Abstract
Objective
Although numerous studies show that preoperative pain catastrophizing is a risk factor for pain after total knee arthroplasty (TKA), little is known about the temporal course of the association between perioperative pain catastrophizing and pain severity. The present study investigated temporal changes and their dynamic associations between pain catastrophizing and pain severity before and after TKA.
Design
A secondary data analysis of a larger observational parent study featuring prospective repeated measurement over 12 months.
Setting
Dual-site academic hospital.
Subjects
A total of 245 individuals who underwent TKA.
Methods
Participants completed pain catastrophizing and pain severity questionnaires at baseline, 6 weeks, and 3, 6, and 12 months after TKA. Cross-lagged panel analysis was conducted with structural equation modeling including age, sex, race, baseline anxiety, and depressive symptoms as covariates.
Results
Reduction in pain catastrophizing from baseline to 6 weeks after TKA was associated with lower pain severity at 3 months after TKA (standardized β = 0.14; SE = 0.07, P = 0.046), while reduction in pain severity at 6 weeks after TKA was not associated with pain catastrophizing at 3 months after TKA (P = 0.905). In the chronic postsurgical period (>3 months), pain catastrophizing at 6 months after TKA predicted pain severity at 12 months after TKA (β = 0.23, P = 0.009) with controlling for auto-correlation and covariates, but not vice versa.
Conclusions
We provide evidence that changes in pain catastrophizing from baseline to 6 weeks after TKA are associated with subsequent pain severity. Future studies are warranted to determine whether targeting pain catastrophizing during the perioperative period may improve clinical outcomes for individuals undergoing TKA.
Keywords: Perioperative Pain, Catastrophizing, Postoperative Pain, Osteoarthritis
Introduction
Osteoarthritis is a leading cause of chronic pain and disability worldwide. Treatment of advanced osteoarthritis with total knee arthroplasty (TKA) is common and typically leads to significant reductions in pain and improvements in physical function for the majority of individuals. However, despite good outcomes, 15–30% of individuals who undergo TKA report persistent postoperative pain and disability [1–9]. Identifying modifiable nonsurgical risk factors during the perioperative period may improve post-TKA clinical outcomes.
Pain catastrophizing, a maladaptive cognitive and affective response to pain, is a modifiable psychological factor that influences TKA outcomes [1, 10–12]. Robust evidence suggests that preoperative pain catastrophizing contributes to post-TKA pain severity and chronicity, above and beyond the effects of depressive and anxiety symptoms [13–15]. In fact, numerous systematic reviews have suggested that preoperative pain catastrophizing adversely affects clinical outcomes after TKA [16–18], and a meta-analysis has provided further evidence that preoperative pain catastrophizing is one of the strongest predictors of chronic post-TKA pain [1].
Changes in pain catastrophizing during the acute postoperative (i.e., <3 months) period may serve as a novel therapeutic target for improving TKA outcomes, yet scant evidence exists on how changes in pain catastrophizing across the perioperative (i.e., preoperative to 3 months postoperative) period may influence long-term post-TKA outcomes [19]. To our knowledge, only one study has examined temporal associations between pain severity and pain catastrophizing after TKA [20]. That study showed that while controlling for depressive and anxiety symptoms, pain severity and pain catastrophizing measured at baseline and 2 months after TKA predicted future pain catastrophizing at 2 and 6 months, respectively. However, the authors did not report whether changes in pain catastrophizing prospectively predicted post-TKA pain severity [20]. Longitudinal studies assessing the potential bidirectional temporal association between changes in pain catastrophizing and pain severity may provide greater understanding of their causality, which may guide the development of more efficacious pain interventions administered in the preoperative and postoperative periods [21].
In the present study, we conducted a cross-lagged panel analysis, which can reveal the direction of potential causality between variables measured across time [22], in a cohort of patients who underwent TKA. The primary aim of the study was to examine whether temporal changes in pain catastrophizing precede changes in pain severity during the perioperative period. Previous studies using cross-lagged panel analysis revealed that changes in pain catastrophizing precede and contribute to subsequent alterations in pain severity across an acute time period (i.e., days to weeks) in both healthy and clinical pain populations [23–25]. Thus, we expected that there would be a unidirectional association between perioperative pain catastrophizing and pain severity. Specifically, we hypothesized that even when controlling for age, sex, anxiety, and depressive symptoms, baseline to 6-week post-TKA changes in pain catastrophizing would predict pain severity at 3 months after TKA. We also hypothesized that pain catastrophizing at 3 and 6 months after TKA would predict subsequent pain severity in the chronic postsurgical period at 6 and 12 months after TKA. However, we did not expect that temporal changes in pain severity would predict future pain catastrophizing.
