Abstract
Aims
Weight management seems to be beneficial for obese atrial fibrillation (AF) patients; however, randomized data are sparse. Thus, this study aimed to investigate the influence of weight reduction on AF ablation outcomes.
Methods and results
SORT-AF is an investigator-sponsored, prospective, randomized, multicentre, and clinical trial. Patients with symptomatic AF (paroxysmal or persistent) and body mass index (BMI) 30–40 kg/m2 underwent AF ablation and were randomized to either weight-reduction (group 1) or usual care (group 2), after sleep–apnoea–screening and loop recorder (ILR) implantation. The primary endpoint was defined as AF burden between 3 and 12 months after AF ablation. Overall, 133 patients (60 ± 10 years, 57% persistent AF) were randomized to group 1 (n = 67) and group 2 (n = 66), respectively. Complications after AF-ablation were rare (one stroke and no tamponade). The intervention led to a significant reduction of BMI (34.9 ± 2.6–33.4 ± 3.6) in group 1 compared to a stable BMI in group 2 (P < 0.001). Atrial fibrillation burden after ablation decreased significantly (P < 0.001), with no significant difference regarding the primary endpoint between the groups (P = 0.815, odds ratio: 1.143, confidence interval: 0.369–3.613). Further analyses showed a significant correlation between BMI and AF recurrence for patients with persistent AF compared with paroxysmal AF patients (P = 0.032).
Conclusion
The SORT-AF study shows that AF ablation is safe and successful in obese patients using continuous monitoring via ILR. Although the primary endpoint of AF burden after ablation did not differ between the two groups, the effects of weight loss and improvement of exercise activity were beneficial for obese patients with persistent AF demonstrating the relevance of life-style management as an important adjunct to AF ablation in this setting.
Trial registration number
Keywords: Atrial fibrillation, Obesity, Catheter ablation, Weight reduction, Atrial fibrillation burden, Implantable loop recorder
What’s new?
The SORT-AF study is the first randomized, multicentre clinical trial to evaluate the effect of weight loss with a structured weight reduction programme on the success rate of atrial fibrillation (AF) ablation in obese patients.
It shows that AF ablation is safe and successful in obese patients using continuous rhythm monitoring via implantable loop recorder (one stroke and no tamponade).
The primary endpoint of AF burden did not differ between the two groups, but the effects of weight loss and improvement of exercise activity were beneficial for obese patients with persistent AF.
SORT-AF underscores the relevance of life-style management as an important adjunct to AF ablation.
Introduction
Atrial fibrillation (AF) is the most frequent cardiac arrhythmia in adults. Prevalence of AF tripled over the last 30 years and further progress is expected.1 Atrial fibrillation is associated with increased mortality and morbidity. Catheter ablation therapy has been proven safe and effective for the treatment of AF and is now a standard therapy.2
Obesity is a widespread disease with increasing incidence over the last years in all industrial nations. Being overweight negatively influences cardiovascular risk and leads to progression of arterial hypertension, diabetes mellitus, and coronary artery disease. Therefore, treatment of obesity plays an important role in the prevention of these diseases.3
Retrospective and observational studies found a similar relationship in atrial fibrillation (AF) in general4–6 and particularly regarding the success rate after AF ablation with a higher recurrence rate of AF in patients with obesity or sleep apnoea.7–9 However, randomized data are sparse. This prospective randomized study aimed to evaluate the role of weight reduction on AF ablation outcomes in obese patients.
Methods
Study design and population
The ‘SORT-AF’—Supervised Obesity Reduction Trial for AF Ablation Patients—study (ClinicalTrials.gov, NCT02064114) is an investigator-sponsored, prospective, two-arm, randomized, open label, active controlled, parallel group and multicentre, clinical trial that was endorsed by DZHK (German Centre for Cardiovascular Research) and supported by Abbott Medical GmbH via a research grant for investigator-sponsored trials. It was conducted at three clinical centres in Germany (see Supplementary material online for list of hospitals S1). The trial complies with the Declaration of Helsinki and was approved by the ethics committees at each participating centre. The authors attest to the accuracy of the data and of all analyses and to the fidelity of this report to the trial protocol, which is available on request as well as all data that support study findings. The funding partner had no influence on the content of the manuscript.
Study participants
To be enrolled in the SORT-AF study, patients had to have symptomatic AF (paroxysmal or persistent AF) with indication for AF ablation and a body mass index (BMI) between 30 and 40. Main exclusion criteria were previous ablation therapy, longstanding persistent atrial fibrillation (continuous episodes >12 months), contraindication for anticoagulation, manifest hyperthyroidism or hypothyroidism, and diseases or conditions prohibiting physical activity. Detailed inclusion and exclusion criteria are listed in the Supplementary material online (see Supplementary material online for inclusion/exclusion criteria S2). Definitions for paroxysmal and persistent AF were according to the guidelines.10,11
Enrolment and risk factor management
All patients provided written informed consent. After enrolment, baseline assessments of the patients were performed, including collection of demographic and medical information, transthoracic echocardiography, electrocardiogram (ECG), ergometry, and blood tests. For diagnosing sleep apnoea, all patients received a polygraphy and filled out the Epworth Sleepiness Scale (ESS) to detect daytime sleepiness. If there was evidence for sleep disordered breathing, patients were transferred to a specialist in sleep medicine for further diagnostics and therapy as recommended by current guidelines.12 Additionally, high blood pressure and newly diagnosed diabetes were treated before AF ablation according to the current guidelines.13,14
Implantable loop recorder and catheter ablation
At a maximum of 4 weeks prior to AF ablation, an implantable loop recorder (ILR, Confirm by Abbott) was implanted. The ILR was programmed to detect all AF episodes with a duration of ≥30 s.
