Abstract
OBJECTIVE:
To examine genomic correlates of conversion to resection (CTR) and overall survival (OS) in patients with initially unresectable colorectal liver metastasis (IU-CRLM) treated with combination systemic and hepatic artery infusion (HAI) chemotherapy.
BACKGROUND:
In patients presenting with IU-CRLM, combination systemic and HAI chemotherapy enables CTR with associated long-term OS in a subset of patients. Genomic correlates of CTR and OS in IU-CRLM have not been previously explored.
METHODS:
Specimens from IU-CRLM patients receiving systemic/HAI chemotherapy (2003-2017) were submitted for next-generation sequencing. Fisher’s Exact test assessed associations with CTR, and Kaplan-Meier/Cox methods assessed associations with OS from HAI initiation.
RESULTS:
Of 128 IU-CRLM patients, 51 (40%) underwent CTR at median 6 months (range:3-35) from HAI initiation. CTR and persistently unresectable (PU) cohorts differed significantly in preoperative systemic chemotherapy exposure, node-positive primary status, and size of largest liver metastasis. Median and 5-year OS was 66 months and 51%. CTR was associated with prolonged survival (time-dependent HR 0.23, 95%CI:0.12-0.46, p<0.001). The most frequently altered genes were APC (81%), TP53 (77%), and KRAS (37%). Oncogenic mutations in SOX9 and BRAF were associated with CTR. BRAF mutations, any RAS pathway alterations, and co-altered RAS/RAF-TP53 mutations were associated with worse survival. Classification and regression tree analysis defined prognostically relevant clusters of genomic risk to reveal co-altered RAS/RAF-TP53 as the highest risk subgroup. Co-altered RAS/RAF-TP53 remained independently associated with worse survival (HR 2.52, 95%CI:1.37-4.64, p=0.003) after controlling for CTR, number of liver metastases, and preoperative extrahepatic disease.
CONCLUSIONS:
Distinct genomic profiles are associated with CTR and survival in patients with IU-CRLM treated with HAI/systemic chemotherapy. Presence of SOX9, BRAF, and co-altered RAS/RAF-TP53 mutations are promising biomarkers that, when validated in larger datasets, may impact treatment of IU-CRLM patients.
MINI ABSTRACT
In patients with initially unresectable colorectal liver metastases (IU-CRLM), combination systemic and hepatic artery infusion chemotherapy enables conversion to resection (CTR) with associated long-term survival in a subset of patients. In the first study exploring genomic correlates of outcomes in IU-CRLM, presence of SOX9, BRAF, and co-altered RAS/RAF-TP53 mutations emerged as novel biomarkers of CTR and survival. In this selected cohort of patients, co-altered RAS/RAF-TP53 was not only the highest risk genomic subgroup, but also remained independently associated with worse survival.
INTRODUCTION
Colorectal cancer (CRC) accounts for an estimated 140,000 new cases and approximately 50,000 deaths in the United States annually. Colorectal liver metastasis (CRLM), either synchronous or metachronous, develop in up to 60% of patients.1 Complete resection of CRLM is associated with a cure rate of 20%.2
At presentation, it is estimated that approximately 80% of patients with CRLM are initially unresectable (IU-CRLM).3 Given that complete resection is a major contributor to long-term survival, significant efforts have focused on expanding the pool of resectable patients. The advent of effective systemic chemotherapy agents (e.g. irinotecan, oxaliplatin),4, 5 modern combination regimens with 5-FU,6 and targeted molecular therapies (e.g. anti- EGFR antibodies for KRAS wild-type tumors)7 have enabled downstaging of IU-CRLM to potentially resectable, with similar 5-year survival compared with upfront resectable patients.3, 8, 9 However, inconsistent reporting of criteria for irresectability and/or responses required for conversion to resection (CTR) in such studies has made interpretation of these studies difficult.10, 11 Moreover, modern chemotherapy regimens—although associated with median overall survival (OS) as high as 30 months12, 13—rarely, if ever, result in long-term cure in IU-CRLM patients. While first line chemotherapy has response rates over 50%, response rates with second or third-line regimens are below 20%.12,13
Hepatic arterial infusion (HAI) chemotherapy, in conjunction with systemic chemotherapy, has evolved as another potential strategy to improve rates of CTR in IU-CRLM.14-17 Intra-arterial delivery of specific agents with high first-pass hepatic extraction (e.g., floxuridine [FUDR]) limits systemic toxicity and allows for concomitant administration of systemic chemotherapy.18 Several studies have demonstrated impressive response rates with combination HAI and systemic chemotherapy, even in heavily pre-treated patients.19 Moreover, up to half of all patients in prospective trials converted to complete resection and/or ablation; importantly, CTR was associated with significantly improved OS and potential for long-term cure.15-17
However, the ability to select which patients with IU-CRLM are more likely to convert to resection and/or live longer following combination HAI and systemic chemotherapy remains elusive. We theorized that tumor genomics may reveal novel insight into this clinical dilemma; as such, the genomic underpinnings of CTR and OS in patients with IU-CRLM have not previously been explored. In the present study, utilizing the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT), a targeted genomic sequencing platform, we hypothesized that distinct molecular profiles are associated with CTR and OS in patients with IU-CRLM receiving HAI and systemic chemotherapy.
