Abstract
Postoperative imaging following orthopaedic surgeries is essential in assessing complications post-surgery and also helps plan further treatment. Combining a high degree of clinical insight with appropriate imaging can guide the treating clinician to the correct diagnosis. Imaging is quite challenging because of surgery-related soft tissue changes, especially in the early postoperative period and the presence of metal implants resulting in image scatter and metal artifacts. Newer modalities and advances in imaging have helped overcome shortcomings and assess better, especially in procedures that involve implants. Collaborative decision-making involving radiologists and clinicians has shown to be beneficial and is the way forward. This narrative review discusses the utility of imaging in evaluating postoperative complications following musculoskeletal surgeries with specific relation to trauma, arthroplasty, and tumour by discussing commonly encountered clinical scenarios.
Keywords: Postoperative imaging, MRI, Metal subtraction, Bone scintigraphy, SPECT-CT, Scintigraphy
Abbreviations
- MRI
Magnetic Resonance Imaging
- CT
Computerized Tomography
- USG
Ultrasonogram
- PET
Positron emission tomography
- ALVAL
Aseptic lymphocyte-dominated vasculitis-associated lesion
- MARS
Metal artifact reduction sequence
1. Introduction
Imaging is a vital part of the work-up and follow-up of patients following orthopaedic surgery to assess their progress and monitor any complications.1,2 Management depends on early and accurate diagnosis, as delayed interventions, especially in complications, have been associated with poorer outcomes. Ideally, decision-making should be achieved without any invasive diagnostic method, if possible, but quickly and reliably. Understanding the respective advantages and disadvantages of each imaging modality is required to deliver optimal patient care. Imaging plays a vital role in i) identifying the exact nature of the complication and ii) helping in formulating an appropriate management plan to address the issue.3, 4, 5 As imaging techniques are always evolving, appropriate knowledge of techniques will guide the treating clinician towards choosing the best modality required for the patient. This narrative review focuses specifically on imaging following musculoskeletal surgeries related to trauma, arthroplasty, and tumour by discussing commonly encountered clinical scenarios.
Challenges in postoperative imaging: Postoperative patients usually present with vague symptoms, majority being non-specific. Imaging is equally challenging due to surgery related soft-tissue and bone changes.6
Soft tissue changes: The major soft issue changes like edema, hematoma or seroma can be expected and will be challenging to distinguish from infection or abscess. Another potential mimic of collection could be a pseudo aneurysm which can be missed without appropriate imaging.7,8 Flaps and graft may have variable appearances which can be problematic for untrained interpreter. Fibrosis or scarring can give rise to confusing imaging picture especially when there is a question of post-operative tumor recurrence.
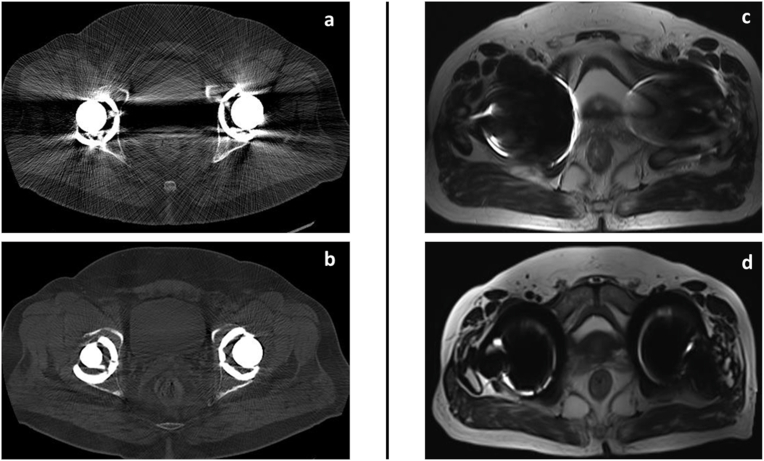
Metal artifacts: Presence of metal is another challenge which results in suboptimal images in CT and MRI scan. Presence of metal results in severe attenuation of the X-ray beam resulting in artifact in the reconstructed image. Similarly, loss of magnetic field homogeneity due to presence of metal will result in image distortion, signal loss and misregistration in MRI.9 Fortunately, there are multiple advances both in CT and MRI to reduce these artifacts to the extent required for adequate information, basic knowledge of which are helpful for both radiologist and referring surgeon in order to collaboratively diagnose most complex complications while being aware of certain limitations. Metal artifact reducing sequences (MARS) are useful advances which assist in clearer evaluation of structures whenever metals or prosthesis are involved (Fig. 1).10,11
Fig. 1.
Role of metal artifact reduction/subtraction techniques: CT scan (a) of both hip axial images following total hip replacement shows significant signal loss due to proton starvation and beam hardening artifacts (arrow) which is drastically reduced by metal artifact reduction techniques as in (b). MRI scan of both hip axial images(c) following total hip replacement shows significant image distortion and loss of signal around the implant(arrow). Substantial improvement of periprosthetic tissue details following artifact reduction techniques as in (d).
This review article aims to discuss the utility of imaging in evaluating postoperative complications following musculoskeletal surgeries with specific relation to trauma, arthroplasty, and tumour by discussing commonly encountered clinical scenarios. We also discuss the role of the time-tested MRI and CT scans (Table 1) in postoperative imaging and the newer modalities, including nuclear medicine (Table 2).
Table 1.
Summary of the recent advances, applications and drawbacks of CT Scan and MRI in postoperative imaging in musculoskeletal surgery.
| Imaging modality | Advances | Applications in post operative imaging | Drawbacks/Disadvantages |
|---|---|---|---|
| CT Scan: CT is readily available, easy to perform and less expensive modality used in post operative period. It is popular imaging tool used mainly in revision arthroplasty to look for bone loss and complex anatomies before revision surgeries. Although soft tissue details pertaining to collections, infection can also be evaluated by CT scan, MRI is preferred for such details. |
|
|
|
|
|
||
|
|
||
|
|
|
|
|
|
|
|
| |||
|
|
|
|
| |||
|
|
||
|
|
|
|
|
|
||
|
|
||
| Magnetic resonance imaging (MRI): Due to its superior soft tissue resolution MRI is increasingly used as a problem-solving modality in various complications like soft tissue collections around the implant, adjacent muscle and tendon pathologies, tumor surveillance. It is well known that ferromagnetic materials will be attracted to the main magnetic field of an MR scanner; however, it is safe to scan patients with orthopedic hardware such as intramedullary nails, screws, malleable plates, and joint prostheses, even in the immediate postoperative period. |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 2.
