Abstract
Tissue factor (TF) is the principal initiator of blood coagulation and is necessary for thrombosis. We previously reported that lysophosphatidic acid (LPA), a potent bioactive lipid, highly induces TF expression at the transcriptional level in vascular smooth muscle cells. To date, however, the specific role of the LPA receptor is unknown, and the intracellular signaling pathways that lead to LPA induction of TF have been largely undetermined. In the current study, we found that LPA markedly induced protein kinase D (PKD) activation in mouse aortic smooth muscle cells (MASMCs). Small-interfering RNA-mediated knockdown of PKD2 blocked LPA-induced TF expression and activity, indicating that PKD2 is the key intracellular mediator of LPA signaling leading to the expression and cell surface activity of TF. Furthermore, our data reveal a novel finding that PKD2 mediates LPA-induced TF expression via the p38α and JNK2 MAPK signaling pathways, which are accompanied by the PKD-independent MEK1/2-ERK-JNK pathway. To identify the LPA receptor(s) responsible for LPA-induced TF expression, we isolated MASMCs from LPA receptor-knockout mice. Our results demonstrated that SMCs isolated from LPA receptor 1 (LPA1)-deficient mice completely lost responsiveness to LPA stimulation, which mediates induction of TF expression and activation of PKD and p38/JNK MAPK, indicating that LPA1 is responsible for PKD2-mediated activation of JNK2 and p38α. Taken together, our data reveal a new signaling mechanism in which the LPA1-PKD2 axis mediates LPA-induced TF expression via the p38α and JNK2 pathways. This finding provides new insights into LPA signaling, the PKD2 pathway, and the mechanisms of coagulation/atherothrombosis.
Keywords: vascular biology, smooth muscle cells, signal transduction, lipid signaling, gene expression, atherosclerosis
Abbreviations: Egr1, early growth response protein 1; ERK, extracellular-signal-regulated kinase; JNK, Jun N-terminal kinases; LDL, low-density lipoprotein; LPA, lysophosphatidic acid; LPA1, LPA receptor 1; LPA2, LPA receptor 2; MAPK, mitogen-activated protein kinase; MASMC, mouse aortic smooth muscle cell; MEK, mitogen-activated protein kinase kinase; oxLDL, oxidized LDL; p38 MAPK, p38 mitogen-activated protein kinases; PKD, protein kinase D; SMC, smooth muscle cell; TF, tissue factor
Tissue factor (TF) is a transmembrane protein that is a key initiator of the extrinsic pathway of the blood coagulation cascade (1, 2) and can trigger both arterial and venous thrombosis (3, 4, 5, 6). TF has also been reported to mediate smooth muscle cell (SMC) migration (7) and proliferation (8), both of which contribute to the development of atherosclerosis. In normal arteries, little or no TF is found in the intima or media; however, in acute arterial injury, SMCs appear to be chief sites of TF expression (9). TF is also abundant in atherosclerotic plaques (10, 11, 12, 13), where it colocalizes with both SMCs and macrophages (11, 14). The amount of TF protein in the plaque correlates with TF activity (15, 16); however, the stimuli responsible for TF expression in cells of arterial lesions and the mechanisms of TF induction within the plaque are still largely unknown.
Previously, we reported that low-density lipoprotein (LDL), oxidized LDL (oxLDL), and their lipid extracts induce TF gene expression in SMCs (17). Native LDL does not induce TF expression as strongly as oxLDL, suggesting that particular lipid components of oxLDL enhance TF gene induction (17). We found that lysophosphatidic acid (LPA) markedly induces the levels of TF mRNA, TF protein, and TF surface activity in rat SMCs (18). LPA has been shown to be a component of oxLDL and to accumulate in human atherosclerotic plaques in vivo (19). LPA levels were increased in culprit coronary arteries in human patients and in experimentally induced mouse atherosclerotic plaques (20, 21). LDL-associated LPA is increased in plasma from high-fat Western diet-fed mice that are genetically prone to hyperlipidemia (22). Our previous study demonstrated that LPA-induced TF expression is controlled at the transcriptional level and that LPA induction of TF gene expression in SMCs depends on the activation of a Gi protein and the subsequent phosphorylation of mitogen-activated protein kinase kinases (MEKs) and extracellular signaling–regulated kinases (ERKs) (18). In the current study, we aimed to explore other essential signaling pathways mediating LPA-induced TF expression and to identify the specific LPA receptor that controls TF expression.
The protein kinase D (PKD) family comprises three members: PKD1, PKD2, and PKD3. This family possesses substrate specificity similar to Ca2+/calmodulin-dependent protein kinases (CaMKs) and structural features reminiscent of PKCs. The structure of PKDs consists of a C-terminal catalytic domain and an N-terminal region with autoinhibitory, regulatory domains such as the diacylglycerol (DAG) binding domain and the pleckstrin homology domain (23). Activated forms of PKD1 and PKD2 (but not PKD3) autophosphorylate at a consensus phosphorylation motif of PKD1 and PKD2 (in a PDZ domain-binding motif) at the extreme C-terminus. This modification regulates interactions with scaffolding proteins, trafficking to distinct cellular subdomains, and the amplitude/tempo of PKD signaling responses (24).
The function of PKDs in LPA-mediated events in vascular biology has not been well explored. PKD1 has been reported to be involved in LPA-promoted angiogenesis (25) and CD36 transcription (26) in endothelial cells. However, the role of the other PKD isoforms in LPA-induced vascular signaling remains undocumented. Also, whether and how PKD mediates LPA signaling leading to the expression of the coagulation initiator TF has not been revealed.
In the current study, our data demonstrate that LPA markedly induces PKD activation in mouse aortic SMCs. Interestingly, we found that PKD2 mediates LPA-induced TF expression via JNK and p38 MAPK pathways. We further identified that PKD2-mediated, LPA-induced TF expression is specifically dependent on the activation of p38α MAPK and JNK2. In addition, using isolated primary SMCs from LPA receptor knockout mice, our data reveal that LPA receptor 1 (LPA1) is the key receptor controlling LPA-induced TF expression. Targeting this signaling cascade could be a novel approach for preventing blood coagulation and thrombus formation. These results offer new insights into the LPA-triggered mechanisms of blood coagulation and atherothrombosis.
Results
Three PKD isoforms are expressed in MASMCs
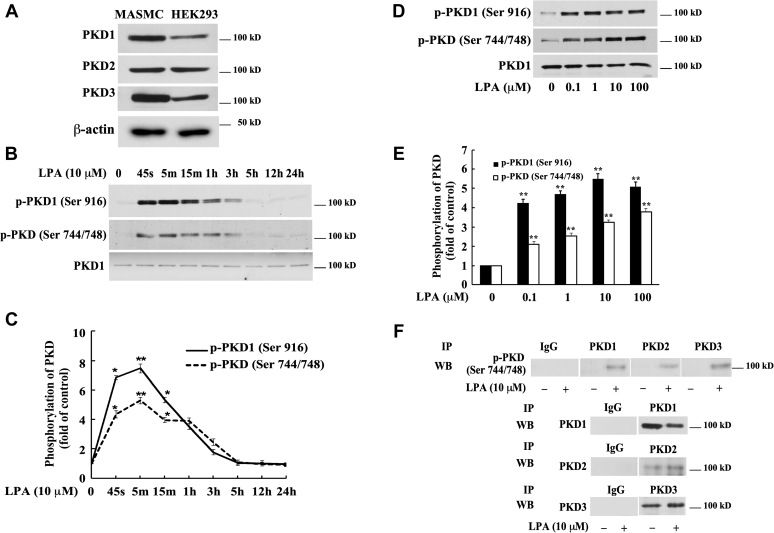
To examine whether PKD mediates LPA signaling in SMCs, we first examined the expression levels of PKD isoforms in mouse aortic SMCs (MASMCs). Cell lysates of MASMCs were collected and analyzed by Western blotting with specific antibodies against PKD family members: PKD1, PKD2, and PKD3. Cell lysate from HEK293 cells was used as a positive control, because all three PKD isoforms are known to be expressed in this cell line (27). As shown in Figure 1A, all three PKD isoforms, PKD1, PKD2, and PKD3, are expressed in MASMCs.
Figure 1.
