Abstract
This study examined the safety, tolerability, and pharmacokinetics (PK) of napabucasin in healthy Asian and non‐Asian participants and investigated the potential for QT/QTc interval prolongation. This five‐part (A–E) study proceeded in a stepwise manner, unless stopping criteria were met. Parts A–D were randomized, double‐blind, placebo‐controlled, and included healthy Asian male and female and non‐Asian male participants. PK parameters were measured following single‐dose napabucasin (80–1200 mg) in the fasted or fed state (Part D). Potential QT/QTc interval prolongation was assessed using digital 12‐lead electrocardiogram (Parts B and C). Part E was open‐label, and examined the PK of single‐dose napabucasin (240–720 mg) in healthy non‐Asian males. Safety and tolerability were measured in Parts A–E. Changes from baseline in the Fridericia‐corrected QT interval (ΔQTcF) and other electrocardiogram parameters were analyzed using a linear mixed‐effects model. Napabucasin was well‐tolerated across the study (n = 70), and no serious adverse events or significant safety issues were reported when administered with or without food. The most frequent treatment‐emergent adverse events were diarrhea and abdominal pain, and these were mild in severity. No prolongation of the QTcF interval was reported following single‐dose napabucasin (240–1200 mg) and changes in other cardiac parameters were negligible. The PK profile of napabucasin was consistent with earlier studies. Single‐dose napabucasin was tolerated in healthy male and female participants, and no significant safety (including no QTcF prolongation) or tolerability issues were identified, irrespective of food intake. Clinical studies of napabucasin in advanced cancers are ongoing.
Keywords: Asian, healthy volunteers, napabucasin, non‐Asian, pharmacokinetics, QT/QTc interval, safety
This study examined the safety, tolerability and pharmacokinetics of napabucasin in healthy participants and investigated the potential for QT/QTc interval prolongation. Napabucasin was well‐tolerated, with only negligible changes in cardiac parameters detected, and no differences in the pharmacokinetic profile of napabucasin were observed between Asian and non‐Asian participants.

Abbreviations
- AEs
adverse events
- BMI
body mass index
- HR
heart rate
- PK
pharmacokinetics
- ROS
reactive oxygen species
- SAEs
serious AEs
1. INTRODUCTION
Cancer has been the leading cause of death in Japan since the 1980s. 1 All cancer incidence has continuously increased, with annual percent changes of 0.6% (95% confidence interval [CI]: 0.5–0.8) between 1985 and 2005, and 1.8% (95% CI: 0.6–2.9) between 2005 and 2010, mainly attributable to increases in prostate cancer in males and breast cancer in females. 2 More recent data show that in 2018, nearly 885 000 people in Japan were diagnosed with cancer, and approximately 410 000 cancer deaths were recorded in the same year. 3
Napabucasin is an orally administered, small molecule reactive oxygen species (ROS) generator that is bioactivated by the antioxidant NAD(P)H:quinone oxireductase 1 (NQO1). 4 Cancer cells, including cancer stem cells, often express high levels of NQO1 compared with healthy cells. 5 Napabucasin exerts its antitumor activity by increasing levels of ROS beyond a cytotoxic threshold, causing cancer cell death. 6 In vitro assays have shown that NQO1‐expressing cancer cells are more sensitive to napabucasin, compared with NQO1‐negative cancer cells.4, 6
Dose‐escalation studies have assessed the safety, pharmacokinetics (PK), and clinical activity of napabucasin monotherapy in patients with advanced cancers.7, 8 In a phase I dose‐escalation study in patients with advanced cancer, napabucasin showed a favorable PK profile with no evidence of dose‐limiting toxicity up to an oral dose of 2000 mg/day. 7 In the first study of napabucasin conducted in Japanese patients with advanced solid tumors, napabucasin was tolerable up to an oral dose of 1440 mg/day and showed a similar PK profile to that observed previously. 8 No substantial differences in plasma napabucasin concentration were observed over the time course of the study, indicating minimal to no accumulation. 8 This study was designed with four main objectives: to examine the safety, tolerability, and PK of single‐dose napabucasin in healthy Asian participants; to assess the potential QT/QTc interval prolongation effect of napabucasin in healthy Japanese participants; to determine the effect of food on the PK of napabucasin; and, finally, to examine the safety, tolerability, and PK profile of napabucasin in healthy non‐Asian males.
2. MATERIALS AND METHODS
This study was conducted in accordance with Pharmaceutical Affairs Law, Good Clinical Practice Ordinance, relevant regulatory notifications, and the ethical principles of the Declaration of Helsinki. The implementation of the study was approved by the Institutional Review Board. All participants provided written informed consent.
2.1. Study design and objectives
Study D8806034 was conducted at two study sites, Souseikai Fukuoka Mirai Hospital and Souseikai Sumida Hospital (Fukuoka and Tokyo, Japan, respectively). The phase I study comprised five parts (A to E, described below; Figure 1). Parts A to D were randomized, double‐blind, placebo‐controlled, and included healthy Asian male (Parts A, B, and D) and female participants (Part C). Part E was open‐label and uncontrolled, and included healthy non‐Asian male participants. Parts A, B, C, and E included a screening period of 25 days (Day −28 to Day −3), a 4‐day inpatient period, and a follow‐up on Day 7, except for Step B4 (no follow‐up). With the exception of Step D4 (in which treatment was administered in the fed state), napabucasin was administered orally with water following a fast state of at least 10‐h duration. Fasting continued for a further 4 h after administration.
FIGURE 1.