METHODS
Patient Selection and Variables
After Institutional Review Board approval, IU-CRLM patients receiving treatment on and off trial protocols at Memorial Sloan Kettering Cancer Center between January 2000 and October 2017, and whose tumors underwent sequencing with MSK-IMPACT, were identified from a prospectively-maintained hepatobiliary database. All human investigations were performed after approval by a local Human Investigations Committee and in accord with an assurance filed with and approved by the Department of Health and Human Services. All patients included in this study underwent informed consent.
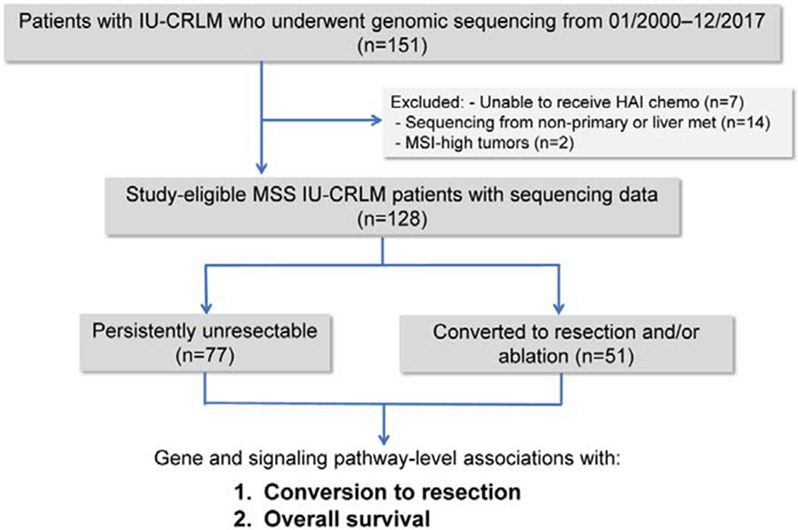
General criteria for irresectability (i.e., biologic and technical) at our institution have been previously defined.15-17 Briefly, irresectability was defined as the inability to perform complete resection and/or ablation while preserving an adequate future liver remnant and/or the presence of unresectable extrahepatic disease. Patients were excluded if no regional chemotherapy was delivered despite HAI pump placement, pathologic analysis of the primary tumor revealed histology other than adenocarcinoma, sequencing was performed on extrahepatic metastatic tissue only, or if tumors were mismatch repair-deficient by immunohistochemistry or microsatellite instability-high by MSIsensor analysis (Figure 1; Figure S1, http://links.lww.com/SLA/C753).20
Figure 1:
CONSORT diagram of study design.
After sufficient tumor response, CTR was defined as complete resection and/or ablation (i.e., either radiofrequency or microwave) of all tumors while leaving an adequate functional liver remnant. Select patients with resected extrahepatic disease were considered for hepatectomy.14
The clinical risk score (CRS) was calculated for all patients with available data. The CRS is a prognostic index incorporating five criteria, including node-positive primary tumor, disease-free interval (DFI) between the primary tumor resection and liver metastasis diagnosis >12 months, >1 hepatic metastasis, largest metastasis >5 cm, and preoperative carcinoembryonic antigen (CEA) level >200 ng/ml.21
Genomic Sequencing
Sequencing of DNA from primary tumor or specified metastasis with matched normal controls was performed with MSK-IMPACT, a hybridization exome capture-based next-generation sequencing assay.22 Sequencing output was processed as previously described23 and in Figure S1, http://links.lww.com/SLA/C753.
Statistical Analysis
We compared clinicodemographic characteristics between CTR and persistently unresectable (PU) cohorts with Fisher's Exact test and the Wilcoxon Rank Sum test, where appropriate. We included point mutations, fusions, and copy number alterations (CNA) including deep deletions and amplifications. We analyzed outcomes for oncogenic driver alterations annotated by OncoKB,24 including recurrent hotspot and 3D hotspot mutations.22 For CTR analyses, we examined mutations present in at least 5% of patients; for overall survival (OS) analyses, we examined mutations present in 10 patients to allow for enough events to occur within groups. When a gene was not present in earlier versions of MSK- IMPACT, only patients with later versions were included in formal analyses. We also assessed the following canonical signaling pathways: Receptor Tyrosine Kinase (RTK)-RAS, Cell cycle, HIPPO, MYC, NOTCH, p53, PI3K, RAS alone, and TGF-β/WNT.25
We compared individual mutation rates between CTR and PU cohorts with Fisher's Exact test and applied a False Discovery Rate (denoted by p-adj) correction for multiple testing. Overall survival (OS) was measured from HAI initiation until death. Patients alive at last follow-up (January 2019) were censored. We used Kaplan-Meier methods to illustrate survival outcomes and compared survival by mutation status with the log-rank test, also adjusting for multiple testing.
Given the recent prognostic association of co-altered RAS/RAF (i.e., any KRAS, NRAS, or BRAF) and TP53 alterations in resectable CRLM,26-29 we assessed the relationship between co-altered RAS/RAF-TP53 mutations and survival in this cohort of IU-CRLM patients. We first checked for a dependent or multiplicative effect between RAS/RAF and TP53 using an interaction term in a logistic or Cox model, where appropriate. Next, we investigated if there was an additive component to RAS/RAF-TP53 co-occurrence with bivariable (TP53 and RAS/RAF) Cox or logistic models as positive for both TP53 and RAS/RAF (pos-pos) versus all other options in univariable analyses, and using classification and regression trees (CART). CART analyses allowed us to explore subgroups that may have been masked through standard modeling procedures. For CART analyses, values were treated as a binary outcome for CTR versus PU, and with an assumed exponential distribution for OS. We also used ten-fold cross validation and cross-complexity pruning on all trees. As above, False Discovery Rate corrections were applied within each outcome and for each mutation category.
