Abstract
Background:
Eosinophilic esophagitis (EoE) is a T cell-mediated disease that is caused by specific foods and results in esophageal dysfunction. Existing allergy testing modalities are not helpful when attempting to identify EoE-causal foods necessitating empiric food elimination and recurrent endoscopy. The goal of this study was to identify and compare allergen-specific immune features that can be assayed in a minimally-invasive manner to predict clinical food allergy in EoE.
Methods:
We obtained blood samples from control subjects (n=17), subjects with clinical EoE milk allergy (n=17), and subjects with IgE-mediated milk allergy (n=9). We measured total and milk-specific plasma IgG4 levels and peripheral memory CD4+ T helper (TH) cell proliferation and cytokine production after stimulation with endotoxin-depleted milk proteins. Sensitivity and specificity for predicting clinical EoE milk allergy was calculated and compared between approaches.
Results:
Total and milk-specific IgG4 levels were not significantly different between control subjects and subjects with clinical EoE milk allergy. Stimulation with milk proteins caused TH lymphocytes from subjects with clinical EoE milk allergy to proliferate more (%P1 of 38.3±4.6 vs 12.7±2.8, P<0.0001), and produce more type 2 cytokines (%IL-4+ of 33.7±2.8 vs 6.9±1.6, P<0.0001), than cells from control subjects. Milk-dependent memory TH cell proliferation (sensitivity and specificity of 88 and 82%, respectively) and IL-4 production (sensitivity and specificity of 100%) most strongly predicted clinical EoE milk allergy.
Conclusions:
Peripheral markers of allergen-specific immune activation may be useful in identifying EoE-causal foods. Assaying milk-dependent IL-4 production by circulating memory TH lymphocytes most accurately predicts clinical EoE milk allergy.
Keywords: Assay, Diagnosis, Eosinophilic Esophagitis, Management, T cell
INTRODUCTION
Eosinophilic esophagitis (EoE) is a chronic, T cell-mediated disease that is caused by specific foods and results in inflammation, odynophagia, and progressive esophageal dysfunction (1,2). Due to variable contribution of food-specific IgE to EoE immunopathology (3), and limited correlation between allergen patch testing and clinical food allergy (4,5), existing testing modalities are of low utility when attempting to identify EoE-causal foods. As such, current clinical management consists of proton-pump inhibitor therapy, empiric elimination diets, and/or swallowed steroids with recurrent endoscopies to assess clinical response (6). Due to the considerable morbidity and time to inflammation resolution associated with current management practices, there is critical need for the development of minimally-invasive assays to aid in the identification of EoE-causal foods (7).
In addition to clinical features including inflammation resolution with food avoidance (8–10), there is ample experimental evidence that EoE is an antigen-driven process (11). Mouse models show correlations between antigen-specific T helper type 2 (TH2) cell responses and esophageal eosinophilia (12–14). Esophageal eosinophilia in these models is dependent on CD4+ TH cells, but not CD8+ T cells, B cells, or IgE (14,15). In human studies, activated TH cells have been detected in the circulation of EoE subjects after polyclonal or allergen-specific stimulation (7,16,17), and in the esophagus during active disease (18). However, it is not known whether allergen-specific memory TH cells are present in patients with EoE.
Additional evidence for antigen-driven inflammation in EoE comes from studies of food-specific IgG4 levels. For example, adults with EoE have higher total and food-specific IgG4 levels in the serum and esophagus (19), while children with EoE have higher food-specific IgG4 levels in the serum (20), as compared with healthy controls. In adults this phenomenon seems to be closely linked to consumption of the allergenic food, and as a result, EoE activity (21,22). It is not known whether a similar relationship exists in children. Taken together, these studies highlight the relevance of antigen-driven inflammation to EoE pathogenesis.
The ability to detect allergen-specific markers of immune activation in EoE raises the potential for the development of minimally-invasive assays to aid in the identification of EoE-causal foods. Such assays would allow for the institution of directed elimination diets, and minimize the number of required endoscopies and time to inflammation resolution (7). In addition, there is emerging evidence that EoE can resolve (clinical remission) in some children with prolonged food avoidance (23). As a result, there is also need for assays that can determine the presence of food-specific immune responses when an individual is avoiding known EoE-causal food(s). Such an assay would be valuable in determining if and when an allergenic food can be reintroduced into a patient’s diet (akin to skin prick testing in IgE-mediated food allergy). As bovine milk is the most common cause of EoE in children and adults (11), we sought to identify peripheral markers of milk-specific immune activation that are independent of allergenic food consumption, and are indicative of clinical EoE milk allergy.
METHODS:
Subject recruitment and IRB approval:
Subjects with clinical EoE milk allergy, IgE-mediated milk allergy, or non-allergic controls were recruited at Children’s Hospital of Philadelphia (CHOP) between January 2019 and January 2021. Subjects were enrolled with subject or guardian consent, and subject assent (when applicable), via CHOP IRB protocol 18–015524.
