Abstract
Improved understanding of host antiviral defense and antitumor immunity have elucidated molecular pathways important to both processes. During viral infection, RNA or DNA in the host cell serves as a danger signal that initiates the antiviral response. Recent studies have elucidated similarities in the signaling pathways activated by viruses and the signaling pathways induced by tumor DNA that is released into the cytoplasm of irradiated tumor cells. Both the host defense to viral infection and the sterile inflammation provoked by radiotherapy induce a type I interferon response that is necessary for pathogen control and immune-mediated tumor control, respectively. These findings have led to the hypothesis that radiotherapy employs a form of viral mimicry.
The immunobiology of chronic viral infection and cancer are closely intertwined, and our mechanistic understanding of virology and host antiviral immunity has substantially contributed to the field of oncology. To this end, programmed cell death protein-1 (PD-1) was initially described as a marker of exhaustion on CD8+ T cells during chronic viral infection and subsequently found to play a fundamental role in tumor immunology. Since the original discovery of PD-1 in the context of viral immunology, therapeutic antibodies targeting PD-1 and its ligands have revolutionized the field of oncology.
The molecular pathways that activate innate immunity and lead to antigen-specific T-cell responses against viral or tumor antigens also share considerable overlap. The presence of viral RNA and DNA nucleic acid species function as pathogen-associated molecular patterns (PAMPs) that are sensed by the host. These signals initiate an antiviral response characterized by the induction of type I interferons (IFNs) and upregulation of interferon-stimulated genes (ISGs). Similarly, the presence of cytosolic tumor DNA resulting from radiation-induced DNA damage can function as a damage-associated molecular pattern, which triggers cellular mechanisms similar to those elicited by viral PAMPs. This review will discuss the convergence of these signaling pathways to highlight connections between the antiviral state and the irradiated state. By drawing parallels between the immune response to viral infection and the antitumor immune response to radiation, we aim to bridge a conceptual gap between the fields of virology, cancer immunology, and radiation oncology.
Parallels Between Chronic Viral Infection and Cancer
The immune system evolved both innate and adaptive arms to eliminate pathogens and subsequently generate long-term protective immunity against a specific pathogen. This protective immunity allows the host to generate a more efficient and expedient memory response when reencountering the same pathogen. The innate immune system discriminates self (host) and non-self (pathogen) by using pattern recognition receptors (PRRs) that sense and bind conserved elements of bacteria, viruses, fungi, and protozoan parasites. Collectively, these conserved elements are termed PAMPs and include proteins and lectins expressed by pathogens, as well as DNA and RNA in the cytoplasm or endosomes during viral replication (1,2). PAMPs bind cytosolic or membrane-bound PRRs on antigen-presenting cells (APCs) such as dendritic cells (DCs) or macrophages, leading to downstream transcriptional changes that upregulate major histocompatibility complex (MHC) molecules and antigen-processing machinery, as well as cytokines and chemokines. APCs load antigen onto MHC molecules and migrate to the draining lymph nodes where they prime antigen-specific T cells.
The initial goal of the immune response is to clear the pathogen and resolve acute infection. In acute viral infection, viral clearance involves the eradication of infected cells by activated CD8+ cytotoxic T lymphocytes (CTLs) and the eventual generation of virus-specific antibodies (3). CTLs engage death receptors on virally infected target cells via the interaction between Fas and Fas ligand. They also secrete cytotoxic granules that contain pore-forming perforins and granzymes which mediate apoptosis. In addition, there is evidence that a small number of viral antigen-specific T cells secrete cytokines that interfere with viral replication without direct lysis of infected cells. For example, hepatitis B virus (HBV)-specific CD8+ T cells secrete IFN gamma (IFN-γ) and tumor necrosis factor α (TNF-α) to eliminate HBV nucleocapsid particles and destabilize viral RNA with the goal of interfering with viral replication (4).
During viral infection, PD-1 is upregulated as an early activation marker. However, repeated engagement of PD-1 can lead to T-cell exhaustion when CD8+ T cells are continuously exposed to viral antigen (5). The expression of immune-inhibitory checkpoint molecules with modulation of hallmark transcriptional programs and epigenetic modifications serves as a marker for exhausted CD8+ T cells that are no longer able to perform cytotoxic effector functions. Since chronic antigen stimulation drives a state of T-cell dysfunction, there is a positive correlation between viral load and level of T-cell exhaustion (6). Antigens present at lower levels in vivo induce functional exhaustion of T cells, whereas antigens present at higher levels may lead to the deletion of T cells (7).
In cancer immunology, tumor-associated antigens or tumor-specific neoantigens (generated via nonsynonymous somatic mutation) can prime antigen-specific CD8+ T-cell responses. Malignant tumors that have escaped immune surveillance are also a chronic source of antigen and thus can induce a state of T-cell exhaustion akin to chronic viral infection. The coexistence of mixed clinical responses, where simultaneous immune-mediated tumor regression and disease progression occur, emphasizes the complexity and dynamic interplay between host immunity and tumor evolution. The evasion mechanisms co-opted in chronic viral infection have provided valuable insight for understanding T-cell dysfunction in cancer as well as the transcriptional and epigenetic programs in T cells that allow them to persist in this hypofunctional state. The reversal of T-cell exhaustion via PD-1 axis blockade to reinvigorate antitumor immunity has been a transformative advance in cancer immunotherapy.
Innate Sensing of Viral Infection and Radiotherapy
Viral Replication Co-Opts Host Replication Machinery
When a virus enters a host cell, the virus replicates and generates the components necessary for progeny viruses to assemble. Viruses are intrinsically parasitic to the host and are dependent on host cell machinery for viral replication. Once assembled, progeny viruses exit the host cell and infect nearby cells.
The mode of viral replication is generally dependent on the genetic material packaged by the infective virus. The viral genome ranges from single-stranded (ss) RNA or DNA to double-stranded (ds) RNA or DNA, and ssRNA viruses can be further subclassified into positive- or negative-sense RNA viruses. These differences in viral genome are relevant because they engage the host antiviral response through different signaling pathways. Radiotherapy predominantly induces genotoxic stress via dsDNA breaks and subsequent cytosolic micronuclei rupture. The presence of these nucleic acid species in the cytoplasm after radiation activates an immune response similar to the antiviral host response to dsDNA viruses.
Viral dsDNA integrates into the host genome in the cytoplasm or the nucleus. In the nucleus, many dsDNA viruses such as HBV require host cell polymerases to replicate, whereas others, such as adenoviruses or herpesviruses, encode their own polymerases (8). Replication of dsDNA viral genomes requires that cells are in a state that allows DNA replication and therefore this replication is dependent on the cell cycle. In certain contexts, viruses integrate into the host genome and initiate aberrant cell division that can lead to malignant transformation due to dysregulation of host cell–intrinsic division and growth. An example of the oncogenic nature of viral infection is human papillomavirus (HPV), which has a dsDNA genome and is associated with virally induced cancers of the head and neck, cervix, and anogenital region.
In contrast to DNA viruses, which generally replicate within the nucleus, RNA viruses replicate in the cytosol. Because the presence of dsRNA in the cytoplasm triggers host defense mechanisms, dsRNA viruses encapsulate their genome in a core particle or capsid. In addition, dsRNA viruses do not require host polymerases to replicate because these capsids contain all of the enzymes required for replication. Whereas replication of many DNA viruses is dependent on the phase of the cell cycle, many RNA viruses replicate independently of the cell cycle (9).
Recognition of Nucleic Acids by Pattern Recognition Receptors and the Inflammasome
PRRs are able to recognize certain DNA- or RNA-based nucleic species that are located outside their normal subcellular locations. PRRs include toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)–like receptors (NLRs), and retinoic acid-inducible gene I (RIG-I)–like receptors (RLRs) that respond to PAMPs located within different subcellular compartments, and there is considerable crosstalk between these innate viral sensing pathways (Figure 1).
