes to the nuclear envelope membrane. It is currently not known whether TPXLs and Aurora interact on the nuclear envelope and require each other for this localization. TPXLs function redundantly in promoting nuclear envelope breakdown and mitotic spindle assembly. In the mitotic spindle, TPXLs target Aurora kinase to microtubules and activate it.
The role of TPXLs in Aurora targeting was thus far addressed only in interphase cells. These experiments were facilitated by the discovery that ectopic expression of GFP–TPX2 or GFP–TPXL3 in Nicotiana benthamiana leaf pavement cells results in labelling of the intranuclear microtubules, whereas TPXL2 and Aurora 1 localize in the nucleoplasm (Petrovska et al., 2013; Boruc et al., 2019). Co-expression of TPXL3 with Aurora 1 was sufficient for targeting Aurora 1 to the intranuclear microtubules; co-expression of TPXL2 and Aurora 1 resulted in both proteins associating with microtubules; and, somewhat surprisingly, Aurora 1 caused dissociation of TPX2 from intranuclear microtubules (Boruc et al., 2019). Transient overexpression experiments in the interphase cells showed microtubule and nuclear localization of TPXL1, TPXL2, TPXL3, TPX4, and TPX8; microtubule only localization of TPXL5 and TPXL6; and nuclear envelope only localization of TPXL7 (Tomastikova et al., 2020). Co-expression of these proteins with Aurora kinase relocated TPXL7 into the nucleus and caused stronger nuclear labelling of other proteins, with the exception of TPXL1 (Tomastikova et al., 2020). This outcome suggests involvement of many TPXLs in Aurora localization and activation (Fig. 4; Supplementary Table S2).
The origin of intranuclear microtubules and their function remain unclear. Although plant nuclei contain tubulin (Schwarzerova et al., 2019), intranuclear microtubules were not reported using conventional live-cell imaging probes. Plausibly, ectopic TPXL expression stimulates microtubule assembly from the nuclear tubulin pool. The next crucial step will be determining how the above findings in vitro and in interphase cells relate to Aurora activation and targeting during mitosis.
TPX2 binds microtubules in aur1/aur2, demonstrating that TPX2 can associate with spindle microtubules independently of Aurora (corroborating findings of studies in animal systems). Aurora 1 localizes to spindle microtubules in tpx2-3 mutant cells, further supporting functional redundancy of canonical TPX2 in plants (Boruc et al., 2019).
In conclusion, of many known TPX2 functions in animals, only activation of Aurora kinase has been examined in plants thus far. Furthermore, at least three plant TPXL proteins appear to be responsible for the mitotic activities of single animal TPX2. Considering profound differences of spindle construction between plants and animals, one of which is lacking structurally defined spindle poles, TPXL proteins might play many unique roles in plant mitosis.
Functions of WDLs in anisotropic cell expansion and signalling
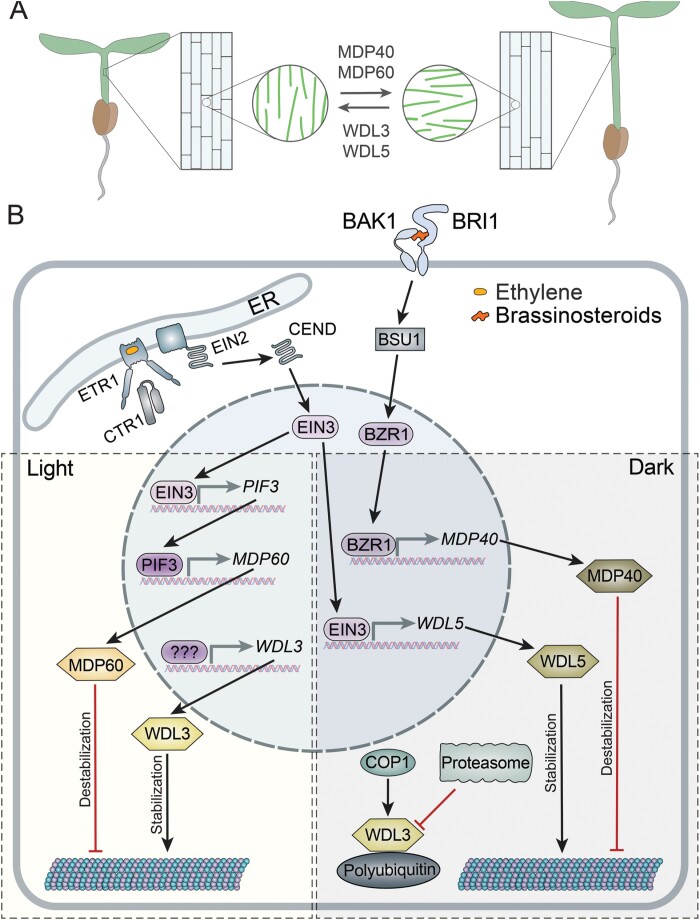
Orientation of cortical microtubules governs the direction of cell expansion by influencing orientation of cellulose microfibril deposition in the cell wall (Burk and Ye, 2002; Bringmann et al., 2012a). In expanding cells, the expansion axis is generally perpendicular to the overall orientation of microtubules, and consequentially the cellulose microfibrils (Crowell et al., 2009). Cortical microtubules influence the orientation of cellulose microfibrils by serving as tracks for movement of cellulose synthase complexes (Paredez et al., 2006; Gutierrez et al., 2009). As cell expansion drives plant morphogenesis, microtubule organization appears to be a key effector in many hormonal pathways (Shaw, 2013; Vineyard et al., 2013; Zhang and Zhang, 2016), though mechanistic understanding of this phenomenon remains limited. Several A. thaliana WDLs, WDL3, WDL5, MDP60, and MDP40, contribute to regulation of microtubule orientation, cell expansion, and hypocotyl elongation in response to ethylene, brassinosteroids, and light (Fig. 5A; Supplementary Table S2).