PBMC isolation and culture:
Peripheral blood samples were obtained from subjects or controls. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient, carboxyfluorescein succinimidyl ester (CFSE) labeled (Invitrogen, REF C34554), and cultured at 1 million cells/mL in OpTimizer SFM (Gibco, REF A10221–01) for 6 days in a 96 well round-bottom plate (Corning Inc. Costar, REF 3799) in the presence or absence of endotoxin-depleted milk proteins (6.25 µg of each of ɑ-lactalbumin, β-lactoglobulin, ɑ-casein, β-casein, and κ-casein per 200k cells; Supplemental Figure 1) or tetanus toxoid (TT; Astarte Biologics, CAT 1002; 0.625 µg per 200k cells).
Flow Cytometry:
Cells were surface and/or intracellularly stained (see online supplement for additional methods) prior to being acquired on an LSR Fortessa (BD) within 24 hours of harvest. The cytometer was compensated using OneComp eBeads (Invitrogen), unstained cells, CFSE stained cells, or Live/Dead Fixable Blue (Invitrogen) stained cells. Fluorescence minus one (FMO) controls were used to establish lower gate bounds. A minimum of 250k events were acquired for each arm, with a range of up to 1 million events depending on initial PBMC recovery. Data was analyzed using FlowJo software (Becton Dickinson). Events were gated by Lymphocytes, Live/Dead negative, CD8−, CD19−, CD3+, CD4+, CD45RA−, CD45RO+ (Supplemental Figure 2).
Data Analysis and Statistics:
Intracellular IL-4 production by memory TH cells was measured by intracellular cytokine staining, and memory TH cell proliferation was determined by identifying CFSEbright and CFSEdim cells as P0 and P1 gates (representing undivided and proliferating cells, respectively). Samples with a TT vs unstimulated % P1 difference of less than two (% P1 TT – % P1 unstimulated < 2) were excluded due to lack of adequate positive control response (Supplemental Figure 3), and significant outliers within each group were determined by extreme studentized deviate method and excluded. Significant differences between experimental groups were determined by unpaired or paired parametric t test, depending on context and as indicated in figure legends. To reduce inter-subject variability, the % P1 milk proteins was normalized to the % P1 TT, for a given sample. Associations between assay outcomes and milk consumption at the time of assay were determined by Pearson’s correlation. The association between milk protein-activation of memory TH cells and clinical sensitivity to milk was measured using a Receiver Operating Characteristic (ROC) curve. A positive test threshold was selected which maximized sensitivity and specificity for correlation with clinical EoE milk allergy. Pairwise comparisons between ROCs were made via Delong’s test.
See online supplement for additional methods.
RESULTS:
We recruited 17 subjects with clinical EoE milk allergy and 17 non-milk-allergic controls between January 2019 and January 2021. For comparison, we recruited 9 subjects with IgE-mediated milk allergy. All subjects met international criteria for the diagnosis of EoE or IgE-mediated food allergy (6), and the majority of clinical EoE milk allergy subjects and all IgE-mediated milk allergy subjects were avoiding milk at the time of enrollment. Subject characteristics are shown in Supplemental Table 1. The average age of enrolled subjects was 13 years, and the clinical EoE milk allergy cohort was predominantly male (65%) and had a high degree of allergic comorbidity, observations that are consistent with prior studies of demographic and clinical characteristics of EoE subjects (25).
As food-specific IgG4 levels have been shown to correlate with clinical EoE food allergy (7,19–22), we first examined total and milk-specific IgG4 levels in the plasma of control or clinical EoE milk allergy subjects. We did not observe a significant difference in total IgG4 levels (Figure 1A), but did observe a non-significant increase in milk-specific IgG4 levels in clinical EoE milk allergy subjects compared with controls (Figure 1B). Similarly, we observed a non-significant increase in the ratio of milk-specific to total IgG4 in clinical EoE milk allergy subjects compared with controls (Figure 1C), an outcome that has been shown to correlate with clinical food allergy in active disease cohorts (7,21). Notably, the ratio of milk-specific to total IgG4 in clinical EoE milk allergy subjects was higher in individuals consuming milk at the time of assay (Supplemental Figure 4, P=0.05). Children with IgE-mediated milk allergy had slightly lower total serum IgG4 levels, and similar milk-specific IgG4 and specific to total IgG4 ratios, as compared with clinical EoE milk allergy subjects (Supplemental Figure 5A–C). When examining all the clinical EoE milk allergy subjects the relative overlap in milk-specific IgG4 levels between our control and experimental cohorts resulted in a moderate retrospective sensitivity and specificity for predicting clinical allergy to milk (77% and 71%, respectively) (Figure 1D).
Figure 1. Plasma total and milk-specific IgG4 levels show non-significant correlations with clinical EoE milk allergy.

(A) Total plasma IgG4 levels in control or clinical EoE milk allergy subjects. (B) Milk-specific plasma IgG4 levels in control or clinical EoE milk allergy subjects. (C) Ratio of milk-specific to total plasma IgG4 levels in control or clinical EoE milk allergy subjects. Clinical EoE milk allergy subjects consuming milk at time of assay in red. (D) Receiver operating characteristic (ROC) curve of the milk-specific to total IgG4 ratio for the clinical EoE milk allergy outcome. N=17. Mean ±SEM shown. Statistics by t-test. ns, not significant.
