Abstract
A 29-year-old man presented with a high-grade fever, headache, and urinary retention, in addition to meningeal irritation and myoclonus in his upper extremities. A cerebrospinal fluid (CSF) examination showed pleocytosis and high adenosine deaminase (ADA) levels with no evidence of bacterial infection, including Mycobacterium tuberculosis. T2-weighted brain magnetic resonance imaging showed transient hyper-intensity lesions at the splenium of the corpus callosum (SCC), bilateral putamen, and pons during the course of the disease. The CSF was positive for anti-glial fibrillary acidic protein (GFAP) antibodies. He was diagnosed with autoimmune GFAP astrocytopathy. The present case shows that the combination of an elevated ADA level in the CSF and reversible T2-weighted hyper-intensity on the SCC supports the diagnosis of autoimmune GFAP encephalopathy.
Keywords: autoimmune GFAP astrocytopathy, glial fibrillary acidic protein (GFAP), adenosine deaminase (ADA), urinary retention, myoclonus, reversible splenial lesion
Introduction
Autoimmune glial fibrillary acidic protein (GFAP) astrocytopathy is a recently emerging autoimmune disease of the central nervous system (CNS) (1,2). The disease is diagnosed by the detection of IgG autoantibodies against GFAP-α, the predominant intermediate filament protein expressed on adult astrocytes, in the cerebrospinal fluid (CSF). Recent studies have shown that clinical manifestations of autoimmune GFAP astrocytopathy include steroid-responsive meningoencephalitis/encephalomyelitis, which often presents as a fever, headache, abnormal vision, ataxia, meningeal sign, consciousness disturbance, autonomic nervous system dysfunction, involuntary movements, hyponatremia, and other symptoms (1-4). However, it is difficult to diagnose the disease in the early stages because these features are common to many forms of autoimmune and infectious CNS diseases.
We herein report a case of autoimmune GFAP astrocytopathy that showed self-remitting elevation of ADA levels in the CSF with peculiar findings on brain magnetic resonance imaging (MRI). Our case suggests that elevation of CSF ADA without Mycobacterium tuberculosis infection supports the possibility of autoimmune GFAP astrocytopathy. In addition, in order to help physicians diagnose this rare CNS disease, features of other autoimmune-related CNS diseases with elevation of CSF ADA levels that have been reported to date are summarized.
Case Report
A 29-year-old man who had no previous illness was admitted to our hospital complaining of a high-grade fever and headache for 1 week. His body temperature was elevated (39.5°C). His consciousness was clear, and a general physical examination showed no abnormalities. A neurological examination showed neck stiffness and Kernig's sign. A blood examination showed a white blood cell count in the normal range (7,300 /μL), slightly elevated C-reactive protein (CRP) (0.29 mg/dL), and mild hyponatremia (127 mEq/L). Lumbar puncture revealed a CSF pressure of 240 mmH2O. The cell count was 46 /μL (neutrophils 13, lymphocytes 33). The CSF protein concentration was elevated (108 mg/dL), and the glucose level was slightly decreased (44 g/dL; blood glucose 92 mg/dL).
A few days after admission, acute urinary retention and constipation developed. He required urinary catheterization and laxative medication. Myoclonus appeared in his upper extremities. His consciousness remained unchanged, and he had no symptoms suggesting myelopathy, such as hyper-reflexia. One week later, we found an elevated CSF ADA level (23.0 IU/L; normal range, 0-1.9 IU/L). The result of polymerase chain reaction (PCR) for tuberculosis was negative, and M. tuberculosis bacilli were not cultured from the CSF. Myelin basic protein was normal (95.6 pg/mL; normal range, <102 pg/mL), and oligoclonal bands were negative. The interferon-γ release assay, which aids in the diagnosis of systemic tuberculosis infection, was negative. No tumor markers were significantly elevated in the blood test, and cytology of the CSF showed no evidence of malignant cells. Tests for anti-aquaporin-4 (AQP4) antibody, anti-myelin-oligodendrocyte glycoprotein (MOG) antibody, anti-N-methyl-D-aspartate receptor (NMDAR) antibody, and other autoantibodies suggesting collagen diseases or vasculitis were all negative. We checked for CSF IgG against GFAP by immunohistochemistry and a cell-based assay, as previously reported (2,4). Strong immunoreactivity with the CSF sample was observed against astrocytes in the cerebellum, pial, subpial, and periventricular regions of the rat brain. We confirmed the presence of CSF IgG against GFAP using a cell-based assay with HEK293 cells expressing GFAP-α.
