Abstract
Objective
Distal sensory polyneuropathy (DSP) is a disabling consequence of human immunodeficiency virus (HIV), leading to poor quality of life and more frequent falls in older age. Neuropathic pain and paresthesia are prevalent symptoms; however, there are currently no known curative treatments and the longitudinal course of pain in HIV-associated DSP is poorly characterized.
Methods
This was a prospective longitudinal study of 265 people with HIV (PWH) enrolled in the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study with baseline and 12-year follow-up evaluations. Since pain and paresthesia are highly correlated, statistical decomposition was used to separate the two symptoms at baseline. Multivariable logistic regression analyses of decomposed variables were used to determine the effects of neuropathy symptoms at baseline on presence and worsening of distal neuropathic pain at 12-year follow-up, adjusted for covariates.
Results
Mean age was 56 ± 8 years, and 21% were female at follow-up. Nearly the entire cohort (96%) was on antiretroviral therapy (ART), and 82% had suppressed (≤50 copies/mL) plasma viral loads at follow-up. Of those with pain at follow-up (n = 100), 23% had paresthesia at the initial visit. Decomposed paresthesia at baseline increased the risk of pain at follow-up (odds ratio [OR] 1.56; 95% confidence interval [CI] 1.18, 2.07), and decomposed pain at baseline predicted a higher frequency of pain at follow-up (OR 1.96 [95% CI 1.51, 2.58]).
Conclusions
Paresthesias are a clinically significant predictor of incident pain at follow-up among aging PWH with DSP. Development of new therapies to encourage neuroregeneration might take advantage of this finding to choose individuals likely to benefit from treatment preventing incident pain.
Keywords: HIV, Neuropathy, Pain, CHARTER Study, Paresthesia
Introduction
Chronic neuropathic pain is one of the most disabling complications among persons with human immunodeficiency virus (HIV, PWH) in older age [1, 2], with a reported frequency between 28% and 97% depending on the instrument utilized [2]. Neuropathic pain is defined as pain, usually distal, bilateral, symmetrical with qualities of burning, shooting or aching, caused by a lesion of peripheral nerve fibers (Aβ, Aγ or C fibers) or central neurons affecting the somatosensory system [3]. To date, there are no FDA-approved treatments for neuropathic pain in HIV-associated DSP despite its debilitating consequences in older PWH, although chronic pain may contribute to dependence on pain medications, such as opioids, among a group of patients who may already be at higher risk for substance dependence [2]. In addition, factors such as psychiatric comorbid conditions, prevalent among PWH [4], lead to barriers to effective treatment of painful conditions and limit treatment options due to interactions with psychiatric medications. In chemotherapy-induced peripheral neuropathy, sensory neuropathy symptoms (sensory loss and paresthesia [pins-and-needles sensation or tingling]) often exist without neuropathic pain symptoms, but the reverse is uncommon, suggesting that numbness and tingling occur first with pain later being a sequela of neuropathy [5]. However, this relationship is unknown in the context of HIV. Developing an improved understanding of the mechanisms and predictors of neuropathic pain may lead to new treatments as well as improved clinical management of HIV-associated DSP.
Symptoms of DSP include neuropathic pain, paresthesia, and sensory loss or numbness usually in a distal symmetric “stocking-and-glove” pattern affecting primarily the feet and toes [6]. They are more common in older individuals and among those with prior exposure to neurotoxic antiretroviral therapy (ART), such as dideoxynucleosides (stavudine, didanosine) [7] and in older age, among females, and those with detectable plasma viral loads and lifetime history of opioid use disorder [8]. Recently, our lab has reported a novel subcortical central nervous system (CNS) mechanism that may explain the link between HIV neuropathic paresthesias and pain via ascending atrophy of white matter tracts leading to atrophy of the cortical posterior cingulate gyrus of the CNS [9, 10]. Although these important findings may represent a pathophysiologic mechanism linking pain and parethesias, they were derived from a cross-sectional study that prevented conclusions from being drawn of the causal relationships between paresthesia and neuropathic pain.