While prior studies in animal models and human subjects have shown that allergen-specific TH cells circulate in the blood of patients with EoE (7,12,14,17), examinations of memory TH cell populations have not been undertaken. We therefore sought to determine whether milk-specific memory TH cells are present in the circulation of clinical EoE milk allergy subjects. As proliferation in response to antigen-specific stimulation is a key feature of memory CD4+ TH cells (26), we labeled PBMCs from control or clinical EoE milk allergy subjects with CFSE prior to ex vivo culture in the absence or presence of tetanus toxoid (TT) or a solution of 5 endotoxin-depleted milk proteins (ɑ/β/κ-casein, ɑ-lactalbumin, and β-lactoglobulin). Consistent with prior tetanus vaccination, we observed that CD4+ CD45RO+ memory TH cells from both control and clinical EoE milk allergy subjects proliferated in response to stimulation with TT (Figure 2A). However, significantly more memory TH cells from clinical EoE milk allergy subjects proliferated in response to stimulation with milk proteins as compared with cells from controls (Figure 2A, B). Children with IgE-mediated milk allergy had higher baseline, but proportionally similar proliferative responses as compared with clinical EoE milk allergy subjects (Supplemental Figure 5D,E). Together, these results indicate that milk-reactive memory TH cells are present in the circulation of children with clinical EoE milk allergy.
Figure 2. Presence of milk-reactive memory T cells in subjects with clinical EoE milk allergy.
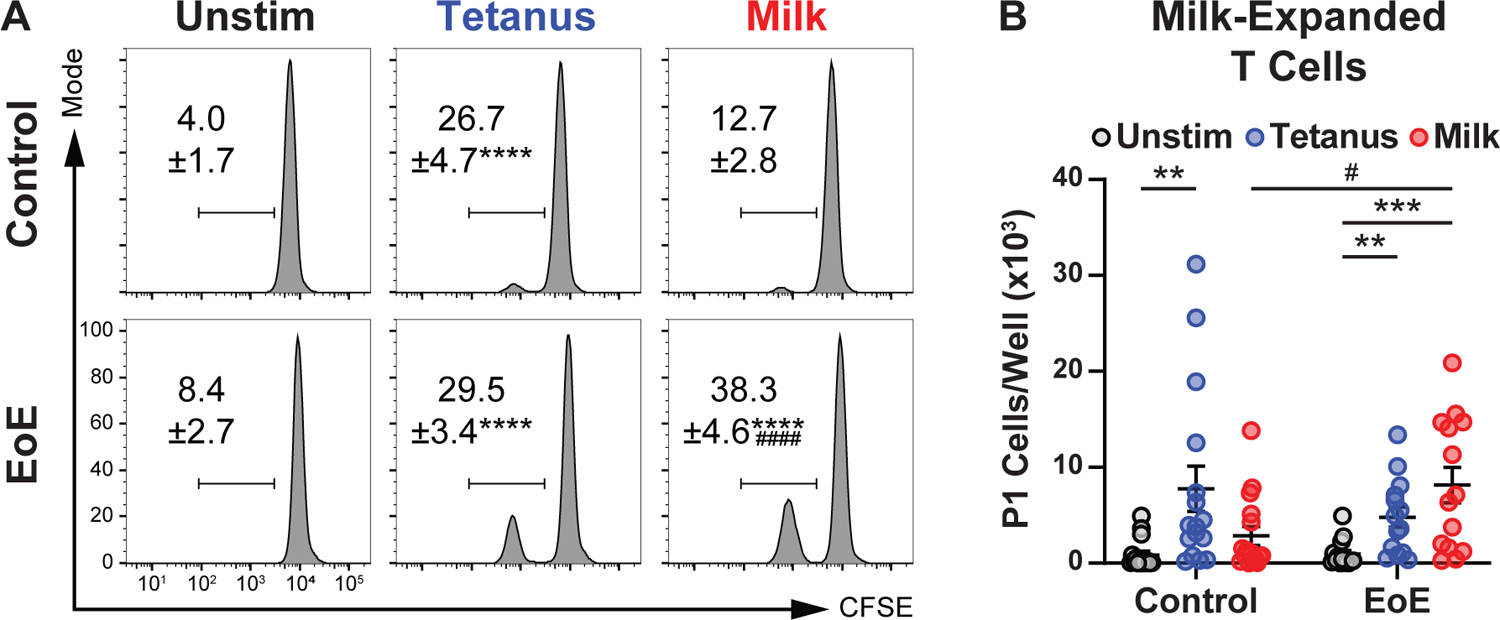
(A) Flow cytometry of CFSE-labeled peripheral memory T cells from control or clinical EoE milk allergy subjects after culture in the absence (Unstim) or presence of tetanus toxoid (Tetanus) or milk proteins (Milk). Gated on live, CD8−, CD19−, CD3+, CD4+, CD45RA−, CD45RO+. (B) Number of CFSE dim (P1) peripheral memory T cells after culture in the absence (Unstim) or presence of tetanus toxoid (Tetanus) or milk proteins (Milk). N= 14–17. Mean ±SEM shown. Statistics by t-test. * indicate comparisons between treatment arms of a single experimental group, # indicate comparisons between experimental groups of a single treatment arm. **, P<0.01; ***, P<0.001, ****, P<0.0001; #, P<0.05; ####, P<0.0001.