LPA induces activation of protein kinase D (PKD) in mouse aortic smooth muscle cells (MASMCs).A, Western blot analysis of the expression of PKD isoforms in MASMCs using antibodies against PKD1, PKD2, and PKD3. Lysates of HEK293 cells were used as a positive control. β-actin serves as a loading control. B, Western blot analysis of the time course of LPA stimulation of PKD phosphorylation in MASMCs. Antibodies against p-PKD1 (Ser916) and p-PKD activation loop (Ser 744/748) were used. PKD1 protein expression is shown in the bottom panel. C: time-dependent PKD phosphorylation was quantified by densitometry. Data are mean ± SD from three experiments. D, Western blot analysis of the concentration dependence of LPA induction of PKD phosphorylation. MASMCs were stimulated with LPA for 5 min. E, concentration-dependent PKD phosphorylation induced by LPA was quantified by densitometry. Data are mean ± SD from three experiments. ∗p < 0.05 and ∗∗p < 0.01 versus control. F, immunoprecipitation results of phospho-PKD three isoforms (PKD1–3). Harvested protein samples were immunoprecipitated with specific PKD1, PKD2, and PKD3 antibodies followed by Western blotting analysis for detection of each of the three PKD activations (first panel) with antibody against p-PKD activation loop (Ser744/748). The lower panels (2–4) indicate the efficiency of the immunoprecipitation with each of the specific PKD antibodies. IgG immunoprecipitation serves as a negative control.
LPA rapidly and markedly induces activation of PKD in MASMCs
To determine whether LPA activates PKD in MASMCs, we next examined PKD phosphorylation in MASMCs. The PKD activation loop is fully conserved in all three mammalian PKD isoforms (Table 1). Hence, PKD “activation loop” phospho-specific antibodies should cross-react equally with PKD1 (phosphorylated on Ser744/Ser748), PKD2 (phosphorylated on Ser707/Ser711), and PKD3 (phosphorylated on Ser730/Ser734) (28). It has been shown that phosphorylation of the serine residue at the C terminus of PKD1 (Ser916) occurs by auto-phosphorylation and correlates with the activation status of PKD1 (29); however, PKD3 lacks an extreme C-terminal phosphorylation site (Table 2) (24, 30, 31). All three isoforms of PKD share phosphorylation sites in the activation loop (23). Cultured MASMCs were starved for 24 h and then treated with 10 μM LPA for various time periods. Specific antibodies against PKD1's C-terminal auto-phosphorylation site (Ser 916) and the PKD activation loop (Ser744/748) were used to detect PKD activation in these cell lysates. As shown in Figure 1, B and C, we observed that PKD1 auto-phosphorylation and PKD activation (phosphorylation at the PKD activation loop) were markedly and rapidly induced, with a peak around 45 s–5 min. PKD1 protein expression showed no change after LPA stimulation. The LPA-induced phosphorylation of serine residues at both the C terminus of PKD1 and the PKD activation loop declined to baseline at around 3 h (Fig. 1, B and C). As shown in Figure 1, D and E, LPA also induced phosphorylation of both the PKD1 C terminus (S916) and the PKD activation loop (S744 and S748 in PKD1) in a dose-dependent manner. These data indicate that LPA markedly induces activation of PKD in MASMCs. In the following studies, the LPA concentration of 10 μM was chosen to stimulate MASMCs because it is in the range of pathological concentrations found in atherosclerotic lesions in vivo (19) and acute myocardial infarction (32).
Table 1.
Highly conserved activation loop kinase domains of the three mouse protein kinase D (PKD) isoforms
| PKD1 (mouse) 722-SADPFPQVKLCDFGFARIIGEKS744FRRS748VVGTPAYLAPEVLRNKGY PKD2 (mouse) 685-SADPFPQVKLCDFGFARIIGEKS707FRRS711VVGTPAYLAPEVLLNQGY PKD3 (mouse) 708-SAEPFPQVKLCDFGFARIIGEKS730FRRS734VVGTPAYLAPEVLRSKGY |
Table 2.
The variable C-terminal amino acid sequences of the three mouse protein kinase D (PKD)
| PKD1 (mouse): extreme c-terminal autophosphorylation site: EREMKALS916ER-918 PKD2 (mouse): extreme c-terminal autophosphorylation site: QGLAERIS873IL-875 PKD3 (mouse): no extreme c-terminal autophosphorylation site: PNPDDMEEDP-889 |
The relevant serine phosphorylation sites are indicated. PKD1 and PKD2 have autophosphorylation sites, but not PKD3.
All three isoforms of PKD are activated by LPA in SMCs
The effect of LPA on the activation of all three PKD isoforms is currently undocumented. Our experiment demonstrated that all three isoforms of PKD are expressed in MASMCs (Fig. 1A) and that LPA markedly induced the phosphorylation of the PKD1 C-terminus and the activation loop of PKD (Fig. 1, B and C). To determine whether all three PKD isoforms were activated (phosphorylated at the activation loop site), we immunoprecipitated PKD1, PKD2, and PKD3 using three isoform-specific PKD antibodies in both untreated and LPA-stimulated MASMCs. We then used phospho-PKD activation loop-specific antibody (against p-Ser744/748 in PKD1) to detect activation loop phosphorylation of each PKD isoform by Western blot analysis. We found that phosphorylation of the PKD activation loop was detected in all three PKD isoforms (PKD1, PKD2, and PKD3) in response to LPA stimulation (Fig. 1F, top panel), The data from the lower three panels of Figure 1F verified the successful immunoprecipitation applied in our experiments, including the use of IgG as a negative control. These results reveal that LPA induces activation of all three isoforms of PKD in MASMCs.
PKD2, but not PKD1 or PKD3, mediates LPA-induced TF expression and TF coagulant activity on cell surface
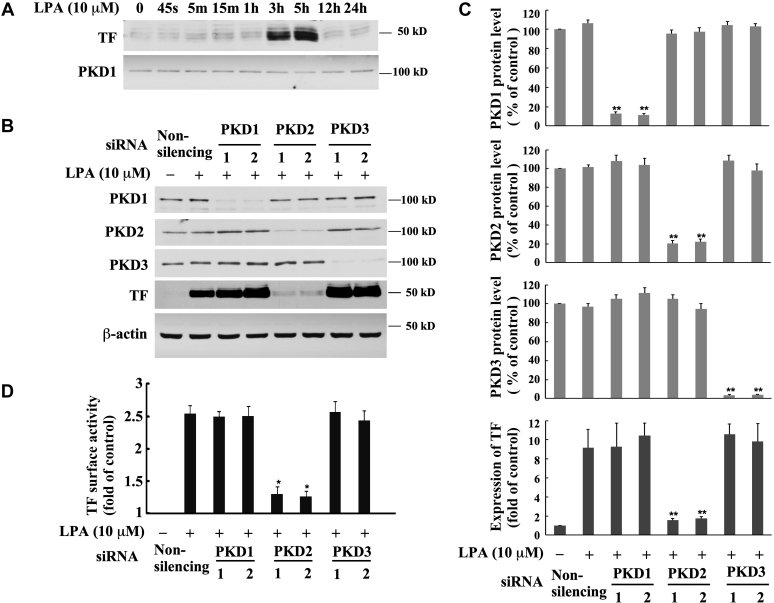
To date, it has been unknown whether any isoforms of PKD mediate LPA signaling, leading to TF expression and TF coagulant activity on cell surface. Our result demonstrated that LPA prominently induces TF expression in MASMCs (Fig. 2A). To determine the role of PKD in LPA-induced TF expression, we examined whether depletion of the expression of each PKD isoform, using specific siRNA knockdown approach, affects TF expression in response to LPA. Our results reveal that knockdown of PKD2 protein expression nearly completely abolished TF expression induced by LPA. However, depletion of PKD1 and PKD3 expression had no effect on TF expression (Fig. 2, B and C), indicating that PKD2 plays an important and specific role in the LPA signaling pathway, controlling TF expression. We next examined the effect that knockdown of the specific PKD isoforms has on TF coagulant activity on cell surface. Our results show that knockdown of PKD2 expression with the specific PKD2 siRNA resulted in remarkably blocking LPA-induced TF surface activity, while knockdown of PKD1 and PKD3 had no effect on the surface activity of TF in MASMCs (Fig. 2D). Collectively, these data strongly support the conclusion that PKD2 predominantly mediates LPA-induced TF expression and TF coagulant activity on cell surface.
Figure 2.
Effect of isoform-specific PKD siRNA transfection on tissue factor (TF) expression and TF surface activity induced by LPA in MASMCs.A, MASMCs were treated with 10 μM of LPA for various time points and TF expression was detected by Western blotting analysis with TF antibody. PKD1 protein expression in the time course was served as an internal control (PKD1 image is the same shown in Fig. 1B). B, effect of knockdown of the expression of PKD isoforms with PKD isoform-specific siRNAs on LPA-induced TF protein expression. Expression of PKDs and TF proteins was examined by Western blot analysis. For each PKD isoform, two specific siRNAs targeting different sequences were utilized. The specific PKD siRNAs (40 nM) were transfected into MASMCs for 48 h followed by serum starvation for 24 h; then cells were stimulated with 10 μM of LPA for 5 h. β-actin serves as a loading control. C, knockdown results of panel B were quantified by densitometry. Data are mean ± SD from three experiments. ∗∗p < 0.01 versus LPA-treated, nonsilencing siRNA group. D, effect of knockdown of the expression of PKD isoforms with PKD isoform-specific siRNAs on LPA-induced TF surface activity. Data are mean ± SD from three experiments. ∗∗p < 0.01 versus LPA-treated, nonsilencing siRNA group.