Although it is known that the prevalence of paresthesia and pain among HIV-associated DSP ranges from 28% to 84%, often with significant overlap [1, 11, 12], the longitudinal effect of paresthesia on development of future neuropathic pain is unknown. We present the first longitudinal study on the effect of HIV-associated neuropathic paresthesia on incident neuropathic pain. In this study, we investigated the effect of baseline DSP-related paresthesia with or without pain on pain outcomes after 12 years.
Methods
Participants
Participants were 265 PWH, mean age 56 ± 8 years (at follow-up), 79% male. The study was conducted from 1998 to 2018, and during this time all participants completed both baseline and 12-year follow-up study visits. The long-term longitudinal cohort was derived from the larger CNS HIV Antiretroviral Therapy Effects Research (CHARTER) cohort, a prospective multi-site cohort of participants with HIV in care at six academic medical centers (Johns Hopkins University [Baltimore, MD]; Icahn School of Medicine at Mount Sinai [New York, NY]; University of California, San Diego [La Jolla, CA]; University of Texas, Galveston [Galveston, TX]; University of Washington [Seattle, WA]; and Washington University [St. Louis, MO]) (13). The study protocol involves comprehensive standardized neuromedical, neuropsychological, and psychiatric assessments that have been previously described (14).
The Human Subjects Protection Committees of each participating institution approved the study procedures. Written informed consent was obtained from all study participants before enrollment into the study.
Materials and Procedures
Diagnosis of HIV-Associated Distal Sensory Polyneuropathy
Using the CHARTER study protocol described in detail elsewhere [13], diagnoses of HIV-associated DSP were made using a standardized neurological examination evaluating signs and symptoms of HIV-associated DSP. Participants were asked about any symptoms of paresthesia, loss of sensation and neuropathic pain, specifically tingling or burning, aching, or stabbing pain in a bilateral, predominantly distal distribution (i.e., fingers and toes only, extending to ankles or wrists, or extending to the knees or elbows). If participants expressed having tingling in any of these bilateral distal distributions, they were considered to have a positive self-report of paresthesia; if they endorsed pain symptoms in these distributions, they were considered to have a positive self-report of DNP; if they endorsed sensory loss or numbness in any of these distributions, they were considered positive for self-report of loss of sensation. Study clinicians further classified neuropathic pain (defined as burning, aching, or shooting symptoms) into five categories of “clinician-rated pain severity”: none, slight (occasional, fleeting), mild (frequent), moderate (frequent, disabling), and severe (constant, daily, disabling, requiring analgesic medication or other pain medication). Neuropathy signs were defined as objective neurological examination findings, such as: diminished ability to distinguish vibration sense or reduced sharp-dull discrimination in the feet and toes bilaterally or reduced ankle reflexes bilaterally. Demographic data including age, gender, race/ethnicity, and years of education were collected. Only a small subset of the CHARTER participants included in this study with longitudinal data over 12 years underwent nerve conduction studies (NCS), quantitative sensory testing (QST), or skin biopsy; thus, these diagnostics were not considered, and the diagnosis of HIV-associated DSP was based on clinical exam findings and structured interview previously used in other CHARTER studies [15, 16[] that correlates with neurophysiologic findings among PWH [1].
The Medical Outcomes Study HIV Health Survey (MOS-HIV) was also used and is a reliable and valid tool for assessing overall quality of life, daily functioning, and physical health [17, 18]. The MOS-HIV contains 36 questions that assess physical and mental dimensions of health. These are scored as summary percentile scales ranging from 0 to 100, with higher scores indicating better health. Increased dependence in instrumental activities of daily living (IADLs) was assessed with a modified version of the Lawton and Brody Scale [19] that asks participants to rate their current and best lifetime levels of independence for 13 major IADLs such as shopping, financial management, transportation, and medication management [20].
