Abstract
Purpose
Initial findings in patients with cancer suggest a lower seroconversion to SARS-CoV-2 vaccination possibly related to myelo-immunosuppressive therapies. We conducted a prospective study to assess factors predicting poor seroconversion and adverse events following immunisation (AEFI) to the BNT162b2 vaccine in patients on active treatment.
Patients and methods
Cancer patients, candidates to two doses of BNT162b2 SARS-CoV-2 vaccination, were enrolled. Patients on active surveillance served as controls. The primary endpoint was poor seroconversion (anti S1/S2 IgG < 25 AU/mL) after 21 days from the second dose.
Results
Between March and July 2021, 320 subjects were recruited, and 291 were assessable. The lack of seroconversion at 21 days from the second dose was 1.6% (95% CI, 0.4–8.7) on active surveillance, 13.9% (8.2–21.6) on chemotherapy, 11.4% (5.1–21.3) on hormone therapy, 21.7% (7.5–43.7) on targeted therapy and 4.8% (0.12–23.8) on immune-checkpoint-inhibitors (ICI). Compared to controls, the risk of no IgG response was greater for chemotherapy (p = 0.033), targeted therapy (0.005) and hormonotherapy (p = 0.051). Lymphocyte count < 1 × 109/L (p = 0.04) and older age (p = 0.03) also significantly predicted poor seroconversion. Overall, 43 patients (14.8%) complained of AEFI, mostly of mild grade. Risk of AEFI was greater in females (p = 0.001) and younger patients (p = 0.009).
Conclusion
Chemotherapy, targeted therapy, hormone therapy, lymphocyte count < 1 × 109/L, and increasing age predict poor seroconversion after two doses of BNT162b2 in up to 20% of patients, indicating the need for a third dose and long-term serological testing in non-responders. AEFI occur much more frequently in women and younger subjects who may benefit from preventive medications.
ClinicalTrials.gov Identifier
Keywords: COVID-19 vaccine in cancer patients, SARS-CoV-2 vaccine, Antibody responses to the BNT162b2 vaccine, SARS-CoV-2 vaccine adverse effects, Cancer chemotherapy, Cancer immunotherapy, Cancer hormone therapy, Cancer target therapy, Cancer biological treatment, Immunogenicity
1. Introduction
SARS-CoV-2 has been associated with increased morbidity and mortality in cancer patients [1,2], indicating the need for prompt preventive intervention in this myelo-immunosuppressed population. However, vaccines trials mostly excluded cancer patients [3]. Initial observational studies on seroconversion in cancer patients with SARS-CoV-2 demonstrated that some subgroups of patients, especially those with haematologic malignancies, have lower rates of seroconversion [[4], [5], [6]].
Cancer patients have been identified as a high-priority subgroup for SARS-CoV-2 vaccinations [[7], [8], [9], [10]]. However, there remain many uncertainties, including their seroconversion ability leading to a safe immunisation and their best timing for patients undergoing cancer treatment [11]. Vaccine safety and immunogenicity information is also incomplete in patients on immune checkpoint inhibitors (ICI), with a few studies suggesting general safety and heightened immunity [12], but others leading to opposite conclusions [13]. Moreover, there is insufficient data on seroconversion under targeted therapies, as well as under hormone therapy for breast and prostate cancer patients, which is generally deemed not to be immunosuppressive [14]. Similar uncertainties remain for the rate of adverse events following immunisation (AEFI) in this frail population, particularly in light of the risk of hemorrhagic events in young females in the general population [15].
To shed light on this knowledge gap, we conducted an observational study to determine the immunogenicity of vaccines in cancer patients undergoing different treatments through evaluation of rates of anti-spike immunoglobulin G (IgG) antibody positivity following vaccination with the BNT162b2 SARS-CoV-2. Specifically, our cohort study aims at assessing the factors that predict poor seroconversion (a proxy of lack of vaccine efficacy) and AEFI in order to plan better prevention strategies in this frail population.
2. Patients and methods
2.1. Study design and participants
We conducted a prospective, observational cohort study in order to assess the antibody titer reactogenicity to the BNT162b2 SARS-CoV-2 vaccine (Pfizer-BioNTech) in cancer patients on active treatment. Inclusion criteria were patients with malignancy aged ≥18 years, cancer treatment ongoing or ended within the last 6 months and lymphocytes count ≥0.5 × 109/L (500/μL) based on the risk of infections in subjects on chronic immunosuppressive therapy with lymphopenia <0.6 × 109/L [16]. The patients with the last treatment >6 months on active surveillance served as the control group. Participants underwent a clinical visit and blood sample collection: (1) at baseline before the first vaccine dose (visit 1), (2) 21 days after the first vaccine dose (visit 2), (3) 42 days after visit 1 (visit 3), and (4) 6 months after visit 1 (visit 4). The trial (ClinicalTrials.gov ID: NCT04932863) was approved by the National Institute for Infectious Diseases, Rome, and the local Ethical Committee. Participants were recruited at Galliera Hospital, Genoa, from March 15 to July 21, 2021.
