Abstract
Background
Most children and youth develop mild or asymptomatic disease during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, a very small number of patients suffer severe Coronavirus induced disease 2019 (COVID-19). The reasons underlying these different outcomes remain unknown.
Methods
We analyzed three different cohorts: children with acute infection (n=550), convalescent children (n=138), and MIS-C (multisystem inflammatory syndrome in children, n=42). IgG and IgM antibodies to the spike protein of SARS-CoV-2, serum-neutralizing activity, plasma cytokine levels, and the frequency of circulating Follicular T helper cells (cTfh) and plasmablasts were analyzed by conventional methods.
Findings
Fifty-eight percent of the children in the acute phase of infection had no detectable antibodies at the time of sampling while a seronegative status was found in 25% and 12% of convalescent and MIS-C children, respectively. When children in the acute phase of the infection were stratified according disease severity, we found that contrasting with the response of children with asymptomatic, mild and moderate disease, children with severe COVID-19 did not develop any detectable response. A defective antibody response was also observed in the convalescent cohort for children with severe disease at the time of admission. This poor antibody response was associated to both, a low frequency of cTfh and a high plasma concentration of inflammatory cytokines.
Interpretation
A weak and delayed kinetic of antibody response to SARS-CoV-2 together with a systemic pro-inflammatory profile characterize pediatric severe COVID-19. Because comorbidities are highly prevalent in children with severe COVID-19, further studies are needed to clarify their contribution in the weak antibody response observed in severe disease.
Funding
National Agency for Scientific and Technological Promotion from Argentina (IP-COVID-19-0277 and PMO-BID-PICT2018-2548).
Keywords: Pediatric COVID-19; Disease severity, antibodies, T cells
Research in context.
Evidence before this study
Most children and youth develop mild or asymptomatic disease during severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection. These patients usually mount a robust antibody response. However, there is very scarce information about the antibody response in children with severe Coronavirus induced disease 2019 (COVID-19).
Added value of this study
We found that children with severe COVID-19 display a poor and delayed antibody response against SARS-CoV-2. This poor antibody response was associated to both, a low frequency of circulating Tfh cells and a systemic inflammatory response that persist during the convalescent phase of infection.
Implications of all the available evidence
Our observations contribute to define a small but vulnerable population of children at higher risk of severe COVID-19. They suggest that early passive immunotherapy with neutralizing antibodies directed to SARS-CoV-2 might represent a useful therapeutic tool in children with severe COVID-19.
Alt-text: Unlabelled box
1. Introduction
Children and youth are less severely affected by the Coronavirus disease 2019 (COVID-19) than adults [1], [2], [3]. For instance, a recent study performed in Spain has shown 0.18 deaths per 100,000 in children aged 0-9 years, and 0.37 deaths per 100,000 in children aged 10-19 years [4]. Epidemiological data from Argentina as of May 5, 2021 have reported more than 290,000 confirmed cases under 20 years and only 0.2% of them were admitted to Pediatric Intensive Care Unit (PICU). In rare cases, infected children suffer a multisystem inflammatory syndrome (MIS-C), an autoimmune condition that develops weeks after the resolution of acute infection [5,6].
The reasons underlying the mild course of COVID-19 in children remain unclear. It could be explained, at least partially, by the fact that compared with adults, children have a decreased expression in the airway epithelium of host factors required for the initiation and spreading of the infection, such as the viral entry receptor angiotensin-converting enzyme 2 and the Transmembrane Serine Protease 2 [7,8]. In spite of this, a recent study showed that SARS-CoV-2 viral load in symptomatic children during the first week of infection was only slightly lower compared with symptomatic adults [9].
Differences in the immune response against SARS-CoV-2 between children and adults might also explain the different outcome of pediatric COVID-19. Several studies have been conducted to analyze the immune response to SARS-CoV-2 infection in adults. However, very few studies have addressed the immune response in pediatric COVID-19. It has been reported that upon SARS-CoV-2 infection children display a stronger and earlier mucosal innate immune response associated to a high expression of IFN response genes, in comparison with adults [10]. In addition, children show a higher absolute number of circulating T cells, and a high proportion of naïve T cells compared with adults, thus enabling an efficient adaptive immune response to previously unrecognized microbial antigens [11]. Regarding the antibody response, it has been reported that asymptomatic or mild symptomatic SARS-CoV-2 infection elicit durable neutralizing antibody responses in children and adolescents [12]. Moreover, Weisberg and coworkers have recently reported that mildly infected children mount an antibody response with a lower neutralizing activity compared with adults that suffer severe COVID-19 [13].
In this study, we focus on the antibody response in children with COVID-19. We found that children with severe COVID-19 display a poor and delayed antibody response against SARS-CoV-2. This feature was associated with a systemic inflammatory response revealed by a high serum concentration of inflammatory cytokines that remained elevated in the convalescent phase of infection.
2. Methods
2.1. Study population
This observational study was conducted in Buenos Aires City and Buenos Aires province, Argentina, between May 2020 and January 2021. We recruited girls, boys and adolescents aged between 3 months and 15 years admitted to the Hospital General de Niños Pedro de Elizalde, Hospital Nacional Prof. Alejandro Posadas, Hospital Municipal Diego Thompson, Clínica del Niño de Quilmes, Hospital Naval Cirujano Mayor Dr. Pedro Mallo, Hospital HIGA Eva Perón, Hospital Universitario Austral, and Hospital General de Agudos Dr. J. A Fernández. Patients were divided into three main cohorts: 1- patients with active SARS-CoV-2 infection confirmed by PCR of nasopharyngeal swabs (acute, n=550), 2- patients in the convalescent phase of the infection between 4–6 weeks following the appearance of symptoms (convalescent patients, n=138) and, 3- MIS-C patients with previous SARS-CoV-2 infection confirmed by PCR of nasopharyngeal swabs and/or SARS-CoV-2 IgG antibody-positive serology (n=42). Disease severity was classified as asymptomatic, mild, moderate or severe, according to the Health Ministry from Argentina and the WHO interim guidance. The cohort of children with active SARS-CoV-2 infection included the following patients. Asymptomatic patients (n=84) that remained without symptoms throughout the course of the infection. They were admitted after SARS-CoV-2 infection was confirmed by PCR. Mild patients (n=358) without evidence of pneumonia or hypoxia. Symptoms were non-specific and could include fever, fatigue, headache, myalgia, cough, abdominal pain and diarrhea. Moderate patients (n=100) with symptoms and signs of non-severe pneumonia (cough or fast breathing and/or chest indrawing), included patients with comorbidities such as cardiac or respiratory disease, immunodeficiency and prematurity. Severe children (n=8) with severe pneumonia, cough, difficulty in breathing, respiratory distress and/or letargy and convulsions. Children during convalescence were also stratified based on their diagnosis at the time of admission (asymptomatic, n=10; mild, n=88; moderate, n=31; and severe n=9). Characteristics of children with COVID-19 are shown in Table 1, S1 and S2. Particular data of children with severe COVID-19 is shown in Table S3. MIS-C was diagnosed according to WHO criteria: -fever during more than 3 days; -two of the following signs (rash or bilateral non-purulent conjunctivitis, mucocutaneous inflammation signs, hypotension, shock, myocardial dysfunction, pericarditis, valvulitis, coronary abnormalities, coagulopathy, and/or acute gastrointestinal symptoms); -elevated markers of inflammation, -no other microbial cause; and -evidence of previous SARS-CoV-2 infection (PCR or serology positive). Clinical features of MIS-C are shown in Table 1. Our control cohort included 22 children age and sex matched that were admitted to the hospitals for routine screening and/or scheduled surgery. They had no history of recent respiratory infection or close-contact and they were negative for IgM and IgG antibodies directed to SARS-CoV-2.