Role of Nuclear Imaging as a modality following musculoskeletal surgery.
| Imaging Modality | Advances | Applications in post operative imaging | Drawbacks/Disadvantages |
|---|---|---|---|
| Bone Scintigraphy Most common radiopharmaceutical are Tc-99 m MDP and Tc-99 m HDP These are bone-seeking agents that undergo adsorption to the hydroxyapatite structure of bone tissue. |
|
Allows for better image contrast and precise lesion localization. | SPECT is an added procedure rather than a stand-alone procedure in that whole body images or limited bone scan are performed initially followed by SPECT imaging |
| Uptake is dependent on blood flow and bone turnover(in osteoblastic areas) Less sensitive for osteolytic lesions |
|
SPECT allows for greater accuracy in the determination of the source of abnormal activity Correlation with CT-morphology allows the exclusion of potentially false positive bone uptake | Increased radiation exposure. Not widely available. |
| Planar whole body images or focal planar images can be acquired. | |||
| Quantification of uptake- the osseous radioactivity concentration is expressed as standardized uptake values (SUV) |
|
Improves attenuation correction around metal implants | 3-phase skeletal scintigraphy is nonspecific and is most useful when study is normal or identifies fracture or other non prosthesis-related cause of symptoms |
|
Localization to regions of increased bone perfusion and osteoblastic response | ||
| Tracers |
|
Normal bone scan has high negative predictive value Rules out infection and loosening | Patients with implants can show false positive uptake up to 3 years after surgery. |
|
Localizes to areas of infection and stress fractures with positive three phase uptake | Compacted marrow from procedure also shows increased activity | |
| Used both in conjunction with bone scans and independent of bone scans for further specificity | Differentiation of metabolically active vascular hypertrophic union and oligotrophic/atrophic non union | False-positive WBC scan in recently “violated” bone likely fracture due to localization in marrow elements | |
| Tagged WBC scan In-111 or Tc-99 m HMPAO WBCs localize to infection | Early detection of heterotrophic ossification | False-negative results occur in chronic infections/aseptic inflammation | |
|
Labeled leukocytes are mostly neutrophils, which are present in infections and not prevalent in aseptically loosened prosthesis | Requires Tc-99 m SC marrow map 24–72 h later if (+) Radiation exposure to the patient is greater | |
|
Usually matches 3-phase bone scan distribution in infection | Radiation exposure is a major disadvantage | |
|
PET/CT & PET/MRI Three-dimensional images are obtained with a circular array of detectors and a CT scanner in the PET/CT bore. |
F-18 FDG PET CT | Better imaging characteristics Used to confirm labeled leukocyte scintigraphy results Localizes to normal marrow, hence discordant activity to | Increased uptake around prosthesis |
| A glucose analogue, F-18-labeled | leukocyte scintigraphy indicates infection | CT based attenuation correction can falsely elevate radiotracer uptake in presence of implant related artifacts | |
| fluorodeoxyglucose is taken up by many types of tumour cells in the glycolysis pathway and is trapped after phosphorylation by hexokinase. | Areas of recurrent tumour show increased activity, particularly in patients with implants | Limited in case of metallic artifacts | |
| PET/MRI is a recent advance which can be used in patients with radiation concern- like pregnancy and young adults | Combined high soft tissue resolution of MRI and metabolic activity with PET increases sensitivity | Not widely available | |
| F-18 NaF PET Second-line modality after nondiagnostic Tc-99 m bone scans. | During shortages of Tc-99 m, F-18 PET bone scans offer an alternative for skeletal imaging | Shows no uptake in sclerotic acellular/small tumor volume in areas of tumor recurrence | |
| The mechanism of uptake is incorporation of fluoride ions into the bone matrix in sites of infection, after trauma/stress, or during inflammation | The resolution and pharmacokinetics are far greater than Tc-99m-based bone agents Fast blood clearance Higher bone uptake High bone to soft tissue uptake ratio | ||
| Shows uptake in osteolytic and osteoblastic lesions | Total exam time is shorter Improved resolution when compared to planar and SPECT or SPECT/CT |
1.1. Assessment of fracture union
Radiographs: Conventional radiographs are the workhorse to assess fracture union. The ability to monitor fracture healing to predict which fractures will go onto non-union accurately is of great value in reducing patient morbidity.12,13 The gradual appearance of the callus and its progressive ossification, as seen in two plain radiographs in a patient with decreased pain, is an indicator of good healing. Oblique radiographs may be helpful in some cases to visualize for callus. Radiographic union score (RUS) and modified RUS were developed to overcome the subjective nature of visual, radiographic evaluation, and clinical correlation in patients with delayed or non-union.14 Studies showed good inter-observer agreement in validation studies, even in complex bone defects and in patients with co-morbidities. These are not routinely used in clinical practice and still suffer from the fundamental limitation of being semiquantitative and subjective. The scoring system can be challenging for beginners and in the presence of metal plate.
CT scan: Computed tomography (CT) with the ability for multiplanar reconstruction and metallic artifact reduction is superior to plain radiography in the assessment of union even in the presence of abundant callus or overlaying cast. Krestan et al. compared MDCT and digital radiography in evaluation of the fracture healing in 43 patients in which 19% of digital radiographs underestimated the extent of bone healing, whereas in another 19% they overestimated the degree of fusion.15 Another study by Bhattacharyya et al. showed that computed tomography has 100% sensitivity for detecting non-union; however, it is limited by a low specificity of 62%, were in there was fibrous union intraoperatively.16
Quantitative and qualitative assessment using CT scan: Investigators have compared quantitative and qualitative changes of fracture healing using both computed tomography and conventional radiography. They found that early manifestations of healing, including blurring of fracture margins and formation of external callus, were observed earlier with CT scan. Most of the discrepancies between X-ray and CT scan findings were in periarticular and metaphyseal injuries.17 Currently, cost and radiation dose of CT scans limit their widespread use as the main clinical assessment tool for fracture healing. However, MDCT with 2D reformatted images can be considered the gold standard for evaluating the suspected non-union not clearly evident on radiographs (Fig. 2, Fig. 3).
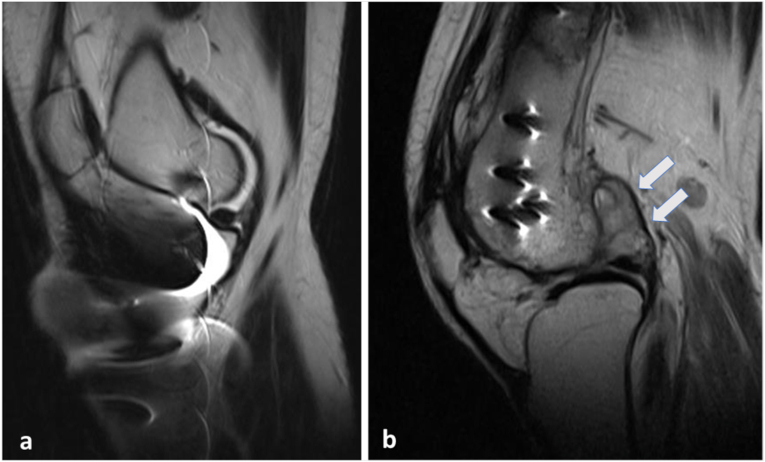
Fig. 2.
Metal artifact reduction sequence (MARS): Postoperative case of proximal tibial fracture with implants obscuring the images on routine T2 sequence, rendering it difficult for the radiologist to assess the underlying pathology (a). Another case with implant in the distal femur imaged using MARS sequence, depicting synovial thickening and intra-articular fluid collection in the knee joint (b).
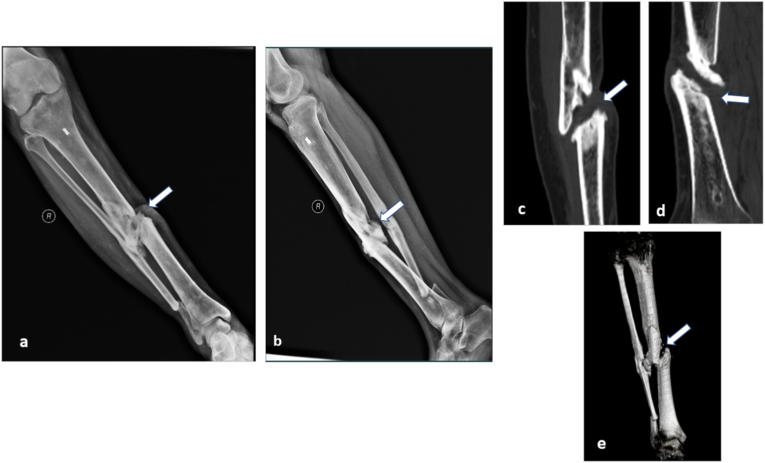
Fig. 3.
