Abstract
Aims and Objectives
More than half of the world’s population is infected with Helicobacter pylori, which can cause chronic gastritis. WHO has regarded clarithromycin-resistant H. pylori as a high priority pathogen. Hence, accurate diagnosis and detection of clarithromycin- and levofloxacin-resistant H. pylori strains is essential for proper management of infection. The objective of this study was to develop and optimize multiplex quantitative PCR assay for detection of mutations associated with clarithromycin and levofloxacin resistance in H. pylori directly from the gastric biopsies.
Materials and Methods
Specific primers and probes were designed to amplify ureA and mutations in 23S rRNA and gyrA genes. Singleplex and triplex qPCR assays were optimized and the assay’s sensitivities and specificities were determined. The optimized multiplex qPCR assay was performed on 571 gastric biopsies.
Results
In this study, 14.7% (84/571) of the gastric biopsies were positive for H. pylori by conventional methods and 23.8% (136/571) were positive by the ureA-qPCR with 96.4% sensitivity and 88.5% specificity, while the +LR and −LR were 8.72 and 0.04, respectively. The ureA-positive samples (n=136) were subjected to multiplex qPCR which detected A2142G and A2143G mutations in the 23S rRNA gene (20.6%, 28/136) conferring clarithromycin resistance and gyrA mutations N87K, N87I, D91N, and D91Y (11.8%, 16/136) leading to levofloxacin resistance. The sensitivity and specificity of qPCR of 23S rRNA gene were 100% and 98.7%, respectively, while 100% and 99.8% for qPCR of gyrA, respectively.
Conclusion
The effectiveness of this qPCR is that it is sensitive in detecting low bacterial load and will help in timely detection of clarithromycin- and levofloxacin-resistant strains, especially in case of mixed infections. Since it is culture independent, it can inform clinicians about antibiotics to be included in the first-line therapy, thereby improving the management of H. pylori infection at a much greater pace.
Keywords: Helicobacter pylori, multiplex qPCR, resistance, mutation, clarithromycin, levofloxacin
Introduction
Helicobacter pylori is a common pathogen that infects nearly 50% of the global population.1 Infection with this pathogen causes chronic gastritis, which can cause chronic gastroduodenal diseases such as gastritis, gastric ulcer, duodenal ulcer, gastric cancer, and gastric mucosa-associated lymphoid tissue (MALT) B-cell lymphoma.2 Although H. pylori is the strongest known risk factor that account for 80% or more of gastric cancers, it is noteworthy that only 3% of infected patients subsequently develop gastric cancer.3
Antibiotic resistance in H. pylori is an important factor affecting the effectiveness of current therapeutic regimens. In the general population, the prevalence of bacterial resistance has been associated with antibiotic consumption and it varies in different geographic areas.4 Antimicrobial resistance is the main factor leading to therapeutic failure, especially in the case of clarithromycin, which can induce a 70% loss of effectiveness in eradication of H. pylori as a part of proton pump inhibitor (PPI)/amoxicillin-based triple therapy.5,6 Levofloxacin has been recommended as salvage therapy and is shown to be more effective than quadruple therapy in second- and third-line treatments of H. pylori infection.7 However, resistance to clarithromycin or levofloxacin is the major reason for eradication failure after clarithromycin- or levofloxacin-based triple therapy, respectively.8
Clarithromycin and levofloxacin resistance are exclusively caused by specific mutations in a small region of the responsible genes, therefore molecular methods offer an alternative to conventional techniques for detection of these mutations.9 Clarithromycin resistance in H. pylori is caused by mutations in the peptidyltransferase region encoded in domain V of the H. pylori 23S ribosomal RNA (rRNA) gene in the 50S ribosomal subunit. Clarithromycin stimulates the release of peptidyl-tRNA from the A site (aminoacyl accepter site), blocking the elongation of the nascent peptide chain during bacterial protein synthesis.10 Mutations in the V domain of the 23S rRNA gene are the main cause of clarithromycin resistance in H. pylori. The most frequent mutations are A2143G, A2142G, and A2142C, which account for more than 80% of clarithromycin resistance in H. pylori. Other mutations such as A2115G, G2141A, C2147G, T2190C, C2195T, A2223G, and C2694A have also been reported, but their role in clarithromycin resistance is unclear.11 The quinolone resistance determining region (QRDR) is a short conserved region within the gyrA (codon 74–113) and gyrB (codon 500–538) genes that has been shown to be associated with levofloxacin resistance.12 Resistance to levofloxacin in H. pylori is mediated by the mutations at amino acid position 87 (Asn87) and 91 (Asp91) in the QRDR of the gyrA gene which has a more critical impact than gyrB.13,14 Mutations in the gyrB are also reported in levofloxacin resistant strains, but often occur along with gyrA mutations.15 The knowledge of resistance mechanisms may contribute to elaborate more rational antibiotic combinations with the aim of improving treatment success. There are various methods used to detect the presence of H. pylori infection and it can be categorized into two types, ie, invasive and non-invasive methods. These methods have their own advantages, and limitations.16 Culture of H. pylori from biopsies is the gold standard for antibiotic susceptibility testing and is the most specific method for H. pylori detection. However, sensitivity of culture may be compromised, by strain-fastidiousness, the effect of therapy causing low cell density or altered morphologic features and loss of viability and/or overgrowth of contaminating microorganisms.16
Development of a rapid and reliable high-throughput method for detection of H. pylori resistance is expected to be highly useful for assisting in H. pylori management. This is particularly relevant in recognizing that culture and susceptibility testing of H. pylori should be mandatory after first treatment failure. Molecular detection of H. pylori using a PCR-based method is able to detect low amounts of bacterial DNA and can aid in detection of H. pylori at a much higher rate. Development of molecular methods for the detection of mutations involved in clarithromycin and/or levofloxacin resistance H. pylori in stool samples has been reported in several studies.17–21 Quantitative PCR (qPCR) represents a highly sensitive and powerful technology for the quantification of Helicobacter DNA in gastrointestinal samples. As compared to other available methods for clinical and research purposes, qPCR is highly reliable, rapid, and sensitive for diagnosis of H. pylori infection.22 To date, qPCR has been used extensively for the evaluation of antibiotic sensitivity of H. pylori in biopsy samples, specifically for the detection of clarithromycin resistance.21,23–25 Conversely, there are only a few available qPCR assays for detection of H. pylori and mutations conferring resistance to both clarithromycin and levofloxacin antibiotics.26,27 Therefore, the objective of this study was to develop and evaluate a multiplex qPCR assay for detection of H. pylori and gene mutations conferring clarithromycin and levofloxacin resistance in H. pylori in gastric biopsy specimens. This assay will provide us with a new tool along with rapid detection method and standard protocols for management and monitoring, and in improving outcomes of H. pylori eradication therapy.
Materials and Methods
Patients and Gastric Biopsy Specimens
Two hundred and eighty-eight patients with chronic dyspepsia undergoing upper oesophagogastroduodenoscopy (OGDS) at the Endoscopy Unit, Universiti Kebangsaan Malaysia Medical Centre (UKMMC), Kuala Lumpur, Malaysia from April 2014 to August 2015 were included in this study. Patients on antibiotics or nonsteroidal anti-inflammatory drugs within 4 weeks prior to OGDS and those with gastric malignancy or immunosuppression were excluded. Three biopsies were obtained from antrum and/or corpus of the stomach from each patient. The biopsies were placed individually in different tubes for determination of H. pylori by conventional methods including culture, rapid urease test, and histological examination, as highlighted in the previous study.28 A total of 571 gastric biopsies were obtained and H. pylori infection was determined in these biopsies by culture (n=571), rapid urease test (n=571), and histological examination (n=569; two biopsies were not suitable).
Clinical H. pylori Isolates
Culture was deemed positive when H. pylori growth was identified as Gram-negative curved/spiral rods by Gram staining and urease, catalase, and oxidase positive.28 A total of 59 H. pylori were isolated by culture. Antibiotic susceptibility testing of clarithromycin and levofloxacin was performed as described in our previous study28 using E-test and mutations were identified by gene sequencing.
Among the 59 H. pylori isolates; H. pylori strains A3, A26, C81, C55, A142, A129, A150, A177, and C192 were used as positive controls. All are curved/spiral shaped rods, urease-, catalase-, and oxidase-positive and positive for glmM,29 as determined by conventional PCR. H. pylori strain C55 was used as a positive control for H. pylori detection by ureA-qPCR. Sequencing confirmed the genetic mutations of the 23S rRNA gene and gyrA of H. pylori in strains A3, A26, C81, A142, A129, A150, A177, and C192, which were used as positive controls for detection of clarithromycin or levofloxacin resistance. H. pylori strains A177 and A142 harboured the A2142G and A2143G mutations, respectively. H. pylori strains A129, A150, and C192 were wild-type strain, strain harbouring the N87K and N87I mutations, respectively. While H. pylori strains A3, A26, and C81 were wild-type strain, strain harbouring the D91N and D91Y mutations, respectively.
DNA Extraction
DNA from 59 H. pylori isolates and 571 gastric biopsies (used for rapid urease test) were extracted using FavorPrepTM Tissue Genomic DNA Extraction Mini Kit (Favorgen Biotech Corporation, Taiwan) according to the manufacturer’s instructions. The extracted DNA was stored at −20°C until use. Concentration and purity of DNA sample were measured by determining the absorbance of optical density at the wavelength of 260 nm and 280 nm using an ultraviolet (UV) spectrophotometer (Multiskan GO, Thermo Fisher Scientific, USA). Samples with low (<1.5) or high (>2.0) OD260/OD280 ratio were not used for qPCR, and the extraction was repeated.