PKD2 mediates LPA-induced phosphorylation of JNK and p38 MAPKs
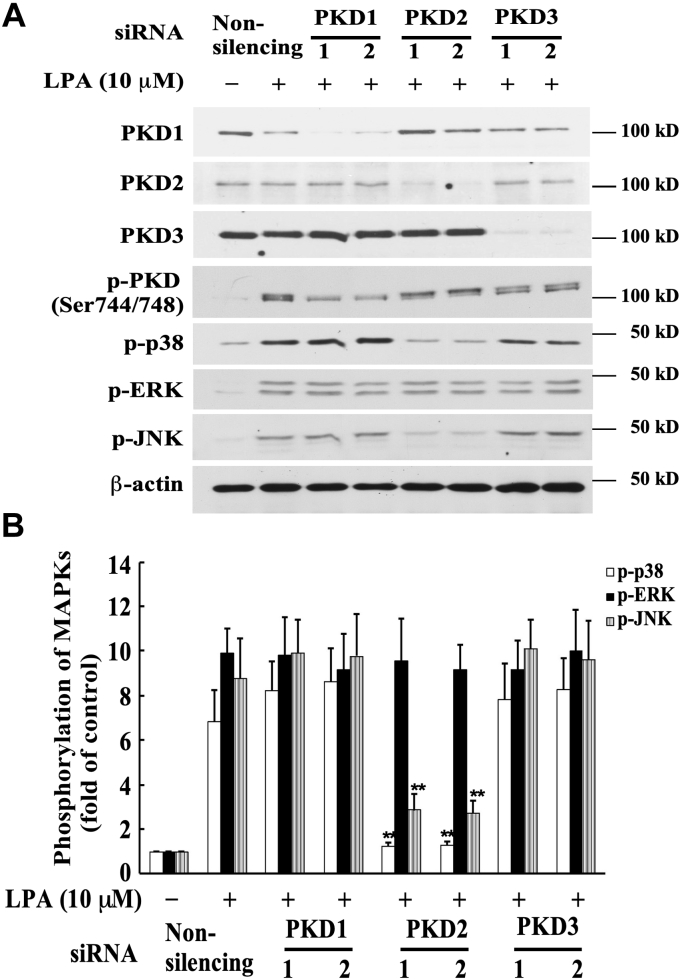
To investigate the downstream signaling molecules in this PKD-mediated signaling pathway, we evaluated the effect of PKD on mitogen-activated protein kinase (MAPK) activation, as LPA has been reported to activate MAPKs (18, 33, 34, 35, 36, 37). We examined the relationship of PKD and MAPKs in this LPA-triggered signaling pathway by evaluating whether depletion of the expression of PKD isoforms, using specific siRNA for PKD1, PKD2, or PKD3, has an effect on phosphorylation of MAPKs. The specific PKD siRNAs were transfected into MASMCs for 48 h, followed by serum starvation for 24 h. Cells were then stimulated with LPA (10 μM) for 5 min. As shown in Figure 3, A and B, transfection of PKD1 and PKD3 siRNAs nearly completely depleted endogenous protein expression of PKD1 and PKD3, but had no significant effect on phosphorylation of ERK, JNK, and p38 MAPKs. However, transfection of PKD2 siRNA, which nearly completely depleted endogenous PKD2 protein expression, markedly inhibited phosphorylation of p38 and JNK MAPKs. In contrast, depletion of PKD2 protein expression had no effect on phosphorylation of ERK MAPK in response to LPA. These results indicate that PKD2, but not PKD1 or PKD3, is required for the activation of p38 and JNK MAPKs in response to LPA stimulation in MASMCs.
Figure 3.
Effect of isoform-specific PKD siRNA on phosphorylation of various MAPKs induced by LPA in MASMCs. The specific PKD siRNAs (40 nM) were transfected into MASMCs for 48 h followed by serum starvation for 24 h; then cells were stimulated with 10 μM of LPA for 5 min. A, effect of knockdown of the expression of PKD isoforms with PKD siRNAs on LPA-induced MAPK phosphorylation. Expression of PKDs and phosphorylation of MAPKs were examined by Western blot analysis. B, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗∗p < 0.01 versus LPA-treated, nonsilencing siRNA group.
PKD-dependent JNK and p38 MAPK pathways and PKD-independent MEK1/2-ERK-JNK pathway mediate LPA-induced TF expression in MASMCs
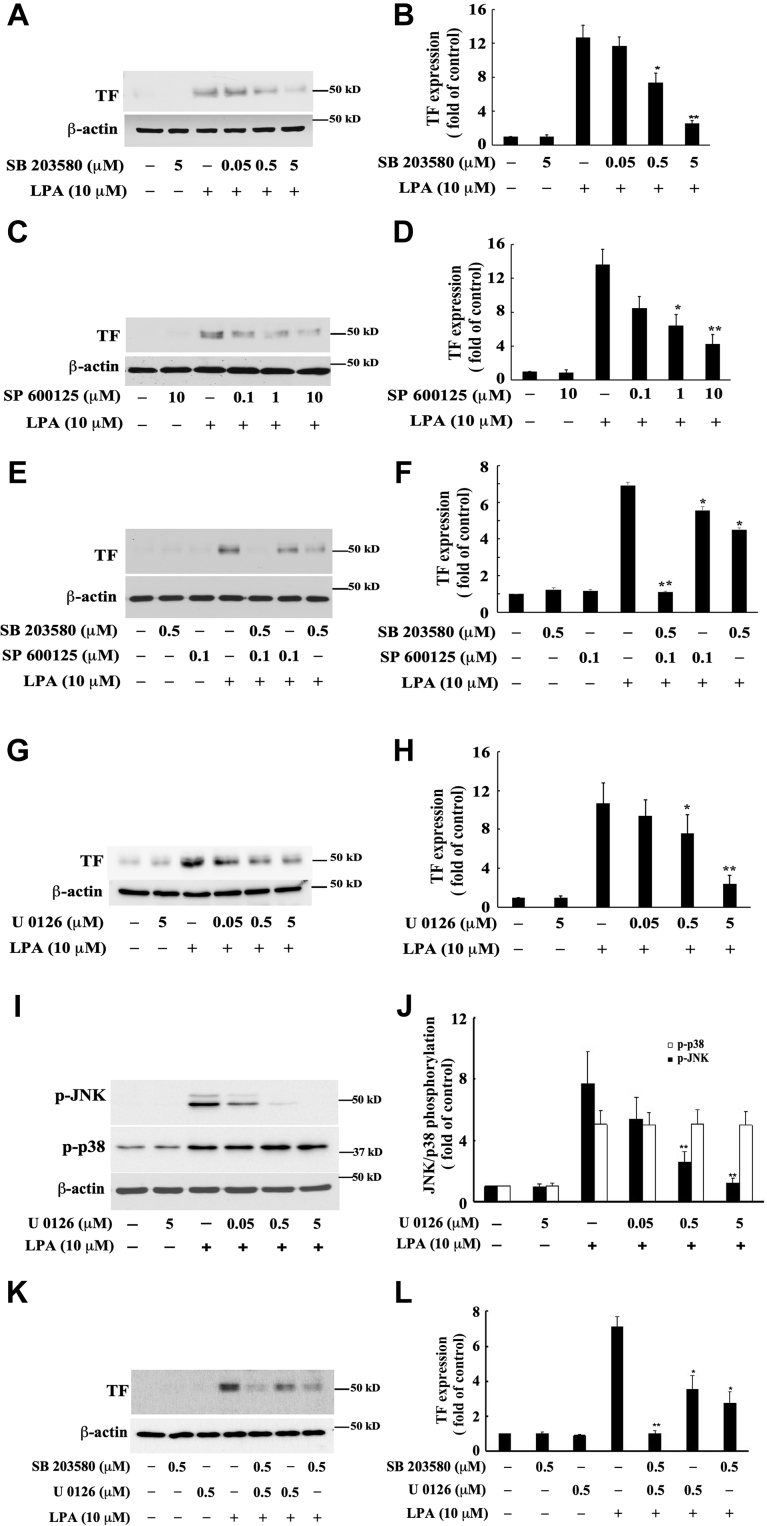
MAPKs have been reported to be involved in TF expression brought about by various inducers (38, 39, 40). To determine whether PKD2-mediated JNK and p38 MAPK activation is required for LPA-induced TF expression in MASMCs, we pretreated cells with the specific JNK inhibitor SP600128 and the specific p38 MAPK inhibitor SB203580 and measured LPA-induced TF expression. We found that both inhibitors SP600128 and SB203580 individually and dose-dependently reduced LPA-induced TF protein expression in MASMCs (Fig. 4, A–D). Furthermore, treatment with a combination of SP600128 and SB203580 showed an additive effect on the inhibition of TF expression and nearly completely blocked LPA-induced TF expression (Fig. 4, E and F). In light of our previous report that MEK1/2-ERK MAPK contributes to LPA-induced TF expression in rat SMCs (18), we reasoned that the PKD-independent MEK1/2-ERK MAPK pathway may merge into the JNK pathway, contributing to LPA-induced TF expression. This speculation was supported by our previously published data that MEK1/2-ERK pathway-mediated JNK activation leads to LPA-induced early growth response protein 1 (Egr-1) expression (37), and by another group's report that activation of ERK1/2 appears to activate its downstream targets such as p90RSK and SAPK/JNK (41), despite the notion that in the majority of cases, the MEK1/2-ERK pathway is parallel to the MKK4/7-JNK pathway (42). Our speculation was confirmed by the following experimental results. We first substantiated that MEK1/2-ERK mediates LPA-induced TF expression in MASMCs by employing the specific MEK1/2 inhibitor U0126. Our results show that pretreatment of U0126 dose-dependently inhibited LPA-induced TF expression in MASMCs (Fig. 4, G and H). Next, we determined whether the MEK1/2-ERK pathway is parallel to or in cross talk with the JNK and p38 MAPK pathways. Interestingly we found that U0126 dose-dependently blocked phosphorylation of JNK but had no effect on p38 activation (Fig. 4, I and J), suggesting that MEK1/2-ERK mediates JNK, but not p38, activation. These data support a notion that the MEK1/2-ERK pathway merges into the JNK pathway (cross talking), but is parallel to the p38 pathway in response to LPA stimulation, leading to LPA-induced TF expression. Furthermore, to test if both the p38 pathway and MEK1/2-ERK-JNK pathway mediate LPA-induced TF expression, we treated MASMCs with the combination of MEK inhibitor U0126 and p38 MAPK inhibitor SB203580 and found an additive effect of both inhibitors on LPA-induced TF expression (Fig. 4, K and L). Taken together, these results suggested a new regulatory paradigm that PKD2-mediated activation of both JNK and p38, along with PKD-independent activation of MEK1/2-ERK-JNK contributes to LPA-induced TF expression in MASMCs.
