Abstract
Background
Interleukin-1-dependent increases in glycolysis promote allergic airways disease in mice and disruption of pyruvate kinase M2 (PKM2) activity is critical herein. Glutathione-S-transferase P (GSTP) has been implicated in asthma pathogenesis and regulates the oxidation state of proteins via S-glutathionylation. We addressed whether GSTP-dependent S-glutathionylation promotes allergic airways disease by promoting glycolytic reprogramming and whether it involves the disruption of PKM2.
Methods
We used house dust mite (HDM) or interleukin-1β in C57BL6/NJ WT or mice that lack GSTP. Airway basal cells were stimulated with interleukin-1β and the selective GSTP inhibitor, TLK199. GSTP and PKM2 were evaluated in sputum samples of asthmatics and healthy controls and incorporated analysis of the U-BIOPRED severe asthma cohort database.
Results
Ablation of Gstp decreased total S-glutathionylation and attenuated HDM-induced allergic airways disease and interleukin-1β-mediated inflammation. Gstp deletion or inhibition by TLK199 decreased the interleukin-1β-stimulated secretion of pro-inflammatory mediators and lactate by epithelial cells. 13C-glucose metabolomics showed decreased glycolysis flux at the pyruvate kinase step in response to TLK199. GSTP and PKM2 levels were increased in BAL of HDM-exposed mice as well as in sputum of asthmatics compared to controls. Sputum proteomics and transcriptomics revealed strong correlations between GSTP, PKM2, and the glycolysis pathway in asthma.
Conclusions
GSTP contributes to the pathogenesis of allergic airways disease in association with enhanced glycolysis and oxidative disruption of PKM2. Our findings also suggest a PKM2-GSTP-glycolysis signature in asthma that is associated with severe disease.
Keywords: Allergic airways disease, House dust mite, Interleukin-1β, S-glutathionylation, Thymic stromal lymphopoietin
Abbreviations
- GSH:
Glutathione
- GST-π/GSTP:
Glutathione S-transferase P
- Glrx:
Glutaredoxin
- AHR:
Airways hyperresponsiveness
- HDM:
House dust mite
- PSSG:
S-Glutathionylation
- IL-1β:
Interleukin 1β
- PKM2:
Pyruvate kinase M2
- NADPH:
Nicotinamide-adenine-dinucleotide phosphate
- WT:
Wild-type
- MTE cells:
Mouse tracheal epithelial cells
- BSA:
Bovine serum albumin
- BALF:
Bronchoalveolar lavage fluid
- HK2:
Hexokinase II
- GAPDH:
Glyceraldehyde 3-phosphate dehydrogenase
- α-SMA:
Alpha smooth muscle actin
- TSLP:
Thymic stromal lymphopoietin
- GM-CSF:
Granulocyte-macrophage colony-stimulating factor
- CCL20:
Chemokine (C-C motif) ligand 20
- CXCL1:
Chemokine (C-X-C motif) ligand 1
- IKKε:
Inhibitory kappa B kinase epsilon
- STAT3:
Signal transducer and activator of transcription 3
1. Introduction
Asthma is a chronic inflammatory disease characterized by airway hyperresponsiveness and remodeling [1], processes that are energy-demanding. We recently demonstrated that changes in cellular metabolism, notably increases in glycolysis occur in allergic airways disease in association with interleukin-1 (IL-1)-signaling, and that increases in airway lactate accompany neutrophilic and steroid-resistant asthma [2,3]. The last, rate-limiting step of glycolysis is the conversion of phosphoenolpyruvate (PEP) to pyruvate, catalyzed by pyruvate kinase M (PKM). The PKM gene consists of two isozymes, PKM2 and PKM1. While PKM1 is constitutively active as a glycolysis kinase and occurs as a tetramer with high binding affinity for the PEP substrate, PKM2 can also exist as dimers or monomers, which have low binding affinity for PEP and have non-canonical alternative functions important in promoting glycolytic reprogramming and inflammation [[4], [5], [6]]. Notably, dimeric PKM2 can translocate into the nucleus and increase the expression of pro-inflammatory cytokines in a signal transducer and activator of transcription 3 (STAT3)- or hypoxia-inducible factor 1-alpha (HIF1α)-dependent manner, thereby enhancing immune cell activation [7,8], including the development of Th17 cells [9,10]. We recently showed that PKM2 is increased in nasal epithelial cells from subjects with asthma as compared to controls [2]. Furthermore, an allosteric activator of PKM2 that stabilizes the tetramer and enhances its canonical glycolysis activity decreased allergic airways disease in mice along with diminished activation of STAT3 [3]. PKM2 can be regulated by a number of post-translational modifications including phosphorylation, acetylation, methylation, and oxidation [[11], [12], [13], [14], [15]]. Oxidation of PKM2 inhibits the tetramer form and glycolytic activity [14,16]. It remains unclear whether oxidation of PKM2 occurs in asthma and how this oxidative process is regulated.
One type of protein oxidation that is a key regulator of (patho)physiological processes is S-glutathionylation (PSSG), the conjugation of glutathione (GSH) to cysteine residues in proteins [17]. GSH-adducts affect protein structure and function [18]. Glutathione S-transferase P (GSTP) is well-known for its role in phase II drug metabolism, but recent studies have shown that GSTP is also a catalyst for PSSG [19,20]. Importantly, GSTP has already been strongly linked to asthma, as polymorphisms in the GSTP gene have been associated with altered susceptibility to asthma development [21,22], with some conflicting reports [23] and an unclear mechanism of action. It remains uncertain whether GSTP contributes to asthma pathophysiology as a catalyst of PSSG and whether this is linked to glycolytic reprogramming. In the present study, we utilized a house dust mite (HDM) mouse model of allergic airway disease or IL-1β-induced acute inflammation in conjunction with airway basal cells that globally lack GSTP or a clinically relevant inhibitor of GSTP. We also evaluated sputum samples and data from the U-BIOPRED severe asthma cohort. Our studies show that GSTP contributes to allergic airways disease in association with enhanced glycolysis, and oxidation and disruption of PKM2. We also demonstrate correlations between GSTP, PKM2 and glycolysis in sputum proteomics and transcriptomics of the U-BIOPRED cohort. These collective observations reveal a putative PKM2-GSTP-glycolysis signature in people with severe asthma and shed insights into the various mechanism whereby GSTP may promote asthma.
2. Methods
2.1. Participant characteristics
Study participants were enrolled at the asthma clinic in CHU Liège (Belgium) (n=40). Healthy subjects were recruited at the hospital and University of Liège, Belgium (n=20). The demographic and functional characteristics of all subjects used in this study are shown in Table S1. The study was approved by the local ethics committee at the University of Liège, Belgium (reference 2005/181). Informed consent was obtained from all participants. Sputum was induced and processed as previously described [2,24,25].
