Abstract
Introduction
In older individuals with cardiovascular diseases, it has been challenging to diagnose osteoporosis due to aortic calcification and degenerative processes in the spine of older adults, especially in very old adults.
Aim
To assess whether the distal forearm BMD with the proximal femur BMD has greater sensitivity for the diagnosis of osteoporosis than the lumbar spine BMD with the proximal femur BMD.
Methods
We evaluated 515 older adults with cardiovascular disease from the SARCOS study and stratified them into under and over 80-year-old age groups and according to gender. Two diagnostic criteria were used to assess osteoporosis, SPF (lumbar spine and proximal femur BMD) and DFF (distal forearm and proximal femur BMD), which were compared with the multiple bone sites (MS) criteria (lumbar spine, distal radius, femoral neck, and total femur BMD).
Results
43.9% were aged ≥80 years. Osteoporosis by SPF was diagnosed in 34% (n = 175), by DFF in 42.2% (n = 216), and by MS in 46.8% (n = 241). The characteristics of the three groups were similar. For every 100 older individuals with osteoporosis based on MS, 27 were not diagnosed by the SPF, and nine were not diagnosed by DFF (p = 0.001). The SPF did not diagnose osteoporosis in 23/100 in older adults aged <80 years, while DFF did not diagnose 16/100 (p.ns). In adults aged ≥80 years, the SPF did not identify osteoporosis in 31/100 older adults, while the DFF failed to identify it in only 5/100 (p < 0.001). In men and women aged ≥80 years, DFF showed higher sensitivity for the diagnosis of osteoporosis compared to the SPF criterion.
Conclusion
In the elderly population with cardiovascular disease evaluated in our study, the use of distal forearm BMD instead of lumbar spine BMD, associated with proximal femur BMD, showed higher sensitivity for the diagnosis of osteoporosis, regardless of gender, and especially among the very older adults.
Keywords: Osteoporosis, Elderly, Distal forearm BMD, Aortic calcification
Highlights
-
•
Diagnosis of osteoporosis (OP) in the elderly with cardiovascular disease is challenging.
-
•
We evaluated the sensitivity of distal forearm (DF) BMD vs. lumbar spine (LS) BMD.
-
•
LS BMD did not diagnose OP in 27/100, and DF BMD in 9/100 (p = 0.001).
-
•
LS BMD did not identify OP in 31/100, and DF BMD in 5/100 (p < 0.001) aged ≥80 years.
-
•
DF BMD showed higher sensitivity for diagnosing osteoporosis, regardless of gender.
1. Introduction
Osteoporosis and cardiovascular diseases are prevalent among the elderly and directly correlate with aging (Schuit et al., 2004; North and Sinclair, 2012). Hypertension and atherosclerotic heart disease (ASHD) have been associated with significant loss of bone mineral density (BMD) of femoral neck (Cappuccio et al., 1999; Hu et al., 2021), while increased atherosclerotic calcification is associated with higher rates of vertebral and hip fractures (Arfai et al., 2004). Fragility fractures are associated with loss of function, institutionalization, higher mortality, and substantial financial and societal burdens (Gullberg et al., 1997; Cauley et al., 2014; Cauley, 2013). In 2020, there will be 2.6 million hip fractures, and by 2050 there will be 4.5 million fractures (Gullberg et al., 1997). Quantification of BMD by dual-source x-ray absorptiometry (DXA) is a simple and highly accurate method for screening individuals with bone loss, facilitating the prevention and treatment of osteoporosis (Kanis et al., 2013). Several medical societies (Kanis et al., 2019; Cosman et al., 2014; Viswanathan et al., 2018; Schousboe et al., 2013) recommend that the diagnosis should be based on BMD measurement in the lumbar spine and the proximal femur. However, in the elderly population and especially among those with cardiovascular diseases, several degenerative processes falsely increase BMD in the lumbar spine, decreasing the sensitivity of the diagnosis of osteoporosis (Liu et al., 1997a; Dalle Carbonare et al., 2000; Kinoshita et al., 1998; Rand et al., 1997). The International Society for Clinical Densitometry (2019) recommended measuring the BMD in the distal forearm when hip and/or spine cannot be measured or interpreted. However, perivertebral degenerative processes, such as aortic calcification, osteoarthritis of the lumbar spine, and vertebral fractures are not systematically evaluated prior to DXA scanning, making it difficult to use the distal forearm as a preferred measurement site rather than the lumbar spine.
Consequently, many older adults with osteoporosis may be undiagnosed, causing difficulties for measures that reduce the incidence of fragility fractures. Because the risk of fractures in the elderly derives from several treatable and untreatable etiologic factors, there is an urgent need to optimize diagnosis methods by measuring BMD. Based on the problems associated with measuring the lumbar spine BMD and the need to optimize the diagnosis of osteoporosis in the elderly with cardiovascular disease, we hypothesized that the distal forearm BMD and the proximal femur BMD would provide greater sensitivity for the diagnosis of osteoporosis than the lumbar spine BMD and the proximal femur BMD and that this difference would increase with age. To test our hypothesis, we measured the prevalence of osteoporosis using two diagnostic criteria in adults above and below 80 years of age. Then, we analyzed the sensitivity of the two diagnostic criteria, comparing them with the diagnostic criteria for osteoporosis with multiple bone sites (i.e., the lumbar spine, total hip, or femoral neck, and distal forearm).
2. Methods
2.1. Study design
This was a cross-sectional analysis of the SARCopenia and OSteoporosis in older adults with cardiovascular diseases study (SARCOS). This one-year prospective cohort study investigated the association of sarcopenia and osteoporosis as a common pathway to disability and physical frailty in older adults in an outpatient community-dwelling setting (Frisoli et al., 2021).