Figure 4.
Effects of the specific p38 MAPK inhibitor, the specific JNK MAPK inhibitor, and the specific MEK1/2 inhibitor on LPA-induced TF expression in MASMCs. The effect of the specific MEK1/2 inhibitor on LPA-induced phosphorylation of JNK and p38 MAPK was also examined. MASMCs were pretreated with the specific inhibitors for 45 min, then stimulated with LPA for 5 h for detection of TF expression. To determine MAPK phosphorylation, cell lysates of MASMCs stimulated by LPA for 5 min after pretreatment of specific ERK inhibitor were collected. TF expression and MAPK phosphorylation were examined by Western blot analysis. A, effect of various doses of the specific p38 MAPK inhibitor SB203580 on LPA-induced TF expression. B, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗p < 0.05 and ∗∗p < 0.01 versus LPA only group. C, effect of various doses of the specific JNK inhibitor SP600125 on LPA-induced TF expression. D, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗p < 0.05 and ∗∗p < 0.01 versus LPA only group. E, effect of combination of SP600125 and SB203580 on LPA-induced TF expression. F, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗p < 0.05 and ∗∗p < 0.01 versus LPA only group. G, effect of various doses of the specific MEK1/2 inhibitor U0126 on LPA-induced TF expression. H, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗p < 0.05 and ∗∗p < 0.01 versus LPA only group. I, effect of various doses of the specific MEK1/2 inhibitor U0126 on LPA-induced phosphorylation of JNK and p38 MAPK. J, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗∗p< 0.01 versus LPA only group. K, effect of combination of U0126 and SB203580 on LPA-induced TF expression. L, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗p < 0.05 and ∗∗p< 0.01 versus LPA only group.
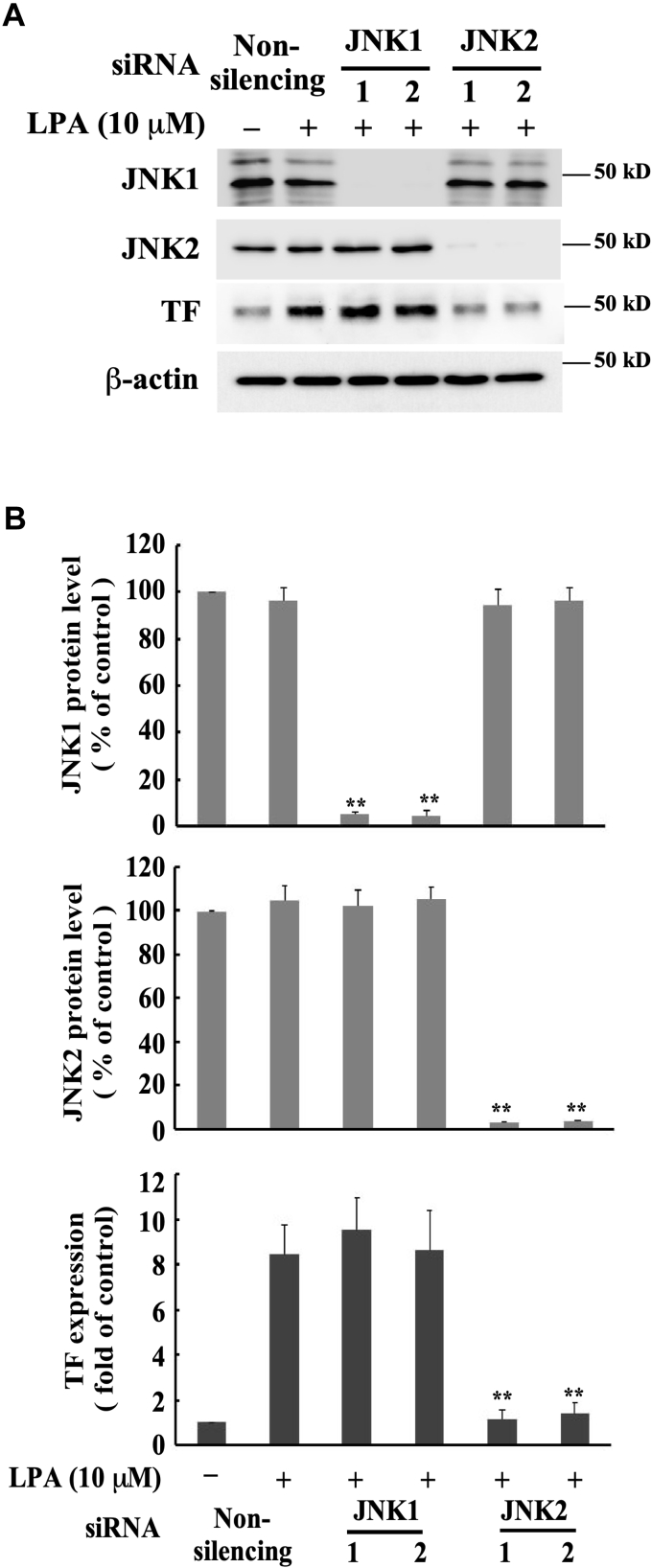
JNK2, but not JNK1, mediates LPA-induced TF expression
We further aimed to identify which JNK isoform mediates LPA-induced TF expression. The JNK family includes three isoforms. JNK1 and JNK2 have broad tissue distributions, while JNK3 is expressed predominantly in CNS neurons (43). Therefore, we tested the role of JNK1 and JNK2 in LPA-induced TF expression by depleting either JNK1 or JNK2 protein expression using specific siRNAs. We observed that depletion of JNK2 protein expression markedly diminished LPA-induced TF expression, while depletion of JNK1 expression had no effect (Fig. 5, A and B), indicating that JNK2, but not JNK1, mediates LPA-induced TF expression in MASMCs.
Figure 5.
JNK2, but not JNK1, mediates LPA-induced TF expression. The specific JNK1 and JNK2 siRNAs (40 nM) were transfected into MASMCs for 48 h followed by serum starvation for 24 h; cells were then stimulated with 10 μM of LPA for 5 h. For each JNK isoform, two siRNAs targeting different sequences were utilized. A, effect of knockdown of the expression of JNK isoforms with the specific JNK1 or JNK2 siRNAs on LPA-induced TF expression. B, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗∗p < 0.01 versus LPA-treated, nonsilencing siRNA group.