Fig. 5.
Antagonistic interactions between WDLs modulate hypocotyl elongation. (A) WDL3 and WDL5 stabilize microtubules, facilitate longitudinal orientation of microtubules, and reduce cell elongation, whereas MDP40 and MDP60 destabilize microtubules, promote transverse microtubule arrays, and stimulate cell elongation. The balance between activity of these proteins could fine-tune hypocotyl length under either light or dark conditions. (B) Regulation of WDL3, WDL5, MDP40, and MDP60 by light and hormones. Ethylene binding to ETR1 in the endoplasmic reticulum (ER) causes inactivation of kinase CTR1. This results in cleavage of EIN2 C-terminal peptide (CEND). CEND is relocated to the nucleus and activates the transcription factor EIN3. Under light conditions, EIN3 promotes transcription of PIF3; PIF3 promotes transcription of MDP60; MDP60 destabilizes microtubules. WDL3 expression under light promotes microtubule stability. It remains unknown which pathway regulates transcription of WDL3. WDL3 could counteract MDP60. In the dark, the cytoplasmic COP1 ubiquitinates WDL3 and triggers its degradation. In parallel, EIN3 promotes transcription of WDL5, and WDL5 stabilizes microtubules. Brassinosteroids bind the BRI1–BAK1 receptor complex that, through several cytoplasmic proteins, including BSU1, activates BZR1. BZR1 promotes transcription of MDP40. Destabilization of microtubules by MDP40 could counteract WDL5. Activating interactions are shown in red and inhibiting interactions are shown in red.
The idea that WDLs govern cell expansion was originally proposed by Yuen and co-authors based on reduction of cell length in roots of A. thaliana plants overexpressing A. thaliana WVD2 or WDL1 (Yuen et al., 2003). Roots in these plants were shorter and leaves, siliques, and etiolated hypocotyls were smaller. Furthermore, the epidermal cells of etiolated hypocotyls exhibited right-handed circumferential rotation and rosette leaves exhibited left-handed circumferential rotation. The knockout phenotype of WVD2 and WDL1 has not been reported thus far, but analysis of mutants in other WDL genes in A. thaliana supports the importance of these proteins in cell expansion.
Biochemical properties of WDLs
All A. thaliana WDLs studied thus far bind microtubules in vivo or in vitro. Eucalyptus WDL3–yellow fluorescent protein (YFP) and WDL3L–GFP localize to cortical microtubules in tobacco leaf pavement cells (Du et al., 2016; Lei et al., 2019). Cotton WDLA2 and WDLA7 also bind cortical microtubules in tobacco leaf pavement cells and interact with α-tubulin TUA2 in yeast two-hybrid assay, whereas WDLA4 and WDLA9 show diffused localization in the cytoplasm and nucleus (Lei et al., 2019).
Arabidopsis thaliana WVD2, WDL3, and WDL5 stabilize microtubules. For example, WDL3 and WDL5 reduce dilution-induced depolymerization of microtubules in vitro, and cortical microtubules in WDL5 knockout or WDL3 knockdown lines are more sensitive to the inhibitor of microtubule polymerization, oryzalin (Liu et al., 2013; Sun et al., 2015). Overexpression of WDL3 in A. thaliana increases microtubule tolerance to oryzalin (Liu et al., 2013). Furthermore, these proteins can also bundle microtubules. Structural illumination microscopy reveals reduced microtubule bundling in cells of A. thaliana wdl5-1 etiolated hypocotyls relative to the wild-type control (Ma et al., 2016). All three proteins produce microtubule bundles in vitro, but electron microscopy shows that microtubules in bundles induced by WDL3 and WVD2 are ‘glued’ together without an apparent linker (Perrin et al., 2007; Liu et al., 2013; Sun et al., 2015). Overexpression of WVD2 in A. thaliana seedlings increases bundling of microtubules (zippering) and reduces the frequency of catastrophe events compared with controls (Perrin et al., 2007).
In contrast, A. thaliana MDP60 and MDP40 destabilize microtubules (Wang et al., 2012; Ma et al., 2018). MDP60 binds microtubules in vitro and in vivo (Ma et al., 2018). Incubation of microtubules with MDP60 in vitro generates somewhat shorter microtubules, and overexpression of MDP60 increases microtubule sensitivity to oryzalin. MDP60 knockout makes microtubules more tolerant to oryzalin treatment, implying that MDP60 acts through direct interaction with the microtubule lattice.
