Abstract
Context
Dyslipidemia and cardiovascular disease are common in shift workers and eating at night may contribute to this pathophysiology.
Objective
To examine the effects of eating at different times of day on lipid profiles.
Design
Two 24-hour baseline days with 8 hours of sleep, 3 meals (breakfast, lunch, dinner) and a snack, followed by a 40-hour constant routine (CR) with hourly isocaloric meals.
Setting
Intensive Physiological Monitoring Unit, Brigham and Women’s Hospital.
Participants
Twenty-one healthy adults [23.4 ± 2.7 years, 5F]
Intervention
Forty-hour CR.
Main Outcome Measures
A standard clinical lipid panel, consisting of total cholesterol, triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), was assayed in blood samples collected 4-hourly across ~4 days.
Results
When participants ate at night, levels of TG were similar to eating during the day, however, these levels at night were reached with consuming approximately half the calories. Additionally, 24-hour levels of TG were 10% higher when meals were consumed hourly across 24 hours compared to consuming a typical 3-meal schedule while awake during the day and sleeping at night. The endogenous circadian rhythms of TG, which peaked at night, were shifted earlier by ~10 hours under baseline conditions, whereas the rhythms in total cholesterol, HDL-C, and LDL-C remained unchanged and peaked in the afternoon.
Conclusions
The time-of-day dependency on postprandial lipid metabolism, which leads to hypersensitivity in TG responses when eating at night, may underlie the dyslipidemia and elevated cardiovascular disease risk observed in shift workers.
Keywords: circadian, chrononutrition, meal timing, lipids, triglyceride
Dyslipidemia is a known risk factor for cardiovascular disease (CVD) (1), a leading cause of death, and emerging evidence shows that shift work is a behavioral risk factor for CVD (2-4). Compared with day workers, shift workers have 40% higher odds of elevated triglyceride (TG) levels (OR 1.40; 95% confidence interval [CI], 1.08-1.83) and double the odds of low high-density lipoprotein (HDL) levels (OR 2.03; 95% CI, 1.18-3.48) (5).
Engaging in behaviors at the wrong biological time (e.g., eating at night and sleeping during the day) has adverse metabolic consequences. For example, TG levels are elevated and postprandial TG kinetics are altered when eating at night compared to eating identical test meals during the day (6-10). Understanding the time-of-day effect on postprandial TG and other lipid metabolism is important given (i) recent evidence suggesting that TGs may play a greater role in CVD risk than previously thought (11), and (ii) recommendations calling for the routine use of nonfasted samples for assessing lipid profiles (12). Prior studies examining time-of-day effects on TGs may be confounded by prolonged fasting or pre-test meals of varying composition. In the current study, therefore, we isolated the effects of eating time on lipid profiles by distributing food intake equally across the 24-hour day. We aimed to test the hypothesis that circulating lipids are under endogenous circadian regulation and that their levels are elevated by nighttime eating.
Methodology
Participants and study design
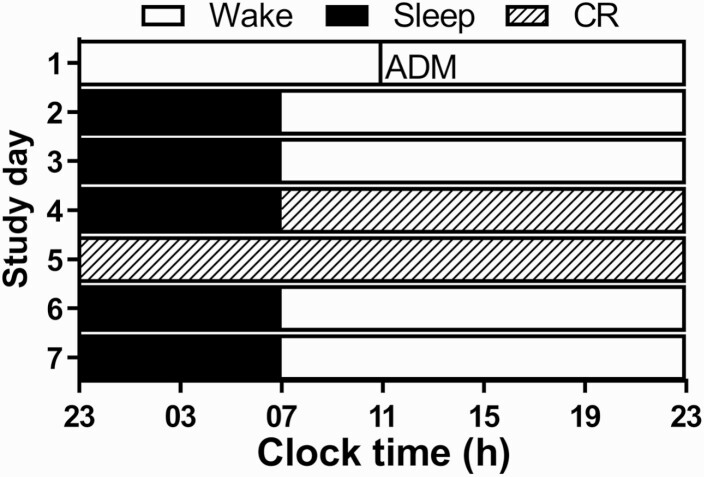
We studied 21 adults (23.4 ± 2.7 years; 5 females) in a 7- to 9-day inpatient study (13). Only data from the first 5 days were analyzed herein. The study was approved by Partners Human Research Committee and participants provided written informed consent. Participants underwent 3 standard ambulatory baseline days with an 8:16 hour sleep-wake/dark-light schedule, followed by a 40-hour constant routine (CR; Fig. 1). During baseline, participants were provided an isocaloric diet with 3 meals and a snack, whereas during the CR, daily nutritional intake was divided into hourly isocaloric portions. Four-hourly plasma samples were collected starting from baseline day 2 (n = 8) or 3 (n = 13) for ~72 to 96 hours from an indwelling IV cannula and assayed for total cholesterol, HDL cholesterol (HDL-C), and TG.