Methods
The present study is a secondary data analysis of a larger dual-site parent study featuring prospective repeated measurements over a 12-month period. The parent study collected preoperative measures (e.g., self-report questionnaires, experimental pain testing, daily diaries, physical functioning test) at baseline (i.e., 1–4 weeks before TKA) and postoperative measures at 6 weeks, 3 months, 6 months, and 12 months after TKA. The main outcome report for the parent study, which examines bio-behavioral risk factors related to the development of persistent pain after TKA, is currently in progress. Previously published studies based on the parent study data do not overlap with the present study in terms of research aims and do not use all the longitudinal assessment time points [26–29].
Participants
Participants were recruited through the Johns Hopkins University School of Medicine (JHU SOM), Baltimore, Maryland, and Brigham and Women’s Hospital (BWH), Boston, Massachusetts, between March 1, 2011, and January 15, 2016, through posted flyers, advertisement letters mailed to patients scheduled for TKA, advertisements in local orthopedic clinics, and announcements on hospital and university clinical research websites. Patients were also directly recruited from orthopedic surgery clinics at both institutions. All study-related procedures were approved by the JHU SOM and BWH Institutional Review Boards, and all participants provided informed consent.
Inclusion criteria included 1) age >45 years; 2) met American College of Rheumatology diagnostic criteria for knee osteoarthritis; 3) scheduled or planning to undergo TKA; 4) proficient in English; and 5) on a stable dose of acetaminophen or nonsteroidal anti-inflammatory drugs for at least 1 month before study enrollment. Exclusion criteria included 1) use of opioids within the prior 30 days; 2) recent history of substance abuse or dependence; 3) meeting diagnostic criteria for certain sleep disorders, such as restless legs syndrome; 4) presence of systemic inflammatory or autoimmune disorders; 5) pregnancy; 6) history of Raynaud’s disease; 7) current infection; 8) moderate-to-severe peripheral neuropathy; 9) history of myocardial infarction or other serious cardiovascular condition in the prior 12 months; 10) current use of oral steroids; and 11) demonstration of delirium, dementia, psychosis, or other cognitive impairments that would prevent the completion of study procedures.
We screened a total of 636 patients. Two hundred forty-eight individuals met the inclusion and exclusion criteria and consented to participate in the study. Three participants dropped out before initiating the study. Thus, a total of 245 participants were included in the present study. Because of an administrative oversight at the beginning of the study, baseline data were missing for 33 participants (13.5% of the total sample). However, as this missing data is most likely missing at random (i.e., no relationship between the missingness and the observed data), we used full-information maximum likelihood (FIML) estimation to address the missing data [30, 31] and included these 33 participants in the main statistical analyses.
Study Measures
Pain Severity
The Brief Pain Inventory (BPI) [32] is a well-validated and widely used self-report measure that asks participants to indicate their level of pain intensity on a standard numeric rating scale, from 0 = no pain to 10 = worst pain you can imagine. Pain severity was the mean of four pain items (worst, least, average, and current pain), with higher scores indicating greater pain. Participants completed the BPI before undergoing any experimental pain assessments at each of the visits.
Catastrophizing
The Pain Catastrophizing Scale (PCS) [13] consists of 13 items rated on a five-point Likert scale ranging from 0 (not at all) to 4 (all the time). The measure assesses three dimensions of pain catastrophizing: rumination, magnification, and helplessness. Participants also completed the PCS before undergoing any experimental pain assessments at each of the visits.
Covariates
A number of covariates pertinent to the experience of pain severity and pain catastrophizing were included in the present study: 1) sex, 2) dichotomized race (White vs. racial minorities), and 3) baseline anxiety, measured by the widely used and validated PROMIS Anxiety and Depression Short Form V1.0 [33].
Data Analytic Plan
Before testing our primary hypothesis, we first conducted a repeated-measures analysis of variance (RM-ANOVA) to examine changes in pain severity and pain catastrophizing from baseline to 12-month post-TKA follow-up. We also created pre- and postoperative change scores of pain catastrophizing and pain severity by subtracting baseline scores from 6-week post-TKA follow-up scores. Negative change scores are indicative of improvement (i.e., decrease in either pain severity or pain catastrophizing from baseline to 6 weeks after TKA).
Next, we conducted a cross-lagged panel model using baseline and 6-week post-TKA change scores of pain catastrophizing and pain severity and 3-, 6-, and 12-month post-TKA scores of pain catastrophizing and pain severity. As findings of raw change scores can be influenced by baseline scores [34], we controlled for baseline preoperative pain severity and pain catastrophizing variables in the cross-lagged model (see Figure 1). We also included age, sex, race, and baseline anxiety and depressive symptoms as additional covariates in the model. Appropriateness of model fit was determined by the comparative fit index (CFI), the root mean square error of approximation (RMSEA), and the standardized root mean residual (SRMR). CFI values >0.90, RMSEA values <0.06, and SRMR values <0.08 indicate good fit to the data [35–37]. Missing data were handled by FIML estimation based on a missing-at-random assumption. FIML estimation is one of the best existing missing-data analytic techniques [30, 31]. Variables that were assessed at concurrent time points were allowed to correlate. Mplus 8.2 (Muthen & Muthen) [38] was used to conduct the cross-lagged panel model.