2.2. Procedures
Vaccine treatment consisted of 30 μg of BNT162b2 (0.3 mL volume per dose) delivered in the deltoid muscle in 2 doses, 21 days apart. Current treatment groups consisted of different combinations, including chemotherapy alone (23.4%), chemotherapy with hormone therapy (4.4%), or targeted therapy (9.6%), or ICI (4.1%); hormone therapy alone, including LHRH analogues, novel antiandrogens and aromatase inhibitors or tamoxifen (17.2%) or with targeted therapy (CDK 4/6 inhibitors, 6.9%); targeted therapy alone, including tyrosine kinase inhibitors, PARP inhibitors, CDK 4–6 inhibitors, and monoclonal antibodies (7.9%); ICI alone (6.9%) or combined with biological (0.3%). We pooled treatments in five groups to facilitate comparisons: active surveillance (no treatment), chemotherapy, hormone therapy, targeted therapy and ICI.
The antibody titer was measured by the LIAISON® SARS-CoV-2 S1/S2 IgG, a chemiluminescent immunoassay (CLIA) for the quantitative detection of IgG antibodies against the S1/S2 domains of the SARS-CoV-2 spike protein in the human serum [17,18]. The analyser calculates SARS-CoV-2 S1/S2 IgG antibody concentrations as arbitrary units (AU/mL; assay range 3.8–400 AU/mL) and grades the results. The diagnostic specificity of the test is 98.9% and sensitivity 96.2% [18], with a positive IgG cut-off of >15.0 AU/mL. However, the threshold of seroconversion was increased to ≥25 AU/mL according to our lab procedures due to a previous preliminary study of correlation between the level of antibodies and concomitant T cell response that further proved immunisation. The main findings are presented using the 25 AU/mL threshold, but Supplementary tables using 15 AU/mL are also provided.
The primary objective was to assess the factors that predicted a poor antibody titer reactogenicity (<25 AU/mL) to BNT162b2 vaccine at 42 days (primary endpoint) and 6 months (co-primary endpoint). The rate of seroconversion at 21 days from the first dose was a secondary endpoint.
The factors predicting the onset of Adverse Events Following Immunisation (AEFI) in cancer patients were also evaluated [19]. The onset of AEFI was evaluated at visit 2, 3 and 4 and was graded as mild, moderate or severe.
2.3. Sample size and statistical analysis
The sample size was calculated with a test of precision assuming a standard error ≤5% in the lack of seroconversion rate (i.e., S1/S2 IgG < 25 AU/mL). Assuming a rate of poor seroconversion at 42 days of 15% [20], with 300 assessable subjects, the rate of poor seroconversion should have an error of ±4%. Median and interquartile range (IQR) for continuous variables and absolute and relative frequencies as summary measures of categorical variables were calculated. Fisher's Exact tests, Wilcoxon Rank tests or the Kruskal–Wallis rank-sum test were performed to investigate the association of seroconversion and AEFI with clinical characteristics and biomarkers. Multivariable logistic and Poisson models were applied to identify independent factors associated with AEFI and seroconversion, respectively. Relative risk (RR) and percentages of IgG non-responders and AEFI are presented with 95% CI. All analyses were carried considering two cut-off points for responders at 25 or 15 AU/mL. All p-values were two-sided with a 5% significance level. Analyses were carried out using the R studio (R version 4.0.0) and STATA (version 14.2) softwares.