As CTR occurred after HAIP, we used time-dependent Cox regression to formally assess the relationship between CTR and OS, which allowed us to account for the time between HAIP and CTR. To help visualize this association, landmark Kaplan Meier plots were constructed from 1-year post-HAI initiation, as the majority of CTR events occurred before 12 months. The survival clock started 12 months post-HAI initiation and only patients who were event-free with greater than 12-month follow up were included in the visualization.
We also used multivariable cox regression to assess the association of co-altered RAS/RAF-TP53 and with OS, while controlling for clinical covariates. Given the number of events, we controlled for the three clinical factors significant in univariable analyses (unadj- p<0.05): resection status (treated as a time-dependent covariate), number of CRLM metastases (log-transformed), and preoperative extrahepatic disease. Since we were only testing this sole relationship, no adjustments were made for multiple testing. Unless otherwise indicated, adjusted two-sided p-values<0.05 were considered statistically significant. Recursive partitioning was performed with the rpart package (version 4.1-13, 2018) in Cran R (Vienna, Austria) and all other analyses were performed with SAS 9.4 (Cary, NC).
RESULTS
Clinical and Survival Characteristics of IU-CRLM Cohort
Of 128 IU-CRLM patients sequenced and included in this study, 51 (40%) converted to resection at a median time between HAI initiation and resection of 6.1 (range:3.2-34.8) months. PU and CTR cohorts differed significantly with respect to preoperative systemic chemotherapy exposure (87% vs. 65%, p=0.004), node-positive primary tumor (87% vs. 71%, p=0.040), median disease-free interval between primary and CRLM diagnosis (0.4 vs. 0.7 months, p=0.002), and size of the largest CRLM (median 36 vs. 51 mm, p=0.032). However, CTR and PU cohorts did not significantly vary by CRS, presentation with synchronous disease, median cycles of pre-HAI chemotherapy, presence of pre-HAI extrahepatic disease, pre-HAI CEA levels, or frequency of grade III/IV HAI-related complications (p=0.20→0.95; Table 1). All 100 (78%) patients selected for pre-HAI chemotherapy received first-line FOLFOX; in addition, 42 (32.8%) received vascular endothelial growth factor (VEGF) inhibitors, 5 (3.9%) received epidermal growth factor receptor (EGFR) inhibitors, and 4 (3.1%) received both EGFR and VEGF inhibitors. Moreover, of patients receiving pre-HAI chemotherapy, 42 (32.8%) progressed on first-line treatment and had initiated second- or third-line regimens prior to pump placement (Figure S2, http://links.lww.com/SLA/C754). Among CTR patients (n=51), median resected tumor size at initial resection was 40 (range:17-88) mm, 35 (69%) underwent R0 resection, and 7 (14%) developed a grade III/IV postoperative complication.
Table 1:
Clinical and demographic characteristics of all patients with initially unresectable colorectal liver metastases (CRLM) who underwent hepatic arterial infusion (HAI) and systemic chemotherapy, stratified by cohorts that converted to resection versus those remaining unresectable (CEA: carcinoembryonic antigen)
| All patients | Converted to Resection |
Persistently unresectable |
p-value | ||
|---|---|---|---|---|---|
| Number of patients | 128 | 51 | 77 | ||
| Gender | Male | 82 (64.1%) | 30 (58.8%) | 52 (67.5%) | 0.35 |
| Female | 46 (35.9%) | 21 (41.2%) | 25 (32.5%) | ||
| Age at Primary Diagnosis, years | Median (range) | 51.5 (25.3–75.3) | 51.7 (28.9–70.7) | 50.9 (25.3–75.3) | 0.94 |
| Age at CRLM Diagnosis, years | Median (range) | 51.7 (25.4–75.3) | 52.3 (29.4–70.9) | 51.2 (25.4–75.3) | 0.84 |
| Location of Primary Tumor | Left Colon | 35 (27.3%) | 10 (19.6%) | 25 (32.5%) | 0.08 |