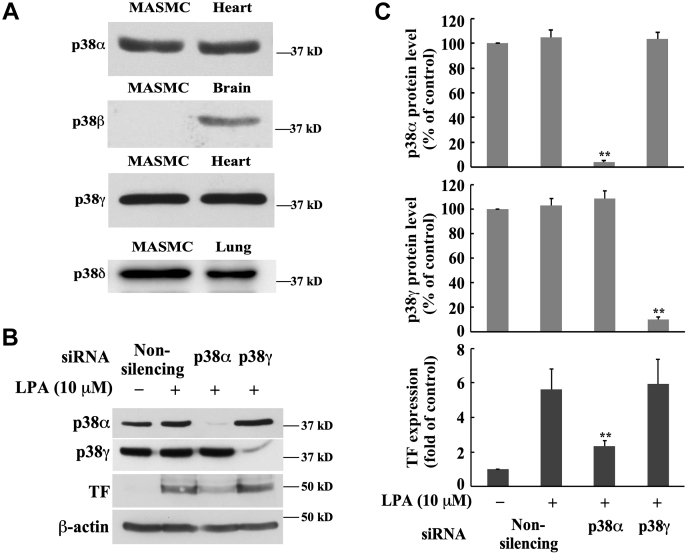
p38α mediates LPA-induced TF expression
Considering that SB 203580 inhibitor dose-dependently blocked LPA-induced TF expression (Fig. 4, A and B), we reasoned that p38α and/or p38β might be involved in TF expression, since it has been reported that SB 203580 inhibitor specifically inhibits the activities of p38α and p38β (44, 45, 46). p38 MAPK family comprises four isoforms: p38α, p38β, p38γ, and p38δ (47). To examine whether p38α and β isoforms are expressed in MASMCs, we detected the protein expression of p38 MAPK isoforms in MASMCs, using specific antibodies against various p38 MAPK isoforms, compared with the positive controls from various mouse organ tissues. We found that p38α, p38γ, and p38δ MAPKs, but not p38β, are expressed in MASMCs (Fig. 6A), suggesting that p38α may mediate TF expression. We then employed the specific siRNA knockdown approach to examine the role of p38α. Specific siRNA against p38α and specific siRNA against p38γ (negative control) were utilized to knock down the protein expression of these two isoforms, and TF protein expression induced by LPA was evaluated. Our data demonstrated that p38γ MAPK had no role in LPA-induced TF expression because the depletion of p38γ protein expression using the specific p38γ siRNA had no effect on LPA-induced TF expression. However, p38α siRNA transfection remarkably reduced LPA-induced TF expression (Fig. 6, B and C). Taken together, these results indicate that p38α MAPK contributes to LPA-induced TF protein expression in MASMCs.
Figure 6.
p38α MAPK mediates LPA-induced TF expression.A, the expression of p38 MAPK isoforms was detected with corresponding antibodies by Western blot analysis. Tissue lysates from mouse heart, brain, and lung were used as positive controls. Twenty microgram protein was loaded to each lane. B, effect of knockdown of the expression of p38 MAPK isoforms with the specific siRNAs on LPA-induced TF expression. The specific p38α and p38γ MAPK siRNAs (40 nM) were transfected into MASMCs for 48 h followed by serum starvation for 24 h; cells were then stimulated with 10 μM of LPA for 5 h. β-actin serves as a loading control. C, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗∗p < 0.01 versus LPA-treated nonsilencing siRNA group.
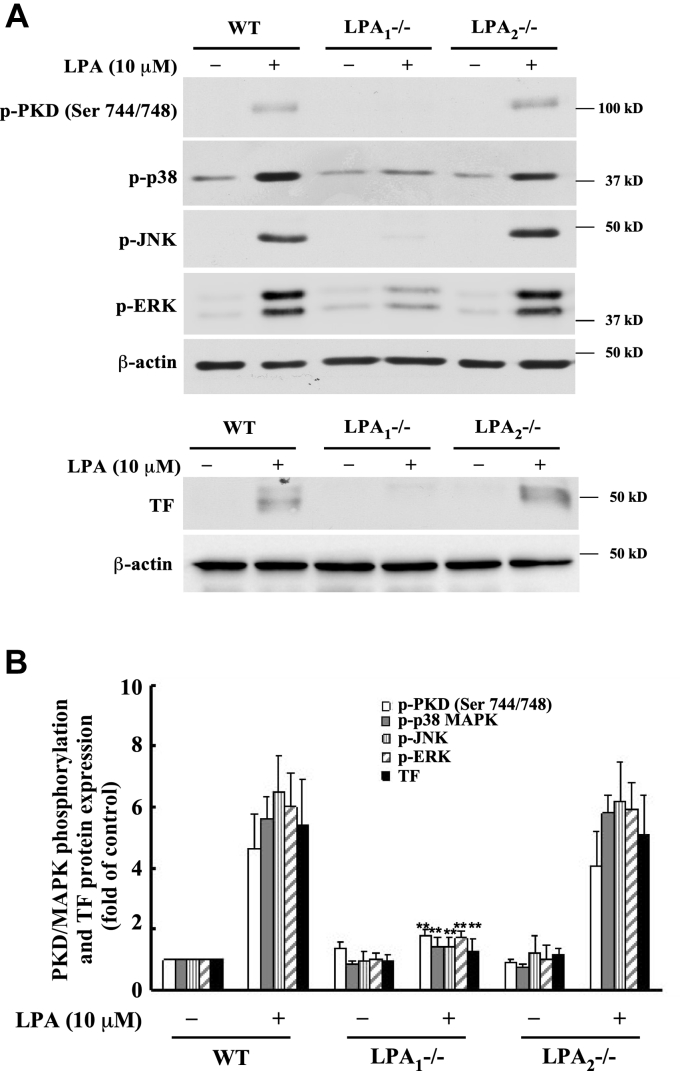
LPA receptor 1 is responsible for LPA induction of PKD phosphorylation, MAPK activation, and TF expression
LPA exerts its function through LPA receptors. Previously we reported LPA receptor 1 (LPA1) and LPA receptor 2 (LPA2) are predominantly expressed in MASMCs (48). We isolated MASMCs from LPA1-/- and LPA2-/- mice and stimulated the cells with LPA. We then detected phosphorylation of PKD, JNK, p38, and ERK MAPKs and expression of TF protein. Our results showed that knockout of LPA1 completely abolished LPA-induced PKD phosphorylation, MAPK phosphorylation, and TF protein expression (Fig. 7, A and B). These results identified that LPA1, but not LPA2, is essential for mediating LPA-induced TF protein expression.
Figure 7.
LPA1is responsible for LPA-induced PKD and MAPK phosphorylation and TF expression.A, wild type (WT), LPA1-/- and LPA2-/- MASMCs were stimulated with 10 μM of LPA for 5 min (kinase activation) or 5 h (TF expression). Phosphorylation of PKD, p38, JNK, and ERK, as well as TF expression, was examined by Western blot analysis. B, results were quantified by densitometry. Data are mean ± SD from three experiments. ∗∗p < 0.01 versus LPA-treated (WT group).
Taken together, our results reveal new pathways leading to LPA induction of TF expression in vascular SMCs, specifically that the receptor LPA1 transduces the LPA signal via PKD2-activated JNK2 and p38α MAPK pathways and regulates TF expression. Our results also suggest that the PKD-independent MEK1/2-ERK pathway merges into the JNK pathway, regulating TF expression.
Discussion
TF-mediated activation of coagulation is critically important for arterial thrombosis in the context of atherosclerotic disease. In addition to its role as the primary initiator of coagulation, TF contributes to cellular inflammation via the generation of downstream coagulation proteases, such as Factor Xa and thrombin. However, the stimuli responsible for TF expression in cells of arterial lesions and the mechanisms of TF induction within the plaque are still largely unknown. LPA has been shown to be a component of oxLDL and to accumulate in human atherosclerotic plaques in vivo (19). LPA levels were increased in culprit coronary arteries in human patients and in experimentally induced mouse atherosclerotic plaques (20, 21). In this study, using SMCs isolated from LPA receptor knockout mice, we identified that LPA receptor 1 (LPA1) is responsible for LPA induction of TF expression in SMCs. Currently there are six bona fide LPA receptors, LPA1-6 (49). We previously identified LPA1 as the key mediator of LPA signaling in SMCs, leading to nuclear early growth response protein 1 (Egr-1) expression and cell migration (37, 48). In a recent in vivo study, we observed LPA1 mediates neointimal formation in mouse carotid arteries in response to LPA stimulation (50). Therefore, LPA1 is essential for mediation of SMC inflammation and vascular lesion formation in response to LPA stimulation.