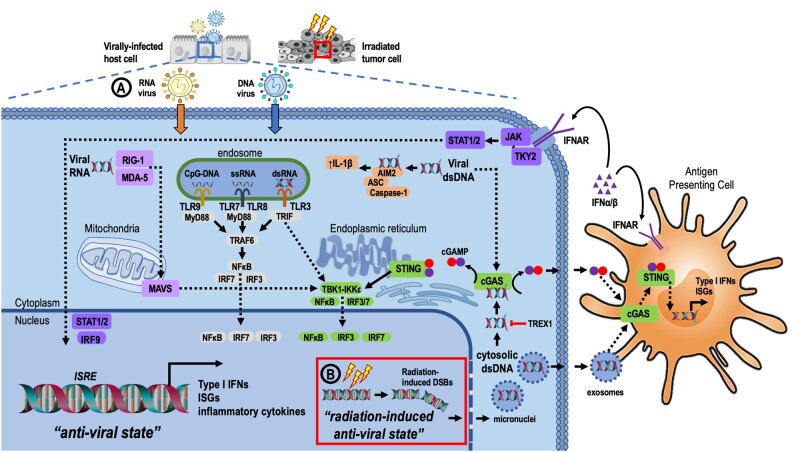
Figure 1.
Innate viral sensing and cellular response to viral infection and host response to radiation-induced inflammation. A) The presence of viral nucleic acids within different cellular compartments activates PRRs. RIG-I and MDA-5 are cytosolic PRRs that sense viral dsRNA in association with the MAVS complex that interacts with TBK1-IKKε. IRF3 and IRF7 are phosphorylated and translocate to the nucleus to initiate transcription of type I IFNs and ISGs. A subset of endosomal TLRs detect nucleic acid species. TLR9 senses unmethylated CpG-DNA, and TLR7 and TLR8 recognize ssRNA. TLR7, 8, and 9 signal through a pathway dependent on the adapter, MyD88. TLR3 senses dsRNA and triggers the TRIF-dependent pathway. Both MyD88 and TRIF-dependent signaling converge to activate TRAF6, whereas TRIF-dependent signaling also involves TBK1-IKKε. Collectively, endosomal TLR signaling leads to Nuclear factor kappa B (NFĸB) activation and transcriptional activation of type I IFN via IRF7 and IRF3. Cytosolic dsDNA can activate the AIM2 inflammasome to induce pro-inflammatory cytokines and caspase-1–mediated pyroptosis. Cytosolic viral dsDNA is also recognized by cGAS, which catalyzes production of cGAMP. cGAMP activates the endoplasmic reticulum-localized adapter, STING, causing STING to dimerize and translocate to nucleus where it activates TBK1, leading to IRF3 phosphorylation and nuclear translocation. NF-ĸB signaling is also activated downstream of STING via IKKε. B) Radiation-induced DNA damage results in double-stranded DNA breaks (DSBs) and micronuclei carrying fragmented dsDNA rupture in the cytoplasm, triggering cGAS-STING signaling and a type I IFN response. Exosomes loaded with radiation-induced dsDNA fragments as well as extracellular cGAMP activate the cGAS-STING pathway and type I IFN signaling. Type I IFNs bind cognate IFNAR complexes leading to phosphorylation of JAK1 and TYK2. This promotes the recruitment of STAT1 and STAT2 and IRF9 to form the ISGF3 complex, which translocates to the nucleus and binds ISREs, leading to transcription of ISGs. AIM2 = absent in melanoma 2; cGAMP = 2’3’-cyclic CMP-AMP; cGAS = cGAMP synthase; dsDNA = double-stranded DNA; dsRNA = double-stranded RNA; IFN = interferon; IFNAR = interferon-alpha/beta receptor; IKK = IĸB kinase; IRF = interferon regulatory factor; ISG = interferon-stimulated genes; ISREs = interferon-stimulated response elements; JAK1 = Janus kinase 1; MAVS = mitochondrial antiviral signaling; MDA-5 = melanoma differentiation-associated protein 5; PRR = pattern recognition receptor; RIG-I = retinoic acid-inducible gene-I; ssRNA = singe-stranded RNA; STAT = signal transducers and activators of transcription; STING = stimulator of interferon genes; TBK1 = TANK binding kinase 1; TLR = toll-like receptor; TRIF = TIR domian containing adapter-inducing interferon-β; TYK2 = tyrosine kinase 2.
TLRs are transmembrane proteins located on the plasma membrane or within endosomes. The TLR family consists of 13 different transmembrane proteins that recognize conserved patterns of microbial components, or PAMPs, including nucleic acids, bacterial glycolipid lipopolysaccharides, and peptidoglycans (10-12). The unique subcellular localization of TLRs allows them to sense different pathogens with unique routes of entry and modes of replication. TLR2 and TLR4 are located on the cell surface where they bind to lipopolysaccharide, and TLR3, TLR7, TLR8, and TLR9 are located inside endosomes where they bind to viruses or nucleic acids that have been endocytosed or phagocytosed. PAMP recognition by TLRs can activate the Myeloid differentiation primary response 88 (MyD88)-dependent pathway or the TRIF (Toll/Interleukin-1 receptor domain containing adapter-inducing interferon-β)–dependent pathway (13,14). For example, TLR9 senses CpG-DNA and signals through the MyD88 adaptor protein complex in conjunction with IRAK-4 (Interleukin-1 receptor associated kinase 4) to activate MAP (Mitogen activated protein)-kinase, NF-κB and interferon regulatory factor (IRF) signaling (15). These TLR-initiated signaling pathways ultimately induce the expression of downstream genes that are critical for the innate immune response.
RLRs are a family of PRRs that detect viral replication within the cytosol and/or the nucleus and include RIG-I (retinoic acid-inducible gene I) and melanoma differentiation–associated protein 5 (2). RLR recognition of RNA in the cytosol leads to the aggregation of the mitochondrial antiviral signaling (MAVS) and activation of TANK-binding kinase 1 IκB kinase-ε (TBK1-IKKε), which activates IRF3, IRF7, and NF-κB.
NLRs are cytosolic receptors that enter the cell via phagocytosis or membrane pores to form protein complexes called inflammasomes, which induce caspase-1–mediated activation of pro-IL-1β. The NLR family of inflammasomes includes NOD (nucleotide-binding oligomerization) and NLRPs (NOD-like leucine rich repeat and pyrin domains) that recognize bacterial, fungal, and parasitic PAMPs, as well as the AIM2 (absent in melanoma 2) receptor that detects bacterial and viral DNA. The NLRP3 inflammasome is activated by efflux of K+ from damaged cells, and the AIM2 inflammasome is activated by cytosolic DNA (16). Specifically, cytosolic dsDNA binding to AIM2 results in recruitment of an adaptor protein, ASC, apoptosis-associated speck-like protein containing a CARD (caspase activation and recruitment domain), which recruits and activates caspase-1 to generate the AIM2 inflammasome.
Innate immune activation by the cytosolic DNA-sensing, cyclic GMP-AMP (cyclic guanosine monophosphate-adenosine monophosphate) synthase/stimulator of interferon genes (cGAS-STING) pathway has been a subject of recent investigation. During infection, cGAS senses viral dsDNA and catalyzes the generation of cGAMP (17). cGAMP then binds STING dimers in the endoplasmic reticulum, where a conformational change allows for binding and activation of TBK1-IKKε. Akin to RLR signaling, cGAS-STING signaling results in the activation of IRF3/NF-κB and type I IFN production. Because IFN-α and -β signaling is important for activation and maturation of DCs, which are required for effective priming of CD8+ T cells (18), the activation of the cGAS-STING IFN-β pathway serves as a critical bridge between innate and adaptive immunity.
Many pathogens have evolved mechanisms to escape host recognition of foreign nucleic acids. For example, cytomegalovirus (CMV) inhibits DNA sensing by cGAS to abrogate the antiviral immune response (19). Some organisms, such as bats, have adapted to chronic viral infection by self-limiting their ability to mount a host antiviral response via downregulation of cGAS-STING signaling (20).