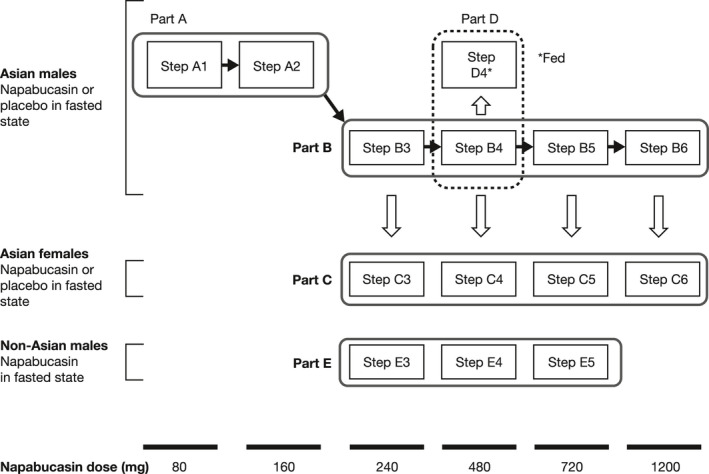
Study design. Part A assessed safety, tolerability, and pharmacokinetics of a single dose of napabucasin or placebo, in Asian males in the fasted state. Parts B and C assessed QT/QTc interval prolongation, safety, tolerability, and pharmacokinetics of a single dose of napabucasin or placebo in Asian males (Steps B3 to B6) and Asian females (Steps C3 to C6) in the fasted state. Part D assessed safety, tolerability, and pharmacokinetics of a single dose of napabucasin in Asian males from Step B4 following a typical Japanese breakfast consumed over 20 min. Part E assessed safety, tolerability, and pharmacokinetics of a single dose of napabucasin in non‐Asian males in the fasted state
In order to ensure the safety of participants, the trial proceeded in a stepwise manner, unless stopping criteria were met (see Supporting Information Methods). Mandatory completion and subsequent steps are shown in Supporting Information Table S1. The Study Investigator and Sponsor performed blinded assessment of safety‐related test results (e.g., physical examination, vital signs, 12‐lead electrocardiogram [ECG], and laboratory tests) to determine whether a participant could proceed to the next step within Parts A and B.
2.1.1. Part A: assessment of the safety and tolerability of napabucasin in healthy Asian male participants
Part A assessed safety, tolerability, and PK following a single dose of napabucasin 80 mg (Step A1) and 160 mg (Step A2), or placebo, in the fasted state.
2.1.2. Parts B and C: assessment of the QT/QTc interval prolongation effect of napabucasin
Potential QT/QTc interval prolongation, PK, safety, and tolerability were assessed in Part B (Steps B3 to B6) and Part C (Steps C3 to C6) following single‐dose administration of napabucasin 240, 480, 720, and 1200 mg, or placebo, in the fasted state in healthy Asian male and female participants.
2.1.3. Part D: assessment of the effect of food on the PK, safety, and tolerability of napabucasin
Participants who received treatment in the fasted state in Step B4 were admitted to Part D4 in a sequential manner after a washout period of at least 6 days. Following a minimum 10‐h fast, participants received oral napabucasin 480 mg 30 min after the start of a typical Japanese breakfast consumed over 20 min.
2.1.4. Part E: assessment of the PK, safety, and tolerability of napabucasin in healthy non‐Asian males
Healthy non‐Asian males received a single dose of napabucasin 240, 480, or 720 mg in the fasted state (Steps E3 to E5).
2.2. Eligibility criteria
Key inclusion criteria included an ability and willingness to fulfill study requirements, aged between 20 and <45 years at the time of informed consent, Asian (Parts A to D) or Caucasian (Part E) race, good general health in the opinion of the Study Investigator, use of appropriate contraception and a negative pregnancy test for female participants of child‐bearing potential, and use of contraception for males with a female partner of child‐bearing potential. Body weight and body mass index (BMI) at screening were required to fall within specified ranges according to race and sex (≥50.0 to ≤80.0 kg and ≥18.5 to <25.0 kg/m2 [Asian males]; ≥40.0 to ≤80.0 kg and ≥16.8 to <25.0 kg/m2 [Asian females]; and ≥50.0 to ≤100.0 kg and ≥18.5 to <30.0 kg/m2 [non‐Asian males]).
Subjects with current disease, or a past history of medical problems (on the basis of physical or other examinations at screening, with the exception of centrally assessed ECG parameters), that were judged to be significant by the Study Investigator, were excluded from the study. Other key exclusion criteria included evidence of cardiovascular disease, cardiac failure or hypokalemia, risk factors for torsade de pointes, severe drug‐related allergy, alcohol abuse, recent hospitalization, or consumption of drugs or substances that may interfere with study outcomes. Subjects who at screening had a 12‐lead analogue ECG that showed evidence of any of the following: heart rate (HR) <50 bpm or >100 bpm, QRS >100 ms, Fridericia‐corrected QT interval (QTcF) >450 ms, or PR interval >200 ms were also excluded from the study.
2.3. Safety assessments
Safety assessments included the reporting of adverse events (AEs) and serious AEs (SAEs), including severity and causal relationship to the study drug, graded according to the Common Terminology Criteria for Adverse Events (v4.03). AEs assessed as possibly, probably, or definitely related to study drug were regarded as adverse drug reactions (ADRs).
In Parts A, B, C, and E, AEs were assessed from the point of study drug administration to Day 7 post‐dose (±1 day). In Part D4, AEs were assessed from the start of Step B4 up to Day 14 (Day 7 post‐dose [±1 day]). Vital signs and laboratory testing (hematology, biochemistry, and urinalysis) were performed regularly throughout the study. Clinically significant abnormal objective findings (including clinical laboratory values, ECG values, and physical examination observations) were also recorded as AEs.
2.4. ECG assessments
Twelve‐lead ECG safety assessments were performed at regular intervals throughout the study.
2.4.1. QT/QTc interval endpoints
In Parts B and C, digital 12‐lead ECG assessments (Holter ECG) were performed at −45, −30, −15 min, and immediately pre‐dose on Day 1, and at 2, 3, 4, 5, 6, 8, 10, 12, and 24 h post‐dose. Participants were supine for at least 10 min before ECG recording. ECGs were extracted, up to ten replicates, for each prespecified time point. The median value from each replicate was calculated, and the mean of all available medians from each time point was used as the subject's reportable value at that time point. ECG data were recorded for at least 5 min. The primary ECG endpoint was the change from baseline in QTcF (ΔQTcF). Secondary ECG endpoints included the change from baseline in HR, PR, and QRS.