2.2. Sample
Our sample consisted of older adults from a geriatric cardiology outpatient clinic at the Universidade Federal de São Paulo, São Paulo, Brazil. We interviewed 632 subjects, and 515 underwent DXA and met eligibility criteria for this analysis. Exclusion criteria included lumbar spine surgery, wrist surgery, bilateral hip surgery, poliomyelitis, unstable medical conditions, cancer in the previous five years, chronic renal failure requiring dialysis, and chronic liver disease. The Ethics Committee at our institution approved the study (number 682659) and written informed consent was obtained from all participants.
2.3. Variables recorded at baseline
2.3.1. Cardiovascular and chronic diseases
Cardiovascular diseases included arterial hypertension, diabetes mellitus, previous myocardial infarction (over six months before the study), angina, heart failure, previous stroke (over six months prior), and peripheral arterial disease. Chronic diseases included osteoarthritis, non-dialytic chronic kidney disease, chronic obstructive pulmonary disease, and a history of fracture over the previous 30 years. All disease information was obtained from medical records.
2.3.2. Lifestyle factors and other information
We gathered demographic data and medications that may interfere with bone metabolism (i.e., bisphosphonate, calcium, vitamin D, testosterone, hormone replacements, and denosumab), other medications, self-reported current or past smoking, and respective pack-years, and self-reported current or past alcohol consumption.
2.3.3. Osteoporosis
The BMD (g/m2) of all patients were obtained from DXA analysis using dual-energy X-ray absorptiometry (GE Lunar; DPX-MD 73477, GE Medical Systems, Madison, WI) of the lumbar spine, distal forearm (33% distal radius), femoral neck, and total femur (the left hip was only used in patients with previous trauma or surgeries in the right hip). The inter operator variability for the distal forearm is 0.023 g/ cm2. The diagnosis of osteoporosis was considered if T-score was −2.5 or below in any bone site (Camacho et al., 2020).
We classified the patients according to three criteria for the diagnosis of osteoporosis: SPF criteria, as recommended by WHO (1994): T-score BMD ≤ -2.5 SD at the lumbar spine or proximal femur (femoral neck or total femur); DFF criteria: T-score BMD ≤ -2.5 SD at the distal forearm or proximal femur (femoral neck or total femur); and multiple-site criteria (MS): T-score BMD ≤ -2.5 SD at the lumbar spine and or distal forearm or proximal femur (femoral neck or total femur). Subjects with osteoporosis by SPF, DFF, or MS criteria were analyzed in groups labeled OP SPF, OP DFF, and OP MS, respectively. We considered the multiple-site criteria as the “gold standard” for diagnosing osteoporosis, from which we performed the sensitivity analyses of the SPF and DFF criteria. The use of the MS criterion as “gold standard” results from the fact that it evaluates, at the same time, all bone sites used for the diagnosis of osteoporosis, in the clinical practice, in addition to being the ones with the highest incidence of fractures.
2.4. Statistical analysis
Analyses were performed on the entire sample population, and we subsequently divided the four groups (under 80 y and 80 y or older, and men and women), to determine if the effect of age and gender were relevant. Qualitative variables were expressed as absolute and relative frequencies.
To compare the groups, the chi-square was used for qualitative variables, and analysis of variance was used for quantitative variables. Generalized estimating equations with the McNeamer test were used to assess the relevance of the sensitivity of the DFF and SPF criteria. Only hypertension, diabetes mellitus, and heart failure were included in the analyses for osteoporosis. SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA) statistical software was used for all analyses. Statistical significance was set at 0.05.
3. Results
The characteristics of our population and the groups with osteoporosis according to the three diagnostic criteria are displayed in Table 1. The mean age of the sample was 78.31 (7.17) yo, and 43.9% were 80 years or older. Osteoporosis by SPF criteria was found in 34%, by DFF criteria it was 42.2%, and by MS criteria it was 46.8%. Overall, females were slightly more common in the OP DFF and OP MS groups than in OP SPF. The three OP groups had similar profiles concerning gender, ethnicity, age, past smoking and alcohol use, past fractures, and bisphosphonate, thiazides and vitamin D use. Mean BMD of total hip, femoral neck, and lumbar spine were similar and higher in the OP DFF and MS groups than the OP SPF group, whereas BMD of the distal forearm was lower.
Table 1.
Demographic characteristics, the prevalence of cardiovascular and other chronic diseases, mean BMD of the lumbar spine, femoral neck, total hip, and distal forearm, of the sample and the groups diagnosed by SPF, DFF, and MS criteria.