3. Results
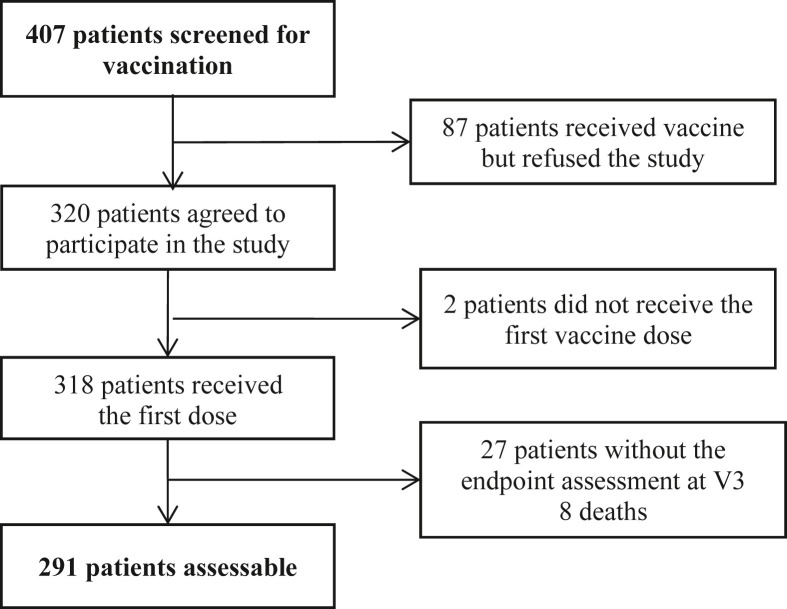
From 15th March 2021 to 21st July 2021, 407 patients were screened for vaccination and offered to participate in the study, of whom 320 agreed to participate, and 291 were assessable for the primary endpoint at 42 days (Fig. 1 ).
Fig. 1.
Participant flow diagram.
The main subject and tumour characteristics of the 291 patients are summarised in Table 1 . The median age was 68.2 years, approximately 60% were females and had stage IV disease, over 20% were treated >6 months ago, nearly 40% were on current chemotherapy, whereas hormone therapy, targeted therapy and ICI were used by 24%, 8% and 7%, respectively. The median (IQR) time from the last treatment cycle to the first vaccine dose was 13 days (0–21).
Table 1.
Main subject and tumour characteristics (n = 291).
| Age, median (IQR) | 68.2 (59.7–75.0) |
| Sex, n (%) | |
| Female | 173 (59.5) |
| Male | 118 (40.5) |
| BMI, median (IQR) | 24.5 (22.2–27.6) |
| Lymphocyte count (× 109/L), median (IQR) | 1.58 (1.13–2.12) |
| Days from last treatment cycle to first vaccine dose, median (IQR) | 13 (0–21) |
| Days from first to second vaccine dose and from second dose to visit 3, median (IQR) | 21 (21–21) |
| Tumour site, n (%) | |
| Digestive | 99 (34.0) |
| Lung | 30 (10.3) |
| Breast | 72 (24.8) |
| Genitourinary and gynaecologic | 79 (27.1) |
| Othera | 11 (3.8) |
| Stage, n (%) | |
| I | 17 (5.9) |
| II | 55 (18.9) |
| III | 42 (14.4) |
| IV | 177 (60.8) |
| Line of treatment, n (%) | |
| Adjuvant/Neoadjuvant | 105 (36.1) |
| 1st | 105 (36.1) |
| 2nd | 45 (15.4) |
| 3rd or more | 36 (12.4) |
| Type of treatment, n (%) | |
| No treatmentb | 62 (21.3) |
| Chemotherapy | 115 (39.5) |
| Hormone therapy | 70 (24.1) |
| Targeted therapy | 23 (7.9) |
| ICI | 21 (7.2) |
Other includes 5 head and neck cancer, 2 choroid melanoma, 2 CLL, 1 multiple myeloma, 1 brain glioma.
Patients with last treatment ≥180 days before the vaccine administration were considered as untreated; ICI, immune checkpoint inhibitors.
Overall, the rate of lack of seroconversion (IgG < 25 AU/mL) at 42 days was 10.7% (95% CI, 7.35–14.78, 31/291, Table 2 ). The median (IQR) IgG AU/mL level at baseline was 3.8 (3.8–4.9), 15.3 (3.8–51.2) at 21 days, 232 (85.1–400) at 42 days. Thirty-three patients had IgG levels above 25 AU/mL at baseline, indicating prior asymptomatic SARS-CoV-2 exposure. There was a significant association between poor seroconversion and current treatment (p = 0.01 versus active surveillance) and by type of therapy, with chemotherapy, targeted therapy and hormone therapy, but not ICI, being significantly associated with a poor IgG response compared with no treatment (Table 2). There was no difference in seroconversion between breast and prostate cancer among hormone therapy users (p = 0.38), nor was there a low response in CDK 4/6 inhibitors users (18/20 responders). There was no effect of corticosteroids on poor seroconversion (16% in corticosteroid users vs 10% in non-users, p = 0.32). Increasing age (above the median age) significantly predicted poor seroconversion, whereas higher disease stage and sex did not (Table 2). Patients with urological or gynaecological cancers had a higher rate of seroconversion than other tumour sites (Table 2). Patients with baseline lymphocyte count <1 × 109/L had a rate of no immunisation of 18.5% versus 8.9% in those with lymphocytes ≥1 × 109/L (p = 0.04).