Atrial fibrillation ablation was performed in the experienced trial centres. It was recommended to perform radiofrequency (RF) ablation in this study. Transoesophageal echocardiography was performed within 24 h before the procedure to rule out the presence of left atrial thrombi. The ablation technique used has been described previously15 and included wide-encircling pulmonary vein ablation with the endpoint of complete electrical isolation of the pulmonary veins (PVI) and unexcitability of the ablation lines in all patients. Further substrate modification was up to the decision of the treating electrophysiologist. This included linear ablation (roofline and/or mitral isthmus) with an endpoint of bidirectional block and/or electrogram guided ablation of fractionated sites. Repeat ablation was offered, if patients developed recurrent symptomatic arrhythmia after the blanking period of 3 months (± 2 weeks). During repeat ablation, re-isolation of the pulmonary veins was attained and further ablation strategies were up to the decision of the treating electrophysiologist. It was recommended, that antiarrhythmic drug (AAD) therapy (except beta-blockers) should be discontinued at the end of blanking period. In case of symptomatic arrhythmia after blanking period, usage of AAD therapy was up to the decision of the treating physician.
Randomization and follow-up
On the day of AF ablation, patients were randomized 1:1 to either intervention for weight reduction (group 1) or usual care (group 2). In addition to usual care, patients in group 1 were enrolled in a structured weight reduction programme with medical attendance twice a month, regular nutrition advice as well as assistance for physical training for the duration of 6 months, as recommended by current guidelines.16 This individual concept for weight reduction took place in a specialized obesity department as an interdisciplinary multimodal concept under the surveillance of a physician specialized for endocrinology. A nutrition log which each patient kept for 2 months monitored patient’s adherence to energy reduction (see Supplementary material online for details on weight reduction programme S3). Compliance to weight reduction programme was noticed. Non-compliance was defined as participation was cancelled or not started.
Regular follow-up for both groups was at 3, 6, and 12 months after AF ablation. The visits consisted of a physical examination, ECG, and ILR interrogation, which was performed at each hospitals’ device department, and not by the physicians performing the ablation procedure. A specialized and well-trained investigator of the study site reviewed each detected AF episode. Atrial fibrillation burden was calculated manually accordingly. The AF burden automatically generated by the device was not used and therefore the presented AF burden was adequately precise. Additionally, a transthoracic echocardiography, ergometry, and blood tests were assessed at 6 and 12 months. The regular follow-up at 3, 6, and 12 months was performed by the cardiologist. Although patient and investigator were not blinded to treatment arms, the cardiologist treated each patient’s arrhythmia symptoms according to the current guidelines. Re-ablation or AAD therapy was only recommended in case of symptomatic AF recurrence during the study, but there was no specific documentation regarding symptoms for each detected episode during the study. The cardiologist was not actively involved in the weight loss programme of the study patients.
Endpoints
The primary endpoint of the study was defined as AF burden between 3 and 12 months after first AF ablation. Atrial fibrillation burden was defined as overall percentage of AF (≥30 s) during the observed period detected by ILR.
Secondary endpoints were (i) AF burden between 0 and 12 months after first AF ablation; (ii) AF burden between 0–3, 3–6, and 6–12 months after first AF ablation; (iii) freedom of AF (after 3 months blanking period); (iv) time to first recurrent AF (after 3 months blanking period); (v) BMI change from baseline to 12 months; (vi) exercise capacity change from baseline to 12 months, evaluated by metabolic equivalent (MET); (vii) mean blood pressure change from baseline to 12 months; (viii) numbers of repeated AF ablation procedures; and (xi) AAD therapy at 12 months. Further data of interest were complications of AF ablation, procedural data, and all serious adverse events occurring during the study.
Additionally, ancillary analyses for the entire study cohort were performed without considering the randomized study design, with the aim to further evaluate the influence of weight loss on AF recurrence after AF ablation.
Statistical analysis
We hypothesized a difference of 3.15% (12.60 ± 5.50 vs. 9.45 ± 7.00) with regard to AF burden between the two groups with a power of 80% and a significance level of α = 0.05. To detect this difference, 64 patients should have been included in each study arm. Allowing for a non-adherence or dropout rate of 10%, 140 patients should have been included in the trial.
Baseline data (demographic and echo data, co-morbidities, type of arrhythmia, and medication data), sleep apnoea data (Epworth Sleepiness Scale, apnoea–hypopnoe index, and continuous positive airway pressure therapy [CPAP] therapy) as well as procedural data, complications, and follow-up data including ILR interrogation of N = 133 patients were collected.
The data are summarized by treatment groups. Continuous variables are described by the lower quartile, the median, and the upper quartile as well as the mean and standard deviation. Categorical data are shown with proportions and frequencies.
Procedural data were compared between the treatment arms. Pearson tests and Fisher’s exact tests were used for categorical data. Wilcoxon tests for continuous variables. Linear mixed models were applied to compare BMI and MET between treatment groups across time. Mean values [and confidence intervals (CIs)] were plotted at each follow-up and for each group.
The AF burden was analysed between treatment groups using a quasibinomial model. As <50% of the patients exhibit a burden >0, the 90th, 80th, and 70th percentiles across time and group are presented graphically.
Atrial fibrillation freedom after a single and after multiple procedures stratified by treatment groups are presented with Kaplan–Meier curves.
A Cox proportional hazards model was used to related treatment group, gender, age, CHA2DS2-VASc score, BMI, and AF type to AF free survival. Interactions between group and BMI as well as AF type and BMI were incorporated in the model. Body mass index was introduced as a time-dependent covariate. Atrial fibrillation recurrences within the blanking period of 12 ± 2 weeks after index procedure were not considered. Effects on AF recurrences are shown with hazard ratios (HRs; 95% CIs) and P-values. Survival estimates for different BMI courses are shown as Kaplan–Meier plots.