| Overall, n (%) | Osteoporosis SPF, n (%) | p | Osteoporosis DFF, n (%) | p | Osteoporosis MS, n (%) | p | |
|---|---|---|---|---|---|---|---|
| Caucasian | 354 (68.7) | 126 (72.0) | 0.047 | 161 (74.5) | 0.003 | 177 (73.4) | 0.008 |
| Afro-descendent | 142 (27.6) | 39 (22.3) | 44 (20.4) | 52 (21.6) | |||
| Asian descendent | 19 (3.7) | 10 (5.7) | 11 (5.1) | 12 (5.0) | |||
| Mean Age, years | 78.31(±7.17) | 80.1 (7.6) | <0.001 | 80.53 (7.3) | <0.001 | 79.78 (7.2) | <0.001 |
| Male | 232 (45) | 62 (35.4) | 0.002 | 67 (31) | <0.001 | 75 (31) | <0.001 |
| Female | 283 (55) | 113 (64.6) | 149 (69) | 166 (68.9) | |||
| Years of education | 3.44 (±3.19) | 3.24 (2.90) | 0.226 | 3.40 (3.03) | 0.664 | 3.33 (2.97) | 0.332 |
| Hypertension | 477 (92.8) | 157 (89.7) | 0.070 | 198 (91.7) | 0.383 | 220 (91.3) | 0.234 |
| Diabetes mellitus | 204 (39.8) | 49 (28.2) | <0.001 | 66 (30.6) | <0.001 | 72 (30.0) | <0.001 |
| Previous osteoporosis | 91 (17.7) | 43 (24.6) | 0.004 | 51(23.6) | 0.002 | 60 (24.9) | <0.001 |
| Previous fracture | 164 (31.8) | 73 (41.7) | 0.001 | 89 (41.2) | <0.001 | 98 (40.7) | <0.001 |
| Osteoarthritis | 181 (35.4) | 58 (33.1) | 0.495 | 67 (31.0) | 0.092 | 80 (33.2) | 0.354 |
| CKD | 87 (16.9) | 31(17.7) | 0.804 | 35 (16.2) | 0.722 | 39 (16.2) | 0.724 |
| Smoke Years | 17.79 (±19.3) | 17.62 (22.36) | 0.026 | 16.48 (22.20) | 0.012 | 16.24 (21.76) | 0.002 |
| Previous smoke | 254 (49.4) | 76 (43.7) | 0.076 | 93 (43.3) | 0.020 | 107 (44.6) | 0.042 |
| COPD | 51 (9.9) | 19 (10.9) | 0.641 | 24 (11.1) | 0.550 | 26 (10.8) | 0.557 |
| Previous alcohol intake | 74 (14.4) | 23 (13.1) | 0.599 | 27 (12.5) | 0.310 | 29 (12.0) | 0.168 |
| Bisphosphonate | 45 (8.7) | 28 (16.0) | <0.001 | 34 (15.7) | <0.001 | 40 (16.6) | <0.001 |
| Vitamin D | 21 (4.1) | 9 (5.1) | 0.481 | 14 (6.5) | 0.024 | 14 (5.8) | 0.075 |
| Thiazides | 128 (24.9) | 38 (21.7) | 0.282 | 46 (21.3) | 0.121 | 51 (21.2) | 0.082 |
| BMI, Kg/m2 |
26.73(±4.59) | 24.98 (4.21) | 0.521 | 25.14 (4.30) | 0.767 | 25.33 (4.26) | 0.839 |
| Lumbar spine BMD, g/cm | 21.084(±0.22) | 0.904 (0.16) | <0.001 | 0.949 (0.16) | <0.001 | 0.943 (0.16) | <0.001 |
| Femoral Neck BMD, g/cm2 | 0.832(±0.170) | 0.695 (0.10) | <0.001 | 0.715 (0.10) | <0.001 | 0.726 (0.102) | <0.001 |
| Total hip BMD, g/cm2 |
(0.875(±0.170) | 0.729 (0.11) | <0.001 | 0.746 (0.10) | <0.001 | 0.758 (0.115) | <0.001 |
| Distal forearm BMD, g/cm2 | 0.697(±0.16) | 0.604 (0.14) | <0.001 | 0.583 (0.13) | <0.001 | 0.596 (0.137) | <0.001 |
BMI – body mass index; CKD – chronic kidney disease; COPD – chronic obstructive pulmonary disease.
3.1. Osteoporosis by SPF, DFF, and MS criteria in all subjects
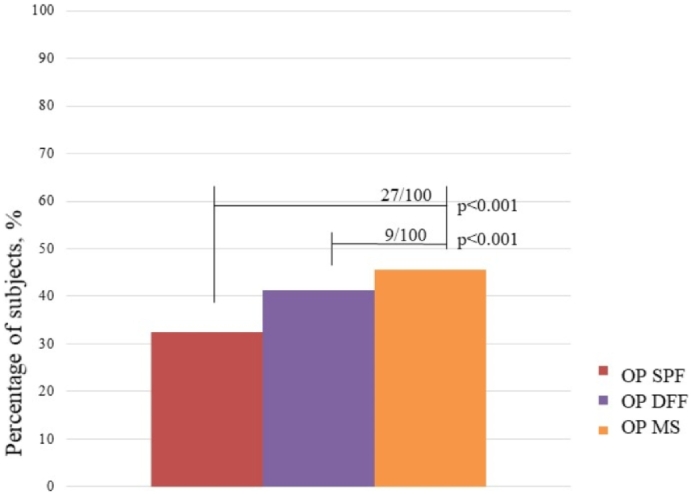
When comparing the OP DFF and OP SPF groups, we observed that, among the elderly in OP DFF, 30.6% (n = 66) were not diagnosed by the SPF criteria, while 12.8% (n = 22) of the OP SPF group were not diagnosed by the DFF criteria (p < 0.001). When comparing the OP SPF and OP DFF groups with the OP MS group (reference group), 27.4% (n = 66) of the subjects in the OP MS group were not diagnosed with osteoporosis using the SPF criteria (p < 0.001), while only 9.2% (n = 22) were diagnosed with the DFF criteria. In clinical practice, this means that, for every 100 older adults with osteoporosis, 27 would not be diagnosed if evaluated according to the SPF criteria, and nine would not be diagnosed according to the DFF criteria (Fig. 1). In the complementary analyses that determined if there was an increase of sensitivity of the DFF criteria in relation to the SPF criteria, we found that there was a significant interaction effect between the criteria in the total sample (p < 0.001), demonstrating significantly greater sensitivity of the DFF criteria than the SPF criteria for the diagnosis of osteoporosis.
Fig. 1.
Prevalence of osteoporosis by SPF, DFF, and multi-site criteria in all individuals and the number of patients with osteoporosis who were not diagnosed by SPF and DFF criteria.
OP SPF – Group of elderly with osteoporosis by SPF criteria
OP DFF – Group of elderly with osteoporosis by DFF criteria
OP MS – Group of elderly with osteoporosis by MS criteria.
