Abstract
Objective
Visceral larva migrans (VLM) caused by Ascaris suum is a major health problem in pig farming regions. The clinical characteristics of pulmonary VLM caused by A. suum, however, are unclear. We assessed the clinico-radiologic features of this disease.
Methods
Medical records, including the results of chest radiography and high-resolution computed tomography (HRCT), were retrospectively reviewed from January 2000 through June 2019, at the University of Miyazaki Hospital and Kyoritsuiin Hospital in Miyazaki Prefecture, Japan.
Results
Seven patients with VLM caused by A. suum were identified. All seven patients had a unique habit of consuming raw foods, such as organic vegetables, chicken, turkey, wild boar, and venison. All but one patient, who had eosinophilic pneumonia with a fever and severe fatigue, had only mild or no respiratory symptoms. All 7 patients had remarkable eosinophilia (median, 1,960 /μL) and high serum IgE levels (median, 1,346 IU/mL). Chest HRCT revealed multiple nodules and multiple nodular ground-glass opacities in 57% and 29% of the patients, respectively. The pulmonary lesions were located predominantly in subpleural areas. All seven patients were treated with albendazole, which led to improvement within two to three months. Neither eggs nor parasites were detected in the feces or sputum of any patient.
Conclusion
Consumption of raw organic vegetables or raw meat is a possible route of A. suum infection. Infected patients exhibit mild respiratory symptoms, and multiple nodules with a halo in the subpleural area are a common finding on chest HRCT. Treatment with albendazole was effective in these cases.
Keywords: Ascaris suum, high-resolution computed tomography, helminth, clinico-radiologic features, raw food
Introduction
Visceral larva migrans (VLM) results from the migration of specific helminth larvae into various human viscera and was first described by Beaver et al. (1). The disease is caused mainly by Toxocara canis, but human VLM due to Ascaris suum has also been reported (2,3). Although A. suum VLM might be overlooked, life-threatening respiratory failure can occur when a large number of eggs are ingested (3).
Raising pigs or using pig manure as a fertilizer confers a high risk of infection, and an outbreak in a pig farming region was reported in Japan in 1996 (4). As other animals besides pigs may be infected with A. suum (5), consumption of these animals, particularly the raw meat, might also be a risk factor for infection (6,7). To our knowledge, the clinico-radiologic features of pulmonary VLM caused by A. suum have not been comprehensively characterized, although there are some reports of the chest computed tomography (CT) findings in this disease (8,9).
We herein report the retrospective evaluation of the symptoms, chest radiography and CT findings, treatment, and possible routes of infection in patients with pulmonary VLM caused by A. suum and summarize in detail our recent experiences with seven patients who developed VLM of the lung due to A. suum.
Materials and Methods
The human ethics review committee of the University of Miyazaki Hospital approved the study protocol. The authors retrospectively reviewed chest radiographs, high-resolution computed tomography (HRCT), and medical records of seven patients with VLM caused by A. suum at two institutions. All patients were confirmed to have A. suum infection by an immunodiagnosis from January 2000 through June 2019.
In brief, anti-immunoglobulin G (IgG) to A. suum was detected by a multiple-dot enzyme-linked immunosorbent assay (ELISA) or multiple-dot ELISA with A. suum-specific IgG antibody titer measurement by a microplate ELISA at the Division of Parasitology, Department of Infectious Diseases, Faculty of Medicine, University of Miyazaki. Details of the immunodiagnostic methods were described previously (10).
All patients were men (median age, 54 years old; range 26 to 72 years old). A full clinical evaluation was performed in all patients before and repeatedly after treatment. Findings of chest radiography and HRCT were available for review. Bronchoscopy with bronchial washing was performed in four of the seven patients. Bronchial wash samples were examined for cytology and the presence of parasite eggs and worms. The samples were also stained with Ziehl-Neelsen and cultured for acid-fast bacilli.