Figure 1.
7-day inpatient study protocol. The study protocol consisted of 2 baseline days followed by a 40-hour constant routine (CR). White bars represent wake in 90 lux, black bars represent sleep (0 lux), and the bar with the hatched diagonal pattern represents the CR. Abbreviation: ADM, admit.
Diet
During baseline, participants were provided a nutritionist-designed, isocaloric diet with 3 meals and a snack [2423.4 ± 267.5 kcal, fat: ~25%, protein: ~15%, carbohydrate: ~60% (Fig. 1D), 150 mEq Na+/100 mEq K+ (± 20%), 2500 mL fluids/24 hours] adjusted for sex, weight, and age using the Mifflin-St Jeor equation with an activity factor of 1.4 × basal energy expenditure. During the CR, daily nutrition intake was divided into hourly isocaloric portions [92.8 ± 8.0 kcal, fat: ~25%, protein: ~15%, carbohydrate: ~60%, 150 mEq Na+/100 mEq K+ (± 20%), isocaloric (basal energy expenditure × 1.3) diet, and 2500 mL fluids/24 hours].
Lipid assays
All assays were conducted using colorimetric tests (Roche Diagnostics Inc., USA) by a CLIA-certified laboratory blinded to the study conditions and sampling time (LabCorp Inc., Burlington, NC). The intra- and inter-assay coefficients of variation were, respectively, 0.8% and 1.7% for cholesterol; 1.0% and 1.3% for HDL-C; and 1.6% and 1.9% for TG. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Freidewald equation and very-low-density lipoprotein cholesterol (VLDL-C) was estimated as TG/5.
Lighting
During the first 2.5 baseline days, maximum ambient fluorescent light during scheduled wake was 48 µW/cm2 (~190 lux) when measured in the horizontal plane at a height of 187 cm and 25 µW/cm2 (~90 lux) when measured in the vertical plane (137 cm). Halfway through day 3, maximum ambient light was decreased to ~0.5 µW/cm2 (~1.5 lux) when measured in the vertical plane and maintained for the remainder of the study analyzed herein except during scheduled sleep. Regular illuminance and irradiance measures were recorded using an IL1400 radiometer/photometer with an SEL-033/Y/W or SEL-033/F/W detector, respectively (International Light, Inc., Newburyport, MA). Lighting was generated from ceiling mounted 4100 K fluorescent lamps (F96T12/41U/HO/EW, 95 W; F32T8/ADV841/A, 32 W F25T8/TL841,25 W; Philips Lighting, The Netherlands) with digital ballasts (Hi-Lume 1% and Eco-10 ballasts, Lutron Electronics Co., Inc., Coopersburg, PA) transmitted through a UV-stable filter (Lexan 9030 with prismatic lens, GE Plastics, Pittsfield, MA).
Data analysis
The 24-hour area under the curve (AUC), as well as AUC for “night” (samples collected between sleep onset and breakfast) and “day” (samples collected between breakfast and sleep onset), was calculated for each lipid using the trapezoid method and compared between conditions using paired t tests. A repeated measures 2-way ANOVA was used to examine the influence of time-of-day (day versus night) and condition (baseline versus CR) on lipid levels. The 2 baseline nights and 2 CR days were collapsed, respectively, as the AUC within each pair was not different (P > 0.3). These statistical tests were conducted in Prism 7 (GraphPad Software, La Jolla CA). In addition, the time at which each lipid rhythm peaked during the 24-hour day was determined for each condition using cosinor regression analysis (14) (SAS 9.4 Inc., Cary, NC). In 8 participants, blood collection started midway through day 3 and therefore they were only included in the CR analysis. A priori levels of significance were set at 0.05 (2-sided) for statistical tests. This study comprises a retrospective analysis of data collected from a study designed to examine the role of wavelength on nonvisual responses to light (13). A priori power analyses were not conducted; however, the sample size is commensurate with previous studies demonstrating an effect of meal timing on lipid responses (6-10).