Innate Viral Sensing as a Framework to Understand the Host Response to Radiotherapy
In response to radiotherapy, tumor cells release nucleic acids into the cytoplasm to activate similar innate signaling pathways as activated by viral infection (13). Radiation generates DNA damage, produces dsDNA breaks, and disrupts the nuclear envelope, allowing small DNA fragments to leak into the cytoplasm. Further, radiation can induce chromosomal aberrations and the formation of abnormal DNA structures such as micronuclei (21). Radiation can also de-repress certain noncoding RNAs that have viral origins, including endogenous retroviruses or retroelements, which can bind to cytoplasmic PRRs, including RIG-I. Of note, radiation also induces molecular hallmarks of immunogenic cell death, which serve as damage-associated molecular patterns, including translocation of calreticulin from the endoplasmic reticulum to cell surface, extracellular release of high mobility group box 1 (HMGB1), and extracellular secretion of ATP (22).
Seminal work by Deng et al. (23) has demonstrated that radiation-mediated antitumor immunity requires cGAS-STING to sense cytosolic DNA and induce IFN-β. Exogenous IFN-β treatment is sufficient to rescue a defect in cross-priming DCs observed in Tmem173-/- mice (23), suggesting that the engulfment of irradiated tumor cells in the context of immunogenic cell death can activate Baft3+ DCs via IFN-β (24). Batf3+ conventional type I DCs (cDC1) play a key role in cross-presentation of tumor-derived antigens, leading to direct priming of CD8+ T cells (18).
Although modulation of cGAS-STING pathway appears to be a promising therapeutic avenue to boost antitumor immunity (25), it is important to remember that this conserved biology largely evolved as a form of antiviral host defense and is exquisitely complex. Initial efforts utilizing STING agonists for tumor control were underwhelming, partly because natural cyclic dinucleotide ligands are metabolically unstable. Recently, multiple research groups have developed synthetic non-nucleotide STING agonists, including linked aminobenzimidazole-based compounds (26), MSA-2 (27), and a cGAMP (cyclic guanosine monophosphate-adenosine monophosphate) mimetic (28) to induce IFN-β and antitumor immunity. However, other evidence suggests that chronic activation of the cGAS-STING axis may yield deleterious effects on viral infection and tumor control. Bakhoum et al. (29) demonstrated that cancer cells with chromosomal instability co-opt cGAS-STING signaling to promote metastatic dissemination. Therefore, compensatory and/or regulatory mechanisms may have evolved to limit deleterious chronic cGAS-STING signaling and prevent excessive host tissue damage.
A relevant example is 3-prime repair exonuclease-1 (TREX1), which was initially discovered for its role in HIV-1 infection, because it degrades HIV-1 DNA to subvert recognition by PRRs and prevent type I IFN production (30). Preclinical work by Vanpouille-Box et al. (31) demonstrated that cytosolic DNA extruded into the cytoplasm following radiation is degraded by the DNA exonuclease activity of TREX1. Importantly, radiation induces TREX1 expression in a dose-dependent manner to dampen cGAS-STING IFN-β signaling. This data has led to the hypothesis that higher fractional doses of radiation may be less immunogenic because of their ability to induce TREX1 expression—a potential mechanism for dose-dependent activation of the immune response.
In addition, the complement system is a system of serum proteins that evolved 3 pathways: the classical pathway, the mannose-binding lectin pathway, and the alternative pathway. The system opsonizes pathogens, recruits effector cells, and forms a “membrane attack complex” to lyse infected cells. Because there is cross talk between TLRs and the complement system, the complement system has been implicated as a key system linking innate and adaptive immunity (32,33). Therefore, it is not surprising that many viruses (such as West Nile virus) have evolved mechanisms to evade or alter the complement system in order to interfere with host defense to viral infections (34-36). In addition, recent work has highlighted a role for components of the complement cascade in the radiation-induced immune response (37). Pro-inflammatory anaphylatoxins C3a and C5a play a role in radiotherapy-induced antitumor immunity, and various forms of radiation lead to an increase in serum levels of complement C1q and C3 (38). Just as the complement system plays a critical role in antiviral immunity, this work suggests that radiation-induced complement activation may boost antitumor immunity.
The Antiviral State
The antiviral state describes the collective changes induced in host cells resulting from activation of type I IFNs (IFN-α and -β) and type III IFN (IFN-λ). This review will focus on type I IFNs, which are secreted by a variety of cells in response to infection, including epithelial cells, natural killer cells (NK cells), B cells, T cells, macrophages, fibroblasts, and endothelial cells. All nucleated cells express the type I IFN receptor (IFNAR) complex, allowing IFN-α and -β to act on a broad range of infected cells. Binding of IFN-α and -β leads to dimerization of IFNAR1 and IFNAR2 subunits, and the resulting phosphorylation activates the receptor-associated kinases, tyrosine kinase 2 and Janus kinase 1 (JAK1). These kinases recruit STAT 1 (signal transducer and activator of transcription 1) and STAT 2. STAT homodimers and heterodimers translocate to the nucleus to initiate transcription of more than 300 ISGs. Some ISGs are nucleic acid-binding proteins that suppress viral replication, whereas other ISGs bind to PRRs to activate innate immunity.
Type I IFN signaling upregulates MHC class I (MHC-I) that is required for antigen presentation and T-cell–mediated elimination of virus-infected cells. Autocrine signaling loops involving IFN-β on DCs promote their activation and maturation, leading to upregulation of antigen presentation machinery and co-stimulatory molecules (CD80 and CD86) (39). Type I IFNs also directly promote effector functions of CD8+ T cells and natural killer cells and modulate the hematopoietic compartment by promoting differentiation of bone marrow progenitors into monocytic DCs (40).
Whereas type I IFN signaling stimulates innate and adaptive immunity to promote the resolution of viral infection (41), various regulatory mechanisms determine whether pathogens are cleared or chronic infection develops (42). Just as viruses encode viral proteins that inhibit cGAS-STING signaling, other viral proteins inhibit IFN-α/-β signaling, highlighting the evolutionary pressure exerted by type I IFN in the control of viral infection. Moreover, during lymphocytic choriomeningitis virus (LCMV) clone-13 infection, elevated type I IFN upregulates negative immune-regulatory molecules like PD-L1 and IL-10, promoting T-cell exhaustion and viral persistence (43,44).
Harnessing Adaptive Immunity and Overcoming T-Cell Exhaustion
Antigen Presentation Propels Adaptive Immunity
The foundation of adaptive immunity is the development of antigen-specific T-cell and B-cell responses. The uptake, processing, and presentation of antigens are the first steps required for antigen-specific immunity against a viral infection or an irradiated tumor (Figure 2). The intracellular antigen-presenting pathway predominates in virally infected cells. Viral proteins undergo proteasomal degradation in the cytosol, and these antigens are trafficked to the endoplasmic reticulum where they are loaded on MHC-I molecules. The newly formed MHC-I–antigen complex is presented on the cell surface and recognized by CD8+ T cells with cognate T-cell receptors (TCRs). Alternatively, the exogenous (extracellular) pathway processes extracellular proteins that are internalized (via endocytosis or phagocytosis) by APCs. These peptides undergo processing within endosomes where they associate with MHC-II and are presented on the surface of APCs and recognized by CD4+ T cells. Importantly, there is cross talk between these pathways in the form of cross-presentation where extracellularly derived antigens are initially processed via the exogenous pathway but subsequently loaded on MHC-I to generate CD8+ T-cell responses. This distinction carries relevance because cross-presentation is an important mechanism by which radiation elicits adaptive immunity (45). This occurs through conventional dendritic cell 1 (cDC1) engulfment of irradiated dying tumor cells and processing of tumor-derived peptides onto MHC-I machinery (46).