3.2. Osteoporosis by SPF, DFF, and MS criteria in older adults less than and greater than 80 years old
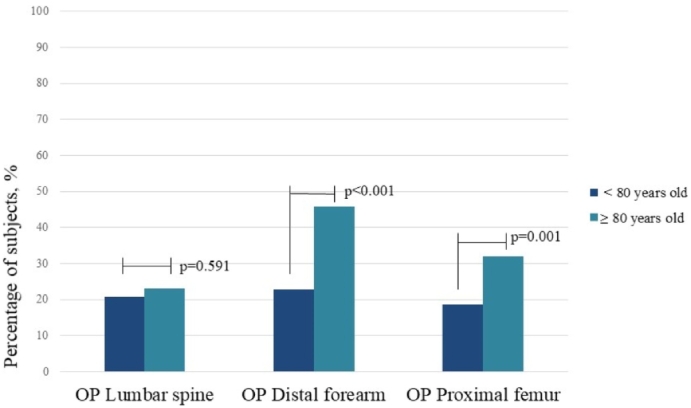
In the analyses of groups under and over 80 years of age, osteoporosis diagnosed by SPF criteria was the least prevalent in both age groups, (29.4% [n = 85] vs. 39.8% [n = 90]; p = 0.015), while osteoporosis according to the DFF criteria was intermediate between these groups (32.1% [n = 92], vs. 55.1% [n = 124]; p < 0.001), while the MS criteria reached the highest value in both groups (38.4% [n = 111] vs. 57.5% [n = 130]; p < 0.001). However, it is noteworthy that the difference in prevalence between age groups was almost twice as high in the OP DFF and OP MS groups than in the OP SPF group (23% and 18.5% and 10.4%, respectively). This was due to the significantly greater prevalence of osteoporosis observed at the distal forearm in the group older than 80 years compared to the younger ones (22.8% [n = 65] vs. 45.9% [n = 100] respectively; p < 0. 001), which followed the trend of proximal femur osteoporosis (18.7% [n = 54] vs. 31.9% [n = 72]; p = 0.001; Fig. 2), while lumbar spine osteoporosis showed no variation between the age groups (20.8% [n = 60] vs. 23% [n = 52] respectively; p = 0.591).
Fig. 2.
Prevalence of osteoporosis in lumbar spine, distal forearm and proximal femur in patients aged less than and more than 80 years old.
OP Lumbar spine – Group of elderly with osteoporosis in the lumbar spine
OP Distal forearm – Group of elderly with osteoporosis in the distal forearm
OP Proximal femur – Group of elderly with osteoporosis in the proximal femur (hip total or femoral neck).
3.3. Comparison of SPF, DFF, and MS prevalence of osteoporosis among age groups and gender
When comparing the prevalence of osteoporosis using the three methods in individuals under 80 years of age, we observed that osteoporosis by the DFF criteria was higher than by the SPF criteria (2.7%; p < 0.001); the prevalence of osteoporosis by the MS criteria was (9.0%; p < 0.001) higher than by the SPF criteria. The MS and DFF criteria comparison showed a 6.3% (p < 0.001) superiority for the MS criteria. Among individuals older than 80 years, the prevalence of osteoporosis by the DFF criteria was 15.3% (p < 0.001) higher than by the SPF criteria, while the MS criteria diagnosed 18.4% (p < 0.001) more osteoporotic individuals than the SPF criteria. Between the MS and DFF criteria, there was a difference of only 2.4% for the MS criteria (p < 0.001). However, in the sensitivity analysis using the McNemar's test, there was no increase in sensitivity in the diagnosis of osteoporosis by the SPF criteria compared to the DFF criteria (p = 0.222) in the age group under 80 years. However, there was an increase among those over 80 years (p < 0.001). These results suggest that the sensitivity of the BMD of the lumbar spine for the diagnosis of osteoporosis decreases significantly in the 80s group, while the sensitivity of the BMD of the distal forearm increases (Fig. 3).
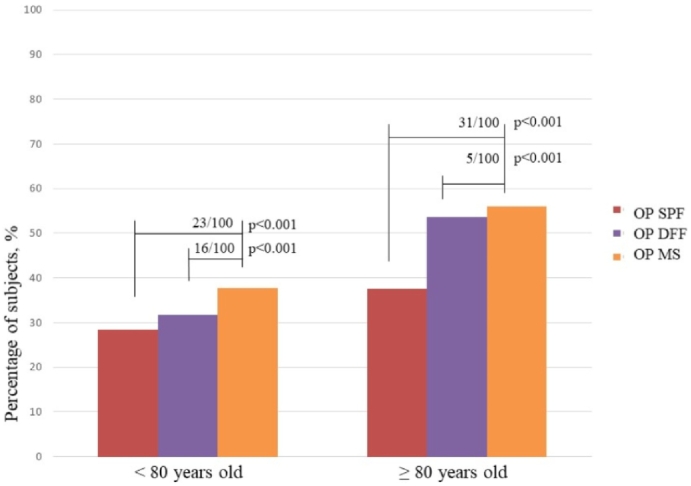
Fig. 3.
Prevalence of osteoporosis by SPF, DFF, and MS criteria in patients aged less than and more than 80 years, and number of patients per 100 with osteoporosis who were not diagnosed by the SPF and DFF criteria
OP SPF – Group of elderly with osteoporosis by SPF criteria
OP DFF – Group of elderly with osteoporosis by DFF criteria
OP MS – Group of elderly with osteoporosis by MS criteria.
Projecting the above data into the screening process for osteoporosis, in absolute numbers of persons aged 60–80 years, the use of the SPF criteria (would fail to diagnose osteoporosis in approximately 23 of 100 older individuals with osteoporosis. By the DFF method, this figure would fall to 16 of 100 (Fig. 3). Among those aged ≥80 years diagnosed using the SPF criteria, this deficit would increase substantially because 31 of 100 individuals with osteoporosis would not be diagnosed. For the DFF criteria, the difference would be only 5 per 100 (Fig. 3). In the complementary analyses for sensitivity using McNemar's test of the SPF and DFF criteria, concerning OP MS, in the group under 80 years of age, OP SPF and OP DFF had a significant difference between them (p < 0.001), as did each one concerning MS (p < 0.001). Among the elderly over 80 years, the difference remained between the SPF and DFF (p < 0.001), between SPF and MS (p < 0.001); however, between DFF and MS, there was no significant difference (p = 0.063), showing similarity between the two criteria.