Knockdown of MDP40 in A. thaliana also makes microtubules more tolerant to oryzalin treatment (Wang et al., 2012). Although MDP40–GFP binds microtubules in vivo, direct interaction between MDP40 and microtubules has not yet been examined (Wang et al., 2012). This indicates that MDP40 could destabilize microtubules by affecting the activity of known microtubule-severing factors or through interaction with other proteins. If the WAND functions as a protein interface, MDP40 could localize to microtubules through interaction with this domain of WDL7, WDL8, or WDL9.
The smallest family member, MAP20, was reported in aspen as a target of the cell wall synthesis herbicide 2,6-dichlorobenzonitrile (Rajangam et al., 2008a). GFP fusions of MAP20 from aspen, Eucalyptus, and B. distachyon were shown to bind microtubules in the interphase cells (Rajangam et al., 2008a; Du et al., 2016; Smertenko et al., 2020). Furthermore, aspen and B. distachyon MAP20 can directly bind and stabilize microtubules in vitro (Rajangam et al., 2008a; Smertenko et al., 2020). Brachypodium distachyon MAP20 suppresses microtubule depolymerization by reducing tubulin loss from microtubule ends (Smertenko et al., 2020). Overexpression of aspen or Eucalyptus MAP20 in Arabidopsis perturbs normal cell expansion and causes deformation and twisting of cotyledon and hypocotyl similarly to the wvd2 phenotype (Yuen et al., 2003; Du et al., 2016). Comparison of B. distachyon MAP20 activity with that of Xenopus TPX2 in the Xenopus egg total protein extract demonstrated that MAP20 does not facilitate microtubule nucleation by γ-TuRC, but promotes microtubule elongation (Smertenko et al., 2020). Collectively, MAP20, WVD2, WDL3, and WDL5 act as microtubule stabilization factors in vitro and in vivo.
Ethylene and light signalling
Hypocotyl length is controlled by light and phytohormone signals, which modulate cell length and microtubule orientation (Feng et al., 2008; Pierik et al., 2009). Dark-induced hypocotyl cell elongation is accompanied by transverse orientation of microtubules, whereas suppression of cell elongation by light results in oblique and longitudinal microtubules (Sambade et al., 2012). Ethylene plays an important role in this process; exposure to ethylene promotes formation of longitudinal microtubules and inhibits cell elongation, resulting in a short, thick, curled plant phenotype (Guzman and Ecker, 1990). Ethylene signalling occurs through a well-studied pathway consisting of endoplasmic reticulum membrane-bound protein EIN2 (Ethylene Insensitive 2). The C-terminal region of EIN2 (CEND) is cleaved and localizes to the nucleus where it activates transcription factors EIN3 and EIL1 that drive the expression of ethylene-responsive genes (reviewed in Dolgikh et al., 2019). Out of these two transcription factors, EIN3 plays an essential role in regulating expression of several TPX2-family proteins.
One of the targets of EIN3 in A. thaliana is WDL5 (Fig. 5B). EIN3 binds to the WDL5 promoter through three conserved motifs and promotes WDL5 transcription in response to ethylene (Sun et al., 2015). Several observations demonstrate that ethylene promotes microtubule stability through WDL5. First, pre-treatment of seedlings with the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) makes microtubules more stable, resulting in lower susceptibility to oryzalin in control but not in wdl5-1 plants (Sun et al., 2015). Secondly, Sun et al. showed that microtubules are less stable in the ethylene-unresponsive mutants ein2-5 and ein3eil1 (Alonso et al., 2003; Wang et al., 2007) and more stable in the constitutively activated ethylene pathway mutant ctr1-1 (Ma et al., 2016).
Impaired microtubule stability in the A. thaliana wdl5-1 knockout allele correlates with abrogation of microtubule response to ethylene. Microtubules in hypocotyl epidermal cells of both ein2-5 and wdl5-1 alleles stay predominantly transverse even after ACC treatment (Ma et al., 2016), whereas microtubules in ctr1-1 are mostly longitudinal, oblique, or random even without ACC treatment (Ma et al., 2016). Epidermis cells of etiolated hypocotyls of wdl5-1 were longer than those of control plants either with or without ACC treatment (Ma et al., 2016). Consequently, etiolated hypocotyls of wdl5-1 were longer, indicating that WDL5 functions in ethylene-dependent inhibition of cell elongation.