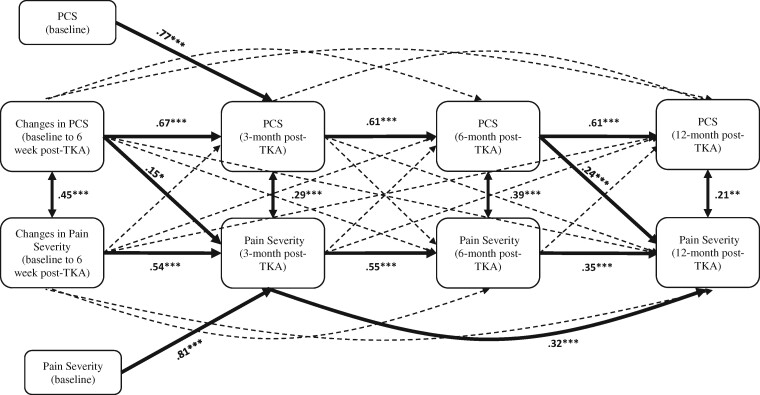
Figure 1.
Cross-lagged model with pain severity and pain catastrophizing. Bold lines indicate statistically significant paths. For visual parsimony, only significant path estimates are shown. All path estimates are standardized regression coefficients. The following covariates were included in the analyses but are not shown for parsimony: age, sex, race, anxiety, and depressive symptoms at baseline. Single-headed arrows indicate regression paths. Double-headed arrows indicate correlations. * P < 0.05, ** P < 0.01, *** P < 0.001.
Results
Participants’ Characteristics
As shown in Table 1, the participants’ mean age was 65.1 years (standard deviation = 8.2), and the majority of participants were female and Caucasian and had at least some college education. Most participants were either married or had a partner and were working full-time or retired.
Table 1.
Baseline sample characteristics
| Variables | N = 245 |
|---|---|
| Age, y, mean ± SD | 65.1 ± 8.2 |
| Sex, female, % (n) | 59.5% (147) |
| Race, % (n) | |
| White | 88.0% (216) |
| Black | 9.1% (22) |
| Others | 2.9% (7) |
| Education, % (n) | |
| Some high school or less | 3.3% (8) |
| High school graduate/tech graduate/GED | 8.7% (21) |
| Some college | 25.6% (63) |
| College graduate | 29.3% (72) |
| Graduate school degree | 33.1% (81) |
| Marital status, % (n) | |
| Married or living with partner | 73.7% (180) |
| Single | 8.2% (21) |
| Separated or divorced | 11.1% (27) |
| Widowed | 7.0% (17) |
| Employment, % (n) | |
| Full-time | 35.1% (86) |
| Part-time | 12.0% (29) |
| Homemaker | 4.5% (11) |
| Retired | 39.3% (96) |
| Unemployed | 3.7% (9) |
| Disability | 5.4% (13) |
SD = standard deviation.
Pain Severity and Pain Catastrophizing from Baseline to 12 Months After TKA
Table 2 indicates pain severity and pain catastrophizing at each time point. As expected, RM-ANOVAs indicated that pain severity decreased significantly from baseline to 12 months after TKA (F[2.815, 315.266] = 61.126, P < 0.001). Similarly, pain catastrophizing also decreased significantly over time (F[2.674, 299.498] = 26.971, P < 0.001).
Table 2.
Means and standard deviations of study variables at each time point
| Variables | Baseline | 6 Weeks After TKA | 3 Months After TKA | 6 Months After TKA | 12 Months After TKA |
|---|---|---|---|---|---|
| Pain catastrophizing | 13.80 ± 12.10 | 9.43 ± 10.39 | 7.87 ± 10.22 | 6.39 ± 9.20 | 5.99 ± 9.35 |
| Pain severity | 3.76 ± 2.20 | 2.75 ± 1.78 | 1.73 ± 1.68 | 1.61 ± 1.73 | 1.61 ± 1.80 |
Findings of the Cross-Lagged Panel Model
The cross-lagged panel model (see Figure 1) had overall good model fit (CFI = 0.987, RMSEA = 0.059, SRMR = 0.016). Changes in baseline to 6-week post-TKA pain catastrophizing significantly (standardized β = 0.15; SE = 0.07, P = 0.046) predicted pain severity at 3 months after TKA while controlling for auto-correlation and covariates. In other words, a decrease in pain catastrophizing from baseline to 6 weeks after TKA was associated with lower pain severity at 3 months after TKA. On the other hand, baseline to 6-week post-TKA changes in pain severity did not predict pain catastrophizing at 3 months after TKA (standardized β = –0.004; SE = 0.07, P = 0.952) while controlling for auto-correlation and covariates.