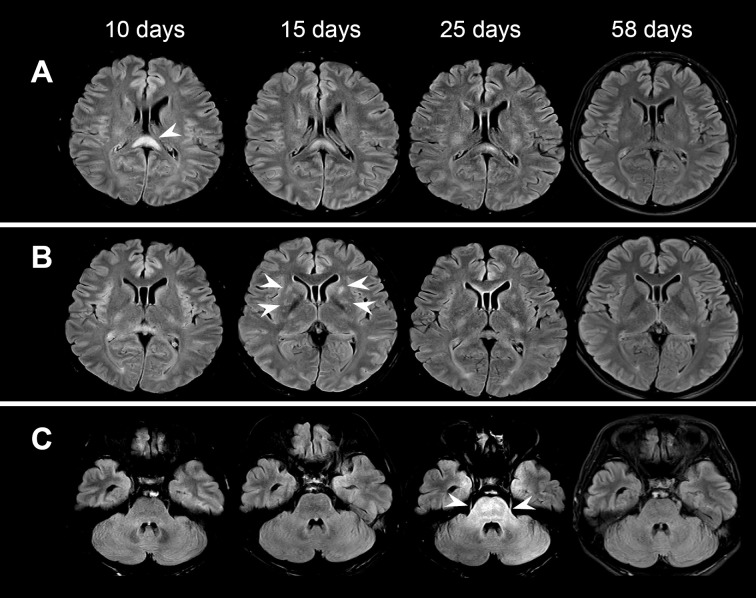
Brain MRI with T2-weighted fluid-attenuated inversion recovery (FLAIR) revealed hyper-intensity lesions in the splenium of the corpus callosum (SCC) on admission, which was 10 days after the onset (Fig. 1A). Follow-up MRI showed gradual remission of the SCC lesions (Fig. 1A) and the transient appearance of hyper-intensity in the bilateral putamen 15 days after the onset (Fig. 1B) and in the pons 25 days after the onset (Fig. 1C). There were no gadolinium-enhanced lesions in the course of the disease. Spinal MRI and computed tomography (CT) of the chest, abdomen, and pelvis with contrast showed no abnormalities.
Figure 1.
Brain MRI with T2-weighted FLAIR at 10, 15, 25, and 58 days after the onset. Arrowheads show hyperintense lesions in the splenium of the corpus callosum (A), bilateral putamen (B), and pons (C).
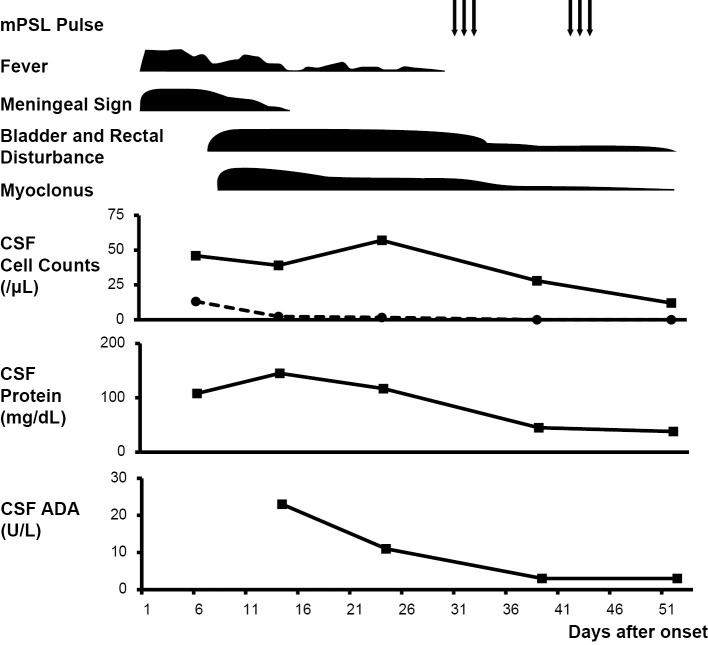
The patient received adjuvant therapy with glycerol and nonsteroidal anti-inflammatory drugs (NSAIDs). His general symptoms gradually improved, and the elevated CSF ADA levels decreased spontaneously (Fig. 2). Although a low-grade fever, minor myoclonus, and urinary retention lasted for one month, additional treatment with methylprednisolone pulse therapy ameliorated these symptoms. Three months later, all symptoms had disappeared completely.