Another target of ethylene is phytochrome-interacting factor 3 (PIF3) (Zhong et al., 2012). Light suppresses hypocotyl elongation by inducing degradation of PIF3 whereas ethylene promotes hypocotyl elongation by up-regulating PIF3 expression. PIF3, in turn, binds the MDP60 promoter (Ma et al., 2018). Consequently, both ethylene and PIF3 up-regulate MDP60 transcription (Fig. 5B). MDP60 promotes assembly of transverse microtubules (Ma et al., 2018). Consequently, the frequency of transverse microtubules in response to light or ACC depends on the MDP60 gene dosage. Hypocotyl epidermal cells and hypocotyls of light-grown mdp60 knockout alleles are shorter than in control seedlings, whereas overexpression of MDP60 in the wild-type background causes longer hypocotyl epidermal cells and longer hypocotyls (Ma et al., 2018). Furthermore, MDP60 overexpression can rescue the shorter hypocotyl phenotype in the pif3 or ethylene-insensitive ein2 mutant backgrounds. Thus, MDP60 mediates ethylene-induced transverse orientation of microtubules and hypocotyl elongation in response to light (Fig. 5A).
COP1-dependent signalling
Another important regulator of light signalling is an ubiquitin E3 ligase CONSTITUTIVE PHOTOMORPHOGENESIS 1 (COP1) (Deng et al., 1992; Lau and Deng, 2012). COP1 represses photomorphogenic responses, such as hypocotyl elongation, by conjugating ubiquitin with positive regulators of photomorphogenesis for degradation by the 26S proteasome (Holm et al., 2002; Seo et al., 2004). In dark-grown plants, COP1 primarily localizes to the nucleus; however, some is also present in the cytoplasm (reviewed in Lau and Deng, 2012). Cytoplasmic COP1 was shown to interact with A. thaliana WDL3 in pull-down, yeast two-hybrid, firefly luciferase complementation imaging, and co-immunoprecipitation assays (Lian et al., 2017). Bimolecular fluorescence complementation and in vitro fluorescent microtubule assays showed interaction between WDL3 and COP1 at the cortical microtubules (Lian et al., 2017).
WDL3 transcript was detected in both light- and dark-grown A. thaliana seedlings; however, WDL3–GFP was only observed in light-grown seedlings. Treatment with a 26S proteasome inhibitor restored WDL3–GFP in dark-grown seedlings, suggesting that the 26S proteasome is responsible for WDL3 degradation (Liu et al., 2013; Lian et al., 2017). COP1 is responsible for ubiquitination of WDL3 prior to the degradation (Fig. 5B).
WDL3 promotes formation of longitudinal microtubule arrays in the cells of light-grown seedlings (Fig. 5A). Consequently, a transverse to longitudinal switch of microtubules in hypocotyl cells during light response was partially abrogated in WDL3 RNAi plants. The light-grown WDL3 RNAi seedlings had longer hypocotyl epidermal cells and longer hypocotyls, while overexpression of WDL3 resulted in shorter cells and shorter hypocotyls. Furthermore, WDL3 knockdown can partially rescue reduced dark-induced hypocotyl elongation in cop1-6 mutants (Lian et al., 2017). Thus, WDL3 is a downstream factor of COP1-mediated hypocotyl cell elongation (Fig. 5A, B).
Brassinosteroid signalling
Brassinosteroids regulate almost all aspects of plant life including cell elongation and hypocotyl growth through the transcription factor BRASSINAZOLE-RESISTANT1 (BZR1) (reviewed in Nolan et al., 2020). Although several upstream components of brassinosteroid signalling have been linked to regulation of hypocotyl growth through BZR1 phosphorylation, the downstream effectors remain less understood (reviewed in Nolan et al., 2020).
BZR1 binds to the MDP40 promoter and up-regulates MDP40 transcription in A. thaliana (Fig. 5B) (Wang et al., 2012). Treatment of seedlings with the brassinosteroid, brassinolide, induces formation of transverse cortical microtubule arrays in cotyledon epidermal cells of the wild type but not in the MDP40 knockdown seedlings grown in the dark. Etiolated hypocotyls of MDP40 RNAi knockdown lines were shorter than those in wild-type seedlings (Wang et al., 2012). Overexpression of MDP40 rescues the short cell phenotype in the hypocotyl of the brassinosteroid synthesis-deficient mutant, det2 (Chory et al., 1991). This indicates that MDP40 contributes to brassinosteroid-induced hypocotyl elongation in the dark.
Stress adaptation
WDLs play an essential role in response and adaptation to environmental stresses. Salt stress was found to activate WDL5 transcription through the ethylene pathway in A. thaliana seedlings (Dou et al., 2018). One of the salt stress phenotypes is depolymerization of microtubules, and plant survival depends on the ability of cells to re-polymerize microtubules (reviewed in Wang and Mao, 2019). Several observations highlight the importance of ethylene in this process. First, microtubules in ein2-5 hypocotyl and cotyledon epidermal cells are hypersensitive to depolymerization under NaCl treatment (Dou et al., 2018). Secondly, ein3eil1 double knockouts failed to reassemble microtubules in cotyledon pavement cells under salt stress. WDL5 appears to be the ethylene effector responsible for re-polymerization of microtubules. wdl5-1 cells frequently failed to reassemble microtubules after 30–48 h of salt stress, whereas overexpression of WDL5 resulted in faster regeneration of the microtubule network and nearly doubled plant survival in medium with 200 mM NaCl. Furthermore, WDL5 overexpression rescues microtubule reassembly defects and the salt susceptibility phenotype of ein3eil1 double knockouts (Dou et al., 2018).