| Left Colon | 47 (36.7%) | 24 (47.1%) | 23 (29.9%) | ||
| Rectum | 45 (35.2%) | 16 (31.4%) | 29 (37.7%) | ||
| Colon, NOS | 1 (0.8%) | 1 (2%) | 0 (0%) | ||
| Primary Tumor Size, mm | Median (range) | 41 (0–130) | 40 (12–130) | 43 (0–112) | 0.88 |
| N Missing | (9) | (1) | (8) | ||
| Primary Pathologic T-classification | T0-T3 | 89 (69.5%) | 43 (84.3%) | 46 (59.7%) | 0.24 |
| T4a-T4b | 3 (2.3%) | 3 (5.9%) | 0 (0%) | ||
| Unknown | 36 (28.1%) | 5 (9.8%) | 31 (40.3%) | ||
| Primary Pathologic Nodal Status | Negative | 24 (18.8%) | 1 14 (27.5%) | 10 (13%) | 0.040 |
| Positive | 103 (80.5%) 1 | 36 (70.6%) | 67 (87%) | ||
| Unknown | 1 (0.8%) | 1 (2%) | 0 (0%) | ||
| Disease Free Interval, months* | Median (range) | 0.5 (0.0 – 25.1) 1 | 0.7 (0.0 – 11.1) | 0.4 (0.0 – 25.1) | 0.002 |
| Pre-HACEA (ng/mL) | Median (range) | 62.9 (0.5–15969) | J 59.0 (0.5–7337) | 64.6 (0.9–15969) | >0.95 |
| N Missing | (2) | (0) | (2) | ||
| Largest CRLM Size, mm | Median (range) | 42 (11–210) | 51 (13–210) | 36 (11–125) | 0.032 |
| Number of CRLM | ≤4 | . 8 (6.3%) | 3 (5.9%) | 5 (6.5%) | >0.95 |
| >4 | 120 (93.8%) | 48 (94.1%) | 72 (93.5%) | ||
| Clinical Risk Score (CRS) | Low Risk (0–2) | 10 (7.8%) | 5 (9.8%) | 5 (6.5%) | 0.52 |
| High Risk (3–5) | 115 (89.8%) | 1 45 (88.2%) 1 | 70 (90.9%) | ||
| Unknown | 3 (2.3%) | 1 (2%) | 2 (2.6%) | ||
| Pre-HAChemotherapy | Yes | 100 (78.1%) | 33 (64.7%) | 67 (87%) | 0.004 |
| No | 28 (21.9%) | 18 (35.3%) | 10 (13%) | ||
| Pre-HAExtrahepatic Disease | Yes | 49 (38.3%) | 1 16 (31.4%) | 33 (42.9%) | 0.20 |
| No | 79 (61.7%) | 35 (68.6%) | 44 (57.1%) | ||
| HAI-related Grade 3–4 Complications | Yes | 11 (8.6%) | 4 (7.8%) | 7 (9.1%) | >0.95 |
| No | 117 (91.4%) | 47 (92.2%) | 70 (90.9%) | ||
| CEA 6 months post-HAI | Median (range) | 6.0 (0.9–1506) | . 3.6 (0.9–1189) 1 | 11.4 (1.6–1506) | <0.001 |
DFI calculated between diagnosis of primary and development of liver metastasis; “0 “ is synchronously detected
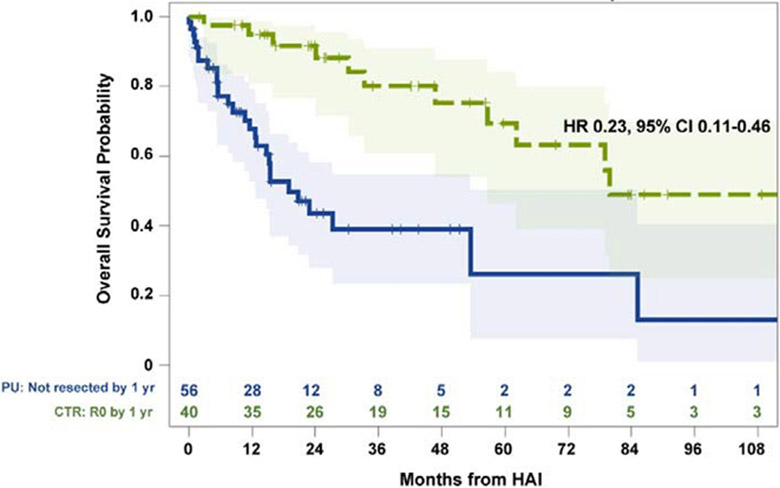
At last follow-up, 48 patients had died with a median OS of 65.5 (95%CI:34.9-91.9) months; 1-year and 5-year OS were 92.2% (95%CI:85.6-95.9%) and 50.9% (95%CI:38.7-61.8%), respectively (Appendix, http://links.lww.com/SLA/C757). Median follow-up time in survivors was 26 (range:2-179) months. Patients with CTR had prolonged survival compared to PU patients (HR 0.23, 95%CI:0.11-0.46, p<0.001; Figure 2).
Figure 2:
Landmark analysis of overall survival in patients with initially unresectable colorectal liver metastases who received hepatic arterial infusion and systemic chemotherapy, stratified by patients who converted to resection (CTR; n=40) and those persistently unresectable (PU; n=56). Time zero refers to 1 year from HAI initiation; 32 patients who had events within 12 months of follow-up were excluded. Number at risk at each time point indicated in adjoining risk table.
A Distinct Genomic Profile Associated with CTR in IU-CRLM Patients
In the overall IU-CRLM cohort, the genes with the most commonly detected alterations were APC (81%, n=103), TP53 (77%, n=99), KRAS (37%, n=47), and PIK3CA (16%, n=21), with a median 3 mutations (range=0-10) per patient. A significantly higher frequency of SOX9 alterations were observed in the CTR compared with the PU cohorts (15.7% [n=8] vs. 1.3% [n=1], p-adj=0.036). In contrast, oncogenic BRAF mutations (i.e., class I and II)30 were significantly more frequent in the PU compared with CTR cohorts (13% [n=10] vs. 0% [n=0], p-adj=0.039)—i.e., no patient with an oncogenic BRAF mutation converted to resection (Figure 3). Of the 10 patients with BRAF mutations, 8 (80%) harbored the V600E mutations. No significant associations between CTR and pathway-level alterations were observed (Figure S3, http://links.lww.com/SLA/C755).