The U-BIOPRED cohort consisted of severe non-smoking asthma (SA.ns), current/ex-smokers with severe asthma (SAs), mild/moderate non-smoking subjects with asthma (MMA) and non-smoking healthy volunteers (HV) [26]. Sputum cells and brushings of the lower airways were obtained as previously described [26]. Sputum cells were studied by the SOMAscan proteomic assay [27,28]. The study was approved by the ethics committee for each participating clinical institution, and adhered to the standards set by International Conference on Harmonisation and Good Clinical Practice, and is registered on ClinicalTrials.gov (NCT01976767). All participants gave written and signed informed consent.
2.2. Mouse studies
Age-matched 8–12 weeks old wild-type (WT) C57BL6/NJ mice (The Jackson Laboratory, Bar Harbor, ME) or global Gstp−/− mice (deficient in Gstp1 and Gstp2) were bred at the University of Vermont. All animal experiments were approved by the Institutional Animal Care and Use Committee. Allergic airways disease was induced as described in Fig. S1A with intranasal administrations of 10 μg HDM protein [2].
In separate experiments, WT and Gstp−/− mice received an intranasal administration of 1 μg of IL-1β (R&D Systems). Control groups received 0.1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), which served as the vehicle for IL-1β. Mice were harvested 6 or 24 h after IL-1β administration.
2.3. Cell experiments
Primary mouse tracheal epithelial (MTE) cells were isolated from C57BL6/NJ WT or Gstp−/− tracheas and cultured as previously described [29]. MTE cells were grown to confluency on 6- or 12-wells transwell inserts (Corning, Corning, NY). Cells were starved overnight in plain DMEM/F12 phenol red free media supplemented with penicillin (50U/mL)-streptomycin (50 μg/mL (P/S) and treated with 1 ng/ml of IL-1β (R&D Systems) at indicated timepoints.
WT MTE cells were also pre-treated with 50 μM of TLK199 (Sigma-Aldrich) for 1 h prior to stimulation with 1 ng/ml of IL-1β (R&D Systems) and re-treated with 50 μM TLK199 8 h post IL-1β treatment. TLK199 was dissolved in DMSO (0.05%) and IL-1β in 0.1% BSA in PBS. MTE cells were treated at the apical and basolateral side. Basolateral medium was collected to assess pro-inflammatory cytokine and lactate levels, and cells were harvested for protein and biochemical analyses.
2.4. Statistical analysis
Cell experiments were performed at least three times with n=3/group/experiment. Significant differences between groups were evaluated by two-way ANOVA with a Tukey post-hoc test for multiple comparison using GraphPad Prism software 8.0 (GraphPad, Inc., La Jolla, CA), unless otherwise noted. Values were considered statistically significant when p < 0.05. Data are shown as means ± S.E.M.
Additional details are provided in the online supplement.
3. Results
3.1. GSTP expression is predominant in the airway epithelium
To investigate the role of GSTP in the pathogenesis of allergic airways disease, C57BL6/NJ WT and Gstp−/− mice were sensitized and challenged with HDM (Figs. S1A–C). Immunofluorescence staining confirmed that GSTP was mainly expressed in the airway epithelium in saline- and HDM-exposed lung tissues (Fig. S1C) confirming a prior report [30]. In contrast to GSTP protein levels which did not change, Gstp mRNA expression decreased in lungs of WT mice subjected to HDM compared to saline controls (Fig. S1D). mRNA expression levels of Gstm and Gstt were also decreased in HDM-exposed mouse lungs compared to saline controls, while Gsto was unaffected. No compensatory changes in mRNA expression of other Gst genes occurred in the lungs of WT or Gstp−/− mice compared with their respective exposure groups, with the exception of a decrease in the mRNA levels of Gstt in HDM-exposed Gstp−/− mice compared to respective WT mice (Fig. S1D).
3.2. Genetic ablation of Gstp attenuates features of HDM-induced allergic airways disease
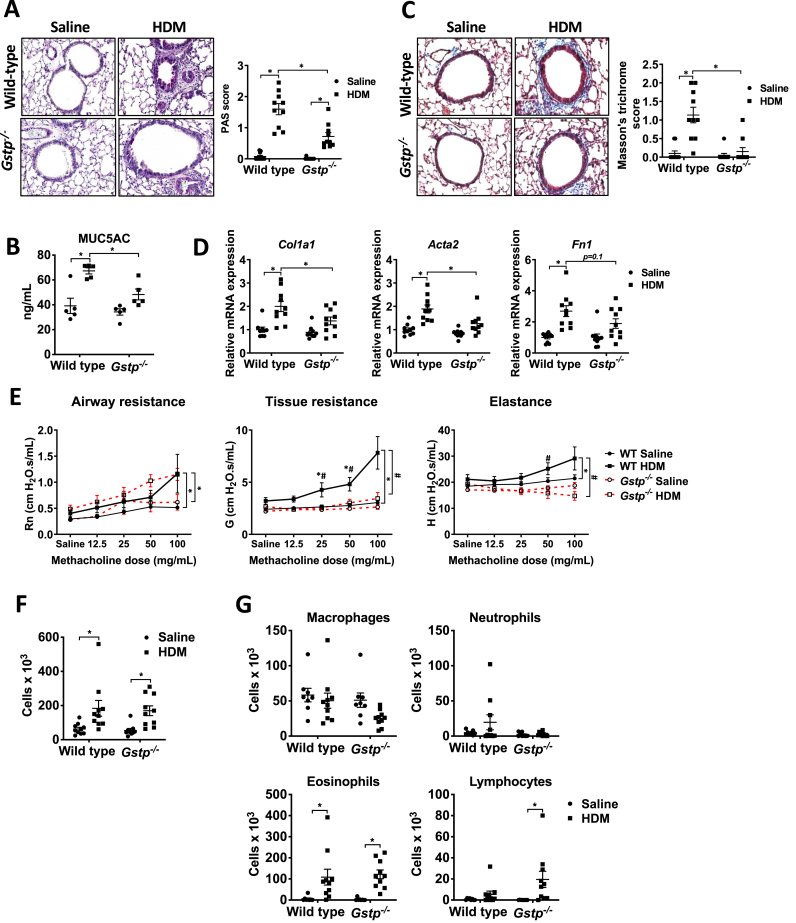
We next assessed whether ablation of Gstp affected HDM-induced airway remodeling or airway hyperresponsiveness (AHR). Gstp ablation diminished HDM-mediated increases in mucus metaplasia, MUC5AC levels in the bronchoalveolar lavage fluid (BALF), and subepithelial collagen deposition, and attenuated mRNA expression of collagen 1a1 (Col1a1), and alpha smooth muscle actin (Acta2) compared to WT counterparts, with trends towards decreases in fibronectin 1 (Fn1) mRNA (Fig. 1A–D). Moreover, HDM-mediated increases in AHR were attenuated in Gstp−/− mice, notably in the tissue resistance and elastance parameters (Fig. 1E). WT and Gstp−/− mice showed similar increases in BAL cell counts in response to HDM compared to the saline controls (Fig. 1F), reflected mainly by increases in eosinophils (Fig. 1G). Together, these findings show that GSTP promotes mucus metaplasia, subepithelial collagen, and AHR, without eliciting a major impact on airway inflammatory cells in response to HDM.