We next sought to quantify the extent to which milk-dependent memory TH cell proliferation associated with clinical EoE milk allergy. We found that there was a high degree of variability in TH cell proliferative responses between subjects that complicated identification of a threshold that distinguished control from clinical EoE milk allergy subjects (Figure 3A). To account for this, we performed intra-sample normalization of milk-induced to tetanus-induced memory TH cell proliferation. This normalization minimized inter-sample variability and allowed more accurate determination of a threshold for assay positivity (Figure 3B). Notably, memory TH cells from clinical EoE milk allergy subjects consuming milk at the time of assay did not significantly differ in their proliferative response to milk stimulation as compared with TH cells from clinical EoE milk allergy subjects avoiding milk at the time of assay (Supplemental Figure 4, P=0.5382). Using this approach, we found that a threshold of 0.86 (ratio of %P1 milk protein-stimulated to %P1 tetanus-stimulated) resulted in a retrospective assay sensitivity and specificity of 88% and 82% for clinical EoE milk allergy, respectively (Figure 3C). Together, these results indicate that milk-dependent proliferation of milk-specific memory TH cells significantly correlates with clinical EoE milk allergy irrespective of whether an individual is consuming milk at the time of the assay.
Figure 3. Proliferation of milk-reactive memory T cells predicts clinical EoE milk allergy.

(A) Variability among T cell proliferative responses. (B) Ratio of the percentage of P1 cells in cultures of milk-expanded as compared with tetanus-expanded PBMCs from control or clinical EoE milk allergy subjects. Clinical EoE milk allergy subjects consuming milk at time of assay in red. (C) Receiver operating characteristic (ROC) curve of the %P1 milk-stimulated to tetanus-stimulated ratio for the clinical EoE milk allergy outcome. N=17. Mean ±SEM shown. Statistics by t-test. ####, P<0.0001.
To test whether we could improve upon the sensitivity and specificity of our milk-specific IgG4 and milk-dependent memory TH cell proliferation assays, we examined whether combining these two assays improved our ability to identify clinical EoE milk allergy. To do so, we multiplied the milk-specific to total IgG4 ratio by the milk to tetanus P1 CFSE ratio (IgG4 x CFSE). The IgG4 x CFSE measure provided an outcome that was significantly different between the control and clinical EoE milk allergy subjects (Figure 4A), and resulted in a modest improvement in the overall specificity for identifying clinical EoE milk allergy (88%) (Figure 4B). Like the ratio of milk-specific to total IgG4 in clinical EoE milk allergy subjects, the combined IgG4 x CFSE measure was higher in individuals consuming milk at the time of assay (Supplemental Figure 4, P=0.0495). A pairwise comparison of each of the ROC curves confirmed that the area under the IgG4 x CFSE curve was greater than that of the milk-specific to total IgG4 ratio curve (Figure 4C, P=0.0178 by DeLong’s test). However, the area under the IgG4 x CFSE curve was not significantly different from that of the milk to tetanus P1 CFSE ratio curve (P=0.6492 by DeLong’s test). Thus, our analysis of milk-dependent memory TH cell proliferation alone is roughly equivalent to our combined analysis of milk-specific IgG4 and milk-dependent memory TH cell proliferation.
Figure 4. Examination of milk-specific IgG4 and milk-reactive memory T cell proliferation improves identification of clinical EoE milk allergy.

(A) Ratio of milk-specific to total plasma IgG4 levels in control or clinical EoE milk allergy subjects multiplied by the ratio of the percentage of P1 cells in cultures of milk-expanded as compared with tetanus-expanded PBMCs from control or clinical EoE milk allergy subjects (IgG4 x CFSE). Clinical EoE milk allergy subjects consuming milk at time of assay in red. (B) Receiver operating characteristic (ROC) curve of the IgG4 x CFSE measure for the clinical EoE milk allergy outcome. (C) Comparison of ROC curves of the IgG4, CFSE, and IgG4 x CFSE measures. N=17. Mean ±SEM shown. Statistics by t-test. #, P<0.05.
Having established that milk-reactive memory TH cell populations are present in the circulation of children with clinical EoE milk allergy, we next sought to identify the activation state of these cells. To do so, we isolated PBMCs from control or clinical EoE milk allergy subjects and stimulated them ex vivo for six days in the absence or presence of TT or milk proteins, prior to assaying intracellular interleukin (IL) −4 production. CD4+ CD45RO+ memory TH cells from both control and clinical EoE milk allergy subjects produced IL-4 in response to stimulation with TT (Figure 5A). However, significantly more memory TH cells from clinical EoE milk allergy subjects produced IL-4 in response to stimulation with milk proteins as compared with cells from control subjects (Figure 5A, B). Children with IgE-mediated milk allergy had higher baseline, but proportionally similar IL-4 production as compared with clinical EoE milk allergy subjects (Supplemental Figure 5F,G). To test whether milk-specific memory TH cells produced other canonical type 2 cytokines, we examined culture supernatant fractions. Milk-stimulated cultures of PBMCs from clinical EoE milk allergy subjects contained higher amounts of IL-4, IL-5, and IL-13 as compared with PBMCs from control subjects (Figure 5C). These results indicate that milk-specific memory TH2 cells circulate in the periphery of children with clinical EoE milk allergy.