2.5. PK measurement
Plasma napabucasin concentrations were measured using validated liquid chromatography‐tandem mass spectrometry (LC–MS/MS) methodology. Napabucasin (AMPAC Analytical) and the internal standard (7‐hydroxyflavone; Sigma‐Aldrich Co. LLC) were extracted from 50 μl human plasma (anticoagulant: K2EDTA) by liquid phase extraction using methyl t‐butyl ether. The extract was evaporated, and the residue dissolved. Reverse‐phase liquid chromatography (Shimadzu Corporation) was performed using a Polaris 5, C18‐A column (2.0 × 50 mm, 5 μM; Agilent) with gradient elution (0.1% formic acid in water/methanol, 70:30 for conditioning, then linear gradient to 5:95 for 0–2.1 min, kept at 5:95 for 2.1–3.1 min, then linear gradient to 70:30 for 3.1–3.2 min, and reconditioning 70:30 for 3.2–4.2 min) at a flow rate of 0.8 ml/min. The precursor to product ion transition for napabucasin (241.1–199.1 m/z) and the internal standard (238.9–137.1 m/z) were monitored in positive electrospray multiple reaction monitoring mode in an ESI 5000 quadrupole mass spectrometry system (AB Sciex). The quantification range for napabucasin was 5–5000 ng/ml. Calibration curves for napabucasin were constructed using quadratic regression with 1/x 2 weighting factor showing good linearity (R 2 of accepted batch ≥0.98). Accuracy and precision were ±10%, within and between runs. Within runs, accuracy and precision were −7.3% to −0.5% and 2.1% to 7.7% for 15, 1000, and 3750 ng/ml napabucasin. Between runs, accuracy and precision were −5.3% to −3.3% and 3.3% to 6.7% for 15, 1000, and 3750 ng/ml napabucasin. All sample analyses satisfied stability conditions. Method validation was conducted at Frontage Laboratories, Inc. Analyst® software (v1.6.2) was used to acquire and process data for this study.
2.6. PK assessments
Blood samples for PK analysis were collected at regular intervals from pre‐dose up to 48 h post‐dose. In Parts B and C, blood samples were collected at the time point of the digital 12‐lead ECG, within 5 min after completion of the ECG recording. PK assessments included maximum plasma concentration (C max), time to maximum plasma concentration (t max), area under the plasma concentration–time curve from time zero to last measurable time point (AUC0‐last), area under the plasma concentration–time curve from time zero to infinity (AUC0‐inf), terminal elimination half‐life (t 1/2), apparent total body clearance (CL/F), mean residence time from time zero to infinity (MRT), and apparent volume of distribution during terminal phase (Vz /F). PK parameters were calculated based on noncompartmental methodology.
2.7. Statistical analysis
The study populations were defined as the safety analysis set (all participants who received at least one dose of study drug), the QT/QTc analysis set (all participants in the safety analysis set who were in Part B or C, and had baseline and ≥1 post‐dose QTcF measurement), and the PK/QTc analysis set (all participants in both the QT/QTc and PK analysis sets with ≥1 pair of post‐dose PK and QTc data from the same time point). The food effect analysis set (Part D) included all participants who had available data on PK food effect parameters for both fasted and fed state administration of the study drug. The target sample size was 70 participants, including four in Part A, 32 in Part B, 16 in Part C, and 18 in Part E. Individual participants could be involved in both Parts B and D, as appropriate.
All statistical analyses were performed using SAS software (v9.4 or newer; SAS Institute, Inc.). Digital ECG data were centrally read (iCardiac Technologies) and 10‐second digital 12‐lead ECG tracings were extracted from continuous ECG recordings. For all ECG parameters, baseline was defined as the average of the measured ECG intervals from the three pre‐dose time points (−45, −30, and −15 min) on Day 1. The QT interval was corrected according to Fridericia's formula, defined as QTcF = QT/RR1/3 and the analysis for QTcF was based on a linear mixed‐effects model with ΔQTcF as the dependent variable, time (categorical), treatment and time‐by‐treatment interaction as fixed effects, and baseline QTcF as a covariate. An unstructured covariance matrix was specified for the repeated measures at post‐dose time points for participants within a given treatment period. Analysis of the change from baseline in HR, PR, and QRS intervals used the same model as described for QTcF. Criteria for the categorical outlier analysis of ECG intervals are described in Supporting Information Table S2. A participant was counted only once for a specified outlier event if they experienced more than episode. The analysis of T‐wave morphology was based on the change from baseline to capture treatment‐emergent changes.
The relationship between plasma napabucasin concentration and ∆QTcF was quantified using a linear mixed‐effects model, with ΔQTcF as the dependent variable, plasma napabucasin concentration and cantered baseline QTcF as covariates, treatment (active or placebo) and time point as categorical factors, and a random intercept and slope per subject. The degrees of freedom for model estimates were determined by the Kenward–Rogers method. The slope and intercept (defined as the difference between active treatment and placebo populations) were estimated using two‐sided 90% CIs.
PK analysis was performed on samples collected from participants in Parts A–E. The dose proportionality of PK outcomes, in terms of C max, AUC0‐last, and AUC0‐inf, was examined for Parts B, C, and E. For Part D, the analysis of food effects was conducted using log‐transformed PK parameters (with the exception of t max). The ratios of C max, AUC0‐last, AUC0‐inf, t 1/2, MRT, and t max following fed state administration to those with fasted state administration were calculated for each participant, and the 90% and 95% CI were calculated.
3. RESULTS
3.1. Baseline demographic characteristics
The baseline characteristics of healthy Asian male and female participants in Parts A, B, and C (safety analysis set, n = 52) were generally comparable with those of the healthy non‐Asian male participants (n = 18) in Part E (Table 1). All Asian participants were of Japanese ethnicity. All participants completed the study. The baseline ECG parameters of the 48 participants from Parts B and C, who comprised the QT/QTc analysis set, are shown in Table 2.
TABLE 1.