Table 2.
Distribution of the 31 non-responders (IgG < 25 AU/mL) at 42 days.
| n | Non-responders n. (%) |
95% CI | P valuea | P valuea | |
|---|---|---|---|---|---|
| Overall | 291 | 31 (10.65%) | 7.4%–14.8% | ||
| Age | 0.03 | ||||
| Age ≤68.2 | 146 | 11 (7.5%) | 3.8%–13.1% | 0.031 | |
| Age >68.2 | 145 | 20 (13.8%) | 8.6%–20.5% | – | |
| Sex | 0.18 | ||||
| Women | 173 | 17 (9.8%) | 5.8%–15.3% | 0.181 | |
| Men | 118 | 14 (11.9%) | 6.6%–19.1% | – | |
| Tumour site | 0.01 | ||||
| Digestive | 99 | 11 (11.3%) | 5.7%–19.0% | 0.078 | |
| Lung | 30 | 4 (13.3%) | 3.8%–30.7% | 0.077 | |
| Breast | 72 | 9 (12.5%) | 5.9%–22.4% | 0.023 | |
| Genitourinary and gynaecologic | 79 | 4 (5.1%) | 1.4%–12.5% | – | |
| Otherb | 11 | 3 (27.3%) | 6.0%–61.0% | 0.001 | |
| Stage | 0.51 | ||||
| I–III | 114 | 9 (7.9%) | 3.7%–14.5% | – | |
| IV | 177 | 22 (12.4%) | 8.0%–18.2% | 0.513 | |
| Type of treatment | 0.01 | ||||
| No treatment | 62 | 1 (1.6%) | 0.4%–8.7% | – | |
| Chemotherapy | 115 | 16 (13.9%) | 8.2%–21.6% | 0.033 | |
| Hormone therapy | 70 | 8 (11.4%) | 5.1%–21.3% | 0.051 | |
| Targeted therapy | 23 | 5 (21.7%) | 7.5%–43.7% | 0.005 | |
| ICI | 21 | 1 (4.8%) | 0.12%–23.8% | 0.600 | |
| Lymphocyte count (× 109/L) | 0.04 | ||||
| <1 | 54 | 10 (18.5%) | 9.3%–31.4% | 0.04 | |
| ≥1 | 237 | 21 (8.9%) | 5.6%–13.2% | – | |
| IgG at baseline, median (IQR) | 3.8 (3.8–4.9) | ||||
| IgG at 21 day median (IQR) | 15.3 (3.8–51.2) | ||||
| IgG at 42 day, median (IQR) | 232 (85.1–400) |
P-values are obtained from fully adjusted models, including age, sex, treatment, stage, tumour site and lymphocyte count. The variable stage was considered with the following coding: 1 = IV stage; 0 = I, II and III stage.
Other includes 5 head and neck, 2 choroid melanoma, 2 CLL, 1 multiple myeloma, 1 brain glioma; ICI, immune checkpoint inhibitors.
Similar associations with treatment, age and tumour site were noted using 15 AU/mL threshold, with the addition of tumour stage IV that became significantly associated with poor seroconversion (Supplementary Table S1).