Results
Clinical features
Clinical features and laboratory data of all patients are shown in Table 1. All patients had consumed raw food, and 6 (86%) patients consumed raw meat, including chicken (57%) and wild boar (57%). Three patients (43%) consumed raw organic vegetables. One patient cultivated commodity crops using pig manure. Only 2 of the 7 patients (29%) had significant symptoms. One patient (patient 5) who had a fever and fatigue had a pneumonia-like shadow and cell differentials of the bronchoalveolar lavage fluid revealed 26% eosinophils. One patient (patient 7) who had a mild cough had idiopathic pulmonary fibrosis. One patient (patient 2) had a solitary nodule in the brain and eosinophils in the cerebrospinal fluid but exhibited no remarkable symptoms. All 7 patients had remarkable eosinophilia (median, 1,960 /μL; range 445 to 7,250 /μL) and elevated serum IgE levels (median, 1,346 IU/mL; range 633 to 24,733 IU/mL). Two patients had multiple liver nodules due to A. suum infection. Neither eggs nor parasites were detected in the feces or sputum of any of the patients.
Table 1.
Summary of the Patient Characteristics.
| Patient | Age, y | Sex | Dietary Habits | Symptoms | WBC, /μL (%Eo) | IgE, IU/mL | Others |
|---|---|---|---|---|---|---|---|
| 1 | 54 | M | raw slices of chicken and wild boar meat | none | 7,600 (29%) | 3,571 | |
| 2 | 57 | M | raw slices of chicken liver, and turkey | none | 6,100 (12%) | 832 | solitary nodule in brain eosinophils in CSF |
| 3 | 26 | M | raw slices of wild boar meat and venison | none | 7,200 (11%) | 926 | |
| 4 | 65 | M | freshwater crab raw organic vegetables | none | 8,800 (45%) | 24,733 | multiple nodules in liver |
| 5 | 52 | M | raw organic vegetables | fever fatigue | 8,900 (5%) | 1,401 | eosinophils in BALF: 26% |
| 6 | 44 | M | raw slices of chicken and wild boar meat | none | 7,000 (28%) | 1,346 | |
| 7 | 72 | M | raw slices of chicken, turkey, wild boar, and venison freshwater crab raw organic vegetables | mild cough | 14,500 (50%) | 633 | eosinophils in BALF: 40% multiple nodules in liver cultivated using pig manure |
WBC: white blood cell, Eo: eosinophils, BALF: bronchoalveolar lavage fluid, CSF: cerebrospinal fluid
Chest radiography and HRCT findings
Chest radiography and HRCT findings are shown in Table 2. In 4 of the 7 patients (57%), the chest radiography findings were normal. Ground-glass nodules were observed on chest radiography in two patients, and non-segmental ground-glass opacity was observed in one patient. The most common finding on HRCT was multiple nodules with or without a halo, which was observed in 4 of the 7 patients (57%). Multiple ground-glass nodules were found in two patients, and a solitary ground-glass nodule was found in one patient. A non-segmental crazy-paving pattern was observed in one patient. In all seven patients, abnormal findings on HRCT were predominantly observed in the subpleural area. Only two patients had multiple lesions distributed in both the central and subpleural areas. Neither pleural effusion nor mediastinal lymph node swelling was detected. Pulmonary lesions of VLM caused by A. suum are typically seen as multiple nodules with a halo distributed in the subpleural area (Figure).
Table 2.
Chest Radiographic and High-resolution CT Findings of the Patients.
| Patient | Chest radiographic findings | Chest high-resolution CT findings |
|---|---|---|
| 1 | Normal findings | Multiple nodules with a halo of ground-glass opacity or without a halo. The nodule size ranges from 5 to 10 mm. |
| The lesions are distributed in the subpleural area. | ||
| 2 | Irregularly marginated ground-glass nodule | Multiple nodules or nodular ground-glass opacities that range in size from 5 to 15 mm. |
| The lesions are distributed in the subpleural area. | ||
| 3 | Ill-defined ground-glass nodule | Solitary nodular ground-glass opacity with ill-defined margins up to 18 mm in size. |
| The lesions are distributed in the subpleural area. | ||
| 4 | Normal findings | Multiple ground-glass opacities with ill-defined margins that range in size from 3 to 5 mm. |
| The lesions are distributed in the subpleural area. | ||
| 5 | Ground-glass opacity in the lower lung field | Non-segmental crazy-paving appearance in the right middle and lower lobe. |
| The lesions are distributed in both central and subpleural areas of the lung. | ||
| 6 | Normal findings | Multiple nodules without a halo. The nodule size ranges from 10 to 15 mm. |
| The nodules are distributed in the subpleural area. | ||
| 7 | Normal findings | Multiple irregularly marginated nodules with a halo of ground-glass opacity. |
| The nodule size ranges from 3 to 20 mm. The nodules are distributed in both central and peripheral areas of the lung. |
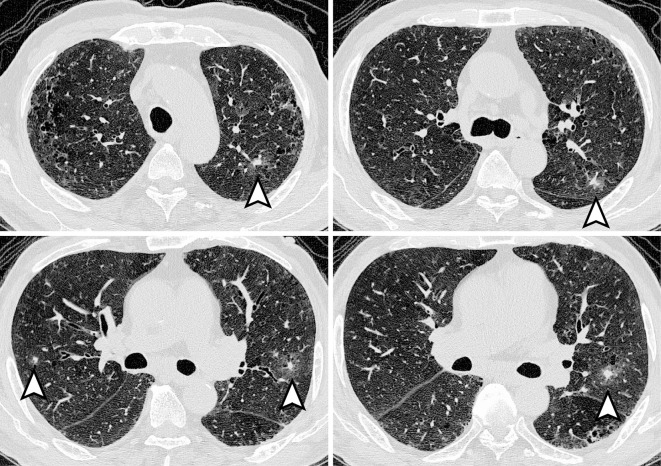
Figure.