Medical Evaluation
The neurological examination was performed by a clinician (MD, NP, or RN) trained in neurological AIDS disorders and experienced in the care of PWH who performed a standardized, targeted neurological examination used to evaluate signs and symptoms of HIV-associated DSP. Clinicians were trained using a standardized protocol in which all examiners are trained by one clinician so that the neurological examination may be standardized to allow for consistency and reproducibility across examiners. Participants underwent structured interviews and medical record reviews to determine HIV disease status and medication history. We obtained information regarding exposure to ART drugs, including current and past exposure to dideoxynucleoside reverse transcriptase inhibitors (e.g., zalcitabine, didanosine, and stavudine), given their associations with neuropathy. Study participants were categorized as currently using, past history of, or never used ART. We queried participants on any current pain treatment with opioids, tricyclic and other antidepressants, or anticonvulsants used for pain management. Blood was collected by venipuncture to quantify plasma HIV concentration by reverse transcription polymerase chain reaction ultrasensitive assay (nominal lower quantitation limit, 50 copies/mL [Amplicor; Roche Diagnostic Systems, Indianapolis, Indiana]). Current peripheral blood CD4+ T-cell concentrations were measured by flow cytometry. Hepatitis C virus (HCV) exposure was determined by serology. Nadir CD4 was determined by participant report with medical record verification when available. Current mood was also assessed by the Beck Depression Inventory (BDI)-II [21]. Past and current DSM-IV diagnoses of major depressive disorder (MDD) and substance use disorders (abuse and dependence) were captured using the Composite International Diagnostic Interview (CIDI), a fully structured, lay-administered instrument [22].
Statistical Decomposition of Neuropathic Pain and Paresthesia
Due to the significant overlap between pain and paresthesia (59%) and variance between pain and paresthesia (r = 0.61), separating out the individual effects of pain and paresthesia is challenging. Variable whitening [23] is a method used to decompose the covariance between variables. The steps involved in decomposing the variables are: 1) determine orthogonal components of the variable in a data matrix through a principal component analysis; 2) determine a rotation of the variables to match the original (nondecomposed) variables. Zero-phase component analysis finds the whitening transformation minimizing the distance between the decomposed and the non-decomposed variable independent to variable scaling [24]. The final decomposed variables have no correlation with each other, but there is maximization of the correlation with the nondecomposed variable. In our data set, nondecomposed and decomposed dichotomous variables are highly correlated for presence or absence of paresthesia (r = 0.911) and pain (r = 0.921), separately. Nondecomposed dichotomous pain did not correlate strongly with decomposed paresthesia (r=−0.288). After data whitening of the baseline pain and paresthesia, no collinearity existed between the decomposed pain (VIF = 1.02) and decomposed paresthesia (VIF = 1.10) against all other variables. We also analyzed decomposed ordinal paresthesia variables (ratings of 0 or 1 or 2 or 3) by severity, with higher numbers signifying worse severity of paresthesia.
Statistical Analyses
Two-sample t-tests and χ2 tests were used to examine the univariable association between neuropathic pain at 12-year follow-up and baseline participant characteristics, including demographic factors (e.g., age, years of education, and sex), HIV disease characteristics (e.g., estimated duration of infection, nadir CD4, viral load), medical comorbidities (e.g., diabetes, hepatitis C infection), psychiatric comorbidities (lifetime and current major depressive disorder), lifetime or current substance use diagnoses (cannabis, cocaine, methamphetamine, opioids), and past use of peripherally neurotoxic agents (e.g., stavudine, didanosine, or zalcitabine) and baseline neurological exam findings (pin sensibility, vibration sense, ankle reflexes). Two models were built to analyze: 1) the effect of baseline decomposed pain and paresthesia (as separate dichotomous variables), and 2) the effect of baseline decomposed pain and paresthesia severity (as ordinal variables) vs. pain at the follow-up visit adjusted for significant covariates known to be associated with neuropathy (age, current CD4, nadir CD4, current plasma viral load, history of diabetes, history of hepatitis C infection, past use of peripherally neurotoxic ART), using an α = .05. JMP version 14.0.0 and R version 3.5.1 was used for all statistical analyses.