Assessment of fracture union: Follow up case of open fracture of tibia managed with external fixator. On follow-up at 6 months, AP (a) and lateral radiographs (b)of the leg showed doubtful union of tibia (arrow in a, b) and fibula. CT scan was done to assess bony union. Sagittal and coronal formats (c and d) and three dimensional volumetric reconstructions (e) showed complete non-union at the tibia (arrow in c, d, e).
MRI: The most promising work comes from the recent development of dynamic contrast-enhanced MRI, which enables the evaluation of the vascularity of a non-union site.18 Presently it has been used more successfully in scaphoid fractures. Another major utility is for physeal fracture healing, where clinicians can accurately detect bony bridging and area or involvement.
Ultrasound: The use of ultrasound was estimated to have 97% positive predictive value, and 100% sensitivity for fracture union when bridging callus was visualized at approximately two months following fixation. Fracture union was determined by ultrasound at a mean of 6.5 weeks versus 19 weeks on radiographs.19 However, USG is not popular as a standard clinical modality, mainly due to its operator dependency, lack of depth resolution in obese patients, and penetration through the bone.
Finite element analysis: Using CT scan images, this approach captures bone and callus tissue location, density, and distribution, and virtual loadings can be applied to simulate various biomechanical scenarios. In one recent study with image-based FEA, virtual stress testing of severely comminuted tibial fractures under clinically relevant loading was able to predict which patients would suffer a failure upon hardware removal with a sensitivity and specificity of 100% and 78%, respectively.20 DEXA and nuclear scans are not used in current practice to evaluate fracture non-union. In addition to clinical examination, we rely on conventional two-plane radiographs to assess union in routine clinical practice. MDCT with 2D reformatted images for suspected non-united fractures or complex anatomy fractures has been beneficial.
Evaluating suspected Prosthesis/Implant malposition: Radiographs are the first-line imaging modality to assess the components and their relationship to the adjacent bone. In certain situations, special radiographic views are used and are comparable to the CT scans; however, when surgery is planned, accurate assessment by CT scan is recommended. For example, in patients with recurrent dislocation post total hip replacement in whom surgery is indicated, obtaining a CT scan can help identify patients who have adequate bone stock which allows surgeons to retain the acetabular shell during revision surgery. Assessment of exact version of native bone is also possible helping plan revision surgeries if indicated.21 Another study by Keil et al. concluded that although Intraoperative 3D imaging is a valuable adjunct in assessing reduction and implant placement in acetabular fractures, it lacks accuracy due to metal artifacts, and hence post-operative CT is indicated to avoid impairment of clinical outcome.22
In our own experience, MDCT with multiplanar reconstruction better depicts the implant status, especially with relation to pelvis and is routinely used when radiographs are equivocal. It also helps to assess the bone quality accurately if revision is planned and gives a 3D view of the anatomy, and related soft tissue and osseous changes (Fig. 4).
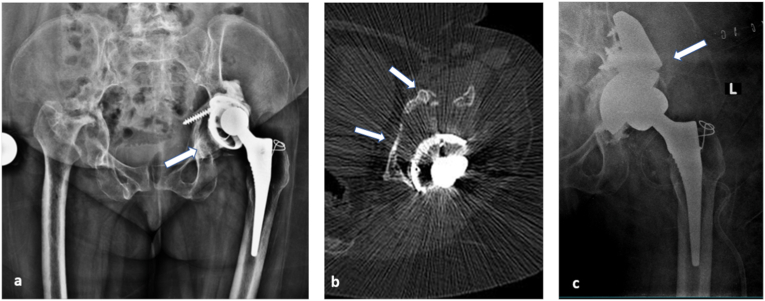
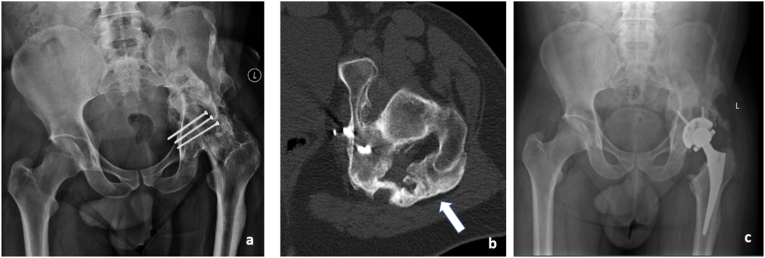
Fig. 4.
Evaluating suspected implant malposition: This 48 year old female with rheumatoid arthritis, who has undergone left total hip replacement one and half years back presented with dull aching left hip pain. Anteroposterior radiograph of pelvis with both hips (a)revealed loosening of the acetabular component and superior migration (arrow in a) with periarticular osteopenia. CT scan (b) confirmed the findings and additionally helped assess the extent of acetabular bone loss in specific regions (arrows in b). Post-surgical radiography showing acetabular cup with augmentation using a posterior column buttress (arrows in c) based on planning done on CT scan images.
1.2. Differentiating between aseptic and septic loosening in joint replacement – which imaging modality is preferable?
In failed prosthetic joint replacements, delineating the mechanism of failure is critical for planning ongoing clinical management.3,23 Clinical evaluation comprises clinical assessment in conjunction with imaging; in the form of plain radiographs, cross-sectional imaging, or nuclear medicine studies.24 Component loosening, whereby the bone-implant interface becomes loosened, is a commonly reported mechanism of implant failure.25 The cause of loosening is broadly divided into either septic or aseptic loosening, with differentiating between these causes a major diagnostic challenge.3 Distinguishing between these causes is clinically relevant; component failure due to aseptic loosening can be managed in a single-stage operation, whereas components loosened by established infection require multi-disciplinary input and more extensive staged operative intervention.
Differentiating between septic and aseptic loosening is not possible on plain radiograph analysis alone, with lysis at the bone-implant interface appearing identical in both aseptic and septic loosening.25,26 The use of additional imaging modalities is required to evaluate the cause of loosening, in conjunction with blood test monitoring and clinical assessment. The work of Cyteval et al. theorises that radiological identification of soft tissue reaction and inflammatory synovium around a loosened implant can point towards infection as the precipitating cause, as opposed to aseptic loosening.27 Intravenous contrast used with computerised tomography (CT) has proved to be an adequate and accessible imaging modality in this instance, with an adequate radiological evaluation of both bone and soft tissue.28 Ultrasound has limited utility in diagnosing deep tissue collection due to the shallow field of imaging afforded by the modality, however, it is a valuable diagnostic tool in evaluating superficial collection without being obscured by metal artifact.1,28 In the event of equivocal findings from either modality described, more sophisticated techniques can be pursued to evaluate the underlying cause of component loosening.
