Abstract
Studies of bacterial meningitis are hampered by the inability to maintain the viability of etiological agents during transport to reference laboratories. The long-term survival rate of 20 isolates of Neisseria meningitidis and Haemophilus influenzae type b (Hib) on Dorset egg medium, supplemented Columbia agar base medium, chocolate agar, and Amies medium was compared with that on 70% GC agar (chocolate) transport medium. N. meningitidis isolates were also inoculated onto 5% horse blood agar, and Hib was inoculated onto Haemophilus test medium. All of the N. meningitidis isolates remained viable on Dorset egg medium for 21 days; viability on the other media was poor after only 7 days. Recovery rates of Hib isolates were similar on Dorset egg and Haemophilus test media (100% after 21 days) and significantly better than on the other media. Dorset egg medium is inexpensive and easy to make and may be invaluable for studies of bacterial meningitis in developing countries.
We previously reported on the use of Dorset egg medium (DE) for the long-term maintenance of Streptococcus pneumoniae at room temperature (6). On this medium, all 45 isolates of pneumococci tested remained viable for 44 days. We have subsequently investigated DE as a possible transport medium for the two other major respiratory pathogens causing meningitis, namely, Neisseria meningitidis and Haemophilus influenzae type b (Hib). Endemic meningococcal disease, primarily meningitis, occurs in 10 to 25/100,000 persons in developing countries, and epidemics of disease continue throughout the world (4). Hib is one of the leading causes of pneumonia and meningitis in young children in developing countries where vaccination is currently not available (3).
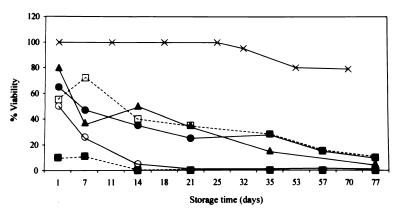
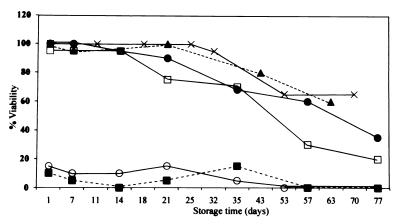
Twenty blood and cerebrospinal fluid isolates of N. meningitidis (14 group A, 4 group B, 1 group Y, and 1 group X) and 20 Hib nasopharyngeal isolates were tested. These isolates had been maintained at −70°C on rabbit blood (N. meningitidis) or skim milk (Hib) prior to testing. Viability rates of the isolates were compared on six different media dispensed in 7-ml glass screw-cap bottles (Bijoux bottles). These media were selected because they are used for transport of other fastidious organisms. N. meningitidis isolates were grown on chocolate agar, and Hib isolates were grown on bacitracin-heated blood agar (BHB) made by autoclaving 78 g of Oxoid Columbia agar in 2,000 ml of demineralized water containing 6 drops of pourite, cooling to 50°C, adding 100 ml of sterile citrated horse blood, heating the medium until chocolate, recooling to 50°C, and then adding 20,000 U of bacitracin dissolved in 10 ml of sterile distilled water. The overnight cultures of each organism were inoculated onto DE prepared by mixing a 1:3 ratio of sterile normal saline and beaten whole hen’s eggs and then inspissating at 80°C for 60 min. N. meningitidis and Hib isolates were also inoculated onto supplemented Columbia agar base medium (CABS), described previously (6); standard chocolate agar (C); Amies (A) medium containing 10 g of activated charcoal (Oxoid), 3 g of NaCl, 1.2 g of Na2HPO4, 0.2 g of KH2PO4, 0.2 g of KCl, 1 g of sodium thioglycolate, 0.1 g of CaCl2 · 2H2O, 0.1 g of MgCl2, and 4 g of Bacto agar (Difco) in 1,000 ml of distilled water (final pH = 7.2) and autoclaved; and on 70% GC agar (chocolate) transport medium (G), consisting of 25 ml of sterile citrated horse blood chocolatized in an autoclaved mixture of 12.6 g of GC agar base (Oxoid) and 475 ml of distilled water and supplemented with 3 μg of vancomycin/ml. In addition to the above-mentioned media, N. meningitidis isolates were tested on standard 5% horse blood agar (B), and the Hib isolates were tested on semisolid Haemophilus test medium (H) prepared by autoclaving 10.5 g of Mueller-Hinton broth (Oxoid), 2.5 g of yeast extract (Difco), and 2.5 g of Bacto agar (Difco) in 500 ml of distilled water (final pH = 7.4) and then supplementing with one vial of HTM supplement (Oxoid) reconstituted with 2 ml of sterile distilled water. The Bijoux bottles were incubated overnight at 37°C in 5% CO2 and were subsequently maintained at room temperature (21°C). Continued viability (at least one visible colony) was ascertained at 1 day and then approximately weekly thereafter, by subculturing a loopful (0.001 ml) of each strain onto blood agar and BHB, as