Table 1.
Characteristics of children with COVID-19 and MIS-C.
| Control | Acute | Convalescent | MIS-C | P | ||
|---|---|---|---|---|---|---|
| n= 22 | n= 550 | n= 138 | n= 42 | |||
| Age, y, median (range) | 1.7 (1.0-5.5) | 3.0 (0.7-10.0) | 5 (1-12) | 5.0 (2.2-9.2) | ||
| <1 y, n (%) | 6 (27) | 186 (33) | 28 (20) | 5 (12) | *Ac vs Co, MI | |
| 1-5 y, n (%) | 11 (50) | 146 (27) | 47 (34) | 18 (43) | ||
| 6-10 y, n (%) | 3 (14) | 76 (14) | 16 (12) | 10 (24) | ||
| >10 y, n (%) | 2 (9) | 142 (26) | 47 (34) | 9 (21) | ||
| Sex, n (%) | ||||||
| Female | 10 (45) | 267 (49) | 67 (49) | 12 (29) | ||
| Male | 12 (55) | 283 (51) | 71 (51) | 30 (71) | ||
| Days after symptom onset (range) | N/A | 1 (1-3) | 31 (22-35) | 4.5 (2-5.7) | *Ac vs MI; Ac, ***MI vs Co | |
| SARS-CoV-2 PCR positivea, n (%) | N/A | 550 (100) | N/A | 17 (40) | ***Ac vs MIS-C | |
| SARS-CoV-2 IgG antibody positive, n (%) | 0 | 139 (25) | 87 (63) | 37 (88) | ****Ac vs Co, MI; **Co vs MI | |
| SARS-CoV-2 IgM antibody positive, n (%) | 0 | 191 (35) | 70 (50) | 23 (55) | ***Ac vs Co; **Ac vs MI | |
| Severity of illnessb, n (%) | N/A | N/A | ||||
| Asymptomatic | 84 (15.3) | 10 (7) | *Ac vs Co | |||
| Mild | 358 (65) | 88 (64) | ||||
| Moderate | 100 (18.2) | 31 (22) | ||||
| Severe | 8 (1.5) | 9 (7) | *Ac vs Co | |||
| Comorbidities, n (%) | ||||||
| None | 17 (77) | 442 (80) | 94 (68) | 35 (83) | *Ac vs Co | |
| Skin disease | 0 | 3 (0.5) | 0 | 0 | ||
| Heart disease | 0 | 9 (1.6) | 4 (3) | 0 | ||
| Renal disease | 0 | 9 (1.6) | 4 (3) | 0 | ||
| Lung disease | 0 | 43 (8) | 19 (14) | 1 (2.4) | *Ac vs Co; *Co vs MI | |
| Prematurity | 0 | 11 (2) | 6 (4.3) | 0 | ||
| Autoimmunity | 0 | 8 (1.5) | 2 (1.4) | 0 | ||
| Cancer | 0 | 14 (2.5) | 9 (7) | 2 (5) | ||
| Obesity | 2 (9) | 23 (4.2) | 7 (5) | 4 (10) | ||
| Undernutrition | 2 (9) | 11 (2) | 7 (5) | 0 | *Ctrl vs Ac, MI; *Ac vs Co | |
| Diabetes | 0 | 7 (1.3) | 2 (1.4) | 0 | ||
| Genetic disorder | 1 (5) | 5 (0.9) | 2 (1.4) | 0 | ||
| Coinfections, n (%) | ||||||
| None | 22 (100) | 527 (95.8) | 127 (92) | 40 (95) | ||
| Bacterial | 0 | 22 (4) | 10 (7.2) | 2 (5) | ||
| Viral | 0 | 1 (0.2) | 1 (0.7) | 0 | ||
| PICU admission, n (%) | N/A | 12 (2) | 12 (9) | 28 (67) | *Ac vs Co; **Ac, Co vs MI | |
| Respiratory status, n (%) | ||||||
| Mechanical ventilation | 0 | 4 (0.6) | 5 (4) | 10 (24) | *Ac vs Co; *Ac, Co vs MI | |
| Oxygen requirement | 0 | 13 (2.4) | 16 (11) | 1 (2.4) | *Ac vs Co | |
| Room air | 22 (100) | 533 (97) | 117 (85) | 31 (73.6) | *Ac vs Co, MI |
Data are expressed as median values (IQR) unless otherwise indicated. Abbreviations: PICU, pediatric intensive care unit. a at time of blood collection. b at hospital admission. Only significant p values are shown. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
2.2. Study approval
This study was conducted in accordance with the Declaration of Helsinki. The Institutional Review Board at institutions participants reviewed and approved the sample collection and the overall study (Hospital General de Niños Pedro de Elizalde protocol reference 1226/20, Hospital Universitario Austral protocol reference 2147/2020 and Hospital General de Agudos Dr. J. A Fernández protocol reference 1720/20).
2.3. Ethics statement
Parents or legal guardians from children under 8 years provided written, informed consent. Children older than 8 years old provided written, informed assent and their parents or legal guardians also provided written, informed consent.
2.4. Blood sample processing
0.5-1 mL of whole blood samples were obtained within 1-4 days of hospital admission. After being centrifuged for 10 min at 1000 rpm, plasma was separated and stored at -80°C until used.
2.5. Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMCs) were obtained from blood samples by Ficoll-Hypaque gradient centrifugation (GE Healthcare Life Sciences, Uppsala, Sweden). PBMCs were washed twice, and suspended in PBS (GIBCO, 10010023).
2.6. Cells and virus
VERO C1008 (clone E6, ATCC® CRL-1586™) were cultured in DMEM (GIBCO, 10313021) supplemented with 5% heat-inactivated fetal bovine serum (FCS, Sigma-Aldrich, F2442), 2mM L-Glutamine (Sigma-Aldrich, G7513), penicillin and streptomycin (Sigma-Aldrich, P0781). The cells were incubated in 95% air and 5% CO2 at 37°C. The SARS-CoV-2 isolate (hCoV-19/Argentina/PAIS-G0001/2020, GISAID Accession ID: EPI_ISL_499083) was kindly provided by Dr. Sandra Gallegos (InViV working group, National University of Córdoba, Argentina). Viral master seed stock was prepared using T175 flasks of Vero E6 cells. Each flask was harvested on day two post-infection, and the supernatant was centrifuged twice at 220 x g for 15 min to remove cellular debris. The virus stock titer was determined by plaque assay on Vero E6 cells and expressed as plaque-forming units per mL. The experiments using the virus were carried out in BSL3 facilities from the School of Medicine at the University of Buenos Aires.