Regarding the intracellular mediators of LPA induction of TF expression, we previously identified that G-protein αi and the MEK/ERK pathway contribute to TF expression in rat SMCs (18). To explore other possible signaling mediators involved in LPA-induced TF expression, in the current study, we identified the role of PKD in LPA-induced TF expression. Our results revealed PKD as a critical intracellular mediator in LPA-induced TF expression. First, all three isoforms of PKD are expressed in mouse SMCs. Second, LPA induces phosphorylation of all three isoforms of PKD. Third, knockdown of PKD2, but not PKD1 or PKD3, blocks LPA-induced TF expression and TF surface activity. Therefore, our study identifies that PKD2 is an essential regulator of LPA induction of TF expression. Furthermore, our data revealed that PKD2 exerts its regulatory effect on TF expression via p38 and JNK MAPKs. In other words, p38 and JNK MAPKs are indispensable downstream mediators of PKD2. We demonstrated that pharmacological inhibitors of p38 and JNK MAPKs dose-dependently block LPA-induced TF protein expression. Knockdown of PKD2, but not other isoforms of PKD, blocked phosphorylation of p38 and JNK MAPKs, indicating that PKD2-induced p38 and JNK MAPK pathways are essential for LPA-induced TF expression.
The role of specific isoforms of p38 and JNK MAPKs in TF expression has not been previously revealed. p38 MAPK comprises p38α, β, γ, and δ isoforms, while JNK consists of JNK1, 2, and 3 isoforms. Our results revealed that p38α, γ, and δ MAPKs and JNK1 and JNK2 are expressed in MASMCs. Utilizing specific inhibitors and silencing, specific MAPK isoforms helped to identify that p38α and JNK2 are responsible for LPA-induced TF expression in MASMCs. Therefore, for the first time, we documented that p38α and JNK2 are essential mediators of TF protein expression in VSMCs.
PKD family isoforms (PKD1–3) are emerging as important mediators of growth factor, lipid, hormonal, oxidative, and metabolic stress signals. LPA has been reported as one of the inducers triggering PKD activation. In the vascular context, PKD1 was found to play a role in LPA-induced endothelial cell functions. LPA suppression of endothelial cell CD36 expression and promotion of angiogenesis were reported to be mediated by PKD1 (25). PKD1-FoxO1 signaling was demonstrated to mediate LPA-induced CD36 transcription in endothelial cells (26). However, the role of the other isoforms of PKD in both LPA-induced vascular signaling and its consequences has been unknown. In the current study, our results reveal that although three isoforms of PKD (PKD1–3) are expressed in SMCs, it is only PKD2, not the other isoforms of PKD, mediating LPA signaling toward TF expression and surface activity. Furthermore, we identified that LPA-mediated PKD2 activation leads to its downstream p38α and JNK2 MAPK activation, which promotes TF expression in vascular SMCs. Regarding PKD2 and its downstream mediators, our previous study revealed that the PKD2-p38 pathway mediates lysophosphatidylcholine-induced migration of monocytic cells and monocytes (27). PKD2 mediation of PMA-induced ERK1/2 MAPK and NF-κB activation has also been reported (51). Our group previously identified that both PKD1 and PKD2 mediate histamine-induced TF expression in human SMCs: PKD1 via a p38 MAPK-independent pathway and PKD2 via a p38 MAPK-dependent pathway (52). To our knowledge, the current study is the first to demonstrate the regulatory relationship between PKD2 and its specific downstream MAPK targets p38α and JNK2, which mediate TF protein expression.
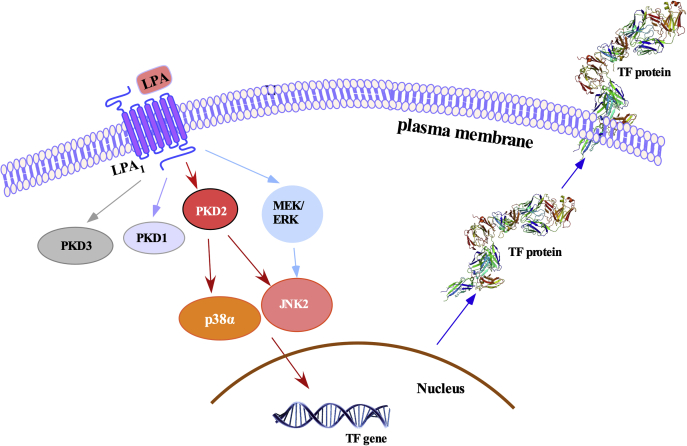
The newly proposed regulatory mechanism of LPA induction of TF in SMCs is illustrated in Figure 8. The current study reveals a new pathway in LPA induction of TF expression, starting with the identification of the specific LPA receptor LPA1 and the PKD2-mediated p38 and JNK pathways. The novel finding is the identification that the specific isoforms p38α and JNK2 function in both the PKD2 pathway and TF expression. The previously reported MEK/ERK pathway (18) is speculated to merge into the JNK pathway because our experimental data shows that inhibition of MEK1/2 activity with U0126 blocked JNK activation (Fig. 4, I and J) in MASMCs and rat SMCs (37). These data suggest a new paradigm that the PKD-dependent JNK and p38 MAPK pathways and the PKD-independent MEK1/2-ERK-JNK pathway mediate LPA-induced TF expression in MASMCs. We speculate that LPA-PKD2 pathway-mediated TF protein expression and activity are likely due to induction of TF gene transcription, as we previously demonstrated that LPA induction of TF is controlled at the transcriptional level in rat SMCs (18). Because of the increased levels of LPA in culprit coronary arteries in human patients and the significant role of TF in atherothrombosis, the discovered new mechanism will contribute to elucidating LPA signaling, TF expression, and atherothrombosis.
Figure 8.
Summary illustration of the signaling pathways for LPA induction of TF expression in SMCs. LPA initiates its signaling pathways through LPA1 and activates intracellular PKDs. Activation of PKD2 further activates downstream p38α and JNK2, leading to induction of TF expression. The previous reported LPA-induced MEK1/2-ERK pathway (18) is found to be PKD-independent, but cross talks with JNK, contributing to TF expression.
Experimental procedures
Reagents
MAPK inhibitors SB203580, SP600125, and U0126 were from Enzo life Science. Antibodies against PKD1, PKD3, p38α MAPK, phospho-PKD-activation loop (Ser744/748 in mouse PKD1), phospho-PKD1 C termini (Ser 916), phospho-ERK, phospho-p38 MAPK, and phospho-JNK were from Cell Signaling Technology. Antibody against PKD2 was from Bethyl Lab. Antibody against β-actin was from Sigma-Aldrich. Antibody against p38γ was from EMD Millipore. Antibody against p38δ MAPK was from R&D systems. Antibodies against mouse TF and p38β were from Santa Cruz. TF surface activity assay kit was from American Diagnostica. Nonsilencing control siRNA, mouse PKD1 siRNA, PKD2 siRNA, PKD3 siRNA, JNK1 siRNA, JNK2 siRNA, p38α siRNA, and p38γ siRNA were from Qiagen. The siRNA transfection reagent RNAi MAX was from Invitrogen.
Cell culture
All animal care and use procedures were performed under a protocol approved by the Institutional Animal Care and Use Committee at the University of Texas Permian Basin. MASMCs were isolated from C57BL/6J mice and cultured in DMEM (Corning) with 10% fetal bovine serum. MASMCs of passages 6–8 were starved in serum-free media for 24 h prior to 10 μM of LPA stimulation. To detect LPA induction of TF protein, cells were stimulated with LPA for 5 h; for detection of PKD and MAPK phosphorylation, starved cells were stimulated with LPA for indicated times.
Western blot analysis
MASMCs were rinsed with cold phosphate-buffered saline (PBS) and lysed in lysis buffer (50 mM Tris-HCl, pH 6.8, 8 M urea, 5% mercaptoethanol, 2% SDS, 1 mM PMSF, 1X protease inhibitors cocktail [Roche], 20 mM NaF, 1 mM Na3VO4) with sonication for 20 s on ice. Cellular proteins were separated by 10% SDS–polyacrylamide gel electrophoresis and were transferred to a polyvinylidene fluoride membrane (Immobilon-P, Millipore-Sigma). The membranes were then probed with the specific antibodies, and the specific protein bands were visualized by ECL-Plus (GE Healthcare). Blot images were taken by ChemiDoc imaging system (Bio-Rad).
siRNA transfection
Nonsilencing siRNA, PKD1, PKD2 and PKD3 siRNAs, JNK1, and JNK2 siRNAs and p38α and p38γ siRNAs were transfected into MASMCs using the RNAi MAX transfection reagent (Invitrogen) according to the manufacturer's instructions. Briefly, siRNAs were mixed with opti-MEM (Gibco) for 5 min, then mixed with RNAi MAX transfection reagent for 20 min. MSAMCs resuspended in DMEM/10% FBS were planted with mixed siRNA-RNAi MAX reagent into cell culture dishes. Final concentration of siRNAs was 40 nM. Nonsilencing siRNA was used as a negative control. Forty-eight hours after transfection, the cells were starved for 24 h followed by treatment with or without LPA.