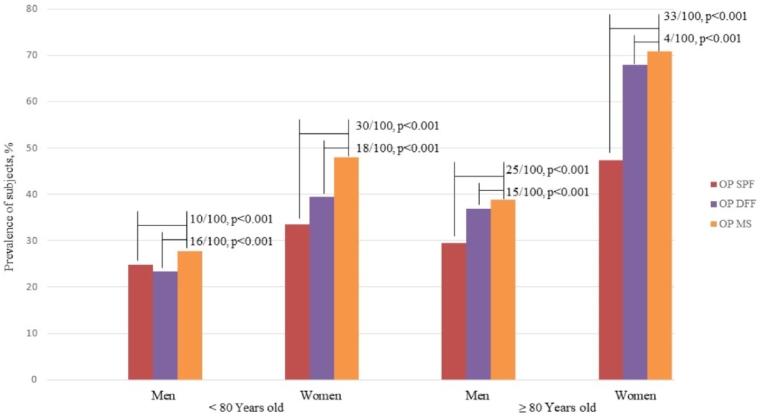
Among individuals under 80 years, the prevalence of osteoporosis in men by the SPF, DFF and MS criteria (24.8% vs. 23.3% vs. 27.7%, respectively, p < 0.001) were very similar. In the group of women, the prevalence of all criteria was higher compared to the group of men (SPF = 33.5% vs. DFF = 39.4% vs. MS = 48%, p < 0.001), as shown in Fig. 4. Among those aged ≥80 years, the prevalence of osteoporosis by DFF criteria was very similar to the prevalence by MS criteria (36.9% vs. 38.9%; p < 0.001) in men and women (67.9% vs. 70.9%; p < 0.001), while the prevalence of SPF was significantly lower in men (SPF = 29.5%; p < 0.001) and women (SPF = 47.3%; p < 0.001) compared MS criteria .
Fig. 4.
Prevalence of osteoporosis by SPF, DFF, and MS criteria in patients aged less than and more than 80 years, according to gender, and number of patients per 100 with osteoporosis who were not diagnosed by the SPF and DFF criteria
OP SPF – Group of elderly with osteoporosis by SPF criteria
OP DFF – Group of elderly with osteoporosis by DFF criteria
OP MS – Group of elderly with osteoporosis by MS criteria.
Projecting the above data into the screening process for osteoporosis, in absolute numbers of persons aged 60–80 years, the use of the SPF criteria would fail to diagnose osteoporosis in approximately 10 of 100 men and 30 of 100 women with osteoporosis. By the DFF method, this figure would rise to 16 of 100 in men and fall to 18 in women (Fig. 4). Among those aged ≥80 years diagnosed using the SPF criteria, this deficit would increase substantially because 25 of 100 men and 33 of 100 women with osteoporosis would not be diagnosed. For the DFF criteria, the gap would decrease for 15 per 100 men and only to 4 per 100 women (Fig. 4). In the complementary sensitivity analyses using McNemar's test for the SPF and DFF criteria, regarding the MS OP, in the men under 80 years of age, the SPF OP had no statistical difference (p = 0.125) while the DFF OP did (p = 0.031). In the women's group, both criteria were statistically different from the MS criteria (SPF p < 0.001 and DFF p = 0.003). Among the elderly older than 80 years, the DFF criteria had no statistical differences from the MS criteria in both men (p = 0.500) and women (p = 0.250), whereas the SPF criteria remained significantly lower in both men and women (p < 0.001). These data suggest that the DFF criteria have greater sensitivity for diagnosing osteoporosis for men and women aged ≥80 years compared to the SPF criteria, and the SPF criteria appear to be better for diagnosing osteoporosis in men under 80 years of age.
4. Discussion
To the best of our knowledge, the present study is the first to demonstrate the importance of BMD of the distal forearm and the poor performance of BMD of the lumbar spine in the diagnosis of osteoporosis in older individuals with cardiovascular disease. This finding occurred because, when we replaced the BMD of the lumbar spine by the BMD of the distal third of the radius, there was a significant increase in the diagnosis of osteoporosis, both in the general sample and in both age groups (although only among those over 80 years of age) the distal forearm, showing significantly superior diagnostic sensitivity. The criteria involving BMD of the distal forearm (DFF) were more efficient as a screening method for osteoporosis in older individuals, as it was able to identify 18 more individuals than the conventional method (SPF) in every 100 with osteoporosis. This assumes greater importance regarding older individuals with cardiovascular diseases and those aged 80 years or more, where the risk of fragility fracture is higher. In this population, the DFF criteria identified 26 more individuals with osteoporosis than the SPF criteria. In clinical practice, this finding suggests that one in four older people diagnosed with osteoporosis and at high risk for fracture were undiagnosed and would not have the opportunity to receive adequate prevention and treatment for osteoporosis.
The loss of sensitivity of the SPF criteria concerning age groups is due to the absence of the expected growth in the prevalence of osteoporosis of the lumbar spine among those below and above the age of 80 years; by contrast, the DFF criteria, which use the BMD of the distal forearm, detected a 70% increase in osteoporosis at this site, similar to the growth observed in the proximal femur (Fig. 2). The phenomenon of no significant variation in BMD of the lumbar spine with increasing age has been described by some authors, regardless of the presence of cardiovascular diseases (Reid et al., 1991; Steiger et al., 1992; Melton et al., 2000; Warming et al., 2002; Looker et al., 2012). This finding is associated with several factors related to the aging process and the accumulation of comorbidities that overestimate the BMD of the lumbar spine assessed in the anterior, posterior projection (Tobias et al., 2007).