Primers and Probes Design
GenBank was searched for sequences of H. pylori ureA gene. The published sequences of H. pylori 23S rRNA gene (Accession no. KC556778), gyrA (Accession no. L29481), the wild type H. pylori ATCC 26695, and sequences from clinical H. pylori strains were aligned using CLUSTAL W Multiple Alignment30 in Bioedit Version 7.2.5, and primers and probes were designed by using the online PrimerQuest Tool on the IDT website (https://sg.idtdna.com/PrimerQuest/Home/Index). Primers and probes for ureA were selected from a region with 100% homology between all published H. pylori ureA encoding sequences. The BLAST search revealed 100% homology to the H. pylori-specific ureA gene only. Primers and probes for the 23S rRNA gene were designed to target the location of the two adjacent adenines where mutations A2142 and A2143 occur conferring resistance to clarithromycin in the 23S rRNA gene. Primers and probes for the gyrA gene were designed targetting mutations in codon positions 87 and 91 of the H. pylori gyrA (N87K and N87I, and D91N and D91Y). A BLAST search was performed to check the specificity of the DNA sequences of the primers and probes (http://www.ncbi.nlm.nih.gov/BLAST/). The properties of primers and probes were assessed for the optimized melting temperatures, secondary structure, base composition, and amplicon lengths. Potential self-annealing and hairpin formation were checked using free oligo self-complementarity check software (http://biotools.nubic.northwestern.edu/OligoCalc.html). The primers and probes were synthesized by Integrated DNA Technologies (Singapore Science Park II, Singapore) and Macrogen (Synapase, Singapore) and are listed in Table 1.
Table 1.
Oligonucleotides Sequence Used in This Study
| Primers/Probes | Sequences (5′→3′) | Targets | Product (bp) |
|---|---|---|---|
| UreA-Fa | AGTTCCTGGTGAGTTGTTCTT | ureA | 120 |
| UreA-Rb | TGGAAGTGTGAGCCGATTT | ||
| Cla-Fa | CTGCGCATGATATTCCCGTT | 23S rDNA | 157 |
| Cla-Rb | CGACCTGCATGATGGCGTA | ||
| Lev-Fa | CGCCATCAATAGAGCCAAAG | gyrA | 136 |
| Lev-Rb | GTGGGTGATGTGATTGGTAAAT | ||
| UreA-Pc | FAM-AAATGTTGGCGACAGACGGTTC-TAMRA | ureA | |
| Cla-PWTc | Cy5-AAGGTCCACGGGGTCTTTCCGTC-BHQ2 | Wild type | |
| Cla-P2142Gc | HEX-AGGTCCACGGGGTCTTCCCGTCT-BHQ2 | A2142G | |
| Cla-P2143Gc | FAM-AGGTCCACGGGGTCTCTCCGTCTT-BHQ2 | A2143G | |
| Lev-N87WTc | HEX-CCG+C+G+TTA+T+CG-IowaBlack®FQ | Wild type | |
| Lev-N87Kc | 6-FAM-CCG+C+T+TT+A+T+CGC-IowaBlack®FQ | N87K | |
| Lev-N87Ic | Cy5-CCG+C+A+AT+A+T+CGC-IowaBlack®RQ-Sp | N87I | |
| Lev-D91WTc | HEX-TA+T+G+AT+GCG+CT-IowaBlack®FQ | Wild type | |
| Lev-D91Nc | Cy5-TTA+T+A+ATG+C+G+CTAGT-IowaBlack®RQ-Sp | D91N | |
| Lev-D91Yc | 6-FAM-TTA+T+T+ATG+C+G+CTAGT-IowaBlack®FQ | D91Y |
Notes: aForward primer, bReverse primer, cTaqMan probe.
Detection of H. pylori in Biopsy Samples by qPCR
For the ureA detection, the reaction mixture was prepared in a final volume of 20 µL at the final concentration containing 10 µL of 1×Probe PCR master mix reaction buffer (contains Tris-Cl, KCl, NH4Cl, MgCl2, dNTP, and QuantiNova DNA Polymerase) (QuantiNovaTM Probe PCR kit, Qiagen, Corbett Robotics, Australia), 0.5 µM primer UreA-F, 0.5 µM primer UreA-R, 0.2 µM of UreA-probe, 2.5 µL template DNA, and RNase free water. The amplification reaction was performed using Rotor-Gene Q (Qiagen, Corbett Robotics, Australia), with preliminary denaturation for 2 minutes at 95°C, followed by 40 amplification cycles of denaturation at 95°C for 5 seconds and combined annealing/extension step for 5 seconds at 60°C. Samples were run in duplicate and were considered positive if both reactions were positive. DNAs of two H. pylori reference strains (ATCC 43526 and J99) and one clinical strain C55 were used as positive with negative controls containing DNA template replaced with sterile distilled water. A standard curve was generated using 10-fold serially diluted samples of DNA extracted from H. pylori strain C55, varying from 100 ng/µL to 1 fg/µL (100 to 10−7) of 1.22×108 CFU/mL which were used as a template for qPCR. The fluorescence reading for each sample was taken at the annealing step green channel [6-carboxyfluorescein (FAM)]. To verify the amplified ureA, all qPCR products were electrophoresed on 1.5% agarose gel and visualized under UV light with GelStain staining.
In order to exclude the effect of human DNA on the H. pylori DNA quantification, the human albumin gene (human genome consists of only one copy of the albumin gene) was amplified to quantify the number of human cells in the biopsy using the following primers: Forward (5´-CTGCATTGCCGAAGTGGAA-3´) and Reverse (5´-CAAACATCCTTACTTTCAACAAAATCA-3´), and the probe (5´-FAM-TGCCTGCTGACTTGCCTTCATTAGCTG-3´-TAMRA).31 DNA extracted from human whole blood was used to determine the DNA concentration and purity, and used as a template for standard curve for human cells. Conversion of DNA template (ng) to copy number (C, copies/μL) in each target was carried out using the formula as described previously.32 H. pylori DNA load in gastric biopsy was expressed as the quotient between the copy number of microorganisms and copy number of human cells in each gastric sample31 multiplied by 100 to provide the number of copies of H. pylori DNA per 100 human cells.33
The limit of detection of the ureA qPCR assay was determined using 10 serially diluted DNA samples of H. pylori strain C55. DNA concentration ranging from 100 ng/µL to 0.01 fg/µL were used as a template for qPCR. The specificity of the primers and probes were investigated by applying the DNA of various bacterial species, including urease producing bacterial strains (clinical isolates) such as Staphylococcus aureus, Acinetobacter spp., Vibrio parahaemolyticus, Proteus mirabilis, Serratia spp., Staphylococcus epidermidis, Enterobacter spp., Klebsiella pneumoniae, and Bacillus subtilis. H. pylori ATCC 43526 and H. pylori J99 were used as positive control.
Detection of Point Mutation in the 23S rRNA Gene of H. pylori in Biopsy Samples by Multiplex qPCR
The ureA-positive samples were subjected to multiplex qPCR for the detection of mutation in 23S rRNA gene. The reaction mixture was prepared in a final volume of 20 µL containing five µL of 1× Multiplex PCR master mix (contains QuantiNova DNA Polymerase, QuantiNova multiplex PCR buffer and dNTP mix) (QuantiNovaTM Multiplex PCR kit, Qiagen, Corbett Robotics, Australia), 0.4 µM primer Cla-Fw, 0.4 µM primer Cla-Rw, 0.25 µM of ClaPWT, Cla-P2142G and Cla-P2143G TaqMan probe, 2.5 µL of template DNA and RNase free water. The amplification was performed using Rotor-Gene Q (Qiagen, Corbett Robotics, Australia) with conditions of preliminary denaturation for 2 minutes at 95°C, followed by 40 amplification cycles of denaturation at 95°C for 5 seconds and combined annealing/extension step for 30 seconds at 60°C. Samples were run in duplicate and were considered positive if both reactions were positive. H. pylori J99 strain and clarithromycin resistant A177 (mutation A2142G) and A142 (mutation A2143G) were used as positive control with sterile distilled water used in negative control. The standard curves were generated individually using 10-fold dilutions of DNA ranging from 72 ng to 7.2 fg of 1.15×108 CFU/mL for wild-type strain (H. pylori J99), 150 ng to 15 fg of 1.1×108 CFU/mL for H. pylori A177, and 100 ng to 10 fg of 1.38×108 CFU/mL for A142. The seven 10-fold serial dilutions of each DNA were used as a template for qPCR. The fluorescence reading for each sample was taken at the annealing step on red channel (Cy5), yellow channel (HEX), and green channel [6-carboxyfluorescein (FAM)]. To verify the amplified 23S rRNA gene sequence, all qPCR products were electrophoresed on 1.5% agarose gel and visualized under UV light with GelStain staining.
Singleplex PCR was performed before conducting multiplex reactions in order to validate the performance of qPCR assay. The reaction mixture was prepared in a final volume of 20 µL containing 5 µL of 1×Multiplex PCR master mix (QuantiNovaTM Multiplex PCR kit, Qiagen, Corbett Robotics, Australia), 0.4 µM primer Cla-Fw, 0.4 µM primer Cla-Rw, 0.25 µM of ClaPWT or Cla-P2142G or Cla-P2143G TaqMan probe, and 2.5 µL of template DNA and RNase free water. For multiplex reaction, the reaction mixture was prepared in a final volume of 20 µL containing 5 µL of 1×Multiplex PCR master mix (QuantiNovaTM Multiplex PCR kit, Qiagen, Corbett Robotics, Australia), 0.4 µM primer Cla-Fw, 0.4 µM primer Cla-Rw, 0.25 µM of ClaPWT, Cla-P2142G, and Cla-P2143G TaqMan probe and 2.5 µL of template DNA and RNase free water.
The above reactions of singleplex and multiplex were proceeded independently and the Cq values of the assay were compared. The values obtained for a given target in the singleplex and multiplex assays should not differ significantly.
In order to evaluate the sensitivity of the multiplex qPCR assay, 10-fold dilutions of DNA ranging from 72 ng to 0.0072 fg of 1.15×108 CFU/mL for wild-type strain (H. pylori J99), 150 ng to 0.0015 fg of 1.1×108 CFU/mL for H. pylori A177 and 100 ng to 0.001 fg of 1.38×108 CFU/mL for H. pylori A142 were prepared independently. The bacterial DNA was used as a template for qPCR.