Within the radiology community, debate exists regarding the modality of choice for differentiating between component loosening due to infectious and non-infectious causes.29 Leukocyte-marrow scintigraphy is currently favoured, with imaging quality not impeded by implant artifact.30 Leukocyte labeled imaging stains neutrophils. Hence the procedure is a robust method of detecting neutrophil-mediated bacterial infection. The literature's reported accuracy rates are quoted at 90% in differentiating between aseptic and septic loosening with this technique.27 This modality is often available in select centers and is both a labour and cost-intensive technique.31
An emerging technique, Fluoride-FDG Positron emission tomography/CT, has been shown to provide less radiation exposure and lower cost than the aforementioned radiolabelling leukocyte labelling techniques, with related studies corroborating robust results findings.32,33 Initial study findings are positive, but current data lacks validation from the broader community. Broadly speaking however, if diagnosis from this modality is inconclusive, leukocyte marrow scintigraphy is often sought to solidify the diagnosis.
MRI has greatest advantage to show soft tissue abnormality, and also in differentiating suspected infection (Fig. 5). Recent study by Galley et al. showed that the presence of periosteal reaction, capsule edema, and intramuscular edema after total hip arthroplasty by MRI had a high accuracy in evaluation of periprosthetic infection.34 Aliprandi et al. were able to use MRI to identify and characterize fluid collections as being serous, purulent, or bloody and to detect soft-tissue edema and fistulous tracts.35 Similarly, for knee arthroplasty, MRI has been shown to be highly sensitive (92%) and specific (99%) to detect infection.36 Clinical examination, patient symptoms, and blood investigations are vital points that help the clinician narrow the diagnosis of infection before imaging in most cases.
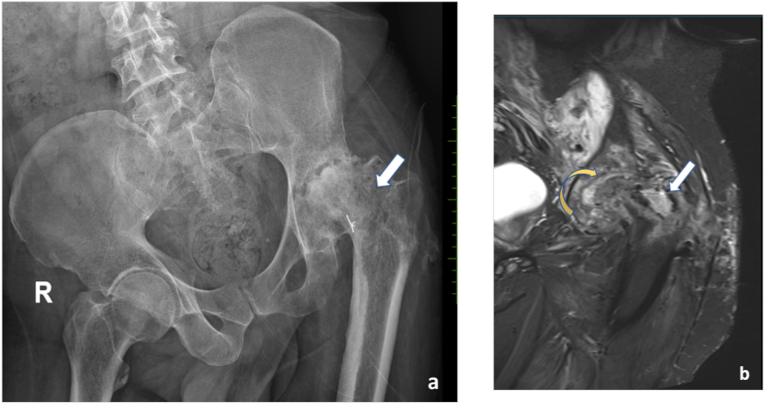
Fig. 5.
To rule out infection: Post implant removal - patient presented with complaints of pain in left hip. Anteroposterior radiograph (a) shows screw tracts (arrows in a) in the left neck of femur with severe arthritic changes (flattening of the left femoral head, articular surface irregularity, joint space reduction and deformity). Elevated inflammatory markers raised suspicion of possible infection. MRI demonstrated - T2 axial (b) and coronal STIR (c) images show significant subarticular marrow edema (curved arrow in b), soft tissue edema (blue arrow in b) and screw tract abscess (white arrow in b), suggestive of infection.
Metallosis/aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL): Radiographs and CT scans are less specific than MRI in the detection and quantification of the ALVAL lesions. 37 MRI can demonstrate ALVAL lesions following metal-on-metal prostheses even in asymptomatic patients. The hallmark appearance of metallosis at MR imaging is a lobulated mass adjacent to the capsule of the joint or bone that shows homogeneous low signal intensity on T2-weighted images and is surrounded by a rim with low T2 signal intensity. On T1-weighted images, the mass shows intermediate-to-high signal intensity, with focal areas of low T1 signal intensity typically at the periphery (Fig. 6).
Fig. 6.
Evaluation of HO: Patient with post traumatic left hip arthritis presented with stiffness and foot drop, AP radiograph of pelvis and both hips (a) shows trans articular screws in situ with deformation of femoral head. severe degenerative changes and periarticular heterotrophic soft tissue ossification. Axial sections of CT scan (b) shows heterotrophic ossification in the posterior aspect of left hip (b)with reduced intermuscular fat plane along course of left sciatic nerve. Post operative radiograph (c)shows-myositis mass excision and implant removal with total hip replacement.
Williams et al. recommended that USG surveillance be performed in all asymptomatic patients to detect ALVAL lesions. 38 Nishii et al. found USG to be 74% sensitive and 92% specific in detecting adverse local tissue reaction compared to the gold standard of MRI of metal-on-metal prostheses. 39
Localized areas of bone resorption around joint prothesis occur due to the release of small particles of cement, polyethylene, or metal. This may lead to osteolysis and subsequent arthroplasty implant failure. The condition may be clinically silent, emphasizing the need for imaging even on slight suspicion. Radiographs are typically the first method of identifying these areas of bone resorption. Special views can be used to supplement the AP radiograph for this assessment. However, considerable bone loss is necessary before lesions are identified with certainty on radiographs. Puri et al. found the sensitivity of radiographs for identifying osteolytic acetabular lesions to be 62% and the specificity 100% in comparison to a CT standard. 40 MRI has been reported to be the most accurate method for detecting and quantifying osteolysis and wear-induced synovitis after hip arthroplasty. A study on cadaver models involving hip replacements showed MRI to be the most sensitive test (95.4%) for detecting periacetabular lesions, although CT was the most accurate for determining lesion volume. 41 On imaging, hallmark features of particle disease include T2-weighted MR imaging demonstrating fluid collections or effusions with intermediate-to-high signal intensity, with specific segmental foci at periphery representing disorganized, irregular synovitis.
1.3. Evaluation of heterotopic ossification (HO)
Radiographs are the standard investigation pf choice for evaluating HO. In many cases, they are incidentally discovered during routine radiographs. CT scans are used to identify and determine the volume of heterotopic bone, the extent of bridging across the joint, and assess the maturity and relationship to neurovascular structures.42 MRI is not the primary modality for diagnosing HO; rather its main use is to differentiate heterotopic ossification from infection.
A three-phase bone scan has shown to be the most sensitive test for detecting heterotopic ossification in patients.43 Flow studies and blood-pool images can detect heterotopic bone approximately 2.5 weeks after injury. However in practice, the performance of bone scanning for determination of the maturity of hetero-topic ossification for surgical resection is not routinely done. In our institute, we prefer to use CT scan with contrast before surgical resection (Fig. 7) as it also acts a useful aid in surgical planning.
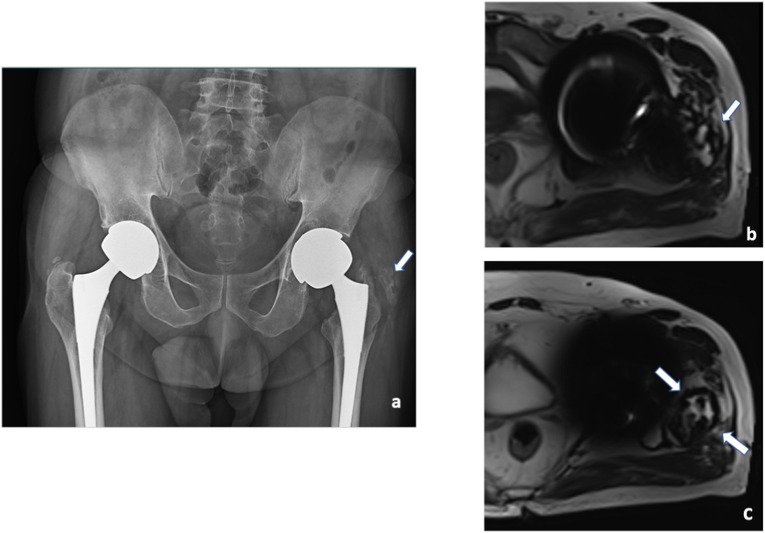
Fig. 7.