TF surface activity assay
Cell-surface TF activity was measured using the Actochrome TF activity assay kit purchased from American Diagnostica. Briefly, starved MASMCs were stimulated with LPA (10 μM) for 5 h. The cells were washed twice with PBS. Assay buffer (300 μl, pH 8.4), 25 μl of factor VIIa, and 25 μl of factor X were added to 12-well cell culture plates, and the plates were stirred on an orbital rotator for 15 min at 37 °C. Then 25 μl of Spectrozyme factor Xa substrate was added and incubated at 37 °C for 20 min with constant stirring. Aliquots of the reaction mixture were pipetted into 96-well plates and read along with the standards provided by American Diagnostica on a Universal microplate reader Synergy HT (Bio-TEK Instruments) at 405 nm.
Data analysis
All data are representative of a minimum of three experiments. Results are expressed as mean ± standard deviation (SD). Comparisons between multiple groups were performed using one-way analysis of variance with post-hoc Dunnett t tests. A single comparison analysis was made using two-tailed, unpaired Student t tests. A p value of 0.05 was considered statistically significant.
Data availability
All data are contained within the article.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are grateful to Dr Jerold Chun, Sanford Burnham Prebys Medical Discovery Institute, for providing LPA1−/− and LPA2−/− mice. This work was supported by the University of Texas System STARs awards.
Author contributions
F. H., Q. L., and M.-Z. C., conceptualization; F. H., Q. L., F. Z., X. X., and M.-Z. C., data curation; F. H., Q. L., X. X., and M.-Z. C., formal analysis; M.-Z. C., funding acquisition; F. H., Q. L., X. X., and M.-Z. C., investigation; F. H., Q. L., F. Z., J. D., A. D., X. X., and M.-Z. C., methodology; F. H., Q. L., and M.-Z. C., project administration; F. H., Q. L., and M.-Z. C., validation; F. H., Q. L., and M.-Z. C., writing–original draft; F. H., Q. L., A. D., X. X., and M.-Z. C., writing–review and editing.
Funding and additional information
This work was supported by National Institutes of Health Grants HL107466 and HL153529 (to M.-Z. C.) and NS095256 (to X. X.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Edited by Henrik Dohlman
Footnotes
Present address for Jiaxin Du: Changchun Medical College, Changchun, China.
References
- 1.Nemerson Y. The phospholipid requirement of tissue factor in blood coagulation. J. Clin. Invest. 1968;47:72–80. doi: 10.1172/JCI105716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach R.R. Initiation of coagulation by tissue factor. CRC Crit. Rev. Biochem. 1988;23:339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 3.Golino P., Cirillo P., Calabro P., Ragni M., D'Andrea D., Avvedimento E.V., Vigorito F., Corcione N., Loffredo F., Chiariello M. Expression of exogenous tissue factor pathway inhibitor in vivo suppresses thrombus formation in injured rabbit carotid arteries. J. Am. Coll. Cardiol. 2001;38:569–576. doi: 10.1016/s0735-1097(01)01350-x. [DOI] [PubMed] [Google Scholar]
- 4.Golino P. The inhibitors of the tissue factor:factor VII pathway. Thromb. Res. 2002;106:V257–265. doi: 10.1016/s0049-3848(02)00079-8. [DOI] [PubMed] [Google Scholar]
- 5.Giesen P.L., Rauch U., Bohrmann B., Kling D., Roque M., Fallon J.T., Badimon J.J., Himber J., Riederer M.A., Nemerson Y. Blood-borne tissue factor: Another view of thrombosis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2311–2315. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Himber J., Kirchhofer D., Riederer M., Tschopp T.B., Steiner B., Roux S.P. Dissociation of antithrombotic effect and bleeding time prolongation in rabbits by inhibiting tissue factor function. Thromb. Haemost. 1997;78:1142–1149. [PubMed] [Google Scholar]
- 7.Sato Y., Asada Y., Marutsuka K., Hatakeyama K., Sumiyoshi A. Tissue factor induces migration of cultured aortic smooth muscle cells. Thromb. Haemost. 1996;75:389–392. [PubMed] [Google Scholar]
- 8.Cirillo P., Cali G., Golino P., Calabro P., Forte L., De Rosa S., Pacileo M., Ragni M., Scopacasa F., Nitsch L., Chiariello M. Tissue factor binding of activated factor VII triggers smooth muscle cell proliferation via extracellular signal-regulated kinase activation. Circulation. 2004;109:2911–2916. doi: 10.1161/01.CIR.0000129312.43547.08. [DOI] [PubMed] [Google Scholar]
- 9.Marmur J.D., Rossikhina M., Guha A., Fyfe B., Friedrich V., Mendlowitz M., Nemerson Y., Taubman M.B. Tissue factor is rapidly induced in arterial smooth muscle after balloon injury. J. Clin. Invest. 1993;91:2253–2259. doi: 10.1172/JCI116452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox J.N., Smith K.M., Schwartz S.M., Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. U. S. A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiruvikraman S.V., Guha A., Roboz J., Taubman M.B., Nemerson Y., Fallon J.T. In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X [published erratum appears in Lab Invest 1997 Feb;76(2):297-9] Lab. Invest. 1996;75:451–461. [PubMed] [Google Scholar]
- 12.Annex B.H., Denning S.M., Channon K.M., Sketch M.H., Jr., Stack R.S., Morrissey J.H., Peters K.G. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation. 1995;91:619–622. doi: 10.1161/01.cir.91.3.619. [DOI] [PubMed] [Google Scholar]
- 13.Grover S.P., Mackman N. Tissue factor in atherosclerosis and atherothrombosis. Atherosclerosis. 2020;307:80–86. doi: 10.1016/j.atherosclerosis.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Moreno P.R., Bernardi V.H., Lopez-Cuellar J., Murcia A.M., Palacios I.F., Gold H.K., Mehran R., Sharma S.K., Nemerson Y., Fuster V., Fallon J.T. Macrophages, smooth muscle cells, and tissue factor in unstable angina. Implications for cell-mediated thrombogenicity in acute coronary syndromes. Circulation. 1996;94:3090–3097. doi: 10.1161/01.cir.94.12.3090. [DOI] [PubMed] [Google Scholar]
- 15.Marmur J.D., Thiruvikraman S.V., Fyfe B.S., Guha A., Sharma S.K., Ambrose J.A., Fallon J.T., Nemerson Y., Taubman M.B. Identification of active tissue factor in human coronary atheroma. Circulation. 1996;94:1226–1232. doi: 10.1161/01.cir.94.6.1226. [DOI] [PubMed] [Google Scholar]
- 16.Ardissino D., Merlini P.A., Ariens R., Coppola R., Bramucci E., Mannucci P.M. Tissue-factor antigen and activity in human coronary atherosclerotic plaques. Lancet. 1997;349:769–771. doi: 10.1016/S0140-6736(96)11189-2. [DOI] [PubMed] [Google Scholar]
- 17.Cui M.Z., Penn M.S., Chisolm G.M. Native and oxidized low density lipoprotein induction of tissue factor gene expression in smooth muscle cells is mediated by both Egr-1 and Sp1. J. Biol. Chem. 1999;274:32795–32802. doi: 10.1074/jbc.274.46.32795. [DOI] [PubMed] [Google Scholar]
- 18.Cui M.Z., Zhao G., Winokur A.L., Laag E., Bydash J.R., Penn M.S., Chisolm G.M., Xu X. Lysophosphatidic acid induction of tissue factor expression in aortic smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:224–230. doi: 10.1161/01.atv.0000054660.61191.7d. [DOI] [PubMed] [Google Scholar]
- 19.Siess W., Zangl K.J., Essler M., Bauer M., Brandl R., Corrinth C., Bittman R., Tigyi G., Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dohi T., Miyauchi K., Ohkawa R., Nakamura K., Kurano M., Kishimoto T., Yanagisawa N., Ogita M., Miyazaki T., Nishino A., Yaginuma K., Tamura H., Kojima T., Yokoyama K., Kurata T. Increased lysophosphatidic acid levels in culprit coronary arteries of patients with acute coronary syndrome. Atherosclerosis. 2013;229:192–197. doi: 10.1016/j.atherosclerosis.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 21.Bot M., de Jager S.C., MacAleese L., Lagraauw H.M., van Berkel T.J., Quax P.H., Kuiper J., Heeren R.M., Biessen E.A., Bot I. Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation. J. Lipid Res. 2013;54:1265–1274. doi: 10.1194/jlr.M032862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraemer M.P., Mao G., Hammill C., Yan B., Li Y., Onono F., Smyth S.S., Morris A.J. Effects of diet and hyperlipidemia on levels and distribution of circulating lysophosphatidic acid. J. Lipid Res. 2019;60:1818–1828. doi: 10.1194/jlr.M093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozengurt E., Rey O., Waldron R.T. Protein kinase D signaling. J. Biol. Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Ruiloba L., Cabrera-Poch N., Rodriguez-Martinez M., Lopez-Menendez C., Jean-Mairet R.M., Higuero A.M., Iglesias T. Protein kinase D intracellular localization and activity control kinase D-interacting substrate of 220-kDa traffic through a postsynaptic density-95/discs large/zonula occludens-1-binding motif. J. Biol. Chem. 2006;281:18888–18900. doi: 10.1074/jbc.M603044200. [DOI] [PubMed] [Google Scholar]