All analyses were applied for the intention to treat population. The P-values were two-sided. A P-value <0.05 was considered significant. All calculations were performed with the statistical analysis software R (R Foundation for Statistical Computing, 2020, Vienna, Austria) (see Supplementary material online for statistical literature S4).
Results
Patient characteristics and procedural data
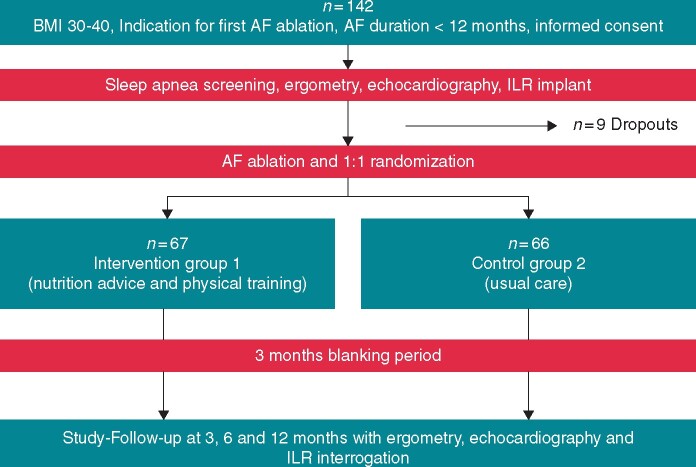
Patient enrolment started on 31 January 2014 and was completed on 16 January 2020. In total, 142 patients were included in the study (Figure 1). There was a dropout of nine patients prior to randomization, which leads to an intention to treat population of 133 patients (see Supplementary material online for details in dropouts S5). Of 133 patients randomized (mean age 60 ± 10 years, 38% women, and 57% persistent AF), 67 were assigned to group 1 for weight reduction program, and 66 to group 2 (usual care without weight reduction program).
Figure 1.
Flowchart of the trial.
Baseline characteristics and baseline measurements were balanced in both groups (Table 1), with the exception of AF type, with 67% of patients having persistent AF in group 1 compared with 48% in group 2 [odds ratio (OR) 2.173, CI 1.084–4.433].
Table 1.
Baseline data (n = 133)
| N | Intervention (N = 67) | Control (N = 66) | |
|---|---|---|---|
| Age (years) | 133 | 58.7 ± 11.6 | 62.1 ± 9.1 |
| Sex (male) | 133 | 0.64 (43) | 0.62 (41) |
| Measures | |||
| Weight, kg | 133 | 111 ± 18 | 110 ± 17 |
| BMI, kg/m2 | 133 | 34.9 ± 2.6 | 34.8 ± 3.0 |
| MET | 123 | 5.5 ± 0.9 | 5.3 ± 1.1 |
| HbA1c, % | 113 | 5.65 (5.30–6.00) | 5.60 (5.40–6.10) |
| Creatinine, mg/dL | 133 | 0.99 (0.88–1.10) | 1.00 (0.82–1.18) |
| Echocardiographic measures | |||
| LVEF, % | 133 | 57.0 (53.0–60.0) | 60.0 (55.0–60.0) |
| LA vol biplane, mL | 80 | 75 (64–93) | 67 (56–78) |
| LV septum, mm | 116 | 11.3 (10.0–12.0) | 11.0 (10.0–12.1) |
| Co-morbidities | |||
| Hypertension | 133 | 0.81 (54) | 0.88 (58) |
| Hyperlipidaemia | 133 | 0.19 (13) | 0.26 (17) |
| Coronary artery disease | 133 | 0.15 (10) | 0.21 (14) |
| Previous stroke | 133 | 0.07 (5) | 0.06 (4) |
| Diabetes mellitus | 133 | 0.13 (9) | 0.21 (14) |
| Nicotine | |||
| Non-smoking | 130 | 0.41 (27) | 0.44 (28) |
| Former smoker | 0.44 (29) | 0.42 (27) | |
| Active smoker | 0.15 (10) | 0.14 (9) | |
| Type of AFa | |||
| Paroxysmal | 133 | 0.33 (22) | 0.52 (34) |
| Persistent | 0.67 (45) | 0.48 (32) | |
| History of AF (months) | 121 | 21 (7– 48) | 24 (10–48) |
| Number of previous electro cardioversions | 133 | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) |
| CHA2DS2-VASc score | 133 | 2.0 (1.0– 3.0) | 2.0 (1.0–3.0) |
| EHRA score | 127 | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) |
| Medication use | |||
| No oral anticoagulation | 133 | 0.12 (8) | 0.05 (3) |
| VKA | 0.16 (11) | 0.23 (15) | |
| NOAC | 0.72 (48) | 0.73 (48) | |
| No antiarrhythmic therapy | 133 | 0.52 (35) | 0.61 (40) |
| Amiodarone | 0.27 (18) | 0.24 (16) | |
| Flecainide | 0.18 (12) | 0.11 (7) | |
| Other (dronedarone, sotalol) | 0.02 (2) | 0.05 (3) | |
| Beta blockers | 133 | 0.78 (52) | 0.76 (49) |
b (a−c) represent the median b with interquartile range [IQR (lower quartile a and the upper quartile c)] for continuous variables. x ± s represents X ± 1 SD. N is the number of non-missing values. Numbers after proportions are frequencies.
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; HbA1c, glycated haemoglobin; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MET, metabolic equivalent; NOAC, novel oral anticoagulant; OR, odds ratio; VKA, vitamin K antagonist.
The difference between the treatment groups was significant (OR 0.4601, CI: 0.2256–0.9226) (see Supplementary material online S6 for abbreviations).