Results
Twenty-four-hour levels were 9.7% higher during CR compared with baseline, even though on average 7.7% ± 4.5% fewer calories were consumed per 24 hours under CR conditions (Table 1).
Table 1.
Peak-Time and 24-Hour Levels of Lipids Under Baseline and Constant Routine Conditionsa
| Lipid | Baseline | Constant routine | ||
|---|---|---|---|---|
| Peak-time (hh:mm) n = 13 |
24-h AUC (mg/dL * h) n = 13 |
Peak-time (hh:mm) n = 21 |
24-h AUC (mg/dL * h) n = 13 |
|
| Total Cholesterol (M ± SE) | 16:14 ± 0:08 | 3677.77 ± 207.0 | 16:04 ± 0:27 | 3613.92 ± 211.7 |
| HDL-C (M ± SE) | 16:48 ± 0:09 | 1174.95 ± 79.9 | 16:09 ± 0:10 | 1132.88 ± 72.9 |
| LDL-C (M ± SE) | 14:39 ± 0:23 | 1949.41 ± 178.1 | 15:45 ± 0:14 | 1867.91 ± 181.4 |
| TG (M ± SE) | 16:55 ± 0:12b | 2774.23 ± 289.2b | 03:26 ± 0:32 | 3043.69 ± 318.9 |
Abbreviations: 24-h AUC, 24-hour area under the curve; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; M, mean; SE, standard error; TG, triglyceride.
a The estimated peak-time of each outcome measure was derived from Cosinor regression analyses of group averaged data under baseline and constant routine conditions. All lipids exhibited significant 24-h rhythms under both conditions (P < 0.02 for all). Peak-times are reported in relative clock time referenced from the group-average scheduled wake time at 0700 h (06:59 ± 00:56 h). The 24-h AUC was calculated from 17:00 to 17:00 h for baseline and CR.
b Significant differences between baseline and constant routine (P < 0.01).
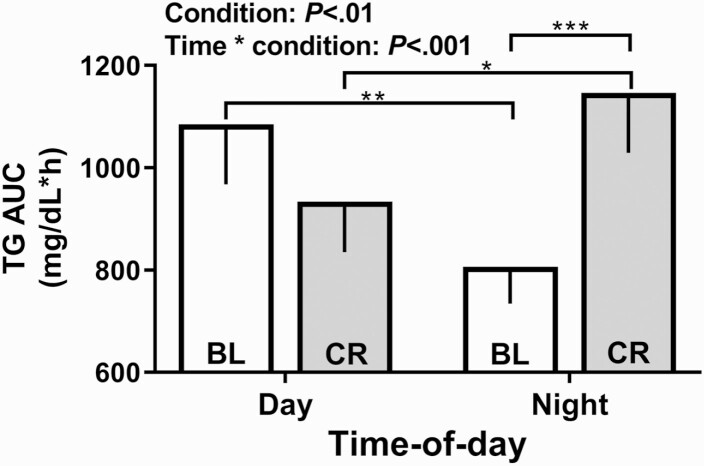
This elevation in TG was associated with eating at night since the levels were similar between conditions during the daytime, but higher under CR than under baseline at night (Fig. 2). Under baseline conditions, TG levels during the ~8-hour overnight sleep-associated fast were ~25% lower compared to the levels observed during the ~8-hour daytime interval, which included a typical breakfast, lunch, and dinner. Compared with the overnight fasted levels of TG under baseline conditions, their levels increased by ~42% when eating small isocaloric snacks at night during the CR condition. Consistent with this temporal effect of feeding on TG levels that was found by contrasting levels under baseline and CR conditions, levels of TG were elevated by 23%, when eating during the nighttime portion of the CR compared to caloric- and nutrient-content matched snacks consumed during the daytime portion of the same CR. Although the levels of TG with nighttime eating were similar to the levels with daytime eating, it took approximately half (46.3%) the caloric content at night (1113.7 ± 93.4 kCal), as compared to the calories consumed during the day (2082.0 ± 237.7 kCal), to reach these levels (Fig. 2). The results were identical for VLDL-C (calculated as TG/5; data not shown). Unlike TG, total cholesterol, LDL-C, and HDL-C differed only marginally between day and night, or between CR and baseline (<8%; Figure S1 (15)).
Figure 2.