Figure 2.
Clinical course and treatment. mPSL: methylprednisolone, CSF: cerebrospinal fluid, ADA: adenosine deaminase
Discussion
We herein report a case of autoimmune GFAP astrocytopathy with self-remitting elevation of CSF ADA levels. In general, elevation of ADA in the CSF is an adjunctive biomarker for a clinical diagnosis of tuberculous meningitis (TBM) (5) and has been applied extensively in clinical practice for decades. According to a previous meta-analysis, CSF ADA levels >8 U/L suggest a diagnosis of TBM, with a sensitivity less than 59% and a specificity greater than 96% (6). Although our patient's CSF ADA levels were higher than the cut-off value, antituberculous medication was withheld because of his good general condition and spontaneous recovery of his symptoms during elevation of CSF ADA.
In addition to its significant value for the diagnosis of TBM, elevated CSF ADA levels can suggest other diseases, such as nontuberculous infectious meningitis (7-13), lymphoproliferative disorders (14,15), and autoimmune-related CNS disease (16-20). In particular, a recent retrospective study found that the elevation of ADA levels was a unique CSF finding in patients with autoimmune GFAP astrocytopathy (4). Along with other characteristic features that have been reported in patients with autoimmune GFAP astrocytopathy (1-4), such as a fever, headache, autonomic nervous system dysfunction, involuntary movement, and hyponatremia, the elevation of the CSF ADA levels led us to this diagnosis in our patient.
Our search of the PubMed database yielded 21 cases of CNS disease with elevated CSF ADA levels caused by autoimmune-related mechanisms. The clinical profiles of these cases are summarized in Table. There were 7 published articles, which reported 14 case series of GFAP astrocytopathy (4), 3 cases of immune-related adverse events (irAE) induced by immune checkpoint inhibitors (16,18), and 1 case each of multiple sclerosis (17), Vogt-Koyanagi-Harada disease (19), sarcoid meningitis (21), and meningoencephalitis of unknown etiology (21). The ADA levels ranged from 7 to 91.41 IU/L. All cases except one showed pleocytosis (median 90.5 cells/μL, range 13-981 cells/μL) and elevated protein levels (range 71-286 mg/dL) in the CSF. All patients were treated with high-dose corticosteroid therapy, and nine patients also received antituberculous medication at the same time or antecedently. All patients responded well to steroid therapy except one patent who died from progression of lung cancer (16). These studies confirmed that CSF ADA was observed in patients with autoimmune-related CNS diseases. There is no doubt about the importance of performing diagnostic treatment for TBM in order to judge the response to antituberculous medication. It is advisable not to delay steroid therapy for patients with suspected steroid-responsive CNS disease. Furthermore, although there is little compelling evidence for the diagnostic value of elevated CSF ADA levels, they might be a supportive finding for physicians suspicious of a rare CNS disease, as in our case. Most cases of autoimmune GFAP astrocytopathy were diagnosed as meningoencephalitis/encephalomyelitis of unknown etiology or acute disseminated encephalomyelitis (ADEM) (4,20). In addition, coexisting neural antibodies, such as anti-NMDAR antibody, anti-AQP4 antibody, and others, have been frequently observed in autoimmune GFAP astrocytopathy (1-4). These data indicate that it is important to assess anti-GFAP antibody in patients with an elevated CSF ADA, even in cases with other diagnoses.