Results
Cohort Characteristics at Follow-up
In the overall cohort (n = 265), 100 (38%) had pain at the 12-year follow-up visit. The group with pain at follow-up was significantly older than the group without follow-up pain (P = .019, Table 1). Forty-five percent of the cohort identified as non-Hispanic Black, 42% non-Hispanic White, 11% Hispanic, and 2% other (Table 1). Those with and without pain at follow-up did not differ with regard to sex, race, or education (P > .05). Presence of diabetes at follow-up was significantly higher in those with follow-up pain versus those without (odds ratio [OR] 2.05, 95% confidence interval [CI] 1.09, 3.87; Table 1). Lifetime cocaine abuse or dependence increased the odds of pain at follow-up (OR 1.70 [95% CI 1.02 to 2.84]). The two groups did not differ significantly in any other lifetime substance use or lifetime or current major depressive disorder. BDI-II results did not differ between pain groups at follow-up (P = .21). DNP at follow-up was associated with a higher prevalence of positive urine toxicology for opiates (OR 4.45 [95% CI 1.82, 12.0]). Participants with paresthesia at follow-up had significantly worse mental and physical health MOS-HIV scores than those without (46.9 ± 12.8 vs 53.1 ± 9.2; P < .0001 and 40.6 ± 12.5 vs 48.2 ± 10.7; P < .0001) and also were more likely to have developed incident dependence of IADLs (21/35 [37.5%] vs 16/66 [19.5%]; OR 2.48 [95% CI 1.15, 5.34]).
Table 1.
Cohort characteristics by presence of pain at follow-up visit (N = 265)
| Mean (SD), Median (IQR), or Count (%) |
Group Comparisons | ||
|---|---|---|---|
| Demographics | Pain at Follow-up (n = 100) | No Pain at Follow-up (n = 165) | P-value |
| Age (at follow-up) | 57.6 (7.4) | 55.2 (8.3) | .019 |
| Education | 13.1 (2.5) | 13.1 (2.5) | .936 |
| Male | 78 (78%) | 128 (78%) | .903 |
| Race/Ethnicity | .998 | ||
| Black (Non-Hispanic) | 45 (45%) | 75 (46%) | — |
| Hispanic | 11 (11%) | 19 (12%) | — |
| White (Non-Hispanic) | 42 (42%) | 68 (41%) | — |
| Missing 2 | Missing 3 | ||
| HIV-disease characteristics | |||
| AIDS history | 77 (77%) | 122 (74%) | .577 |
| Current CD4 count | 569 (368, 789) | 561 (324, 782) | .897 |
| Nadir CD4 count | 108 (22, 218) | 104 (16, 201) | .491 |
| Antiretroviral therapy (ART) | 95 (95%) | 160 (97%) | .415 |
| Plasma viral load detectable a | 9 (9.7%) | 27 (18%) | .079 |
| Months exposure to ART | 190 (77) | 181 (73) | .353 |
| Past use of peripherally neurotoxic ART | 67 (67%) | 93 (56%) | .086 |
| Past medical or psychiatric history | |||
| Hepatitis C infection | 39 (39%) | 54 (32.7%) | .230 |
| Diabetes | 25 (25%) | 24 (14.5%) | .025 |
| Current major depressive disorder | 6 (6%) | 11 (6.7%) | .831 |
| Lifetime major depressive disorder | 62 (62%) | 100 (61%) | .835 |
| Lifetime substance use disorder | |||
| Any substance use disorder | 79 (79%) | 115 (70%) | .084 |
| Alcohol Use Disorder | 54 (54%) | 87 (52.7%) | .854 |
| Cannabis Use Disorder | 40 (40%) | 48 (30.4%) | .069 |
| Cocaine Use Disorder | 49 (49%) | 60 (36%) | .041 |
| Methamphetamine Use Disorder | 15 (15%) | 25 (15%) | .967 |
| Opioid Use Disorder | 79 (79%) | 115 (70%) | .084 |
Table 2.
Relationship of baseline decomposed symptom variables vs 12-year follow-up distal neuropathic pain (N = 265)
| Year 0 vs Year 12 | Odds Ratios 95% CI | P-value | |
|---|---|---|---|
| Objective neuropathy signs | 1.29 | 0.94, 1.79 | .113 |
| Baseline neuropathic pain | 1.97 | 1.51, 2.58 | <.0001* |
| Baseline sensory loss | 1.23 | 0.93, 1.62 | .147 |
| Baseline paresthesia | 1.56 | 1.18, 2.07 | .0017* |
| Age at baseline | 1.04 | 0.75, 1.44 | .7785 |
| Nadir CD4 | 1.02 | 0.68, 1.52 | .9271 |
| Absolute CD4 | 1.00 | 0.67, 1.49 | .9865 |
| D-Drugs ever | 1.31 | 0.97, 1.78 | .0799 |
| Plasma viral load (rnlog10) | 1.02 | 0.74, 1.41 | .8881 |
| HCV | 1.13 | 0.85, 1.50 | .3873 |
| Diabetes | 1.06 | 0.79, 1.41 | .7038 |
Comparing HIV disease characteristics between the two follow-up pain groups, there were no significant differences in current or nadir CD4, detectable plasma viral load, history of AIDS status, months on lifetime ART (Table 1, all P > .05). Nearly all participants (96%) were on ART at the time of 12-year follow-up. A majority of the cohort (60%) endorsed past use of peripherally neurotoxic ART, which also conveyed a marginally higher odds of pain at follow-up approaching significance (OR 1.6 [95% CI 0.94 to 2.6]).
