Abstract
Background
While previous studies indicate that the zonae reticularis (ZR) and glomerulosa (ZG) diminish with aging, little is known about age-related transformations of the zona fasciculata (ZF).
Objectives
To investigate the morphological and functional changes of the adrenal cortex across adulthood, with emphasis on (i) the understudied ZF and (ii) sexual dimorphisms.
Methods
We used immunohistochemistry to evaluate the expression of aldosterone synthase (CYP11B2), visinin-like protein 1 (VSNL1), 3β-hydroxysteroid dehydrogenase type II (HSD3B2), 11β-hydroxylase (CYP11B1), and cytochrome b5 type A (CYB5A) in adrenal glands from 60 adults (30 men), aged 18 to 86. Additionally, we employed mass spectrometry to quantify the morning serum concentrations of cortisol, 11-deoxycortisol (11dF), 17α-hydroxyprogesterone, 11-deoxycorticosterone, corticosterone, and androstenedione in 149 pairs of age- and body mass index–matched men and women, age 21 to 95 years.
Results
The total cortical area was positively correlated with age (r = 0.34, P = 0.008). Both the total (VSNL1-positive) and functional ZG (CYP11B2-positive) areas declined with aging in men (r = −0.57 and −0.67, P < 0.01), but not in women. The CYB5A-positive area declined with age in both sexes (r = −0.76, P < 0.0001). In contrast, the estimated ZF area correlated positively with age in men (r = 0.59, P = 0.0006) and women (r = 0.49, P = 0.007), while CYP11B1-positive area remained unchanged across ages. Serum cortisol, corticosterone, and 11-deoxycorticosterone levels were stable across ages, while 11dF levels increased slightly with age (r = 0.16, P = 0.007).
Conclusion
Unlike the ZG and ZR, the ZF and the total adrenal cortex areas enlarge with aging. An abrupt decline of the ZG occurs with age in men only, possibly contributing to sexual dimorphism in cardiovascular risk.
Keywords: adrenal, adrenal cortex, aging, zona fasciculata, zona glomerulosa, zona reticularis
Introduction
Like other organs, the adrenal gland undergoes a series of morphological and functional changes with aging. The human adrenal cortex consists of three histologically distinct zones, each equipped with key enzymes for the productions of 3 major groups of steroid hormones: the outer zona glomerulosa (ZG) produces mineralocorticoids, such as aldosterone; the intermediate zona fasciculata (ZF) produces glucocorticoids, particularly cortisol; and the innermost zona reticularis (ZR) produces androgen precursors. The most abundant adrenal androgen precursors, dehydroepiandrosterone and its sulfate, decline gradually after their peak in mid-20s, to concentrations approximately 5-fold lower by age 80 (1). Reflecting these changes, the ZR displays a parallel involution with aging (2,3). Age-related transformations also occur within the functional ZG: while the aldosterone synthase (CYP11B2)-expressing area is continuous in young individuals, this area decreases and becomes fragmented with aging (4).
The ZF is the largest cortical zone and the source of the single adrenal hormone essential for life. The evolution of the ZF across adult ages, however, has been minimally studied. With a reduction of the ZR and functional ZG with aging, we asked if the dimensions of the ZF are also altered across adulthood, either similarly with or disparately from its flanking zones. The implications of aging on cortisol synthesis remain incompletely understood. Cortisol synthesis is highly dynamic and tightly controlled by the hypothalamic-pituitary axis. Cortisol synthesis displays characteristic circadian rhythmicity, and its secretion is enhanced by a variety of stressors (5,6). Several alterations in the hypothalamic-pituitary-adrenal (HPA) axis regulations have been reported with aging, including an elevated nocturnal nadir of adrenocorticotropic hormone (ACTH) and cortisol (7,8), which affects sleep and memory function in the elderly (9,10). In addition, the HPA axis has been shown to become more sensitive to CRH stimulation and more resistant to dexamethasone suppression in elderly as compared with young adults (11-14). The patterns of baseline cortisol synthesis across ages in humans have varied among reports (8,15,16). To assess the impact of aging on the adrenal cortex itself, rather than on the hypothalamic-pituitary regulation, we herein investigated the morphological changes of the adrenal cortical zones across the adult lifespan in both men and women, with focus on the understudied ZF.
Materials and Methods
Adrenals tissue
Adrenal glands were obtained from 60 adults (30 women), with ages between 18 and 86 years (17). Tissue was collected from deceased kidney transplant donors (n = 44) and from patients with renal cancer who underwent en bloc nephrectomy and adrenalectomy (n = 16). Deceased organ donor tissue was obtained through the Gift of Life program; all renal cancer patients provided written informed consent. These studies were conducted under protocols approved by the institutional review boards of the University of Michigan and Augusta University.