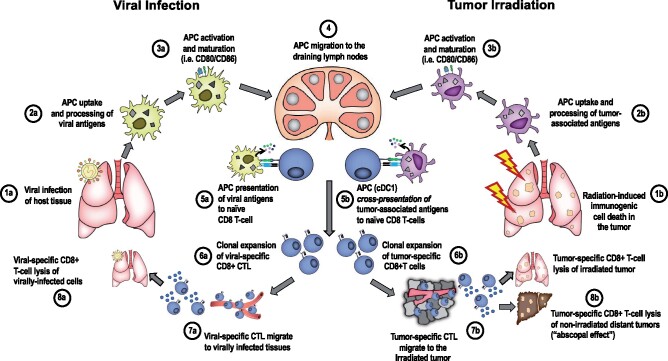
Figure 2.
Parallels between the adaptive immune response to viral infection and radiation-driven antitumor immunity. A) In the context of viral infection, (1a) viral entry into host cells leads to detection of PAMPs by PRRs; (2a) IFN-α and -β production, which recruits APCs that engulf viral antigens and process via the MHC-I antigen presentation pathway; (3a) APC activation and maturation induces expression of costimulatory molecules (4). Activated APCs loaded with viral antigen migrate to draining lymph nodes where they (5a) prime naïve T cells via 3 signals: (6a) signal 1 (TCR-antigen–MHC-I), signal 2 (co-stimulation; CD28-CD80/CD86), and signal 3 (cytokines), which result in clonal expansion of viral-antigen–specific CD8+ T cells, which then (7a) migrate to virally infected tissues. 8a) Antigen-specific CD8+ T cells lyse infected cells. B) In the context of radiation-induced antitumor immunity, (1b) tumor irradiation induces DNA damage and activates the cGAS-STING pathway leading to IFN-I signaling. (2b) cDC1 are recruited to the irradiated TME where they engulf and process antigen for cross-presentation on MHC-I. 3b) DC activation, maturation, and (4) trafficking to tumor-associated draining lymph nodes. 5b) DCs cross-present antigens to CD8+ T cells via TCR binding to antigen–MHC-I. 6b) Clonal expansion occurs as described in 6a. 7b) Tumor antigen-specific effector CD8+ T cells migrate to the irradiated tumor and (8b) infiltrate the irradiated TME where they recognize tumor cells expressing cognate antigen and lyse tumor cells. They also migrate to nonirradiated tumor sites and recognize shared epitopes that drive T-cell mediated tumor lysis. APC = antigen-presenting cell; cDC1 = conventional dendritic cell 1; cGAS-STING = cyclic GMP-AMP synthase; CTL = cytotoxic T lymphocyte; DC = dendritic cell; IFN-1 = type I interferon; ISG = interferon-stimulated gene; MHC-I = MHC class I; PAMPs = pathogen-associated molecular patterns; PRR = pattern recognition receptor; STING = stimulator of interferon genes; TCR = T-cell receptor; TME = tumor microenvironment;.
Additionally, there is emerging data that radiation may drive a cellular program in tumor cells that enhances transcription and translation of mutant peptides, which are recognized as neoantigens (47). Because immunogenic tumor neoantigens derived from somatic mutations appear to be important targets of T-cell–directed immunotherapy, the ability of radiation to “expose” immunogenic neoantigens to the immune system may prove to be an important mechanism of radiation-mediated adaptive immunity (48,49).
Vaccination and Memory CD8+ T-Cell Formation
The first successful vaccine against smallpox, developed by Dr Edward Jenner in 1796, was based on the demonstration that prior inoculation with cowpox could prevent infection upon subsequent challenge with smallpox. Ultimately, all vaccines are designed to educate the host immune system to confer antigen-specific immunity against a pathogen using an antigen and an adjuvant. Vaccines trigger host adaptive immunity by providing an antigen that cross-primes T cells via MHC-antigen binding to the TCR (signal 1), and an adjuvant that functions as a PAMP to activate PRRs and induce co-stimulation (signal 2) and pro-inflammatory cytokines (signal 3).
A hallmark of vaccination is the generation of long-lasting, protective immunologic memory. LCMV is a useful preclinical model to study memory T-cell responses because different strains can induce acute infection (Armstrong) or chronic infection (clone-13) in vivo, with similarities in memory T-cell formation observed in both viral infection models (50,51). Mechanistic studies on memory T-cell formation have illustrated that the T-cell response to viral infection is a highly orchestrated process defined by 3 phases: expansion, contraction, and memory formation (52,53). Seminal studies of adoptively transferred LCMV-specific, TCR-transgenic CD8+ T cells have shown that a population of viral antigen-specific T cells initially undergoes massive clonal expansion in response to acute infection before migrating throughout the body to kill virally infected cells and eradicate systemic infection (Figure 2) (45,55). After the acute viral infection resolves, a contraction phase leads to rapid reduction in the number of virus-specific T cells, allowing the host to return to a more energetically efficient state. Following this contraction phase, a subpopulation of effector CD8+ T cells (approximately 5%-10%) become memory T cells, which persist at low levels to defend against rechallenge by the same pathogen (3,48,56).
Memory CD8+ T cells are a heterogeneous population that exists along a spectrum of differentiation states dictated by the integration of intrinsic transcriptional programs, epigenetic modulation, and environmental cues (58,59). There are 3 broad classes of memory T cells—effector memory (TEM), central memory (TCM), and tissue-resident memory (TRM) cells—which serve complementary functions to protect the host from re-infection. TEM circulate between tissues and secondary lymphoid organs to provide baseline effector function, and TCM reside in secondary lymphoid organs and rapidly expand in the context of re-exposure to antigen. TRM cells are located in peripheral tissues and provide rapid protection at sites of infection. Recent work has highlighted the growing complexity in defining various TRM populations because differences in organ site and tissue specificity also shape T-cell fate (60). Further, using single-cell RNA sequencing, Milner et al. (61) dissected the TRM landscape by identifying distinct subsets defined by B lymphocyte-induced maturation protein-1 and Id3 expression with contrasting effector-like and memory-like properties. Importantly, the distinct subsets of TRM that were identified in response to LCMV infection were also observed in the tumor microenvironment. Jansen et al. (62) recently reported the presence of distinct tumor-infiltrating CD8+ T-cell populations in human kidney tumors that recapitulate the terminally differentiated state or the CXCR5-enriched “stem-like state” that has been described in preclinical LCMV models. Taken together, these findings highlight the strong parallels between T cell biology in cancer and viral infection.
Evolving Understanding of T-Cell Exhaustion
Persistent antigenic stimulation drives functional impairment of CD8+ T cells. This dysfunctional state of T-cell exhaustion is a dynamic process characterized by progressive loss of effector functions and increased expression of immune-inhibitory checkpoints (ie, PD-1, LAG-3, TIM3) in a hierarchical fashion. PD-1 is the prototypical immune-inhibitory receptor, and its role in T-cell exhaustion was originally established in the context of LCMV infection (63). Mice with chronic viral infection upregulate PD-1 on virus-specific T cells, and these PD-1+ virus-specific T cells are functionally exhausted T cells (TEX). TEX demonstrate alterations in TCR and cytokine signaling, differential expression of genes involved in T-cell migration and homing, as well as distinct transcriptional and metabolic programs (64). Interestingly, blockade of PD-1 is able to restore TEX function and promote viral clearance (65).