2.7. Quantitation of plasma SARS-CoV-2–specific IgG and IgM antibodies
Plasma levels of IgG and/or IgM antibodies directed to the spike protein of SARS-CoV-2 were analyzed by ELISA (COVIDAR IgG and COVIDAR IgM), as described [14]. Briefly, samples were diluted 1:10 in PBS-T containing 0.8% casein and incubated for 1 h at 37°C. After washing with PBS-T, HRP-conjugated goat mouse anti-human IgG antibodies was added and incubated for 30 min at 37°C, followed by TMB Substrate Reagent. The absorbance (OD) was measured at 450 nm. The cut-off was calculated as the mean OD of the negative sera plus 0.2. Samples with OD lower than the cut-off were considered negative for anti-SARS-CoV-2 antibodies. IgG antibody titers were determined by endpoint titration. Samples were two-fold serially diluted in FCS and then analyzed by ELISA COVIDAR IgG.
2.8. Neutralization assay
Serum samples were heat-inactivated (56°C for 30 min) and serial dilutions (1/2 to 1/1024) were incubated at 37°C for 1 h, in the presence of WT SARS-CoV-2 (MOI=0.01) in DMEM 2% FBS. Fifty µL of the mixtures were then deposited over Vero E6 cell monolayers for 1 h at 37°C. Infectious media was removed and replaced for DMEM supplemented with 2% FCS. After 72 h of culture, cells were fixed with 4% paraformaldehyde (Sigma-Aldrich, 158127) at 4°C during 20 min and stained with crystal violet solution in methanol (Sigma-Aldrich, 34860). Neutralization titer was determined as the inverse of the last serum dilution with 80% cytopathic effect inhibition (CI80).
2.9. Flow cytometry
PBMCs (1×106 cells) were stained with the antibody cocktail mix for 20 min at room temperature. After being washed with PBS 0.1% BSA, cells were fixed using Fixation buffer (BD Biosciences, 554655). The following conjugated-monoclonal antibodies were used: anti-CD3 (PerCP, 300428), anti-CD4 (Brilliant Violet 510™,317444), anti-CD45RA (PECy7, 304126), anti-CXCR5 (Alexa Fluor 647, 356906), anti-CD19 (APCCy7, 302218), anti-CD38 (FITC, 356610), anti-CD27 (PE, 356406), all from Biolegend. Control samples were incubated with an isotype-matched antibody. Statistical analyses were based on at least 100,000 events gated on the population of interest. Data were acquired using a FACSCanto II (Becton Dickinson) and analyzed with FlowJov10.6.2.
2.10. LegendPlex assay
Levels of IL-1β, IFN-α2, IFN-γ, TNF-α, MCP-1 (CCL2), IL-6, IL-8 (CXCL8), IL-10, IL-12p70, IL-17A, IL-18 and IL-33 in plasma were analyzed by flow cytometry according to the manufacturer's instructions (Human Inflammation Panel 1, Biolegend, 740809). The lowest detection limits for each cytokine were as follows (pg/mL): IL-1β, 2.7; IFN-α2, 3.2; IFN-γ, 2.2; TNF-α, 2.8; MCP-1, 2.4; IL-6, 2.4; IL-8, 2.7; IL-10, 2.7; IL-12p70, 3.4; IL-17A, 0.8; IL-18, 4.2; and IL-33, 24.4.
2.11. Statistics
Clinical characteristics were summarized using descriptive statistics. Categorical variables are reported as numbers and percentages. Quantitative variables are reported as medians and interquartile ranges and presented as medians and min to max in the figures. The normality of experimental data was evaluated by the Shapiro-Wilk test. Two groups were compared using the Wilcoxon signed-rank test or Mann-Whitney U test. Three or more groups were compared using the Kruskall-Wallis test followed by Dunn's multiple comparison test, Pearson's Chi-square or Fisher's exact test followed by Two sample Z test of proportions with Bonferroni correction for multiple comparisons (the exact method used is stated in the figure legends). Significance between 2 continuous variables was calculated by using a Spearman correlation test. To further explore severity effects on IgG we conducted Ordinary Least Squares regression models, including the severity groups as dichotomized variables, and age, gender, and comorbidities as covariates. These analyses were conducted on the software environment R (R Core Team, 2021). Statistical significances are indicated in the figures by asterisks as follows * p< 0.05, **p< 0.01, ***p< 0.001 or ****p< 0.0001. Analysis and visualizations were performed using GraphPad Prism.v.8 (GraphPad Software) and SPSS software v.19.0 (SPSS Corp).
2.12. Role of the funding source
The funders had no role in study design, data collection, analysis and interpretation, writing and submission of the manuscript.
3. Results
3.1. Study cohorts
We recruited a total of 730 children and adolescents admitted to different hospitals from Buenos Aires city and surroundings with SARS-CoV-2 infection between May 2020 and January 2021. Children with acute COVID-19 (n=550) included asymptomatic (n=84), mild (n=358), moderate (n=100) and severe (n=8) patients that were stratified according to the Health Ministry from Argentina and the WHO interim guidance. The median age and IQR of acute COVID-19 group was 3 years (0.7-10), of whom 49% (n=267) were girls. The median time since onset of symptoms until hospital admission was 1.0 day (IQR, 1.0-3.0) and there were no differences in the length of symptoms in relation to the severity of disease. Most children had no co-infections, having 4% (n=22) bacterial and 0.2% (n=1) viral co-infections. Almost all children who developed severe COVID-19 disease had an existing comorbidity (87.5%, n=7). Only 2% (n=12) of children with acute COVID-19 were admitted to PICU. Among the convalescence cohort (n=138), the median age and IQR was 5 years (1-12) and 49% (n=67) were girls. The median time since onset of symptoms until testing was 31 days (IQR, 22-35) and there were no differences according to disease severity. Nine percent (n=12) of convalescent children required PICU admission and 7.2% (n=10) had bacterial co-infections. Ten of them had no symptoms (7%), 88 had mild symptoms (64%), 31 had moderate symptoms (22%) and 9 had severe disease (7%) at diagnosis. Regarding the MIS-C group (n=42), the median age and IQR was 5 years (2.2-9.2), of whom 29% (n=12) were girls. The median time since onset of symptoms until hospital admission was 4.5 days (IQR, 2.0-5.7). Most of MIS-C had no comorbidities. More than half of MIS-C patients required intensive care and 24% (n=10) required mechanical ventilation. Two children were deceased under acute COVID-19. No fatal outcome was observed in convalescent children or in MIS-C. Tables 1, S1, S2 and S3 summarize the characteristics of all children included.