Immunohistochemistry
To evaluate global and zonal adrenal cortex differences across ages, we performed hematoxylin and eosin staining (H&E) and immunohistochemistry (IHC) for several cortical proteins: CYP11B2, which marks the aldosterone-producing cells in the ZG (18); visinin-like protein 1 (VSNL1), a marker of the ZG (19); 3β-hydroxysteroid dehydrogenase type II (HSD3B2), which is expressed within the ZG and ZF;11β-hydroxylase (CYP11B1), an enzyme that catalyzes the last step in cortisol synthesis, found in the ZF and ZR (18, 20); and cytochrome b5 type A (CYB5A), a 17α-hydroxylase/17,20-lyase (CYP17A1) cofactor, expressed exclusively in the ZR (21). Serial sections of formalin-fixed paraffin-embedded adrenal tissue blocks with a thickness of 5 μm were used for H&E and IHC, which were conducted as previously described (4). Primary antibodies used and conditions for each staining are summarized as reported (17).
Digital image analysis
All stained sections were scanned as digital images with PathScan Enabler IV (Meyer Instruments, Houston, TX, USA) for analysis. To accurately evaluate the adrenal cortex with digital image analysis, we manually cropped out the capsular and adrenal medulla in each staining image based on H&E, using PhotoScape X (MOOII Tech, Korea). The cropped images were subsequently analyzed with ImageJ (US National Institutes of Health).
As no cortical enzyme is expressed exclusively within the ZF, the ZF area was calculated by subtracting the VSNL1-postive area (marking the ZG) from the HSD3B2-expressing area (marking both ZG and ZF). Because in some of the formalin-fixed paraffin-embedded sections small areas were lost during fixation, all IHC-positive areas were expressed as percentage of the cortical area for each section.
Measurement of circulating steroid concentrations across ages
We employed liquid chromatography-tandem mass spectrometry (LC-MS/MS) to quantify the concentration of six major Δ 4 cortical steroids across adulthood. Morning (7:00 am-9:00 am) serum samples were obtained in outpatient settings from adults without major comorbidities and who were not taking hormonal therapy, as part of a previous study with focus on 11-oxyandrogens (22). A subset of 298 patients, 149 for each sex, matched for age and body mass index (BMI) were included in this study. We measured 3 steroid hormones derived from the ZF: cortisol, 11-deoxycortisol (11dF), and 17α-hydroxyprogesterone (17OHP4); 2 produced in the ZG: 11-deoxycorticosterone, and corticosterone; and androstenedione (A4), made in the ZR. Steroid extraction and quantification with LC-MS/MS was performed as previously reported (22,23).
Statistical analysis
We used the Mann-Whitney U test and the Wilcoxon signed-rank test to compare continuous variables between two independent or paired groups, respectively. The chi-squared test was employed to compare categorical variables between groups. Associations between age and studied variables were assessed with the Spearman or Pearson correlation tests, as appropriate. All statistical analyses were performed with Prism (version 8.0; GraphPad Software, La Jolla, CA, USA). Statistical significance was set at 2-tailed P value below 0.05.
Results
Adrenal cortex differences between sexes
Adrenal tissue was obtained from 30 women and 30 men with median age of 50 years (Table 1) (17). Overall, the adrenal cortical area was slightly, albeit not significantly, larger in men than in women [42.7 (24.1, 51.8) vs 29.9 (21.2, 43.6) mm2, P = 0.07]. The expression of most proteins evaluated were comparable between the 2 sexes, with the exception of CYP11B1-positive area, which was modestly larger in men as compared to women (P = 0.01, Table 1).
Table 1.
Pathological parameters of studied adrenal glands
| Total | Men | Women | P-value | |
|---|---|---|---|---|
| N | 60 | 30 | 30 | |
| Age | 50 (18-86) | 50 (18-86) | 50 (25-85) | 0.94 |
| 18–30 years (n) | 10 | 5 | 5 | |
| 31–40 years (n) | 10 | 5 | 5 | |
| 41–50 years (n) | 10 | 5 | 5 | |
| 51–60 years (n) | 10 | 5 | 5 | |
| 61–70 years (n) | 10 | 5 | 5 | |
| >70 years (n) | 10 | 5 | 5 | |
| Adrenal cortex area (mm2) | 35.8 [23.4, 49.3] | 42.7 [24.1, 51.8] | 29.9 [21.2, 43.6] | 0.07 |
| Proportion of CYP11B2-expressing area (%) | 1.25 [0.82, 3.09] | 1.27 [0.67, 4.40] | 1.25 [0.88, 2.54] | 0.90 |
| Proportion of VSNL1-expressing area (%) | 13.7 [8.9, 19.0] | 11.8 [7.8, 17.9] | 14.5 [10.1, 20.8] | 0.16 |
| Proportion of HSD3B2-expressing area (%) | 56.8 [48.2, 63.3] | 56.5 [49.2, 60.6] | 57.1 [47.0, 66.2] | 0.63 |
| Proportion of CYB5A-expressing area (%) | 19.2 [9.9, 26.7] | 19.8 [9.9, 27.1] | 16.7 [9.6, 26.1] | 0.54 |
| Proportion of CYP11B1-expressing area (%) | 45.1 [40.5, 51.3] | 47.6 [44.0, 53.5] | 43.1 [38.3, 48.0] | 0.01 |
| Proportion of estimated ZF (%) | 42.9 [33.1, 49.7] | 44.0 [32.8, 48.5] | 40.5 [33.2, 52.2] | 0.87 |
| Ratio of CYP11B2 per VSNL1 expression (%) | 12.2 [5.0, 27.3] | 15.7 [5.0, 34.1] | 11.4 [5.0, 18.3] | 0.24 |
All enzymatic areas are represented as percentage of total cortical area. The estimated ZF proportion was calculated by subtracting the percentage of VSNL1-positive area from the percentage of HSD3B2-positive area. Data are shown as median and range (for age) or interquartile range (other variables). Differences between men and women were assessed with the Mann-Whitney U test.