Figure 5. Milk-reactive memory TH2 cells are present in the circulation of children with clinical EoE milk allergy.

(A) Flow cytometry of intracellular IL-4 expression in PBMCs from control or clinical EoE milk allergy subjects after culture in the absence (Unstim) or presence of tetanus toxoid (Tetanus) or milk proteins (Milk). Gated on live, CD8−, CD19−, CD3+, CD4+, CD45RA−, CD45RO+. (B) Number of IL-4+ memory TH2 cells after culture in the absence (Unstim) or presence of tetanus toxoid (Tetanus) or milk proteins (Milk). (C) IL-4, IL-5, and IL-13 amounts in culture superannuates of PBMCs from control or clinical EoE milk allergy subjects after culture in the absence (Unstim) or presence of milk proteins (Milk). N=7–8. Mean ±SEM shown. Statistics by t-test. * indicate comparisons between treatment arms of a single experimental group, # indicate comparisons between experimental groups of a single treatment arm. *, P<0.05; **, P<0.001; ****, P<0.0001; #, P<0.05; ##, P<0.01; ####, P<0.0001.
Finally, we sought to quantify the extent to which peripheral milk-specific memory TH2 cell frequency associated with clinical EoE milk allergy. When examining the association between the percent of milk-specific memory TH cells that express IL-4 (Figure 6A), and clinical EoE milk allergy, a positive cutoff threshold of >13.1% had a retrospective sensitivity and specificity of 100% for identifying clinical EoE milk allergy subjects (Figure 6B). Notably, TH cells from clinical EoE milk allergy subjects consuming milk at the time of assay did not significantly differ in their production of IL-4 in response to milk stimulation as compared with TH cells from clinical EoE milk allergy subjects avoiding milk at the time of assay (Supplemental Figure 4, P=0.2896). In sum, these results indicate that assaying milk-dependent memory TH2 cell IL-4 production has the potential to identify clinical EoE milk allergy with a very high degree of accuracy, irrespective of whether an individual is consuming milk at the time of the assay.
Figure 6. Examination of milk-reactive memory TH2 cells predicts clinical EoE milk allergy.
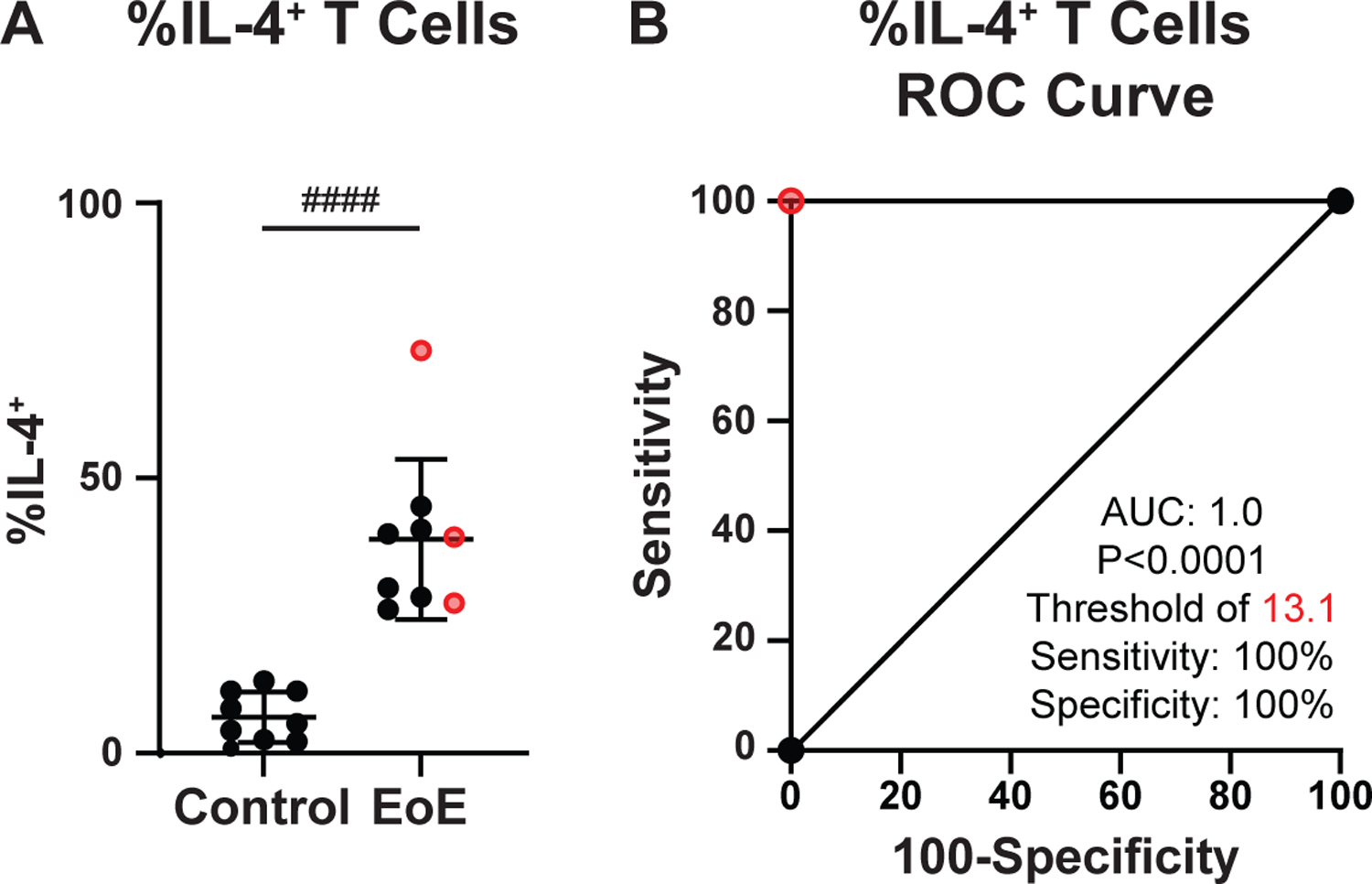
(A) %IL-4+ CD45RO+ TH2 cells from control or clinical EoE milk allergy subjects after stimulation with milk proteins. Clinical EoE milk allergy subjects consuming milk at time of assay in red. (B) Receiver operating characteristic (ROC) curve of the %IL4+ of the CD45RO+ population for the clinical EoE milk allergy outcome. N=8–9. Mean ±SEM shown. Statistics by t-test. ####, P<0.0001.