MDP60 contributes to hypocotyl elongation in response to submergence stress in A. thaliana. Submergence causes a microtubule orientation switch from longitudinal to transverse (Wang et al., 2020). Two facts suggest that MDP60 is essential for this response: MDP60 transcription increases after 2 h of seedling submergence, and MDP60 knockout partially abrogates this microtubule reorientation. Up-regulation of MDP60 transcription and microtubule re-orientation is suppressed in the ethylene-insensitive mutant ein2, suggesting similarities between regulation of microtubule orientation in response to light and stress (Wang et al., 2020).
MAP20 appears to be essential for adaptation to drought in B. distachyon. MAP20 localizes to the edges of vascular pits in developing xylem cells (Smertenko et al., 2020). Pits play a dual role in facilitating transport of solutions through the xylem during the rainy season and preventing the spread of air pockets (embolisms) under drought (Zwieniecki and Holbrook, 2000; Choat et al., 2008; Pittermann, 2010). Knockdown of MAP20 in B. distachyon resulted in larger pits with thinner pit membranes and greater drought susceptibility (Smertenko et al., 2020). However, the cell wall thickness and cellulose content in the mutant was reduced only slightly, suggesting a non-linear relationship between the MAP20 expression level and cell wall synthesis (Smertenko et al., 2020). Determining pit architecture in xylem cells is likely to be one of many MAP20 functions.
Concluding remarks
TPX2-family proteins deserve attention for their exciting evolutionary history and functional diversity. Gaining insight into the activity of these proteins will advance our knowledge about regulation of microtubules in the context of different processes while enabling the development of predictive models of plant responses to environmental and developmental cues. Sustaining progress in analysis of these proteins requires addressing the following challenges. (i) Characterize the functions of all members of TPX2-family proteins (Supplementary Table S2). (ii) Determine how TPX2-family proteins promote microtubule polymerization and organization both in vivo and in vitro; how MDP60 and MDP40 destabilize microtubules. (iii) Advance understanding of the relationships between Aurora kinase and TPXL proteins; determine why some TPXLs have lost TPX2-C; what is special about the functions of TPXL3; what are the functions of TPXL phosphorylation by Aurora. In addition, understanding the role of plant TPX2 protein if it is not the main factor targeting Aurora to spindle microtubules. (iv) Determine whether TPXLs are regulated by importins, can interact with kinesins, can activate γ-TuRC, and can nucleate microtubules. (v) Characterize WAND and find out why MDP40 contains a WAND but has lost all TPX2 domains.
Another intriguing question is whether WDL proteins have activities beyond regulation of microtubule stability during interphase, such as activation and targeting to microtubules of proteins kinases or signalling factors.
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Positions of conserved domains in Arabidopsis TPX2-family proteins.
Table S2. Functions of TPXL/WDL-family proteins.
Acknowledgements
The authors appreciate helpful insights from Miyoung Lee. Research in Andrei Smertenko’s laboratory is supported by National Science Foundation-CAREER award #1751204 and United States Department of Agriculture - National Institute of Food and Agriculture grants #2017-67013-26200 and #1015621.
Author contributions
All authors contributed equally to developing ideas, writing the manuscript draft, and subsequent editing of the draft.
Data availability
All protein sequence data and alignment are available freely upon request.
References
- Alfaro-Aco R, Thawani A, Petry S. 2017. Structural analysis of the role of TPX2 in branching microtubule nucleation. Journal of Cell Biology 216, 983–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. 2003. Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proceedings of the National Academy of Sciences, USA 100, 2992–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI. 2001. On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215, 150–171. [DOI] [PubMed] [Google Scholar]
- Baskin TI. 2005. Anisotropic expansion of the plant cell wall. Annual Review of Cell and Developmental Biology 21, 203–222. [DOI] [PubMed] [Google Scholar]
- Bird AW, Hyman AA. 2008. Building a spindle of the correct length in human cells requires the interaction between TPX2 and Aurora A. Journal of Cell Biology 182, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleti H, Karsenti E, Vernos I. 1996. Xklp2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell 84, 49–59. [DOI] [PubMed] [Google Scholar]