The specificity of the 23S rRNA gene qPCR assay was determined by testing other bacterial strains including Acinetobacter spp., B. subtilis, S. flexneri, P. mirabilis, Serratia spp, S. typhimurium, E. coli, S. epidermidis, P. aeruginosa, Enterobacter spp., K. pneumoniae, V. parahaemolyticus, V. cholera, S. aureus, and Flavobacterium spp. H. pylori ATCC 43526 and H. pylori J99 were used as positive controls.
Detection of Point Mutation in gyrA from Biopsy Samples by Multiplex qPCR
The ureA-positive samples were subjected to multiplex qPCR for detection of the two different sites of mutation in gyrA (amino acids positions 87 and 91). For gyrA amplification at amino acid position 87 (136 bp), the reaction mixture was prepared in a final volume of 20 µL containing 5 µL of 1×Multiplex PCR master mix (QuantiNovaTM Multiplex PCR kit, Qiagen, Corbett Robotics, Australia), 0.4 µM primer Levo-Fw, 0.4 µM primer Levo-Rw, 0.25 µM of Levo-N87WT, Levo-N87K and Levo-N87I TaqMan probe, 2.5 µL of template DNA and RNase free water. H. pylori A129 (the wild-type), H. pylori A150 (levofloxacin-resistant harbouring mutation N87K) and H. pylori C192 (levofloxacin-resistant harbouring mutation N87I) were used as positive controls with sterile distilled water in the negative control. The standard curves were generated individually using 10-fold dilutions of DNA ranging from 100 ng to 10 fg of 1.28×108 CFU/mL for wild-type strain (H. pylori A129), 160 ng to 16 fg of 1.45×108 CFU/mL for H. pylori A150, and 145 ng to 14.5 fg of 1.31×108 CFU/mL for H. pylori A192. The 10-fold serial dilutions of each DNA were used as a template for qPCR.
For gyrA amplification at amino acid position 91 (136 bp), the reaction mixture was prepared in a final volume of 20 µL at a final concentration containing 5 µL of 1×Multiplex PCR master mix (QuantiNovaTM Multiplex PCR kit, Qiagen, Corbett Robotics, Australia), 0.4 µM primer Levo-Fw, 0.4 µM primer Levo-Rw, 0.25 µM of Levo-D91WT, Levo-D91N and Levo-D91Y TaqMan probe, 2.5 µL of template DNA, and RNase free water. H. pylori strains A3 (wild-type), A26 (levofloxacin-resistant harbouring mutation D91N), and C81 (levofloxacin-resistant harbouring D91Y) were used as positive controls with sterile distilled water added in the negative control. The standard curves were generated individually using 10-fold dilutions of DNA ranging from 120 ng to 12 fg of 1.59×108 CFU/mL for H. pylori strain A3, 110 ng to 11 fg of 1.25×108 CFU/mL for H. pylori A26, and 180 ng to 18 fg of 1.98×108 CFU/mL for H. pylori C81. The 10-fold serial dilutions of each DNA were used as a template for qPCR.
The amplification reaction was performed using Rotor-Gene Q (Qiagen, Corbett Robotics, Australia) with conditions of preliminary denaturation for 2 minutes at 95°C, followed by 40 amplification cycles of denaturation at 95°C for 5 seconds and combined annealing/extension step for 30 seconds at 60°C. Samples were run in duplicate and were considered positive if both reactions were positive. The fluorescence reading for each sample was taken at the annealing step on red channel (Cy5), yellow channel (HEX), and green channel [6-carboxyfluorescein (FAM)]. To verify the detected gryA sequence, all qPCR products were electrophoresed on 1.5% agarose gel and visualized under UV light with GelStain staining.
In order to validate the gyrA-N87 and gryA-D91 multiplex qPCR assay, the primer optimization, the performance of singleplex and multiplex reactions, the sensitivity and specificity of the multiplex qPCR assay, and the performance of multiplex qPCR to detect the multiple strains in the same biopsy sample were performed following the protocol as described above for detection of mutations in the 23S rRNA gene.
The discrepancy of results between qPCR and E-test were analyzed by sequencing using BigDye terminator v3.1 sequencing kit on an ABI 3730×L sequencer (MyTACG Bioscience Enterprise, Selangor, Malaysia). The primers used were Cla primers for detection of clarithromycin and Lev primers for detection of levofloxacin resistance (Table 1). After sequencing, a BLAST search was performed to check the sequence similarity/identity of the DNA sequences.
qPCR Parameters and Acceptance Criteria
The qPCR measures the fluorescence at each annealing step. Rotor-Gene Q series software was used to analyze the data. An amplification plot was generated showing the increase in the reporter dye fluorescence (Rn) with each cycle of PCR. Quantification of input target amount was analyzed by quantification cycle (Cq) value that is the point at which the sample of PCR amplification plot crosses the threshold. An acceptable standard curve was considered if a difference of −3.1±0.5 cycles was demonstrated between each of the 10-fold dilutions, and highly efficient and reproducible reactions if the correlation coefficient (R2) from all independent experiments were in the range of 0.90–1.0 (R2=0.90–1).
Statistical Analysis
Statistical analysis was performed using SPSS software version 23 (SPSS Inc, Chicago, IL, USA). Differences between groups of the categorical data were statistically evaluated using Pearson Chi-square (χ2) test. The independent samples t-test is used to determine the positivity results between singleplex and multiplex qPCR. Differences of qPCR-negative and qPCR-positive were statistically evaluated using one sample Chi-square (χ2) test. Kappa and McNemar tests were used to compare consistency between different detection methods. Differences were considered significant when the P-value was <0.05. The accuracy, sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (-LR) of the qPCR results were calculated.
Results
Detection of H. pylori in Biopsy Samples by qPCR
The specificity of the species-specific primers and qPCR protocol was optimized for the reference strains of H. pylori (H. pylori ATCC 43526 and H. pylori J99) and nine urease-producing strains. The H. pylori reference strains yielded specific qPCR amplification at Cq≤37 cycles with expected amplicon of 120 bp. The species of different genera show amplification at Cq≥37 cycles (Supplement 1) suggesting that other bacterial DNA of different species did not cross-react with the primers, hence our primers were specific for H. pylori. The standard curve for quantification was generated using the optimized qPCR amplification assay conditions based on DNA concentration ranging from 100 ng to 0.01 fg. The standard curves showed correlation coefficients (R2) of 0.990, and the quantification detection limits were 1 fg per reaction (Supplement 1). If one bacterium corresponds to 1.8 fg of DNA (1,667,867 bp) per genome,34 so 1 fg equals 0.56 bacterial cell. Agarose gel electrophoresis of the qPCR products (120 bp) confirmed the desired quality of amplification. The qPCR products were sequenced to verify the amplified ureA with 100% homology with H. pylori ureA in GenBank.
The optimized qPCR was performed to amplify ureA fragments of H. pylori in all 571 gastric biopsies. The cut-off values that distinguish the positive and negative detection were calculated using Receiver Operating Characteristic (ROC) curve analysis for the normalized counts (bacterial density) (Supplement 2). The area under the ROC curve (AUC) was obtained (Table 2). The optimal cut-off points were calculated by definition of a positive result as the number of microorganisms that maximized the weighted combination of sensitivity and specificity (ie, that maximized the Youden index (J)=sensitivity+specificity−1).39 Based on the curve coordinates obtained, a sample was considered positive if the value off 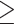 0.5 copies number per 100 human cells was chosen to maintain the highest Youden index of 98.5% (Table 2). The area under the ROC curve was 0.999 (95% confidence interval: 0.998–0.999) (Table 2), which indicates a good predictive of qPCR using this cut-off value.
0.5 copies number per 100 human cells was chosen to maintain the highest Youden index of 98.5% (Table 2). The area under the ROC curve was 0.999 (95% confidence interval: 0.998–0.999) (Table 2), which indicates a good predictive of qPCR using this cut-off value.
Table 2.
Performance of the qPCR of ureA for Detection of H. pylori in 571 Biopsy Samplesa
| AUCb (95% Cl) | Cut-Off | J (%) | No. of Biopsies with the Following Result: | Sp (%) (95% Cl) | Se (%) (95% Cl) | PPV (95% Cl) | NPV (95% Cl) | +LR | –LR | Accuracy (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | ||||||||||
| 0.999 (0.998–0.999) | 0.5 | 98.5 | 80 | 432 | 56 | 3 | 88.5 (85.3–91.1) | 96.4 (89.1–99.1) | 58.8 (50.1–67.1) | 99.3 (97.8–99.8) | 8.72 | 0.04 | 89.7 |
Notes: aTrue-positive (TP) biopsies were defined as biopsies positive by both the qPCR and conventional methods. True-negative (TN) biopsies were defined as biopsies negative by both the qPCR and conventional methods. False-positive (FP) biopsies were defined as biopsies negative by conventional and positive by the qPCR technique. False-negative (FN) biopsies were defined as biopsies positive by conventional and negative by qPCR. bAUC=Area under the curve ROC, Cut-off denotes the cut-off points considering as positive any number of microorganisms (qPCR≥0.5 copy number/100 human cells) that is maximized the weighted combination of sensitivity and specificity (ie, that maximized the Youden index). J, Youden index=sensitivity+specificity-1. Kappa=0.67, P (McNemar)<0.0001.
Abbreviations: Sp, specificity; Se, sensitivity; PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; –LR, negative likelihood ratio.
The distribution of Cq values among qPCR-positive and -negative biopsy samples were analyzed to determine the Cq cut-off (Supplement 3). The distribution of Cq values allowed us to define an optimal cut-off at ≤30 cycles. The cut-off point at 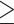 0.5 copies number is confirmed by the fact that Cq values obtained from all positive biopsy samples (n=136) were lower than and equal to 30 cycles. No biopsy samples that were positive for qPCR of ureA exhibit at Cq values higher than 30 cycles (Supplement 3). Therefore, the qPCR result was considered positive if it resulted in an amplification plot with Cq≤30, ie,
0.5 copies number is confirmed by the fact that Cq values obtained from all positive biopsy samples (n=136) were lower than and equal to 30 cycles. No biopsy samples that were positive for qPCR of ureA exhibit at Cq values higher than 30 cycles (Supplement 3). Therefore, the qPCR result was considered positive if it resulted in an amplification plot with Cq≤30, ie, 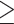 0.5 copies number per 100 human cells.