Abbreviations: CYB5A, cytochrome b5 type A; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; HSD3B2, 3β-hydroxysteroid dehydrogenase type II; VSNL1, visinin-like 1; ZF, zona fasciculata.
Adrenal cortical area across ages
The cortical area was positively correlated with age (r = 0.34, P = 0.008), and the correlation achieved statistical significance only in men (r = 0.41, P = 0.02 vs r = 0.29, P = 0.12 in women; Table 2).
Table 2.
Correlations between age and area of immunoreactivity for steroidogenic enzymes
| All (N = 60) | Men (N = 30) | Women (N = 30) | ||||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | |
| Adrenal cortex area | 0.34 | 0.0008 | 0.41 | 0.02 | 0.29 | 0.12 |
| CYP11B2-positive area | −0.50 | <0.0001 | −0.67 | <0.0001 | −0.31 | 0.09 |
| VSNL1-positive area | −0.42 | 0.0007 | −0.57 | 0.001 | −0.34 | 0.06 |
| HSD3B2-positive area | 0.35 | 0.007 | 0.34 | 0.07 | 0.35 | 0.06 |
| CYB5A-positive area | −0.76 | <0.0001 | −0.74 | <0.0001 | −0.77 | <0.0001 |
| CYP11B1-positive area | 0.02 | 0.86 | 0.11 | 0.56 | −0.06 | 0.75 |
| Estimated ZF area | 0.53 | <0.0001 | 0.59 | 0.0006 | 0.49 | 0.007 |
| Ratio of CYP11B2 per VSNL1 area | −0.31 | 0.02 | −0.47 | 0.009 | −0.11 | 0.57 |
All enzymatic areas are represented as % of total cortical area. The estimated ZF proportion was calculated by subtracting the % VSNL1-positive area from the % HSD3B2-positive area. Spearman’s rank correlation coefficients were used to evaluate the associations between age and each parameter.
Abbreviations: CYB5A, cytochrome b5 type A; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; HSD3B2, 3β-hydroxysteroid dehydrogenase type II; VSNL1, Visinin-like 1; ZF, zona fasciculata.
Overall, the proportions of both VSNL1- and CYP11B2-espressing areas, marking the entire and the functional ZG, respectively, declined with aging (VSNL1, r = −0.42 and P = 0.0007; CYP11B2, r = −0.50 and P < 0.0001; Table 2). However, while men displayed an abrupt decline in both VSNL1 and CYP11B2 areas (VSNL1, r = −0.57, P = 0.001; CYP11B2, r = −0.67, P < 0.0001), women only exhibited nonsignificant trends of decline across ages for both proteins (VSNL1, r = −0.34, P = 0.06; CYP11B2, r = −0.31, P = 0.09; Table 2). Accordingly, the decline of CYP11B2-expressing area was significant even relative to that of VSNL1 in men (CYP11B2/VSNL1: r = −0.47 and P = 0.001), but not in women (Table 2). When comparing sexes younger and older than 50 years old (the average age of menopause), CYP11B2 expression was higher in men than in women [4.11 (1.33, 7.64) vs 1.38 (1.07, 3.70) %, P = 0.07] in the younger group, whereas this ratio was similar in the older individuals [0.87 (0.25, 1.21) in men vs 1.06 (0.66, 2.47) % in women, P = 0.17]. In contrast, the proportions of VSNL1 were similar between men and women in individuals both younger and older than 50.
In line with previous studies, the proportions of CYB5A-expressing areas, marking the ZR, correlated negatively with age (r = −0.76, P < 0.0001), and these correlations were observed in both sexes (r = −0.74 for men and −0.77 for women; P < 0.0001 for both).