- Boruc J, Deng X, Mylle E, et al. 2019. TPX2-LIKE PROTEIN3 is the primary activator of α-aurora kinases and is essential for embryogenesis. Plant Physiology 180, 1389–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann M, Landrein B, Schudoma C, Hamant O, Hauser MT, Persson S. 2012a. Cracking the elusive alignment hypothesis: the microtubule–cellulose synthase nexus unraveled. Trends in Plant Science 17, 666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann M, Li E, Sampathkumar A, Kocabek T, Hauser MT, Persson S. 2012b. POM-POM2/cellulose synthase interacting1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. The Plant Cell 24, 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Sardon T, Zimmerman T, Wittmann T, Pepperkok R, Karsenti E, Vernos I. 2004. Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts. Molecular Biology of the Cell 15, 5318–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk DH, Ye Z-H. 2002. Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. The Plant Cell 14, 2145–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk DH, Zhong R, Ye Z-H. 2007. The katanin microtubule severing protein in plants. Journal of Integrative Plant Biology 49, 1174–1182. [Google Scholar]
- Carazo-Salas RE, Guarguaglini G, Gruss OJ, Segref A, Karsenti E, Mattaj IW. 1999. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 400, 178–181. [DOI] [PubMed] [Google Scholar]
- Choat B, Cobb AR, Jansen S. 2008. Structure and function of bordered pits: new discoveries and impacts on whole-plant hydraulic function. New Phytologist 177, 608–625. [DOI] [PubMed] [Google Scholar]
- Chory J, Nagpal P, Peto CA. 1991. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. The Plant Cell 3, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell EF, Bischoff V, Desprez T, Rolland A, Stierhof YD, Schumacher K, Gonneau M, Höfte H, Vernhettes S. 2009. Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. The Plant Cell 21, 1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XW, Matsui M, Wei N, Wagner D, Chu AM, Feldmann KA, Quail PH. 1992. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell 71, 791–801. [DOI] [PubMed] [Google Scholar]
- Dolgikh VA, Pukhovaya EM, Zemlyanskaya EV. 2019. Shaping ethylene response: the role of EIN3/EIL1 transcription factors. Frontiers in Plant Science 10, 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou L, He K, Higaki T, Wang X, Mao T. 2018. Ethylene signaling modulates cortical microtubule reassembly in response to salt stress. Plant Physiology 176, 2071–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du P, Kumar M, Yao Y, Xie Q, Wang J, Zhang B, Gan S, Wang Y, Wu AM. 2016. Genome-wide analysis of the TPX2 family proteins in Eucalyptus grandis. BMC Genomics 17, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard JL, Pieuchot L, Vos JW, Vernos I, Schmit AC. 2009. Plant TPX2 and related proteins. Plant Signaling & Behavior 4, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G, et al. 2008. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G. 2011. Identification of a TPX2-like microtubule-associated protein in Drosophila. PLoS One 6, e28120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW. 2001. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell 104, 83–93. [DOI] [PubMed] [Google Scholar]
- Gruss OJ, Wittmann M, Yokoyama H, Pepperkok R, Kufer T, Silljé H, Karsenti E, Mattaj IW, Vernos I. 2002. Chromosome-induced microtubule assembly mediated by TPX2 is required for spindle formation in HeLa cells. Nature Cell Biology 4, 871–879. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Lindeboom JJ, Paredez AR, Emons AM, Ehrhardt DW. 2009. Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nature Cell Biology 11, 797–806. [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadzipasic A, Wilson C, Nguyen V, Kern N, Kim C, Pitsawong W, Villali J, Zheng Y, Kern D. 2020. Ancient origins of allosteric activation in a Ser-Thr kinase. Science 367, 912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T. 2014. Microtubule organization and microtubule-associated proteins in plant cells. International Review of Cell and Molecular Biology 312, 1–52. [DOI] [PubMed] [Google Scholar]
- Hashimoto T. 2013. A ring for all: γ-tubulin-containing nucleation complexes in acentrosomal plant microtubule arrays. Current Opinion in Plant Biology 16, 698–703. [DOI] [PubMed] [Google Scholar]
- Holm M, Ma LG, Qu LJ, Deng XW. 2002. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes & Development 16, 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolev AV, Buschmann H, Doonan JH, Lloyd CW. 2007. AtMAP70-5, a divergent member of the MAP70 family of microtubule-associated proteins, is required for anisotropic cell growth in Arabidopsis. Journal of Cell Science 120, 2241–2247. [DOI] [PubMed] [Google Scholar]
- Korolev AV, Chan J, Naldrett MJ, Doonan JH, Lloyd CW. 2005. Identification of a novel family of 70 kDa microtubule-associated proteins in Arabidopsis cells. The Plant Journal 42, 547–555. [DOI] [PubMed] [Google Scholar]
- Kozgunova E, Yoshida MW, Goshima G. 2020. Spindle position dictates division site during asymmetric cell division in moss. bioRxiv, 2020.2003.2003.975557. [Preprint]. [Google Scholar]
- Kufer TA, Silljé HH, Körner R, Gruss OJ, Meraldi P, Nigg EA. 2002. Human TPX2 is required for targeting Aurora-A kinase to the spindle. Journal of Cell Biology 158, 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Deng XW. 2012. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends in Plant Science 17, 584–593. [DOI] [PubMed] [Google Scholar]