Area under the curve by time-of-day under baseline and constant routine conditions for triglyceride. The area under the curve (AUC ± SEM) for triglyceride (TG) is shown for the day (left) and night (right) under baseline (BL) and CR conditions. Significant post hoc tests with a Holm-Sidak correction are denoted by the horizontal bars (*P < 0.05, **P < 0.01 and ***P < 0.001).
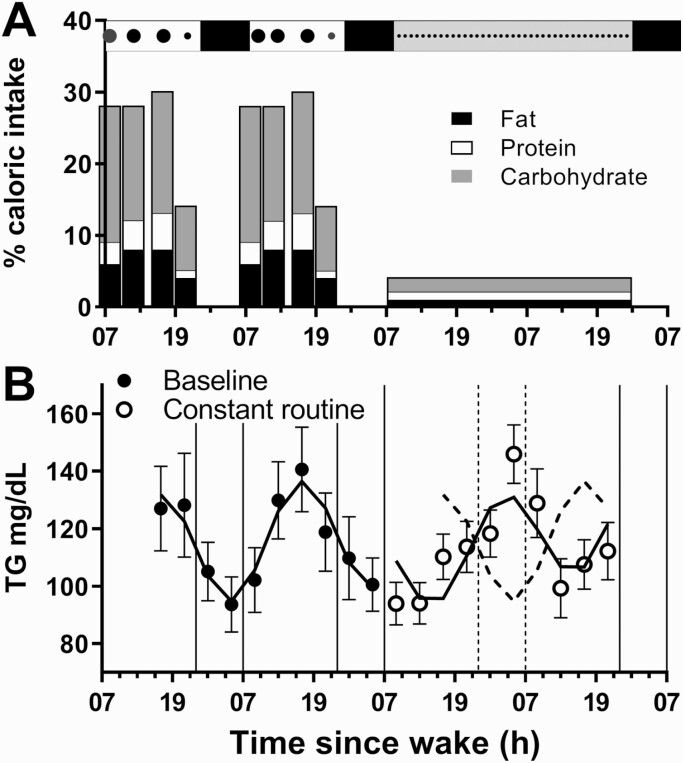
Analyzing the time-series data showed significant 24-hour rhythms under baseline conditions for total cholesterol, LDL-C, HDL-C, and TG, which peaked during the afternoon (peak-time range, 14:39 to 17:29 hours; Table 1) coinciding with food intake (Fig. 3A). Significant 24-hour rhythms were also observed under CR conditions for cholesterol, LDL-C, and HDL-C, which also peaked during the afternoon (range, 15:45 to 16:09 hours; Table 1, Figure S1 (15)), demonstrating endogenous circadian regulation, and similar timing to baseline. In contrast, the TG rhythm was reversed at night during CR, peaking 10.5 hours later compared to baseline (Fig. 3B). VLDL-C (calculated as TG/5) exhibited the same rhythm timing and amplitude as TG (data not shown).
Figure 3.
Meal timing and 24-hour rhythms in triglyceride under baseline and constant routine conditions. The study protocol is represented at the top of panel A, where meal timing and relative sizes are indicated by the black circles. The percentage of daily caloric intake for each meal (breakfast, lunch, dinner and snack) is shown during baseline and for each CR snack, including the percentage of fat (black), protein (white) and carbohydrates (gray). The group-mean (± SEM) of unadjusted data and group-mean fitted cosinor regressions (—) for all participants under baseline (●) and CR (○) conditions are shown for TG (B). Baseline data are re-plotted (---) during the CR for comparative purposes.
Discussion
Our study shows that the levels and timing of circulating TG are adversely affected by eating at night. When calories were distributed evenly across the day, TG increased by 10% relative to a standardized 3-meal schedule. Furthermore, TG levels were ~1.5 times higher when eating at night relative to fasting but were equivalent to daytime levels when eating standardized meals despite consuming only half the calories.