Fig. 1.
Genetic ablation of Gstp attenuates mucus metaplasia, subepithelial collagen, markers of airway remodeling, and airway hyperresponsiveness in mice with HDM-induced allergic airway disease. A, Assessment and quantification of mucus metaplasia by PAS staining intensity (original magnification, ×200). B, MUC5AC protein content in BAL fluid. n=5 per group. C, Assessment and quantification of collagen deposition by Masson's trichrome staining (original magnification, ×200). D, mRNA expression of Col1a1, Fn1, and Acta2 (α-smooth muscle actin) normalized to Ppia.E, Assessment of airway responsiveness to inhaled methacholine using a Flexivent small animal ventilator. F, Total, and G, Differential cell counts in BAL fluid. n=8–10 per group unless otherwise noted. * respective saline vs HDM group; # WT HDM vs Gstp−/− HDM. */#p < 0.05 analyzed by two-way ANOVA.
3.3. Ablation of Gstp attenuates HDM-mediated lactate secretion and promotes resistance to IL-1β-induced glycolysis and neutrophilic inflammation
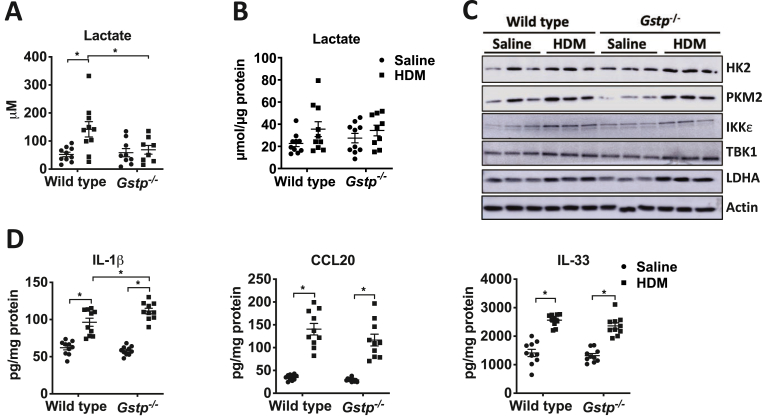
Based on our prior findings that enhanced glycolysis promotes HDM-induced AHR and remodeling [2,3], we next assessed if GSTP absence also dampened parameters of glycolytic reprogramming. Ablation of Gstp attenuated the HDM-mediated increases in lactate in BALF (Fig. 2A). No increases in lactate were observed in lung tissue (Fig. 2B). In agreement with previous results [2], HDM led to increased expression of a number of proteins involved in glycolysis including PKM2, hexokinase 2 (HK2), lactate dehydrogenase A (LDHA), inhibitory kappa B kinase epsilon (IKKε), and TANK-binding Kinase 1 (TBK1) in lung tissue (Fig. 2C). However, no differences in expression of these proteins were found between HDM-exposed WT and Gstp−/− mice in lung lysates (Fig. 2C). IL-1β is a major driver of glycolysis in lungs of mice with HDM-induced allergic airways disease [2]. Surprisingly, IL-1β levels slightly increased in Gstp−/− mice exposed to HDM compared to WT mice, while those of CCL20 and IL-33 did not differ (Fig. 2D).
Fig. 2.
Genetic ablation of Gstp attenuates HDM-mediated lactate secretion. Measurements of lactate levels in A, BAL fluid and B, lung tissue. C, Representative Western blot analyses for HK2, PKM2, LDHA, TBK1, IKKε. β-actin is used as the loading control. D, Measurements of IL-1β, CCL20, and IL-33 in lung tissue homogenates by ELISA. n=9–10 per group. *p < 0.05 analyzed by two-way ANOVA.
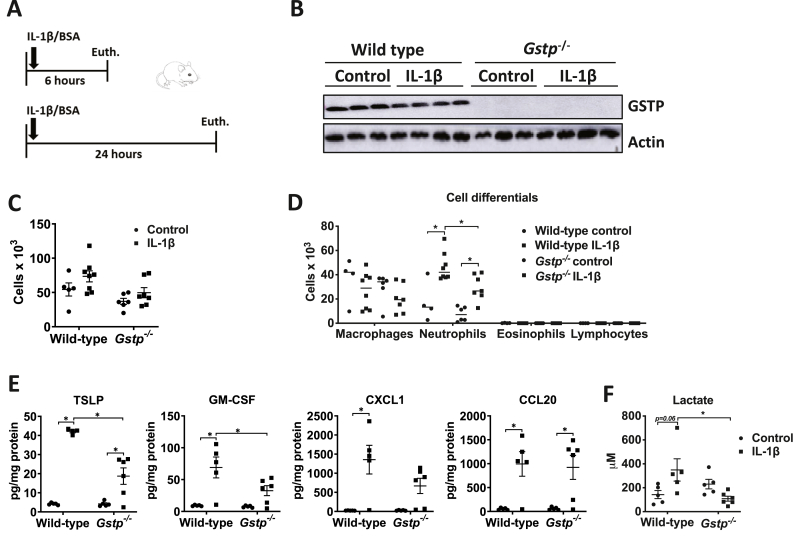
The decreases in airway lactate following ablation of Gstp may occur independently or downstream of IL-1 signaling. To further address this, we directly administered IL-1β intranasally to WT or Gstp−/- mice (Fig. 3A). GSTP expression in lung tissue was marginally affected by IL-1β (Fig. 3B). Although the IL-1β-mediated increases in overall BAL cell counts were not different, a significant decrease in neutrophil counts occurred in Gstp deficient mice compared to WT counterparts administered IL-1β (Fig. 3C–D). Moreover, the IL-1β-mediated increases in the pro-inflammatory cytokines TSLP, GM-CSF, and CXCL1 in lung tissue were attenuated in Gstp−/- mice compared to WT mice, while increases in CCL20 were not affected (Fig. 3E). Lastly, ablation of Gstp also abrogated the IL-1β-mediated increases in lactate in the BALF (Fig. 3F). Collectively, these results demonstrate that GSTP is a critical mediator that promotes IL-1β-induced pro-inflammatory signals and lactate secretion in lung tissue.