3.2. Antibody response in children with COVID-19
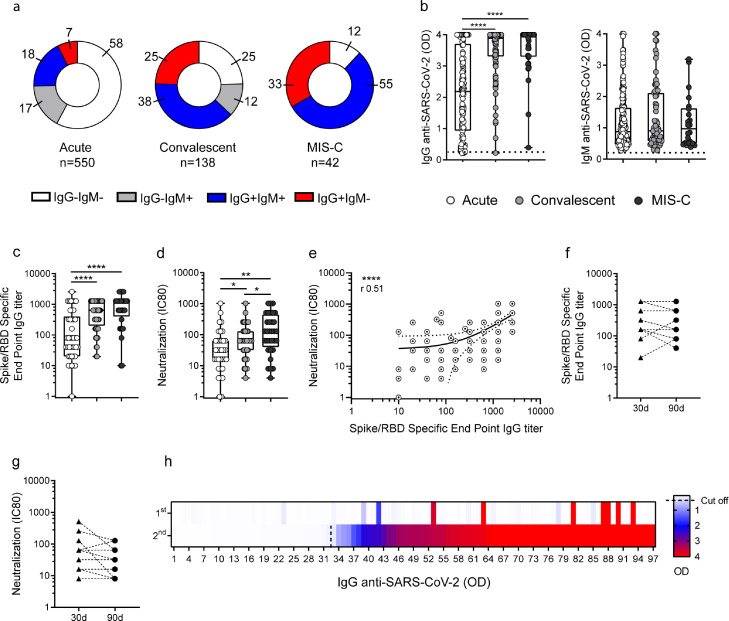
We analyzed the antibody response to SARS-CoV-2 in children with acute COVID-19 (n=550), convalescent children (n=138) and MIS-C (n=42). Blood samples from children with acute COVID-19 and MIS-C were obtained within 1-4 days of being admitted while samples from convalescent children were obtained within 4-6 weeks after the disease onset. As expected, more than half of the children in the acute phase of infection (n=318) had no detectable antibodies at the time of sampling while 25% (n=34) and 12% (n=5) of convalescent and MIS-C children, respectively, showed a seronegative status. IgM antibodies directed to SARS-CoV-2 were detected in 35%, 50% and 55% of the children with acute infection, convalescents and MIS-C, respectively. On the other hand, IgG antibodies directed to SARS-CoV-2 were detected in 25%, 63%, and 88% of children from these cohorts, respectively. We found that the frequency of children in the acute phase of the infection with detectable IgG and/or IgM antibody levels (42%) were significantly lower compared with either convalescents (75%) or MIS-C (88%, p<0.0001; Fig. 1a).
Fig. 1.
Antibody response against SARS-CoV-2 in children with COVID-19 and MIS-C. (a) Donuts graphs show the frequency of children in each cohort that produced IgG and/or IgM antibodies to the Spike/RBD protein of SARS-CoV-2. Pearson's Chi square test, p<0.0001. (b) Plasma levels of IgG and IgM antibodies directed to the Spike/RBD protein in SARS-CoV-2-seropositive children are shown (n=139 and n=191, n=87 and n=70, n=37 and n=23 for IgG and IgM levels in acute, convalescents and MIS-C, respectively). (c) IgG titers defined by end point dilution in SARS-CoV-2-seropositive children in the acute (n=43), convalescent (n=56) and MIS-C (n=32) cohorts. (d) Neutralization antibody titers determined by the reciprocal IC80 in SARS-CoV-2-seropositive children from the acute (n=34), convalescent (n=44) and MIS-C (n=32) cohorts. (e) Correlation between the titers of IgG antibodies to the Spike/RBD and the neutralizing titers of antibodies (n=110). (f-g) Titers of IgG antibodies to the Spike/RBD protein of SARS-CoV-2 and (f) neutralizing antibody titers (g) detected in 13 patients at 30 and 90 days post diagnostic. (h) Heat map of IgG antibodies directed to the Spike/RBD protein quantified in paired plasma samples from 97 children. Each cell represents an individual patient. The first sample was taken between 1-4 days after admission and the second was obtained between 4-6 weeks after admission. Red represents high and blue low antibody levels, as indicated on the right. Dotted line indicates the cut-off value in b. Median and min to max of n donors are shown in b-d. P values were determined by Pearson's Chi square test, Kruskal-Wallis test, Mann-Whitney U test and Spearman correlation test: * p<0.05, ** p<0.01, **** p<0.0001. Acute (white circle), Convalescent (light grey circle), MIS-C (dark grey circle).
Among children with a seropositive status, the levels of IgG antibodies directed to SARS-CoV-2 were significantly higher in convalescent children and MIS-C compared with children in the acute phase of infection [2.1 (0.9-3.7), 3.8 (3.3-4) and 4.0 (3.3-4), p<0.0001; median (IQR) in acute (n=139), convalescents (n=87) and MIS-C (n=37), respectively)]. No differences were found when the levels of IgM antibodies to SARS-CoV-2 were analyzed (Fig. 1b). Consistent with these data, we found that the titers of IgG antibodies to the spike protein of SARS-CoV-2 were higher for convalescent and MIS-C children compared with children in the acute phase of infection [80 (20-400), 640 (200-1280), 1280 (400-1280), p<0.0001; median (IQR) in acute, convalescents and MIS-C, respectively, Fig. 1c]. Similar trends were observed when we analyzed the antibody neutralization titers [32 (14-64), 64 (32-128), 128 (40-448); median (IQR), p<0.05 and p<0.01 for convalescent and MIS-C vs children with acute infection, respectively], as shown in Fig. 1d. As expected, a clear correlation was found when the titers of both, IgG antibodies and neutralizing antibodies were analyzed (n=110, r=0.51, p<0.0001; Fig. 1e). Moreover, by analyzing paired samples in a small cohort of our patients (n=13) we found that the titers of IgG antibodies against SARS-CoV-2 and the titers of neutralizing antibodies remained relatively stable for at least 90 days (Fig. 1f-g). To better understanding the kinetic of seroconversion in children with COVID-19, we analyzed 97 children who were followed since hospital admission (first time of sampling) until 4-6 weeks after admission (second time of sampling). We observed that 67% of them produced IgG directed to SARS-CoV-2 during the first 4-6 weeks after diagnosis (Fig. 1h). The median (IQR) time to seropositivity was 31 days (26-36).
3.3. Children with severe COVID-19 develop a lower and delayed production of IgG antibodies directed to SARS-CoV-2
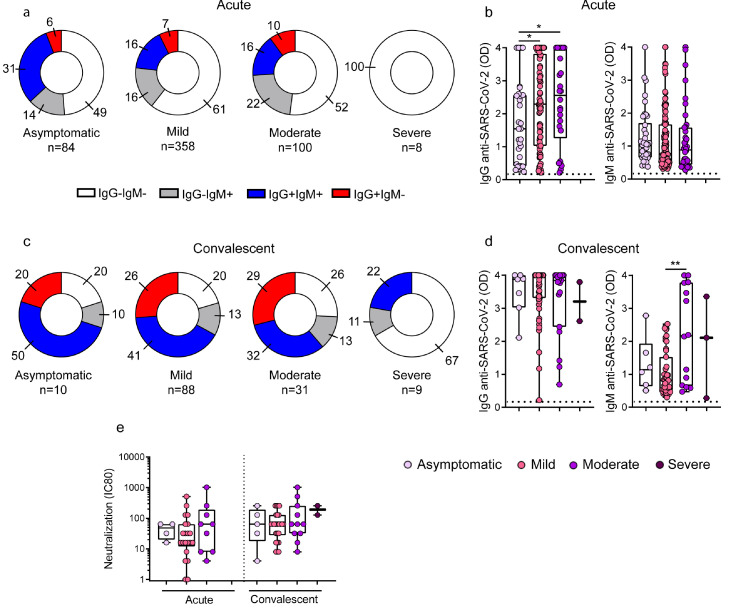
Given the heterogeneous antibody response observed in children with COVID-19, we analyzed whether it could be related to differences in the age, sex and /or comorbidities of patients. No differences were found when IgG and IgM antibodies against SARS-CoV-2 were stratified according to age, sex or comorbidities (Fig. S1, S2 and S3). Then, we analyzed in a cohort of 550 children with acute COVID-19 whether the antibody response differed according to disease severity. Importantly, and contrasting with the observations made in children with asymptomatic, mild and moderate disease, we found that children with severe COVID-19 did not develop any detectable antibody response in the course of acute infection (Fig. 2a). The analysis of antibody levels among seropositive patients during the acute phase also showed that asymptomatic children (n=31) produced lower levels of IgG antibodies against SARS-CoV-2 compared with mild (n=82) and moderate (n=26) patients [1.5 (0.4-2.6), 2.3 (1-3.8) and 2.6 (1.2-4), p<0.05; median (IQR) in asymptomatic, mild and moderate patients, respectively; Fig. 2b]. No differences were found when the levels of IgM antibodies to SARS-CoV-2 were analyzed under the acute phase of infection.