Our results are consistent with previous studies comparing lipid responses between identical test meals consumed during the day versus night (6, 8-10, 16, 17). Consistent with these previous studies, we found that eating at night increases circulating TG levels. By giving identical meals each hour, however, rather than several discrete boluses, we were able to unmask the time course of lipids and show that TGs were most elevated in the early morning. Remarkably, the 24-hour AUC of TG increased by 10% under CR, which included being awake and eating at night, despite consuming 10% less calories across 24 hours as compared to a 24-hour interval under baseline conditions. Additionally, the levels of TG were ~42% higher with eating at night compared to fasting while asleep. Furthermore, it took half the amount of calories consumed at night to reach the same circulating levels of TG as it did during the daytime. Taken together, our results suggest that circulating lipid levels are moderately sensitive to mistimed meals relative to the sleep schedule and circadian rhythms. This effect of nighttime eating on TG levels may be driven by several mechanisms related to circadian control of lipolysis and mobilization as well as lipid utilization and storage (18-20). For example, blood flow to adipose tissue and lipolysis is higher at night (20) with concurrently low rates of fat oxidation during the early morning hours (21, 22) corresponding with the highest concentrations in circulating TG concentrations in the current study.
Based on our findings, we hypothesize that consuming meals at night that are larger than the hourly snacks given in the current study would lead to even greater increases in TG. In support of this hypothesis, Morris and colleagues showed that TG levels rose to ~200 mg/dL, the standard clinical threshold for hypertriglyceridemia in a sample of normolipidemic young adults who were awake and ate standardized meals at night and slept during the day in a simulated shift work protocol (23). This increase in TG was accompanied by changes in insulin sensitivity with time-of-day (23). These changes in insulin sensitivity may explain why we observed that TG, but not other lipids, showed perturbation when eating at night in the current study. These results, when amplified by eating chronically at night, are consistent with epidemiological evidence of elevated TG and disrupted lipid metabolism in shift workers (5).
While the limited sample size of these carefully screened healthy individuals and small proportion of females limits the overall generalizability of these findings and assessment of potential sex-based differences (10), the uniform distribution and careful delineation of the time-of-day effect of meals on lipids provide novel inights into how time of eating can drastically alter circulating lipid levels. The reversal of the peak timing of TG and VLDL-C when staying awake overnight compared to typical sleep-wake and feeding-fasting schedules, a change not observed in other lipids, highlights the importance of assessing the differential effects of behavioral and environmental factors, as well as the timing of feeding, on these lipids when examining the metabolic consequences of diet or sleep. Future research, however, should directly measure, rather than estimate, LDL and VLDL concentrations, particularly when studying these outcomes in hyperlipidemic populations. Although the hourly meals given in the current study do not readily reflect real-world eating patterns, shift workers tend to spread their food intake in small and more frequent meals across the entire 24-hour day (24). Future laboratory simulations and field-based shift work trials are warranted to confirm the clinical consequences of these findings under naturalistic conditions, however. Future studies with a more detailed characterization of the specific types of dietary fats, carbohydrates and proteins are needed to further explore the role of meal timing and content on lipids.
Overall, our study shows divergent impact of behavioral choices, including eating and sleeping, on different types of lipids. The acute elevation of TG observed following nighttime food consumption may contribute to the increased risk of dyslipidemia and CVD in shift workers, notwithstanding the possibility that other risk factors (such as socioeconomic status or heritability of CVD), may be nonuniformly distributed in shift workers compared to nonshift workers. Understanding the most vulnerable time to eat with respect to lipid responses should prompt healthy eating guidelines for shift workers, for example by eating before or early into a nightshift rather than in the early morning hours.
Acknowledgments
We thank the technical, dietary and laboratory staff, nurses and physicians, participant recruiters and the study participants at the Center for Clinical Investigation and Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital.
Financial Support: This work was supported by the National Space Biomedical Research Institute (Lockley: HPF01301) through NASA NCC 9–58 and by the National Center for Research Resources through grants to Brigham and Women’s Hospital General Clinical Research Center (NCRR M01 RR02635) and the Harvard Clinical and Translational Science Center (NCRR UL1 RR025758).