Demographic characteristics in Asian (Parts A, B, and C) and non‐Asian (Part E) subjects (safety analysis set)
| Asian subjects (Parts A, B, C) | Napabucasin (mg) | Placebo (pooled) | |||||
|---|---|---|---|---|---|---|---|
| 80 mg | 160 mg | 240 mg | 480 mg | 720 mg | 1200 mg | ||
| (N = 1) | (N = 1) | (N = 9) | (N = 9) | (N = 9) | (N = 9) | (N = 14) | |
| Sex (male), n (%) | 1 (100.0) | 1 (100.0) | 6 (66.7) | 6 (66.7) | 6 (66.7) | 6 (66.7) | 10 (71.4) |
| Age (y) | |||||||
| Mean (SD) | 21 (N/A) | 22 (N/A) | 23.9 (5.3) | 27.8 (4.3) | 24.6 (4.3) | 28.0 (5.3) | 26.0 (6.2) |
| Median (range) | N/A | N/A | 21 (20–33) | 27 (20–34) | 23 (20–31) | 26 (23–37) | 25 (20–38) |
| Not Hispanic or Latino ethnicity, n (%) | 1 (100.0) | 1 (100.0) | 9 (100.0) | 9 (100.0) | 9 (100.0) | 9 (100.0) | 14 (100.0) |
| Asian race, n (%) | 1 (100.0) | 1 (100.0) | 9 (100.0) | 9 (100.0) | 9 (100.0) | 9 (100.0) | 14 (100.0) |
| Weight (kg) | |||||||
| Mean (SD) | 76.2 (N/A) | 61.3 (N/A) | 57.1 (4.6) | 57.7 (8.5) | 56.9 (8.1) | 61.4 (3.5) | 61.6 (6.8) |
| Median (range) | N/A | N/A | 55.7 (50.5–64.2) | 56.1 (45.1–74.4) | 57.9 (45.3–68.8) | 61.5 (55.3–66.8) | 63.3 (50.4–72.1) |
| BMI (kg/m2) | |||||||
| Mean (SD) | 24.0 (N/A) | 22.6 (N/A) | 20.7 (1.1) | 21.1 (2.2) | 20.7 (1.3) | 22.0 (1.7) | 21.6 (1.7) |
| Median (range) | N/A | N/A | 20.8 (19.1–22.2) | 20.3 (18.6–25.0) | 20.8 (19.3–22.9) | 22.7 (19.5–24.2) | 22.0 (18.9–24.0) |
| Non‐Asian subjects (Part E) | (N = 6) | (N = 6) | (N = 6) | ||||
|---|---|---|---|---|---|---|---|
| Sex (male), n (%) | 6 (100.0) | 6 (100.0) | 6 (100.0) | ||||
| Age (y) | |||||||
| Mean (SD) | 22.5 (4.0) | 29.2 (7.6) | 29.0 (4.3) | ||||
| Median (range) | 24 (22–33) | 26.5 (22–40) | 30 (23–33) | ||||
| Hispanic or Latino ethnicity, n (%) | 2 (33.3) | N/A | 1 (16.7) | ||||
| Not Hispanic or Latino ethnicity, n (%) | 4 (66.7) | 6 (100.0) | 5 (83.3) | ||||
| Caucasian race, n (%) | 6 (100.0) | 6 (100.0) | 6 (100.0) | ||||
| Weight (kg) | |||||||
| Mean (SD) | 65.0 (7.6) | 77.2 (11.0) | 75.1 (10.0) | ||||
| Median (range) | 63.5 (58.6–79.6) | 78.4 (60.2–90.8) | 75.0 (63.0–87.2) | ||||
| BMI (kg/m2) | |||||||
| Mean (SD) | 20.9 (2.4) | 22.1 (1.5) | 24.0 (2.6) | ||||
| Median (range) | 20.6 (18.3–24.3) | 22.7 (19.7–23.7) | 23.8 (20.7–27.2) | ||||
Abbreviations: BMI, body mass index; N/A, not available; SD, standard deviation.
TABLE 2.
Baseline ECG parameters (QT/QTc analysis set)
| Napabucasin (mg) |
Placebo (N = 12) |
||||
|---|---|---|---|---|---|
|
240 mg (N = 9) |
480 mg (N = 9) |
720 mg (N = 9) |
1200 mg (N = 9) |
||
| HR (bpm) | |||||
| Mean (SD) | 56.4 (5.1) | 62.7 (6.2) | 58.5 (6.0) | 59.6 (7.0) | 57.5 (5.6) |
| SE | 1.7 | 2.1 | 2 | 2.3 | 1.6 |
| 90% CI | (53.2–59.5) | (58.9–66.5) | (54.7–62.2) | (55.3–64.0) | (54.6–60.4) |
| Median (range) | 54.6 (51–66) | 63.7 (52–70) | 60.6 (51–67) | 60.3 (46–68) | 56.2 (49–66) |
| QTcF (ms) | |||||
| Mean (SD) | 401.1 (15.1) | 393.3 (20.9) | 398.9 (20.1) | 403.7 (18.2) | 404.6 (21.3) |
| SE | 5 | 7 | 6.7 | 6.1 | 6.2 |
| 90% CI | (391.8–410.5) | (380.3–406.3) | (386.4–411.4) | (392.4–414.9) | (393.5–415.7) |
| Median (range) | 405.5 (381–426) | 390.9 (353–426) | 392.8 (372–429) | 395.7 (384–434) | 398.3 (371–435) |
| PR (ms) | |||||
| Mean (SD) | 143.9 (26.7) | 149.2 (17.6) | 150.7 (19.7) | 145.6 (21.2) | 158.9 (17.5) |
| SE | 8.9 | 5.9 | 6.6 | 7.1 | 5.1 |
| 90% CI | (127.4–160.5) | (138.3–160.1) | (138.4–162.9) | (132.5–158.7) | (149.9–168.0) |
| Median (range) | 143.8 (111–200) | 147.2 (110–168) | 150.7 (121–175) | 136.1 (120–179) | 151.7 (132–189) |
| QRS (ms) | |||||
| Mean (SD) | 106.4 (5.7) | 105.5 (2.9) | 106.0 (5.6) | 107.6 (7.5) | 104.3 (4.5) |
| SE | 1.9 | 1 | 1.9 | 2.5 | 1.3 |
| 90% CI | (102.9–110.0) | (103.7–107.4) | (102.6–109.5) | (102.9–112.2) | (102.0–106.7) |
| Median (range) | 106.3 (99–114) | 104.9 (101–110) | 104.8 (100–115) | 104.7 (102–124) | 103.8 (97–113) |
Abbreviations: CI, confidence interval; ECG, electrocardiogram; HR, heart rate; QTc, QT interval corrected for heart rate; QTcF, QT interval corrected for heart rate using Fridericia's formula; SD, standard deviation; SE, standard error.