Fig. 2.
Antibody response against SARS-CoV-2 across the clinical spectrum of COVID-19 in the course of acute and convalescent phases of infection. (a-e) Children in the acute or convalescent phases of SARS-CoV-2 infection were grouped based on their diagnosis at hospital admission as asymptomatic, mild, moderate or severe. (a) Donuts graphs show the frequency of children that produced IgG and/or IgM antibodies to the Spike/RBD protein of SARS-CoV-2 in the course of acute infection. Pearson's Chi square test, p<0.05 (IgG+IgM+, asymptomatic vs mild; IgG-IgM-, asymptomatic vs severe). (b) Plasma levels of IgG and IgM antibodies directed to the Spike/RBD protein were measured in SARS-CoV-2-seropositive children during acute phase (n=31 and n=38, n=82 and n=115, n=26 and n=38 for IgG and IgM levels in asymptomatic, mild and moderates, respectively). (c) Donuts graphs show the frequency of children that produced IgG and/or IgM antibodies to the Spike/RBD protein of SARS-CoV-2 in the convalescent phase of the infection (IgG-IgM-, mild vs severe, p<0.05). (d) Plasma levels of IgG and IgM antibodies directed to the Spike/RBD protein were measured in SARS-CoV-2-seropositive children during the convalescent phase of the infection (n=7 and n=6, n=59 and n=47, n=19 and n=14, n=2 y n=3 for IgG and IgM levels in asymptomatic, mild, moderates and severe, respectively). (e) Neutralization antibody titers in the course of acute (asymptomatic, n=4; mild, n=21; and moderate, n=9) and convalescent phases (asymptomatic, n=5; mild, n=26; moderate, n=11 and severe, n=2) of the infection. Dotted line indicates the cut-off value in b and d. Median and min to max of n donors are shown in b, d, and e. P values were determined by Pearson's Chi square test or Fisher's exact test, Kruskal-Wallis test and Mann-Whitney U test: * p<0.05, ** p<0.01. Acute (white circle), Convalescent (light grey circle), MIS-C (dark grey circle). Asymptomatic (lilac circle), mild (pink circle), moderate (violet circle), severe (purple circle).
Taking into account the striking finding indicating that severe COVID-19 in children is associated with a defective antibody response during acute infection, we then analyzed whether these children showed a detectable antibody response when studied under the convalescent phase of COVID-19. To this aim, the convalescent cohort of children was stratified according to the severity at the time of hospital admission (Table S2). We found that only 22% (2 out of 9 patients) and 33% (3 out of 9 patients) of children with severe COVID-19 seroconverted for IgG and IgM antibodies against SARS-CoV-2, respectively, at 3-5 weeks after admission. This seroconversion pattern was markedly lower compared with the one observed in asymptomatic children as well as in children with mild and moderate symptoms at admission (Fig. 2c).
Given that 7 out of 9 convalescent children with severe COVID-19 had previous diseases, we analyzed whether the impaired antibody response in these children could be related to underlying diseases rather than COVID-19 severity. The full spectrum of comorbidities in convalescent children stratified according the severity of disease at the time of admission is shown in Table S2. The analysis of the frequency of convalescent children carrying comorbidities that seroconverted was 50% for asymptomatic (2/4), 73% for mild (8/11), 64% for moderate (14/22), but only 29% (2/7) for children with severe COVID-19 (p<0.05 for severe COVID-19 vs mild or moderate COVID-19). Moreover, we also analyzed the number of convalescent children under 6 months that produced antibodies and we observed that 100% of asymptomatic (2/2), 71% of mild (5/7), 50% of moderate (2/4) and only 33% of severe-COVID-19 patients (1/3) produced IgG antibodies directed to SARS-CoV-2.
Moreover, the analysis of seropositive children showed no differences among the different groups of patients including children with severe COVID-19 regarding the levels of IgG antibodies against SARS-CoV-2 or the neutralizing antibody titers. The fact that they finally reached comparable levels to those detected in children with asymptomatic, mild or moderate infection, suggests that some children with severe COVID-19 could mount a delayed but effective antibody response (Fig. 2d-e).
To further analyze the relationship between disease severity and the antibody response, we conducted ad hoc multiple regression analysis on the convalescent cohort of children with IgG levels as a function of patients’ severity group (transformed into three dichotomized variables representing the asymptomatic, mild and moderate groups). When conducting this regression model on the convalescent cohort, we found a significant difference between the mild group and the severe group, B=1.67, p=0.01, and between the asymptomatic group and the severe group, B=1.72, p=0.04. For the comparison between the moderate group and the severe group, differences did not reach statistical significance, B=1.29, p=0.06. In all the cases, the severe group showed lower levels of IgG than the others. When conducting the same model but including the three covariates (age, sex, and comorbidities), the difference between the mild and severe group remaining significant, B=1.70, p=0.02. However, the difference between the severe and the asymptomatic group (B=1.56, p=0.08) and between the severe and the moderate group (B=1.25, p=0.08), did not reach statistical significance.