Figure 3:
OncoPrint representation of the 15 most frequently altered genes in patients with initially unresectable colorectal liver metastases (n=128) who converted to resection (n=51) or persistently unresectable (n=77) following treatment with hepatic arterial infusion and systemic chemotherapy. Types of gene alterations grouped by putative driver mutations, variants of undetermined significance, or structural alterations are shown in the adjoining color legend. Somatic alteration frequencies in recurrently altered genes corresponding to specific genes are shown in the adjacent histograms. Red asterisks represent significantly different (p-adj<0.05) genes between CTR and PU cohorts.
A Distinct Genomic Profile is Associated with Survival in IU-CRLM Patients
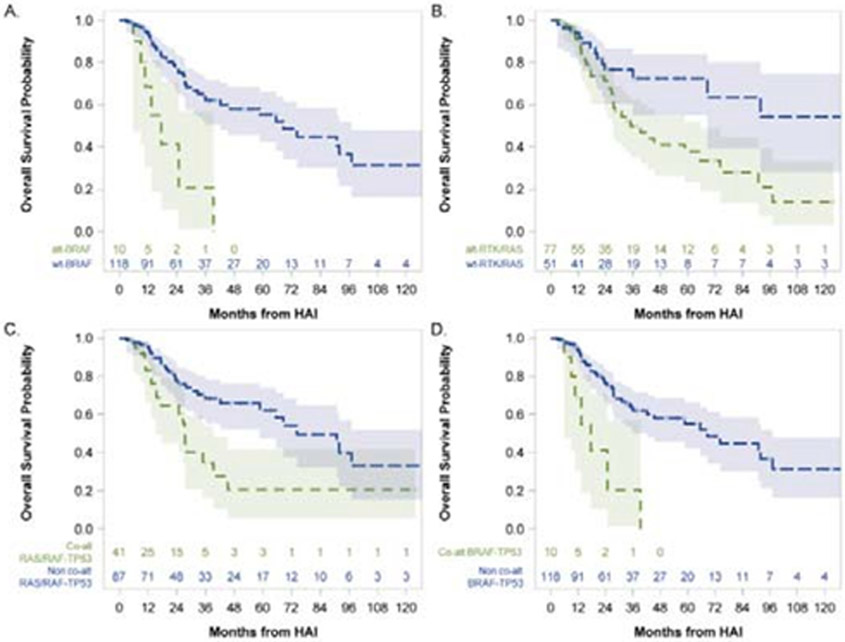
Next, we interrogated the association of oncogenic alterations in individual genes, signaling pathways (Figure S4, http://links.lww.com/SLA/C756), and biologically relevant gene combinations with OS in this cohort of IU-CRLM patients. When examining OS at the individual gene level, patients with class I/II BRAF-mutated (n=10) tumors had significantly shorter OS compared with patients with BRAF-wild type (WT; n=118) tumors (median 17.6 vs. 68.6 months, p-adj<0.001; Figure 4A). Oncogenic alterations in KRAS alone (5-year OS 43.6% altered vs. 55.4% WT, p-adj=0.66) or TP53 alone (5-yr OS 49.2% mutated vs. 57.3% WT, p-adj=0.66) were not significantly associated with OS.
Figure 4:
Stratification of overall survival analysis in patients with initially unresectable colorectal liver metastases who received hepatic arterial infusion and systemic chemotherapy (n=128) by presence or absence of alterations in: A. BRAF only; B. RTK-RAS signaling pathway; C. RAS/RAF-TP53 co-alteration; D. BRAF-TP53 co-alteration. Number at risk at each time point indicated in adjoining risk table.
When examining the association of mutations in signaling pathways with OS, mutations in the RTK-RAS pathway – but not in p53, TGF-β, WNT/β-catenin, PI3K, NOTCH, or MYC pathways – were associated with worse OS (5-year 37.8% mutated [n=77] vs. 72.5% WT [n=51], p-adj=0.032; Figure 4B).
We have recently demonstrated the association of any RAS/RAF (i.e., either KRAS, NRAS, or BRAF) mutation co-altered with TP53 mutation with extremes of survivorship and divergent patterns of metastasis in a broad cohort of CRLM patients.29 To assess the relationship between co-altered RAS/RAF-TP53 and OS in this cohort of IU-CRLM patients, we first checked for an interaction; no significant interaction between RAS/RAF and TP53 with respect to OS (p-adj=0.77) was observed. Next, bivariable models were built in which RAS/RAF (HR: 2.66, 95%CI:1.45-1.91, p-adj=0.006), but not TP53 (HR: 1.86, 95%CI:0.92-3.77, p-adj=0.24), was significantly associated with OS. Notably, patients with co-mutated RAS/RAF-TP53 (n=41) demonstrated significantly worse OS compared with patients without co-mutated RAS/RAF-TP53 (n=87) (5-year 20.7% vs. 62.2%, p-adj=0.002; Figure 4C). This effect appeared to be driven predominantly by co-occurrent BRAF-TP53 mutations, with no patient with co-altered BRAF-TP53 (n=10) surviving more than 4 years (5-year 0% vs. 55.1%; p-adj<0.001; Figure 4D). Of note, no significant association was found between co-occurrent KRAS-TP53 mutations and OS (p-adj=0.24).