Table.
Summary of Autoimmune-related CNS Diseases with Elevated CSF ADA Levels.
| Reference | n | Sex/Age | Clinical diagnosis | CSF | Brain MRI lesion | Antituberculous agents/ Response |
Main treatment | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cell counts (/μL) |
Protein level (mg/dL) |
Highest level of ADA (IU/L) |
Glucose level (mg/dL) |
|||||||
| Our case | 1 | M/29 | Autoimmune GFAP astrocytopathy |
46 | 108 | 23 | 44 | splenial region, basal ganglia, brainstem |
- | mPSL pulse |
| 4 | 14 | F(n=6), M(n=8)/ 44[18-66]* |
Autoimmune GFAP astrocytopathy [ME(n=5), ADEM(n=4), MEM(n=3), NMDAR(n=1), neurosyphilis(n=1)] |
148 [25-378]* |
195 [71-286]* |
13 [7-16.2]* |
ND | basal ganglia(n=8), thalamus(n=6), cerebral white matter(n=5), brain stem(n=4), and others. |
5/ND | mPSL pulse |
| 18 | 1 | M/61 | IrAE assosiated with pembrolizumab |
79 | 209.2 | 21.9 | 43 | cerebral white matter, brainstem |
+/- | mPSL pulse, oral PSL |
| 16 | 2 | M/70 | IrAE assosiated with nivolumab |
50 | 166 | 31.2 | ND | no lesions | +/- | mPSL pulse, oral PSL |
| M/75 | IrAE assosiated with nivolumab and ipilimumab |
13 | 163 | 12.3 | ND | no lesions | - | mPSL pulse, oral PSL, IVIg, rituximab |
||
| 17 | 1 | M/80 | MS | normal | 73 | 20.2 | 64 | cerebral white matter |
- | mPSL pulse |
| 19 | 1 | M/67 | Vogt-Koyanagi- Harada disease |
75 | 81 | 12.9 | 77 | ND | +/- | mPSL |
| 20 | 1 | M/34 | Cerebral symptoms caused by an immune mechanism following TBM diagnosed clinically |
981 | 171 | 91.41 | 45 | splenial region, basal ganglia, brainstem |
+/+ | mPSL |
| 21 | 1 | M/24 | Sarcoid meningitis | 12 | 171 | 23 | 10 | ND | - | dexamethasone |
*Data are given as median [range]. n: number of patients, CSF: cerebrospinal fluid, ADA: adenosine deaminase, M: male, F: female, GFAP: glial fibrillary acidic protein, ME: meningoencephalitis, ADEM: acute disseminated encephalomyelitis, MEM: meningoencephalomyelitis, NMDAR: anti-N-methyl-D-aspartate receptors encephalitis, IrAE: immuno-related adverse events, MS: multiple sclerosis, TBM: tuberculous meningitis, mPSL: methylprednisolone, PSL: prednisolone, IVIg: intravenous immunoglobulin, ND: not described in detail
The mechanism underlying the elevation of CSF ADA in our case and other reported cases remains unclear. ADA has a major role in the proliferation and differentiation of T-lymphocytes, which play a central role in the cell-mediated immune response (22-24). Interestingly, the CSF ADA levels in our patient gradually decreased spontaneously, accompanied by the remission of his symptoms. This self-remitting elevation of ADA is in contrast with that in patients with TBM, wherein the levels remain high and are reduced by antituberculous treatment (25). Although further analyses are needed, it is reasonable to assume that disease-specific cascades related to the cell-mediated immune response in the early stage of autoimmune GFAP astrocytopathy cause the elevation of CSF ADA.