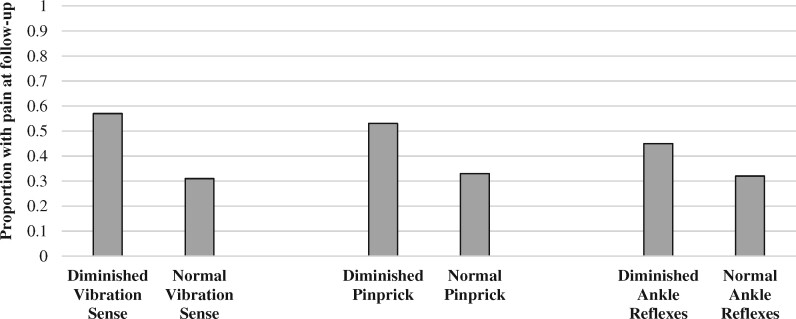
Seventy percent of participants had one or more objective signs of neuropathy at their follow-up visit. Of the 186 individuals with objective neuropathy signs, half had paresthesia, 40% had sensory loss, and 42% had neuropathic pain at the follow-up, with significant overlap among symptoms. The remainder had signs of neuropathy but were asymptomatic. Few participants had either pain alone (2.6%), loss of sensation alone (3.7%), or paresthesia alone (9%). Among the 76 participants without objective neuropathy signs on exam at follow-up visit, 30% had neuropathic pain, 34% had paresthesia, and 14% had some degree of loss of sensation with some overlap of symptoms (data not shown). Comparing the two pain groups at follow-up, those with objective neuropathy signs on neurological exam (diminished ankle reflexes, reduced vibratory sensation, reduced pinprick sensation) were more likely to have pain at follow-up (Figure 1).
Figure 1.
Baseline clinical exam findings vs distal neuropathic pain at 12-year follow-up.
Association of Neuropathy Symptoms at Baseline with 12-Year Follow-up Visit: statistically Decomposed Variables
The nondecomposed paresthesia and pain variables were significantly correlated (R2= 0.615). Of the 100 patients with neuropathic pain at the baseline visit, 87% also had neuropathic paresthesia but only 13 had neuropathic pain without paresthesia (13%). Statistical decomposition successfully eliminated collinearity between baseline pain and paresthesia. Patients with baseline decomposed paresthesia were significantly more likely to have pain at the 12-year follow-up (OR 1.56 [95% CI 1.18, 2.06]), adjusted for age, plasma viral load, nadir and absolute current CD4, past or current peripherally neurotoxic ART use, history of HCV or diabetes. Having decomposed pain at baseline was associated with increased risk of pain at follow-up (OR 1.97 [95% CI 1.51, 2.58]), but decomposed sensory loss at baseline did not predict pain at follow-up (OR 1.23 [95% CI 0.93, 1.62]). All other baseline covariates (age, nadir CD4, absolute CD4, plasma viral load, history of HCV, or diabetes) did not predict pain (all P > .05). A sensitivity analysis was performed excluding those with a detectable plasma viral load at follow-up (n = 49), the relationship between baseline paresthesia and follow-up pain lost significance, however the odds ratios remained similar when results were compared to the full model including all participants at follow-up (OR 1.67 [95% CI 0.78, 3.60]). Past use of peripherally neurotoxic ART increased the odds of pain at follow-up but did not reach significance (OR 1.31 [95% CI 0.97, 1.78], P = .08). Using the two separate models of dichotomous or categorical (ordinal) paresthesia variables, the same results were obtained of a statistically significant increased odds ratio for paresthesia in predicting pain at follow-up independent of the model utilized. Worse paresthesia severity increased the odds of pain at follow-up (OR 1.46 [95% CI 1.09, 1.95], P = .01), and worse baseline pain severity predicted greater odds of pain at follow-up (OR 2.26 [95% CI 1.65, 3.23]).