Classification and Regression Tree Analysis of Genomic Correlates of Survival
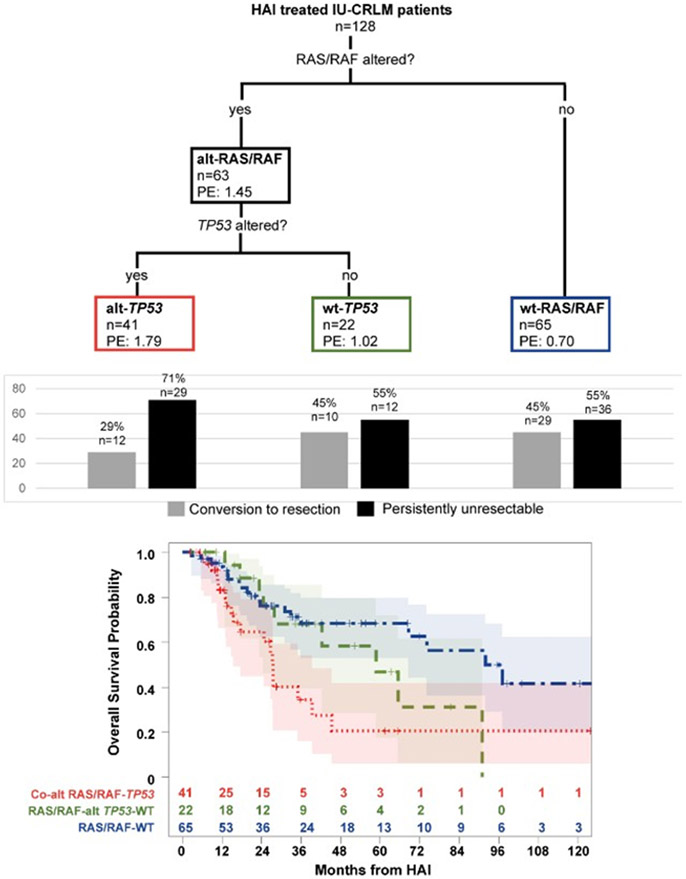
We next performed a CART analysis31 to better understand the effect of co-altered RAS/RAF-TP53 alterations on OS, but lack of significant interaction or significance of TP53 mutation in bivariable models. After pruning, the first survival split occurred on RAS/RAF-mutations with parameter estimates of 1.45 for RAS/RAF-mutated and 0.70 for RAS/RAF-WT, indicating a higher risk of death for RAS/RAF-mutated patients. The second split occurred on TP53 mutations with parameter estimates of 1.79 for TP53-altered and 1.02 for TP53-WT. Therefore, three terminal nodes associated with OS were established: co-mutated RAS/RAF-TP53 (n=41), RAS/RAF-mutated/TP53-WT (n=22), and RAS-WT (irrespective of TP53 status; n=65). Patients with co-mutated RAS/RAF-TP53 demonstrated the worst OS (5-year 20.7%, 95%CI:5.9-41.6%), followed by RAS/RAS-mutated/TP53-WT (5-year 46.7%, 95%CI:17.4-71.8%), and RAS/RAF-WT (5-year 68.3%, 95%CI:53.0-79.6%) (Figure 5).
Figure 5:
Classification and regression tree analysis revealing three terminal nodes of genomic risk (colored boxes) in patients with initially unresectable colorectal liver metastases who received hepatic arterial infusion and systemic chemotherapy (n=128); PE: parameter estimate; Histogram showing relative proportion of patients in each genomic risk subgroup who converted to resection and were persistently unresectable> Stratification of overall survival by three genomic risk subgroups: co-altered RAS/RAF-TP53, RAS/RAF-altTP53-WT, and RAS/RAF-WT. Number at risk at each time point indicated in adjoining risk table.
Cox Regression Analysis of Predictors of Survival in IU-CRLM
Next, on multivariable Cox regression analysis in this cohort, conversion to resection (HR: 0.24, 95%CI:0.12-0.49, p<0.001) and co-altered RAS/RAF-TP53 (HR: 2.52, 95%CI:1.37-4.64, p=0.003), but not number of liver metastases (HR: 1.31, p=0.17) or presence of extrahepatic disease (HR: 1.71, p=0.11), were independently associated with OS (Table 2).
Table 2:
Cox proportional hazards regression model for overall survival in patients with initially unresectable colorectal liver metastasis
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Number of liver metastases | 1.75 (1.18–2.61) | 0.006 | 1.31 (0.89–1.93) | 0.170 |
| Extrahepatic disease | 1.83 (1.02–3.29) | 0.042 | 1.71 (0.89–3.27) | 0.110 |
| Conversion to resection | 0.23 (0.12–0.46) | <0.001 | 0.24 (0.12–0.49) | <0.001 |
| Co-altered RAS/RAF-TP53 | 2.55 (1.41–4.62) | 0.002 | 2.52 (1.37–4.64) | 0.003 |
DISCUSSION
The present study uncovers distinct molecular profiles associated with conversion to resection (CTR) and overall survival in patients with initially unresectable colorectal liver metastasis (IU-CRLM) treated with combination systemic and hepatic artery infusion chemotherapy. Oncogenic mutations in BRAF and SOX9 were associated with CTR. Moreover, BRAF mutations, RTK-RAS pathway-level alterations, and co-occurrent mutations in RAS/RAF and TP53 were associated with worse survival in this selected cohort of IU-CRLM patients. In an effort to define prognostically relevant clusters of genomic risk in this cohort of IU-CRLM patients, we utilized a classification and regression tree analysis which revealed co-mutated RAS/RAF-TP53 as the highest risk genomic subgroup and indicated a synergistic nature to RAS/RAF-TP53 cooperativity. Further, co-mutated RAS/RAF-TP53 remained independently associated with worse survival after controlling for conversion to resection, number of liver metastases, and presence of extrahepatic disease.