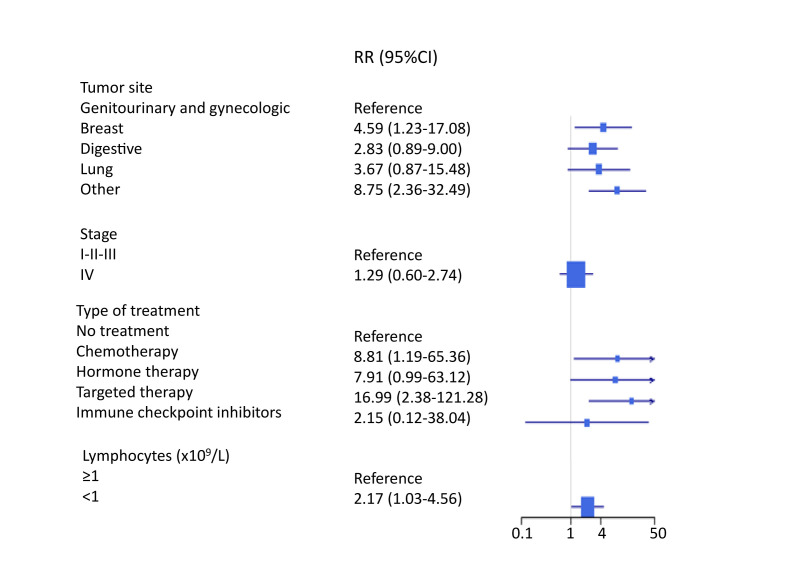
Compared with the untreated group, the multivariate RR of no IgG response according to treatment type was 8.81 (95% CI, 1.19–65.36) for chemotherapy, 7.91 (0.99–63.12) for hormone therapy, 16.99 (2,38–121.28) for targeted therapy and 2.15 (0.12–38.04) for ICI (Fig. 2 ). There was a trend for a higher risk of poor seroconversion in patients with breast cancer and patients with miscellaneous (other) tumours compared with genitourinary and gynaecologic cancers and no association with tumour stage (Fig. 2). Patients with baseline lymphocyte count <1 × 109/L had a two-fold risk of no seroconversion compared with lymphocytes ≥1 × 109/L (OR = 2.17, 1.03–4.56).
Fig. 2.
Forest plot of the relative risk (RR) of poor seroconversion (<25 AU/mL) according to tumour site, stage and treatment. RR and 95% CI are obtained from fully adjusted Poisson model, including age, sex, treatment, stage, tumour site and baseline lymphocyte count.
The rate of poor seroconversion (<25 AU/mL) after the first dose at 21 days was very high overall (59.8%, 174/291) and varied by treatment similar to the pattern at 42 days with the exception of ICI, which also showed a very high rate of poor seroconversion (Supplementary Table S2).
Overall, 14.8% (43/291) of the patients complained of multiple AEFI (n = 100), mostly of mild grade, a few of moderate grade, none severe. The frequency of AEFI was higher in females, non-smokers (current or former) and younger subjects (under median age 68.2, Table 3 ).
Table 3.
Description of adverse events following immunisation (AEFI).
| Number of patient with AEFI, n. (%) |
43 (14.78%) |
|
| Number of AEFI, n |
100a |
|
| Grade |
Mild, n. |
Moderate, n. |
| AEFI | ||
| Grade of adverse event | 69 | 31 |
| Local reaction | 8 | 1 |
| Fatigue | 6 | 6 |
| Headache | 14 | 6 |
| Chills | 8 | 2 |
| Pain | 15 | 10 |
| Sick | 0 | 1 |
| Diarrhoea | 1 | 0 |
| Lymphadenopathy | 1 | 0 |
| Pyrexia ≥38cc | 11 | 2 |
| Other | 5 | 3 |
| Age | ||
| Age ≤ 68.2 | 56 | 31 |
| Age > 68.2 | 13 | 0 |
| Sex | ||
| Women | 60 | 31 |
| Men | 9 | 0 |
| Smoking | ||
| No smokers | 67 | 31 |
| Smokers | 2 | 0 |
AEFI may be multiple and repeated in a single patient.
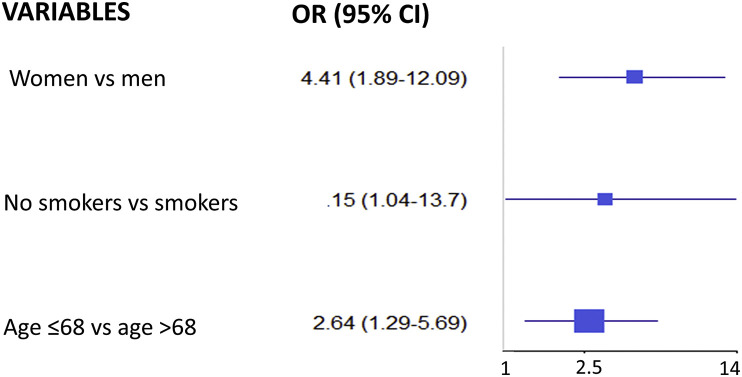
The multivariable models for the association with AEFI indicate a significant increased risk in females versus males (OR = 4.41, 95% CI, 1.89–12.09; P = 0.001), in younger versus older patients (OR = 2.64, 1.19–5.69), and in non-smokers versus smokers (OR = 3.15, 1.04–13.70; P = 0.07) (Fig. 3 ).
Fig. 3.
Forest plot of the Odds Ratios (OR) for adverse events following immunisation from a multivariable logistic model adjusted for sex, age and smoke.