3.2. Safety assessments
No SAEs, fatal AEs, or AEs leading to discontinuation or dose reduction were observed in Parts A‐E (Table 3). Across all parts of the study, the most frequently reported treatment‐emergent AEs were diarrhea and abdominal pain, followed by chromaturia (Parts A, B, C combined), abdominal pain (Part D), and nausea (Part E). All AEs were of Grade 1 severity and resolved. Individual clinically significant laboratory abnormalities included blood creatine phosphokinase increase in one of 52 participants (Parts A, B, and C); and lymphocyte count decrease, monocyte count increase, neutrophil count increase, and white blood cell count increase, each in one participant in Part D. No significant changes in body temperature, pulse rate, or blood pressure were observed, except for one participant with hypertension of Grade 1 severity. No significant differences were observed in AEs following single‐dose napabucasin administration in the fasted or fed state.
TABLE 3.
Frequency of AEs and ADRs, and AEs by SOC and preferred term in Parts A, B, C, D, and E combined (safety analysis set)
| Napabucasin (mg) |
Pooled placebo (N = 14) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
80 mg (N = 1) |
160 mg (N = 1) |
240 mg (N = 15) |
480 mg (N = 15) |
720 mg (N = 15) |
1200 mg (N = 9) |
480 mg fed (N = 6) |
||
| AEs, n (%) [number of events] | 0 | 0 | 12 (80.0) [31] | 12 (80.0) [26] | 15 (100.0) [35] | 9 (100.0) [23] | 5 (83.3) [11] | 1 (7.1) [1] |
| AEs leading to death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Serious AEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ADRs, n (%) [number of events] | 0 | 0 | 11 (73.3) [18] | 12 (80.0) [25] | 15 (100.0) [35] | 9 (100.0) [21] | 4 (66.7) [4] | 1 (7.1) [1] |
| ADRs leading to death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Serious ADRs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AE—SOC, n (%) | ||||||||
| Cardiac disorders | – | – | 2 (13.3) | – | – | – | – | – |
| Atrioventricular block first degree | 1 (6.7) | |||||||
| Sinus bradycardia | 1 (6.7) | |||||||
| Gastrointestinal disorders | – | – | 10 (66.7) | 12 (80.0) | 15 (100.0) | 9 (100.0) | 5 (83.3) | 1 (7.1) |
| Diarrhea | 7 (46.7) | 11 (73.3) | 14 (93.3) | 8 (88.9) | – | 1 (7.1) | ||
| Abdominal pain | 7 (46.7) | 9 (60.0) | 8 (53.3) | 5 (55.6) | 4 (66.7) | – | ||
| Nausea | 1 (6.7) | 1 (6.7) | 4 (26.7) | 1 (11.1) | – | – | ||
| Abdominal distension | – | – | – | 1 (11.1) | – | – | ||
| Gingival pain | – | – | – | – | 1 (16.7) | – | ||
| General disorders/administration site conditions | – | – | – | – | 1 (6.7) | 1 (11.1) | 1 (16.7) | – |
| Application site erythema | – | – | 1 (16.7) | |||||
| Application site pruritus | – | 1 (11.1) | – | |||||
| Malaise | – | 1 (11.1) | – | |||||
| Pyrexia | 1 (6.7) | – | – | |||||
| Investigations | – | – | 1 (6.7) | – | – | – | 1 (16.7) | – |
| Blood creatine phosphokinase increased | 1 (6.7) | – | ||||||
| Lymphocyte count decreased | – | 1 (16.7) | ||||||
| Monocyte count increased | – | 1 (16.7) | ||||||
| Neutrophil count increased | – | 1 (16.7) | ||||||
| White blood cell count increased | – | 1 (16.7) | ||||||
| Nervous system disorders | – | – | – | – | 2 (13.3) | 1 (11.1) | – | – |
| Presyncope | 1 (6.7) | 1 (11.1) | ||||||
| Headache | 1 (6.7) | – | ||||||
| Renal and urinary disorders | – | – | 3 (20.0) | 3 (20.0) | 4 (26.7) | 5 (55.6) | – | – |
| Chromaturia | 3 (20.0) | 3 (20.0) | 4 (26.7) | 5 (55.6) | ||||
| Vascular disorders | – | – | – | 1 (6.7) | 1 (6.7) | – | 1 (16.7) | – |
| Hypertension | 1 (6.7) | – | 1 (16.7) | |||||
| Hot flush | – | 1 (6.7) | – | |||||
Abbreviations: ADR, adverse drug reaction; AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities; SOC, system organ class.
AEs coded according to MedDRA v19.1.
3.3. ECG assessments
Among participants receiving napabucasin 240 mg in Parts A–C, a total of two AEs were reported under the cardiac disorders system order class, one under the preferred term (PT) of first‐degree atrioventricular block and one under the PT of sinus bradycardia (Table 3). These AEs were not serious, and were not considered to be related to the study drug and resolved.
The analysis of ECG assessments in Parts B and C showed small mean changes from baseline in HR in both napabucasin and placebo treatment groups, with no outliers observed (Figure 2). Single‐dose administration of napabucasin up to 1200 mg did not appear to affect cardiac conduction, as measured by changes in PR and QRS intervals, and no outliers were reported. Mean placebo‐corrected ∆PR (∆∆PR) were within ±6.0 ms at all post‐dose time points, with few, not dose‐related exceptions. Mean change from baseline QRS (∆QRS) was very small across treatment groups, and the mean placebo‐corrected ∆QRS was within ±1.0 ms at all doses and all post‐dose time points. Mean change from baseline in QTcF over time was comparable between treatment groups, and placebo‐corrected from baseline changes (ΔΔQTcF) revealed no dose dependency of effect (Figure 3). In one participant receiving napabucasin 1200 mg, ΔΔQTcF was >30 and ≤60 ms at one time point (Supporting Information Table S3). In terms of T‐wave morphology, there were no categorical outliers from the predefined range observed in any participant in the QT/QTc analysis set (data not shown).
FIGURE 2.