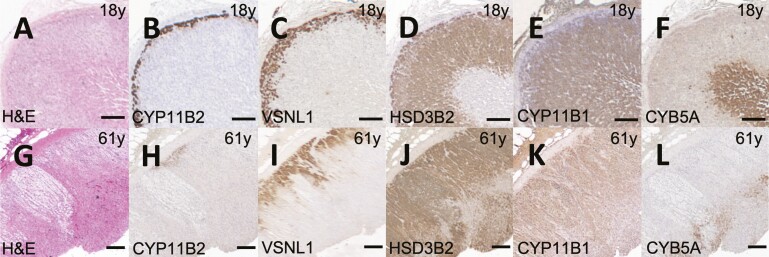
In contrast with the ZG- and the ZR-specific enzymes, the proportions of HSD3B2 expression increased with aging (r = 0.35, P = 0.007), and this trend was observed in both men and women (r = 0.34 and 0.35, P = 0.07 and 0.06, respectively). In addition, the expression of CYP11B1 remained stable across ages (r = 0.02, P = 0.86), despite a decline in the ZR component. The estimated ZF proportion (obtained by subtracting the VSNL1-positive area from the HSD3B2-positive area) demonstrated a strong positive correlation with aging (r = 0.53, P < 0.0001), and this association was present in both men (r = 0.59, P = 0.0006) and women (r = 0.49, P = 0.007). Representative adrenal cortex sections from an 18-year-old man and a 61-year-old man are shown in Fig. 1.
Figure 1.
Representative adrenal images from young and old individuals. Adrenal images of an 18-year-old man (A-F) and a 61-year-old man (G-L), respectively are shown: hematoxylin and eosin staining (H&E, A and F), and immunohistochemistry staining for aldosterone synthase (CYP11B2, B and H); visinin-like 1 (VSNL1, C and I); 3β-hydroxysteroid dehydrogenase type II (HSD3B2, D and J); 11β-hydroxylase (CYP11B1, E and K); and cytochrome b5 type A (CYB5A, F and L). Scale bars indicate 200 μm.
Serum steroid concentrations across ages
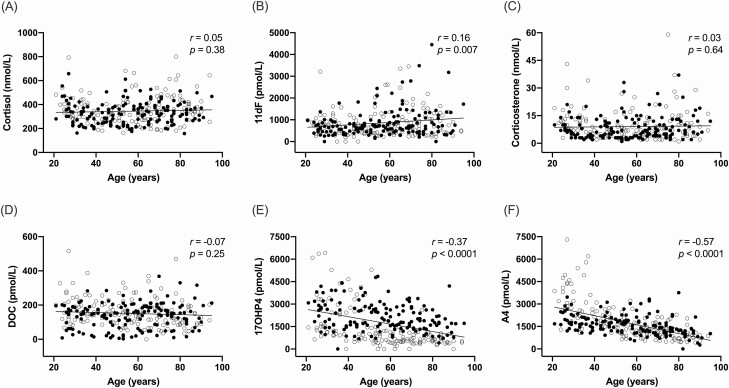
We measured the concentrations of 6 Δ 4 cortical steroids in the peripheral circulation of 298 adults, half men, with a median age of 57 years (range 21-95), and median BMI of 26 kg/m2 (Table 3). The prevalence of hypertension, diabetes mellitus, and dyslipidemia was similar in men and women (Table 3). Serum cortisol levels remained relatively stable across ages (P = 0.38, Fig. 2A), while levels of 11dF, a precursor of cortisol, increased slightly with age (r = 0.16 and P = 0.007, Fig. 2B). A subtle increase of 11dF with aging was observed in men (r = 0.23, P = 0.005), but not in women (P = 0.26). The mineralocorticoid pathway steroids, corticosterone and 11-deoxycorticosterone, remained stable across ages (P = 0.64 and 0.25, respectively, Fig. 2C and 2D). As previously reported (16,22), 17OHP4 and A4 declined with aging (17OHP4, r = −0.37 and P < 0.0001; A4, r = −0.37 and P < 0.0001; Fig. 2E and 2F), and this phenomenon was common to men and women.
Table 3.
Clinical characteristics and morning steroid concentrations in 298 adults
| Total | Men | Women | p | |
|---|---|---|---|---|
| N | 298 | 149 | 149 | |
| Age, years (range) | 57 (21-95) | 57 (21-95) | 57 (21-94) | 0.87 |
| BMI, kg/m2 | 26.1 [23.7, 30.0] | 26.4 [24.0, 30.0] | 26.0 [23.6, 30.0] | 0.84 |
| Hypertension, n (%) | 146/296 (49.3) | 77/147 (52.4) | 69/149 (46.3) | 0.30 |
| Diabetes mellitus, n (%) | 36/265 (13.6) | 17/130 (13.1) | 19/135 (14.1) | 0.81 |
| Dyslipidemia, n (%) | 164/275 (59.6) | 87/140 (62.9) | 76/135 (56.3) | 0.27 |
| Cortisol, nmol/L | 333 [269, 403] | 322 [255, 381] | 346 [276, 415] | 0.001 |
| 11dF, pmol/L | 692 [437, 1010] | 681 [453, 957] | 700 [404, 1104] | 0.67 |
| Corticosterone, nmol/L | 7 [4, 12] | 6 [4, 11] | 7 [4, 12] | 0.17 |
| DOC, pmol/L | 149 [94, 193] | 149 [85, 188] | 147 [101, 198] | 0.03 |
| 17OHP4, pmol/L | 1453 [806, 2534] | 1887 [1450, 2737] | 908 [527, 1453] | <0.0001 |
| A4, pmol/L | 1476 [1078, 2107] | 1550 [1185, 1935] | 1394 [976, 2268] | 0.16 |
Continuous variables are shown as median and range (for age) or interquartile range (all other variables). Differences between men and women were assessed with the Wilcoxon signed-rank test (for continuous variables) and the chi-squared test (for categorical variables).