There were no significant cross-lagged paths from 3 months after TKA to 6 months after TKA while controlling for auto-correlation and all other covariates. Although baseline pain catastrophizing did not predict pain severity at 6 months, baseline anxiety symptoms significantly predicted pain catastrophizing at 6 months after TKA (standardized β = 0.24; SE = 0.08, P = 0.002), and baseline depressive symptoms significantly predicted pain severity at 6 months after TKA (standardized β = 0.19; SE = 0.08, P = 0.019).
With regard to 12-month post-TKA outcomes, pain catastrophizing measured at 6 months after TKA significantly predicted pain severity at 12 months after TKA (standardized β = 0.24; SE = 0.09, P = 0.006) while controlling for auto-correlation and covariates. On the other hand, pain severity measured at 6 months after TKA did not significantly predict pain catastrophizing at 12 months after TKA (standardized β = 0.13; SE = 0.08, P = 0.106) while controlling for auto-correlation and covariates.
Discussion
This study investigated whether changes in pain catastrophizing prospectively influence subsequent pain severity, or vice versa, in individuals who underwent TKA. Using a cross-lagged panel model, we found that reduction in pain catastrophizing from baseline to 6 weeks after TKA was associated with decreased pain severity at 3 months after TKA. These findings were observed even when controlling for baseline to 6-week changes in pain severity, as well as baseline pain severity, pain catastrophizing, anxiety and depressive symptoms, age, and sex. In contrast, changes in pain severity from baseline to 6 weeks after TKA did not predict pain catastrophizing 3 months after TKA. In addition, when we examined the long-term bidirectional temporal association of pain severity and pain catastrophizing beyond 3 months after TKA, pain catastrophizing predicted subsequent pain severity at 12 months after TKA, whereas pain severity did not predict subsequent pain catastrophizing. Collectively, these results provide further support that perioperative changes in pain catastrophizing (i.e., elevations or minimal reductions in high catastrophizers) may be an important clinical target for reducing chronic postsurgical pain (CPSP) after TKA.
Our findings are consistent with numerous studies demonstrating that the greatest reductions in pain severity and pain catastrophizing occur within 3 months after TKA [20, 39–44], and these reductions are sustained over long-term follow-up [20, 39, 45]. Our results also add to a growing body of literature suggesting that changes in catastrophizing may precede changes in pain severity [23–25]. To date, only one study has examined temporal associations between pain severity and catastrophizing in a population of patients undergoing TKA [20]. Contrary to our findings, that study found that baseline and 2-month post-TKA pain severity predicted subsequent pain catastrophizing through 6-month follow-up, though the association of pain catastrophizing with subsequent pain was not reported. Further research is needed to elucidate the mechanisms underpinning these mixed findings. Participants’ education level, a proxy for socioeconomic status, is a notable difference between these studies. Only 10% of participants in our study had a high school education or less, compared with one third of participants in the previous study [20]. Lower socioeconomic status is associated with greater preoperative pain and catastrophizing in individuals undergoing TKA [46], yet the extent to which socioeconomic status impacts acute and long-term outcomes after TKA has been difficult to discern [47, 48]. Future studies that examine the impact of socioeconomic risk factors on catastrophizing, pain expectations, treatment access, and chronic stressors [49, 50] may help elucidate underlying mechanisms influencing the temporal relationship of catastrophizing and pain.
To our knowledge, this is the first study to demonstrate that reduction in pain catastrophizing in the acute (i.e., 6-week) post-TKA period predicts subsequent pain reduction at the acute-to-chronic postoperative transition (i.e., 3-month post-TKA) period. This finding is important because individuals who experience acute post-TKA pain are at greater risk of developing CPSP [51–54], and progression to CPSP predicts worse long-term outcomes [40, 55, 56]. A recent systematic review that evaluated the effectiveness of psychological interventions for managing post-TKA pain highlighted the need for more rigorous studies that assess the effect of timing of intervention delivery on clinical outcomes, including during the acute-to-chronic pain transition period [57]. Few studies [39, 58] have examined the effectiveness of delivering postoperative adjunctive psychological interventions. Our findings that acute post-TKA changes in pain catastrophizing influence pain severity around the time of onset of CPSP buttress support for more empirical research on when to deliver adjunctive interventions.