The standard practice in this difficult-to-treat subset of IU-CRLM patients is to initiate combination systemic chemotherapy, which yields hepatic response rates of approximately 60% and CTR rates of 13-24%.3, 10 Patients who progress on first-line therapy, however, have limited treatment options. Our and other institutions have applied liver-directed in combination with systemic chemotherapy in these patients with encouraging results.16, 17, 32 However, there are no reproducible clinical or pathologic predictors to select which patients are more likely to convert to resection or demonstrate long-term disease control. The findings here may allow a more nuanced biologic understanding of these processes—presumed surrogates of underlying treatment response to liver-directed/systemic chemotherapy—beyond that predicted by standard clinicopathologic features alone. Moreover, identification of genomic profiles predicting low likelihood of CTR and poor survival in the present study reveal subsets of IU-CRLM patients in whom application of liver-directed chemotherapy may perhaps be an ineffective detour. Prospective studies are warranted to investigate these hypotheses further.
The classification and regression tree and multivariable Cox regression models revealed co-mutated RAS/RAF-TP53 as the highest risk genomic subgroup in this cohort of IU-CRLM patients. These findings deepen our understanding of the clinical implications of pivotal driver alterations beyond the isolated contribution of oncogenic KRAS in metastatic CRC. Moreover, this oncogenic cooperativity between p53 tumor suppressor loss and oncogenic RAS/RAF activation is supported mechanistically by preclinical evidence. Beyond sufficiency to induce tumorigenesis, co-occurrence of RAS/RAF signaling and TP53 mutations abrogate cellular senescence, engender metastasis-defining processes such as invasion and motility, and promote metastatic outgrowth in vivo.33, 34 The findings here justify further investigation into the association between RAS-p53 cooperativity and response to regional/systemic chemotherapy (particularly 5-FU) in metastatic colorectal cancer.
Another novel finding described here is the association between oncogenic SOX9 mutations and CTR. The SOX9 transcription factor has putative tumor suppressor function that regulates cellular proliferation, senescence, and lineage commitment in colorectal tumorigenesis.35 Moreover, recent evidence suggests that SOX9 dosage modulates the balance between actively proliferating and reserve intestinal stem cells; as such, overexpression of Sox9 inhibits the stemness of colon cancer cells.36 Intriguingly, Javier and colleagues demonstrated that SOX9 truncating mutations — the predominant variant observed in the CTR cohort in the present study (Figure 3)— result in an overexpressed Sox9 protein, and was associated with improved overall survival in a cohort of patients with metastatic CRC.37 While hypothesis-generating only, the present data may justify further investigation into the role of SOX9 alterations in chemotherapy responsiveness in CRC.
Our study has several limitations. First, given the retrospective design and inclusion of IU-CRLM patients on and off trial protocols at our institution, we cannot eliminate biases in patients selected for resection following HAI. A related confounder potentially impacting the findings here is the fact that conversion to resection if often a subjective decision, often not a clear-cut binary outcome, and not always a barometer of chemotherapy effectiveness. Second, given our design of including only patients who were selected for genomic sequencing a priori, it is possible that these findings reflect an unintended enrichment of tumors with higher-risk biology—as evidenced by a relatively high proportion of patients with extrahepatic disease in the study cohort (38%)—prompting sequencing. Third, it remains to be seen if the genomic correlates described here are validated in an independent cohort of patients treated with HAI and systemic chemotherapy, or reproduced in IU-CRLM patients receiving systemic chemotherapy alone. Fourth, while this study addresses only genomic-level molecular data associated with CTR and OS, future studies should investigate the transcriptomic, epigenomic, and immunomic underpinnings of these clinical endpoints in IU-CRLM. Finally, it bears mentioning that the clinical outcomes (e.g., median 5-year OS 50.9%) reported in this cohort of IU-CRLM patients undoubtedly reflect the contributions of biologic and physiologic selection in addition to effects of HAI/systemic chemotherapy and ensuing CTR.
Notwithstanding, our report describes novel and prognostic genomic correlates of CTR and overall survival in IU-CRLM. Not only do these findings illuminate avenues for translational investigation in the future, but also have ready implications for patient selection and shared clinical decision-making for liver-directed therapies in this high-risk subset of patients. Finally, such molecular stratification should be incorporated into future trial design to allow more precise selection of IU-CRLM patients for elements of multimodality therapy, with the goal of improving contemporary survival outcomes.
Supplementary Material
Funding:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number P30 CA240139, a KL2 career development grant by the Miami Clinical and Translational Science Institute (CTSI) under NIH award number UL1TR002736, the American College of Surgeons’ Franklin H. Martin Fellowship, and the Stanley Glaser Foundation (all to J. Datta).