appropriate, and then incubating overnight. At each time point the N. meningitidis and Hib isolates were also tested by standard laboratory methods to confirm the identity and serogroup or serotype of the organism. The results are shown in Fig. 1 and 2. All of the N. meningitidis isolates remained viable on DE for 3 weeks, and 95% were viable up to 32 days (Fig. 1). Viability of N. meningitidis isolates maintained on the other media was poor within 1 to 7 days of room temperature storage. As measured by using a proportional hazards model (2, 5) to statistically compare the long-term viability rates of the organisms on each medium, survival on DE was significantly longer than on the other media (P = 0.0001). Recovery of Hib isolates was similar on DE and H, with all of the isolates remaining viable for at least 21 days (Fig. 2), and 95 and 80% were recovered on DE and H after 32 and 43 days, respectively. Survival rates on DE and H were not significantly different (P ≥ 0.05). Viability on C and CABS was at least 95% for the first 14 days after storage and then dropped to 70% by 30 days at room temperature. Recovery of organisms was significantly better on either DE or H than on the other media (P = 0.03 to 0.0001). To ensure the viability of the isolates on DE during actual transport, 20 N. meningitidis isolates (six group A, four group B, four group C, four group Y, and two group W135) and 20 Hib isolates were inoculated onto DE as described above, shipped 1 h by air freight to a regional laboratory, left in the unopened box at room temperature for 1 week, and then shipped back by air freight to our laboratory. All the N. meningitidis and Hib isolates remained viable.
FIG. 1.
Percentage of N. meningitidis isolates viable on DE (X), CABS (□), C (●), A (■), G (○), and B (▴) after incubation at room temperature. Contaminated isolates were not included in calculations of viability.
FIG. 2.
Percentage of Hib isolates viable on DE (X), CABS (□), C (●), A (■), G (○), and H (▴) after incubation at room temperature. Contaminated isolates were not included in calculations of viability.
Based on these results and on those we reported previously (6), DE is the optimal medium for the transport of respiratory pathogens, particularly fastidious organisms such as N. meningitidis and S. pneumoniae. Either DE or H can be used for transport of Hib, although the lower cost and ease of preparation of DE might make it preferable. Trans-Isolate (T-I) medium has also been used for transport of these organisms (1). The recovery rates of N. meningitidis pneumococci and Hib after 2 to 4 weeks at room temperature on T-I were 81, 92, and 38%, respectively. Contamination prevented recovery of 8% of the Hib and N. meningitidis isolates. In our experience, contamination on DE is minimal (<1%), and DE is much simpler and less expensive to make than the diphasic T-I medium. We would, therefore, recommend the use of DE for transport of respiratory pathogens between laboratories. This medium may be invaluable for studies of bacterial meningitis in developing countries.
REFERENCES
- 1.Ajello G W, Feeley J C, Hayes P S, Reingold A L, Bolan G, Broome C V, Phillips C J. Trans-Isolate medium: a new medium for primary culturing and transport of Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae. J Clin Microbiol. 1984;20:55–58. doi: 10.1128/jcm.20.1.55-58.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dean A G, Dean J A, Coulombier D, et al. Epi Info, version 6: a word processing, database, and statistics program for epidemiology on microcomputers. Atlanta, Ga: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 3.Moxon E R, Wilson R. The role of Haemophilus influenzae in the pathogenesis of pneumonia. Rev Infect Dis. 1991;13(Suppl. 6):S518–S527. doi: 10.1093/clinids/13.supplement_6.s518. [DOI] [PubMed] [Google Scholar]
- 4.Riedo F X, Plikaytis B D, Broome C V. Epidemiology and prevention of meningococcal disease. Pediatr Infect Dis J. 1995;14:634–657. doi: 10.1097/00006454-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 5.SAS Institute Inc. SAS/STAT user’s guide, version 6. 4th ed. Vol. 2. Cary, N.C: SAS Institute Inc; 1989. [Google Scholar]
- 6.Wasas A D, Huebner R E, De Blanche M, Klugman K P. Long-term survival of Streptococcus pneumoniae at room temperature on Dorset egg medium. J Clin Microbiol. 1998;36:1139–1140. doi: 10.1128/jcm.36.4.1139-1140.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]