Case of bilateral total hip replacement with complaints of hip pain for 2 months.
AP radiographs of pelvis and both hips (a) shows suspected heterotopic ossification (arrows in a) along the greater trochanter. MRI axial T2 weighted images (b) periprosthetic fluid collection with central hypointense foci and capsule (arrows in b and c), suggestive of metallosis.
Evaluation of suspected tendon, muscle, bursal pathologies and nerve injury: Superficial tendon, muscles as well as neurovascular injuries and bursal pathologies can be easily detected by ultrasound (Fig. 8); however, deeper structures require MRI.44
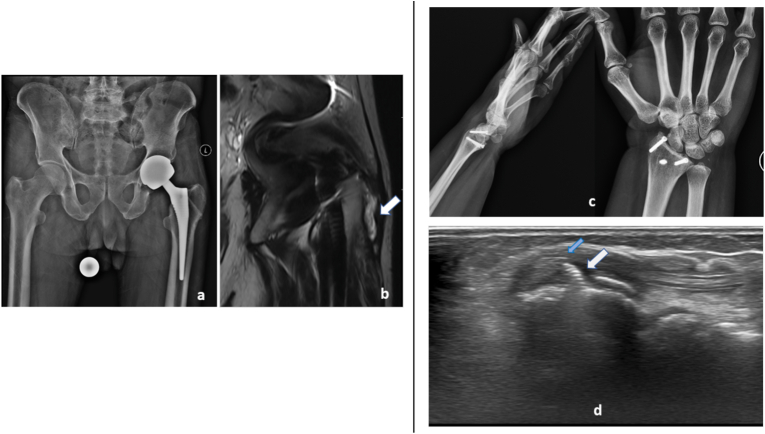
Fig. 8.
Evaluating implant related muscle, tendon and bursal pathologies: Patient with left hip implant 8 year post surgery with left hip pain AP radiograph (a) shows no significant abnormality. MRI T2 coronal image (b) shows left greater trochanteric bursitis (arrows in b). Patient with history of injury to the wrist 6 years back, post-surgical status, presented with complaints of pain in the wrist for past 2 years. AP and Lateral radiographs of wrist showed no significant abnormality. Ultrasound image the tip of implant (white arrows in c) at the level of listers tubercle impinging on the extensor digitorum tendon (blue arrow in d), which is thickened with fluid accumulation within the tendon sheath.
1.4. Evaluation of suspected tumour recurrence post-surgery
Evaluation starts from proper history-taking and physical examination during every follow-up visit. Tumour characteristics including location and grade have a substantial impact on the local recurrence and imply the need for follow-up imaging.45
Routine follow-up of these patients, especially involving bone tumour, is mainly by serial radiographs. In the immediate post-operative period, the baseline radiograph is extremely important since it shows the osseous and soft tissue changes following surgery. USG is helpful to detect soft tissue mass, especially in superficial regions, and useful in biopsies.46 MRI is the modality of choice to differentiate residual or recurrent tumors from post-surgical scars in the post-operative period. Many tumour mimics can complicate the imaging. Fortunately, there are many advancements in MR imaging that can be problem-solving along with routine sequences. The details of imaging appearances of recurrence and mimics are shown in Table 3.
Table 3.
Common postoperative complications and their respective imaging modalities.
| Complication | MRI Findings | Role of advanced Imaging | Pitfalls and precautions |
|---|---|---|---|
| Hematoma/Seroma | Signal intensity varies with age of hematoma | Gradient-echo imaging shows areas of blooming in the region of hematoma. These areas will not show any post contrast enhancement. | When haemorrhage occurs within the tumour it may not be possible to differentiate. |
| Seroma is well defined, Homogeneous area with T1 hypointense and T2 hyperintense with low-intensity rim caused by deposition of hemosiderin | DWI: ADC is significantly higher compared to recurrent tumour | Recurrent myxomas are T2 hyperintense- show post contrast enhancement and mimic seroma | |
| Post-surgical hypertrophic scar/Pseudotumor | Can be bulky mass- like progressively enlarging, irregular shape | DWI: Increased ADC values | Early inflammatory pseudotumors with granulation tissue might show T2 hyper intensity and enhancement pattern mimicking tumour |
| T1: Hypointense | DCE: Almost never shows arterial phase enhancement | ||
| T2: Hypointense | |||
| Little/no post contrast enhancement | |||
| Pseudo-progression | Post radiation/post chemotherapy | The residual tumour will show persistent enhancement and diffusion restriction. The pseudo-progression will show blooming in gradient sequences with non-enhancement and no diffusion restriction. | Avoid imaging for 4–6weeks following therapy to allow differentiation form recurrence. |
| Heterogeneous signal intensity | |||
| Enlargement of central non-enhancing component with reduced solid enhancing areas | |||
| Post radiation reactive changes in soft tissue, flaps and bone | Soft tissue: Diffuse edema with preserved architecture (“muscle texture sign or feathering sign” on T1 weighted images) limited to the radiation field is seen within the muscles and the flap. | DWI: Low signal on DWI and high signal on ADC | Follow up imaging muscle atrophy with fat overgrowth. The overall flap size will decrease. |
| Radiation osteitis: T2/STIR hyperintense areas in the marrow | DCE: Delayed enhancement | New mass or bone destruction within radiation field, | |
| Radiation osteonecrosis: New heterogeneous area within irradiated area | Heterogenous signal intensity lesion with matrix calcification can be sarcoma and should not be mistaken for osteonecrosis. | ||
| T1 hyperintense | |||
| T2 intermediate signal intensity | |||
| Tumour recurrence Bone/soft tissue | Focal progressively enlarging mass with heterogeneous signal intensity | Shows Post contrast enhancement with increase diffusion restriction and decreased ADC values. | Certain Densely ossified tumors and fibrous tumors may remain T2 hypointese. |
| Replacement of normal muscle texture | Diffusion quantification also known as tumor ADC histograms represent the distribution of ADC values within the tumor. Malignant tumor components generally show ADC values less than 1.0 x 10-3 mm2/s | No optimal cut off value between different vendors for ADC parameters. | |
| T1 hypointensity | DCE: Early arterial phase enhancement with washout or plateau in highly vascular recurrent tumor as seen in time intensity curves. | Time consuming for routine clinical use and may not be available in all centers | |
| T2 hyperintensity | |||
| Similar in signal intensity to primary tumor |
DCE – Dynamic contrast enhancement; DWI – Diffusion weighted image.