- Lei K, Liu AY, Fan SM, et al. 2019. Identification of TPX2 gene family in upland cotton and its functional analysis in cotton fiber development. Genes 10, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian N, Liu X, Wang X, Zhou Y, Li H, Li J, Mao T. 2017. COP1 mediates dark-specific degradation of microtubule-associated protein WDL3 in regulating Arabidopsis hypocotyl elongation. Proceedings of the National Academy of Sciences, USA 114, 12321–12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom JJ, Nakamura M, Hibbel A, Shundyak K, Gutierrez R, Ketelaar T, Emons AM, Mulder BM, Kirik V, Ehrhardt DW. 2013. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342, 1245533. [DOI] [PubMed] [Google Scholar]
- Liu X, Qin T, Ma Q, Sun J, Liu Z, Yuan M, Mao T. 2013. Light-regulated hypocotyl elongation involves proteasome-dependent degradation of the microtubule regulatory protein WDL3 in Arabidopsis. The Plant Cell 25, 1740–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Sun J, Mao T. 2016. Microtubule bundling plays a role in ethylene-mediated cortical microtubule reorientation in etiolated Arabidopsis hypocotyls. Journal of Cell Science 129, 2043–2051. [DOI] [PubMed] [Google Scholar]
- Ma Q, Wang X, Sun J, Mao T. 2018. Coordinated regulation of hypocotyl cell elongation by light and ethylene through a microtubule destabilizing protein. Plant Physiology 176, 678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumayer G, Helfricht A, Shim SY, et al. 2012. Targeting protein for Xenopus kinesin-like protein 2 (TPX2) regulates γ-histone 2AX (γ-H2AX) levels upon ionizing radiation. Journal of Biological Chemistry 287, 42206–42222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan TM, Vukašinović N, Liu D, Russinova E, Yin Y. 2020. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. The Plant Cell 32, 295–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozlü N, Srayko M, Kinoshita K, Habermann B, O’Toole ET, Müller-Reichert T, Schmalz N, Desai A, Hyman AA. 2005. An essential function of the C. elegans ortholog of TPX2 is to localize activated aurora A kinase to mitotic spindles. Developmental Cell 9, 237–248. [DOI] [PubMed] [Google Scholar]
- Paredez AR, Somerville CR, Ehrhardt DW. 2006. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312, 1491–1495. [DOI] [PubMed] [Google Scholar]
- Perrin RM, Wang Y, Yuen CY, Will J, Masson PH. 2007. WVD2 is a novel microtubule-associated protein in Arabidopsis thaliana. The Plant Journal 49, 961–971. [DOI] [PubMed] [Google Scholar]
- Petrovská B, Cenklová V, Pochylová Z, Kourová H, Doskočilová A, Plíhal O, Binarová L, Binarová P. 2012. Plant Aurora kinases play a role in maintenance of primary meristems and control of endoreduplication. New Phytologist 193, 590–604. [DOI] [PubMed] [Google Scholar]
- Petrovská B, Jerábková H, Kohoutová L, et al. 2013. Overexpressed TPX2 causes ectopic formation of microtubular arrays in the nuclei of acentrosomal plant cells. Journal of Experimental Botany 64, 4575–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry S, Groen AC, Ishihara K, Mitchison TJ, Vale RD. 2013. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Djakovic-Petrovic T, Keuskamp DH, de Wit M, Voesenek LA. 2009. Auxin and ethylene regulate elongation responses to neighbor proximity signals independent of gibberellin and della proteins in Arabidopsis. Plant Physiology 149, 1701–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittermann J. 2010. The evolution of water transport in plants: an integrated approach. Geobiology 8, 112–139. [DOI] [PubMed] [Google Scholar]
- Rajangam AS, Kumar M, Aspeborg H, et al. 2008. a. MAP20, a microtubule-associated protein in the secondary cell walls of hybrid aspen, is a target of the cellulose synthesis inhibitor 2,6-dichlorobenzonitrile. Plant Physiology 148, 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajangam AS, Yang H, Teeri TT, Arvestad L. 2008b. Evolution of a domain conserved in microtubule-associated proteins of eukaryotes. Advances and Applications in Bioinformatics and Chemistry 1, 51–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid TA, Schuster BM, Mann BJ, Balchand SK, Plooster M, McClellan M, Coombes CE, Wadsworth P, Gardner MK. 2016. Suppression of microtubule assembly kinetics by the mitotic protein TPX2. Journal of Cell Science 129, 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roostalu J, Cade NI, Surrey T. 2015. Complementary activities of TPX2 and chTOG constitute an efficient importin-regulated microtubule nucleation module. Nature Cell Biology 17, 1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambade A, Pratap A, Buschmann H, Morris RJ, Lloyd C. 2012. The influence of light on microtubule dynamics and alignment in the Arabidopsis hypocotyl. The Plant Cell 24, 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz CA, Santarella R, Hoenger A, Karsenti E, Mattaj IW, Gruss OJ, Carazo-Salas RE. 2003. Importin α-regulated nucleation of microtubules by TPX2. The EMBO Journal 22, 2060–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzerová K, Bellinvia E, Martinek J, Sikorová L, Dostál V, Libusová L, Bokvaj P, Fischer L, Schmit AC, Nick P. 2019. Tubulin is actively exported from the nucleus through the Exportin1/CRM1 pathway. Scientific Reports 9, 5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HS, Watanabe E, Tokutomi S, Nagatani A, Chua NH. 2004. Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes & Development 18, 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL. 2013. Reorganization of the plant cortical microtubule array. Current Opinion in Plant Biology 16, 693–697. [DOI] [PubMed] [Google Scholar]