Fig. 3.
Gstp absence attenuates the release of pro-inflammatory cytokines and decreases airway neutrophilia following intranasal administration of IL-1β. A, Schematic depicting the intranasal administration of 1 μg of IL-1β for either 6 or 24 h. The total cell count and cell differentials in the BAL fluid reflect 24 h post IL-1β treatment, the other results shown are obtained 6 h post IL-1β administration. B, Representative western blots for total GSTP levels. β-actin; loading control. C, Total, and D, Differential cell counts in BAL fluid. E, Levels of the pro-inflammatory cytokines TSLP, GM-CSF, CXCL1 and CCL20 in lung tissue homogenates by ELISA. F, Measurements of lactate levels in BAL fluid. n=4–8 per group. *p < 0.05 analyzed by two-way ANOVA.
3.4. Ablation or TLK199-mediated inhibition of Gstp attenuates IL-1β-induced lactate and pro-inflammatory mediators in airway epithelial cells
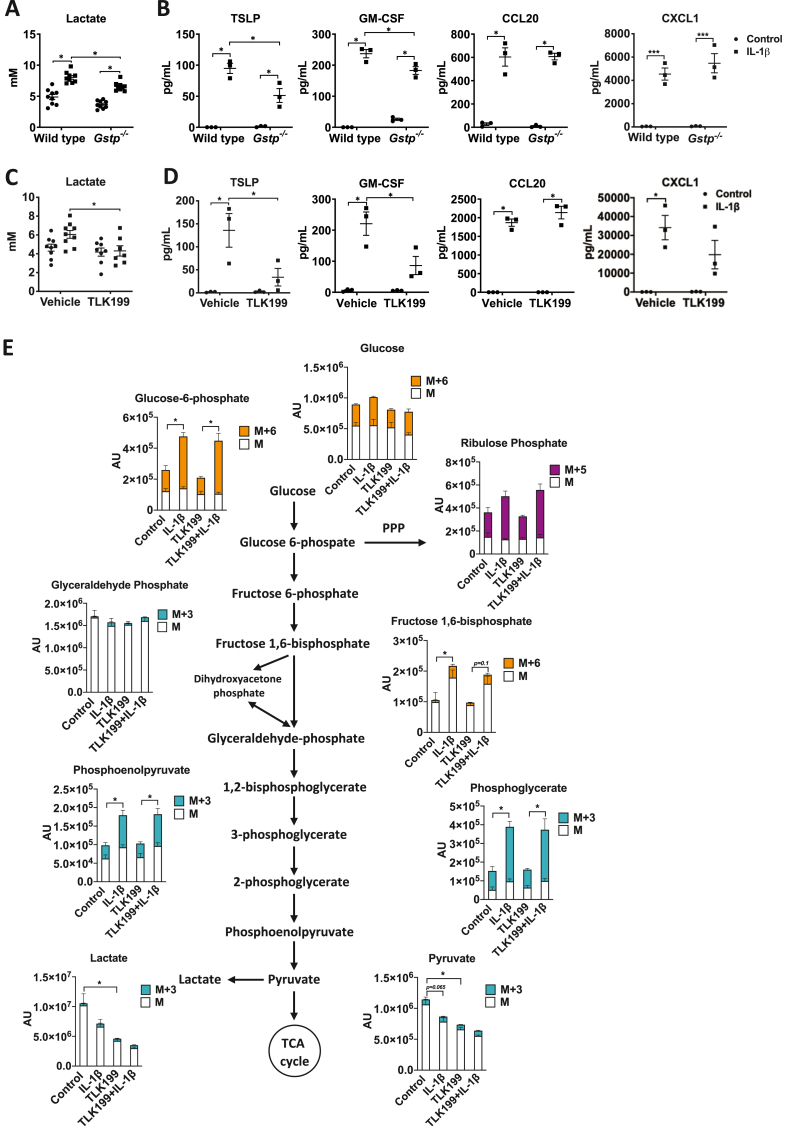
As GSTP is highly expressed in airway epithelia, the impact of its ablation may have been dampened in the analysis of whole lung tissues. We therefore next examined whether GSTP also promotes IL-1(β)-induced glycolysis and proinflammatory mediator release from airway epithelial cells. MTE cells isolated from WT mice exposed to 1 ng/mL IL-1β showed increased lactate levels and pro-inflammatory cytokine secretion in a time-dependent manner (Figs. S2A–B). Gstp deficiency attenuated the IL-1β-induced lactate secretion, as well as the release of TSLP and GM-CSF, while CCL20 and CXCL1 were unaffected (Fig. 4A–B). We next sought to address whether pharmacological inhibition of GSTP elicited similar responses. TLK199 is the most specific GSTP inhibitor to date, with a binding affinity greater than GSH itself and is selectively for GSTP over 50-fold greater than the GSTM and GSTA classes (inhibition constant [Ki] = 0.4 μM) [31]. Administration of TLK199, prior to IL-1β exposure also decreased IL-1β-induced lactate secretion and markedly decreased TSLP, GM-CSF and CXCL1 without affecting CCL20 (Fig. 4C–D). Pre-treatment of MTE cells with TLK199 did not affect survival, demonstrating that the results described were not due to a loss of cell viability (not shown). To more closely examine the effect of GSTP on the glycolysis pathway, MTE cells were cultured in the presence of 13C-glucose and treated with TLK199 in combination with IL-1β. IL-1β led to increases in most intracellular metabolites in the glycolysis pathway, while no clear changes were observed in TCA cycle intermediates (Fig. 4E, Fig. S2C). In IL-1β-exposed cells that were also treated with TLK199, glycolytic intermediates including 13C-PEP were increased similarly to the vehicle IL-1β group. However, a shift in the glycolysis cascade was observed in IL-1β-exposed cells in the last step of the pathway, the conversion from PEP to pyruvate, which is catalyzed by PKM. Interestingly, in cells exposed to TLK199 both total pyruvate and lactate decreased. In response to IL-1β, intracellular pyruvate and lactate were decreased compared to controls, consistent with increases in expression of Ldha in IL-1β-stimulated cells [2] which mediates the conversion of pyruvate to lactate which is then secreted (Fig. 4C). These findings point to a potential disruption of the glycolysis cascade following GSTP inhibition at the level of pyruvate kinase. Collectively, these data show that GSTP promotes IL-1β-induced glycolysis and pro-inflammatory cytokine secretion from epithelial cells.
Fig. 4.