Moderate to severe sleep apnoea was diagnosed by polygraphy in 32% of patients in group 1 (severe sleep apnoea in 16%) and in 41% in group 2 (severe sleep apnoea in 12%) (P = 0.442), though ∼37% of patients in both groups had an indication for CPAP therapy after further polysomnography, according to current guidelines.9 Compliance for CPAP therapy was 62% in group 1 and 71% in group 2 (P = 0.465) (Table 2).
Table 2.
Sleep apnoea data (n = 133)
| N | Intervention (N = 67) | Control (N = 66) | |
|---|---|---|---|
| ESS (score) | 96 | 6.0 (3.0–10.2) | 5.0 (3.0–8.0) |
| ESS (grouped) | |||
| No symptoms (ESS <5) | 96 | 0.39 (22) | 0.48 (19) |
| Symptoms (ESS 5–15) | 0.59 (33) | 0.48 (19) | |
| Severe symptoms (ESS >15) | 0.02 (1) | 0.05 (2) | |
| Apnoea–hypopnoe index | 96 | 7.0 (3.0–23.1) | 10.0 (4.9–21.1) |
| Apnoea–hypopnoe index (grouped) | |||
| No sleep apnea (AHI ≤5) | 96 | 0.42 (23) | 0.27 (11) |
| Mild sleep apnea (AHI 6–15) | 0.25 (14) | 0.32 (13) | |
| Moderate sleep apnea (AHI 16–30) | 0.16 (9) | 0.29 (12) | |
| Severe sleep apnea (AHI >30) | 0.16 (9) | 0.12 (5) | |
| Oxygen desaturation index | 94 | 8.9 (4.7–22.0) | 11.8 (6.5–22.6) |
| CPAP therapy recommended | 121 | 0.36 (22) | 0.37 (22) |
| Non-compliance | 42 | 0.38 (8) | 0.24 (5) |
| Daily use | 42 | 0.62 (13) | 0.71 (15) |
b (a−c) represent the median b with interquartile range [IQR (lower quartile a and the upper quartile c)] for continuous variables. N is the number of non-missing values. Numbers after proportions are frequencies.
AHI, apnoea–hypopnoe index; CPAP, continuous positive airway pressure; ESS, Epworth Sleepiness Scale.
Procedural data of AF ablation were comparable (Table 3). The majority of patients received a PVI-only procedure (75% group 1 and 76% group 2), whereas additional left atrial ablation beyond PVI was performed in 25% and 24% (P = 0.88). Overall, RF ablation was performed in 94% and Cryo-PVI in 6%.
Table 3.
Procedural data and complications (n = 133)
| N | Intervention (N = 67) | Control (N = 66) | P valuea | |
|---|---|---|---|---|
| Ablation: PVI + additional lines | 133 | 0.25 (17) | 0.24 (16) | 0.88a |
| PVI only | 133 | 0.75 (50) | 0.76 (50) | |
| Procedure duration, min | 133 | 130 (110–152) | 120 (108–150) | 0.42b |
| Fluoroscopy time, min | 133 | 13.6 (9.1–18.7) | 10.2 (8.1–16.6) | 0.055b |
| Fluoroscopy dose, cGycm2 | 131 | 915 (503–3083) | 880 (489–3153) | 1b |
| Complications: no | 133 | 0.94 (63) | 0.92 (61) | 0.47a |
| Major complications | 0.01 (1) | 0.00 0 | ||
| Minor complications | 0.04 (3) | 0.08 (5) |
b (a−c) represent the median b with interquartile range [IQR (lower quartile a and the upper quartile c)] for continuous variables. x ± s represents X ± 1 SD. N is the number of non-missing values. Numbers after proportions are frequencies.
PVI, pulmonary veins.
Tests used: Pearson test/Fisher’s exact test.
Tests used: Wilcoxon test.
Complications related to AF ablation were rare, with no statistical significance between the groups (P = 0.47). There was one major complication (stroke) and no pericardial tamponade. Minor complications were vascular complications (groin access site) with the need of intervention in five patients, one phrenic nerve palsy after Cryo-PVI, and two aspirations (one asymptomatic; one with mild pneumonia without the necessity of intensive care treatment).
Outcomes
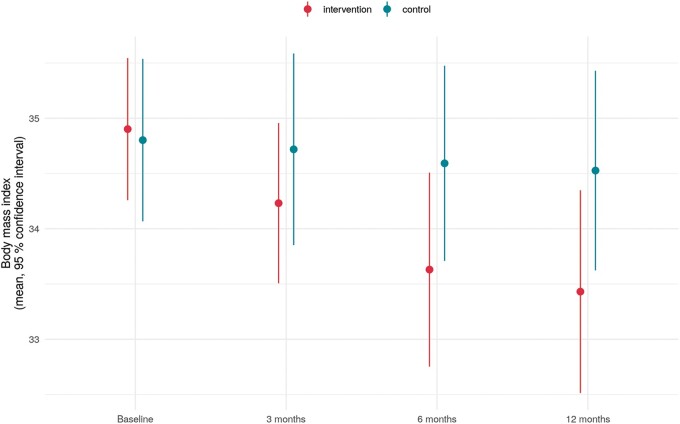
Regarding the change in BMI from baseline to 12 months, a significant reduction of BMI from 34.9 ± 2.6 to 33.4 ± 3.6 was observed in group 1 compared to a stable BMI of 34.8 ± 3.0 to 34.5 ± 3.6 in group 2 (P < 0.001 between groups; Figure 2). Similar were the results for change in kg. A reduction was observed for group 1 from 111 ± 18 kg to 106 ± 16 kg but not for group 2 (110 ± 17 to 109 ± 18), with a significant difference between the groups (P < 0.001).
Figure 2.
Body mass index by treatment group (intervention group 1 and control group 2) and follow-up: from baseline (BL) to 12 months (12 M), showing a significant difference between the groups (P < 0.001).