Chest HRCT findings of patient No. 7 before treatment. Multiple irregularly marginated nodules with a halo can be seen in the periphery of both lungs (arrowheads).
Treatment
All 7 patients were treated with albendazole (600 mg/day) for 1 to 4 weeks. No serious side effects were reported. Treatment with albendazole led to the resolution of the symptoms as well as the abnormal findings of nodules and ground-glass opacities on chest radiography or HRCT within two to three months. The multiple nodules in the liver of patient 4 and the solitary nodule in the brain of patient 2 also disappeared after treatment. Peripheral blood eosinophilia and elevated serum IgE levels returned to normal ranges.
Discussion
We herein report the clinico-radiologic characteristics of seven patients with VLM caused by A. suum. VLM is a zoonotic disease caused by the larvae of specific helminths, such as Toxocara canis, T. cati, and A. suum (11,12). The larvae of the helminths can infect, but not mature, in humans, and the larvae migrate to various organs, where they cause eosinophilic inflammation and tissue damage. Toxocara spp. are the most common species involved in VLM. Based on antibody testing, Yoshida et al. concluded that the proportion of ascariasis in VLM cases in Japan is between 8.3% and 15.0% (10). Although a large number of VLM cases are caused by Toxocara spp., an outbreak of VLM due to A. suum was reported in 1996 in southern Kyushu in Japan, where pig farms are abundant and pig manure is commonly used for cultivation (4). It is important to clarify the clinical features of VLM caused by A. suum.
Common features of chest radiographs obtained from patients with VLM of A. suum are bilateral infiltrates, which can be migratory, or nodular lesions (4,9). Sakai et al. (8) reported that five of six patients with VLM had normal chest radiography findings, but chest CT showed small nodules. In our case series, over half the patients had normal chest radiography findings. Chest radiography revealed nodular lesions in only two of the seven patients and diffuse infiltration in one patient.
The main HRCT findings in the present study were multiple nodules with or without a halo, nodular ground-glass opacities, or diffuse infiltrates. These findings are similar to those in previous reports. Sakai et al. (8) reported that nodules with a halo or ground-glass attenuation are common chest CT features of VLM, observed in more than 60% of patients. Okada et al. (9) reported that multifocal subpleural nodules with a halo or ground-glass opacities and ill-defined margins are the most common abnormalities in patients with VLM caused by A. suum. A comparison of CT abnormalities with the pathologic findings suggests that ground-glass attenuations and nodules surrounded by a halo in CT correspond with the accumulation of inflammatory cells, especially eosinophils, and that nodules with halos correspond to necrosis and eosinophilic infiltrations (9). The most common HRCT features of patients with VLM caused by A. suum are multiple nodules with a halo of ground-glass opacity distributed in the subpleural area (Figure).
The main risk factors for zoonotic ascariasis are the use of manure as a fertilizer, living in close proximity to pig farms, and age under five years old; cross-infection from pigs is the most probable source of infection (5,13). Izumikawa et al. (7) recently reported cases with A. suum VLM caused by eating the raw meat of infected animals - most likely chicken, boar, deer, cattle, poultry, or horse - or raw vegetables grown in soil contaminated with A. suum eggs. In our study, only one patient who used pig manure for cultivation had had direct contact with pigs or pig manure, and the possible sources of infection in the other six were thought to be raw chicken; wild boar, turkey, or venison meat; or organic vegetables.