0.5 copies number per 100 human cells.
For the detection of H. pylori by conventional methods for comparison, a biopsy sample was regarded as positive when it was positive by either culture, rapid urease test, or histology. The positive detection rate for qPCR assay was significantly higher than conventional methods [23.8%, (136/571) vs 14.7% (84/571), P<0.0001]. A total of 136 H. pylori positive biopsies by ureA-qPCR were detected in 92 out of 288 patients. These biopsies were from antrum and/or corpus stomach of the patients. The qPCR using biopsies from different sites of the stomach showed consistent results. The performances of qPCR in comparison to the results of conventional methods as the gold standard were analyzed. Based on the optimal cut-off value for qPCR, the sensitivity, specificity, and accuracy of the qPCR were 96.4% (95% confidence interval [CI]=89.1–99.1%), 88.5% (95% CI=85.3–91.1%) and 89.7%, respectively (Table 2). The qPCR results in the present study show acceptable sensitivity, specificity, and accuracy for detection of H. pylori in biopsy samples. The positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), negative likelihood ratio (−LR) and were 58.8% (95% CI=50.1–67.1%), 99.3% (95% CI=97.8–99.8%), 8.72, and 0.04, respectively (Table 2). The Kappa value indicates a substantial degree of agreement (Kappa=0.67, P<0.0001) of the qPCR assay and conventional methods for detection of H. pylori in gastric biopsy samples.
The qPCR assay enabled detection and quantification of H. pylori in biopsy samples. A total of the 571 biopsies were tested, 23.8% (136/571) were positive by qPCR based on the optimal cut-off value (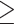 0.5 copies number per 100 human cells at the Cq≤30 value), while 76.2% (435/571) were negative for H. pylori (<0.5 copy number in the sample at the Cq>30 value). The H. pylori-positive biopsies by qPCR yielded amplification products with a predicted size of 120 bp. The sequences alignment showed 100% homology with published H. pylori ureA in GenBank. There was a significant difference among the H. pylori-positive and H. pylori-negative samples detection by qPCR (p<0.0001).
0.5 copies number per 100 human cells at the Cq≤30 value), while 76.2% (435/571) were negative for H. pylori (<0.5 copy number in the sample at the Cq>30 value). The H. pylori-positive biopsies by qPCR yielded amplification products with a predicted size of 120 bp. The sequences alignment showed 100% homology with published H. pylori ureA in GenBank. There was a significant difference among the H. pylori-positive and H. pylori-negative samples detection by qPCR (p<0.0001).
Optimization of multiplex qPCR of 23S rRNA gene, multiplex qPCR of gyrA87, and multiplex qPCR of gyrA91
The parameters used for validation of multiplex qPCR of 23S rRNA gene, qPCR of gyrA87, and qPCR of gyrA91 were primer concentration, singleplex qPCR reaction, triplex qPCR reaction, and singleplex versus triplex qPCR reactions. The optimum of triplex qPCR reaction determined the limit of triplex qPCR detection, specificity of triplex qPCR, and detection of multiple strains in the same biopsy samples. The key factors examined were the performance of singleplex versus triplex qPCR reaction. The results showed that multiplex reaction of 23S rRNA gene (Supplement 4), qPCR of gyrA87 (Supplement 5) and gyrA91 (Supplement 6) gave the same efficiency as the singleplex reaction. The tested H. pylori strains displayed only one Cq value for one genotype which showed no cross-reactivity with other non-targeted strains. There was no statistical significance (independent t-test P-value>0.05) of Cq value obtained for a given target in the singleplex and multiplex assays (Supplement 7–9) which proved that the probe for detection of 23S rRNA gene and gyrA are specific for each mutation. No fluorescence signal from other bacterial strains tested was obtained in all fluorescence colors (Supplement 10–12) and no PCR band was amplified.
Detection of Point Mutation in the 23S rRNA Gene of H. pylori in Biopsy Samples by Multiplex qPCR
The optimized multiplex qPCR of 23S rRNA gene was performed to amplify 23S rDNA fragments of H. pylori in 136 positive samples. The detection of point mutations in 23S rRNA gene by multiplex qPCR is shown in Table 3. Overall, wild-type strains were detected in 79.4% (108/136) of the biopsies and 20.6% (28/136) of the biopsies were infected with H. pylori-resistant strains. Among the 28 biopsies, 22 biopsies were infected with single genotype (A2142G genotype in three biopsies and A2143G genotype in 19 biopsies) while six biopsies were infected with mix genotype (wild-type and mutation strains). A mixture of resistant and susceptible was found in six biopsies, in which 66.7% (4/6) of the biopsies had wild-type and A2142G strains, while 33.3% (2/6) biopsies harboured wild type and A2143G strains. The performance of the multiplex qPCR of 23S rRNA gene is shown in Table 4. The result was obtained when multiplex qPCR of the 23S rRNA gene was compared with E-test as the gold standard. The sensitivity, specificity, and accuracy of qPCR were 100%, 98.7%, and 98.8%, respectively. Positive and negative predictive values were 75% and 100%, respectively.
Table 3.
Detection of Clarithromycin Susceptibility Testing by E-Test, Sequencing and the Multiplex qPCR of 23S rDNA
| No. of Samples | Culture | E-Testb | Sequencingb (No. of Samples) | qPCR of ureA | Multiplex qPCR of 23S rDNAc (No. of Samples) | Median of Bacterial Load (Bacteria) |
|---|---|---|---|---|---|---|
| 38 | Positive | Sensitive | T2182 (34) WTa (4) |
Positive | WTa (38) | 103 |
| 21 | Positive | Resistance | A2142G (2) A2143G (19) |
Positive | A2142G (2) A2143G (19) |
102 103 |
| 70 | Negative | Negative | Negative | Positive | WTa (70) | 103 |
| 7 | Negative | Negative | Negative | Positive | A2142G (1) A2142G + WTa (4) A2143G + WTa (2) |
102 102 (A2142), 103 (WT) 102 (A2143), 103 (WT) |
Notes: aNo mutation at nucleotide position 2142 and 2143. bE-test and sequencing were performed on H. pylori-positive isolates by culture (59 clinical isolates). cMultiplex qPCR of 23S rDNA was performed on H. pylori-positive samples by qPCR of ureA (136 biopsy samples).
Table 4.
Performance of the Multiplex qPCR of 23S rDNA and Multiplex qPCR of gyrA Assays for Detection of Clarithromycin and Levofloxacin Resistance in Biopsy Samplesa
| No. of Biopsies with the Following Result: | Sp (%) (95% Cl) | Se (%) (95% Cl) | PPV (95% Cl) | NPV (95% Cl) | Accuracy (%) | Kappa | P (McNemar) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | ||||||||
| Clarithromycin resistance | 21 | 542 | 7 | 0 | 98.7 (97.3–99.4) | 100 (80.8–100) | 75 (54.8–88.6) | 100 (99.1–100) | 98.8 | 0.851 | 0.016 |
| Levofloxacin resistance | 15 | 554 | 1 | 0 | 99.8 (98.9–99.9) | 100 (74.7–100) | 93.8 (67.7–99.7) | 100 (99.1–100) | 99.8 | 0.967 | 1.000 |
Notes: aTrue-positive (TP) biopsies were defined as biopsies resistant by both the qPCR and E-test. True-negative (TN) biopsies were defined as biopsies susceptible by both the qPCR and E-test. False-positive (FP) biopsies were defined as biopsies susceptible by E-test and resistant by the qPCR. False-negative (FN) biopsies were defined as biopsies susceptible by qPCR and resistant by E-test.
Abbreviations: Sp, specificity; Se, sensitivity; PPV, positive predictive value; NPV, negative predictive value.
Detection of Point Mutation in the gyrA Gene in Biopsy Samples by Multiplex qPCR-gyrA
The detection of point mutation in the gyrA gene in 136 positive samples by multiplex qPCR of gyrA is shown in Table 5. Overall, wild-type strains detected in biopsies were 88.2% (120/136) compared to 11.8% (16/136) with levofloxacin resistant H. pylori. Among the 16 biopsies, one biopsy was infected with a single genotype while 15 biopsies had a mixed genotype. The performance of the multiplex qPCR of gyrA assay is shown in Table 4. The sensitivity, specificity, and accuracy were 100%, 99.8%, and 99.8%. Positive and negative predictive values were 93.8% and 100%, respectively.
Table 5.
Detection of Levofloxacin Susceptibility Testing by E-Test and Sequencing to the Multiplex qPCR of gyrA
| No. of Samples | Culture | E-Testd | Sequencingd (No. of samples) | qPCR of ureA | Multiplex qPCR gyrAe (No. of Samples) | Median of Bacterial Load (Bacteria) |
|---|---|---|---|---|---|---|
| 44 | Positive | Sensitive | WTa (44) | Positive | N87WTb+D91WTc (44) | 105 (N87WT), 106 (D91WT) |
| 15 | Positive | Resistance | N87I (2) N87K (8) D91N (2) D91Y (3) |
Positive | N87K (1) N87WTb+D91N (2) N87WTb+D91Y (2) N87K+D91Y (1) N87K+D91WTc (5) N87I+D91WTc (2) N87WTb+N87K+D91WTc (2) |
101 105 (N87WT), 105 (D91N) 101 (N87WT), 106 (D91Y) 101 (N87K), 106 (D91Y) 104 (N87K), 107 (D91WT) 104 (N87I), 106 (D91WT) 104 (N87WT), 103 (N87K), 106 (D91WT) |
| 76 | Negative | Negative | Negative | Positive | N87WTb+D91WTc (76) | 105 (N87WT), 106 (D91WT) |
| 1 | Negative | Negative | Negative | Positive | N87K +D91WTc (1) | 101 (N87K), 105 (D91WT) |
Notes: aNo mutation at amino acid position 87 and 91; bNo mutation at amino acid position 87; cNo mutation at amino acid position 91. dE-test and sequencing were performed on H. pylori-positive isolates by culture (59 clinical isolates). eMultiplex qPCR of gyrA was performed on H. pylori-positive samples by qPCR of ureA (136 biopsy samples).