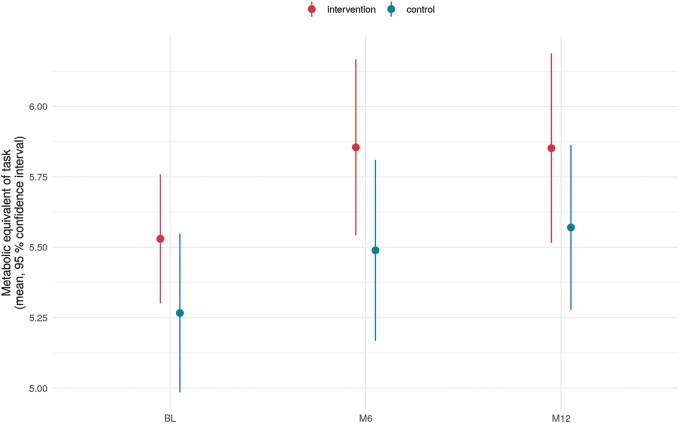
As shown in Figure 3, there was no significant difference between the groups regarding exercise capacity (MET) (P = 0.822). Both groups improved slightly over time.
Figure 3.
Exercise capacity (MET) by treatment group (intervention group 1 and control group 2) and follow-up: from baseline (BL) to 12 months (12 M), showing no significant difference between the groups (P = 0.822). MET, metabolic equivalent.
With regard to group 1, non-compliance to intervention (structured weight reduction programme) was 19% at 3 months, 21% at 6 months, and 33% at 12 months of follow-up.
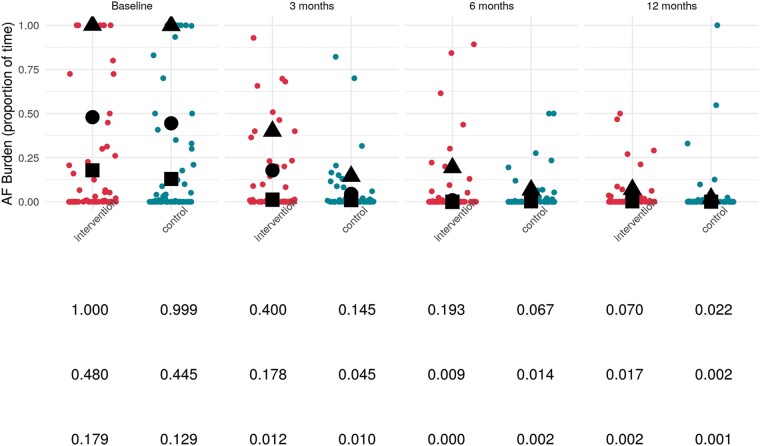
The mean AF burden was reduced in both groups after AF ablation, from 21.55 ± 36.03% (group 1) and 22.4 ± 36.78% (group 2) before ablation to 3.70 ± 12.54% (group 1) vs. 4.21 ± 11.28% (group 2) 3–12 months after ablation (P < 0.001). Figure 4 shows the 90th, 80th, and 70th percentile of AF burden from baseline to 12-month follow-up. The primary endpoint, AF burden between 3 and 12 months after the index AF ablation did not differ among the two groups (P = 0.815, OR: 1.143, CI: 0.369–3.613). The between group difference of primary endpoint AF Burden 3–12 months, as difference in means (95% CI) was 0.00507% (−0.0373, 0.0475).
Figure 4.
Burden of atrial fibrillation: triangles, circles, and squares represent 90th, 80th, and 70th percentile.
Likewise, there was no difference between the groups regarding the secondary endpoints (i) AF burden between 0 and 12 months (P = 0.815) and (ii) AF burden between 0–3, 3–6, and 6–12 months (P = 0.118, 0.263, and 0.993).
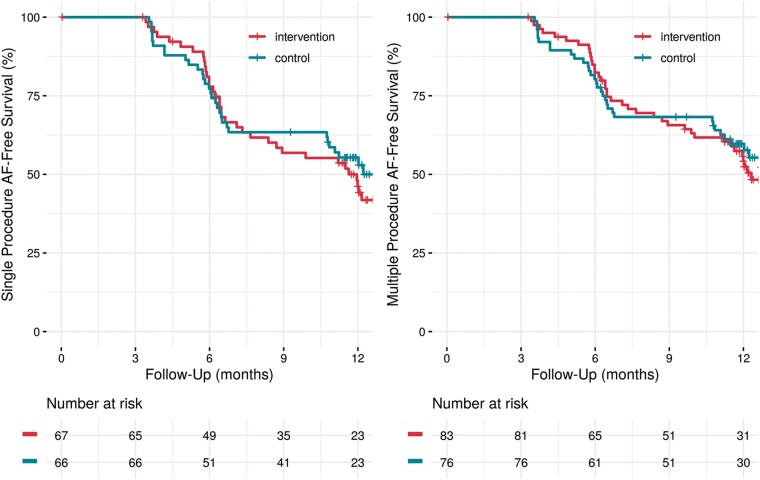
Among the secondary endpoints (iii) freedom of AF (after 3 months blanking period) and (iv) time to first recurrent AF (after 3 months blanking period), there was also no significant difference between the treatment groups (P = 0.5777) (Figure 5). Re-ablation was performed in 20% (group 1) and 13% (group 2) during the observational period. Figure 5 shows multiple procedure recurrence free survival with no significant difference between the groups (P = 0.7027). Usage of AAD therapy was the same in both groups with 16% at 6-month follow-up and 11% at the end of study.
Figure 5.
Recurrence free survival showing no significant difference between the groups (recurrence = AF episodes ≥30 s in ILR): single procedure (P = 0.5777); multiple procedure (P = 0.7027). AF, atrial fibrillation; ILR, implantable loop recorder.