Abbreviations: 11dF, 11-deoxycortisol; 17OHP4, 17-hydroxyprogesterone; A4, androstenedione; BMI, body mass index; DOC, 11-deoxycorticosterone.
Figure 2.
Morning serum steroid concentrations across ages. Associations between morning serum steroid concentrations and age are shown in 248 patients: 149 of each sex, matched by age and body mass index. Closed circles represent men and open circles represent women. r represents the Pearson correlation coefficient.
Abbreviations: 11dF, 11-deoxycortisol; 17OHP4, 17α-hydroxyprogesterone; A4, androstenedione; DOC, 11-deoxycorticosterone.
Discussion
This study reveals that in contrast with the ZR and the ZG areas, which both decrease with aging, ZF enlarges as a function of age. Intriguingly, we found that this ZF expansion exceeded the loss of ZR across adulthood, such that the total cortical area increased with aging.
In a recent study of adrenals from deceased Japanese patients with ages between infancy to 104 years, the adrenal weight was found to increase up to age 65 years in males, with a subsequent inverse correlation between adrenal weight and age in older men (24). In contrast, adrenal weight remained relatively stable in females across ages. Using contrast-enhanced multidetector computed tomography, Schneller et al found that the in vivo adrenal volume and the adrenal width increased slightly with age, and these dimensions were also larger in men and on the left side (25). The adrenal cortex volume and the ZF were shown to be larger in older as compared to younger rodents (26). The ZF is the largest adrenal component (27). Comprehensive studies of adrenal cortical zone changes across aging humans have been lacking. A small Japanese study of autopsy human adrenal tissue showed that the ratio of HSD3B2-positive area to the total cortical area was larger in individuals younger vs older than age 20, but the number of adults included in the study was small (28). Our study of adults aged 18 to 86 years found a linear increase of the adrenal cortical HSD3B2 expression and estimated ZF area with aging in both men and women.
The physiological significance of the ZF and adrenal cortex expansion with aging deserves further study. ACTH has corticotropic effects, and it is the primary regulator of glucocorticoid synthesis (29). Sustained ACTH elevations lead to hypertrophy of the adrenal cortex, while suppression of ACTH or knockout of its receptors cause adrenal atrophy (30-32). In addition, ACTH is a key regulator of steroidogenic enzymes gene expression, including HSD3B2, CYP17A1, and CYP11B1 (33-35). Several age- and sex-dependent alterations within the HPA axis have been reported (12,36-39), many pointing toward some degree of HPA hyperactivity in elderly. A higher integrated ACTH production might explain the expansion of the ZF in aging individuals (7,40). From a ZF functionality aspect, while the morning serum cortisol changes across ages have varied among published reports (8,16,41), 24-h mean cortisol and urinary free cortisol increase with aging (42,43), primarily due to increased cortisol production between midnight and early morning (40,43).
Intriguingly, we found that the expression of CYP11B1, which involves the ZF and outer ZR, remained unaltered with aging, despite a profound reduction of the ZR. In a study of 590 patients (319 men), age 18 to 97 years, we found that unlike their precursors, the CYP11B1 metabolites of A4 and testosterone, 11β-hydroxyandrostenedione and 11β-hydroxytestosterone, respectively, did not decline with aging (22). Considering the dramatic reduction of the ZR and subtle uptrend of 11dF with aging, we speculate that CYP11B1 might be preferentially using the nascent A4 generated at the ZF/ZR interface over 11dF. In vitro CYP11B1 kinetic studies will be needed to further understand the mechanisms of adrenal hormones physiology in aging.
In agreement with previous reports (4,28,44-46), we found that CYP11B2 and CYB5A expression diminished with aging. Notably, while the decline in ZR was common to both sexes, the ZG, and the CYP11B2/VSNL1 ratio declined abruptly with aging in men, but not in women. Generally, aldosterone production is regulated via the renin-angiotensin-aldosterone system, which is impacted by the salt intake (47). Previous studies suggest that aldosterone secretion from aldosterone-producing micronodules (formerly known as aldosterone-producing cell clusters) (48) gradually increases until around age 50, but the number of aldosterone-producing micronodules subsequently declines with aging (49-51). Existing data indicate a blunting in aldosterone response to sodium intake with aging and an increased salt sensitivity in the elderly compared with young adults (4,52). The impact of gender on aldosterone production remains poorly understood (53-55). Reproductive age women have higher levels of renin and aldosterone during the luteal phase compared with the follicular phase (56). Some reports suggest that women have higher salt sensitivity and greater response of aldosterone and blood pressure to angiotensin II than men (57,58). The significance of CYP11B2 decline in aging men but not women deserves further investigation.