DISCUSSION:
A minimally-invasive assay that clinicians could use to guide food elimination would minimize the need for endoscopies and represent a significant step forward for EoE clinical care. Prior work has suggested that food-specific IgG4 levels in the serum and/or the esophagus may be a viable method to identify EoE-causal foods (19–22). In our cohort, this approach had a relatively low sensitivity and specificity for distinguishing clinical EoE milk allergy. This could be due to the fact that we studied a pediatric cohort, assayed plasma antibody levels as opposed to those of esophageal tissue, or because most of the clinical EoE milk allergy subjects in this study were avoiding milk at the time of sample acquisition. Indeed, both disease activity and/or consumption of allergenic foods may influence total or antigen-specific IgG4 levels, as well as circulating memory TH2 cell responses (21,22). Consistent with this, we found a higher degree of correlation between food-specific IgG4 levels and milk allergy when examining the subset of subjects that were consuming milk at the time of assay. Larger studies of the correlation between food-specific IgG4 levels, clinical allergens, and disease activity in children are warranted.
Efforts to combine examinations of esophageal allergen-specific IgG4 levels with total CD4+ allergen-dependent TH cell proliferation have also been undertaken, and have yielded a combined sensitivity and specificity for detecting milk-allergic EoE of 64% and 25%, respectively (7). In our cohort, examination of milk-dependent memory TH cell proliferation alone has an 88% sensitivity and 82% specificity for identifying children with clinical EoE milk allergy. When we combine assays of milk-specific IgG4 and TH cell proliferation, we found that the overall specificity for identifying clinical EoE milk allergy of 88%, though the ROC curve for this approach was not significantly different from that of the TH cell proliferation assay alone. As a result, there may be limited benefit to assaying plasma milk-specific IgG4 in combination with milk-dependent memory TH cell proliferation.
There are likely three technical advances that have improved the sensitivity and specificity of our TH cell proliferation assay over prior studies (7). These include 1) depletion of endotoxin from milk proteins, 2) assaying CD4+ CD45RO+ memory TH cells as opposed to total TH cell populations, and 3) intra-sample normalization of milk-dependent to TT-dependent proliferative responses (though this final technical advance limits potential use of this assay to individuals who have been adequately immunized against tetanus). The fact that we observe that all of the milk proteins tested are able to signal via hTLR4 highlights the importance of accounting for endotoxin contamination in TH cell activation assays. Further, while we observed significant reductions in hTLR4 signaling for all five proteins after endotoxin depletion some hTLR4 signaling capacity persisted. This was particularly relevant for β-lactoglobulin which was relatively resistant to endotoxin removal when compared to the other milk proteins. This could be the result of intrinsic ability for β-lactoglobulin to signal via hTLR4 (27), or inherent endotoxin binding capacity of this protein as is the case with other mammalian milk-derived proteins (28,29).
Our TH cell proliferation assay approach has the additional benefit of being able to be performed solely using a peripheral blood sample, and thus qualifies as a minimally-invasive assay that could be performed by most clinical laboratories. An important finding is that this approach is able to identify foods for which the immune system has an established memory TH cell response, irrespective of whether the individual is actively consuming the food in question. This is of particular interest as this or similar assays could assist in determining not only foods to avoid but also the timing of food reintroduction, a useful feature given emerging evidence that some children can outgrow EoE after periods of prolonged causal food avoidance (23).
In an attempt to further improve our ability to identify clinical EoE milk allergy, we first established the existence of milk-specific memory TH2 cells in the circulation of children with clinical EoE milk allergy. The existence of allergen-reactive memory TH2 cells in the circulation of EoE patients is a novel finding, but also consistent with observations in mouse models that have shown that skin sensitization can lead to the development of antigen-specific TH2 cell responses and esophageal eosinophilia (12–14). This finding is also consistent with our understanding of the role that epicutaneous sensitization plays in IgE-mediated food allergy (30), the high degree of clinical comorbidity between IgE-mediated food allergy and EoE (31), and EoE being a member of the allergic march (25). Ultimately, the establishment of milk-specific memory TH2 cells in the circulation of children with clinical EoE milk allergy improves our understanding of EoE immunopathology.