In our case, MRI findings also supported the diagnosis of autoimmune GFAP astrocytopathy. T2-weighted hyper-intensity involving the basal ganglia and brainstem is common in autoimmune GFAP astrocytopathy (3,4). Furthermore, a recent report described a pediatric patient with autoimmune GFAP astrocytopathy presenting with reversible lesions of the SCC (26). Reversible splenial lesions have been reported in patients with heterogeneous pathogenesis triggered by viral infection, hypoglycemia, antiepileptic drug treatment, seizure, trauma, and other causes; these lesions are more common in children and young adults than in older adults (27,28). Taken together with the findings from one previous pediatric case, our present findings suggest that reversible splenial lesions should be considered as a radiological feature in pediatric and young adult patients with autoimmune GFAP astrocytopathy. However, it is noteworthy that there were rare cases presenting with reversible splenial lesions among patients with TBM (29), and after ruling out TBM, the combination of reversible splenial lesions and an elevated CSF ADA was deemed to strongly suggest a diagnosis of autoimmune GFAP astrocytopathy.
Of note, the abnormalities on MRI that emerged and disappeared in the course of the disease were not necessarily coincident with the patient's symptoms. Although previous reports revealed neuropathological features of patients with autoimmune GFAP astrocytopathy that showed inflammation, including intramyelinic edema or inflammatory infiltrate around small vessels (3), further analyses are needed to elucidate the pathologic mechanisms underlying the various MRI features of autoimmune GFAP astrocytopathy.
Conclusion
We herein report a case of autoimmune GFAP astrocytopathy wherein the combination of self-remitting elevation of CSF ADA and reversible T2-weighted hyper-intensity on SCC supported the diagnosis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful to Dr. Toshiyuki Takahashi (Department of Neurology, Tohoku University Graduate School of Medicine, Miyagi, Japan) for examining anti-myelin-oligodendrocyte glycoprotein (MOG) antibody in our patient.
References
- 1.Fang B, McKeon A, Hinson SR, et al. Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol 73: 1297-1307, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan EP, Hinson SR, Lennon VA, et al. Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: analysis of 102 patients. Ann Neurol 81: 298-309, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Shan F, Long Y, Qiu W. Autoimmune glial fibrillary acidic protein astrocytopathy: a review of the literature. Front Immunol 9: 2802, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura A, Takekoshi A, Yoshikura N, Hayashi Y, Shimohata T. Clinical characteristics of autoimmune GFAP astrocytopathy. J Neuroimmunol 332: 91-98, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Pettersson T, Klockars M, Weber TH, Somer H. Diagnostic value of cerebrospinal fluid adenosine deaminase determination. Scand J Infect Dis 23: 97-100, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Tuon FF, Higashino HR, Lopes MI, et al. Adenosine deaminase and tuberculous meningitis--a systematic review with meta-analysis. Scand J Infect Dis 42: 198-207, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimori M, Imadome KI, Tomii S, Yamamoto K, Miura O, Arai A. Cerebrospinal fluid findings in chronic active Epstein-Barr virus infection with central nervous system involvement. Rinsho Ketsueki (Jpn J Clin Hematol) 59: 367-372, 2018. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, Satomi K. Cryptococcal meningitis associated with increased adenosine deaminase in the cerebrospinal fluid. Springerplus 5: 2093, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alborizi A, Hosseini-nasab A, Zeyaeian M, Sanaei A, Kashef S. A case of hypogammaglobulinemia with enteroviral meningoencephalitis, associated with increased adenosine deaminase in cerebrospinal fluid. Iran J Allergy Asthma Immunol 8: 117-119, 2009. [PubMed] [Google Scholar]