REFERENCES
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017; 67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007; 25(29):4575–80. [DOI] [PubMed] [Google Scholar]
- 3.Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 2009; 27(11):1829–35. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18(16):2938–47. [DOI] [PubMed] [Google Scholar]
- 5.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000; 343(13):905–14. [DOI] [PubMed] [Google Scholar]
- 6.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22(2):229–37. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351(4):337–45. [DOI] [PubMed] [Google Scholar]
- 8.Alberts SR, Horvath WL, Sternfeld WC, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. J Clin Oncol 2005; 23(36):9243–9. [DOI] [PubMed] [Google Scholar]
- 9.Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg 2009; 249(3):420–5. [DOI] [PubMed] [Google Scholar]
- 10.Lam VW, Spiro C, Laurence JM, et al. A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol 2012; 19(4):1292–301. [DOI] [PubMed] [Google Scholar]
- 11.Mokdad A, Choti MA, Yopp AC. Conversion Chemotherapy for Unresectable Colorectal Liver Metastases: Are We Making a Difference? Current Colorectal Cancer Reports 2015; 11(4):160–167. [Google Scholar]
- 12.Peeters M, Price TJ, Cervantes A, et al. Final results from a randomized phase 3 study of FOLFIRI {+/−} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol 2014; 25(1):107–16. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg ML, Eckardt JR, Kuhn JG, et al. Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J Clin Oncol 1996; 14(4):1128–35. [DOI] [PubMed] [Google Scholar]
- 14.Ammori JB, Kemeny NE, Fong Y, et al. Conversion to complete resection and/or ablation using hepatic artery infusional chemotherapy in patients with unresectable liver metastases from colorectal cancer: a decade of experience at a single institution. Ann Surg Oncol 2013; 20(9):2901–7. [DOI] [PubMed] [Google Scholar]
- 15.D'Angelica MI, Correa-Gallego C, Paty PB, et al. Phase II trial of hepatic artery infusional and systemic chemotherapy for patients with unresectable hepatic metastases from colorectal cancer: conversion to resection and long-term outcomes. Ann Surg 2015; 261(2):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009; 27(21):3465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pak LM, Kemeny NE, Capanu M, et al. Prospective phase II trial of combination hepatic artery infusion and systemic chemotherapy for unresectable colorectal liver metastases: Long term results and curative potential. J Surg Oncol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ensminger WD, Rosowsky A, Raso V, et al. A clinical-pharmacological evaluation of hepatic arterial infusions of 5-fluoro-2'-deoxyuridine and 5-fluorouracil. Cancer Res 1978; 38(11 Pt 1):3784–92. [PubMed] [Google Scholar]
- 19.Karanicolas PJ, Ko YJ. Hepatic Arterial Infusion for Unresectable Liver Metastases from Colorectal Cancer: The Dawn of a New Era? Ann Surg Oncol 2017; 24(1):6–7. [DOI] [PubMed] [Google Scholar]
- 20.Niu B, Ye K, Zhang Q, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics 2014; 30(7):1015–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230(3):309–18; discussion 318-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015; 17(3):251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018; 33(1):125–136 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol 2017; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Vega F, Mina M, Armenia J, et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018; 173(2):321–337 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun YS, Passot G, Yamashita S, et al. Deleterious Effect of RAS and Evolutionary High-risk TP53 Double Mutation in Colorectal Liver Metastases. Ann Surg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi Y, Kopetz S, Newhook TE, et al. Mutation Status of RAS, TP53, and SMAD4 is Superior to Mutation Status of RAS Alone for Predicting Prognosis after Resection of Colorectal Liver Metastases. Clin Cancer Res 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang H, Baumgart J, Heinrich S, et al. Extended Molecular Profiling Improves Stratification and Prediction of Survival After Resection of Colorectal Liver Metastases. Ann Surg 2019; 270(5):799–805. [DOI] [PubMed] [Google Scholar]
- 29.Datta J, Smith JJ, Chatila WK, et al. Coaltered Ras/B-raf and TP53 Is Associated with Extremes of Survivorship and Distinct Patterns of Metastasis in Patients with Metastatic Colorectal Cancer. Clin Cancer Res 2020; 26(5):1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Z, Torres NM, Tao A, et al. BRAF Mutants Evade ERK-Dependent Feedback by Different Mechanisms that Determine Their Sensitivity to Pharmacologic Inhibition. Cancer Cell 2015; 28(3):370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman JH, Meulman JJ. Multiple additive regression trees with application in epidemiology. Stat Med 2003; 22(9):1365–81. [DOI] [PubMed] [Google Scholar]
- 32.D'Angelica M, Fong Y, DeMatteo RP, et al. Hepatic arterial infusion chemotherapy for metastases from colorectal cancer: is it really the end of an era? J Clin Oncol 2008; 26(16):2788–9; author reply 2789-90. [DOI] [PubMed] [Google Scholar]
- 33.Morton JP, Timpson P, Karim SA, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A 2010; 107(1):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng S, El-Naggar AK, Kim ES, et al. A genetic mouse model for metastatic lung cancer with gender differences in survival. Oncogene 2007; 26(48):6896–904. [DOI] [PubMed] [Google Scholar]
- 35.Mori-Akiyama Y, van den Born M, van Es JH, et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology 2007; 133(2):539–46. [DOI] [PubMed] [Google Scholar]
- 36.Roche KC, Gracz AD, Liu XF, et al. SOX9 maintains reserve stem cells and preserves radioresistance in mouse small intestine. Gastroenterology 2015; 149(6):1553–1563 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javier BM, Yaeger R, Wang L, et al. Recurrent, truncating SOX9 mutations are associated with SOX9 overexpression, KRAS mutation, and TP53 wild type status in colorectal carcinoma. Oncotarget 2016; 7(32):50875–50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.