Abstract
Lemmel's syndrome is a rare cause of obstructive jaundice in the absence of stones or tumors, commonly due to periampullary duodenal diverticulum (PAD). CT scan with the oral and intravenous administration of iodinated contrast media, was crucial to exclude the presence of stones or tumours, but esophagogastroduodenoscopy (EGD) confirms the diagnosis. We report a case of a 72 -year-old woman affected by Lemmel's syndrome secondary to a giant PAD, suffering from right upper abdominal quadrant pain and pancreaticobiliary disease. Subsequently we proceed to analyse the pathogenesis of PAD, and later to discuss pitfalls, tips and tricks useful to make a correct diagnosis, in order to achieve an accurate management.
Keywords: Lemmel's syndrome, Periampullary duodenal diverticulum, Pancreaticobiliary disease, Cholangitis, Pancreatitis
Introduction
Periampullary duodenal diverticulum (PAD) is the most common cause of Lemmel's syndrome, with an incidence of 1%-27%. It arises within a 2-3 cm radius from the ampulla of Vater. PAD is usually asymptomatic, but in about 5% of cases it can show complications, such us right upper quadrant pain, elevated bilirubin, liver enzymes and/or pancreatic enzymes levels [1]. Despite endoscopic retrograde cholangiopancreatography (ERCP) is the gold standard diagnostic test, ultrasound and CT represent the first and fastest diagnostic step. Asymptomatic and incidental PAD requires no treatment or intervention, while surgery should be performed in more severe cases [2]. We report a case of symptomatic Lemmel's syndrome secondary to a giant PAD, arrived at our radiology department from the Emergency Room with acute abdominal pain due to suspected pancreatitis. Ultrasound (US) and contrast-enhanced CT (CECT) was performed. PAD was detected and the patient underwent an esophagogastroduodenoscopy (EGD) to confirm the diagnosis.
Case report
A 72-year-old woman arrived to our emergency department with acute pain in the upper abdominal quadrants, she had a medical history of poorly controlled diabetes mellitus type II and hypertension. An initial evaluation revealed she had fever (t. 38°C) and her laboratory tests presented elevated levels of amylase (1370 U/l v.n. 28-100 U/l), lipase (1362 U/l v.n. 8-57 U/l), aspartate aminotransferase (348 U/l v.n. 0-32 U/l), alanine aminotransferase (161 U/l v.n. 0-32 U/l), gamma-glutamyltransferase (179 U/l v.n. 5-36 U/l), alkaline phosphatase (113 U/l v.n. 8-57 U/l), total bilirubin (1,27 mg/dl v.n. 0,1-1,0 mg/dl) determined by the increase of direct bilirubin (0,94 mg/dl v.n. 0,1-0,3 mg/dl), white blood cells (14,9 × 10³/L v.n. 4,00-10,00 × 10³/L). Abdominal US showed an increase in size of the pancreatic cephalic region with hypoechoic echotexture, and an overdistension of the gallbladder with biliary sludge (Fig. 1). Therefore, the patient was admitted to the internal medicine department with a diagnosis of acute pancreatitis and started the appropriate therapy. During the hospitalization, the patient underwent an abdominal CECT with the oral and intravenous administration of iodinated contrast media (Fig 2, Fig 3), which demonstrated a 4 cm diverticulum of the second portion of the duodenum. It was distended and filled of ingested food, so it produced a mild mass effect on the extraepatic bile duct, which was dilatated upstream (choledochus diameter: 12 mm). The overdistended gallbladder with biliary sludge, and the inhomogeneous density of the pancreatic head due to edema, were confirmed. Consequently, the patient underwent esophagus-gastro-duodenoscopy which confirmed a large perivaterian diverticulum in the second portion of the duodenum, filled by ingested food material (Fig. 4). After the normalization of blood tests and a surgical consultation, the patient was discharged with the prescription of a light diet and periodic follow-ups.
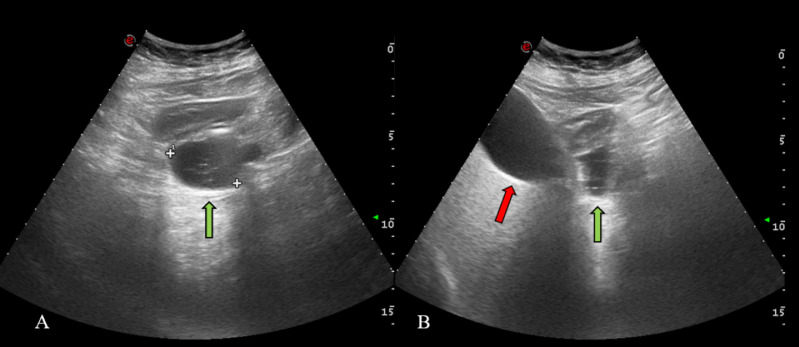
Fig. 1.