When examining the utility of measuring allergen-dependent memory TH2 cell cytokine production in identifying EoE-causal foods we found that this approach has a very high sensitivity and specificity for identifying clinical EoE milk allergy. The high accuracy of this approach likely also derives from the depletion of endotoxin from milk proteins, and examination of memory TH cell populations. This approach can be performed using a peripheral blood sample, and also seems to be useful irrespective of whether an individual is actively consuming the allergic food in question lending itself to determining the timing of food reintroduction in the case of clinical remission. However, this approach has the disadvantage of intracellular IL-4 staining being a technically challenging protocol for some clinical labs.
Finally, it is notable that milk-specific immune responses in children with IgE-mediated milk allergy were grossly similar to those observed in children with clinical EoE milk allergy. The presence of circulating, allergen-activated TH cells in IgE-mediated milk allergy is consistent with both our understanding of the immunopathology of this condition, as well as our hypotheses as to the clinical and immunopathological relationship between IgE-mediated food allergy and EoE (31,32). Further, this observation may have implications for understanding EoE that occurs after outgrowing IgE-mediated food allergy (33), or initiation of oral immunotherapy (34). Notable differences in the immune responses between these two related conditions include the generally lower level of total IgG4 in children with IgE-mediated milk allergy, and the higher baseline proliferation and IL-4 production exhibited by circulating TH cells obtained from IgE-mediated milk allergy subjects. These latter observations could be indicative of important mechanistic differences between these two conditions. Regardless, the implications of these observations to the potential clinical assays described herein are likely inconsequential as IgE-mediated food allergy and EoE are easily distinguished clinically.
In sum, we report the identification of milk-reactive memory TH2 cells in the circulation of children with clinical EoE milk allergy. This approach provides a minimally-invasive assay that accurately predicts clinical EoE milk allergy with high sensitivity and specificity. Further, the success of these milk assays may indicate that the approach is generalizable to the development of assays for other common EoE allergens. However, further studies are needed to determine if these assays will apply to other EoE-causal foods. While potential for non-invasive testing modalities to aid in the identification of EoE-causal foods is of great clinical utility, future prospective studies are required to determine the true applicability of these approaches in EoE management.
Supplementary Material
ACKNOWLEDGMENTS, FUNDING:
We thank Benjamin Wright, MD for assistance with development of the IgG4 ELISA assays. This work was supported by the National Institutes of Health (K08DK116668), the American Academy of Allergy, Asthma, and Immunology, the American Partnership for Eosinophilic Disorders, and a Children’s Hospital of Philadelphia Research Institute Frontier Award.
Abbreviations:
- EoE
Eosinophilic Esophagitis
- PBMCs
Peripheral blood mononuclear cells
- TT
Tetanus Toxoid
- CFSE
Carboxyfluorescein succinimidyl ester
- TH2
T helper type 2
- ROC
Receiver Operating Characteristic
- IL
Interleukin
Footnotes
DISCLOSURES:
The authors have no relevant conflicts of interest to disclose.
REFERENCES:
- (1).Hill DA, Spergel JM. The Immunologic Mechanisms of Eosinophilic Esophagitis. Curr Allergy Asthma Rep 2016. January;16(2):9–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Davis BP, Rothenberg ME. Mechanisms of Disease of Eosinophilic Esophagitis. Annu Rev Pathol 2016. May 23;11:365–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy 2016. May;71(5):611–620. [DOI] [PubMed] [Google Scholar]
- (4).Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. J Allergy Clin Immunol 2007. February;119(2):509–511. [DOI] [PubMed] [Google Scholar]
- (5).Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol 2012. June;129(6):1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology 2018. October 01;155(4):1022–1033.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Dellon ES, Guo R, McGee SJ, Hamilton DK, Nicolai E, Covington J, et al. A Novel Allergen-Specific Immune Signature-Directed Approach to Dietary Elimination in Eosinophilic Esophagitis. Clin Transl Gastroenterol 2019. November 25. [DOI] [PMC free article] [PubMed]
- (8).Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol 2012. August;130(2):461–7.e5. [DOI] [PubMed] [Google Scholar]
- (9).Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 2012. June;142(7):1451–5. [DOI] [PubMed] [Google Scholar]
- (10).Molina-Infante J, Arias A, Barrio J, Rodriguez-Sanchez J, Sanchez-Cazalilla M, Lucendo AJ. Four-food group elimination diet for adult eosinophilic esophagitis: A prospective multicenter study. J Allergy Clin Immunol 2014. November 01;134(5):1093–9.e1. [DOI] [PubMed] [Google Scholar]