The primary factors are atherosclerotic calcification of the aorta, calcification of the intervertebral ligaments, vertebral osteophytosis, and vertebral fractures. These associations were elegantly demonstrated by Liu et al. (Liu et al., 1997b), SPF showed that 75% of men and 61.1% of women aged 60–99 years had vertebral osteophytes, joint space narrowing, and bone sclerosis of the lumbar spine and hip on radiographs. Regression analysis using these factors indicated that lumbar osteophytes explained 16.6% of the variation in lumbar spine BMD in women and 22.4% in men. In another study, Muraki et al. (Muraki et al., 2004) found that in 630 women aged 60 years or older, the vertebral osteophytes, bone sclerosis, and disk space narrowing were independently correlated with BMD of the lumbar spine. In contrast, they were not correlated with BMD of the femoral neck, suggesting that the osteoarthrosis process interfered primarily with BMD of the lumbar spine and not the proximal femur. Aortic calcification can also significantly interfere in the quantification of lumbar spine BMD in our study, primarily because it is more frequent among older people with cardiovascular diseases and those aged over 80 years (Banks et al., 1994; Frye et al., 1992; Bristow et al., 2019).
Aoyagi et al. (Aoyagi et al., 2001) described an almost exponential increase in the prevalence of aortic calcification in lateral lumbar spine radiographs among women under the age of 55 to those over 75 years, whereas Vogt et al. (Vogt et al., 1997) reported that 96% of women aged 85 years and older had the same problem. They also observed that aortic calcification was positively associated with body mass index, systolic blood pressure, diabetes, current smoking, and thiazide use. These same authors and others also observed an inverse correlation between the intensity of calcification of the aorta and the BMD of bone sites other than the lumbar spine and a direct correlation with fracture risk, especially among those with cardiovascular disease (Bristow et al., 2019; Aoyagi et al., 2001; Vogt et al., 1997; Flipon et al., 2012; Maghraoui et al., 2012; Kim et al., 2012; Szulc et al., 2013).
Although there remains no consensus regarding when to use lumbar spine BMD to diagnose osteoporosis, our results corroborate those studies that found that lumbar spine BMD in older age groups significantly loses its sensitivity and should be replaced by other bone sites and not merely subtracted. This is because, in addition to the lumbar spine BMD losing its discriminatory capacity for the diagnosis of osteoporosis in older age groups, it has been questioned regarding its predictive value for non-hip and hip fractures. Kanis et al. in 2006 (Kanis et al., 2006) and Leslie et al. in 2007 (Leslie et al., 2007), when prospectively following approximately 16,000 and 19,000 individuals aged over 50 years, respectively, found that the use of the minimum value of the lumbar spine BMD with the femoral neck did not exceed the predictive value for fractures compared to femoral neck BMD alone. In these studies, the authors suggested that the use of lumbar spine BMD in addition to femoral neck BMD would be of little clinical relevance for osteoporosis. However, these studies did not reveal how many patients were not diagnosed with osteoporosis if one used only the femoral neck BMD. The question that arises is whether one should consider osteoporosis as a risk factor, regardless of the bone site, as suggested by some societies (Camacho et al., 2020), or should clinicians identify the bone sites that have the greatest predictive value for fracture. Our study was not designed to measure the incidence of fractures, and therefore, we did not obtain data that would show that the model with the distal third of the radius BMD would be superior to lumbar spine BMD in predicting fractures. However, we understand that improving the diagnosis is of paramount importance to identify those at risk for fractures and adopt preventive or curative measures. Finally, despite the numerous reports of the decreased sensitivity of the lumbar spine BMD for the diagnosis of osteoporosis in the elderly, most consensuses and guidelines still recommend that the lumbar spine BMD be used and replaced by the distal third of the BMD. Radiographic images are obtained only under conditions where artifacts that make measurement difficult are present. The replacement of the lumbar spine BMD by the distal forearm, as a routine in the osteoporosis screening, is more advantageous for older individuals because it is more sensitive in diagnosis, does not introduce the possibility of greater exposure to radiation due to the need to repeat the examination in cases in which artifacts are visualized, and the distal radius does not present artifacts that promote significant variation with aging.
Our study has limitations resulting from the type of population because it comes from a cardiovascular disease outpatient clinic and cannot be generalized to other populations of older people in general. Despite being a prospective cohort, this study is a cross-sectional analysis that does not permit inferences regarding the risk of fracture. We did not perform lateral lumbar spine radiography, and we were unable to determine how much aortic calcification (considered a cardiovascular disease) may have influenced our results. The diagnostic cutoff for osteoporosis of the distal forearm of -2.5 SD has been widely used in clinical practice and research, but there is still controversy about the validation of this value. Therefore, data concerning for the osteoporosis of the distal forearm still need further studies to be able to make broader conclusions. Serum vitamin D levels were not measured, and this also prevented us from measuring the overlap between osteomalacia and osteoporosis, often found among individuals over 80 years of age.
5. Conclusion
In the elderly population with cardiovascular disease evaluated in our study, the use of distal forearm BMD instead of lumbar spine BMD, associated with proximal femur BMD, showed higher sensitivity for the diagnosis of osteoporosis, regardless of gender, and especially among the very older adults.
5.1. Implications
Distal forearm BMD in conjunction with proximal femur BMD appears to be an alternative for diagnosing osteoporosis in very older adults with cardiovascular disease. Further studies with different populations are still needed before we can consolidate this statement.
Funding
-
o
The authors declare that they did not receive funding for this study.
CRediT authorship contribution statement
-
•
Study concept and design: Alberto Frisoli Jr.
-
•
Acquisition of data: Alberto Frisoli Junior, Amanda Diniz Kimura and Elaine Azevedo
-
•
Analysis and interpretation of data: Alberto Frisoli Jr., Angela Paes.
-
•
Drafting of the manuscript: Alberto Frisoli Jr.
-
•
Critical revision of the manuscript for important intellectual content: Alberto Frisoli Jr., Valdir Ambrosio and Angela Paes.