- 25.Ren B., Hale J., Srikanthan S., Silverstein R.L. Lysophosphatidic acid suppresses endothelial cell CD36 expression and promotes angiogenesis via a PKD-1-dependent signaling pathway. Blood. 2011;117:6036–6045. doi: 10.1182/blood-2010-12-326017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren B., Best B., Ramakrishnan D.P., Walcott B.P., Storz P., Silverstein R.L. LPA/PKD-1-FoxO1 signaling Axis mediates endothelial cell CD36 transcriptional repression and proangiogenic and proarteriogenic reprogramming. Arterioscler. Thromb. Vasc. Biol. 2016;36:1197–1208. doi: 10.1161/ATVBAHA.116.307421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan M., Hao F., Xu X., Chisolm G.M., Cui M.Z. Lysophosphatidylcholine activates a novel PKD2-mediated signaling pathway that controls monocyte migration. Arterioscler. Thromb. Vasc. Biol. 2009;29:1376–1382. doi: 10.1161/ATVBAHA.109.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews S.A., Navarro M.N., Sinclair L.V., Emslie E., Feijoo-Carnero C., Cantrell D.A. Unique functions for protein kinase D1 and protein kinase D2 in mammalian cells. Biochem. J. 2010;432:153–163. doi: 10.1042/BJ20101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews S.A., Rozengurt E., Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/Protein kinase Cmu. J. Biol. Chem. 1999;274:26543–26549. doi: 10.1074/jbc.274.37.26543. [DOI] [PubMed] [Google Scholar]
- 30.Rykx A., De Kimpe L., Mikhalap S., Vantus T., Seufferlein T., Vandenheede J.R., Van Lint J. Protein kinase D: A family affair. FEBS Lett. 2003;546:81–86. doi: 10.1016/s0014-5793(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 31.Durand N., Borges S., Storz P. Protein kinase D enzymes as regulators of EMT and cancer cell invasion. J. Clin. Med. 2016;5 doi: 10.3390/jcm5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X., Yang X.Y., Wang N.D., Ding C., Yang Y.J., You Z.J., Su Q., Chen J.H. Serum lysophosphatidic acid concentrations measured by dot immunogold filtration assay in patients with acute myocardial infarction. Scand. J. Clin. Lab. Invest. 2003;63:497–503. doi: 10.1080/00365510310003265. [DOI] [PubMed] [Google Scholar]
- 33.Faure M., Bourne H.R. Differential effects on cAMP on the MAP kinase cascade: Evidence for a cAMP-insensitive step that can bypass Raf-1. Mol. Biol. Cell. 1995;6:1025–1035. doi: 10.1091/mbc.6.8.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seufferlein T., Withers D.J., Mann D., Rozengurt E. Dissociation of mitogen-activated protein kinase activation from p125 focal adhesion kinase tyrosine phosphorylation in Swiss 3T3 cells stimulated by bombesin, lysophosphatidic acid, and platelet-derived growth factor. Mol. Biol. Cell. 1996;7:1865–1875. doi: 10.1091/mbc.7.12.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao F., Tan M., Xu X., Han J., Miller D.D., Tigyi G., Cui M.Z. Lysophosphatidic acid induces prostate cancer PC3 cell migration via activation of LPA(1), p42 and p38alpha. Biochim. Biophys. Acta. 2007;1771:883–892. doi: 10.1016/j.bbalip.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao F., Tan M., Wu D.D., Xu X., Cui M.Z. LPA induces IL-6 secretion from aortic smooth muscle cells via an LPA1-regulated, PKC-dependent, and p38alpha-mediated pathway. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H974–983. doi: 10.1152/ajpheart.00895.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iyoda T., Zhang F., Sun L., Hao F., Schmitz-Peiffer C., Xu X., Cui M.Z. Lysophosphatidic acid induces early growth response-1 (Egr-1) protein expression via protein kinase Cdelta-regulated extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) activation in vascular smooth muscle cells. J. Biol. Chem. 2012;287:22635–22642. doi: 10.1074/jbc.M111.335695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steffel J., Akhmedov A., Greutert H., Luscher T.F., Tanner F.C. Histamine induces tissue factor expression: Implications for acute coronary syndromes. Circulation. 2005;112:341–349. doi: 10.1161/CIRCULATIONAHA.105.553735. [DOI] [PubMed] [Google Scholar]
- 39.Xuereb J.M., Sie P., Boneu B., Constans J. Inhibition of tissue factor synthesis by disruption of ERK kinases and PKC signaling pathways in human vascular SMCs. Thromb. Haemost. 2000;84:129–136. [PubMed] [Google Scholar]
- 40.Nishibe T., Parry G., Ishida A., Aziz S., Murray J., Patel Y., Rahman S., Strand K., Saito K., Saito Y., Hammond W.P., Savidge G.F., Mackman N., Wijelath E.S. Oncostatin M promotes biphasic tissue factor expression in smooth muscle cells: Evidence for Erk-1/2 activation. Blood. 2001;97:692–699. doi: 10.1182/blood.v97.3.692. [DOI] [PubMed] [Google Scholar]
- 41.Samuel W., Kutty R.K., Sekhar S., Vijayasarathy C., Wiggert B., Redmond T.M. Mitogen-activated protein kinase pathway mediates N-(4-hydroxyphenyl)retinamide-induced neuronal differentiation in the ARPE-19 human retinal pigment epithelial cell line. J. Neurochem. 2008;106:591–602. doi: 10.1111/j.1471-4159.2008.05409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arthur J.S., Ley S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A., Singh U.K., Kini S.G., Garg V., Agrawal S., Tomar P.K., Pathak P., Chaudhary A., Gupta P., Malik A. JNK pathway signaling: A novel and smarter therapeutic targets for various biological diseases. Future Med. Chem. 2015;7:2065–2086. doi: 10.4155/fmc.15.132. [DOI] [PubMed] [Google Scholar]
- 44.Cuenda A., Rouse J., Doza Y.N., Meier R., Cohen P., Gallagher T.F., Young P.R., Lee J.C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S., McDonnell P.C., Gum R.J., Hand A.T., Lee J.C., Young P.R. Novel homologues of CSBP/p38 MAP kinase: Activation, substrate specificity and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 1997;235:533–538. doi: 10.1006/bbrc.1997.6849. [DOI] [PubMed] [Google Scholar]
- 46.Goedert M., Cuenda A., Craxton M., Jakes R., Cohen P. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 1997;16:3563–3571. doi: 10.1093/emboj/16.12.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pramanik R., Qi X., Borowicz S., Choubey D., Schultz R.M., Han J., Chen G. p38 isoforms have opposite effects on AP-1-dependent transcription through regulation of c-Jun. The determinant roles of the isoforms in the p38 MAPK signal specificity. J. Biol. Chem. 2003;278:4831–4839. doi: 10.1074/jbc.M207732200. [DOI] [PubMed] [Google Scholar]
- 48.Wu D.D., Zhang F., Hao F., Chun J., Xu X., Cui M.Z. Matricellular protein Cyr61 bridges lysophosphatidic acid and integrin pathways leading to cell migration. J. Biol. Chem. 2014;289:5774–5783. doi: 10.1074/jbc.M113.533042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blaho V.A., Chun J. ‘Crystal’ clear? Lysophospholipid receptor structure insights and controversies. Trends Pharmacol. Sci. 2018;39:953–966. doi: 10.1016/j.tips.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao F., Zhang F., Wu D.D., An D., Shi J., Li G., Xu X., Cui M.Z. Lysophosphatidic acid-induced vascular neointimal formation in mouse carotid arteries is mediated by the matricellular protein CCN1/Cyr61. Am. J. Physiol. Cell Physiol. 2016;311:C975–C984. doi: 10.1152/ajpcell.00227.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J., Giridhar K.V., Zhang L., Xu S., Wang Q.J. A protein kinase C/protein kinase D pathway protects LNCaP prostate cancer cells from phorbol ester-induced apoptosis by promoting ERK1/2 and NF-{kappa}B activities. Carcinogenesis. 2011;32:1198–1206. doi: 10.1093/carcin/bgr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao F., Wu D.D., Xu X., Cui M.Z. Histamine induces activation of protein kinase D that mediates tissue factor expression and activity in human aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H1344–1352. doi: 10.1152/ajpheart.00500.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article.