3.4. Children with severe COVID-19 show a low frequency of circulating T follicular helper cells
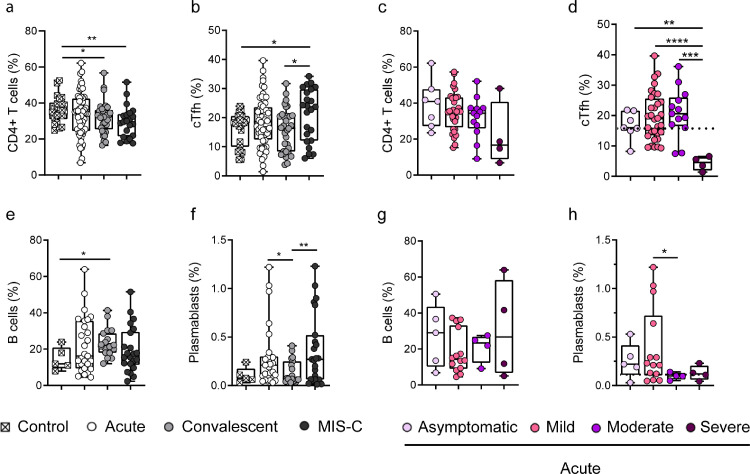
Peripheral blood contains low frequencies of CXCR5+ cTfh cells, and recent studies have shown that they are directly related to Tfh cells found in lymph nodes [15,16]. To gain insight into the mechanisms underlying the poor antibody response in children with severe COVID-19, we analyzed the frequency of cTfh. Children with acute COVID-19 showed frequencies of CD4+ T cells comparable to healthy donors, while a significant decrease was found in convalescent children and MIS-C [35.5% (31.0-40.4), 34.9% (24.5-43.5), 32.4% (25.3-36.2), and 29.5% (21.5-34), median (IQR) in controls (n=22), acute (n=56), convalescents (n=33) and MIS-C (n=23), respectively, p<0.05 and p<0.01; Fig. 3a]. The gating strategy used to identify cTfh by flow cytometry is shown in Fig. S4. Healthy donors [18.07% (9.9-20.5)], children under acute [18.6% (12.5-23.5)] and convalescent phases of infection [16.3% (8.3-20.6)], had similar percentages of cTfh, while increased frequencies were found in MIS-C [23.7% (12.1-30) median (25-75 IQR), p<0.05], Fig. 3b). There was no difference in the frequency of CD4+ T cells when children with acute COVID-19 were analyzed according disease severity (Fig. 3c). Interestingly, we found that children with severe COVID-19 [4.5% (1.9-6.3)] displayed significantly lower frequencies of cTfh compared to children with asymptomatic [16% (15-21.6)], mild [18.9% (13.5-25.6)] and moderate disease [20.5% (16.5-25.9), median (IQR), p<0.01, p<0.0001 and p<0.001 for asymptomatic, mild and moderate disease vs severe disease, respectively, Fig. 3d].
Fig. 3.
Frequency of cTfh cells and plasmablasts in children with COVID-19 and MIS-C. (a-b) Frequency of CD4+ T cells (a) and cTfh (b) analyzed by flow cytometry in healthy children (n=22), children in the acute (n=56) and convalescent (n=33) phases of infection, and MIS-C (n=23). (c-d) Frequency of CD4+ T cells (c) and cTfh (d) in children with acute COVID-19 classified according to disease severity: asymptomatic, n=7; mild, n=32; moderate, n=13; severe, n=4. (e-f) Frequency of B cells (e) and plasmablasts (f) analyzed by flow cytometry in healthy children (n=5), children in the acute (n=27) and convalescent (n=19) phases of the infection, and MIS-C (n=23). (g-h) Frequency of B cells (g) and plasmablasts (h) during acute infection in children with COVID-19 classified according to disease severity: asymptomatic, n=5; mild, n=14; moderate, n=4; severe, n=4. Dotted line depicts the median values found in healthy controls. Median and min to max of n donors are shown. P values were determined by Mann-Whitney U test: * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. Control (white square), Acute (white circle), Convalescent (light grey circle), MIS-C (dark grey circle). Asymptomatic (lilac circle), mild (pink circle), moderate (violet circle), severe (purple circle).
We then analyzed the circulating B cell compartment. The frequency of B cells was similar in healthy donors [11.9% (9.4-20.9)], children with acute infection [16.2% (9.3-35.6)] and MIS-C [16.4% (11.3-29.3)], while convalescent children [21.6% (18-28.7) median (IQR)] showed a higher frequency of B cells, compared with healthy donors (p<0.05; Fig. 3e). The gating strategy used to identify plasmablasts is shown in Fig. S4. The frequency of plasmablasts was significantly increased in both, children with acute infection [0.21% (0.1-0.3)] and MIS-C [0.3% (0.06-0.52)] compared with convalescent children [0.08% (0.05-0.2) median (IQR), p<0.05 and p<0.01 for acute and MIS-C vs convalescents respectively, Fig. 3f]. Analysis based on disease severity showed no differences in the frequency of B cells (Fig. 3g). However, children with moderate [0.1% (0.06-0.1)] and severe [0.1% (0.06-0.2)] acute COVID-19 had lower frequencies of plasmablasts compared with asymptomatic [0.2% (0.1-0.4)] and mild patients [0.2% (0.1-0.7) median (IQR)] (Fig. 3h).
3.5. Children with severe COVID-19 display high levels of inflammatory cytokines in plasma
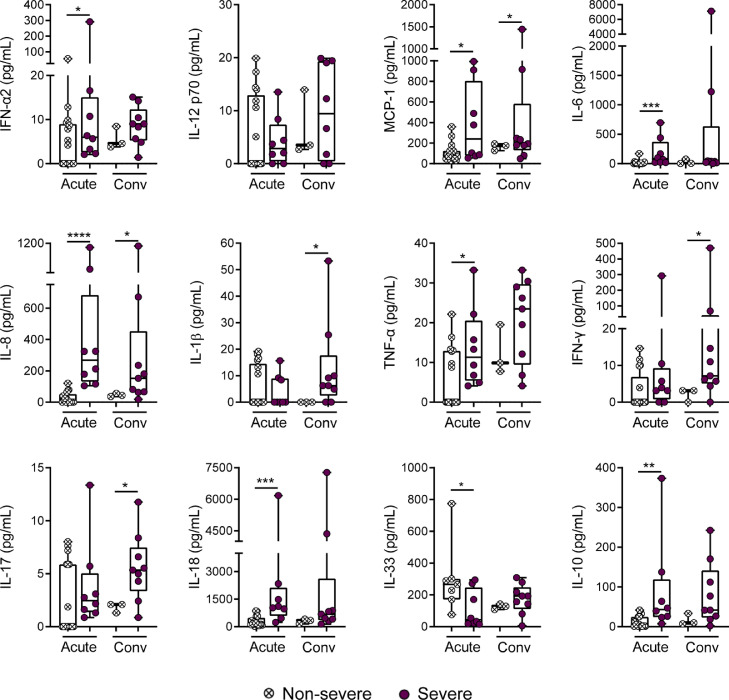
It is well established that severe COVID-19 in adults is strongly associated to the development of a strong and systemic inflammatory response [17], [18], [19]. We then analyzed the profile of inflammatory cytokines in the plasma of infected children. Patients were divided into two groups: non-severe children, including those patients with asymptomatic, mild and moderate disease; and severe patients. We found that disease severity was clearly associated with a systemic inflammatory response. We found a high plasma concentration of several inflammatory cytokines such as: IFN-α2 (6.7 pg/mL ± 3.2 vs 42.3 pg/mL ± 35.6, p<0.05), MCP-1 (109.9 pg/mL ± 21.1 vs 386.2 pg/mL ± 135.6, p<0.05), IL-6, (17.7 pg/mL ± 9.7 vs 192.1 pg/mL ± 87, p<0.001), IL-8 (25.6 pg/mL ± 9 vs 399.9 pg/mL ± 131.5, p<0.0001), TNF-α (5.65 pg/mL ± 1.8 vs 13.7 pg/mL ± 3.5, p<0.05), IL-18 (289.7 pg/mL ± 65 vs 1724 pg/mL ± 675, p<0.001), and the anti-inflammatory cytokine IL-10 (11.5 pg/mL ± 3.3 vs 89.7 pg/mL ± 42, p<0.01, for mean ± SEM in non-severe vs severe respectively). By contrast, IL-33 levels were lower in children with severe disease (301.1 pg/mL ± 84.2 vs 110.1 pg/mL ± 41.2, p<0.05). Interestingly, a number of cytokines such as MCP-1, IL-8, IL-1β, IL-17A, and IFN-γ remained significantly elevated in the severe group in the convalescent phase of infection (p<0.05; Fig. 4).