As of 13th September 2021, there were 13 deaths related to cancer and 3 cases of SARS-CoV-2 infection, all developed after the second dose, with no IgG response at 21 days and IgG titer below 50 AU/mL at 42 days.
4. Discussion
Cancer patients develop severe COVID 19 infection with higher mortality [1,2] and were therefore considered a highly frail population by Health Authorities requiring priority in accessing vaccination [10]. The immunogenicity of cancer patients is also lower than the general population after contracting SARS-CoV-2 infection [[4], [5], [6]], so that the ability to develop a response to the vaccine was also expected to be different in cancer patients compared to healthy subjects, who attained a 95% efficacy with BNT162b2 vaccine [3]. Patients with solid tumours exhibited a higher seroconversion compared with those with haematologic malignancies after the first dose of vaccine [4].
Our study was designed to identify factors predicting a poor seroconversion to BNT162b2 vaccine in patients under treatment for solid cancers in an attempt to guide strategies in non-responders. Moreover, we aimed at identifying predictors of higher risk of AEFI to manage their occurrence and prevent them in future subjects. Our findings indicate that up to 20% of patients under current treatment with chemotherapy, targeted therapy and hormone therapy show no seroconversion after two doses of BNT162b2 vaccine, whereas patients on ICI or patients on clinical surveillance had a >95% IgG response. Importantly, patients with G3 lymphopenia (<1 × 109/L or 1000/μL) had a nearly 20% prevalence and a 2-fold risk of poor seroconversion compared with lymphocyte count ≥1000/uL. Poor seroconversion to COVID 19 infection has been shown in patients with low lymphocyte count [21], and lymphopenia is predictive of infection in patients on chronic immunosuppressive therapy [16] and is associated with severe COVID-19 disease [22]. Increasing age and, to a lesser extent, advanced stage were also predictors of poor seroconversion. It is plausible that patients on those anticancer treatments who develop lymphopenia, as well as older patients, have a lower antibody response to the vaccine due to immunosenescence [23,24]. Our findings strongly indicate the need for long-term serological testing and a third dose or other approaches in this important fraction of non-responding cancer patients [25].
While the immunosuppressive effects of chemotherapy and targeted therapy are plausible, the effect of hormone therapy was quite surprising and in contrast with recent findings [14]. These results appear to be related to a general immunosuppressive mechanism of hormone deprivation as there were no differences between breast and prostate cancer, nor was there an additive effect of CDK 4/6 inhibitors. Female hormones are known to potentiate the immune system [26], and sex steroids have been associated with differences in the immune response to SARS-CoV-2 [27]. Hence, a hormone deprivation therapy may also induce immunosenescence in this population [28].
We noted a low response rate (nearly 60% of poor seroconversion) to the first vaccine dose in line with previous studies [4,5]. Therefore, despite considerations to extend the interval between the two vaccine doses for the healthy population, cancer patients should respect the 21-day distance between the two doses, including subjects receiving ICI who showed a slow seroconversion [4,5].
Lowering the IgG positivity cut-off from 25 to the manufacturer threshold of 15 AU/mL did not affect the pattern of predictors of poor seroconversion, with higher risk for patients undergoing chemotherapy or targeted therapy, as well as patients with older age and advanced stage. Notably, patients undergoing corticosteroids did not exhibit a higher risk of poor seroconversion, thus supporting recommendations to offer the SARS-CoV-2 vaccination to patients taking chronic steroid therapy [29], although a recent study showed a lower immunisation in patients with cancer under corticosteroid therapy [30].
Younger age, female sex and non-smoking status were significant predictors of higher risk of AEFI. Overall, the vaccine was found to be safe since mainly mild AEFI were reported. These observations are consistent with Polack et al. in which AEFI were observed more frequently in young subjects [3]. The reason why women are at higher risk of AEFI is unclear but again points to immune–endocrine interactions. This is important given the known risk of hemorrhagic events in young females in the general population following vaccines [15]. Differences in the response to vaccines between males and females are already known, as women seem to develop a greater antibody response after antiviral and antibacterial vaccines, suggesting that effective doses for women may be lower [31]. This could also explain the greater occurrence of adverse events in women, perhaps linked to a sort of ‘overdose’ of the vaccination. Interestingly, current and former smokers, who are at higher risk for severe SARS-CoV-2 infection, seem to be protected from developing AEFI, possibly related to the immunosuppressive effect of smoking [32,33]. Preventive medications to minimise AEFI onsets, such as paracetamol and antihistamines, should be discussed in these subgroups to minimise adverse events.