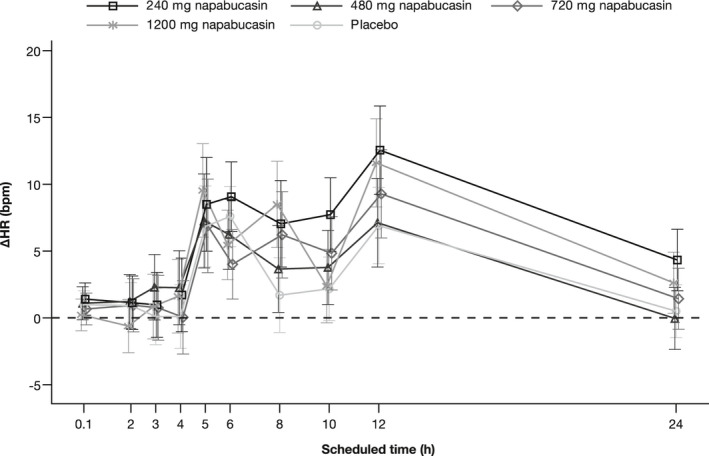
Change from baseline in HR over time (QT/QTc analysis set)a,b. aThe 0.1 time point correlates with the pre‐dose time point. bData points represent LSMean with SE error bars. ∆, change from baseline; HR, heart rate; LSMean, least square means; QTc, QT interval corrected for heart rate; SE, standard error
FIGURE 3.
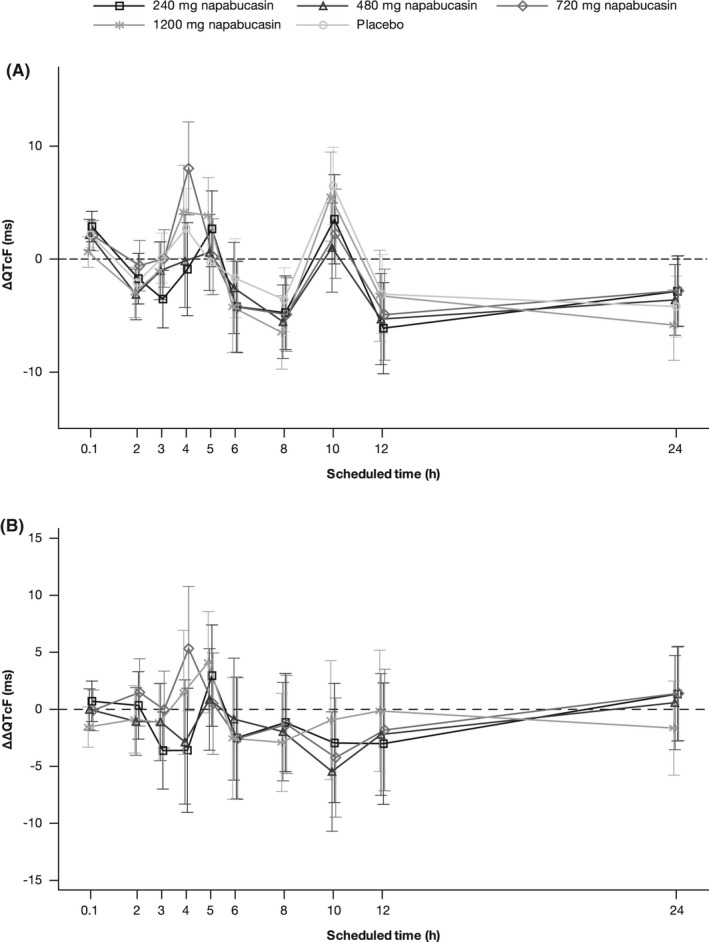
(A) Change from baseline in QTcF over time; (B) Placebo‐corrected change from baseline over time (QT/QTc analysis set)a,b. aThe 0.1 time point correlates with the pre‐dose time point. bData points represent LSMean with SE error bars. ∆, change from baseline; HR, heart rate; LSMean, least square means; QTc, QT interval corrected for HR; QTcF, QT interval corrected for HR using Fridericia's formula; SE, standard error
3.4. PK assessments
One participant in Part E did not have a blood sample taken for PK assessments and was therefore not included in the PK analysis set (n = 55). PK parameters derived from blood samples taken following oral administration of napabucasin during Parts A–E are presented in Table 4. Maximum plasma napabucasin concentration was reached approximately 3–5 h after oral administration, followed by an immediate decrease, with wide interindividual variation (Supporting Information Figure S1). The PK profile of napabucasin did not show dose proportionality, in terms of C max and AUC0‐last, in either male or female participants in the fasted state (Parts B and C) (Supporting Information Figure S2). Individual female participants exhibited higher blood concentrations of napabucasin, but the reason for this is unclear. No dose proportionality was observed in either Asian or non‐Asian male fasted participants (Parts B and E) (Supporting Information Figure S2).
TABLE 4.
PK parameters (mean, SD) measured across Parts A‐E (PK analysis set)
| Dose (mg) | Race | Sex | Fasted/fed state | n | C max (ng/ml) | AUC0‐last (h*ng/ml) | AUC0‐inf (h*ng/ml) | T max (h) | t 1/2 (h) | MRT (h) | CL/F (L/h) | V z/F (L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Part A | ||||||||||||
| 80 | Asian | Male | Fasted | 1 | 151 | 419 | 436 | 5 | 2.2 | 5.3 | 183.4 | 592.4 |
| 160 | Asian | Male | Fasted | 1 | 414 | 1118 | 1261 | 5 | 6.2 | 6.8 | 126.9 | 1141.8 |
| Part B | ||||||||||||
| 240 | Asian | Male | Fasted | 6 | 295.5 (161.5) | 1262.4 (519.5) | 1324.9 (522.1) | 4.2 (1.0) | 3.6 (2.0) | 6.5 (2.6) | 199.5 (57.6) | 1029.6 (734.4) |
| 480 | Asian | Male | Fasted | 6 | 403.5 (77.4) | 2375.3 (863.0) | 2494.7 (884.3) | 3.8 (1.1) | 5.5 (4.8) | 8.2 (4.3) | 216.5 (87.8) | 1572.9 (1260.2) |
| 720 | Asian | Male | Fasted | 6 | 382.8 (127.6) | 2308.9 (690.9) | 2392.8 (683.7) | 3.9 (1.5) | 4.7 (2.0) | 7.0 (2.4) | 326.3 (108.6) | 2113.1 (670.7) |
| 1200 | Asian | Male | Fasted | 6 | 390.0 (98.9) | 2528.5 (465.1) | 2813.8 (622.2) | 3.2 (0.7) | 5.6 (1.7) | 8.8 (2.0) | 445.1 (101.8) | 3445.8 (744.8) |
| Part C | ||||||||||||
| 240 | Asian | Female | Fasted | 3 | 392.3 (29.7) | 1721.2 (631.7) | 1846.3 (750.7) | 3.4 (1.2) | 3.4 (1.2) | 5.4 (0.9) | 143.0 (48.1) | 685.2 (331.1) |
| 480 | Asian | Female | Fasted | 3 | 420.3 (172.7) | 2762.3 (1246.4) | 3050.3 (1125.0) | 4.4 (0.6) | 7.8 (4.5) | 11.0 (3.1) | 172.8 (64.4) | 2032.3 (1277.3) |
| 720 | Asian | Female | Fasted | 3 | 753.3 (299.0) | 5174.3 (2955.5) | 5337.0 (3027.9) | 3.7 (0.6) | 4.5 (0.3) | 7.3 (0.5) | 163.0 (76.2) | 1085.7 (575.2) |
| 1200 | Asian | Female | Fasted | 3 | 514.7 (133.7) | 4203.2 (2112.5) | 8572.4 (9115.6) | 3.2 (0.9) | 15.7 (20.5) | 26.4 (28.2) | 303.5 (267.9) | 2590.6 (877.8) |
| Part D | ||||||||||||
| 480 | Asian | Male | Fasted | 6 | 403.5 (77.4) | 2375.3 (863.0) | 2494.7 (884.3) | 3.8 (1.1) | 5.5 (4.8) | 8.2 (4.3) | 216.5 (87.8) | 1572.9 (1260.2) |
| 480 | Asian | Male | Fed | 6 | 460.7 (71.6) | 3823.3 (1095.5) | 3911.5 (1081.6) | 5.2 (1.7) | 4.5 (1.0) | 8.7 (1.7) | 133.8 (50.1) | 871.8 (394.0) |
| Part E | ||||||||||||