Abdominal US. Hypo-anechoic, rounded, 4 cm mass (green arrow) with multiple hyperechoic spots inside; gallbladder on the right of the PAD (red arrow) (Color version of figure is available online)
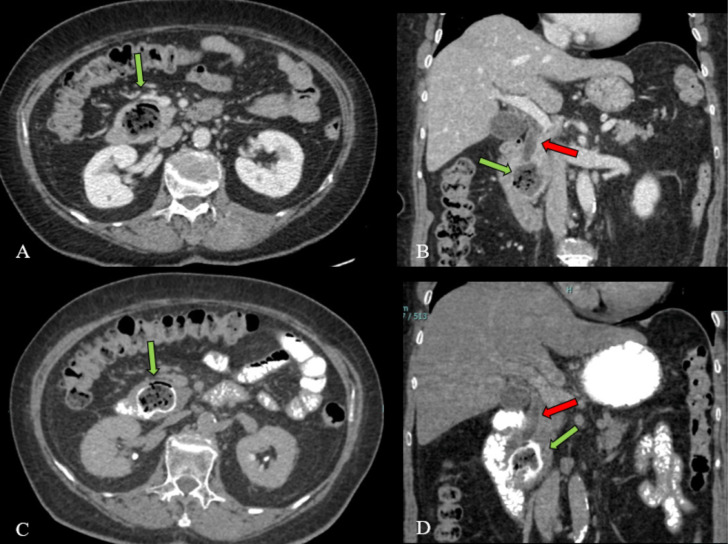
Fig. 2.
NECT (A-B) and CECT with oral contrast administration (C-D). 4 cm PAD filled by food (green arrow). Oral contrast (C-D) was administrated to better identify diverticulum. Coronal reconstructions (B-D) show the bile duct dilatation (red arrow) due to extrinsic compression of PAD (green arrow) (Color version of figure is available online)
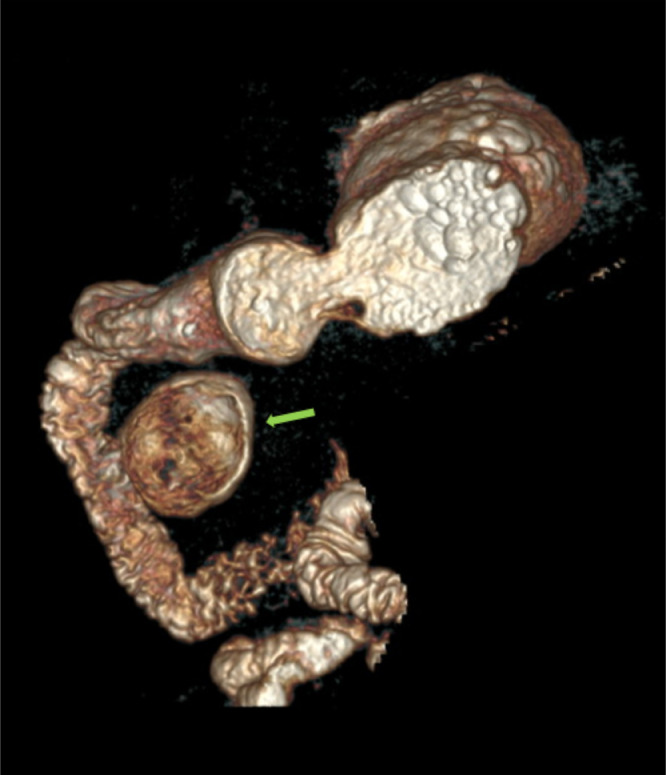
Fig. 3.
Three-dimensional CT reconstruction with volume rendering (3D-VR), after oral contrast administration, shows PAD (green arrow) (Color version of figure is available online)
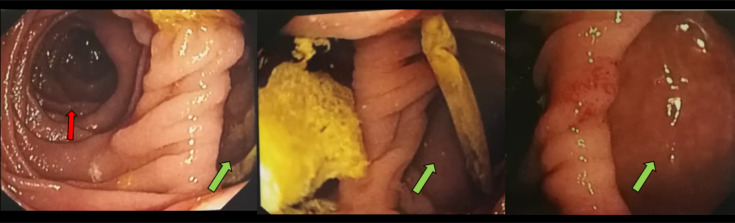
Fig. 4.
EGDS. Images show the large PAD (green arrow) in the second portion of the duodenum (red arrow) (Color version of figure is available online)
Discussion
Duodenal Diverticulum (DD) was first reported by Chomell in 1710, but the first radiological demonstration was performed in 1913 by JT Case. Duodenum is the second most common site of diverticula in alimentary tract, after colon [3]. Peak incidence is between 50 and 60 years, without gender predilection. The pathogenesis of DD is unclear, but it possibly may form through a locus minoris resistentiae that develops through the wall of duodenum at the passage of the biliary and pancreatic duct and blood vessels, in absence of an adequate muscle coat or heterotrophic pancreatic tissue. DD are classified as primary or true diverticula, and secondary or false. These latter are due to chronic duodenal ulceration, so they are also recognized as pre-stenotic diverticula. Furthermore, duodenal diverticulum can be also classified as intraluminal or extraluminal. The secondary false extraluminal variant is the most common. [4,5]. Most cases of DD are asymptomatic and accidentally detected on EGD, but in some cases may occur pancreaticobiliary complications, non-specific abdominal pain, discomfort, haemorrhage, fistula, perforation, enterolith formation with small bowel obstruction or duodenal obstruction by the intra-luminal diverticulum. [6,7].