- 10.Ijyuuin T, Umehara F. Case of Streptococcus salivarius bacteremia/meningoencephalitis leading to discovery of early gastric cancer. Rinsho Shinkeigaku (Clin Neurol) 52: 360-363, 2012. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 11.Escalza-Cortina I, Azkune-Calle I, Rodriguez-Sainz A, Gomez-Beldarrain M, Vicente-Olabarría I, Garcia-Monco JC. Pearls & Oy-sters: chronic mumps meningoencephalitis with low CSF glucose and acute hydrocephalus in an adult. Neurology 82: e41-e43, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Nakae Y, Kuroiwa Y. A case of listeria meningitis showed high levels of adenosine deaminase in cerebrospinal fluid. Rinsho Shinkeigaku (Clin Neurol) 49: 590-593, 2009. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 13.Jakka SR, Veena S, Atmakuri RM, Eisenhut M. Characteristic abnormalities in cerebrospinal fluid biochemistry in children with cerebral malaria compared to viral encephalitis. Cerebrospinal Fluid Res 3: 8, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berciano J, Jiménez C, Figols J, et al. Primary leptomeningeal lymphoma presenting as cerebellopontine angle lesion. Neuroradiology 36: 369-3671, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi S, Niwa J, Tokui K, Nishikawa T, Ichikawa Y, Doyu M. Potent primary leptomeningeal lymphoma masquerading as tuberculous meningitis - a case report. Rinsho Shinkeigaku (Clin Neurol) 52: 416-420, 2012. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara S, Mimura N, Yoshimura H, et al. Elevated adenosine deaminase levels in the cerebrospinal fluid in immune checkpoint inhibitor-induced autoimmune encephalitis. Intern Med 58: 2871-2874, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuraki M, Sakai K, Odake Y, et al. Multiple sclerosis showing elevation of adenosine deaminase levels in the cerebrospinal fluid. Mult Scler Relat Disord 13: 44-46, 2017. [DOI] [PubMed] [Google Scholar]
- 18.Yonenobu Y, Ishijima M, Toyooka K, Fujimura H. A case of meningoencephalitis associated with pembrolizumab treated for squamous cell lung cancer. Rinsho Shinkeigaku (Clin Neurol) 59: 105-108, 2019. (in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 19.Hiraga A, Takatsuna Y, Sakakibara R, Kamitsukasa I, Minamide M, Kuwabara S. Vogt-Koyanagi-Harada disease with meningitis-retention syndrome and increased CSF adenosine deaminase levels. Clin Neurol Neurosurg 127: 42-43, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Hirotani M, Yabe I, Hamada S, Tsuji S, Kikuchi S, Sasaki H. Abnormal brain MRI signals in the splenium of the corpus callosum, basal ganglia and internal capsule in a suspected case with tuberculous meningitis. Intern Med 46: 505-509, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Moncó C, Berciano J. Sarcoid meningitis, high adenosine deaminase levels in CSF and results of cranial irradiation. J Neurol Neurosurg Psychiatry 51: 1594-1596, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong RP, Kameoka J, Hegen M, et al. Characterization of adenosine deaminase binding to human CD26 on T cells and its biologic role in immune response. J Immunol 156: 1349-1355, 1996. [PubMed] [Google Scholar]
- 23.Richard E, Arredondo-Vega FX, Santisteban I, Kelly SJ, Patel DD, Hershfield MS. The binding site of human adenosine deaminase for CD26/Dipeptidyl peptidase IV: the Arg142Gln mutation impairs binding to cd26 but does not cause immune deficiency. J Exp Med 192: 1223-1236, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristalli G, Costanzi S, Lambertucci C, et al. Adenosine deaminase: functional implications and different classes of inhibitors. Med Res Rev 21: 105-128, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Habib A, Amin ZA, Raza SH, Aamir S. Diagnostic accuracy of cerebrospinal fluid adenosine deaminase in detecting Tuberculous Meningitis. Pak J Med Sci 34: 1215-1218, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oger V, Bost C, Salah L, et al. Mild encephalitis/encephalopathy with reversible splenial lesion syndrome: an unusual presentation of anti-GFAP astrocytopathy. Eur J Paediatr Neurol 26: 89-91, 2020. [DOI] [PubMed] [Google Scholar]
- 27.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 63: 1854-1858, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Feraco P, Porretti G, Marchiò G, Bellizzi M, Recla M. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) due to cytomegalovirus: case report and review of the literature. Neuropediatrics 49: 68-71, 2018. [DOI] [PubMed] [Google Scholar]
- 29.Oztoprak I, Engin A, Gümüs C, Egilmez H, Oztoprak B. Transient splenial lesions of the corpus callosum in different stages of evolution. Clin Radiol 62: 907-913, 2007. [DOI] [PubMed] [Google Scholar]