Discussion
Our results highlight the importance of neuropathic paresthesia, a previously underemphasized symptom of DSP. We found that distal neuropathic paresthesia at baseline predicted increased risk of incident pain or persistence of pain at a 12-year follow-up visit among PWH. Recently, our group found that paresthesia, but not neuropathic pain, was associated with subcortical atrophy of the midbrain and medial posterior thalamus, among PWH with DSP [9]. Cortical atrophy of the posterior cingulate gyrus and smaller total cerebral gray matter volumes have been attributed to distal neuropathic pain in HIV independent of the presence of paresthesia [10, 15], thus providing a potential pathophysiologic mechanism linking paresthesia and pain within the CNS [9, 10]. Together with our previous findings, our observations in the present study support that clinical paresthesia may play a role in sensory processing and in clinical outcomes of neuropathic pain many years later.
In our study, those with pain at follow-up were older than those without pain, but there were no other demographic differences between groups. This is not surprising given HIV-associated DSP is more commonly encountered in older age, leading to worse quality of life among the elderly [1, 12]. Older age did not confound the relationship between paresthesia and incident pain. There was a significantly greater proportion of participants with a lifetime cocaine use diagnosis in the group with pain at follow-up, which has not been associated specifically with neuropathy, however cocaine is known to lead to vasculopathy that can cause peripheral neuropathic pain among those without HIV [25], thus may contribute to pain outcomes among older PWH and past cocaine use. No HIV characteristic (nadir CD4, current CD4, plasma viral load, length of exposure to ART) was associated with presence of neuropathic pain at follow-up, consistent with previous findings [12, 13]. We included paresthesia or sensory loss as a predictor even in those without DSP signs. This approach is supported by previous reports that self-reported characteristic patterns of both paresthesia and pain are associated with abnormal total neuropathy scores, with a positive predictive value of 96%, indicating that symptoms consistent with DSP correspond to objective evidence of HIV-associated DSP on neurological examination [1].
The clinical relationship between paresthesia and neuropathic pain has been previously established in the chemotherapy literature [5]. In those with chemotherapy-induced peripheral neuropathy, sensory neuropathy symptoms (numbness and tingling) often exist without neuropathic pain symptoms, but the reverse is uncommon, suggesting that numbness and tingling occur first with pain later becoming a sequela of neuropathy [5]. A similar mechanism may exist in sensory processing in HIV, but this has not previously been shown. Allodynia and paresthesia have been correlated with chronic neuropathic pain associated with electrophysiological changes of both small and large peripheral nerve fibers in HIV-associated DSP [26]. Similarly, in a Simian macaque model, simian immunodeficiency virus (SIV) was found to alter the conduction of small, unmyelinated peripheral nerves leading to neuropathic pain [27]. Although it is unknown which fibers are affected first in HIV-associated DSP, one study suggested that HIV-associated DSP preferably affects myelinated fibers, leading to paresthesia, and DSP due to neurotoxic ART therapy affects unmyelinated fibers instead [28]. Our study also suggests that myelinated fibers may be affected first prior to unmyelinated fibers, given that clinical paresthesia may precede incident neuropathic pain years later.
Our study has limitations. At follow-up, 82% of the cohort was virologically suppressed, which is still lower than many other modern cohorts. However, odds ratios were similar after excluding participants with detectable plasma viral loads. Our study did not include electrophysiological analysis nor a skin biopsy for confirmation of HIV-associated DSP as only a small subset of participants included in this study with longitudinal data spanning 12 years. Since we did not collect skin biopsies, we cannot confirm that individuals with neuropathic pain without exam signs of neuropathy have HIV small fiber peripheral neuropathy. Paresthesia is usually highly correlated with pain, as was seen in our study, but the use of decomposed variables eliminated collinearity between the variables and allowed us to demonstrate the importance of paresthesia as a predictive tool for pain years later.