Regarding the secondary endpoint mean blood pressure change from baseline to 12 months, there was no significant difference between the groups (P = 0.685). Considering the co-morbidities diabetes mellitus and renal failure, there was no difference over time between the groups for glycated haemoglobin (HbA1c) (P = 0.111) and Creatinine (P = 0.920).
Ancillary analyses
Regarding different types of AF (paroxysmal and persistent), ancillary analyses for the entire study cohort showed a significant correlation between BMI and AF recurrence for patients with persistent AF compared to patients with paroxysmal AF, which indicates that BMI reduction was associated with a significant decrease in AF recurrence in persistent AF patients compared to paroxysmal AF patients (OR 1.154, CI 1.028–1.297; P = 0.032).
Additionally, males had a 1.9-fold increased risk for AF recurrence compared to females (HR 1.888, CI 1.145–3.115), and a 2% increased risk for recurrence per year of advancing age was noted [HR 1.018, CI 0.996–1.039). Patients with persistent AF and an increase in exercise capacity had a 23% reduced risk for AF recurrence compared to patients with paroxysmal AF (HR 0.771, CI 0.640–0.928).
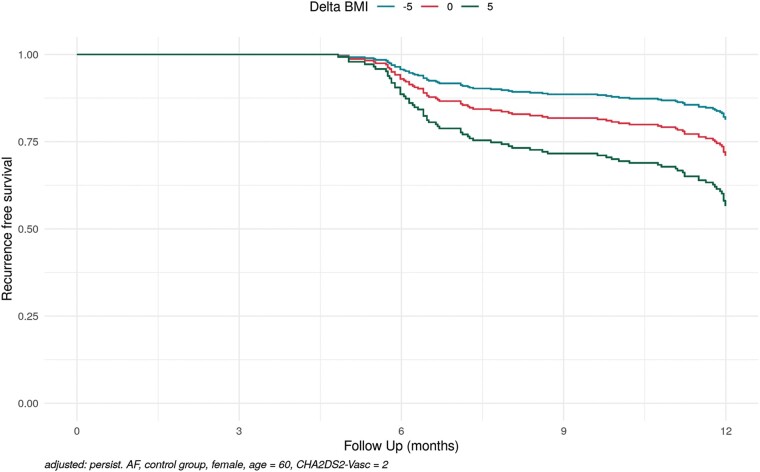
These ancillary results were used for a model-based estimate to visualize these findings for a persistent AF patient with three possible scenarios of BMI change after AF ablation (Delta BMI of −5, 0, and +5) showing the positive effect of BMI reduction on AF free survival after AF ablation in persistent AF patients (Figure 6).
Figure 6.
Model-based estimates of BMI change on recurrence free survival in persistent AF (adjusted: persistend AF, control group, female, age = 60, CHA2DS2-VASc score = 2). AF, atrial fibrillation; BMI, body mass index.
Discussion
The ‘SORT-AF’—Supervised Obesity Reduction Trial for AF Ablation Patients—study is the first randomized clinical trial to evaluate the effect of weight loss with a structured weight reduction programme on the success rate of AF ablation in obese patients. Detection of AF was based on ILR, which allowed a continuous rhythm monitoring with assessment of an overall AF burden. Regarding the primary endpoint, there was no difference observed with regard to the overall AF burden between the two groups after ablation. In subsequent ancillary analyses, we showed that weight loss and improvement in exercise activity is beneficial for obese persistent AF patients with respect to freedom from recurrence. Furthermore, the SORT-AF study proved AF ablation to be safe and successful in obese patients.
Previous observational studies including the landmark LEGACY study, report that sustained weight loss is associated with a significant reduction in AF burden measured by annual 7-day Holter monitoring.5 The CARDIO-FIT study observed the same effect on AF for improvement of exercise capacity.6 Atrial fibrillation therapy varied in both studies and compromised conservative and invasive strategies, such as AAD use, AF ablation, and others.5
In the context of AF ablation, Providencia et al.7 detected BMI as an independent predictor for relapse, especially in patients with BMI >35. The study was observational, follow-up for AF monitoring was performed via 12-lead ECG and 24-h Holter monitoring at 3, 6, and 12 months after the procedure and the study was performed without any weight loss interventions. In the ARREST-AF study, patients undergoing AF ablation were offered a structured programme for risk factor management (RFM) and were divided into an intervention (RFM) and a control group (no RFM), depending on the patient’s decision and motivation. Follow-up was performed every 3–6 months by clinic review and 7-day Holter monitoring showing that aggressive RFM improved the long-term success of AF ablation.8 Donnellan et al.9 showed in a retrospective observational cohort study that patients with prior bariatric surgery had significant lower AF recurrence rates after AF ablation compared to patients without prior bariatric surgery.
Now, SORT-AF provides additional data in conjunction with some limitations of prior published data regarding this topic. Atrial fibrillation ablation was the cornerstone of AF therapy and mandatory for all patients, AF detection was done continuously by ILR, and furthermore, SORT-AF was primarily investigating the effect of a structured weight loss programme in obese patients undergoing AF ablation in a randomized trial.