Fig. 4.
Plasma levels of cytokines in children with non-severe and severe COVID-19. Plasma levels of IFN-α2, IL-12p70, MCP-1 (CCL2), IL-6, IL-8, IL-1β, TNF-α, IFN-γ, IL-17A, IL-18, IL-33, and IL-10 were quantified by multiplex flow cytometric bead array in the acute (non-severe, n=17; severe, n=8) and convalescent (non-severe, n=3; severe, n=9) phases of infection. Median and min to max of n donors are shown. P values were determined by Mann-Whitney U test: * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001. Non-severe (white circle), severe (purple circle).
4. Discussion
Our study reveals that severe COVID-19 in children is associated with a poor and delayed production of IgG antibodies directed to the spike protein of SARS-CoV-2. This was observed in both, the acute and convalescence phases of the infection. Severe COVID-19 in children is rare being the clinical course of the infection typically mild and often asymptomatic. The percentage of children infected by SARS-COV-2 who develop a critical disease ranges from 2% to 7% across different studies [20], [21], [22]. Our cohort of 550 children with acute COVID-19 includes only eight children with severe disease (1.5%). Although our data was obtained from a multicentric study including a large cohort of children, our cohorts should not be considered as representative of the distribution of cases in our country.
Recent reports have analyzed the antibody response in children with COVID-19. Weisberg and coworkers [13] reported differences between the antibody response in adults with severe COVID-19 and infected children, mostly asymptomatic or with mild/moderate disease. The antibody response in the adult cohort was characterized by the production of IgG, IgM, and IgA antibodies directed to the spike and the nucleocapsid proteins of SARS-CoV-2. Children showed a reduced breadth of anti-SARS-CoV-2 antibodies characterized by the presence of IgG antibodies directed to the spike protein but not to the nucleocapsid protein. Moreover, they reported that infected children showed a reduced neutralizing activity compared to adults with severe disease. By studying the immune response in 69 children and adolescents with asymptomatic or mild disease, Garrido and coworkers [12] found a robust IgM, IgG, and IgA antibody responses to a broad array of SARS-CoV-2 antigens during acute infection as well as at 2-4 months after the onset of disease. The authors also reported that the antibody response and the serum neutralizing activity in children and adolescent were comparable or even higher than the response found in adults with mild symptomatic infection. In addition, Hachim and coworkers [23] showed that children with no or mild symptoms have lower levels of IgG antibodies directed to the spike and nucleocapsid proteins compared with adults, but they display a stronger antibody response directed to accessory proteins, i.e., NSP1 67 and Open Reading Frames. Finally, Anderson and coworkers [24] focused on the production of IgG antibodies directed to the spike and nucleocapsid proteins in children with mild and severe COVID-19 disease. By studying 10 children with asymptomatic or mild disease and 9 children with severe disease, the authors found that the first group of patients produced variable levels of IgG antibodies while the majority of children with severe disease had undetectable levels of IgG antibodies directed to either, the spike or the nucleocapsid proteins. However, the comparison between both groups did not reach statistical significance, perhaps reflecting the high dispersion of values found in the asymptomatic and mild group.
Previous studies indicated that adults with severe COVID-19 display a robust, yet delayed response humoral response compared to adults with milder disease [25], [26], [27], [28]. Our present results show that severe COVID-19 in children is associated with a very poor antibody response. In fact, contrasting with the observations made in asymptomatic and children with mild and moderate disease, no children with severe disease seroconverted under the acute phase of infection. Moreover, only 33% of children with severe COVID-19 (3 out of 9) seroconverted in the convalescent phase of infection while mildly infected children showed a seroconversion rate higher than 75%. In addition, within the cohort of children with severe COVID-19 we identified two different patterns. Two out of the 8 children studied seroconverted when analyzed in the convalescence period, reaching values similar to those found in patients with mild disease. By contrast, other 6 children remained seronegative. This suggests that a fraction of children with severe disease might mount a robust, yet delayed antibody response. Interestingly, the Iwasaki lab has recently reported that mortality in adults with COVID-19 does not correlate with antibody levels but rather with a delayed kinetic of neutralizing antibody production [28]. Due to the small size of our cohorts of children with severe COVID-19, our observations need to be confirmed on larger cohorts.
The subset of Tfh cells play a critical role in regulating the germinal center reaction and the production of both, high affinity IgG antibodies and memory B cells. Peripheral blood contains a low frequency of cTfh directly related to those found in lymph nodes [29]. To our knowledge, no previous studies have analyzed the frequency of cTfh in children with COVID-19 according their severity, although contradictory data have been reported in MIS-C [5,30]. Consistent with the poor antibody response observed in children with severe COVID-19, we found a low frequency of cTfh in the 4 patients analyzed. By contrast, a higher frequency of cTfh was found in MIS-C compared with healthy donors. In line with these findings, the analysis of circulating plasmablasts revealed a lower frequency in children with moderate and severe COVID-19 and a higher frequency of these cells in MIS-C, however differences with healthy donors or asymptomatic patients did not reach statistical significance.
Several studies have revealed that the hyper-inflammatory response induced by SARS-CoV-2 is a major cause of disease severity and death in adults [31,32]. Consistent with previous reports [6,[33], [34], [35]], and contrasting with our observations made in children with mild or moderate COVID-19, we found that severe disease was associated to a systemic inflammatory profile, revealed by the presence of high concentrations of inflammatory cytokines in plasma. Some of these cytokines remained elevated in the convalescent phase of infection, as observed in adults with severe COVID-19 [19,36]. Contrasting with findings in adults with COVID-19 [37] we found that children with severe COVID-19 did not increase the production of the alarmin IL-33, but rather they showed lower levels of IL-33 compared with children with non-severe COVID-19.
There are a number of limitations in our study. This work was conducted in a localized area of our country; hence, we cannot assume that our patient cohorts adequately reflect the general picture of our country. Moreover, as an observational study, there may be potential confounding factors. Hence, causality should not be inferred. The small number of patients in the cohort of children with severe COVID-19 require that our observations should be confirmed in a multicentric study including a large cohort of patients and the results should not be generalized until such work is done. Moreover, because comorbidities are highly prevalent in children with severe COVID-19, their contribution to the weak antibody response in severe diseases should be clarified. On the other hand, the small blood sample size collected from patients limited the performance of studies aimed at characterizing the phenotype and function of CD4+ T cell populations, including cTfh. Finally, neither chest CT scans nor laboratory proofs directed to depict the systemic inflammatory status of our cohorts (PCR, LDH, D-dimer, Ferritin) were available.
In summary, our study establishes a link between a weak and delayed antibody response and disease severity in children. This observation might contribute not only to define the basis of the protective immune response against SARS-CoV-2 infection in children, but also to define a small but vulnerable population of children at higher risk of severe COVID-19.