VSNL1 is a membrane calcium-sensor, which is highly expressed in the ZG (19), including steroidogenically active and inactive cells (59). The VSNL1 gene expression is acutely suppressed by ACTH (19), but the chronic effects are less understood. VSNL1 is reportedly upregulated in aldosterone-producing adenomas and prevents apoptosis, particularly those with KCNJ5 somatic mutations (60,61). The role of VSNL1 in normal adrenals remains elusive. A recent study of pigs exposed to variable dietary sodium reported that VSNL1 is regulated by sodium intake, were other ZG markers, such as KCNJ5 and CYP11B (62). We found that, like CYP11B2, VSNL1 expression declined with aging, particularly in men. Further studies are needed to elucidate the role of VSNL1 in human physiology, including aging.
Among the limitations of our study are its cross-sectional design, both for the histological and serum steroids studies, the limited clinical information on the deceased kidney donors, and the lack of integrated 24-h steroid production data. In addition, plasma renin, aldosterone, dietary salt intake, and hypertension status were not available in this study to allow a direct correlation of morphological and functional data. Nevertheless, this study included a substantial number of patients across a broad adult age range, and it allowed us to identify sex differences in adrenal cortical zone transformation with aging. The age-related expansion of the ZF and adrenal cortex are in stark contrast with the previously described decline of the ZG and ZR. If this phenomenon is a direct consequence of the chronic HPA-activation observed in elderly or an independent process remains to be elucidated. Moreover, the sexual dimorphism of the ZG changes with aging may be key in the understanding of cardiovascular risk in aging men and women.
Acknowledgments
We are indebted to Dr. Celso Gomez-Sanchez for the generous gift of antibodies against CYP11B1, CYP11B2, and HSD3B2. We thank Patrick O’Day for his assistance with steroid assays by LC-MS/MS and Michelle Vinco and Farah Keyoumarsi for assistance with adrenal tissue procurement.
Financial Support: JR was supported by the National Center for Advancing Translational Sciences (NCATS)-MICHR grant UL1TR002240. WER was supported by grants R01DK069950 and R01DK43140 from the NIDDK. AFT was supported by grant 1K08DK109116 from the NIDDK.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39(4):327-348. [DOI] [PubMed] [Google Scholar]
- 2. Parker CR Jr. Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids. 1999;64(9):640-647. [DOI] [PubMed] [Google Scholar]
- 3. Parker CR Jr, Slayden SM, Azziz R, et al. . Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000;85(1):48-54. [DOI] [PubMed] [Google Scholar]
- 4. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. 2017;136(4):347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin V1 antagonist. J Neurosci. 1996;16(17):5555-5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. 2019;15(9):525-534. [DOI] [PubMed] [Google Scholar]
- 7. Dodt C, Theine KJ, Uthgenannt D, Born J, Fehm HL. Basal secretory activity of the hypothalamo-pituitary-adrenocortical axis is enhanced in healthy elderly: an assessment during undisturbed night-time sleep. Eur J Endocrinol. 1994;131(5):443-450. [DOI] [PubMed] [Google Scholar]
- 8. Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81(7):2468-2473. [DOI] [PubMed] [Google Scholar]
- 9. Ferrari E, Casarotti D, Muzzoni B, et al. . Age-related changes of the adrenal secretory pattern: possible role in pathological brain aging. Brain Res Brain Res Rev. 2001;37(1-3):294-300. [DOI] [PubMed] [Google Scholar]
- 10. Buckley TM, Schatzberg AF. Aging and the role of the HPA axis and rhythm in sleep and memory-consolidation. Am J Geriatr Psychiatry. 2005;13(5):344-352. [DOI] [PubMed] [Google Scholar]
- 11. O’Brien JT, Schweitzer I, Ames D, Tuckwell V, Mastwyk M. Cortisol suppression by dexamethasone in the healthy elderly: effects of age, dexamethasone levels, and cognitive function. Biol Psychiatry. 1994;36(6):389-394. [DOI] [PubMed] [Google Scholar]
- 12. Born J, Ditschuneit I, Schreiber M, Dodt C, Fehm HL. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur J Endocrinol. 1995;132(6):705-711. [DOI] [PubMed] [Google Scholar]
- 13. Dodt C, Dittmann J, Hruby J, et al. . Different regulation of adrenocorticotropin and cortisol secretion in young, mentally healthy elderly and patients with senile dementia of Alzheimer’s type. J Clin Endocrinol Metab. 1991;72(2):272-276. [DOI] [PubMed] [Google Scholar]
- 14. Heuser IJ, Gotthardt U, Schweiger U, et al. . Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol Aging. 1994;15(2):227-231. [DOI] [PubMed] [Google Scholar]