Ablation or inhibition of Gstp, by TLK199, attenuates IL-1β-induced lactate and pro-inflammatory responses in primary MTE cells. A, Measurements of lactate and B, pro-inflammatory cytokines TSLP, GM-CSF, CXCL1, and CCL20 in cell culture supernatants of wild-type and Gstp−/- MTE cells. n=3–9 per group. C, Wild-type MTE cells were pre-treated with 50 μM TLK199 for 1 h followed by stimulation of 1 ng/mL IL-1β for 24 h. Lactate levels, and D, levels of pro-inflammatory cytokines in cull culture supernatants. n=3–9 per group. E, Metabolomics data of total levels (unlabeled fraction and labelling with 13C glucose) of metabolites in the glycolysis pathway. n=5–6 per group. The heavy metabolites are presented with solid color panels and are depicted as M+3 (green/blue), M+5 (purple), and M+6 (orange). The unlabeled portion of each metabolite is depicted as M and presented with solid white color panels. *p < 0.05 analyzed by two-way ANOVA. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5. GSTP-linked S-glutathionylation of PKM2 disrupts its tetramer configuration and attenuates PKM2's glycolytic activity
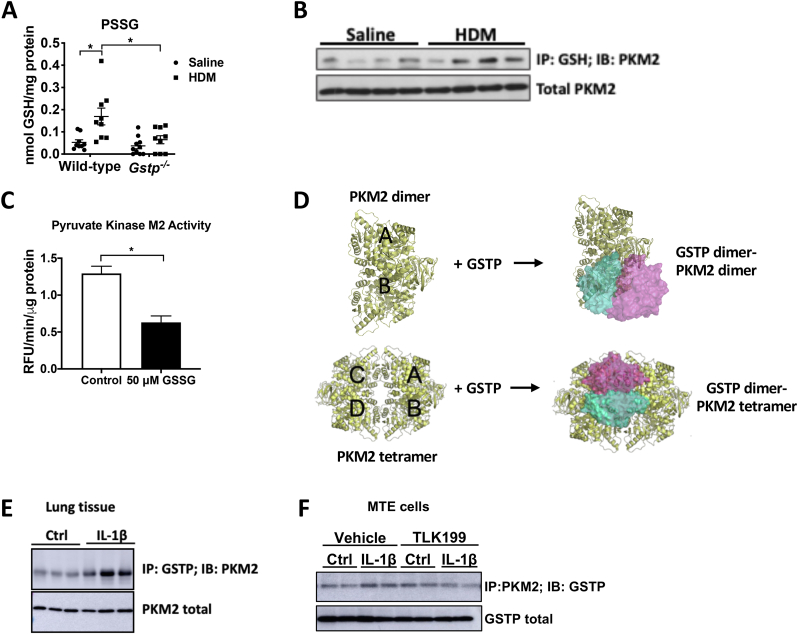
As stated earlier, GSTP catalyzes the forward PSSG reaction [18]. We therefore investigated whether GSTP contributes to increases in PSSG in HDM-induced allergic airways disease [32]. As expected, HDM led to increases in PSSG in WT mice, while PSSG was attenuated in HDM-exposed lungs from Gstp−/− mice compared to the respective WT group (Fig. 5A). Based upon our results suggesting that TLK199 interferes in the glycolysis cascade at the pyruvate kinase step (Fig. 4E), our prior findings that the small molecule allosteric activator of PKM2, TEPP46, dampens allergic airway inflammation in mice [3], and previous observations that PKM2 contains reactive cysteines that can be oxidized to disrupt function [14,16], we next addressed whether PKM2 could be S-glutathionylated by GSTP. Immunoprecipitation of S-glutathionylated proteins followed by western blotting of PKM2 showed increases in S-glutathionylation of PKM2 (PKM2-SSG) in HDM-exposed mouse lungs (Fig. 5B). Direct exposure of recombinant PKM2 to oxidized GSH (GSSG), a condition that induces PSSG, was sufficient to cause a decrease of PKM2 glycolytic activity (Fig. 5C). We next addressed whether GSTP can directly interact with PKM2, via protein-protein docking with crystal structures of PKM2 and GSTP, and confirmation of complex stability by conventional molecular dynamics (MD) simulations (Fig. 5D, Fig. S3). Docking studies and binding affinity calculations predicted that both dimeric and tetrameric PKM2 can interact with GSTP, with a more likely interaction between GSTP with PKM2 tetramers as compared to PKM2 dimers due to the larger interface and more contacts (Table S2, Fig. S3). Co-immunoprecipitation of GSTP and PKM2 confirmed an increased interaction between GSTP and PKM2 in IL-1β exposed mouse lungs (6 h), as well as in IL-1β-treated lung epithelial cells compared to the control groups (Fig. 5E–F). Interestingly, the GSTP inhibitor TLK199 tended to decrease the interaction between GSTP and PKM2 (Fig. 5F). Altogether, these data suggest a model wherein GSTP binds to PKM2 resulting in its S-glutathionylation, thereby disrupting the tetramer and decreasing its canonical glycolysis activity.
Fig. 5.
GSTP-linked S-glutathionylaton of Pyruvate Kinase M2 attenuates its glycolysis activity. A, Total PSSG in lung tissue from saline- and HDM-exposed WT and Gstp−/- mice, analyzed by DTNB assay. n=9–10 per group. B, Representative Western blot of PKM2-SSG in saline vs HDM-exposed mouse lungs. C, Pyruvate kinase enzymatic activity assay of 10 ng recombinant PKM2 incubated with 50 μM oxidized glutathione (GSSG). D, Complex models and interactions between dimeric or tetrameric PKM2 with a GSTP dimer by protein-protein docking. PDB structures used for modeling: 1T5A (PKM2 tetramer) and 1AQW (GSTP dimer). The most likely models are illustrated here, and all predicted models are shown in a cartoon in the supplemental information. E, Representative western blots of GSTP-PKM2 interaction via co-immunoprecipitation and total PKM2 levels in IL-1β-treated mouse lung tissues. F, Representative Western blot of PKM2-GSTP (IP: PKM2; IB; GSTP) and total PKM2 levels in wild type MTE cells pre-treated with 50 μM TLK199 for 1 h followed by stimulation with 1 ng/ml IL-1β for 2 h *p < 0.05 analyzed by two-way ANOVA.
3.6. Gstp ablation or inhibition reduces phosphorylation of STAT3
The glycolysis inactive monomer/dimer form of PKM2 has alternative functions as a protein kinase, transcriptional activator, and also can exert functions following its secretion [3,7,11,33]. PKM2 promotes inflammation in part via phosphorylation of STAT3 (p-STAT3) [7,8]. We recently showed that STAT3 contributes to IL-1β-induced lung inflammation in association with disruption of PKM2's canonical glycolysis activity, and that the allosteric activator of PKM2, TEPP46, attenuated p-STAT3 [3]. We therefore addressed whether GSTP status also affects p-STAT3. HDM- or IL-1β-mediated increases in p-STAT3 in lung tissue [3] or MTE cells were abolished following ablation of Gstp (Figs. S4A–B). Inhibition of GSTP with TLK199 also decreased the IL-1β-induced p-STAT3 in MTE cells (Fig. S4C). Collectively, these results indicate that STAT3 is one of the downstream targets that promotes IL-1β-induced airway inflammation following the GSTP-mediated S-glutathionylation.