Mounting evidence suggests that myriad interventions, including surgery itself, can reduce pain catastrophizing in TKA candidates [45, 59]. Nevertheless, results of psychological interventions targeting individuals with elevated pre-TKA pain catastrophizing remain equivocal. In one study, patients with elevated preoperative pain catastrophizing who received an eight-session pain coping skills intervention within 8 weeks after TKA showed clinically significant reductions in pain and catastrophizing when compared to a historical usual care group [60]. However, these results were not replicated in a subsequent larger multisite clinical trial [39] in which patients received an eight-session pain coping skills intervention that began 2 weeks preoperatively and continued through the acute post-TKA period. The authors suggested that a moderate PCS score at baseline may not be a viable predictive tool for TKA outcomes. Similarly, a randomized controlled trial that investigated the effectiveness of a 10-session postoperative motivational interviewing intervention delivered up to 6 months after TKA for individuals with elevated preoperative catastrophizing did not yield functional improvement compared with usual care [58].
Our findings support the possibility that preoperative pain catastrophizing alone may not provide sufficient utility in predicting which individuals would benefit from adjunctive interventions. It is worth speculating that elevated or unchanged pain catastrophizing at 6–8 weeks after TKA may potentially serve as a more nuanced target for adjunctive interventions to help reduce the development of CPSP [61]. Indeed, the recent adaptation of perioperative pain clinics in North America and Europe [62–66] has demonstrated the necessity and effectiveness of identifying and treating patients who experience intense pain and elevated distress both before and after surgery. As many surgical patients enter perioperative pain programs postoperatively [63, 67], empirical research is needed to investigate whether psychological interventions targeting elevated or unchanged acute post-TKA pain catastrophizing may facilitate adaptation of more tailored real-world treatments.
Although pain catastrophizing predicts poor outcomes independent of the effects of anxiety and depressive symptoms, preoperative anxiety and depressive symptoms are also significantly associated with pain severity 1 to 2 years after TKA [68–70], and psychological distress has adverse effects on post-TKA outcomes in older patients [71]. Consistent with the extant literature, we found that baseline anxiety symptoms predicted pain catastrophizing at 3 months and 6 months after TKA, and baseline depressive symptoms predicted pain severity at 6 months after TKA. Collectively, these findings provide further evidence that early-phase trials are needed to adapt perioperative psychological interventions targeting the modifiable factors that are associated with worse post-TKA outcomes.
These findings must be interpreted in the context of study limitations. First, the sample was largely Caucasian, college educated, employed, and married; thus, our findings may not be generalizable across all individuals undergoing TKA. Additionally, as we were unable to ascertain the total number of TKAs conducted at both study sites during the enrollment period, our findings may not reflect the overall potential sample of patients undergoing TKA within our academic centers. Second, the sample excluded patients with opioid use within 3 months of TKA and represented a cohort with low levels of pain catastrophizing; thus, our findings may not be generalizable across a significant minority of patients on preoperative opioids or with high baseline catastrophizing (i.e., PCS ≥16 [39, 72]). Third, a study administration error resulted in some missing data at baseline assessment. However, we used a modern missing-data analysis technique (i.e., FIML) to adequately handle the missing data. Fourth, it was beyond the scope of this study to examine participants’ use of pharmacological and nonpharmacological analgesia treatments after TKA. It is possible that various treatments could have influenced the temporal relationship of pain severity and pain catastrophizing. Despite these limitations, we think our study contributes important clinical results that warrant further investigation.
In conclusion, we found that changes in pain catastrophizing from baseline to 6 weeks after TKA significantly predict pain severity at the transition from acute to chronic postoperative pain and that pain catastrophizing predicted subsequent pain severity in the chronic postoperative period up to 12 months. Conversely, changes in pain severity did not predict subsequent pain catastrophizing, and pain severity did not predict future pain catastrophizing in the chronic postoperative period up to 12 months. Future studies are warranted to determine how changes in pain catastrophizing during the perioperative period may serve as prognostic indicators for TKA outcomes and how postoperative pain catastrophizing may guide the use of tailored adjunct interventions to improve clinical outcomes for individuals undergoing TKA.
Funding sources: This research was funded by R01 AG034982 (RRE), F32DA049393 (CJM), T32 NS070201 (TJS), and the Johns Hopkins Bayview Medical Campus Clinical Research Unit.