- 15. Piazza JR, Almeida DM, Dmitrieva NO, Klein LC. Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci. 2010;65(5):513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenhofer G, Peitzsch M, Kaden D, et al. . Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: Impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin Chim Acta. 2017;470:115-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tezuka Y, Atsumi N, Blinder AR, et al. Supplemental data for “The age-dependent changes of the human adrenal cortical zones are not congruent.” Deposited 30 November 2020. 10.5281/zenodo.4298765 [DOI] [PMC free article] [PubMed]
- 18. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. . Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trejter M, Hochol A, Tyczewska M, et al. . Visinin-like peptide 1 in adrenal gland of the rat. Gene expression and its hormonal control. Peptides. 2015;63:22-29. [DOI] [PubMed] [Google Scholar]
- 20. Gomez-Sanchez CE, Lewis M, Nanba K, Rainey WE, Kuppusamy M, Gomez-Sanchez EP. Development of monoclonal antibodies against the human 3β-hydroxysteroid dehydrogenase/isomerase isozymes. Steroids. 2017;127:56-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun N, Wu Y, Nanba K, et al. . High-resolution tissue mass spectrometry imaging reveals a refined functional anatomy of the human adult adrenal gland. Endocrinology. 2018;159(3):1511-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davio A, Woolcock H, Nanba AT, et al. . Sex differences in 11-oxygenated androgen patterns across adulthood. J Clin Endocrinol Metab. 2020;105(8):e2921-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wright C, O’Day P, Alyamani M, Sharifi N, Auchus RJ. Abiraterone acetate treatment lowers 11-oxygenated androgens. Eur J Endocrinol. 2020;182(4):413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nonaka K, Aida J, Takubo K, et al. . Correlation between telomere attrition of zona fasciculata and adrenal weight reduction in older men. J Clin Endocrinol Metab. 2020;105(3):e200-210. [DOI] [PubMed] [Google Scholar]
- 25. Schneller J, Reiser M, Beuschlein F, et al. . Linear and volumetric evaluation of the adrenal gland–MDCT-based measurements of the adrenals. Acad Radiol. 2014;21(11):1465-1474. [DOI] [PubMed] [Google Scholar]
- 26. Reaven E, Kostrna M, Ramachandran J, Azhar S. Structure and function changes in rat adrenal glands during aging. Am J Physiol. 1988;255(6 Pt 1):E903-E911. [DOI] [PubMed] [Google Scholar]
- 27. Ross MHa, Pawlina Wa.. Histology: A Text And Atlas: With Correlated Cell and Molecular Biology. 7th ed. Wolters Kluwer Health. [Google Scholar]
- 28. Nakamura Y, Fujishima F, Hui XG, et al. . 3βHSD and CYB5A double positive adrenocortical cells during adrenal development/aging. Endocr Res. 2015;40(1):8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gallo-Payet N. 60 years of POMC: adrenal and extra-adrenal functions of ACTH. J Mol Endocrinol. 2016;56(4):T135-T156. [DOI] [PubMed] [Google Scholar]
- 30. Thomas M, Keramidas M, Monchaux E, Feige JJ. Dual hormonal regulation of endocrine tissue mass and vasculature by adrenocorticotropin in the adrenal cortex. Endocrinology. 2004;145(9):4320-4329. [DOI] [PubMed] [Google Scholar]
- 31. Rebuffat P, Cavallini L, Belloni AS, et al. . A morphometric study of the reversal of ACTH-induced hypertrophy of rat adrenocortical cells after cessation of treatment. J Submicrosc Cytol Pathol. 1989;21(1):73-81. [PubMed] [Google Scholar]
- 32. Chida D, Nakagawa S, Nagai S, et al. . Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc Natl Acad Sci U S A. 2007;104(46):18205-18210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Roy C, Li JY, Stocco DM, Langlois D, Saez JM. Regulation by adrenocorticotropin (ACTH), angiotensin II, transforming growth factor-beta, and insulin-like growth factor I of bovine adrenal cell steroidogenic capacity and expression of ACTH receptor, steroidogenic acute regulatory protein, cytochrome P450c17, and 3beta-hydroxysteroid dehydrogenase. Endocrinology. 2000;141(5):1599-1607. [DOI] [PubMed] [Google Scholar]
- 34. Rege J, Nakamura Y, Wang T, Merchen TD, Sasano H, Rainey WE. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. J Clin Endocrinol Metab. 2014;99(3):E518-E527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xing Y, Edwards MA, Ahlem C, et al. . The effects of ACTH on steroid metabolomic profiles in human adrenal cells. J Endocrinol. 2011;209(3):327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Veldhuis JD, Sharma A, Roelfsema F. Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol Metab Clin North Am. 2013;42(2):201-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferrari E, Magri F, Dori D, et al. . Neuroendocrine correlates of the aging brain in humans. Neuroendocrinology. 1995;61(4):464-470. [DOI] [PubMed] [Google Scholar]
- 38. Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83-98. [DOI] [PubMed] [Google Scholar]