Over the past few decades, a working model of T-cell exhaustion has been continually refined in an effort to better characterize the different subsets of TEX and their underlying biology. These TEX subsets exist along a spectrum of differentiation states and display considerable plasticity. However, TEX can cross a threshold into a state of terminal differentiation where exhaustion becomes irreversible (66). This underlies the rationale for PD-1 axis blockade in cancer and, to a lesser extent, chronic viral infection, since blocking the PD-1 axis can reverse T-cell exhaustion and restore T-cell effector functions before irreversible exhaustion occurs. Both transcriptional programs and epigenetic mechanisms regulate TEX fate and the transition between different exhaustion states. For example, the transcription factor, T-cell factor 1 (TCF1), is expressed on TCF1+ TEX progenitors, and this expression is lost as TEX move toward a more terminally exhausted state. Recently, thymocyte selection-associated high mobility group box (TOX) has been identified as a critical transcription factor that enforces T-cell exhaustion in both chronic viral infection and cancer (67,68). TOX expression is induced by persistent antigen presentation and results in the upregulation of immune-inhibitory receptors and decrease in cytokine production via chromatin remodeling and transcriptomic modulation. A developmental framework has recently been proposed to describe these different TEX subsets by integrating molecular, transcriptomic, and epigenetic information pertinent to chronic viral infection and cancer. These subsets are distinguished by Ly108 (Slamf6) and CD69 expression, and the transition of TEX subsets is regulated by transcriptional and epigenetic checkpoints that involve TCF1, Tbet, and TOX. TCF1 predominates in less terminally differentiated subsets, whereas TOX predominates in the terminally exhausted TEX irreversible state (66).
The Irradiated State: How Does Radiotherapy Boost Antitumor Immunity?
Radiotherapy has emerged as an ideal partner for immunotherapy given its direct immunomodulatory effects on the tumor microenvironment and its ability to augment both innate and adaptive immunity. The notion that focal tumor irradiation can incite systemic regression of nonirradiated tumors via immune-based mechanisms (abscopal response) has gained substantial interest, yet it is rarely observed in the clinic. Using the principles of the innate and adaptive host antiviral response as a conceptual framework, we discuss potential mechanisms and review clinical evidence of radiation-mediated immunogenicity (Figure 3).
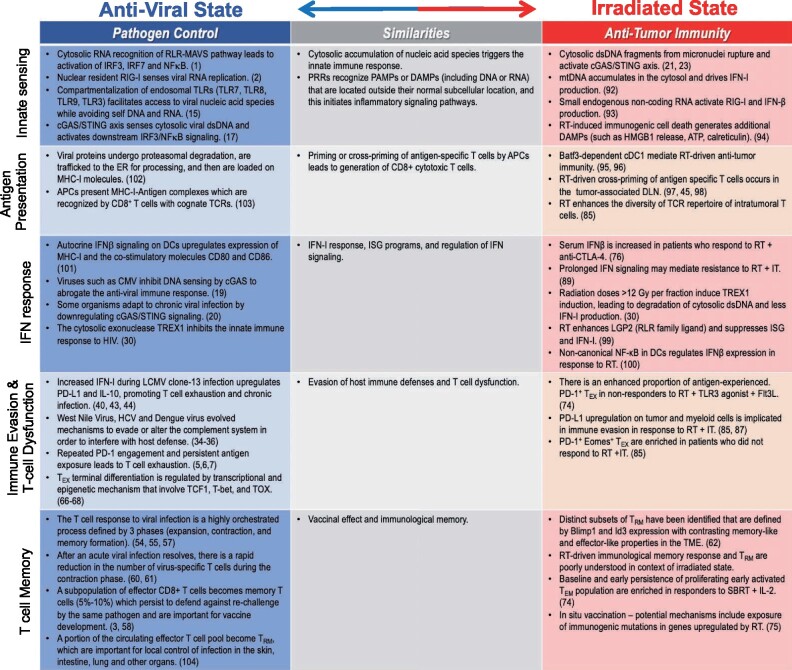
Figure 3.
Similarities between the antiviral state and the irradiated state. 1) Cytosolic accumulation of nucleic acids triggers the innate immune response. PRRs recognize PAMPs or DAMPs (including DNA or RNA) that are located outside their normal subcellular location, and this initiates inflammatory signaling pathways; 2) Priming or cross-priming of antigen-specific T cells by APCs leads to generation of cytotoxic CD8+ T cells; 3) IFN-I response, ISG programs, and regulation of IFN signaling; 4) Evasion of host immune defenses and T-cell dysfunction; 5) Vaccine effect and immunologic memory. APC = antigen-presenting cell; ATP = adenosine triphosphate; Batf3 = basic leucine zipper transcription factor ATF-like 3; Blimp1 = B lymphocyte-induced maturation protein–1; cDC1 = conventional type 1 dendritic cells; cGAS/STING = cyclic GMP-AMP synthase/stimulator of interferon genes; CMV = cytomegalovirus; CTLA-4 = cytotoxic T-lymphocyte associated protein 4; DAMP = damage-associated molecular pattern; DLN = draining lymph node; dsDNA = double-stranded DNA; Eomes = Eomesodermin; ER = endoplasmic reticulum; Flt3L = FMS-like tyrosine kinase 3 ligand; HCV = hepatitis C virus; HMBG1 = high mobility box group protein 1; ICD = immunogenic cell death; Id3= inhibitor of DNA binding 3; IFN- = interferon beta; IFN-I = type I interferon; IL-2 = interleukin-2; IRF = interferon regulatory factor; ISG = interferon-stimulated genes; IT = immunotherapy; LCMV = lymphocytic choriomeningitis virus; LGP2 = laboratory of genetics and physiology 2; MAVS = mitochondrial antiviral signaling; MHC-I = major histocompatibility complex class I; mtDNA = mitochondrial DNA; NFkB = nuclear factor kappa light chain enhancer of activated B cells; PAMP = pathogen-associated molecular pattern; PD-1 = programmed cell death protein 1; PD-L1 = programmed death ligand 1; PRR = pattern recognition receptor; RIG-I = retinoic acid inducible gene-I; RLR = RIG-I–like receptor; RT = radiotherapy; SBRT = stereotactic body radiation therapy; TCR = T-cell receptor; TCF1 = T-cell factor 1; T-bet = T-box transcription factor; TEM = effector memory T cells; TEX = exhausted T cells; TLR = toll-like receptor; TME = tumor microenvironment; TOX = thymocyte selection-associated high mobility group box gene; TREX = 3-prime repair exonuclease 1; TRM = resident memory T cells.
Can Radiotherapy Convert the Irradiated Tumor Into a Personalized in Situ Vaccine?
The concept of in situ vaccination with radiotherapy was introduced at the turn of the 21st century. Early studies demonstrated that the combination of radiotherapy and Flt3 (FMS-like tyrosine kinase 3) ligand achieved local and abscopal tumor control in a tumor-specific, T cell dependent manner (69,70). These preclinical investigations led to the hypothesis that focal tumor irradiation can generate an in vivo vaccination based on the release of tumor antigens in response to radiation—thus, each tumor represents an opportunity to generate a personalized tumor-specific vaccine. Further, immunogenic modulation of both intratumoral myeloid and T-cell compartments by radiation can promote antigen presentation and T-cell activation. Radiotherapy alone generates a very modest vaccine effect as evidenced by extremely rare occurrences of abscopal responses in the clinic, however, occasional systemic responses have been observed when combining radiotherapy with various immunomodulatory agents.
Effective vaccination to generate antigen-specific immunity and memory formation generally requires both antigen and adjuvant. To enhance the adjuvanticity of radiation-driven antigen release, several investigators have attempted to stimulate innate immune signaling with PAMPs in combination with focal irradiation. Proof-of-concept clinical studies of intratumoral TLR9 agonist administration with low-dose radiotherapy in advanced lymphoma have demonstrated systemic disease regression and formation of tumor-specific memory T-cell responses, as evidenced by post-treatment enrichment of CD137+ CD45RO+ CD8+ T cells in the blood (71,72).