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1. Lewis GN, Rice DA, McNair PJ, Kluger M.. Predictors of persistent pain after total knee arthroplasty: A systematic review and meta-analysis. Br J Anaesth 2015;114(4):551–61. [DOI] [PubMed] [Google Scholar]
- 2. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN.. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag 2009;14(4):307–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lingard EA, Katz JN, Wright EA, Sledge CB, Kinemax Outcomes Group. Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am 2004;86(10):2179–86. [DOI] [PubMed] [Google Scholar]
- 4. Baker PN, van der Meulen JH, Lewsey J, Gregg PJ, National Joint Registry for England and Wales. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br 2007;89-B(7):893–900. [DOI] [PubMed] [Google Scholar]
- 5. Carr AJ, Robertsson O, Graves S, et al. Knee replacement. Lancet 2012;379(9823):1331–40. [DOI] [PubMed] [Google Scholar]
- 6. Heck DA, Robinson RL, Partridge CM, Lubitz RM, Freund DA.. Patient outcomes after knee replacement. Clin Orthop Relat Res 1998;(356):93–110. [DOI] [PubMed] [Google Scholar]
- 7. Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P.. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2(1):e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wylde V, Dieppe P, Hewlett S, Learmonth ID.. Total knee replacement: Is it really an effective procedure for all? Knee 2007;14(6):417–23. [DOI] [PubMed] [Google Scholar]
- 9. Becker R, Döring C, Denecke A, Brosz M.. Expectation, satisfaction and clinical outcome of patients after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 2011;19(9):1433–41. [DOI] [PubMed] [Google Scholar]
- 10. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster J-Y.. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am 2004;86(5):963–74. [DOI] [PubMed] [Google Scholar]
- 11. Singh JA, Lewallen DG.. Predictors of use of pain medications for persistent knee pain after primary total knee arthroplasty: A cohort study using an institutional joint registry. Arthritis Res Ther 2012;14(6):R248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruyère O, Ethgen O, Neuprez A, et al. Health-related quality of life after total knee or hip replacement for osteoarthritis: A 7-year prospective study. Arch Orthop Trauma Surg 2012;132(11):1583–7. [DOI] [PubMed] [Google Scholar]
- 13. Sullivan MJL, Bishop SR, Pivik J.. The pain catastrophizing scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 14. Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LM.. Psychological aspects of persistent pain: Current state of the science. J Pain 2004;5(4):195–211. [DOI] [PubMed] [Google Scholar]
- 15. Quartana PJ, Campbell CM, Edwards RR.. Pain catastrophizing: A critical review. Expert Rev Neurother 2009;9(5):745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorel JC, Veltman ES, Honig A, Poolman RW.. The influence of preoperative psychological distress on pain and function after total knee arthroplasty: A systematic review and meta-analysis. Bone Joint J 2019;101-B(1):7–14. [DOI] [PubMed] [Google Scholar]
- 17. Burns LC, Ritvo SE, Ferguson MK, Clarke H.. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: A systematic review. J Pain 2015;8:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hernández C, Díaz-Heredia J, Berraquero ML, et al. Pre-operative predictive factors of post-operative pain in patients with hip or knee arthroplasty: A systematic review. Reumatol Clin 2015;11(6):361–80. [DOI] [PubMed] [Google Scholar]
- 19. Riddle DL, Jensen MP, Ang D, et al. Do pain coping and pain beliefs associate with outcome measures before knee arthroplasty in patients who catastrophize about pain? A cross-sectional analysis from a randomized clinical trial. Clin Orthop Relat Res 2018;476(4):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wade JB, Riddle DL, Thacker LR.. Is pain catastrophizing a stable trait or dynamic state in patients scheduled for knee arthroplasty? Clin J Pain 2012;28(2):122–8. [DOI] [PubMed] [Google Scholar]
- 21. Broderick JE, Keefe FJ, Bruckenthal P, et al. Nurse practitioners can effectively deliver pain coping skills training to osteoarthritis patients with chronic pain: A randomized, controlled trial. Pain 2014;155(9):1743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Selig JP, Little TD.. Autoregressive and cross-lagged panel analysis for longitudinal data. In: Laursen B, ed. Handbook of Developmental Research Methods, vol. 788. New York, NY: The Guilford Press; 2012:265–78. [Google Scholar]
- 23. Campbell CM, McCauley L, Bounds SC, et al. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: Cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res Ther 2012;14(5):R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campbell CM, Quartana PJ, Buenaver LF, Haythornthwaite JA, Edwards RR.. Changes in situation-specific pain catastrophizing precede changes in pain report during capsaicin pain: A cross-lagged panel analysis among healthy, pain-free participants. J Pain 2010;11(9):876–84. [DOI] [PubMed] [Google Scholar]