- 39. Wilkinson CW, Petrie EC, Murray SR, Colasurdo EA, Raskind MA, Peskind ER. Human glucocorticoid feedback inhibition is reduced in older individuals: evening study. J Clin Endocrinol Metab. 2001;86(2):545-550. [DOI] [PubMed] [Google Scholar]
- 40. Deuschle M, Gotthardt U, Schweiger U, et al. . With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61(22):2239-2246. [DOI] [PubMed] [Google Scholar]
- 41. Mezzullo M, Di Dalmazi G, Fazzini A, et al. . Impact of age, body weight and metabolic risk factors on steroid reference intervals in men. Eur J Endocrinol. 2020;182(5):459-471. [DOI] [PubMed] [Google Scholar]
- 42. Moffat SD, An Y, Resnick SM, Diamond MP, Ferrucci L. Longitudinal change in cortisol levels across the adult life span. J Gerontol A Biol Sci Med Sci. 2020;75(2):394-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89(1):281-287. [DOI] [PubMed] [Google Scholar]
- 44. Aiba M, Fujibayashi M. Alteration of subcapsular adrenocortical zonation in humans with aging: the progenitor zone predominates over the previously well-developed zona glomerulosa after 40 years of age. J Histochem Cytochem. 2011;59(5):557-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nanba AT, Rege J, Ren J, Auchus RJ, Rainey WE, Turcu AF. 11-oxygenated C19 steroids do not decline with age in women. J Clin Endocrinol Metab. 2019;104(7):2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dharia S, Slane A, Jian M, et al. . Effects of aging on cytochrome b5 expression in the human adrenal gland. J Clin Endocrinol Metab. 2005;90(7):4357-4361. [DOI] [PubMed] [Google Scholar]
- 47. Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350(2):151-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Williams TA, Gomez-Sanchez CE, Rainey WE, et al. . International histopathology consensus for unilateral primary aldosteronism. J Clin Endocrinol Metab. 2021;106(1):42-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nanba K, Vaidya A, Rainey WE. Aging and adrenal aldosterone production. Hypertension. 2018;71(2):218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hayashi T, Zhang Z, Al-Eyd G, et al. . Expression of aldosterone synthase CYP11B2 was inversely correlated with longevity. J Steroid Biochem Mol Biol. 2019;191:105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Omata K, Anand SK, Hovelson DH, et al. . Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1(7):787-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ando K, Takahashi K, Shimosawa T, Isshiki M, Nagase M, Fujita T. Effect of aging on salt sensitivity of blood pressure in patients with essential hypertension. Clin Exp Nephrol. 1999;3(1):18–22. [Google Scholar]
- 53. Danser AH, Derkx FH, Schalekamp MA, Hense HW, Riegger GA, Schunkert H. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J Hypertens. 1998;16(6):853-862. [DOI] [PubMed] [Google Scholar]
- 54. Solanki P, Gwini SM, Doery JCG, et al. . Age- and sex-specific reference ranges are needed for the aldosterone/renin ratio. Clin Endocrinol (Oxf). 2020;93(3):221-228. [DOI] [PubMed] [Google Scholar]
- 55. Toering TJ, Gant CM, Visser FW, et al. . Sex differences in renin-angiotensin-aldosterone system affect extracellular volume in healthy subjects. Am J Physiol Renal Physiol. 2018;314(5):F873-F878. [DOI] [PubMed] [Google Scholar]
- 56. O’Donnell E, Floras JS, Harvey PJ. Estrogen status and the renin angiotensin aldosterone system. Am J Physiol Regul Integr Comp Physiol. 2014;307(5):R498-R500. [DOI] [PubMed] [Google Scholar]
- 57. Shukri MZ, Tan JW, Manosroi W, et al. . Biological sex modulates the adrenal and blood pressure responses to angiotensin II. Hypertension. 2018;71(6):1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Giacché M, Vuagnat A, Hunt SC, et al. . Aldosterone stimulation by angiotensin II: influence of gender, plasma renin, and familial resemblance. Hypertension. 2000;35(3):710-716. [DOI] [PubMed] [Google Scholar]
- 59. Kuppusamy M, Ishimwe J, Williams TA, Mulatero P, Gomez-Sanchez EP, Gomez-Sanchez CE. Abstract 054: visinin like protein 1 regulation of aldosterone biosynthesis. Hypertension 2017;70(Suppl 1):A054-A054. [Google Scholar]
- 60. Williams TA, Monticone S, Crudo V, Warth R, Veglio F, Mulatero P. Visinin-like 1 is upregulated in aldosterone-producing adenomas with KCNJ5 mutations and protects from calcium-induced apoptosis. Hypertension. 2012;59(4):833-839. [DOI] [PubMed] [Google Scholar]
- 61. Williams TA, Monticone S, Morello F, et al. . Teratocarcinoma-derived growth factor-1 is upregulated in aldosterone-producing adenomas and increases aldosterone secretion and inhibits apoptosis in vitro. Hypertension. 2010;55(6):1468-1475. [DOI] [PubMed] [Google Scholar]
- 62. Vohra T, Kemter E, Sun N, et al. . Effect of dietary sodium modulation on pig adrenal steroidogenesis and transcriptome profiles. Hypertension 2020:76(6):1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.