The strength of our study is the large sample size obtained in a single centre, with minimal variability in clinical and laboratory parameters. A limitation is the use of antibody titration alone as a method for testing the immune response to the vaccine, since the association between antibody binding titers and antibody effector function is still poorly understood, but is also dependent upon the activation of other specific immune cells, such as T lymphocytes and the generated inflammatory response.
In conclusion, our study has important clinical and public health implications as it shows that except for ICI, there is a lack of seroconversion after two doses of BNT162b2 vaccine in up to 20% of cancer patients under active treatment or patients with lymphocyte count <1000/μL, strongly indicating the need for long-term serological testing and a third vaccine dose or passive immunisation in vaccine non-responders. Adverse events occur more significantly in women, younger subjects and non-smokers who may benefit from preventive medications such as paracetamol and antihistamines.
Contributors
TBW, NP, EM and ADC contributed to the study conception and design. TBW, NP, MM, MU, MB, MC, MD, CD, AG, MM, RDP, FP, NM, LI, NS and IC contributed to data acquisition. TBW, ADC, MM and MU contributed to project administration. ADC contributed to funding acquisition. GS, OD and SG provided data analyses. All authors contributed to the interpretation of data. TBW, NP, SG and ADC wrote the first draft of the manuscript with assistance from IMB, GS and OD. All authors reviewed and approved the final manuscript.
Data sharing
Individual participant data are not publicly available because this requirement was not anticipated in the study protocol. Tania Buttiron Webber, Andrea DeCensi, Giacomo Siri, Oriana D'ecclesiis and Sara Gandini had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Data may be shared upon request for collaborative studies.
Funding
Supported in part by the Association WeCare and the Lions Club of Genoa Sant'Agata and the Italian Ministry of Health – Ricerca Corrente and 5 × 1000 funds (IEO IRCCS). These funding sources had no role in the design, execution, analyses, interpretation of the data, or decision to submit results.
Conflict of interest statement
We declare no competing interests.
Acknowledgements
This study is supported by E.O. Ospedali Galliera, Genoa, Italy, Associazione WeCare and Lions Club Genoa Sant'Agata, Italy. The European Institute of Oncology, Milan, Italy is partially supported by the Italian Ministry of Health with Ricerca Corrente and 5 × 1000 funds.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.09.030.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020 Jun 20;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. PMID: 32473681; PMCID: PMC7255743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee L.Y.W., Cazier J.B., Starkey T., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020 Oct;21(10):1309–1316. doi: 10.1016/S1470-2045(20)30442-3. PMID: 32853557; PMCID: PMC7444972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. PMID: 33301246; PMCID: PMC7745181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021 Jun;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. PMID: 33930323; PMCID: PMC8078907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palich R., Veyri M., Marot S., et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol. 2021 Aug;32(8):1051–1053. doi: 10.1016/j.annonc.2021.04.020. Epub 2021 Apr 29. PMID: 33932501; PMCID: PMC8081573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrière J., Chamorey E., Adjtoutah Z., et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021 Aug;32(8):1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garassino M.C., Vyas M., de Vries E.G.E., et al. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol. 2021 May;32(5):579–581. doi: 10.1016/j.annonc.2021.01.068. PMID: 33582237; PMCID: PMC7879154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forni G., Mantovani A., COVID-19 Commission of Accademia Nazionale dei Lincei, Rome COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021 Feb;28(2):626–639. doi: 10.1038/s41418-020-00720-9. PMID: 33479399; PMCID: PMC7818063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Veldt A.A.M., Oosting S.F., Dingemans A.C., et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med. 2021 Apr;27(4):568–569. doi: 10.1038/s41591-021-01240-w. PMID: 33589821. [DOI] [PubMed] [Google Scholar]
- 10.https://www.gazzettaufficiale.it/eli/id/2021/03/24/21A01802/sg.