3.7. GSTP and PKM2 expression are increased in sputum of asthmatics with high levels of IL-1β and lactate
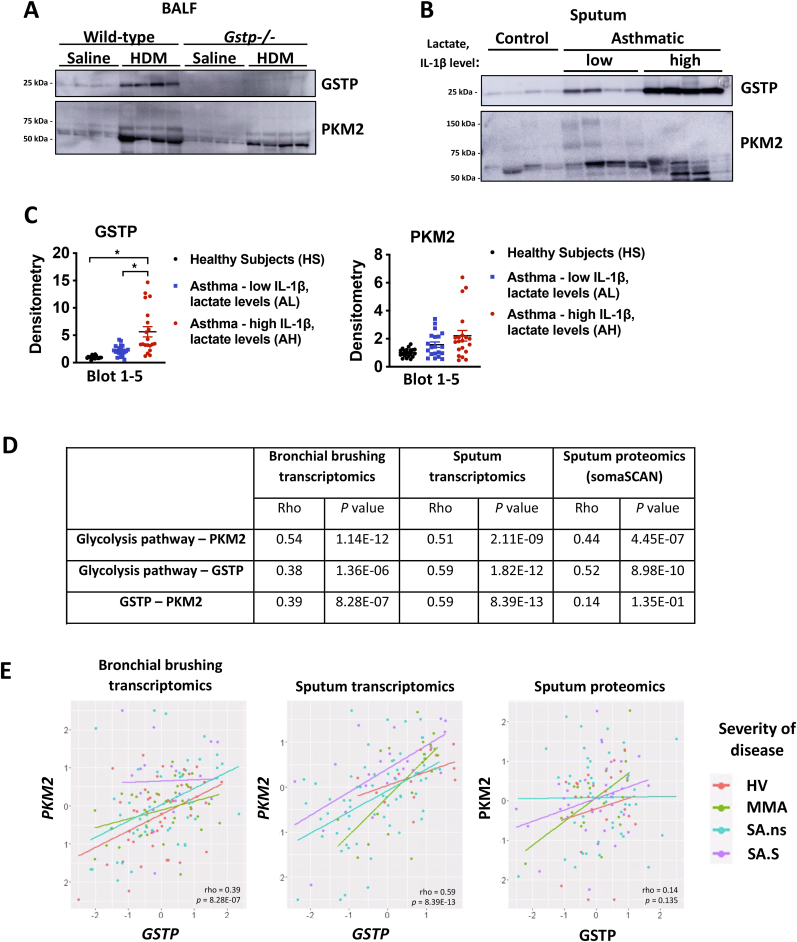
We recently reported that sputum lactate and IL-1β levels were elevated in patients with neutrophilic asthma in association with severe disease and diminished FEV1 [2]. IL-1 has been linked to neutrophilic inflammation, airflow obstruction and steroid resistance in subjects with severe asthma [34,35]. Given the present demonstration that GSTP and PKM2 govern pro-inflammatory responses to IL-1β, and that GSTP and PKM2 can be localized extracellularly [33,36], we next examined whether GSTP and PKM2 could be detected in mouse BALF or induced sputum samples of healthy controls or people with asthma and could serve as potential biomarkers of disease. Interestingly, GSTP and PKM2 were both increased in BALF of mice exposed to HDM (Fig. 6A). In Gstp−/− mice, BAL PKM2 was decreased, suggesting that GSTP promoted the secretion of PKM2. We next evaluated GSTP and PKM2 in sputum samples of controls (n=20) and participants with asthma [2], and distinguished asthma subjects into subgroups having low sputum lactate and low IL-1β (n=20) vs high sputum lactate and high IL-1β (n=20) (Fig. 6B–C, Table S1). Despite some variability between participants, GSTP levels were highest in the subgroup of asthma subjects having high sputum IL-1β and high sputum lactate, while PKM2 trended towards being increased in both asthma groups (Fig. 6B and C and Fig. S5). Interestingly, the subgroup of asthma subjects having high sputum IL-1β and high sputum lactate also displayed more sputum neutrophils compared to the asthma subjects with low sputum IL-1β and lactate (p=0.001). We next evaluated GSTP and PKM2 in a larger subject cohort by evaluating bronchial brush transcriptomics, sputum transcriptomics, and sputum proteomics in the U-BIOPRED adult severe asthma cohort [26]. In all databases, the KEGG glycolysis pathway was significantly correlated with the expression of GSTP and PKM2 (Fig. 6D). Intriguingly, a significant correlation was also observed between GSTP and PKM2 mRNA in bronchial brushings as well as in sputum (Fig. 6D and E).
Fig. 6.
GSTP and PKM2 expression are increased in HDM-exposed BALF and in sputum of people with asthma with high levels of IL-1ß and lactate and correlates with disease severity. A, Representative western blots of extracellular GSTP and PKM2 in BALF from HDM-exposed WT and Gstp−/- mice. B, Representative western blots of extracellular GSTP and PKM2 from induced sputum samples of 4 healthy subjects (HS), 4 participants with asthma (AL) with low sputum IL-1β (<20 pg/mL) and low sputum lactate levels (<33 μM), and 4 participants with asthma (AH) with high sputum IL-1β (>30 pg/mL) and sputum lactate (>80 μM) levels. Each lane represents an individual participant. C, Quantification of GSTP (left) and PKM2 (right) immunoreactivity from 20 HS, AL and AH subjects. Individual western blots of GSTP and PKM2 from the additional 16 HS, AL, and AH subject are shown in Fig. S5. D, Spearman correlation analysis of bronchial brushings transcriptomics, sputum transcriptomics and sputum proteomics datasets, derived from the U-BIOPRED cohort. Shown are Rho and p values of the correlations between glycolysis and PKM2, glycolysis and GSTP, as well as PKM2 and GSTP. E, Figure showing the correlation between GSTP and PKM2 in patients of the different datasets: bronchial brushing transcriptomics (left), sputum transcriptomics (middle), and sputum proteomics (right). HV: healthy volunteers, MMA: mild/moderate non-smoking asthma subjects, SA.ns: severe non-smoking participants with asthma, SA.S: current/ex-smokers with severe asthma. *p < 0.05 analyzed by one-way ANOVA with Tukey's multiple comparisons test.