Patients in group 1 achieved a mean weight reduction of 4.6 ± 8.6 kg (3.91% of their initial body weight), which is a reasonable result compared to others studies. For example, a meta-analysis of 18 randomized trials showed an average 3.34 kg loss of body weight after a combination of dietary changes and increased physical activity after 2 years,17 and Slentz et al.18 reported 4.8 kg weight reduction in the intervention group of a randomized trial evaluating the impact of exercise on body weight. However, the success of weight loss might have been too little to show an effect on AF burden. Considering the LEGACY study, results showed the greatest effect on freedom from AF in patients who lost ≥10% of their body weight.5 We believe this to be a major factor why the intention-to-treat analysis was negative. Nevertheless, it demonstrates a real-life scenario because all eligible patients were included, regardless of their motivation for weight loss. We observed 33% non-compliance to weight reduction programme after 12 months in the intervention group, although all patients were offered a structured weight reduction programme performed in a specialized obesity department, under the surveillance of a physician specialized for endocrinology. It included medical attendance twice a month, regular nutrition advice (plus a nutrition diary), cognitive behaviour therapy as well as assistance for physical training for the duration of 6 months. Weight reduction is heavily dependent on patients’ motivation and a challenging task even in a randomized trial with close patient monitoring. These findings underline the recommendations, that all treating physicians, including the cardiologist need to address these issues when counselling patients and that a network of all treating physicians, nurses, and nutritionists are needed to treat patients’ obesity.16
Ancillary analyses for the entire study cohort showed that BMI reduction was associated with a significant decrease in AF recurrence particularly in persistent AF patients compared to paroxysmal AF patients. Similar results were seen for exercise capacity. Patients with persistent AF and an increase in exercise capacity had a 23% reduced risk for AF recurrence compared to patients with paroxysmal AF. These observations are in line with the results of the CARDIO-FIT study,6 but could not be confirmed in the randomized setting, as both groups improved slightly over the time without a significant difference.
A reason for the positive effect of weight reduction particularly in persistent AF patients might be the observed association of obesity with a more advanced atrial substrate.19,20,21 Recently, Mahajan et al.22 reported weight reduction to be associated with structural and electrophysiological reverse remodelling and a reduced propensity for AF in an obese ovine model. Our findings strengthen the hypothesis of a similar mechanism in humans and underline the importance of weight management in patients with AF.
In line with the results of Donnellan et al.,23 the SORT-AF study demonstrates that AF ablation is safe in obese patients up to a BMI of 40, especially reflected by the fact that no tamponades were observed. All procedural data were comparable to a normal weight population as for example reported in the ‘FIRE AND ICE’ trial including procedure duration and fluoroscopy time.24 Before transferring these results to a general recommendation, it has to be taken into account that all procedures were performed in experienced centres. Additionally, all patients were carefully examined prior to ablation with echocardiography, ergometry, blood test, and sleep apnoea screening via polygraphy. All risk factors were treated before AF ablation and in case of severe sleep apnoea patients were instructed to use CPAP therapy and received invasive ventilation during the ablation if necessary.
Another important finding of the SORT-AF study is that AF ablation is effective in obese patients. Even though most patients had multiple risk factors for AF (e.g. obesity, sleep apnoea, hypertension), the AF burden was reduced significantly in both groups including patients with paroxysmal and persistent AF. As continuous monitoring via ILR was used, a single AF episode of at least 30 s counted as AF recurrence including symptomatic and asymptomatic episodes. Yet, these results are in line with other studies for normal weight patients, like e.g. ‘STOP Persistent AF’ with 54.8% arrhythmia free survival based on Holter-ECG follow-up.25 A meta-analysis from 19 studies (including 6167 AF ablation patients) showed a single-procedure freedom from atrial arrhythmia of 53.1% overall, and 54.1% in paroxysmal AF, and 41.8% in non-paroxysmal AF.26
Despite the accurate detection of AF via ILR during the study, the decision for repeat ablation was made on clinical basis, according to current guidelines.10,11 As a result, patients were only referred to additional AF ablation in case of symptomatic AF recurrence, which was only the case in 15% of all patients. Likewise, 89% of all patients were without any antiarrhythmic medication at the end of study.
Study limitations
Information about AF burden and its variability was limited and restricted to only small studies at the planning stage of the trial, which led to the limitation that we hypothesized a higher difference with regard to the primary endpoint between the two groups than finally observed.
The primary outcome was changed from ‘AF burden, defined as the recurrence of sustained atrial fibrillation (>30 s) after atrial fibrillation ablation’ to ‘AF burden between 3 and 12 months after first AF ablation’ to be more precise with regard to AF ablation outcome and blanking period. This had no impact on the results.
Reduction in BMI or weight was less pronounced compared to the results of observational studies, which we assume to be a reason why the primary endpoint did not differ between the groups. There was no study-determined target for weight loss but an individualized approach. However, it would have been difficult to achieve a specific weight target in a randomized setting with an intervention that is heavily dependent on patients’ motivation and compliance. Lack of patient compliance for the weight reduction programme was an issue during the trial, with 33% of non-compliant patients in group 1 despite regular contact to each patient and usage of patients’ diaries.
Baseline characteristics were comparable except in the type of AF with more persistent AF in group 1. This may be received as a significant confounder, but cox proportional hazards models excluded this.
Additionally, blinding of treatment or sham strategies were not applied and seem hardly feasible in this setting. Furthermore, weight management started after AF ablation and the effect of weight loss might have been clearer, if the weight loss would have been achieved by the time of ablation and not afterwards. However, this would have been a 6-month delay to ablation for the time of intervention, which would have led to progression of AF with known worsening of success rates. Nevertheless, an effect of weight reduction in the persistent AF patients was seen, which may have been even clearer in a larger group of patients.
Conclusion
The SORT-AF study shows that AF ablation is safe and successful in obese patients using continuous rhythm monitoring via ILR. Although the primary endpoint of AF burden after ablation did not differ between the two groups, the effects of weight loss and improvement of exercise activity were beneficial for obese patients with persistent AF demonstrating the relevance of life-style management as an important adjunct to AF ablation in this setting.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgments
The authors thank the patients for their participation in the SORT-AF study. We also thank the DZHK (German Centre for Cardiovascular Research) for support, and Abbott for financing the study via research grant for investigator sponsored trials. Finally, yet importantly we send many thanks to all SORT-AF investigators, study coordinators and members of the study teams.
Funding
This work was endorsed by DZHK (German Centre for Cardiovascular Research) and supported by Abbott Medical GmbH via a research grant for investigator-sponsored trials.