| 240 | Non‐Asian | Male | Fasted | 6 | 436.5 (110.7) | 2060.9 (569.6) | 2142.1 (578.3) | 4.5 (0.8) | 6.4 (1.4) | 7.0 (1.1) | 119.5 (33.9) | 1149.5 (516.5) |
| 480 | Non‐Asian | Male | Fasted | 6 | 454.0 (195.7) | 2153.0 (1040.1) | 2272.8 (1048.0) | 3.3 (1.2) | 3.9 (1.4) | 6.2 (0.6) | 249.2 (110.3) | 1313.4 (605.6) |
| 720 | Non‐Asian | Male | Fasted | 5 | 309.4 (126.3) | 2411.0 (853.0) | 2896.3 (611.9) | 3.0 (1.2) | 8.6 (5.0) | 12.6 (5.9) | 257.1 (51.3) | 3340.1 (2339.4) |
Abbreviations: AUC0‐inf, area under the plasma concentration–time curve from time zero to infinity; AUC0‐last, area under the plasma concentration–time curve from time zero to last measurable time point; CL/F, apparent total body clearance; C max, maximum plasma concentration; MRT, mean residence time; PK, pharmacokinetics; SD, standard deviation; t 1/2, terminal elimination half‐life; t max, time to maximum plasma concentration; V z/F, apparent volume of distribution during terminal phase.
The administration of napabucasin in the fed state (Step D4) was associated with higher mean plasma napabucasin concentrations 6–10 h after administration, compared with administration in the fasted state (Step B4) (Supporting Information Figure S3). C max increased by approximately 15%, AUC0‐last increased by approximately 60%, and t max decreased by approximately 1.4 h following administration in the fed state, compared with the fasted state (Supporting Information Table S4).
3.5. Exposure‐response analysis
The estimated slope of the concentration–QTc relationship was very shallow (0.007 ms per ng/ml [90% CI: 0.0015 to 0.0131]) and with a ∆ΔQTcF intercept of −2.42 ms (90% CI: −4.6561 to −0.1858), representing a minor difference between treatment (napabucasin) and control (placebo) groups (Figure 4). The predicted ∆ΔQTcF effect was 0.92 ms (90% CI: −1.66 to 3.50) and 0.63 ms (90% CI: −1.81 to 3.08) at the observed geometric mean peak plasma napabucasin concentrations for the 720 and 1200 mg doses (457 and 418 ng/ml), respectively. Based on this exposure‐response analysis, a QT effect (∆ΔQTcF) exceeding 10 ms can be excluded within the observed range of plasma napabucasin concentrations, up to ~1000 ng/ml.
FIGURE 4.
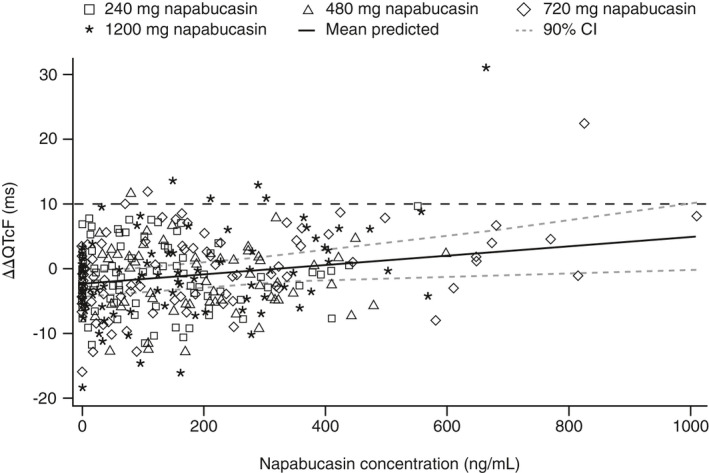
Scatterplot of observed napabucasin plasma concentrations and ΔΔQTcF by healthy participants (PK/QTc analysis set). CI, confidence interval; HR, heart rate; QTcF, QT interval corrected for HR using Fridericia's formula
4. DISCUSSION
This study was designed to assess the safety, tolerability, and PK profile of napabucasin in healthy Asian and non‐Asian participants, and to determine any potential effect on QT/QTc interval prolongation. Previous PK studies of napabucasin have been limited to patients with advanced cancers,7, 8 and this is the first PK study to be conducted in healthy adult volunteers. Napabucasin, at single doses of up to 1200 mg, was tolerated in this study, with no SAEs, fatal AEs, or AEs leading to discontinuation or dose reduction. The most frequently reported AEs were diarrhea and abdominal pain, which is consistent with the known tolerability profile of napabucasin derived from phase I dose‐escalation studies in patients with advanced cancers, in which maximum dose exposures were 1440 mg/day and 2000 mg/day, respectively. In these studies, the most common AEs were predominantly gastrointestinal in nature and included diarrhea, nausea, anorexia, vomiting, and dehydration, and did not necessitate dose reduction.7, 8 Consistent with these studies, AEs and ADRs were of mild intensity in the current study, with no events exceeding Grade 1 severity. The tolerability profile of napabucasin in healthy participants is also similar to that reported in Japanese patients with advanced/recurrent gastric cancer following administration of napabucasin (480 mg twice daily) in combination with paclitaxel. 9 In the current study, no significant safety issues were observed with single‐dose administration with or without food, leading to the conclusion that napabucasin may be dosed irrespective of food.