- 11.Desai A., Gainor J.F., Hegde A., et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021 May;18(5):313–319. doi: 10.1038/s41571-021-00487-z. PMID: 33723371; PMCID: PMC7957448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021 May;22(5):581–583. doi: 10.1016/S1470-2045(21)00155-8. Epub 2021 Apr 1. PMID: 33812495; PMCID: PMC8016402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terpos E., Zagouri F., Liontos M., et al. Low titers of SARS-CoV-2 neutralizing antibodies after first vaccination dose in cancer patients receiving checkpoint inhibitors. J Hematol Oncol. 2021 May 31;14(1):86. doi: 10.1186/s13045-021-01099-x. PMID: 34059088; PMCID: PMC8165511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakkar A., Gonzalez-Lugo J.D., Goradia N., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021 Jun 5 doi: 10.1016/j.ccell.2021.06.002. S1535-S6108(21)00285-3. PMID: 34133951; PMCID: PMC8179248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cari L., Fiore P., Naghavi Alhosseini M., Sava G., Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: an analysis of European data. J Autoimmun. 2021 Aug;122:102685. doi: 10.1016/j.jaut.2021.102685. PMID: 34174723; PMCID: PMC8220408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glück T., Kiefmann B., Grohmann M., Falk W., Straub R.H., Schölmerich J. Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J Rheumatol. 2005 Aug;32(8):1473–1480. PMID: 16078322. [PubMed] [Google Scholar]
- 17.Bonelli F., Sarasini A., Zierold C., et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020 Aug 24;58(9) doi: 10.1128/JCM.01224-20. PMID: 32580948; PMCID: PMC7448652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National SARS-CoV-2 Serology Assay Evaluation Group Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020 Dec;20(12):1390–1400. doi: 10.1016/S1473-3099(20)30634-4. PMID: 32979318; PMCID: PMC7511171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi.
- 20.Thakkar A., Pradhan K., Jindal S., et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat Cancer. 2021;2:392–399. doi: 10.1038/s43018-021-00191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irfan A., Muhammad Khuram S., Muhammad Q., et al. Lymphocyte as a predictive marker for seronegativity of COVID-19. Biomed J Sci Tech Res. 2020;32(4) BJSTR. MS.ID.005279. [Google Scholar]
- 22.Zhao Q., Meng M., Kumar R., et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. 2020 Jul;96:131–135. doi: 10.1016/j.ijid.2020.04.086. Epub 2020 May 4. PMID: 32376308; PMCID: PMC7196544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh E.E., Frenck R.W., Jr., Falsey A.R., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020 Dec 17;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. PMID: 33053279; PMCID: PMC7583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collier D.A., Ferreira I.A.T.M., Kotagiri P., et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021 Jun 30 doi: 10.1038/s41586-021-03739-1. PMID: 34192737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrière J., Re D., Peyrade F., Carles M. Current perspectives for SARS-CoV-2 vaccination efficacy improvement in patients with active treatment against cancer. Eur J Cancer. 2021. Sep;154:66–72. doi: 10.1016/j.ejca.2021.06.008. Epub 2021 Jun 18. PMID: 34243079; PMCID: PMC8260097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauvais-Jarvis F., Klein S.L., Levin E.R. Estradiol, progesterone, immunomodulation, and COVID-19 outcomes. Endocrinology. 2020 Sep 1;161(9):bqaa127. doi: 10.1210/endocr/bqaa127. PMID: 32730568; PMCID: PMC7438701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gadi N., Wu S.C., Spihlman A.P., Moulton V.R. What's sex got to do with COVID-19? Gender-based differences in the host immune response to coronaviruses. Front Immunol. 2020 Aug 28;11:2147. doi: 10.3389/fimmu.2020.02147. PMID: 32983176; PMCID: PMC7485092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straub R.H., Miller L.E., Schölmerich J., Zietz B. Cytokines and hormones as possible links between endocrinosenescence and immunosenescence. J Neuroimmunol. 2000 Sep 1;109(1):10–15. doi: 10.1016/s0165-5728(00)00296-4. PMID: 10969175. [DOI] [PubMed] [Google Scholar]
- 29.Chakravarthy K., Strand N., Frosch A., et al. Recommendations and guidance for steroid injection therapy and COVID-19 vaccine administration from the American Society of Pain and Neuroscience (ASPN) J Pain Res. 2021 Mar 5;14:623–629. doi: 10.2147/JPR.S302115. PMID: 33716511; PMCID: PMC7944369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gounant V., Ferré V.M., Soussi G., et al. Efficacy of SARS-CoV-2 vaccine in thoracic cancer patients: a prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. medRxiv Preprint. 2021 doi: 10.1101/2021.08.12.21261806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016 Oct;16(10):626–638. doi: 10.1038/nri.2016.90. Epub 2016 Aug 22. PMID: 27546235. [DOI] [PubMed] [Google Scholar]
- 32.Qiu F., Liang C.L., Liu H., et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget. 2017 Jan 3;8(1):268–284. doi: 10.18632/oncotarget.13613. PMID: 27902485; PMCID: PMC5352117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002 May;2(5):372–377. doi: 10.1038/nri803. PMID: 12033743. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.