Declaration of competing interest
-
o
Alberto Frisoli Jr. declares that there is no conflict of interest.
-
o
Angela T Paes declares that there is no conflict of interest.
-
o
Amanda Diniz Kimura declares that there is no conflict of interest.
-
o
Elaine Azevedo declares that there is no conflict of interest
-
o
Valdir Ambrosio declares that there is no conflict of interest.
-
o
Previous publication
-
o
We declare that the current version of this study has not been published in any medical journal.
Acknowledgments
The authors would like to thank Eliene Lima and Neusa Maria for help with the administrative aspects and organization of our study.
Contributor Information
Alberto Frisoli Jr, Email: alberto.junior@einstein.br.
Valdir Ambrosio, Email: vmoises@unifesp.br.
References
- Aoyagi K., Ross P.D., Orloff J., Davis J.W., Katagiri H., Wasnich R.D. Low bone density is not associated with aortic calcification. Calcif Tissue Int. 2001;69(1):20–24. doi: 10.1007/s002230020003. Jul. [DOI] [PubMed] [Google Scholar]
- Arfai K., Liu X., Sayre J., Gilsanz V., Schulz E. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89(9):4246–4253. doi: 10.1210/jc.2003-030964. Sep. [DOI] [PubMed] [Google Scholar]
- Banks L.M., Lees B., Mac Sweeney J.E. Stevenson JC effect of degenerative spinal and aortic calcification on bone density measurements in post-menopausal women: links between osteoporosis and cardiovascular disease? Eur. J. Clin. Investig. 1994;24:813–817. doi: 10.1111/j.1365-2362.1994.tb02024.x. [DOI] [PubMed] [Google Scholar]
- Bristow S.M., Gamble G.D., Horne A.M., Reid I.R. Longitudinal changes in bone mineral density, bone mineral content and bone area at the lumbar spine and hip in postmenopausal women, and the influence of abdominal aortic calcification. Bone Rep. 2019;10:100190. doi: 10.1016/j.bonr.2018.100190. Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho P.M., Petak S.M., Binkley N., Diab D.L., Eldeiry L.S., Farooki A., Harris S.T., Hurley D.L., Kelly J., Lewiecki E.M., Pessah-Pollack R., Wimalawansa S.J., Watts N.B., American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis 2020 upto date. Endocr Pract. 2020;26(Suppl. 1):1–46. doi: 10.4158/GL-2020-0524SUPPL. May. [DOI] [PubMed] [Google Scholar]
- Cappuccio F.P., Meilahn E., Zmuda J.M., Cauley J.A. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of osteoporotic fractures research group. Lancet. 1999;354(9183):971–975. doi: 10.1016/s0140-6736(99)01437-3. Sep 18. [DOI] [PubMed] [Google Scholar]
- Cauley J.A. Public health impact of osteoporosis. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(10):1243–1251. doi: 10.1093/gerona/glt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley J.A., Chalhoub D., Kassem A.M., Fuleihan G.E.-H. Geographic and ethnic disparities in osteoporotic fractures. Nat. Rev. Endocrinol. 2014;10(6):338–351. doi: 10.1038/nrendo.2014.51. [DOI] [PubMed] [Google Scholar]
- Cosman F., de Beur S.J., LeBoff M.S., Lewiecki E.M., Tanner B., Randall S., Lindsay R. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Carbonare L., Giannini S., Ciuffreda M., Silva-Netto F., Arlot M.E., Crepaldi G., Sartori L., Nobile M. Lumbar osteoarthritis, bone mineral density, and quantitative ultrasound. Aging (Milan) 2000;12(5) doi: 10.1007/BF03339861. 360–365 3. [DOI] [PubMed] [Google Scholar]
- Flipon E., Liabeuf S., Fardellone P., Mentaverri R., Ryckelynck T., Grados F., Kamel S., Massy Z.A., Dargent-Molina P., Brazier M. Is vascular calcification associated with bone mineral density and osteoporotic fractures in ambulatory, elderly women? Osteoporos. Int. 2012;23:1533–1539. doi: 10.1007/s00198-011-1762-3. [DOI] [PubMed] [Google Scholar]
- Frisoli A., Jr, Paes A.T., Borges J., Ingham S.M., Cartocci M., Lima E., Carvalho A. The association between low lean mass and osteoporosis increases the risk of weakness, poor physical performance, and frailty in Brazilian older adults: data from DFF study. Eur J Clin Nutr. 2021;75(3):446–455. doi: 10.1038/s41430-020-00753-w. Mar. [DOI] [PubMed] [Google Scholar]
- Frye M.A., Melton L.J., 3rd, Bryant S.C., Fitzpatrick L.A., Wahner H.W. Osteoporosis and calcification of the aorta. Bone Miner. 1992;19:185–194. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- Gullberg B., Johnell O., Kanis J.A. World-wide projections for hip fracture. Osteoporos. Int. 1997;7(5):407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- Hu Z., YangK Hu Z., Li M., Wei H., Tang Z., Chen B., Su C., Cai D., Xu J. Determining the association between hypertension and bone metabolism markers in osteoporotic patients. Medicine (Baltimore) 2021;100(24) doi: 10.1097/MD.0000000000026276. Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J.A., Johnell O., Odén A., Johansson H., Eisman J.A., Fujiwara S., Kroger H., Honkanen R., Melton L.J., 3rd, O’Neill T., Reeve J., Silman A., Tenenhouse A. The use of multiple sites for the diagnosis of osteoporosis. Osteoporos. Int. 2006;17:527–534. doi: 10.1007/s00198-005-0014-9. [DOI] [PubMed] [Google Scholar]