PET(Positron emission tomography) -CT can be used as a problem-solving tool when MRI is equivocal for tumour recurrence, shows extensive artifacts, or is contraindicated. Tumour residue or recurrence appears as hypermetabolic tissue with increased standardized uptake values (SUV). Patients with implants, however can show false positive uptake scans.47 Discussion of suspected cases of recurrence will benefit from MDT (multi-disciplinary team) discussion, and allows better judgement making in complex cases.48
Biopsy of suspicious areas might be necessary in equivocal cases to confirm the presence of recurrence and initiate appropriate treatment. DCE-MRI (Fig. 9) and PET-CT can target highly perfused and metabolically active tissue, which will increase the chances of a positive diagnostic yield of the planned biopsy. US-MR fusion and MRI-guided wire localization are other options that have been tried for targeting tumour tissue.49, 50, 51
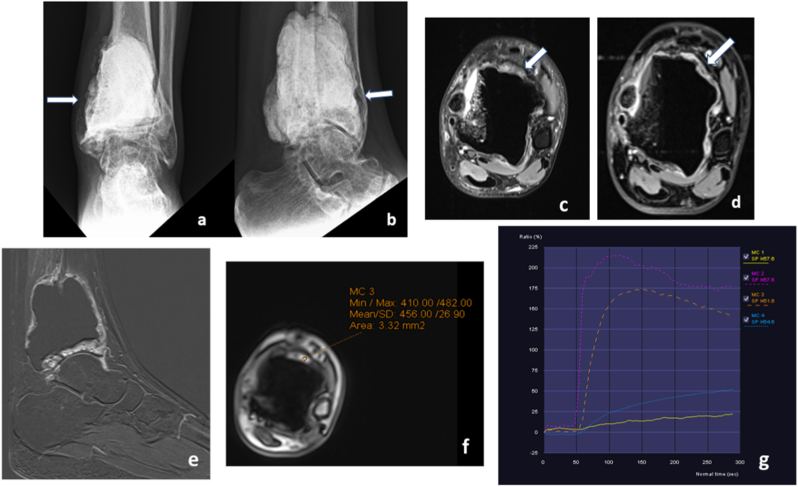
Fig. 9.
Evaluating suspected tumour recurrence: Follow up case of giant cell tumour of tibia-post excision and augmentation with bone cement- AP and lateral radiographs (a and b) reveal mild cortical irregularities with lucency in the medial and anterior aspect of tibia. MRI was done to rule out recurrence. Proton density fat saturated axial images (c) showed suspicious areas of T2 hypointensity with postcontrast enhancement (d) along the anterior aspect of bone cement(arrows), better seen in subtraction image (e). Time-signal intensity curves of dynamic contrast enhanced images with region of interest on suspicious area (orange curve in g) shows type I enhancement (rapid wash in and wash out of contrast) which is similar to the arterial washout pattern (Pink curve in g). Compare with normal soft tissue and scar (blue and yellow curves in g).
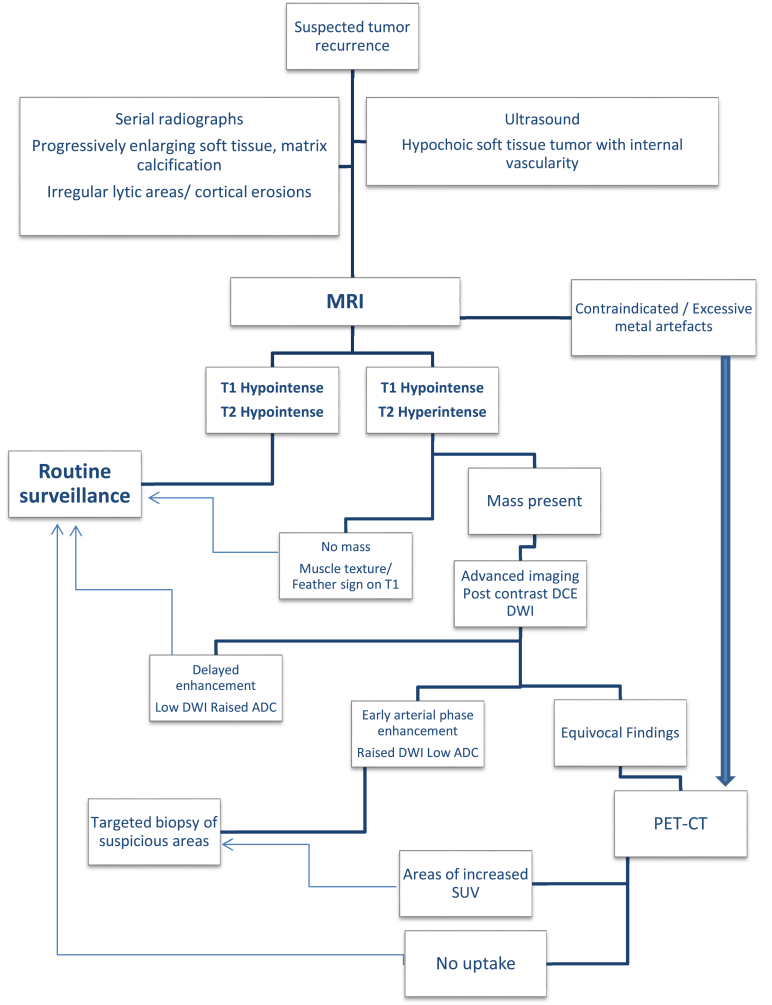
Advanced imaging techniques including MR elastography, MR spectroscopy, and recent advances in artificial intelligence like machine learning and feature extraction in radionics have shown promising results for evaluating post-treatment soft tissue tumour recurrence. However, these are still in research areas. Depending on the tumour characteristics and available sources, clinicians can tailor the follow-up strategies in these patients as illustrated in the flow chart (Fig. 10).
Fig. 10.
Flowchart depicting the imaging modalities for a patient with suspected tumour recurrence post-surgery.
2. Limitations & future directions
Despite recent advances in imaging, there remains no proven ‘gold standard’ imaging modality following musculoskeletal surgery to detect complications. The investigations of choice are also based on availability and affordability, especially in the developing world. Standardization of protocols of imaging would go a long way in making clinicians diagnose better. Improving image quality, especially by reducing implant artifacts, would be a fertile ground for future research. Nuclear medicine has shown promise in the early detection of tumour recurrence and differentiating inflammatory changes from sinister features. This modality could increasingly be employed if resources are available. However, irrespective of the investigation modality, clinical examination is of utmost importance and can help clinch the exact diagnosis in most cases. Formulation of multi-disciplinary clinics in institutions, coordinated discussion of findings, and combined clinics involving orthopaedic surgeons and radiologists could benefit all involved.
Imaging in post operative period following musculoskeletal surgeries can be challenging due to soft tissue changes and the presence of graft and implants. Although radiographs play a primary role in post-operative imaging, cross-sectional imaging and nuclear medicine imaging with a plethora of recent advances are valuable and vital problem-solving modalities. Ultrasound (US) has a limited role in the assessment of most complications. Still, it is a simple helpful modality to identify post-surgery hematoma, peri-prosthetic fluid collections, the presence of soft tissue sinus tracts, and also tendon and ligament status. Plain radiographs are still the first-line investigation of choice to assess early complications related to bone and implants. CT scan is simple to perform and readily available, making it an excellent tool to supplement radiographs when evaluating bone status, periprosthetic soft tissue ossifications, and implant positioning. MRI with evolved metal artifact reduction techniques (MARS) has become an essential diagnostic tool for assessing soft tissue abnormalities and is particularly useful in identifying adverse local tissue reactions in metal on metal implants. CT and MRI are accurate in determining most causes of the complications except infection, for which leukocyte-marrow scintigraphy is considered the modality of choice. Single-photon emission computed tomography with CT (SPECT-CT) is an emerging modality that has been shown to combine the sensitivity that bone scintigraphy offers with the high specificity of CT and has the advantage of showing the bone's metabolic activity. Based on the clinical scenarios, clinicians can choose imaging modalities depending on choice and availability in the local site. Knowledge of the advantages and limitations of advanced imaging is crucial for both radiologists and surgeons for collaborative diagnosis, ultimately benefitting patient care.