Comparison of Clarithromycin and Levofloxacin Susceptibility Testing by E-Test and qPCR
Clarithromycin and levofloxacin susceptibility testing by E-test and sequencing methods were performed on 59 H. pylori isolates, while qPCR was carried out on 136 H. pylori-positive biopsies. Comparison of the methods (Table 4) showed that Kappa values were 0.85 (P=0.016) and 0.97 (P=1.00), indicating the multiplex qPCR of 23S rRNA gene and qPCR of gyrA methods had a substantial degree of agreement with the gold standard of E-test for detection of clarithromycin and levofloxacin resistance. For detection of clarithromycin-resistance, 59 biopsy samples were positive by culture and multiplex qPCR of 23S rRNA gene. From 571 biopsy samples, 77 were negative by culture but had amplification of H. pylori DNA and 23S rRNA gene by qPCR. Among the 77 multiplex qPCR of 23S rRNA gene-positive samples, 90.9% (n=70) had clarithromycin-susceptible strains, 1.3% (n=1) were clarithromycin-resistant (A2142G mutation) and 7.8% (n=6) contained mixed genotypes (four had wild-type+A2142G mutation and two had wild-type+A2143G mutation) (Table 3).
The 59 culture-positive H. pylori samples had 38 wild-type and 21 mutant genotypes detected by sequencing were also detected by multiplex qPCR for the 23S rRNA gene. Among the seven samples infected with resistant genotype as detected by multiplex qPCR of 23S rRNA gene, the median of bacterial load of the A2142G strains in the biopsies was 102, while biopsies with mixed genotype had 103 and 102 bacterial load of the wild type and the mutant genotypes, respectively (Table 3).
Detection of levofloxacin resistance by qPCR was concordant with E-test method where 44 out of 136 positive biopsies showed the presence of wild-type strains (Table 5). Fifteen samples had a single mutant genotype as determined by sequencing, however multiplex qPCR of gyrA showed a mixed genotype in 14 samples. Seventy-seven culture-negative biopsies had mixed infection by multiplex qPCR of gyrA assay. In 76 multiplex qPCR gyrA-positive samples, the median bacterial load in biopsies with mixed infection of wild type N87 and D91 were 105 and 106, respectively. On culture-negative biopsy, the median of bacterial load of mixed genotype strains of wild type D91 and N87K strains were 105 and 101, respectively.
Detection of Mixed Genotypes
Table 6 shows the detection of different resistance genotypes in biopsies from different anatomical sites. A mixed resistance genotype was identified in eight patients, of which five patients had different genotypes of clarithromycin, while three had different levofloxacin resistance genotypes. For the clarithromycin resistance genotype, there was a predominant infection at corpus site of the stomach with H. pylori strains than the antrum site. For levofloxacin, the antrum site showed mixed infection with wild type and mutant resistance genotype, while the corpus site was infected with either single wild type or mutant genotype.
Table 6.
Detection of Mixed Resistance Genotype from Antrum and Corpus Biopsies
| Patient | Biopsy No. | Resistance Genotype | |
|---|---|---|---|
| Antrum | Corpus | ||
| 1 | ETP34 | Cla-wild type | A2142G mutation |
| 2 | ETP40 | Cla-wild type | A2142G mutation |
| 3 | ETP177 | A2142G mutation | Cla-wild type |
| 4 | ETP245 | Cla-wild type | A2142G mutation |
| 5 | ETP250 | Cla-wild type | A2142G mutation |
| 6 | ETP111 | N87K mutation, D91-wild type | Lev-wild type |
| 7 | ETP274 | N87-wild type, N87K mutation, D91-wild type | N87K mutation |
| 8 | ETP275 | N87-wild type, N87K mutation, D91-wild type | N87K mutation, D91Y mutation |
Abbreviations: Cla, clarithromycin; Lev, levofloxacin.
Discussion
Diagnostic methods for detection of H. pylori infection are available, diverse, and the choice of one method or another depends on their advantages and disadvantages in each technique. The limitations associated with conventional techniques such as low concentration of H. pylori in biopsy sample is one of the main causes of failure to detect this bacterium by culture and other conventional methods. The molecular approaches based on DNA amplification have been used for H. pylori detection in clinical and environmental samples. The ureA gene has been reported as highly specific in Helicobacter spp. detection.35 Therefore, in the present study, the ureA gene was selected for detection by qPCR method because of the highly conserved region and no cross-homologies to other closely related bacteria or subspecies of Helicobacter.
The successful quantification, limit of detection, and specificity of qPCR assay were investigated. In the present study, the lowest H. pylori DNA concentration detected was one femtogram. The correlation coefficient obtained by linear regression analysis from this experiment was R2=0.99, indicating highly efficient and reproducible reactions. The limit of qPCR in this study (one fg) was lower compared to the previous report by Ribeiro et al,36 who reported 10 fg. The ureA qPCR assay is also specific for H. pylori detection because it positively detected H. pylori strains at Cq values of ≤30 with no fluorescence signal from other urease-producing bacterial strains detected at these Cq values. Experimental testing of the primer pair of qPCR assay resulted in high specificity for detection of H. pylori strains. The result showed that the qPCR positive result was detected in each tested H. pylori strains and negative for other tested bacterial species. This result suggested that the proposed primer sets gave a specific detection of H. pylori clinical strains and might be useful for diagnosis of H. pylori infection directly from gastric biopsies.
The cut-off point was determined and used to distinguish positive from negative results. The optimal cut-off point was defined as positive by the presence of any number of microorganisms (real-time PCR>0 microorganism/human cell) proposed by Saez et al,31 while Al-Moayad et al33 suggested a cutoff value of one bacterial per 100 human cells to distinguish cases from controls. Due to differences in laboratory practices, it is not feasible to directly compare the suggested cut-off point. Therefore, in this study the cut-off value was calculated using ROC curve analysis to distinguish positive from negative qPCR assays. Based on the obtained ROC curve coordinates, the value of 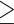 0.5 copies per 100 human cells was chosen to be a cut-off point that has the highest sensitivity and specificity (highest Youden index of 98.5%). Moreover, the distribution of Cq values clearly shows the Cq values of all positive biopsy samples were lower than and equal to 30 cycles (Supplement 3). Thus, biopsy samples with
0.5 copies per 100 human cells was chosen to be a cut-off point that has the highest sensitivity and specificity (highest Youden index of 98.5%). Moreover, the distribution of Cq values clearly shows the Cq values of all positive biopsy samples were lower than and equal to 30 cycles (Supplement 3). Thus, biopsy samples with 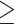 0.5 copies which were reproducible at Cq values ≤30 were considered positive by qPCR.
0.5 copies which were reproducible at Cq values ≤30 were considered positive by qPCR.
With the new optimal cut-off point (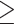 0.5 copies number) obtained from this study, qPCR method used for detecting H. pylori in biopsy samples is a reliable test because of its acceptable sensitivity (96.4%) and specificity (88.5%) with an AUC of 0.999.
0.5 copies number) obtained from this study, qPCR method used for detecting H. pylori in biopsy samples is a reliable test because of its acceptable sensitivity (96.4%) and specificity (88.5%) with an AUC of 0.999.
The prevalence of H. pylori infection in Malaysia varied from 14.1–30.4%.37–39 Positive and negative predictive values are directly related to the prevalence of the disease/infection in the population.40 In the present study, we observed a lower prevalence rate of H. pylori infection (16.7%) by conventional methods compared to previous studies. In 2010, 30.8% prevalence rate was obtained by conventional methods, while 19.2% (positivity was determined by culture) was reported in 2013.41,42 Thus, the low prevalence rate of H. pylori infection in the present study might reflect the PPV and NPV of the test. Likelihood ratio is more informative to measure the clinically useful of qPCR in diagnosis of H. pylori infection because this parameter is independent of prevalence. In the present study, +LR of 8.72 indicates a 9-fold increase in the odds of H. pylori-infected patient has a positive qPCR result. The value of +LR greater than one and –LR is smaller than one obtained by qPCR compared to conventional methods indicate qPCR developed this study is very useful for detection of H. pylori directly from gastric biopsies.