Other studies have attempted to optimize in situ vaccination with radiotherapy by providing stimuli that enhance DC maturation as an immune adjuvant. A proof-of-principle trial combining radiotherapy with granulocyte-macrophage colony-stimulating factor met its prespecified primary endpoint of observed abscopal responses in 26.8% of patients, defined as an out-of-field response in at least one nonirradiated lesion (73). Alternatively, cytokine therapy with IL-2 and IFN-α have demonstrated activity in patients with both melanoma and renal cell carcinoma. Seung et al. (74) combined IL-2 with stereotactic body radiotherapy in a pilot study of renal cell carcinoma and melanoma patients, reporting an objective response rate in nonirradiated lesions of 71.4% and 60%, respectively. These preliminary findings compare favorably with response rates observed with IL-2 alone, suggesting that cytokines that activate T cells may also enhance radiation-mediated in situ vaccination. Interestingly, in this study, a population of proliferating TEM (Ki67+CD45RA+CD8+ T cells) were enriched in the peripheral blood of responders at baseline and early time points, suggesting these patients may have pre-existing immunity that was enhanced by radiation and IL-2.
Emerging evidence that radiation exposes neoantigens is an intriguing opportunity to harness adaptive antitumor immunity. Reits et al. (75) provided the first evidence that radiation enhances the MHC-I immunopeptidome to increase antigen presentation. These investigators demonstrated that radiation increases the antigen pool via degrading existing peptides as well as enhanced translation. Intriguingly, several uniquely expressed radiation-induced peptides were identified by mass spectrometry but were potentially nonimmunogenic. Nearly 15 years later, Formenti et al. (76) provided clinical evidence that radiation increases exposure of immunogenic neoantigens that drive antitumor immunity. Immunogenomic profiling of an exceptional responder to radiotherapy and anti–cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) identified rapid in vivo expansion of CD8+ T-cell clones recognizing an immunogenic neoantigen encoded by a somatic mutation in Karyopherin alpha 2 (KPNA2)—a gene that is directly upregulated by radiotherapy. The potential to develop a vaccination strategy incorporating radiation-induced neoantigens opens a novel line of investigation that could form the basis for a truly personalized in situ vaccine.
However, recent data highlight the complexity and challenges of radiation-induced in situ vaccination. Hammerich et al. (77) conducted a trial combining a radiotherapy with a TLR3 agonist [poly(I: C), a dsRNA analogue] with the addition of Flt3 ligand to mobilize cross-priming cDC1s. This multimodal approach achieved systemic regression of bulky disease in a subset of patients, with 2 complete responses out of 11 treated patients. Among the nonresponders, in situ vaccination appeared to enhance the proportion of antigen-experienced TEX (PD-1+TIGIT+HLA-DR+CD45A-), implicating T-cell exhaustion as an important escape mechanism. This was validated in a preclinical model where the addition of PD-1 blockade to in situ vaccination extended median survival.
Combining Radiotherapy With Immune Checkpoint Inhibition to Overcome T-Cell Exhaustion
Although the potential of radiation-driven in situ vaccination is yet to be realized, radiotherapy can promote antitumor immunity by enhancing preexisting immunity to overcome T-cell exhaustion and other immune evasion programs. Radiotherapy modifies the tumor microenvironment and induces production of chemokines and cytokines that recruit CTLs and other immune cells into the tumor microenvironment (78-80). Furthermore, radiation promotes immunogenic modulation of tumor cells, so that the surviving fraction of irradiated tumor cells undergoes phenotypic and transcriptional modifications that enhance immune recognition and susceptibility to CTL-mediated lysis (81). Several studies have demonstrated that radiation upregulates MHC-I, cell death receptors, tumor-associated antigens, and immune checkpoint molecules (82,83).
There is increasing interest in combining radiotherapy and immune checkpoint inhibitors to re-invigorate preexisting antitumor immunity (84), and multiple preclinical studies suggest that combining these therapies activates synergistic CD8+ T-cell–dependent mechanisms. Twyman-Saint Victor et al. (85) studied resistance mechanisms of melanoma patients undergoing combination treatment with stereotactic body radiotherapy and anti–CTLA-4 and noted that a T-cell exhaustion signature (PD-1+Eomes+ TEX) was enriched in nonresponders, whereas PD-L1 expression was upregulated on both tumor cells and myeloid cells as a dominant immune evasion mechanism. These researchers extended their clinical observations in a preclinical model and found that the addition of PD-L1 blockade significantly improved survival. Mechanistically, PD-L1 blockade re-invigorated TEX cells, and radiotherapy enhanced the diversity of the intratumoral TCR repertoire. Other groups have reported similar adaptive resistance mechanisms in response to radiotherapy and a similar capacity of radiation to expand relevant tumor-specific CD8+ clonotypes within the tumor and the periphery (86,87).
On the other hand, radiotherapy may be able to restore responsiveness to immunotherapy. Using an anti-PD-1-refractory lung cancer model, Wang et al. (88) demonstrated that radiation-induced type I IFN and upregulation of MHC-I on tumor cells was sufficient to overcome defects in antigen presentation. These findings support a potential role for radiotherapy to reignite antitumor immunity in tumors resistant to immune checkpoint inhibition. However, caution is warranted because Benci et al. (89) have demonstrated that chronic IFN signaling in tumor cells drives resistance to immune checkpoint blockade through upregulation of T-cell inhibitory receptors and epigenetic reprogramming of T cells. This is further supported by Chen et al. (90), who reported that radiation-induced type I IFN may also have a tumor-protective role that limits CTL-mediated cytotoxicity. Taken together, these data highlight the complexity and context-dependence of radiation-driven IFN signaling, which has a dual role in promoting and restraining antitumor immunity.
The direct cytotoxic effects of radiotherapy are often overlooked as immunomodulatory. As discussed previously, high tumor burden is a source of chronic antigen exposure that promotes TEX dysfunction. Therefore, cytotoxic therapies that reduce tumor burden and antigen load may facilitate antitumor immunity and reverse T-cell exhaustion. Huang et al. (6) described a correlation between tumor burden and reinvigoration of TEX (Ki-67+PD-1+CD8+) in a cohort of advanced melanoma patients treated with anti–PD-1 therapy. These investigators reported that a lower ratio of reinvigoration-to-tumor burden was associated with inferior survival suggesting that TEX reinvigoration may be insufficient in the context of high tumor burden. Integrating radiotherapy as a form of cytoreduction to potentiate responses to immunotherapy is being tested in clinical studies, and early evidence suggests this may be a promising approach (91).
Combining immune checkpoint inhibition and radiotherapy remains an intriguing strategy. However, it is necessary to understand mechanisms of resistance and determinants of response to design rational combination studies. Moreover, it is likely that multimodal approaches are needed to achieve clinical benefit in tumors that lack preexisting endogenous immunity. Although PD-1 axis blockade and CD8+ T-cell exhaustion have been the focus of this discussion, additional resistance mechanisms intrinsic to the tumor microenvironment as well as radiation-induced immune suppression must be addressed to optimize patient outcomes in the future.
Conclusion
Our understanding of radiation-induced immunogenicity remains in its nascency. Decades of work in viral immunology deciphering immunological mechanisms of the host antiviral response have provided valuable insight for the field of oncology to understand how radiotherapy can activate innate and adaptive immunity to improve local control as well as distant tumor control. It has become clear that radiotherapy can employ a form of viral mimicry by coopting innate sensing pathways to drive a type I IFN program that parallels the antiviral state. Additional similarities exist between ways that viral antigens and radiation-induced antigens activate T cells to direct cellular immunity. Vaccine development has outlined the elements required to augment both antigenicity and adjuvanticity of radiotherapy as well as the immunological barriers such as T-cell exhaustion that must be overcome to successfully convert the irradiated tumor into a personalized in situ vaccine.
Funding
This work was supported by R01 AI123210 to JRT and a Salk Women & Science Grant to HMM.
Notes
Role of the funder: Funding helped support corresponding authors HMM and JRT.
Disclosures: HMM served on an advisory board for AstraZeneca and currently serves as a consultant for RefleXion. SMK serves on the advisory board for Celsius Therapeutics and has stock options in Celsius. AMM has clinical trial funding and/or serves on the advisory board for BMS, Merck, Astra-Zeneca, Genentech, EMD-Serono, Incyte, Dynavax, Zosano, and Transgene. AEM, AMC, and JRT have no competing interests.