- 25. Burns JW, Kubilus A, Bruehl S, Harden RN, Lofland K.. Do changes in cognitive factors influence outcome following multidisciplinary treatment for chronic pain? A cross-lagged panel analysis. J Consult Clin Psychol 2003;71(1):81–91. [DOI] [PubMed] [Google Scholar]
- 26. Abrecht CR, Cornelius M, Wu A, et al. Prediction of pain and opioid utilization in the perioperative period in patients undergoing primary knee arthroplasty: Psychophysical and psychosocial factors. Pain Med 2019;20(1):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lazaridou A, Martel MO, Cornelius M, et al. The association between daily physical activity and pain among patients with knee osteoarthritis: The moderating role of pain catastrophizing. Pain Med 2019;20(5):916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nandi M, Schreiber KL, Martel MO, et al. Sex differences in negative affect and postoperative pain in patients undergoing total knee arthroplasty. Biol Sex Differ 2019;10(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mun CJ, Letzen JE, Nance S, et al. Sex differences in interleukin-6 responses over time following laboratory pain testing among patients with knee osteoarthritis. J Pain 2020;21(5-6):731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Enders CK, Bandalos DL.. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Modeling 2001;8(3):430–57. [Google Scholar]
- 31. Enders CK. Applied Missing Data Analysis. New York: Guilford Press; 2010. [Google Scholar]
- 32. Cleeland CS, Ryan KM.. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23(2):129–38. [PubMed] [Google Scholar]
- 33. Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63(11):1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Norman GR. Issues in the use of change scores in randomized trials. J Clin Epidemiol 1989;42(11):1097–105. [DOI] [PubMed] [Google Scholar]
- 35. Bentler PM, Kano Y.. On the equivalence of factors and components. Multivariate Behav Res 1990;25(1):67–74. [DOI] [PubMed] [Google Scholar]
- 36. Byrne BM. Structural equation modeling with AMOS: Basic concepts, applications, and programming. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. [Google Scholar]
- 37. Kline RB. Software review: Software programs for structural equation modeling: Amos, EQS, and LISREL. J Psychoeduc Assess 1998;16(4):343–64. [Google Scholar]
- 38. Muthén LK, Muthén BO.. Mplus User’s Guide, Eighth Edition. Los Angeles, CA: Muthen & Muthen; 2017. [Google Scholar]
- 39. Riddle DL, Keefe FJ, Ang DC, et al. Pain coping skills training for patients who catastrophize about pain prior to knee arthroplasty: A multisite randomized clinical trial. J Bone Joint Surg Am 2019;101(3):218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dumenci L, Perera R A, Keefe F J, et al. . Model-based pain and function outcome trajectory types for patients undergoing knee arthroplasty: a secondary analysis from a randomized clinical trial. Osteoarthritis Cartilage 2019;27(6):878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wylde V, Dennis J, Beswick AD, et al. Systematic review of management of chronic pain after surgery. Br J Surg 2017;104(10):1293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Riddle DL, Perera RA, Stratford PW, Jiranek WA, Dumenci L.. Progressing toward, and recovering from, knee replacement surgery: A five-year cohort study. Arthritis Rheum 2013;65(12):3304–13. [DOI] [PubMed] [Google Scholar]
- 43. Lape EC, Selzer F, Collins JE, Losina E, Katz JN.. Stability of measures of pain catastrophizing and widespread pain following total knee replacement (TKR). Arthritis Care Res 2020;72(8):1096–103. [DOI] [PubMed] [Google Scholar]
- 44. Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJL, Gross M.. Prospective relation between catastrophizing and residual pain following knee arthroplasty: Two-year follow-up. Pain Res Manag 2008;13(4):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Høvik LH, Winther SB, Foss OA, Gjeilo KH.. Preoperative pain catastrophizing and postoperative pain after total knee arthroplasty: A prospective cohort study with one year follow-up. BMC Musculoskelet Disord 2016;17(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feldman CH, Dong Y, Katz JN, Donnell-Fink LA, Losina E.. Association between socioeconomic status and pain, function and pain catastrophizing at presentation for total knee arthroplasty. BMC Musculoskelet Disord 2015;16(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keeney BJ, Koenig KM, Paddock NG, et al. Do aggregate socioeconomic status factors predict outcomes for total knee arthroplasty in a rural population? J Arthroplasty 2017;32(12):3583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goodman SM, Mandl LA, Parks ML, et al. Disparities in TKA outcomes: Census tract data show interactions between race and poverty. Clin Orthop Relat Res 2016;474(9):1986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meints SM, Cortes A, Morais CA, Edwards RR.. Racial and ethnic differences in the experience and treatment of noncancer pain. Pain Manag 2019;9(3):317–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carriere JS, Martel MO, Meints SM, Cornelius MC, Edwards RR.. What do you expect? Catastrophizing mediates associations between expectancies and pain-facilitatory processes. Eur J Pain 2019;23(4):800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kehlet H, Jensen TS, Woolf CJ.. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006;367(9522):1618–25. [DOI] [PubMed] [Google Scholar]