The qPCR assay targeting ureA gene used to detect H. pylori in biofilm and drinking water has been conducted previously.35,43 Saez et al31 showed the sensitivity of qPCR targeting ureA gene used to detect H. pylori in the gastric antrum in bleeding and non-bleeding patients was 97.9% and 94.7%, respectively, while it was 68.4% in non-bleeding and 91.5% in bleeding patients for the gastric corpus. The specificity in both antrum (53.1% versus 73%) and corpus (56.3% versus 78.4%) was less in bleeding than in non-bleeding patients. It was found that the sensitivity and/or specificity obtained using ureA-qPCR in this study was higher than in previous reports.21,31,33,44 A higher +LR (8.72 versus 4.3) and good –LR (0.04 versuss 0.63) was obtained in this study compared with that of Ramirez-Lazaro et al.45
The qPCR method shows a statistically significant higher percentage of H. pylori positivity compared to conventional methods (23.8% versus 14.7%, p<0.0001). Agreement between the tests according to Kappa analysis was at substantial degree (0.67) for qPCR and conventional methods. Our study showed that the false-negative and false-positive cases were determined as a low density of H. pylori by conventional methods, so the accuracy of qPCR may be decreased. The lower detection rate by conventional methods due to low concentration of H. pylori in biopsy samples and the microaerobic growth characteristic of H. pylori strains46 are causes of failure to detect by culturing and other conventional techniques. The sensitivity of the qPCR method in this study was higher than that of the standard conventional methods, supporting the findings of other studies.21,33,44 The lower specificity refers to more false positive results which was found in the test, however good specificity value is obtained (>80%). The high number of false positive cases found by qPCR was probably due to detection of H. pylori DNA in the samples with low bacterial count, while the microorganism was not detected using conventional methods.33 Therefore, by using a hypothetical ideal gold standard, the specificity of qPCR would be greater.47 Similarly, He et al48 reported that H. pylori DNA was detected by qPCR in 24 samples out of 27 that were negative by culture. Lascols et al49 detected H. pylori DNA by qPCR in nine of the cases that were negative by both culture and histology. The sensitivity and specificity of ureA qPCR method depend on the optimum cut-off point. The cut-off value will improve the specificity of the assay, although sensitivity was slightly affected.33 The ability to detect bacteria at very low counts by qPCR method can compromise its specificity when other techniques are used as gold standard.33 This becomes particularly evident when the proportion of study subjects harbouring low counts is high, which was probably the case in the present study. An increase in the number of biopsy specimens taken might increase the bacterial load in the samples which the cut-off value needs to be recalculated and this improved the specificity of the assay.31 In addition, a possible reason for the differences of detection with various methods is the topographic distribution of H. pylori colonization in the stomach causing the bias in sampling. This might interfere with the detection rate of H. pylori in each method45 and sampling errors that might generate differences in colonization density.36 Several studies have shown that the eradication of H. pylori in the treatment for some gastrointestinal diseases can be affected by bacterial density or localization.50,51
The primer pairs and probes were designed for multiplex qPCR assay to detect the two targets of point mutations in 23S rRNA gene and one target of wild-type sequence in the biopsy samples. In this study, multiplex assays require validation and optimization to confirm the results obtained from multiplex reaction was the same with the results if the reactions were performed separately. The quantitative sensitivity of multiplex qPCR of 23S rRNA gene assay was <10 bacteria per reaction tube, ie, <20 copies of the 23S rRNA gene (two gene copies of 23S rRNA gene present in genome of H. pylori) could be detected. These results are similar to previously published reports.48,52 The results confirm that qPCR can accurately quantify the H. pylori density in gastric mucosa, while other conventional methods are unable for quantification. Multiplex qPCR of 23S rRNA gene assay is specific for H. pylori detection because there was no fluorescence signal from other bacterial species tested obtained in all three fluorescence colours and no PCR band was amplified. Multiplex qPCR of the 23S rRNA gene able to detect the two strains in a mixture with the lowest proportion of the mutant DNA was 1% (99/1). This result is better than those published for real-time PCR assays using allele-specific scorpion (5%, 95/5)53 and using LightCycler (10%).54–56
In this study, a total of 35.6% (21/59) of strains were resistant to clarithromycin by E-test. The multiplex qPCR of 23S rRNA gene on the biopsy samples enabling detection of clarithromycin resistance in 20.6% (28/136) of the samples. Among these samples, 75% (21/28) were H. pylori positive detected by both culture and qPCR. In seven H. pylori-negative biopsies by culture, the qPCR was able to detect the presence of both single mutated genotype and mixed genotype. The median of bacterial load of H. pylori mixed genotype (strains harbouring both A2142G and A2143G mutations) is high, with a bacterial density in the range of 102 bacterial cells. This result indicates that the qPCR assay could be used to detect H. pylori clarithromycin resistance and possible to determine the number of microorganisms present in the biopsy samples. Other techniques are also useful for bacterial quantification; however, each technique has weaknesses. Culture is only semiquantitative and it is time-consuming.49 Histology is also semiquantitative, but its accuracy is relatively weak.47 Therefore, qPCR technique allows diagnosis of H. pylori and antibiotic susceptibility testing in the same day, which is much more rapid than culture which requires at least 5–10 days. The discrepant result between culture and qPCR among the H. pylori-negative biopsies were analyzed using sequencing method. Although sequencing was unable to detect mixed genotypes on the same alleles, it shows only mutated genotypes either A2142G or A2143G in the biopsy samples indicates that this mutant strain might be an infective strain. A reasonable explanation could be that strains of H. pylori harbour two copies of the 23S rRNA gene in the chromosomal DNA with a mutation in one gene copy is shown to confer resistance to clarithromycin.57 Seven biopsies with single mutated (n=1) and mixed genotype (n=6) of H. pylori strains were considered as false negative by culture. This might be due to the low concentration of bacterial density, probable dead cells or decreased viability of the microorganisms present in the specimens that could not be detected by culture.58 H. pylori exist in viable non cultivable (coccoid) form cannot be cultured or detected by rapid urease test or histology, however the nonviable bacterial DNA were available for PCR amplification.36,48,59 This result shows that the genotypic method is more sensitive in detecting bacterial populations in the same samples than the phenotypic method.58
The high performances of multiplex qPCR of 23S rRNA gene (sensitivity, 100%; specificity 98.7%) obtained in this study are comparable with previous reports which showed the range of the sensitivity and specificity were 90–100% and 92.5–99%, respectively.22,49,56,60,61 The potential factors that could explain low specificity, including negative culture might be due to low density of bacterial cells and presence of dead bacterial cells. Compared with phenotypic clarithromycin resistance tested by E-test, mutations detected by qPCR revealed almost perfect concordance with E-test, since all of the resistance detected by E-test was able to be detected by qPCR (Kappa=0.85). The only discrepancy concerned was the mixture of susceptible and resistant genotypes, which were detected by qPCR than E-test. This approach allows us to recommend its use for detection of clarithromycin resistance directly on biopsy samples.
Development of molecular method to detect gyrA mutations is expected to be highly useful for management of the H. pylori eradication therapy with fluoroquinolones-containing regimens. In this study, the quantitative sensitivity of gyrA qPCR assay was <10 bacteria per reaction indicates that qPCR can accurately quantify the H. pylori density in gastric mucosa. The multiplex qPCR-gyrA able to detect the two strains in a mixture with the lowest proportion of the mutant DNA (1%; 99/1 ratio). The available commercial genotyping tests for levofloxacin resistance in H. pylori were not designed to detect the N87 and D91 mutations using triplex gyrA qPCR.62 As such, because the N87 and D91 mutation were common in strains resistant to levofloxacin tested in this study, therefore the inability to detect these resistance strains would likely result in treatment failure.62 Therefore, new primers were designed to detect both mutations (N87 and D91) in gyrA gene. Validation study of the multiplex qPCR-gyrA suggested that the proposed primer sets resulted in a specific detection of gyrA gene mutations in H. pylori clinical strains.
In this study, resistance to levofloxacin was observed in 25.4% (15/59) of the samples as determined by E-test. However, 11.7% (16/136) was resistance to levofloxacin as determined by multiplex qPCR of gyrA. Among these samples, 93.8% (15/16) were H. pylori positive detected by both culture and qPCR. In one H. pylori-negative biopsy by culture, the qPCR was able to detect the presence of mixed genotype (D91 wild-type with N87K mutation). Interestingly, it found that the mixtures of susceptible (D91 wild-type) and resistant (N87K) strain had high bacterial load (D91 wild-type, 105; N87K, 101 bacterial cells). This has been confirmed by sequencing which shows the presence of both genotypes (D91 wild-type and N87K mutation). The culturing of H. pylori from gastric biopsies is often difficult and does not always recover all the bacterial cells present in the sample. This finding shows that the genotypic method is more sensitive than the phenotypic method.58
This study reports very high performances of multiplex qPCR of gyrA (with sensitivity, 100%; specificity, 99.8%) for detection of levofloxacin resistance. Agreement between the tests according to Kappa analysis was almost perfect degree (0.97) for qPCR and E-test, indicating a qPCR result revealed a good concordance with E-test. There is only a discrepancy concerning whether the qPCR was better for detection of the mixtures between susceptible and resistant genotypes than by E-test. These results indicate that multiplex qPCR of gyrA exhibited high performance for detection of H. pylori levofloxacin resistance in biopsy samples.
The present study reveals that the presence of more than one genotype in a single biopsy specimen might be due to either multiple strains which coexist in the same site in the gastric mucosa or the presence of mutated and wild-type alleles of the same strain.62–64 Kargar et al24 revealed that acquisition of antibiotic resistance may be caused by the horizontal transferring from resistant cells to susceptible cells of the same strain which results in increasing the population of resistant strains. Hetero-resistance will also be misdetected unless a specific method to detect sequence diversity in resistance-related genes is employed.65 In this study, the qPCR was able to detect the presence of mixed genotypes in H. pylori-negative biopsies by culture and mixed genotypes are still giving the bacterial load (101 bacterial cells for N87K and 105 bacterial cells for D91WT). A reasonable explanation could be that these mixed sequences are due to mixed infection27 because there is a single-copy of gyrA gene presence in the H. pylori genome.34 The presence of a mixed H. pylori population in the same sample detected by qPCR is an advantage when compared with culture.
Analysis of the resistance genotype in different anatomical sites of gastric biopsies (antrum and corpus) shows the presence of mixed genotypes of resistance H. pylori in patients. We identified that clarithromycin resistance strains predominantly infected the corpus site, while levofloxacin resistance strains infected both the antrum and corpus. In addition, the mixed genotype of levofloxacin resistance (wild type and mutant strains) were detected in the antrum site. Although the number of samples with mixed infection were small, it supports the hypothesis that the existence of heterogenous H. pylori strains in different anatomical sites of the stomach occurs.66 This shows multiplex qPCR assay in this present study is able to detect different resistance genotypes of H. pylori strains which cannot be detected by culture method. There is a possibility that the mixed genotypes of antibiotic resistance might not be found in a single individual biopsy and lead to undetected antibiotic resistance if a single biopsy is analyzed for antimicrobial susceptibility testing. The heterogeneity of H. pylori has important clinical significance and suggests that a single biopsy site might be unable to be considered representative of an antimicrobial susceptibility profile.65 Several authors have indicated that multiple gastric biopsies from both the antrum and corpus should be taken for the assessment of antibiotic resistant H. pylori strains.66–69
In conclusion, we have developed a multiplex qPCR assay that permits reliable, accurate, fast, cost-effective detection and could easily be performed and quantify H. pylori infection directly on gastric biopsies as well as providing determination of the mutation resistance to clarithromycin and levofloxacin in a rapid manner. The qPCR test for ureA for H. pylori detection and two evaluated multiplex qPCR tests for detection of mutations in 23S rRNA gene and gyrA can overcome the weaknesses of culture and other conventional methods. Therefore, the results of the present study might be important for clinical management of the infection. Detection of a mixed population in the same biopsy sample by qPCR is an advantage to overcome the misinterpretation of the overall antibiotic susceptibility profile of the strain. The good performance of the developed multiplex qPCR assay of the present study is useful for diagnosis of H. pylori infection and detection of mutations in clarithromycin- and levofloxacin-resistance strains. These will help in improving the management of H. pylori infection by reducing the development of secondary resistance which has a negative impact on eradication of H. pylori infection.