- Smertenko T, Turner G, Fahy D, Brew-Appiah RAT, Alfaro-Aco R, Engler JD, Sanguinet KA, Smertenko A. 2020. Brachypodium distachyon MAP20 functions in metaxylem pit development and contributes to drought recovery. New Phytologist 227, 1681–1695. [DOI] [PubMed] [Google Scholar]
- Sun JB, Ma QQ, Mao TL. 2015. Ethylene regulates the Arabidopsis microtubule-associated protein WAVE-DAMPENED2-LIKE5 in etiolated hypocotyl elongation. Plant Physiology 169, 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomastikova E, Demidov D, Jerabkova H, Binarova P, Houben A, Dolezel J, Petrovska B. 2015. TPX2 protein of Arabidopsis activates Aurora kinase 1, but not Aurora kinase 3 in vitro. Plant Molecular Biology Reporter 33, 1988–1995. [Google Scholar]
- Tomastikova ED, Rutten T, Dvorak P, Tugai A, Ptoskova K, Petrovska B, van Damme D, Houben A, Dolezel J, Demidov D. 2020. Functional divergence of microtubule-associated TPX2 family members in Arabidopsis thaliana. International Journal of Molecular Sciences 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twell D, Park SK, Hawkins TJ, Schubert D, Schmidt R, Smertenko A, Hussey PJ. 2002. MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nature Cell Biology 4, 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme D, De Rybel B, Gudesblat G, Demidov D, Grunewald W, De Smet I, Houben A, Beeckman T, Russinova E. 2011. Arabidopsis α Aurora kinases function in formative cell division plane orientation. The Plant Cell 23, 4013–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineyard L, Elliott A, Dhingra S, Lucas JR, Shaw SL. 2013. Progressive transverse microtubule array organization in hormone-induced Arabidopsis hypocotyl cells. The Plant Cell 25, 662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos JW, Pieuchot L, Evrard JL, Janski N, Bergdoll M, de Ronde D, Perez LH, Sardon T, Vernos I, Schmit AC. 2008. The plant TPX2 protein regulates prospindle assembly before nuclear envelope breakdown. The Plant Cell 20, 2783–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P. 2015. TPX2. Current Biology 25, R1156–R1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma Q, Wang R, Wang P, Liu Y, Mao T. 2020. Submergence stress-induced hypocotyl elongation through ethylene signaling-mediated regulation of cortical microtubules in Arabidopsis. Journal of Experimental Botany 71, 1067–1077. [DOI] [PubMed] [Google Scholar]
- Wang X, Mao T. 2019. Understanding the functions and mechanisms of plant cytoskeleton in response to environmental signals. Current Opinion in Plant Biology 52, 86–96. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang J, Yuan M, Ehrhardt DW, Wang Z, Mao T. 2012. Arabidopsis microtubule destabilizing protein40 is involved in brassinosteroid regulation of hypocotyl elongation. The Plant Cell 24, 4012–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu C, Li K, et al. 2007. Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Molecular Biology 64, 633–644. [DOI] [PubMed] [Google Scholar]
- Wasteneys GO. 2002. Microtubule organization in the green kingdom: chaos or self-order? Journal of Cell Science 115, 1345–1354. [DOI] [PubMed] [Google Scholar]
- Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, Wasteneys GO. 2001. MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610–613. [DOI] [PubMed] [Google Scholar]
- Wieczorek M, Bechstedt S, Chaaban S, Brouhard GJ. 2015. Microtubule-associated proteins control the kinetics of microtubule nucleation. Nature Cell Biology 17, 907–916. [DOI] [PubMed] [Google Scholar]
- Willems E, Dedobbeleer M, Digregorio M, Lombard A, Lumapat PN, Rogister B. 2018. The functional diversity of Aurora kinases: a comprehensive review. Cell Division 13, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T, Boleti H, Antony C, Karsenti E, Vernos I. 1998. Localization of the kinesin-like protein Xklp2 to spindle poles requires a leucine zipper, a microtubule-associated protein, and dynein. Journal of Cell Biology 143, 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T, Wilm M, Karsenti E, Vernos I. 2000. TPX2, a novel Xenopus MAP involved in spindle pole organization. Journal of Cell Biology 149, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen CY, Pearlman RS, Silo-Suh L, Hilson P, Carroll KL, Masson PH. 2003. WVD2 and WDL1 modulate helical organ growth and anisotropic cell expansion in Arabidopsis. Plant Physiology 131, 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhang W. 2016. Regulation of developmental and environmental signaling by interaction between microtubules and membranes in plant cells. Protein & Cell 7, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Shi H, Xue C, Wang L, Xi Y, Li J, Quail PH, Deng XW, Guo H. 2012. A molecular framework of light-controlled phytohormone action in Arabidopsis. Current Biology 22, 1530–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki MA, Holbrook NM. 2000. Bordered pit structure and vessel wall surface properties. Implications for embolism repair. Plant Physiology 123, 1015–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All protein sequence data and alignment are available freely upon request.