- Kanis J.A., EV McCloskey, Johansson H., Cooper C., Rizzoli R., Reginster J.Y., Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis J.A., Cooper C., Rizzoli R., Reginster J. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019;30(1):3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.J., Kim K.M., Park K.H., Choi H.S., Rhee Y., Lee Y.H., Cha B.S., Kim M.J., Oh S.M., Brown J.K., Lim S.K. Aortic calcification and bone metabolism: the relationship between aortic calcification, BMD, vertebral fracture, 25-hydroxyvitamin D, and osteocalcin. Calcif. Tissue Int. 2012;91:370–378. doi: 10.1007/s00223-012-9642-1. [DOI] [PubMed] [Google Scholar]
- Kinoshita H., Tamaki T., Hashimoto T., Kasagi F. Factors influencing lumbar spine bone mineral density assessment by dual-energy X-ray absorptiometry: comparison with lumbar spinal radiogram. J Orthop Sci. 1998;3(1) doi: 10.1007/s007760050015. 3–9 4. [DOI] [PubMed] [Google Scholar]
- Leslie W.D., Lix L.M., Tsang J.F., Caetano P.A. Single-site vs multisite bone density measurement for fracture prediction. Arch. Intern. Med. 2007;167:1641–1647. doi: 10.1001/archinte.167.15.1641. [DOI] [PubMed] [Google Scholar]
- Liu G., Peacock M., Eilam O., Dorulla G., Braunstein E., Johnston CC effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos Int. 1997;7(6):564–569. doi: 10.1007/BF02652563. [DOI] [PubMed] [Google Scholar]
- Liu G., Peacock M., Eilam O., Dorulla G., Braunstein E., Johnston C.C. Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos. Int. 1997;7(6):564–569. doi: 10.1007/BF02652563. [DOI] [PubMed] [Google Scholar]
- Looker A.C., Melton L., III, Borrud L.G., Shepherd J.A. Lumbar spine bone mineral density in US adults: demographic patterns and relationship with femur neck skeletal status. Osteoporos. Int. 2012;23:1351–1360. doi: 10.1007/s00198-011-1693-z. [DOI] [PubMed] [Google Scholar]
- Maghraoui A., Rezqi A., Mounach A., Achemlal L., Bezza A., Ghozlani I. Relationship between vertebral fracture prevalence and abdominal aortic calcification in men. Rheumatology (Oxford) 2012;51:1714–1720. doi: 10.1093/rheumatology/kes126. [DOI] [PubMed] [Google Scholar]
- Melton L.J., 3rd, Khosla S., Atkinson E.J., O’Connor M.K., O’Fallon W.M. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteoporos. Int. 2000;11:592–599. doi: 10.1007/s001980070080. [DOI] [PubMed] [Google Scholar]
- Muraki S., Yamamoto S., Ishibashi H., Horiuchi T., Hosoi T., Orimo H., Nakamura K. Impact of degenerative spinal diseases on bone mineral density of the lumbar spine in elderly women. Osteoporos Int. 2004;15(9):724–728. doi: 10.1007/s00198-004-1600-y. Sep. [DOI] [PubMed] [Google Scholar]
- North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand T., Schneider B., Grampp S., Wunderbaldinger P., Migsits H., Imhof H. Influence of osteophytic size on bone mineral density measured by dual X-ray absorptiometry. Acta Radiol. 1997;38(2):210–213. doi: 10.1080/02841859709172051. [DOI] [PubMed] [Google Scholar]
- Reid I.R., Evans M.C., Ames R., Wattie D.J. The influence of osteophytes and aortic calcification on spinal mineral density in postmenopausal women. J. Clin. Endocrinol. Metab. 1991;72:1372–1374. doi: 10.1210/jcem-72-6-1372. [DOI] [PubMed] [Google Scholar]
- Schousboe J.T., Shepherd J.A., Bilezikian J.P., Baim S. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom. 2013;16(4):455–466. doi: 10.1016/j.jocd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Schuit S.C., Klift M., Weel A.E., de Laet C.E., Burger H., Seeman E., Hofman A., Uitterlinden A., van Leeuwen J., Pols H. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. 34(1) 2004. pp. 195–202. Jan. [DOI] [PubMed] [Google Scholar]
- Steiger P., Cummings S.R., Black D.M., Spencer N.E. Genant HK age-related decrements in bone mineral density in women over 65. J. Bone Miner. Res. 1992;7:625–632. doi: 10.1002/jbmr.5650070606. [DOI] [PubMed] [Google Scholar]
- Szulc P., Samelson E.J., Sornay-Rendu E., Chapurlat R., Kiel D.P. Severity of aortic calcification is positively associated with vertebral fracture in older men-a densitometry study in the STRAMBO cohort. Osteoporos. Int. 2013;24:1177–1184. doi: 10.1007/s00198-012-2101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias J.H., Hutchinson A.P., Hunt L.P., McCloskey E.V. Stone MD use of clinical risk factors to identify postmenopausal women with vertebral fractures. Osteoporos. Int. 2007;18:35–43. doi: 10.1007/s00198-006-0209-8. [DOI] [PubMed] [Google Scholar]
- Viswanathan M., Reddy S., Berkman N., Cullen K., Middleton J.C., Nicholson W.K., Kahwati L.C. Screening to prevent osteoporotic fractures: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;319(24):2532–2551. doi: 10.1001/jama.2018.6537. [DOI] [PubMed] [Google Scholar]
- Vogt M.T., San Valentin R., Forrest K.Y., Nevitt M.C., Cauley J.A. Bone mineral density and aortic calcification: the study of osteoporotic fractures. J Am Geriatr Soc. 1997;45(2):140–145. doi: 10.1111/j.1532-5415.1997.tb04498.x. Feb. [DOI] [PubMed] [Google Scholar]
- Warming L., Hassager C., Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos. Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 18.International Society for Clinical Densitometry Official Positions Adult . 2019. https://iscd.org/learn/official-positions/adult-positions. [Google Scholar]
- 21.WHO Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a SPF Study Group. World Health Organization technical report series. 1994;843:1–129. [PubMed] [Google Scholar]