Conclusion
Distal neuropathic pain is known to lead to worse quality of life [6, 29, 30] and more frequent falls and balance disturbances [31] among older PWH increasing moribidity. Thus, identifying ways to target populations with high risk of incident neuropathic pain before its onset would be of benefit. Distal neuropathic pain is a challenging clinical condition because interventions like opioid drugs and other neuropathic pain agents (e.g., anti-epileptics) are often ineffective, can lead to substantial morbidity. The findings suggest that paresthesia is a harbinger of central reorganization of sensory processing, increasing the risk of developing incident neuropathic pain or worsening of neuropathic pain longitudinally. The development of new therapies to encourage neuroregeneration might take advantage of the finding that paresthesias predict subsequent pain to choose individuals most likely to benefit from treatment to prevent incident pain. Indeed, the recent discovery that muscarinic M1 receptor antagonism enhances mitochondrial activity and drives nerve regeneration and neuroprotection has been confirmed in multiple cell and animal models of peripheral neuropathies [32–35], providing a novel treatment and preventive strategy.
Funding sources: Financial support for this CHARTER study was provided by the National Institutes of Health (NIMH RFA 00-AI-0005).
Conflicts of interest: There are no conflicts of interest to report.
References
- 1. Robinson-Papp J, Morgello S, Vaida F, et al. Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy. Pain 2010;151(3):732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basu S, Bruce RD, Barry DT, Altice FL.. Pharmacological pain control for human immunodeficiency virus-infected adults with a history of drug dependence. J Subst Abuse Treat 2007;32(4):399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017;3(1):17002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jallow A, Ljunggren G, Wandell P, Wahlstrom L, Carlsson AC.. HIV infection and psychiatric illnesses––A double edged sword that threatens the vision of a contained epidemic: The Greater Stockholm HIV Cohort Study. J Infect 2017;74(1):22–8. [DOI] [PubMed] [Google Scholar]
- 5. Wolf SL, Barton DL, Qin R, et al. The relationship between numbness, tingling, and shooting/burning pain in patients with chemotherapy-induced peripheral neuropathy (CIPN) as measured by the EORTC QLQ-CIPN20 instrument, N06CA. Support Care Cancer 2012;20(3):625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Girach A, Julian TH, Varrassi G, Paladini A, Vadalouka A, Zis P.. Quality of life in painful peripheral neuropathies: A systematic review. Pain Res Manag 2019;2019:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pettersen JA, Jones G, Worthington C, et al. Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: Protease inhibitor-mediated neurotoxicity. Ann Neurol 2006;59(5):816–24. [DOI] [PubMed] [Google Scholar]
- 8. Malvar J, Vaida F, Sanders CF, et al. Predictors of new-onset distal neuropathic pain in HIV-infected individuals in the era of combination antiretroviral therapy. Pain 2015;156(4):731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keltner JR, Tong A, Visser E, et al. Evidence for a novel sub-cortical mechanism for posterior cingulate cortex atrophy in HIV peripheral neuropathy. J Neurovirol 2020;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keltner JR, Connolly CG, Vaida F, CHARTER Group, et al. HIV distal neuropathic pain is associated with smaller ventral posterior cingulate cortex. Pain Med 2017;18(3):428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adoukonou TA, Kouna-Ndouongo P, Kpangon A, et al. Distal sensory polyneuropathy among HIV-infected patients at Parakou University Hospital, Benin, 2011. Med Sante Trop 2017;27(2):190–4. [DOI] [PubMed] [Google Scholar]
- 12. Morgello S, Estanislao L, Simpson D, et al. HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: The Manhattan HIV Brain Bank. Arch Neurol 2004;61(4):546–51. [DOI] [PubMed] [Google Scholar]