Acknowledgment
We would like to thank the Universiti Kebangsaan Malaysia for providing both the permission and the facilities to conduct and publish this research. A great thank to the supporting staffs at Department of Medical Microbiology and Immunology, Faculty of Medicine, UKM for their technical support during the study. We would also like to thank Dr. Bruno S. Lopes from the University of Aberdeen, United Kingdom for critically reviewed the manuscript. The research was funded by a grant from Universiti Kebangsaan Malaysia under the Economic Transformation Programme Research Fund Scheme (grant no. ETP-2013-042).
Data Sharing Statement
Data will be shared and available from the corresponding author on reasonable request.
Ethical Approval and Informed Consent
The study was approved by the Medical Research Ethic Committee of the University (UKM 1.5.3.5/244/ETP-2013-042) and written informed consent was obtained from each patient. The study was conducted in accordance with the Declaration of Helsinki.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Abadi ATB, Kusters JG. Management of Helicobacter pylori infections. BMC Gastroenterol. 2016;16(1):94. doi: 10.1186/s12876-016-0496-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175–1186. doi: 10.1056/NEJMra020542 [DOI] [PubMed] [Google Scholar]
- 3.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128(6):1567–1578. doi: 10.1053/j.gastro.2005.03.037 [DOI] [PubMed] [Google Scholar]
- 4.Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010;8(1):59–70. doi: 10.1586/eri.09.113 [DOI] [PubMed] [Google Scholar]
- 5.Cuadrado-Lavín A, Salcines-Caviedes JR, Carrascosa MF, et al. Antimicrobial susceptibility of Helicobacter pylori to six antibiotics currently used in Spain. J Antimicrob Chemother. 2012;67(1):170–173. doi: 10.1093/jac/dkr410 [DOI] [PubMed] [Google Scholar]
- 6.Megraud F. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut. 2004;53(9):1374–1384. doi: 10.1136/gut.2003.022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112(2):212–239. doi: 10.1038/ajg.2016.563 [DOI] [PubMed] [Google Scholar]
- 8.Liou JM, Chang CY, Sheng WH, et al. Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob Agents Chemother. 2011;55(3):1123–1129. doi: 10.1128/AAC.01131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishizawa T, Suzuki H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Front Mol Biosci. 2014;1(19). doi: 10.3389/fmolb.2014.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrido L, Toledo H. Novel genotypes in Helicobacter pylori involving domain V of the 23S rRNA gene. Helicobacter. 2007;12(5):505–509. doi: 10.1111/j.1523-5378.2007.00506.x [DOI] [PubMed] [Google Scholar]
- 11.Thung I, Aramin H, Vavinskaya V, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–533. doi: 10.1111/apt.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh P, Jain A, Dixit P, et al. Prevalence of gyrA and B gene mutations in fluoroquinolone-resistant and -sensitive clinical isolates of Mycobacterium tuberculosis and their relationship with MIC of ofloxacin. J Antibiot. 2015;68(1):63–66. doi: 10.1038/ja.2014.95 [DOI] [PubMed] [Google Scholar]
- 13.Contreras M, García-Amado MA, Michelangeli F, Contreras M, Peña J. Point mutations at gyrA and gyrB genes of Levofloxacin-Resistant Helicobacter pylori isolates in the esophageal mucosa from a Venezuelan population. Am J Trop Med Hyg. 2018;98(4):1051–1055. doi: 10.4269/ajtmh.17-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LH, Cheng H, Hu FL, Li J. Distribution of gyrA mutations in fluoroquinolone-resistant Helicobacter pylori strains. World J Gastroenterol. 2010;16(18):2272–2277. doi: 10.3748/wjg.v16.i18.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung JW, Lee GH, Jeong JY, et al. Resistance of Helicobacter pylori strains to antibiotics in Korea with a focus on fluoroquinolone resistance. J Gastroenterol Hepatol. 2012;2012;27:(3):493–497. doi: 10.1111/j.1440-1746.2011.06874.x [DOI] [PubMed] [Google Scholar]
- 16.Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, et al. Diagnostic methods for Helicobacter pylori infection: ideals, options, and limitations. Eur J Clin Microbiol Infect Dis. 2019;38(1):55–66. doi: 10.1007/s10096-018-3414-4 [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Talarico S, Yao L, et al. Droplet digital PCR-based detection of clarithromycin resistance in Helicobacter pylori isolates reveals frequent heteroresistance. J Clin Microbiol. 2018;56(9):e00019–18. doi: 10.1128/JCM.00019-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckman E, Saracino I, Fiorini G, et al. A novel stool PCR test for Helicobacter pylori may predict clarithromycin resistance and eradication of infection at a high rate. J Clin Microbiol. 2017;55(8):2400–2405. doi: 10.1128/JCM.00506-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beer-Davidson G, Hindiyeh M, Muhsen K. Detection of Helicobacter pylori in stool samples of young children using real-time polymerase chain reaction. Helicobacter. 2018;23(1):e12450. doi: 10.1111/hel.12450 [DOI] [PubMed] [Google Scholar]
- 20.Losurdo G, Giorgio F, Pricci M, et al. Helicobacter pylori primary and secondary genotypic resistance to clarithromycin and levofloxacin detection in stools: a 4-year scenario in Southern Italy. Antibiotics (Basel). 2020;9(10):723. doi: 10.3390/antibiotics9100723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichon M, Pichard B, Barrioz T, et al. Diagnostic accuracy of a noninvasive test for detection of Helicobacter pylori and resistance to clarithromycin in stool by the amplidiag, H. pylori+ClariR real-time PCR assay. J Clin Microbiol. 2020;58(4):e01787–19. doi: 10.1128/JCM.01787-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schabereiter-Gurtner C, Hirschl AM, Dragosics B, et al. Novel real-time PCR assay for detection of Helicobacter pylori infection and simultaneous clarithromycin susceptibility testing of stool and biopsy specimens. J Clin Microbiol. 2004;42(10):4512–4518. doi: 10.1128/JCM.42.10.4512-4518.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monno R, Giorgio F, Carmine P, Soleo L, Cinquepalmi V, Ierardi E. Helicobacter pylori clarithromycin resistance detected by Etest and TaqMan real-time polymerase chain reaction: a Comparative Study. APMIS. 2012;120(9):712–717. doi: 10.1111/j.1600-0463.2012.02896.x [DOI] [PubMed] [Google Scholar]
- 24.Kargar M, Doosti A, Ghorbani-Dalini S. Detection of four clarithromycin resistance point mutations in Helicobacter pylori: comparison of real-time PCR and PCR-RFLP methods. Comp Clin Pathol. 2013;22(5):1007–1013. doi: 10.1007/s00580-012-1519-1 [DOI] [Google Scholar]
- 25.Jaka H, Rüttgerodt N, Bohne W, et al. Helicobacter pylori mutations conferring resistance to Fluoroquinolones and clarithromycin among dyspeptic patients attending a tertiary hospital, Tanzania. Can J Gastroenterol Hepatol. 2019;2019:7. doi: 10.1155/2019/8481375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Lv T, He C, et al. Evaluation of multiplex ARMS-PCR for detection of Helicobacter pylori mutations conferring resistance to clarithromycin and levofloxacin. Gut Pathog. 2020;12(1):1–12. doi: 10.1186/s13099-020-00373-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egli K, Wagner K, Keller PM, Risch L, Risch M, Bodmer T. Comparison of the diagnostic performance of qPCR, sanger sequencing, and whole-genome sequencing in determining clarithromycin and levofloxacin resistance in Helicobacter pylori. Front Cell Infect Microbiol. 2020;10:596371. doi: 10.3389/fcimb.2020.596371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfizah H, Hasyanee B, Raja Affendi RA, Isa MR, Lopes BS. Molecular characterization and prevalence of antibiotic resistance in Helicobacter pylori isolates in Kuala Lumpur, Malaysia. Infect Drug Resist. 2019;12:3051–3061. doi: 10.2147/IDR.S219069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahamat M, Alavi M, Watts JE, et al. Development of two PCR-based techniques for detecting helical and coccoid forms of Helicobacter pylori. J. Clin. Microbiol. 2004;42(8):3613–3619. doi: 10.1128/JCM.42.8.3613-3619.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson JD, Higgins DG, Gibson TJ, W C. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saez J, Belda S, Santibáñez M, et al. Real-time PCR for diagnosing Helicobacter pylori infection in patients with upper gastrointestinal bleeding: comparison with other classical diagnostic methods. J Clin Microbiol. 2012;50(10):3233–3237. doi: 10.1128/JCM.01205-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gangisetty O, Reddy DS. The optimization of TaqMan real-time RT-PCR assay for transcriptional profiling of GABA-A receptor subunit plasticity. J Neurosci Methods. 2009;181(1):58–66. doi: 10.1016/j.jneumeth.2009.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Moayad EE, Alghalibi SM, Al-Shamahy HA, Nasher AT, Al-hebshi NN. Normalized real-time PCR for diagnosis of H. pylori infection. Qatar Med J. 2014;2:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomb JF, White O, Kerlavage AR, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388(6642):539–547. doi: 10.1038/41483 [DOI] [PubMed] [Google Scholar]