Funding statement
No funding granted or received.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ricketts D., Rogers R.A., Roper T., Ge X. Recognising and dealing with complications in orthopaedic surgery. Ann R Coll Surg Engl. 2017;99(3):185–188. doi: 10.1308/rcsann.2016.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubba R., Taylor J.S., Marks K.E. Cutaneous complications of orthopedic implants. A two-year prospective study. Arch Dermatol. 1981;117(9):554–560. [PubMed] [Google Scholar]
- 3.Lohmann C.H., Rampal S., Lohrengel M., Singh G. Imaging in peri-prosthetic assessment: an orthopaedic perspective. EFORT Open Rev. 2017;2(5):117–125. doi: 10.1302/2058-5241.2.160058. Published 2017 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rego J., Tan K. Advances in imaging-the changing environment for the imaging specialist. Perm J. 2006;10(1):26–28. doi: 10.7812/tpp/05-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thippeswamy P.B., Rajasekaran R.B. Imaging in polytrauma - principles and current concepts. J Clin Orthop Trauma. 2020;16:106–113. doi: 10.1016/j.jcot.2020.12.006. Published 2020 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim H.Y., Kim S.T., Kim H.J. Differentiation of postoperative changes and residual tumors in dynamic contrast-enhanced sella MRI after transsphenoidal resection of pituitary adenoma. Medicine (Baltim) 2019;98(27) doi: 10.1097/MD.0000000000016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stacy G.S., Kapur A. Mimics of bone and soft tissue neoplasms. Radiol Clin. 2011;49(6):1261–vii. doi: 10.1016/j.rcl.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Hayeri M.R., Ziai P., Shehata M.L., Teytelboym O.M., Huang B.K. Soft-tissue infections and their imaging mimics: from cellulitis to necrotizing fasciitis. Radiographics. 2016;36(6):1888–1910. doi: 10.1148/rg.2016160068. [DOI] [PubMed] [Google Scholar]
- 9.Lee M.J., Kim S., Lee S.A. Overcoming artifacts from metallic orthopedic implants at high-field-strength MR imaging and multi-detector CT. Radiographics. 2007;27(3):791–803. doi: 10.1148/rg.273065087. [DOI] [PubMed] [Google Scholar]
- 10.Müller G.M., Månsson S., Müller M.F. MR imaging with metal artifact-reducing sequences and gadolinium contrast agent in a case-control study of periprosthetic abnormalities in patients with metal-on-metal hip prostheses. Skeletal Radiol. 2014;43(8):1101–1112. doi: 10.1007/s00256-014-1893-7. [DOI] [PubMed] [Google Scholar]
- 11.Jennings J.M., Czuczman G.J., Johnson R.M., Dennis D.A. Metal artifact reduction sequence magnetic resonance imaging abnormalities in asymptomatic patients with a ceramic-on-ceramic total hip replacement. J Arthroplasty. 2021;36(2):612–615. doi: 10.1016/j.arth.2020.07.082. [DOI] [PubMed] [Google Scholar]
- 12.Rajasekaran R.B., Palanisami D.R., Natesan R., Jayaramaraju D., Rajasekaran S. Megaprosthesis in distal femur nonunions in elderly patients-experience from twenty four cases. Int Orthop. 2020;44(4):677–684. doi: 10.1007/s00264-019-04383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanakeshwar R.B., Jayaramaraju D., Agraharam D., Rajasekaran S. Management of resistant distal femur non-unions with allograft strut and autografts combined with osteosynthesis in a series of 22 patients. Injury. 2017;48(Suppl 2):S14–S17. doi: 10.1016/S0020-1383(17)30488-6. [DOI] [PubMed] [Google Scholar]
- 14.Christiano A.V., Goch A.M., Leucht P., Konda S.R., Egol K.A. Radiographic union score for tibia fractures predicts success with operative treatment of tibial nonunion. J Clin Orthop Trauma. 2019;10(4):650–654. doi: 10.1016/j.jcot.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krestan C.R., Noske H., Vasilevska V. MDCT versus digital radiography in the evaluation of bone healing in orthopedic patients. AJR Am J Roentgenol. 2006;186(6):1754–1760. doi: 10.2214/AJR.05.0478. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya T., Bouchard K.A., Phadke A., Meigs J.B., Kassarjian A., Salamipour H. The accuracy of computed tomography for the diagnosis of tibial nonunion [published correction appears in J Bone Joint Surg Am. 2014 Nov 5;96(21):e182] J Bone Joint Surg Am. 2006;88(4):692–697. doi: 10.2106/JBJS.E.00232. [DOI] [PubMed] [Google Scholar]
- 17.Grigoryan M., Lynch J.A., Fierlinger A.L. Quantitative and qualitative assessment of closed fracture healing using computed tomography and conventional radiography. Acad Radiol. 2003;10(11):1267–1273. doi: 10.1016/s1076-6332(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 18.Schoierer O., Bloess K., Bender D. Dynamic contrast-enhanced magnetic resonance imaging can assess vascularity within fracture non-unions and predicts good outcome. Eur Radiol. 2014;24(2):449–459. doi: 10.1007/s00330-013-3043-3. [DOI] [PubMed] [Google Scholar]
- 19.Moed B.R., Subramanian S., van Holsbeeck M. Ultrasound for the early diagnosis of tibial fracture healing after static interlocked nailing without reaming: clinical results. J Orthop Trauma. 1998;12(3):206–213. doi: 10.1097/00005131-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Petfield J.L., Hayeck G.T., Kopperdahl D.L. Virtual stress testing of fracture stability in soldiers with severely comminuted tibial fractures. J Orthop Res. 2017;35(4):805–811. doi: 10.1002/jor.23335. [DOI] [PubMed] [Google Scholar]
- 21.Ghelman B., Kepler C.K., Lyman S., Della Valle A.G. CT outperforms radiography for determination of acetabular cup version after THA. Clin Orthop Relat Res. 2009;467(9):2362–2370. doi: 10.1007/s11999-009-0774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keil H., Beisemann N., Schnetzke M. Intraoperative assessment of reduction and implant placement in acetabular fractures-limitations of 3D-imaging compared to computed tomography. J Orthop Surg Res. 2018;13(1):78. doi: 10.1186/s13018-018-0780-7. Published 2018 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mushtaq N., To K., Gooding C., Khan W. Radiological imaging evaluation of the failing total hip replacement. Front Surg. 2019;6:35. doi: 10.3389/fsurg.2019.00035. Published 2019 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz Santiago F., Santiago Chinchilla A., Ansari A. Imaging of hip pain: from radiography to cross-sectional imaging techniques. Radiol Res Pract. 2016;2016:6369237. doi: 10.1155/2016/6369237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirakawa K., Jacobs J.J., Urban R., Saito T. Mechanisms of failure of total hip replacements: lessons learned from retrieval studies. Clin Orthop Relat Res. 2004;420:10–17. doi: 10.1097/00003086-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Apostu D., Lucaciu O., Berce C., Lucaciu D., Cosma D. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty: a review. J Int Med Res. 2018;46(6):2104–2119. doi: 10.1177/0300060517732697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cyteval C., Bourdon A. Imaging orthopedic implant infections. Diagn Interv Imaging. 2012;93(6):547–557. doi: 10.1016/j.diii.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Mushtaq N., To K., Gooding C., Khan W. Radiological imaging evaluation of the failing total hip replacement. Front Surg. 2019;6:35. doi: 10.3389/fsurg.2019.00035. Published 2019 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar R., Kumar R., Kumar V., Malhotra R. Potential clinical implication of (18) F-FDG PET/CT in diagnosis of periprosthetic infection and its comparison with (18) F-Fluoride PET/CT. J Med Imaging Radiat Oncol. 2016;60(3):315–322. doi: 10.1111/1754-9485.12444. [DOI] [PubMed] [Google Scholar]