Author contributions: Heather M. McGee: Conceptualization; Writing-Original Draft; Writing-Reviewing and Editing; Visualization; Project Administration/Supervision; Ariel E. Marciscano: Writing-Original Draft; Visualization; Writing-Reviewing and Editing; Allison M. Campbell: Conceptualization; Writing-Original Draft; Arta M. Monjazeb: Writing-Reviewing and Editing; Susan M. Kaech: Conceptualization; John R. Teijaro; Writing-Original Draft; Writing-Reviewing and Editing
Acknowledgments: Thank you to members of our radiation oncology and immunology departments for interesting discussions on this interdisciplinary topic. Thank you to Annelise Snyder for help with the figures.
Data Availability
Given that this article is a review paper, all of the data mentioned in the paper has already been published and all references have been cited appropriately. Although some of the concepts are new, no new data were generated or analyzed in support of this review paper.
References
- 1. Schmidt A, Endres S, Rothenfusser S.. Pattern recognition of viral nucleic acids by RIG-I-like helicases. J Mol Med. 2011;89(1):5–12. [DOI] [PubMed] [Google Scholar]
- 2. Liu G, Lu Y, Thulasi Raman SN, et al. Nuclear-resident RIG-I senses viral replication inducing antiviral immunity. Nat Commun. 2018;9(1):3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oldstone MB, Tishon A, Chiller JM, Weigle WO, Dixon FJ.. Effect of chronic viral infection on the immune system. I. Comparison of the immune responsiveness of mice chronically infected with LCM virus with that of noninfected mice. J Immunol. 1973;110(5):1268–1278. [PubMed] [Google Scholar]
- 4. Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV.. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4(1):25–36. [DOI] [PubMed] [Google Scholar]
- 5. Diskin B, Adam S, Cassini MF, et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020;21(4):442–454. [DOI] [PubMed] [Google Scholar]
- 6. Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R.. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77(8):4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang L, Sheraz M, McGrane M, Chang J, Guo JT.. DNA polymerase alpha is essential for intracellular amplification of hepatitis B virus covalently closed circular DNA. PLoS Pathog. 2019;15(4):e1007742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma, A and Gupta, SP. "Fundamentals of Viruses and Their Proteases." from the book: Viral Proteases and Their Inhibitors. 2018. p.1-24.
- 10. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA.. Recognition of double-stranded RNA and activation of NF-κB by toll-like receptor 3. Nature. 2001;413(6857):732–738. [DOI] [PubMed] [Google Scholar]
- 11. Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via till-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. [DOI] [PubMed] [Google Scholar]
- 12. Hoshino K, Takeuchi O, Kawai T, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 2020;162(7):3749–3752. [PubMed] [Google Scholar]
- 13. Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198(7):1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamamoto M, Sato S, Hemmi H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. [DOI] [PubMed] [Google Scholar]
- 15. Barton GM, Kagan JC, Medzhitov R.. Intracellular localization of toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7(1):49–56. [DOI] [PubMed] [Google Scholar]
- 16. Cunha LD, Silva ALN, Ribeiro JM, et al. AIM2 engages active but unprocessed caspase-1 to induce noncanonical activation of the NLRP3 inflammasome. Cell Rep. 2017;20(4):794–805. [DOI] [PubMed] [Google Scholar]
- 17. Sun L, Wu J, Du F, Chen X, Chen ZJ.. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339(6121):786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang Z-F, Zou H-M, Liao B-W, et al. Human cytomegalovirus protein UL31 inhibits DNA sensing of cGAS to mediate immune evasion. Cell Host Microbe. 2018;24(1):69–80.e4. [ [DOI] [PubMed] [Google Scholar]
- 20. Xie J, Li Y, Shen X, et al. Dampened STING-dependent interferon activation in bats. Cell Host Microbe. 2018;23(3):297–301.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Durante M, Formenti SC.. Radiation-induced chromosomal aberrations and immunotherapy: micronuclei, cytosolic DNA, and interferon-production pathway. Front Oncol. 2018; May 29; 8:192. doi: 10.3389/fonc.2018.00192. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamazaki T, Vanpouille-Box C, Demaria S, Galluzzi L, Immunogenic cell death driven by radiation—impact on the tumor microenvironment. Cancer Treat Res. 2020;180:281–296. doi: 10.1007/978-3-030-38862-1_10. [DOI] [PubMed] [Google Scholar]
- 23. Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corrales L, Glickman LH, McWhirter SM, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11(7):1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li W, Lu L, Lu J, et al. cGAS-STING-mediated DNA sensing maintains CD8+ T cell stemness and promotes antitumor T cell therapy. Sci Transl Med. 2020;.2020 Jun 24;12(549):eaay9013. doi: 10.1126/scitranslmed.aay9013. [DOI] [PubMed] [Google Scholar]
- 26. Ramanjulu JM, Pesiridis GS, Yang J, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564(7736):439–443. [DOI] [PubMed] [Google Scholar]
- 27. Pan BS, Perera SA, Piesvaux JA, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020;369(6506): [DOI] [PubMed] [Google Scholar]
- 28. Chin EN, Yu C, Vartabedian VF, et al. Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic. Science. 2020;369(6506):993–999. [DOI] [PubMed] [Google Scholar]
- 29. Bakhoum SF, Ngo B, Laughney AM, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553(7689):467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J.. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11(11):1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8(1):15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dempsey PW, Allison MED, Akkaraju S, Goodnow CC, Fearon DT.. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271(5247):348–350. [DOI] [PubMed] [Google Scholar]
- 33. Hajishengallis G, Lambris JD.. Crosstalk pathways between toll-like receptors and the complement system. Trends Immunol. 2010;31(4):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mellors J, Tipton T, Longet S, Carroll M.. Viral evasion of the complement system and its importance for vaccines and therapeutics. Front Immunol. 2020; 11: 1450. Published online 2020 Jul 9. doi: 10.3389/fimmu.2020.01450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwon YC, Ray R, Complement regulation and immune evasion by hepatitis C virus. Methods Mol Biol. 2019;1911:337-347.doi: 10.1007/978-1-4939-8976-8_23. [DOI] [PubMed] [Google Scholar]
- 36. Conde JN, da Silva EM, Allonso D, et al. Inhibition of the membrane attack complex by dengue virus NS1 through interaction with vitronectin and terminal complement proteins. J Virol. 2016;90(21):9570–9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Surace L, Lysenko V, Fontana AO, et al. Complement is a central mediator of radiotherapy-induced tumor-specific immunity and clinical response. Immunity. 2015;42(4):767–777. [DOI] [PubMed] [Google Scholar]
- 38.Montay-Gruel, P. Markarian, M. Allen, BD, Baddour, JD, Giedzinski, E, Jorge, PG, Petit, Benoit, Bailet, C, Vozenin, M-C, Limoli, C; Acharya, M. Ultra-High-Dose-Rate FLASH Irradiation Limits Reactive Gliosis in the Brain. Radiat Res. 2020 Aug 27. doi: 10.1667/RADE-20-00067.1. [DOI] [PMC free article] [PubMed]
- 39. Montoya M, Schiavoni G, Mattei F, et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99(9):3263–3271. [DOI] [PubMed] [Google Scholar]
- 40. Hahm B, Trifilo MJ, Zuniga EI, Oldstone MBA.. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 2005;22(2):247–257. [DOI] [PubMed] [Google Scholar]
- 41. Lindahl P, Gresser I, Leary P, Tovey M.. Interferon treatment of mice: enhanced expression of histocompatibility antigens on lymphoid cells. Proc Natl Acad Sci USA. 1976;73(4):1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Teijaro JR. Type I interferons in viral control and immune regulation. Curr Opin Virol. 2016 Feb;16:31-40.doi: 10.1016/j.coviro.2016.01.001. Epub 2016 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Teijaro JR, Ng C, Lee AM, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340(6129):207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilson EB, Yamada DH, Elsaesser H, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340(6129):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3(4):345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Woo S-R, Fuertes MB, Corrales L, et al. The STING pathway mediates innate immune sensing of immunogenic tumors. Immunity. 2014;41(5):830–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lhuillier C, Rudqvist NP, Elemento O, Formenti SC, Demaria S.. Radiation therapy and anti-tumor immunity: exposing immunogenic mutations to the immune system. Genome Med. 2019;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McGranahan N, Furness AJS, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Youngblood B, Hale JS, Kissick HT, et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017;552(7685):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mueller SN, Langley WA, Li G, García-Sastre A, Webby RJ, Ahmed R.. Qualitatively different memory CD8 + T cells are generated after lymphocytic choriomeningitis virus and influenza virus infections. J Immunol. 2010;185(4):2182–2190. [DOI] [PubMed] [Google Scholar]