4. Discussion
Enhanced glycolysis is a process that accompanies allergic airway disease and promotes airway remodeling in mice [2]. Increases in the oxidative environment promote glycolysis and accompany asthma [32,37,38]. Here, we demonstrate a close link between glycolysis and oxidative changes and illuminate a role for GSTP, a catalyst of S-glutathionylation, in promoting glycolytic reprogramming. Notably, we show that GSTP contributes to IL-1-induced glycolysis and pro-inflammatory signaling in epithelial cells and that GSTP binds to and disrupts PKM2's canonical glycolysis activity. GSTP levels were increased in the sputum of people with severe asthma, and sputum proteomics and transcriptomics also revealed strong correlations between GSTP, PKM2, and glycolysis. Together with our previous observations showing that inactivation of PKM2's canonical glycolysis function contributes to HDM-induced allergic airways disease [3], these findings point to a putative PKM2-GSTP-glycolysis signature in asthma associated with severe disease.
An involvement of GSTs in asthma was first suggested by findings that polymorphisms in GSTP, GST-mu, and GST-omega showed links to asthma susceptibility, and led to numerous studies to date that have provided inconclusive evidence about the linkage of these polymorphisms with asthma susceptibility and/or severity [21,23,39,40]. Previous studies showed that Gstp transcript levels were down-regulated in lung tissues from mice challenged with HDM [41], in an ovalbumin model of allergic airways disease [42], and in nasal epithelial cells from children with asthma [41]. The latter findings are in agreement with decreases in Gstp transcripts in lung tissue in response to HDM shown herein that occurred in the absence of changes in GSTP protein levels. Our results show that GSTP contributes to the pathogenesis of HDM-induced allergic airways disease and promotes mucus metaplasia, airway remodeling, and AHR. Our results contrast a study using ovalbumin that demonstrated enhanced AHR, eosinophilia, and goblet cell hyperplasia in Gstp−/− mice. The latter findings depended on the genetic background [42] and collectively suggest strain- and allergen-dependent effects of Gstp ablation.
Our results provide new insights into the potential mechanism(s) whereby GSTP promotes asthma by the demonstrations that GSTP contributes to IL-1β-induced glycolysis and pro-inflammatory signaling in epithelial cells, and that GSTP disrupts the canonical glycolysis function of PKM2 while increasing its pro-inflammatory function. The molecular details whereby GSTP binds and S-glutathionylates PKM2 and the cysteine(s) in PKM2 that are oxidized will require further study. Oxidation of Cys358 or Cys424 of PKM2 disrupt the tetramer and promote dimer formation [14,16,43] suggesting that these cysteines also may be involved in GSTP-linked oxidation. PKM2 has emerged as a key regulator of immune cell activation by acting as a “moonlighting” enzyme in the dimer state. While disruption of the PKM2 tetramer slows the conversion of PEP to pyruvate, paradoxically, disrupted PKM2 promotes glycolytic reprogramming by acting as a protein kinase and transcriptional regulator to augment expression of glycolysis genes. Nuclear dimeric PKM2 phosphorylates STAT3 [7,8] and increases the transcriptional activation of HIF1a target genes to collectively promote glycolytic reprogramming, and the latter non-canonical function also elicits pro-inflammatory responses. A role of PKM2 in the differentiation of Th17 cells has been demonstrated [10] and was shown to involve activation of STAT3 [9]. Administration of the small molecule activator, TEPP46, which stabilizes the PKM2 tetramer, attenuated HDM- and IL-1β-mediated allergic airways disease and pro-inflammatory signaling, in association with diminished p-STAT3 [3]. The decrease of IL-1-induced pro-inflammatory mediators and STAT3 phosphorylation in the absence of Gstp or upon its inhibition described herein are similar to the effects of TEPP46. The possibility that TEPP46 protects PKM2 from inactivation caused by GSTP-mediated S-glutathionylation awaits further study.
Besides PKM2, other targets of GSTP-mediated S-glutathionylation also have the potential to contribute to allergic airways disease. For example, GSTP increases the proteolytic activity of the protease Der p1 in HDM [44]. GSTP can also glutathionylate AMPK, SRC and IKK-β, leading to decreases of their activity [[45], [46], [47]]. It is worthy of mention that IL-1β itself is a target for S-glutathionylation which enhances its biological activity [48]. Moreover, GSTP may also regulate protein function following binding to client proteins. One well-described binding partner of GSTP is c-Jun N-terminal kinase (JNK), which promotes allergic airways disease [49,50]. Further studies to unravel the proteins that GSTP interacts with, the cell types wherein this occurs, along with the targets of S-glutathionylation will provide a more complete description of the proteins and processes affected by GSTP in settings of allergic inflammation [17].
Altogether, these data demonstrate that GSTP contributes to HDM-induced allergic airways disease in association with enhanced glycolysis. Our findings illuminate that one important target of GSTP-controlled S-glutathionylation is PKM2, a major regulator of glycolytic reprogramming and inflammation. Lastly, the demonstration that GSTP increases IL-1-induced pro-inflammatory signaling, combined with the role of IL-1β in neutrophilic severe asthma [2,34,35], suggest that targeting the GSTP/PKM2 axis may offer potential novel treatment strategies for patients with difficult-to-treat asthma. TLK199 has been used clinically with demonstrated therapeutic efficacy in patients with myelodysplastic syndrome [51,52], findings that warrant its clinical redevelopment. Furthermore, clinical trials employing a small molecule activator of PK are currently ongoing for different clinical indications (NCT02476916, NCT04000165, Clinicaltrials.gov), endeavors that collectively point to putative strategies to harness PKM2 and GSTP in patients with severe asthma in the future.
Declaration of competing interest
Yvonne Janssen-Heininger and Niki Reynaert hold patents: United States Patent No. 8,679,811, “Treatments Involving Glutaredoxins and Similar Agents” (YJ-H, NR), United States Patent No. 8,877,447, “Detection of Glutathionylated Proteins” (YJ-H, NR), United States Patents 9,907,828 and 10,688,150 “Treatments of oxidative stress conditions” (YJ-H). Yvonne Janssen-Heininger has received consulting fees from Celdara Medical LLC for the contributions to the proposed commercialization of glutaredoxin for the treatment of pulmonary fibrosis.
Acknowledgments and funding
This work was supported by grants NIH R01 HL137268, R35HL135828 (Y-JH), an unrestricted grant from Chiesi (EW), and Cancer Center Support Grant P30CA046934 (AD’A). We acknowledge the technical expertise of J. Bates, N. Daphtary and M. Aliyeva with AHR analysis. Imaging was performed in the Microscopy Imaging Center at the University of Vermont (RRID# SCR_018821), and we also acknowledge the Larner College of Medicine Shared Instrumentation Award. U-BIOPRED was supported by an Innovative Medicines Initiative Joint Undertaking (No.115010), resources from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in-kind contribution (www.imi.europa.eu). The computational resources were provided by the Vermont Advanced Computer Core.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102160.