- 30.Aksoy S.Y., Asa S., Ozhan M. FDG and FDG-labelled leucocyte PET/CT in the imaging of prosthetic joint infection. Eur J Nucl Med Mol Imag. 2014;41(3):556–564. doi: 10.1007/s00259-013-2597-2. [DOI] [PubMed] [Google Scholar]
- 31.Blanc P., Bonnet E., Giordano G., Monteil J., Salabert A.S., Payoux P. The use of labelled leucocyte scintigraphy to evaluate chronic periprosthetic joint infections: a retrospective multicentre study on 168 patients. Eur J Clin Microbiol Infect Dis. 2019;38(9):1625–1631. doi: 10.1007/s10096-019-03587-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwee T.C., Basu S., Torigian D.A., Zhuang H., Alavi A. FDG PET imaging for diagnosing prosthetic joint infection: discussing the facts, rectifying the unsupported claims and call for evidence-based and scientific approach. Eur J Nucl Med Mol Imag. 2013;40(3):464–466. doi: 10.1007/s00259-012-2319-1. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi N., Inaba Y., Tezuka T. Evaluation of local bone turnover in painful hip by 18F-fluoride positron emission tomography. Nucl Med Commun. 2016;37(4):399–405. doi: 10.1097/MNM.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 34.Galley J., Sutter R., Stern C., Filli L., Rahm S., Pfirrmann C.W.A. Diagnosis of periprosthetic hip joint infection using MRI with metal artifact reduction at 1.5 T. Radiology. 2020;296(1):98–108. doi: 10.1148/radiol.2020191901. [DOI] [PubMed] [Google Scholar]
- 35.Aliprandi A., Sconfienza L.M., Randelli F., Bandirali M., Di Leo G., Sardanelli F. Magnetic resonance imaging of painful total hip replacement: detection and characterisation of periprosthetic fluid collection and interobserver reproducibility. Radiol Med. 2012;117(1):85–95. doi: 10.1007/s11547-011-0706-5. [DOI] [PubMed] [Google Scholar]
- 36.Plodkowski A.J., Hayter C.L., Miller T.T., Nguyen J.T., Potter H.G. Lamellated hyperintense synovitis: potential MR imaging sign of an infected knee arthroplasty. Radiology. 2013;266(1):256–260. doi: 10.1148/radiol.12120042. [DOI] [PubMed] [Google Scholar]
- 37.Yanny S., Cahir J.G., Barker T. MRI of aseptic lymphocytic vasculitis-associated lesions in metal-on-metal hip replacements. AJR Am J Roentgenol. 2012;198(6):1394–1402. doi: 10.2214/AJR.11.7504. [DOI] [PubMed] [Google Scholar]
- 38.Williams D.H., Greidanus N.V., Masri B.A., Duncan C.P., Garbuz D.S. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;93(23):2164–2171. doi: 10.2106/JBJS.J.01884. [DOI] [PubMed] [Google Scholar]
- 39.Nishii T., Sakai T., Takao M., Yoshikawa H., Sugano N. Is ultrasound screening reliable for adverse local tissue reaction after hip arthroplasty? J Arthroplasty. 2014;29(12):2239–2244. doi: 10.1016/j.arth.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Puri L., Wixson R.L., Stern S.H., Kohli J., Hendrix R.W., Stulberg S.D. Use of helical computed tomography for the assessment of acetabular osteolysis after total hip arthroplasty. J Bone Joint Surg Am. 2002;84(4):609–614. doi: 10.2106/00004623-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 41.Walde T.A., Weiland D.E., Leung S.B. Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res. 2005;437:138–144. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]
- 42.Cahir J.G., Toms A.P., Marshall T.J., Wimhurst J., Nolan J. CT and MRI of hip arthroplasty. Clin Radiol. 2007;62(12):1163–1173. doi: 10.1016/j.crad.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Freed J.H., Hahn H., Menter R., Dillon T. The use of the three-phase bone scan in the early diagnosis of heterotopic ossification (HO) and in the evaluation of Didronel therapy. Paraplegia. 1982;20(4):208–216. doi: 10.1038/sc.1982.39. [DOI] [PubMed] [Google Scholar]
- 44.Ruangchaijatuporn T., Gaetke-Udager K., Jacobson J.A., Yablon C.M., Morag Y. Ultrasound evaluation of bursae: anatomy and pathological appearances. Skeletal Radiol. 2017;46(4):445–462. doi: 10.1007/s00256-017-2577-x. [DOI] [PubMed] [Google Scholar]
- 45.Dammerer D., Van Beeck A., Schneeweiss V., Schwabegger A. Follow-up strategies for primary extremity soft-tissue sarcoma in adults: a systematic review of the published literature. In Vivo. 2020;34(6):3057–3068. doi: 10.21873/invivo.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arya S., Nagarkatti D.G., Dudhat S.B., Nadkarni K.S., Joshi M.S., Shinde S.R. Soft tissue sarcomas: ultrasonographic evaluation of local recurrences. Clin Radiol. 2000 Mar;55(3):193–197. doi: 10.1053/crad.1999.0343. PMID: 10708612. [DOI] [PubMed] [Google Scholar]
- 47.Park S.Y., Chung H.W., Chae S.Y., Lee J.S. Comparison of MRI and PET-CT in detecting the loco-regional recurrence of soft tissue sarcomas during surveillance. Skeletal Radiol. 2016;45(10):1375–1384. doi: 10.1007/s00256-016-2440-5. [DOI] [PubMed] [Google Scholar]
- 48.Rajasekaran R.B., Whitwell D., Cosker T.D.A., Gibbons C.L.M.H., Carr A. Will virtual multidisciplinary team meetings become the norm for musculoskeletal oncology care following the COVID-19 pandemic? - experience from a tertiary sarcoma centre. BMC Muscoskel Disord. 2021;22(1):18. doi: 10.1186/s12891-020-03925-8. Published 2021 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noebauer-Huhmann I.M., Amann G., Krssak M. Use of diagnostic dynamic contrast-enhanced (DCE)-MRI for targeting of soft tissue tumour biopsies at 3T: preliminary results. Eur Radiol. 2015;25(7):2041–2048. doi: 10.1007/s00330-014-3576-0. [DOI] [PubMed] [Google Scholar]
- 50.Chang C.D., Wei J., Goldsmith J.D., Gebhardt M.C., Wu J.S. MRI guided needle localization in a patient with recurrence pleomorphic sarcoma and post-operative scarring. Skeletal Radiol. 2017;46(7):975–981. doi: 10.1007/s00256-017-2614-9. [DOI] [PubMed] [Google Scholar]
- 51.Martins P., Costa F., Lopes F., Canella C. Advanced MR imaging and ultrasound fusion in musculoskeletal procedures. Magn Reson Imag Clin N Am. 2018;26(4):571–579. doi: 10.1016/j.mric.2018.06.012. [DOI] [PubMed] [Google Scholar]