- 52. Ahmed R, Gray D.. Immunological memory and protective immunity: understanding their relation. Science. 1996;272(5258):54–60. [DOI] [PubMed] [Google Scholar]
- 53. Zinkernagel RM, Hengartner H.. Correlates of protective viruses damaging to HIV infection. Science. 1996;272(5266):1362a. [DOI] [PubMed] [Google Scholar]
- 54. Kaech SM, Hemby S, Kersh E, Ahmed R.. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111(6):837–851. [DOI] [PubMed] [Google Scholar]
- 55. Wherry EJ, Ahmed R.. Memory CD8 T-cell differentiation during viral infection. J Virol Immunol. 2004;78(11):5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jamieson BD, Ahmed R.. T cell memory. Long-term persistence of virus-specific cytotoxic T cells. J Exp Med. 1989;169(6):1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Butz EA, Bevan MJ.. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8(2):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Akondy RS, Fitch M, Edupuganti S, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017;552(7685):362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Plumlee CR, Sheridan BS, Cicek BB, Lefrançois L.. Environmental cues dictate the fate of individual CD8+ T cells responding to infection. Immunity. 2013;39(2):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Milner JJ, Toma C, He Z, et al. Heterogenous populations of tissue-resident CD8+ T cells are generated in response to infection and malignancy. Immunity. 2020;52(5):808–824.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jansen CS, Prokhnevska N, Master VA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ahn E, Araki K, Hashimoto M, et al. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci USA. 2018;115(18):4749–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hudson WH, Gensheimer J, Hashimoto M, et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity. 2019;51(6):1043–1058.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. [DOI] [PubMed] [Google Scholar]
- 66. Beltra JC, Manne S, Abdel-Hakeem MS, et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity. 2020;52(5):825–841.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Khan O, Giles JR, McDonald S, et al. TOX transcriptionally and epigenetically programs CD8+ T cell exhaustion. Nature. 2019;571(7764):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scott AC, Dündar F, Zumbo P, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019 Jul;571(7764):270–274.doi: 10.1038/s41586-019-1324-y. Epub 2019 Jun 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. [DOI] [PubMed] [Google Scholar]
- 70. Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59(24):6028–6032. [PubMed] [Google Scholar]
- 71. Brody JD, Ai WZ, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28(28):4324–4332. [ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Frank MJ, Reagan PM, Bartlett NL, et al. In situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 2018;8(10):1258–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16(7):795–803. [DOI] [PubMed] [Google Scholar]
- 74. Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2—tumor and immunological responses. Sci Transl Med. 2012;4(137):137ra74. [DOI] [PubMed] [Google Scholar]
- 75. Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203(5):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018 Dec;24(12):1845–1851.doi: 10.1038/s41591-018-0232-2. Epub 2018 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hammerich L, Marron TU, Upadhyay R, et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat Med. 2019;25(5):814–824. [DOI] [PubMed] [Google Scholar]
- 78. Lim JYH, Gerber SA, Murphy SP, Lord EM.. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8+ T cells. Cancer Immunol Immunother. 2014;63(3):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71(7):2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gameiro SR, Ardiani A, Kwilas A, Hodge JW.. Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. Oncoimmunology. 2014;3(5):e28643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Garnett CT, Palena C, Chakarborty M, Tsang KY, Schlom J, Hodge JW.. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64(21):7985–7994. [DOI] [PubMed] [Google Scholar]
- 83. Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170(12):6338–6347. [DOI] [PubMed] [Google Scholar]
- 84. Crittenden MR, Zebertavage L, Kramer G, et al. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Sci Rep. 2018;8(1):7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated radiation therapy stimulates antitumor immunity mediated by both resident and infiltrating polyclonal T-cell populations when combined with PD-1 blockade. Clin Cancer Res. 2017;23(18):5514–5526. [DOI] [PubMed] [Google Scholar]
- 87. Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang X, Schoenhals JE, Li A, et al. Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res. 2017;77(4):839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Benci JL, Johnson LR, Choa R, et al. Opposing functions of interferon coordinate adaptive and innate immune responses to cancer immune checkpoint blockade. Cell. 2019;178(4):933–948.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen J, Cao Y, Markelc B, Kaeppler J, Vermeer JAF, Muschel RJ.. Type I IFN protects cancer cells from CD8+ T cell-mediated cytotoxicity after radiation. J Clin Invest. 2019;129(10):4224–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yamazaki T, Kirchmair A, Sato A, et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat Immunol. 2020;21(10):1160–1171. [DOI] [PubMed] [Google Scholar]
- 93. Ranoa DRE, Parekh AD, Pitroda SP, et al. Cancer therapies activate RIG-I-like receptor pathway through endogenous non-coding RNAs. Oncotarget. 2016;7(18):26496–26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Golden EB, Frances D, Pellicciotta I, Demaria S, Barcellos-Hoff MH, Formenti SC.. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3(4):e28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Blair TC, Bambina S, Alice AF, et al. Dendritic cell maturation defines immunological responsiveness of tumors to radiation therapy. J Immunol. 2020;204(12):3416–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res. 2016;76(20):5994–6005. [DOI] [PubMed] [Google Scholar]
- 97. Takeshima T, Chamoto K, Wakita D, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with TH1 cell therapy. Cancer Res. 2010;70(7):2697–2706. [DOI] [PubMed] [Google Scholar]
- 98. Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res. 2018;24(20):5058–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Widau RC, Parekh AD, Ranck MC, et al. RIG-I-like receptor LGP2 protects tumor cells from ionizing radiation. Proc Natl Acad Sci USA. 2014 Jan 28;111(4):E484-91. doi: 10.1073/pnas.1323253111. Epub 2014 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hou Y, Liang H, Rao E, et al. Non-canonical NF-κB antagonizes STING sensor-mediated DNA sensing in radiotherapy. Immunity. 2018;49(3):490–503.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nice TJ, Osborne LC, Tomov VT, Artis D, Wherry EJ, Virgin HW.. Type I interferon receptor deficiency in dendritic cells facilitates systemic murine norovirus persistence despite enhanced adaptive immunity. PLoS Pathog. 2016;12(6):e1005684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. McCarthy MK, Malitz DH, Molloy CT, et al. Interferon-dependent immunoproteasome activity during mouse adenovirus type 1 infection. Virology. 2016 Nov;498:57-68.doi: 10.1016/j.virol.2016.08.009. Epub 2016 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yang Y, Xiang Z, Ertl HCJ, Wilson JM.. Upregulation of class I major histocompatibility complex antigens by interferon γ is necessary for T-cell-mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92(16):7257–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kok L, Dijkgraaf FE, Urbanus J, et al. A committed tissue-resident memory T cell precursor within the circulating CD8+ effector T cell pool. J Exp Med. 2020 Oct 5;217(10):e20191711.doi: 10.1084/jem.20191711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Given that this article is a review paper, all of the data mentioned in the paper has already been published and all references have been cited appropriately. Although some of the concepts are new, no new data were generated or analyzed in support of this review paper.