- 52. Puolakka PAE, Rorarius MGF, Roviola M, et al. Persistent pain following knee arthroplasty. Eur J Anaesthesiol 2010;27(5):455–60. [DOI] [PubMed] [Google Scholar]
- 53. Masselin-Dubois A, Attal N, Fletcher D, et al. Are psychological predictors of chronic postsurgical pain dependent on the surgical model? A comparison of total knee arthroplasty and breast surgery for cancer. J Pain 2013;14(8):854–64. [DOI] [PubMed] [Google Scholar]
- 54. Lavand’homme P, Thienpont E.. Pain after total knee arthroplasty: A narrative review focusing on the stratification of patients at risk for persistent pain. Bone Joint J 2015;97-B(10_Supple_A):45–8. [DOI] [PubMed] [Google Scholar]
- 55. Yang HY, Losina E, Lange JK, Katz JN, Collins JE.. Longitudinal trajectories of pain and function improvement following total knee replacement. ACR Open Rheumatol 2019;1(5):308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Singh JA, Lemay CA, Nobel L, et al. Association of early postoperative pain trajectories with longer-term pain outcome after primary total knee arthroplasty. JAMA Netw Open 2019;2(11):e1915105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Whale K, Wylde V, Beswick A, et al. Effectiveness and reporting standards of psychological interventions for improving short-term and long-term pain outcomes after total knee replacement: A systematic review. BMJ Open 2019;9(12):e029742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Losina E, Collins JE, Wright J, et al. Postoperative care navigation for total knee arthroplasty patients: A randomized controlled trial. Arthritis Care Res 2016;68(9):1252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gibson E, Sabo MT.. Can pain catastrophizing be changed in surgical patients? A scoping review. Can J Surg 2018;61(5):311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Riddle DL, Keefe FJ, Nay WT, et al. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: A quasi-experimental study. Arch Phys Med Rehabil 2011;92(6):859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Terradas-Monllor M, Ochandorena-Acha M, Salinas-Chesa J, Ramírez S, Beltran-Alacreu H.. Assessment of postoperative health functioning after knee arthroplasty in relation to pain catastrophizing: A 6-month follow-up cohort study. PeerJ 2020;8:e9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Katz J, Weinrib AZ, Clarke H.. Chronic postsurgical pain: From risk factor identification to multidisciplinary management at the Toronto General Hospital Transitional Pain Service. Can J Pain 2019;3(2):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shechter R, Speed TJ, Blume E, et al. Addressing the opioid crisis one surgical patient at a time: Outcomes of a novel perioperative pain program. Am J Med Qual 2020;35(1):5–15. [DOI] [PubMed] [Google Scholar]
- 64. Hanna MN, Speed TJ, Shechter R, et al. An innovative perioperative pain program for chronic opioid users: An academic medical center’s response to the opioid crisis. Am J Med Qual 2019;34(1):5–13. [DOI] [PubMed] [Google Scholar]
- 65. Katz J, Weinrib A, Fashler SR, et al. The Toronto General Hospital Transitional Pain Service: Development and implementation of a multidisciplinary program to prevent chronic postsurgical pain. J Pain Res 2015;8:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tiippana E, Hamunen K, Heiskanen T, et al. New approach for treatment of prolonged postoperative pain: APS Out-Patient Clinic. Scand J Pain 2016;12(1):19–24. [DOI] [PubMed] [Google Scholar]
- 67. Azam MA, Weinrib AZ, Montbriand J, et al. Acceptance and commitment therapy to manage pain and opioid use after major surgery: Preliminary outcomes from the Toronto General Hospital Transitional Pain Service. Can J Pain 2017;1(1):37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brander VA, Stulberg SD, Adams AD, et al. Ranawat Award Paper: Predicting total knee replacement pain: A prospective, observational study. Clin Orthop Relat Res 2003;(416):27–36. [DOI] [PubMed] [Google Scholar]
- 69. Lingard EA, Riddle DL.. Impact of psychological distress on pain and function following knee arthroplasty. J Bone Joint Surg Am 2007;89(6):1161–9. [DOI] [PubMed] [Google Scholar]
- 70. Hirschmann MT, Testa E, Amsler F, Friederich NF.. The unhappy total knee arthroplasty (TKA) patient: Higher WOMAC and lower KSS in depressed patients prior and after TKA. Knee Surg Sports Traumatol Arthrosc 2013;21(10):2405–11. [DOI] [PubMed] [Google Scholar]
- 71. Faller H, Kirschner S, König A.. Psychological distress predicts functional outcomes at three and twelve months after total knee arthroplasty. Gen Hosp Psychiatry 2003;25(5):372–3. [DOI] [PubMed] [Google Scholar]
- 72. Birch S, Stilling M, Mechlenburg I, Hansen TB.. No effect of cognitive behavioral patient education for patients with pain catastrophizing before total knee arthroplasty: A randomized controlled trial. Acta Orthop 2020;91(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]