The analysis of ECG data revealed no prolongation of the QT or QTc interval following oral single‐dose administration of napabucasin 240–1200 mg in healthy Asian male and female participants. Only negligible changes in HR were observed following napabucasin administration. Cardiac conduction, as measured by changes in the PR and QRS intervals, did not appear to be affected.
The PK profile of napabucasin observed in this study showed considerable variation among individual participants, again, consistent with earlier studies which have found high inter‐patient variability. 9 In general, time to reach maximum plasma napabucasin concentration ranged from 3 to 5 h after oral administration. Dose proportionality was not observed in terms of C max and AUC0‐last, and no marked differences were observed between male or female, and Asian or non‐Asian groups. Plasma napabucasin concentrations were increased under fed state conditions.
The limitations of this study are common to those of any phase I study. All observations were made in healthy volunteers, and as such will need to be assessed in specific patient populations. The inclusion of larger groups of participants may have reduced the variability in PK parameters, but the observations made were generally consistent with previously published data.
In conclusion, single‐dose administration of napabucasin up to 1200 mg was tolerated in healthy Asian male and female participants, and no significant safety or tolerability issues were identified. No differences in the PK profile of napabucasin were observed between Asian and non‐Asian participants. Clinical studies of napabucasin in patients with various types of cancer are ongoing.
DISCLOSURE
N.N., T.T., Y.Y., Y.H., S.I., and H.K. are employees of Sumitomo Dainippon Pharma Co., Ltd. S.M. and Y.O. have no competing interests.
AUTHOR CONTRIBUTIONS
Naoto Noda, Shuichi Iino and Hiroyoshi Kakuyama were involved in the concept and design of the study. Yuzo Horibuchi, Yoichiro Ogama, and Shunji Matsuki were involved in the provision of study materials or subjects, and Takeshi Takagaki and Yasuhide Yodo were involved in statistical analysis. All authors were involved in data analysis and interpretation, manuscript writing, and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Supplementary Material
Fig S1
Fig S2
Fig S3
ACKNOWLEDGEMENTS
The authors thank the study participants and investigators. Medical writing support, under the direction of the authors, was provided by Neil Cockburn, BSc, and Eleanor Finn, PhD, CMC Connect, McCann Health Medical Communications, and was funded by Sumitomo Dainippon Pharma. Co,. Ltd, Japan, in accordance with Good Publication Practice (GPP3) guidelines.
The authors also thank Borje Darpo, MD, PhD, eResearch Technology, Inc, USA, for providing expert cardiac reports, funded by Sumitomo Dainippon Pharma Co., Ltd, Japan.
Noda N, Takagaki T, Yodo Y, et al. Effects of a reactive oxygen species generator, napabucasin (BBI608), on tolerability, safety, pharmacokinetics, and QT/QTc interval in healthy volunteers. Pharmacol Res Perspect. 2021;9:e00874. doi: 10.1002/prp2.874
Principal Investigator statement: The authors confirm that the Principal Investigators for this paper are Shunji Matsuki and Yoichiro Ogama and that they had direct clinical responsibility for volunteers.
Funding information
This study was sponsored by Sumitomo Dainippon Pharma Co., Ltd.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Sumitomo Dainippon Pharma, Co., Ltd. The policy on data sharing is found at https://www.ds‐pharma.com/rd/clinical/clinical_study_data.html.
REFERENCES
- 1. Matsuda T, Saika K. Cancer burden in Japan based on the latest cancer statistics: need for evidence‐based cancer control programs. Ann Cancer Epidemiol. 2018;2:1‐15. [Google Scholar]
- 2. Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol. 2015;45:390‐401. [DOI] [PubMed] [Google Scholar]
- 3. Global Cancer Observatory . GLOBOCAN 2018: Japan factsheet. 2018. Accessed April 6, 2021. https://gco.iarc.fr/today/data/factsheets/populations/392‐japan‐fact‐sheets.pdf.
- 4. Chang A‐Y, Hsu E, Patel J, et al. Evaluation of tumor cell‐tumor microenvironment component interactions as potential predictors of patient response to napabucasin. Mol Cancer Res. 2019;17:1429‐1434. [DOI] [PubMed] [Google Scholar]
- 5. Madajewski B, Boatman MA, Chakrabarti G, Boothman DA, Bey EA. Depleting tumor‐NQO1 potentiates anoikis and inhibits growth of NSCLC. Mol Cancer Res. 2016;14:14‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Froeling FEM, Swamynathan MM, Deschênes A, et al. Bioactivation of napabucasin triggers reactive oxygen species‐mediated cancer cell death. Clin Cancer Res. 2019;25:7162‐7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Langleben A, Supko JG, Hotte SJ, et al. A dose‐escalation phase I study of a first‐in‐class cancer stemness inhibitor in patients with advanced malignancies. J Clin Oncol. 2013;15(suppl):2542. [Google Scholar]
- 8. Kawazoe A, Kuboki Y, Bando H, et al. Phase 1 study of napabucasin, a cancer stemness inhibitor, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2020;85:855‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shitara K, Yodo Y, Iino S. A phase I study of napabucasin plus paclitaxel for Japanese patients with advanced/recurrent gastric cancer. In Vivo. 2019;33:933‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Fig S1
Fig S2
Fig S3
Data Availability Statement
The data that support the findings of this study are available from Sumitomo Dainippon Pharma, Co., Ltd. The policy on data sharing is found at https://www.ds‐pharma.com/rd/clinical/clinical_study_data.html.