Author Contributions: All authors have contributed to and approved this manuscript. C.A.C., S.W.L. and S.A.R. contributed to the initial concept and design of the studies. S.W.L. and S.A.R. contributed to, or oversaw, the collection of the data. L.K.G., C.A.C., S.W.L. and S.A.R. contributed to the analysis of the data. All authors contributed to the interpretation of the data and drafting of the manuscript. L.K.G. and S.A.R. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
Abbreviations
- AUC
area under the curve
- CI
confidence interval
- CR
constant routine
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- TG
triglyceride
- VLDL-C
very-low-density lipoprotein cholesterol
Additional Information
Disclosure Summary: The authors report no conflicts of interest related to the results reported in this manuscript, but in the interest of full disclosure: L.K.G. has nothing to declare. C.A.C. received travel support from Annenberg Center for Health Sciences at Eisenhower (2018), Aspen Brain Institute (2018), Bloomage International Investment Group, Inc. (2018, 2019), UK Biotechnology and Biological Sciences Research Council (2019), Bouley Botanical (2017, 2018, 2019), Dr. Stanley Ho Medical Development Foundation (2019), European Biological Rhythms Society (2017, 2019), German National Academy of Sciences (Leopoldina) (2019), Illuminating Engineering Society (2018), National Safety Council (2017, 2018, 2019), National Sleep Foundation (2017, 2018, 2019), Society for Research on Biological Rhythms (2018), Sleep Research Society Foundation (2018), Stanford Medical School Alumni Association (2019), Tencent Holdings Ltd (2019), University of Zurich (2018), and Vanda Pharmaceuticals Inc (2017, 2018, 2019), Ludwig-Maximilians-Universität München (2018), National Highway Transportation Safety Administration (2018), Office of Naval Research (2018), Salk Institute for Biological Studies/Fondation Ipsen (2018), The National Academy of Sciences, Engineering, and Medicine (2017), The Wonderful Company (2017), Department of Defense (2017); Dr. Czeisler is/was an expert witness in legal cases, including those involving Advanced Power Technologies, Aegis Chemical Solutions LLC (2019), Amtrak (2019); Casper Sleep Inc (2019), C&J Energy Services (2019), Complete General Construction Co (2017), Dallas Police Association (2019), Enterprise Rent-A-Car (2019), Espinal Trucking/Eagle Transport Group LLC/Steel Warehouse Inc (2017, 2018, 2019), FedEx, Greyhound Lines Inc/Motor Coach Industries/FirstGroup America (2017, 2018, 2019), Pomerado Hospital/Palomar Health District (2017, 2018), PAR Electrical Contractors Inc (2019), Product & Logistics Services LLC/Schlumberger Technology Corp/Gelco Fleet Trust (2019), Puckett Emergency Medical Services LLC (2019), South Carolina Central Railroad Company LLC (2017, 2018), Union Pacific Railroad (2019), United Parcel Service/UPS Ground Freight Inc (2017, 2018), and Vanda Pharmaceuticals (2019, 2020); and serves as the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. Dr. Czeisler’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Mass General Brigham in accordance with their conflict of interest policies. S.W.L. has had a number of commercial interests in the last 24 months (2018-2020) unrelated to the study reported herein but are reported in the interests of full disclosure. SWL has received consulting fees from the BHP Billiton, EyeJust Inc., Noble Insights, Rec Room and Team C Racing; honoraria and/or paid travel from Emory University, Estee Lauder, IES, Ineos, Mental Workout, MIT, Roxbury Latin School, Solemma and Wiley; has current consulting contracts with Akili Interactive; Apex 2100 Ltd.; Consumer Sleep Solutions; Headwaters Inc.; Hintsa Performance AG; Light Cognitive; Lighting Science Group Corporation; Mental Workout; PlanLED; Six Senses; Stantec; and KBR Wyle Services; has received unrestricted equipment gifts from F. Lux Software LLC; royalties from Oxford University Press; and has served as a paid expert in legal proceedings related to light, sleep and health. He is an unpaid Board Member of the Midwest Lighting Institute (non-profit) and was the Program Leader for ‘Safety and Productivity Improvements’ in the CRC for Alertness, Safety and Productivity from 2015-2019. These interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. S.A.R. holds patents for Prevention of Circadian Rhythm Disruption by Using Optical Filters and Improving sleep performance in subject exposed to light at night; SAR owns equity in Melcort Inc.; has provided paid consulting services to Sultan & Knight Limited, Bambu Vault LLC, Lucidity Lighting Inc.; and has received honoraria as an invited speaker and travel funds from Starry Skies Lake Superior, University of Minnesota Medical School, PennWell Corp., and Seoul Semiconductor Co. Ltd. These interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Data Availability
Data described in this manuscript and analytic code will be made available upon request. Execution of a Materials Transfer Agreement is required if the data will be used in research supported by a for-profit company, per Partners Healthcare Institutional Review Board policy.