5. Contributors
I.S., C.R., V.S., A.V., M.P.H., and L.A. carried out experiments and analyzed data. S.C.R., S.C.A., M.U., N.D.C., D.F., M.S., E.C., S.V.N., M.J.R., P.C., J.L., and F.F. performed the ethical approval of the study, conceived the pediatric clinical cohorts and provided useful discussions. N.D.C. conceived the healthy pediatric cohort. N.A.G., S.D.L., H.C., M.J.C., C.N.M, G.M., M.M.P, L.I.G., G.G., A.L.A., C.D., M.J.B., N.S., M.F.P., V.N., C.B., L.A., M.R., P.T., M.T.A., H.P., P.S., E.M.T., and M.G. performed coordinated asymptomatic, mild, moderate and severe patient enrollment, collected all relevant clinical information and sample acquisition. M.S. performed the generation of the virus and cell line growing. C.P. carried out serologic studies. A.V. performed the neutralization assays. A.C. and M.L.P. offered suggestions for experiments and useful discussions. M.N., and J.M.G.P contributed to statistical analyses. I.S., S.C.R., J.G. and L.A. conceived the study, designed experiments, conducted data analyses, interpretation and draft the manuscript. S.C.R., J.G. and L.A. performed funding acquisition.
5.1. Data sharing statement
Any data not published within the article will be shared in anonymized format by request from any qualified investigator. If desired, please contact the corresponding author of this article.
Declaration of Competing Interest
None.
Acknowledgments
We express our sincerest thanks to the physicians, nurses, respiratory therapists, and advanced practice providers who cared for these patients for their contribution. Most of all, we are indebted to all the participating children and their families. See Supplemental Acknowledgments for consortium details.
This work was supported by grants from the National Agency for Promotion of Science and Technology, Argentina (IP-COVID-19-0277 and PMO BID PICT 2018-2548 to L.A.).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103615.
Appendix. Supplementary materials
References
- 1.Liu W., Zhang Q., Chen J. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. New England J. Med. 2020;382(14):1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Y., Mo X., Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 3.Lu X., Zhang L., Du H. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagarro A., Garcia-Salido A., Martinez E.V., Vega-Piris L., Mellado M.J. Low COVID-19 mortality in Spanish children. Lancet Child Adolescent Health. 2021;5(6) doi: 10.1016/S2352-4642(21)00125-5. e24-e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consiglio C.R., Cotugno N., Sardh F. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4) doi: 10.1016/j.cell.2020.09.016. 968-81 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diorio C., Henrickson S.E., Vella L.A. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130(11):5967–5975. doi: 10.1172/JCI140970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunyavanich S., Do A., Vicencio A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA. 2020;323(23):2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sajuthi S.P., DeFord P., Li Y. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun. 2020;11(1):5139. doi: 10.1038/s41467-020-18781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellon M., Baggio S., Bausch F.J. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America (NY) 2021. SARS-CoV-2 viral load kinetics in symptomatic children, adolescents and adults; p. ciab396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierce C.A., Sy S., Galen B. Natural mucosal barriers and COVID-19 in children. JCI Insight. 2021;6(9) doi: 10.1172/jci.insight.148694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrido C., Hurst J.H., Lorang C.G. Asymptomatic or mild symptomatic SARS-CoV-2 infection elicits durable neutralizing antibody responses in children and adolescents. JCI Insight. 2021 doi: 10.1172/jci.insight.150909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisberg S.P., Connors T.J., Zhu Y. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22(1):25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ojeda D.S., Gonzalez Lopez Ledesma M.M., Pallares H.M. Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina. PLoS Pathog. 2021;17(1) doi: 10.1371/journal.ppat.1009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vella L.A., Buggert M., Manne S. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest. 2019;129(8):3185–3200. doi: 10.1172/JCI125628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenna E., Davydov A.N., Ladell K. CD4(+) T follicular helper cells in human tonsils and blood are clonally convergent but divergent from Non-Tfh CD4(+) Cells. Cell Rep. 2020;30(1) doi: 10.1016/j.celrep.2019.12.016. 137-52 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wauters E., Van Mol P., Garg A.D. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res. 2021;31(3):272–290. doi: 10.1038/s41422-020-00455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulte-Schrepping J., Reusch N., Paclik D. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell. 2020;182(6) doi: 10.1016/j.cell.2020.08.001. 1419-40 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Valle D.M., Kim-Schulze S., Huang H.H. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou B., Yuan Y., Wang S. Risk profiles of severe illness in children with COVID-19: a meta-analysis of individual patients. Pediatr Res. 2021 doi: 10.1038/s41390-021-01429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey L.C., Razzaghi H., Burrows E.K. Assessment of 135794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatrics. 2021;175(2):176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zare-Zardini H., Soltaninejad H., Ferdosian F., Hamidieh A.A., Memarpoor-Yazdi M. Coronavirus Disease 2019 (COVID-19) in children: prevalence, diagnosis, clinical symptoms, and treatment. Int J General Med. 2020;13:477–482. doi: 10.2147/IJGM.S262098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hachim A., Gu H., Kavian O., The SARS-CoV-2 antibody landscape is lower in magnitude for structural proteins, diversified for accessory proteins and stable long-term in children. medRxiv, 2021, (Jan 4):2021.01.03.21249180.
- 24.Anderson E.M., Diorio C., Goodwin E.C. SARS-CoV-2 antibody responses in children with MIS-C and mild and severe COVID-19. J Ped Infect Dis Soc. 2020:piaa161. doi: 10.1093/jpids/piaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathew D., Giles J.R., Baxter A.E. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508) doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuri-Cervantes L., Pampena M.B., Meng W. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5(49) doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Beltran W.F., Lam E.C., Astudillo M.G. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184(2) doi: 10.1016/j.cell.2020.12.015. 476-88 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas C., Klein J., Sundaram M.E. Delayed production of neutralizing antibodies correlates with fatal COVID-19. Nat Med. 2021;27:1178–1186. doi: 10.1038/s41591-021-01355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita R., Schmitt N., Bentebibel S.E. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vella L.A., Giles J.R., Baxter A.E. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci Immunol. 2021;6(57):eabf7570. doi: 10.1126/sciimmunol.abf7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thwaites R.S., Sanchez Sevilla Uruchurtu A., Siggins M.K. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci Immunol. 2021;6(57):eabg9873. doi: 10.1126/sciimmunol.abg9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadjadj J., Yatim N., Barnabei L. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun D., Li H., Lu X.X. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Ped. 2020;16(3):251–259. doi: 10.1007/s12519-020-00354-4. WJP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venkataraman A., Kumar N.P., Hanna L.E. Plasma biomarker profiling of PIMS-TS, COVID-19 and SARS-CoV2 seropositive children - a cross-sectional observational study from southern India. EBioMed. 2021;66 doi: 10.1016/j.ebiom.2021.103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu W., Yang L., Li X. Early immune responses and prognostic factors in children with COVID-19: a single-center retrospective analysis. BMC Pediatrics. 2021;21(1):181. doi: 10.1186/s12887-021-02561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mann E.R., Menon M., Knight S.B. Longitudinal immune profiling reveals key myeloid signatures associated with COVID-19. Sci Immunol. 2020;5(51):eabd6197. doi: 10.1126/sciimmunol.abd6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang Y., Ge Y., Sun J. IL-33 in COVID-19: friend or foe? Cellular Mol Immunol. 2021;18:1602–1604. doi: 10.1038/s41423-021-00685-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.