- 35.McDaniels A, Wymer L, Rankin C, Haugland R. Evaluation of quantitative real time PCR for the measurement of Helicobacter pylori at low concentrations in drinking water. Water Res. 2005;39(19):4808–4816. doi: 10.1016/j.watres.2005.09.030 [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro ML, Ecclissato CC, Mattos RG, Mendonca S, Pedrazzoli JJ. Quantitative real-time PCR for the clinical detection of Helicobacter pylori. Genet Mol Biol. 2007;30(2):431–434. doi: 10.1590/S1415-47572007000300022 [DOI] [Google Scholar]
- 37.Hooi JK, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and metaanalysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 38.Sasidharan S, Ghayethry B, Ravichandran M, et al. Prevalence of Helicobacter pylori infection among patients referred for endoscopy: gender and ethnic differences in Kedah, Malaysia. Asian Pac J Trop Dis. 2012;2(1):55–59. doi: 10.1016/S2222-1808(12)60013-9 [DOI] [Google Scholar]
- 39.Yu CJ, Yan P, Yee LY. Prevalence of Helicobacter pylori infection among patients attending gastroenterology endoscopy unit at Serdang Hospital. Malaysian J Med Health Sci. 2015;11(1):11–17. [Google Scholar]
- 40.Parikh R, Mathai A, Parikh S, Sekhar GC, Thomas R. Understanding and using sensitivity, specificity and predictive values. Indian J Ophthalmol. 2008;56(1):45. doi: 10.4103/0301-4738.37595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alfizah H, Rizal A, Isa M, Aminuddin A, Jasmi A, Ramelah M. Four year analysis of Helicobacter pylori infection among patients with dyspepsia in Universiti Kebangsaan Malaysia Medical Centre. Med Health. 2010;5:13–21. [Google Scholar]
- 42.Hamat R, Emran NA, Osman M, et al. Ethnic differences in the prevalence, clinical outcome and cag pathogenicity island (cagPAI) virulence dene profiles of Helicobacter pylori strains from Malaysia. Pertanika J Trop Agric Sci. 2013;36:289–298. [Google Scholar]
- 43.Linke S, Lenz J, Gemein S, Exner M, Gebel J. Detection of Helicobacter pylori in biofilms by real-time PCR. Lnt J Hyg Environ Health. 2010;213(3):176–182. doi: 10.1016/j.ijheh.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 44.Kumar A, Imran M, Singh A, Chandel D, Talwar A. Detection of Helicobacter pylori in gastroduodenal diseases by real-time PCR. J Biomed Sci Res. 2010;2(3):170–178. [Google Scholar]
- 45.Ramirez-Lazaro MJ, Lario S, Casalots A, et al. Real-time PCR improves Helicobacter pylori detection in patients with peptic ulcer bleeding. PLoS One. 2011;6(5):e20009. doi: 10.1371/journal.pone.0020009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng L, He XY, Tang B, Xiang Y, Yue JJ. An improved quantitative real-time polymerase chain reaction technology for Helicobacter pylori detection in stomach tissue and its application value in clinical precision testing. BMC Biotechnol. 2020;20(1):33. doi: 10.1186/s12896-020-00624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abadi ATB. Diagnosis of Helicobacter pylori using invasive and noninvasive approaches. J Pathog. 2018;9064952. doi: 10.1155/2018/9064952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Q, Wang JP, Osato M, Lachman LB. Real-time quantitative PCR for detection of Helicobacter pylori. J Clin Microbiol. 2002;40(10):3720–3728. doi: 10.1128/JCM.40.10.3720-3728.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lascols C, Lamarque D, Costa JM, et al. Fast and accurate quantitative detection of Helicobacter pylori and identification of clarithromycin resistance mutations in H. pylori isolates from gastric biopsy specimens by real-time PCR. J Clin Microbiol. 2003;41(10):4573–4577. doi: 10.1128/JCM.41.10.4573-4577.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leodolter A, Wolle K, Malfertheiner P. Current standards in the diagnosis of Helicobacter pylori infection. Dig Dis. 2001;19(2):116–122. doi: 10.1159/000050665 [DOI] [PubMed] [Google Scholar]
- 51.Vaira D, Vakil N, Menegatti M, et al. The stool antigen test for detection of Helicobacter pylori after eradication therapy. Ann Intern Med. 2002;136(4):280–287. doi: 10.7326/0003-4819-136-4-200202190-00007 [DOI] [PubMed] [Google Scholar]
- 52.Shukla S, Prasad K, Tripathi A, Ghoshal U, Krishnani N, Nuzhat H. Quantitation of Helicobacter pylori ureC gene and its comparison with different diagnostic techniques and gastric histopathology. J Microbiol Methods. 2011;86(2):231–237. doi: 10.1016/j.mimet.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 53.Burucoa C, Garnier M, Silvain C, Fauchère JL. Quadruplex real-time pcr assay using allele-specific scorpion primers for detection of mutations conferring clarithromycin resistance to Helicobacter pylori. J Clin Microbiol. 2008;46(7):2320–2326. doi: 10.1128/JCM.02352-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elviss NC, Lawson AJ, Owen RJ. Application of 3′-mismatched reverse primer PCR compared with real-time PCR and PCR-RFLP for the rapid detection of 23S rDNA mutations associated with clarithromycin resistance in Helicobacter pylori. Int J Antimicrob Agents. 2004;23(4):349–355. doi: 10.1016/j.ijantimicag.2003.09.015 [DOI] [PubMed] [Google Scholar]
- 55.Francesco VD, Margiotta M, Zullo A, et al. Primary clarithromycin resistance in Italy assessed on Helicobacter pylori DNA sequences by TaqMan real‐time polymerase chain reaction. Aliment Pharmacol Ther. 2006;23(3):429–435. doi: 10.1111/j.1365-2036.2006.02769.x [DOI] [PubMed] [Google Scholar]
- 56.Oleastro M, Ménard A, Santos A, et al. Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori. J Clin Microbiol. 2003;41(1):397–402. doi: 10.1128/JCM.41.1.397-402.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Binyamin D, Pastukh N, On A, Paritsky M, Peretz A. Phenotypic and genotypic correlation as expressed in Helicobacter pylori resistance to clarithromycin and fluoroquinolones. Gut Pathog. 2017;9(1):48. doi: 10.1186/s13099-017-0198-5 [DOI] [Google Scholar]
- 58.Agudo S, Alarcón T, Urruzuno P, Martínez MJ, López-Brea M. Detection of Helicobacter pylori and clarithromycin resistance in gastric biopsies of pediatric patients by using a commercially available real-time polymerase chain reaction after NucliSens semiautomated DNA extraction. Diagn Microbiol Infect Dis. 2010;67(3):213–219. doi: 10.1016/j.diagmicrobio.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 59.Atkins KL, Atkinson RM, Shanks A, Parvin CA, Dunne WM, Gross G. Evaluation of polymerase chain reaction for group B streptococcus detection using an improved culture method. Obstet Gynecol. 2006;108(3):488–491. doi: 10.1097/01.AOG.0000228961.42272.31 [DOI] [PubMed] [Google Scholar]
- 60.Bilgilier C, Stadlmann A, Makristathis A, et al. Prospective multicenter clinical study on inter- and intrapatient genetic variability for antimicrobial resistance of Helicobacter pylori. Clin Microbiol Infect. 2018;24(3):267–272. doi: 10.1016/j.cmi.2017.06.025 [DOI] [PubMed] [Google Scholar]
- 61.Chisholm SA, Owen RJ. Frequency and molecular characteristics of ciprofloxacin- and rifampicin-resistant Helicobacter pylori from gastric infections in the UK. J. Med. Microbiol. 2009;58(10):1322–1328. doi: 10.1099/jmm.0.011270-0 [DOI] [PubMed] [Google Scholar]
- 62.Trespalacios AA, Rimbara E, Otero W, Reddy R, Graham DY. Improved allele-specific PCR assays for detection of clarithromycin and fluoroquinolone resistant of Helicobacter pylori in gastric biopsies: identification of N87I mutation in gyrA. Diagn Microbiol Infect Dis. 2015;81(4):251–255. doi: 10.1016/j.diagmicrobio.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noguchi N, Rimbara E, Kato A, et al. Detection of mixed clarithromycin-resistant and-susceptible Helicobacter pylori using nested PCR and direct sequencing of DNA extracted from faeces. J Med Microbiol. 2007;56(9):1174–1180. doi: 10.1099/jmm.0.47302-0 [DOI] [PubMed] [Google Scholar]
- 64.Van Der Ende A, Van Doorn LJ, Rooijakkers S, Feller M, Tytgat G, Dankert J. Clarithromycin-susceptible and –resistant H. pylori isolates with identical randomly amplified polymorphic DNA-PCR genotypes cultured from single gastric biopsy specimens prior to antibiotic therapy. J Clin Microbiol. 2001;39(7):2648–2651. doi: 10.1128/JCM.39.7.2648-2651.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen T, van Helden PD, Wilson D, et al. Mixed-strain Mycobacterium tuberculosis infections and the implications for tuberculosis treatment and control. Clin Microbial Rev. 2012;25(4):708–719. doi: 10.1128/CMR.00021-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pichon M, Tran CT, Motillon G, et al. Where to biopsy to detect Helicobacter pylori and how many biopsies are needed to detect antibiotic resistance in a human stomach. J Clin Med. 2020;9(9):2812. doi: 10.3390/jcm9092812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mi M, Wu F, Zhu J, et al. Heterogeneity of Helicobacter pylori strains isolated from patients with gastric disorders in Guiyang,China. Infect Drug Resist. 2021;14:535–545. doi: 10.2147/IDR.S287631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Satoh K, Kimura K, Taniguchi Y, et al. Biopsy sites suitable for the diagnosis of Helicobacter pylori infection and the assessment of the extent of atrophic gastritis. Am J Gastroenterol. 1998;93(4):569–573. doi: 10.1111/j.1572-0241.1998.166_b.x [DOI] [PubMed] [Google Scholar]
- 69.Masuda H, Hiyama T, Yoshihara M, et al. Necessity of multiple gastric biopsies from different sites for detection of clarithromycin-resistant Helicobacter pylori strains. Scand J Gastroenterol. 2003;38(9):942–946. doi: 10.1080/00365520310004731 [DOI] [PubMed] [Google Scholar]