Conflict of interest: S.W. reports grants and personal fees from Abbott and personal feels from Abbott, Boston Scientific, Boehringer Ingelheim, Bristol Myers Squibb, Bayer Vital, Acutus, and Daiichi Sankyo. D.S. has received personal fees from Abbott, grants from Abbott and Boston Scientific, and other support from Biotronik. R.O.A. has received honoraria for lectures from Biosense Webster, Abbott; and educational grants from Biosense Webster. C.M. reports personal fees from Abbott, Bayer, Biosense Webster, Biotronik, BMS/Pfizer, Boehringer, Boston Scientific, and Daiichi Sankyo; grants from Abbott and Boston Scientific. A.S. has received honoraria for lectures from Medtronic, Pfizer, Bayer, and Abbott; honoraria for advisory board activities from Medtronic; and educational grants from Biosense Webster, Abbott, Boston Scientific, and Medtronic. R.T. has received personal fees from Biotronik, Abbott, and Biosense Webster; grants from Abbott and Biosense Webster. C.E. has received grants and personal fees from Abbott, Biosense Webster, Biotronik, Boeringer Ingelheim, Bristol Myers Squibb Boston-Scientific, and Daiichi Sankyo. J.L. has received honoraria for lectures from Medtronic, Bayer Vital and Abbott, and a research grant from Medtronic. The other authors report no conflicts.
References
- 1. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV. et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 2. Willems S, Meyer C, de Bono J, Brandes A, Eckardt L, Elvan A. et al. Cabins, castles, and constant hearts: rhythm control therapy in patients with atrial fibrillation. Eur Heart J 2019;40:3793–3799c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sassi F. Obesity and the Economics of Prevention: Fit Not Fat. OECD Publishing, Edward Elgar Publishing; 2010. [Google Scholar]
- 4. Feng T, Vegard M, Strand LB, Laugsand LE, Mørkedal B, Aune D. et al. Metabolically healthy obesity and risk for atrial fibrillation: the HUNT study. Obesity (Silver Spring) 2019;27:332–8. [DOI] [PubMed] [Google Scholar]
- 5. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX. et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up Study (LEGACY). JACC 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 6. Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R. et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT Study. JACC 2015;66:985–96. [DOI] [PubMed] [Google Scholar]
- 7. Providencia R, Adragao P, de Asmundis C, Chun J, Chierchia G, Defaye P. et al. Impact of body mass index on the outcomes of catheter ablation of atrial fibrillation: a European Observational Multicenter Study. J Am Heart Assoc 2019;8:e012253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D. et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]
- 9. Donnellan E, Wazni OM, Kanj M, Baranowski B, Cremer P, Harb S, McCarthy CP. et al. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace 2019;21:1476–83. [DOI] [PubMed] [Google Scholar]
- 10. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr. et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–76. [Erratum in: J Am Coll Cardiol 2014;64:2305–7]. [DOI] [PubMed] [Google Scholar]
- 11. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C. et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 12. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP. et al. ; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 13. Cuspidi C, Tadic M, Grassi G, Mancia G.. Treatment of hypertension: the ESH/ESC guidelines recommendations. Pharmacol Res 2018;128:315–21. [DOI] [PubMed] [Google Scholar]
- 14. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V. et al. ; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 15. Schaeffer B, Willems S, Meyer C, Lüker J, Akbulak RÖ, Moser J. et al. Contact force facilitates the achievement of an unexcitable ablation line during pulmonary vein isolation. Clin Res Cardiol 2018;107:632–41. [DOI] [PubMed] [Google Scholar]
- 16. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D. et al. ; Obesity Management Task Force of the European Association for the Study of Obesity. European guidelines for obesity management in adults. Obes Facts 2015;8:402–24. [Erratum in: Obes Facts 2016;9:64]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu T, Gao X, Chen M, van Dam RM.. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev 2009;10:313–23. [DOI] [PubMed] [Google Scholar]
- 18. Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP. et al. Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE–a randomized controlled study. Arch Intern Med 2004;164:31–9. [DOI] [PubMed] [Google Scholar]
- 19. Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M. et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 2013;10:90–100. [DOI] [PubMed] [Google Scholar]
- 20. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JPM. et al. Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 2015;66:1–11. [DOI] [PubMed] [Google Scholar]
- 21. Middeldorp ME, , PathakRK, , MeredithM, , MehtaAB, , ElliottAD, , Mahajan Ret al. . PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace 2018;20:1929–35. [DOI] [PubMed] [Google Scholar]
- 22. Mahajan R, Lau DH, Brooks AG, Shipp NJ, Wood JPM, Manavis J, Samuel CS. et al. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. JACC Clin Electrophysiol 2021;S2405-500X:31197-X. Doi: 10.1016/j.jacep.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 23. Donnellan E, Wazni O, Kanj M, Hussein A, Baranowski B, Lindsay B. et al. Outcomes of atrial fibrillation ablation in morbidly obese patients following bariatric surgery compared with a nonobese cohort. Circ Arrhythm Electrophysiol 2019;12:e007598. [Erratum in: Circ Arrhythm Electrophysiol. 2020;13:e000047]. [DOI] [PubMed] [Google Scholar]
- 24. Kuck K-H, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KRJ. et al. Cryoballon or radiofrequency ablation for paroxysmal atrial fibrillation: FIRE AND ICE trial. N Engl J Med 2016;374:2235–45. [DOI] [PubMed] [Google Scholar]
- 25. Su WW, Reddy VY, Bhasin K, Champagne J, Sangrigoli RM, Braegelmann KM. et al. ; STOP Persistent AF Investigators. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP Persistent AF trial. Heart Rhythm 2020;17:1841–7. [DOI] [PubMed] [Google Scholar]
- 26. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS. et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.