- 13. Ellis RJ, Rosario D, Clifford DB, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: The CHARTER Study. Arch Neurol 2010;67(5):552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heaton RK, Clifford DB, Franklin DR Jr; For the CHARTER Group, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75(23):2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keltner JR, Fennema-Notestine C, Vaida F; For the CHARTER Group, et al. HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter. J Neurovirol 2014;20(3):209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellis RJ, Diaz M, Sacktor N; For the CNS Antiretroviral Therapy Effects Research (CHARTER) Study Group, et al. Predictors of worsening neuropathy and neuropathic pain after 12 years in people with HIV. Ann Clin Transl Neurol 2020;7(7):1166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wachtel T, Piette J, Mor V, Stein M, Fleishman J, Carpenter C.. Quality of life in persons with human immunodeficiency virus infection: Measurement by the Medical Outcomes Study instrument. Ann Intern Med 1992;116(2):129–37. [DOI] [PubMed] [Google Scholar]
- 18. Wu AW, Revicki DA, Jacobson D, Malitz FE.. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res 1997;6(6):481–93. [DOI] [PubMed] [Google Scholar]
- 19. Lawton MP, Brody EM.. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969;9(3):179–86. [PubMed] [Google Scholar]
- 20. Heaton RK, Marcotte TD, Mindt MR; HNRC Group, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004;10(3):317–31. [DOI] [PubMed] [Google Scholar]
- 21. Beck AT, Steer RA, Ball R, Ranieri W.. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996;67(3):588–97. [DOI] [PubMed] [Google Scholar]
- 22. Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D; Participants in the Multicentre WHO/Adamha Field Trials. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA Field Trials. Br J Psychiatry 1991;159(5):645–53. 58. [DOI] [PubMed] [Google Scholar]
- 23. Kessy A, Lewin A, Strimmer K.. Optimal whitening and decorrelation. The American Statistician 2018;72(4):309–14. [Google Scholar]
- 24. Eldar YC, Oppenheim AV.. MMSE whitening and subspace whitening. IEEE Trans Information Theory 2003;49(7):1846–51. [Google Scholar]
- 25. Subesinghe S, van Leuven S, Yalakki L, Sangle S, D'Cruz D.. Cocaine and ANCA associated vasculitis-like syndromes: A case series. Autoimmun Rev 2018;17(1):73–7. [DOI] [PubMed] [Google Scholar]
- 26. Bouhassira D, Attal N, Willer JC, Brasseur L.. Painful and painless peripheral sensory neuropathies due to HIV infection: A comparison using quantitative sensory evaluation. Pain 1999;80(1):265–72. [DOI] [PubMed] [Google Scholar]
- 27. Mangus LM, Dorsey JL, Laast VA, et al. Unraveling the pathogenesis of HIV peripheral neuropathy: Insights from a simian immunodeficiency virus macaque model. Ilar J 2014;54(3):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kokotis P, Schmelz M, Skopelitis EE, Kordossis T, Karandreas N.. Differential sensitivity of thick and thin fibers to HIV and therapy-induced neuropathy. Auton Neurosci 2007;136(1-2):90–5. [DOI] [PubMed] [Google Scholar]
- 29. Galantino ML, Kietrys DM, Parrott JS, Stevens ME, Stevens AM, Condoluci DV.. Quality of life and self-reported lower extremity function in adults with HIV-related distal sensory polyneuropathy. Phys Ther 2014;94(10):1455–66. [DOI] [PubMed] [Google Scholar]
- 30. Keltner JR, Vaida F, Ellis RJ, et al. Health-related quality of life “well-being” in HIV distal neuropathic pain is more strongly associated with depression severity than with pain intensity. Psychosomatics 2012;53(4):380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakabumi DZ, Moore RC, Tang B, Delaney PA, Keltner JR, Ellis RJ.. Chronic distal sensory polyneuropathy is a major contributor to balance disturbances in persons living with HIV. J Acquir Immune Defic Syndr 2019;80(5):568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jolivalt CG, Frizzi KE, Han MM, et al. Topical delivery of muscarinic receptor antagonists prevents and reverses peripheral neuropathy in female diabetic mice. J Pharmacol Exp Ther 2020;374(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saleh A, Sabbir MG, Aghanoori MR, et al. Muscarinic toxin 7 signals via Ca2+/Calmodulin-dependent protein kinase β to augment mitochondrial function and prevent neurodegeneration. Mol Neurobiol 2020;57(6):2521–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sabbir MG, Calcutt NA, Fernyhough P.. Muscarinic acetylcholine type 1 receptor activity constrains neurite outgrowth by inhibiting microtubule polymerization and mitochondrial trafficking in adult sensory neurons. Front Neurosci 2018;12:402.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calcutt NA, Smith DR, Frizzi K, et al. Selective antagonism of muscarinic receptors is neuroprotective in peripheral neuropathy. J Clin Invest 2017;127(2):608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]