- (11).Spergel J, Aceves SS. Allergic components of eosinophilic esophagitis. J Allergy Clin Immunol 2018. July 01;142(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Akei HS, Mishra A, Blanchard C, Rothenberg ME. Epicutaneous antigen exposure primes for experimental eosinophilic esophagitis in mice. Gastroenterology 2005. September;129(3):985–994. [DOI] [PubMed] [Google Scholar]
- (13).Mondoulet L, Dioszeghy V, Larcher T, Ligouis M, Dhelft V, Puteaux E, et al. Epicutaneous immunotherapy (EPIT) blocks the allergic esophago-gastro-enteropathy induced by sustained oral exposure to peanuts in sensitized mice. PLoS One 2012;7(2):e31967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med 2013. August;19(8):1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Mishra A, Schlotman J, Wang M, Rothenberg ME. Critical role for adaptive T cell immunity in experimental eosinophilic esophagitis in mice. J Leukoc Biol 2007. April;81(4):916–924. [DOI] [PubMed] [Google Scholar]
- (16).Bullock JZ, Villanueva JM, Blanchard C, Filipovich AH, Putnam PE, Collins MH, et al. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2007. July;45(1):22–31. [DOI] [PubMed] [Google Scholar]
- (17).Cianferoni A, Ruffner MA, Guzek R, Guan S, Brown-Whitehorn T, Muir A, et al. Elevated expression of activated TH2 cells and milk-specific TH2 cells in milk-induced eosinophilic esophagitis. Ann Allergy Asthma Immunol 2018. February;120(2):177–183.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Wen T, Aronow BJ, Rochman Y, Rochman M, Kc K, Dexheimer PJ, et al. Single-cell RNA sequencing identifies inflammatory tissue T cells in eosinophilic esophagitis. J Clin Invest 2019. April 08;129(5):2014–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014. September;147(3):602–609. [DOI] [PubMed] [Google Scholar]
- (20).Schuyler AJ, Wilson JM, Tripathi A, Commins SP, Ogbogu PU, Kruzsewski PG, et al. Specific IgG4 antibodies to cow’s milk proteins in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol 2018. July 01;142(1):139–148.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Wright BL, Kulis M, Guo R, Orgel KA, Wolf WA, Burks AW, et al. Food-specific IgG4 is associated with eosinophilic esophagitis. J Allergy Clin Immunol 2016. October 01;138(4):1190–1192.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Peterson K, Lin E, Saffari H, Qeadan F, Pyne A, Firszt R, et al. Food-specific antibodies in oesophageal secretions: association with trigger foods in eosinophilic oesophagitis. Aliment Pharmacol Ther 2020;52(6):997–1007. [DOI] [PubMed] [Google Scholar]
- (23).Ruffner MA, Brown-Whitehorn TF, Verma R, Cianferoni A, Gober L, Shuker M, et al. Clinical tolerance in eosinophilic esophagitis. J Allergy Clin Immunol Pract 2018. April 01;6(2):661–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kallas EG, Gibbons DC, Soucier H, Fitzgerald T, Treanor JJ, Evans TG. Detection of intracellular antigen-specific cytokines in human T cell populations. J Infect Dis 1999. May 01;179(5):1124–1131. [DOI] [PubMed] [Google Scholar]
- (25).Hill DA, Grundmeier RW, Ramos M, Spergel JM. Eosinophilic Esophagitis Is a Late Manifestation of the Allergic March. J Allergy Clin Immunol Pract 2018. June 13. [DOI] [PMC free article] [PubMed]
- (26).MacLeod MK, Kappler JW, Marrack P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology 2010. May 01;130(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Umekita K, Miyauchi S, Nomura H, Umeki K, Okayama A. Neutrophil-derived lactoferrin induces the inflammatory responses of rheumatoid arthritis synovial fibroblasts via Toll-like receptor 4. Clin Exp Rheumatol 2019. October 01;37(5):834–841. [PubMed] [Google Scholar]
- (28).Na YJ, Han SB, Kang JS, Yoon YD, Park SK, Kim HM, et al. Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int Immunopharmacol 2004. September 01;4(9):1187–1199. [DOI] [PubMed] [Google Scholar]
- (29).Kilar A, Kocsis B, Kustos I, Kilar F, Hjerten S. CE to monitor endotoxins by protein complexation. Electrophoresis 2006. November 01;27(21):4188–4195. [DOI] [PubMed] [Google Scholar]
- (30).Brough HA, Nadeau KC, Sindher SB, Alkotob SS, Chan S, Bahnson HT, et al. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy 2020. September 01;75(9):2185–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hill DA, Dudley JW, Spergel JM. The Prevalence of Eosinophilic Esophagitis in Pediatric Patients with IgE-Mediated Food Allergy. J Allergy Clin Immunol Pract 2017;5(2):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Capucilli P, Hill DA. Allergic Comorbidity in Eosinophilic Esophagitis: Mechanistic Relevance and Clinical Implications. Clin Rev Allergy Immunol 2019. March 22. [DOI] [PMC free article] [PubMed]
- (33).Maggadottir SM, Hill DA, Ruymann K, Brown-Whitehorn TF, Cianferoni A, Shuker M, et al. Resolution of acute IgE-mediated allergy with development of eosinophilic esophagitis triggered by the same food. J Allergy Clin Immunol 2014. May;133(5):1487–1489.e1. [DOI] [PubMed] [Google Scholar]
- (34).Cafone J, Capucilli P, Hill DA, Spergel JM. Eosinophilic esophagitis during sublingual and oral allergen immunotherapy. Curr Opin Allergy Clin Immunol 2019. August 01;19(4):350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