Usually, laboratory tests show leukocytosis, high bilirubin levels (direct and total), an elevated count of liver enzymes, gamma-glutamyl transferase, alkalin phosphatase and inflammatory markers. Higher levels of pancreatic enzymes are also observed if the pad compresses the ampulla of Vater [8].
In our case, the patient was admitted to the internal medicine department with a diagnosis of acute pancreatitis, supported by symptoms, laboratory tests and US imaging, as reported in literature [9]. Diagnosing Lemmel's syndrome is often particularly challenging: CT scan with the oral and intravenous administration of iodinated contrast media, was crucial to exclude the presence of stones or tumours. ERCP can help to demonstrate the presence of a PAD and it is considered the gold standard for diagnosis [10]. In our case EGD was performed to confirm the diagnosis. Diverticulectomy is the standard of care; conservative medical management, endoscopic sphincterotomy or papillary balloon dilatation are possible therapeutic options [11]. After PAD diagnosis was confirmed, the patient was treated with conservative medical management and invited to undergo periodic follow-ups.
In conclusion, diagnosing Lemmel's syndrome can be challenging. PAD may be incidentally found or be suspected in patients with obstructive jaundice, pancreaticobiliary disease, non-specific abdominal pain in the absence of stones or tumours. Being able to make a correct diagnosis is crucial to achieve an accurate patient care management, and to avoid complication of delayed management.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Patient consent: Written informed consent for publication of their clinical details and clinical images was obtained from the patients.
References
- 1.Bernshteyn M, Rao S, Sharma A, Masood U, Manocha D. Lemmel's syndrome: usual presentation of an unusual diagnosis. cureus. 2020;12(4):e7698. doi: 10.7759/cureus.7698. [DOI] [PMC free article] [PubMed]
- 2.Vitturi N, Simoni F, De Stefano F, Orlando R, Lirussi F, Realdi G. Paravaterian diverticula presenting as acute cholangitis in two very elderly patients. J Gastrointestin Liver Dis. 2010;19(2):220–221. [PubMed] [Google Scholar]
- 3.Pimparkar BD. In: Gastroenterology. 3rd (Ed) Bockus Henry L, editor. WB Saunders Co; Philadelphia: 1976. Diverticulosis of the small intestine In; pp. 437–458. (Ed) [Google Scholar]
- 4.Cheshire NJ, Diverticula Glezer G. In: Maingoats Abdominal operation. 10th (Ed) Zinner MJ, Schwartz SI, Ellis H, editors. Prentice Hall International Ince (UK) Limited; London: 2021. volvulus, superior mesenteric artery syndrome and foreign bodies. In. (Ed) 916-2. [Google Scholar]
- 5.Mahajan Sanjay, Rajesh Kashyap, K Chandel, Mokta Jatinder, S Minhas. (2004). Duodenal diverticulum: Review of literature. Ind J Surge. 2004;66:140–145. Num 366. [Google Scholar]
- 6.Alzerwi, Nasser A.N. MBBS, SBGS (Saudi Board of General Surgery). Recurrent ascending cholangitis with acute pancreatitis and pancreatic atrophy caused by a juxtapapillary duodenal diverticulum. Medicine. 2020;99 doi: 10.1097/MD.0000000000021111. July 02Issue 27 - p e21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harthun NL, Morse JH, Shaffer HA, Jr, Minasi JS. Duodenal obstruction caused by intraluminal duodenal diverticulum and annular pancreas in an adult. Gastrointest Endosc. 2002;55(7):940–943. doi: 10.1067/mge.2002.124210. Jun. [DOI] [PubMed] [Google Scholar]
- 8.Venkatanarasimha N, Yong YR, Gogna A, Tan BS. Case 265: Lemmel syndrome or biliary obstruction due to a periampullary duodenal diverticulum. Radiology. 2019;291:542–545. doi: 10.1148/radiol.2019162375. [DOI] [PubMed] [Google Scholar]
- 9.Gwozdz GP, Steinberg WM, Werner M, Henry JP, Pauley C. Comparative evaluation of the diagnosis of acute pancreatitis based on serum and urine enzyme assays. Clin Chim Acta. 1990;187(3):243–254. doi: 10.1016/0009-8981(90)90109-6. [DOI] [PubMed] [Google Scholar]
- 10.Karayiannakis AJ, Bolanaki H, Courcoutsakis N, Kouklakis G, Moustafa E, Prassopoulos P. Common bile duct obstruction secondary to a periampullary diverticulum. Case Rep Gastroenterol. 2012;6(2):523–529. doi: 10.1159/000341955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouet J, Gaujoux S, Ronot M, Palazzo M, Cauchy F, Vilgrain V. Lemmel's syndrome as a rare cause of obstructive jaundice. Clin Res Hepatol Gastroenterol. 2012;36(6):628–631. doi: 10.1016/j.clinre.2012.05.002. DecEpub 2012 Jul 4. [DOI] [PubMed] [Google Scholar]