Several limitations associated with the present study warrant mention. For a definitive diagnosis of VLM, the larvae must be identified in the patient's body. As larvae are rarely isolated from patients' clinical samples, such as sputum, bronchoalveolar lavage fluid, lung biopsy specimens, or stool (3), immunoserologic methods are used for the diagnosis. The detection of antigen-specific IgG in a patient's serum is commonly used as the basis for the diagnosis of VLM because of the usefulness and convenience of this test, but the possibility of false-positive and false-negative results cannot be excluded (2). Although the abnormalities observed on chest imaging resolved after treatment of A. suum with albendazole, a definitive diagnosis of VLM by A. suum was not confirmed. Second, because we did not investigate the possible food sources of the larvae in the present study, the sources or routes of infection could not be confirmed.
Conclusion
We reported the clinico-radiologic features of VLM caused by A. suum in seven patients. Most of the patients exhibited mild or no clinical symptoms, and chest radiographic findings were normal. On chest HRCT, multiple nodules with a halo in the subpleural area were commonly identified. Eating raw meat or organic vegetables fertilized with pig manure is a potential source of infection.
This study was approved by ethics committee of the University of Miyazaki Hospital
The authors state that they have no Conflict of Interest (COI).
References
- 1.Beaver PC, Snyder CH, Carrera GM, Dent JH, Lafferty JW. Chronic eosinophilia due to visceral larva migrans; report of three cases. Pediatrics 9: 7-19, 1952. [PubMed] [Google Scholar]
- 2.Glickman L, Schantz P, Dombroske R, Cypess R. Evaluation of serodiagnostic tests for visceral larva migrans. Am J Trop Med Hyg 27: 492-498, 1978. [DOI] [PubMed] [Google Scholar]
- 3.Phills JA, Harrold AJ, Whiteman GV, Perelmutter L. Pulmonary infiltrates, asthma and eosinophilia due to Ascaris suum infestation in man. N Engl J Med 286: 965-970, 1972. [DOI] [PubMed] [Google Scholar]
- 4.Maruyama H, Nawa Y, Noda S, Mimori T, Choi WY. An outbreak of visceral larva migrans due to Ascaris suum in Kyushu, Japan. Lancet 347: 1766-1767, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Nejsum P, Betson M, Bendall RP, Thamsborg SM, Stothard JR. Assessing the zoonotic potential of Ascaris suum and Trichuris suis: looking to the future from an analysis of the past. J Helminthol 86: 148-155, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Sakakibara A, Baba K, Niwa S, et al. Visceral larva migrans due to Ascaris suum which presented with eosinophilic pneumonia and multiple intra-hepatic lesions with severe eosinophil infiltration--outbreak in a Japanese area other than Kyushu. Intern Med 41: 574-579, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Izumikawa K, Kohno Y, Izumikawa K, et al. Eosinophilic pneumonia due to visceral larva migrans possibly caused by Ascaris suum: a case report and review of recent literatures. Jpn J Infect Dis 64: 428-432, 2011. [PubMed] [Google Scholar]
- 8.Sakai S, Shida Y, Takahashi N, et al. Pulmonary lesions associated with visceral larva migrans due to Ascaris suum or Toxocara canis: imaging of six cases. Am J Roentgenol 186: 1697-1702, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Okada F, Ono A, Ando Y, et al. Pulmonary computed tomography findings of visceral larva migrans caused by Ascaris suum. J Comput Assist Tomogr 31: 402-408, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida A, Hombu A, Wang Z, Maruyama H. Larva migrans syndrome caused by Toxocara and Ascaris roundworm infections in Japanese patients. Eur J Clin Microbiol Infect Dis 35: 1521-1529, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogilvie BM, Savigny D. Immune responses to nematodes. In: Immunology of Parasitic Infections. 2nd ed. Cohen S, Warren KS, Eds. Blackwell, Oxford, UK, 1982: 715-757. [Google Scholar]
- 12.Inatomi Y, Murakami T, Tokunaga M, Ishiwata K, Nawa Y, Uchino M. Encephalopathy caused by visceral larva migrans due to Ascaris suum. J Neurol Sci 164: 195-199, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet 391: 252-265, 2018. [DOI] [PubMed] [Google Scholar]