References
- 1. Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barger LK, Rajaratnam SMW, Cannon CP, et al. Short sleep duration, obstructive sleep apnea, shiftwork, and the risk of adverse cardiovascular events in patients after an acute coronary syndrome. JAMA. 2017;6(10):e006959. doi:10.1161/JAHA.117.006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajaratnam SM, Barger LK, Lockley SW, et al. ; Harvard Work Hours, Health and Safety Group . Sleep disorders, health, and safety in police officers. JAMA. 2011;306(23):2567-2578. [DOI] [PubMed] [Google Scholar]
- 4. Vetter C, Devore EE, Wegrzyn LR, et al. Association between rotating night shift work and risk of coronary heart disease among women. JAMA. 2016;315(16):1726-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76(6):424-430. [DOI] [PubMed] [Google Scholar]
- 6. Al-Naimi S, Hampton SM, Richard P, Tzung C, Morgan LM. Postprandial metabolic profiles following meals and snacks eaten during simulated night and day shift work. Chronobiol Int. 2004;21(6):937-947. [DOI] [PubMed] [Google Scholar]
- 7. Hadjadj S, Paul JL, Meyer L, et al. Delayed changes in postprandial lipid in young normolipidemic men after a nocturnal vitamin A oral fat load test. J Nutr. 1999;129(9):1649-1655. [DOI] [PubMed] [Google Scholar]
- 8. Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001;171(3):557-564. [DOI] [PubMed] [Google Scholar]
- 9. Romon M, Le Fur C, Lebel P, Edmé JL, Fruchart JC, Dallongeville J. Circadian variation of postprandial lipemia. Am J Clin Nutr. 1997;65(4):934-940. [DOI] [PubMed] [Google Scholar]
- 10. Sopowski MJ, Hampton SM, Ribeiro DC, Morgan L, Arendt J. Postprandial triacylglycerol responses in simulated night and day shift: gender differences. J Biol Rhythms. 2001;16(3):272-276. [DOI] [PubMed] [Google Scholar]
- 11. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626-635. [DOI] [PubMed] [Google Scholar]
- 12. Nordestgaard BG, Langsted A, Mora S, et al. ; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) joint consensus initiative . Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37(25):1944-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37(2):271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rahman SA, Grant LK, Gooley JJ, Rajaratnam SMW, Czeisler CA, Lockley SW. Endogenous circadian regulation of female reproductive hormones. J Clin Endocrinol Metab. 2019;104(12):6049-6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grant LK, Czeisler CA, Lockley SW, Rahman SA. Data from: time-of-day and meal size effects on clinical lipid markers. figshare. Deposited July 14, 2020. https://figshare.com/s/50f4f2176cf010cd65e1. [DOI] [PMC free article] [PubMed]
- 16. Holmbäck U, Forslund A, Forslund J, et al. Metabolic responses to nocturnal eating in men are affected by sources of dietary energy. J Nutr. 2002;132(7):1892-1899. [DOI] [PubMed] [Google Scholar]
- 17. Le Fur C, Romon M, Lebel P, et al. Influence of mental stress and circadian cycle on postprandial lipemia. Am J Clin Nutr. 1999;70(2):213-220. [DOI] [PubMed] [Google Scholar]
- 18. Bonham MP, Kaias E, Zimberg I, et al. Effect of night time eating on postprandial triglyceride metabolism in healthy adults: a systematic literature review. J Biol Rhythms. 2019;34(2):119-130. [DOI] [PubMed] [Google Scholar]
- 19. Shostak A, Meyer-Kovac J, Oster H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes. 2013;62(7):2195-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagström-Toft E, Bolinder J, Ungerstedt U, Arner P. A circadian rhythm in lipid mobilization which is altered in IDDM. Diabetologia. 1997;40(9):1070-1078. [DOI] [PubMed] [Google Scholar]
- 21. Zitting KM, Vujovic N, Yuan RK, et al. Human resting energy expenditure varies with circadian phase. Curr Biol. 2018;28(22):3685-3690.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rynders CA, Morton SJ, Bessesen DH, Wright KP, Broussard JL. Circadian rhythm of substrate oxidation and hormonal regulation of energy balance. Obesity. 2020;28(Suppl 1):S104-S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112(17):E2225-E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Assis MA, Kupek E, Nahas MV, Bellisle F. Food intake and circadian rhythms in shift workers with a high workload. Appetite. 2003;40(2):175-183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in this manuscript and analytic code will be made available upon request. Execution of a Materials Transfer Agreement is required if the data will be used in research supported by a for-profit company, per Partners Healthcare Institutional Review Board policy.