Author contributions
C.vdW. designed and performed experiments, analyzed data and wrote the manuscript. A.M.M., R.A., X.Q., C.E., M.M., provided help in performing experiments. M.S., G.C., J.L. performed and analyzed MD simulations. I.A. and N.ZK. analyzed U-BIOPRED data. F.S., R.L. provided patient sputum samples. E.B., A.D. provided and analyzed metabolomics data. E.F.W, N.L.R., C.G.I. assisted with experimental design, edited the manuscript and provided helpful discussion. C.R.W., C.J.H. generated and characterized the Gstp−/− mice. L.K.L provided help with the AHR analysis. M.E.P., A.E.D., A.vdV., J.L.vdV. provided scientific input and discussion. Y. M. W. J-H designed the experiments and was responsible for overall supervision of the project and wrote the manuscript.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fahy J.V. Type 2 inflammation in asthma--present in most, absent in many. Nat. Rev. Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian X. IL-1/inhibitory kappaB kinase epsilon-induced glycolysis augment epithelial effector function and promote allergic airways disease. J. Allergy Clin. Immunol. 2018;142(2):435–450 e10. doi: 10.1016/j.jaci.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Wetering C. Pyruvate kinase M2 promotes expression of proinflammatory mediators in house dust mite-induced allergic airways disease. J. Immunol. 2020;204(4):763–774. doi: 10.4049/jimmunol.1901086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int. J. Biochem. Cell Biol. 2011;43(7):969–980. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Christofk H.R. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 6.Anastasiou D. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012;8(10):839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao X. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell. 2012;45(5):598–609. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirai T. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 2016;213(3):337–354. doi: 10.1084/jem.20150900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damasceno L.E.A. PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation. J. Exp. Med. 2020;217(10) doi: 10.1084/jem.20190613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kono M. Pyruvate kinase M2 is requisite for Th1 and Th17 differentiation. JCI Insight. 2019;4(12) doi: 10.1172/jci.insight.127395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christofk H.R. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452(7184):181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 12.Hitosugi T. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2009;2(97):ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhardwaj A., Das S. SIRT6 deacetylates PKM2 to suppress its nuclear localization and oncogenic functions. Proc. Natl. Acad. Sci. U.S.A. 2016;113(5):E538–E547. doi: 10.1073/pnas.1520045113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anastasiou D. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F. PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat. Cell Biol. 2017;19(11):1358–1370. doi: 10.1038/ncb3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masaki S. The cysteine residue at 424th of pyruvate kinase M2 is crucial for tetramerization and responsiveness to oxidative stress. Biochem. Biophys. Res. Commun. 2020;526(4):973–977. doi: 10.1016/j.bbrc.2020.03.182. [DOI] [PubMed] [Google Scholar]
- 17.van de Wetering C. Glutathione S-transferases and their implications in the lung diseases asthma and chronic obstructive pulmonary disease: early life susceptibility? Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janssen-Heininger Y.M. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes J.D., Strange R.C. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic. Res. 1995;22(3):193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- 20.Townsend D.M. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009;284(1):436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamer L. Glutathione-S-transferase gene polymorphisms (GSTT1, GSTM1, GSTP1) as increased risk factors for asthma. Respirology. 2004;9(4):493–498. doi: 10.1111/j.1440-1843.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 22.Joubert B.R. Evaluation of genetic susceptibility to childhood allergy and asthma in an African American urban population. BMC Med. Genet. 2011;12:25. doi: 10.1186/1471-2350-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minelli C. Glutathione-S-transferase genes and asthma phenotypes: a Human Genome Epidemiology (HuGE) systematic review and meta-analysis including unpublished data. Int. J. Epidemiol. 2010;39(2):539–562. doi: 10.1093/ije/dyp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delvaux M. Nebulised salbutamol administered during sputum induction improves bronchoprotection in patients with asthma. Thorax. 2004;59(2):111–115. doi: 10.1136/thorax.2003.011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maes T. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J. Allergy Clin. Immunol. 2016;137(5):1433–1446. doi: 10.1016/j.jaci.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Shaw D.E. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur. Respir. J. 2015;46(5):1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 27.Gold L. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5(12) doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi K. Sputum proteomics and airway cell transcripts of current and ex-smokers with severe asthma in U-BIOPRED: an exploratory analysis. Eur. Respir. J. 2018;51(5) doi: 10.1183/13993003.02173-2017. [DOI] [PubMed] [Google Scholar]
- 29.Alcorn J.F. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J. Cell Sci. 2008;121(Pt 7):1036–1045. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anttila S. Immunohistochemical localization of glutathione S-transferases in human lung. Cancer Res. 1993;53(23):5643–5648. [PubMed] [Google Scholar]
- 31.McMillan D.H. Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase pi. JCI Insight. 2016;1(8) doi: 10.1172/jci.insight.85717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman S.M. Ablation of glutaredoxin-1 modulates house dust mite-induced allergic airways disease in mice. Am. J. Respir. Cell Mol. Biol. 2016;55(3):377–386. doi: 10.1165/rcmb.2015-0401OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang C. Secreted pyruvate kinase M2 promotes lung cancer metastasis through activating the integrin Beta1/FAK signaling pathway. Cell Rep. 2020;30(6):1780–17897 e6. doi: 10.1016/j.celrep.2020.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Evans M.D. Sputum cell IL-1 receptor expression level is a marker of airway neutrophilia and airflow obstruction in asthmatic patients. J. Allergy Clin. Immunol. 2018;142(2):415–423. doi: 10.1016/j.jaci.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim R.Y. Role for NLRP3 inflammasome-mediated, IL-1beta-dependent responses in severe, steroid-resistant asthma. Am. J. Respir. Crit. Care Med. 2017;196(3):283–297. doi: 10.1164/rccm.201609-1830OC. [DOI] [PubMed] [Google Scholar]
- 36.Coursin D.B. Immunolocalization of antioxidant enzymes and isozymes of glutathione S-transferase in normal rat lung. Am. J. Physiol. 1992;263(6 Pt 1):L679–L691. doi: 10.1152/ajplung.1992.263.6.L679. [DOI] [PubMed] [Google Scholar]
- 37.Winnica D. Bioenergetic differences in the airway epithelium of lean versus obese asthmatics are driven by nitric oxide and reflected in circulating platelets. Antioxidants Redox Signal. 2019;31(10):673–686. doi: 10.1089/ars.2018.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaeloudes C. Role of metabolic reprogramming in pulmonary innate immunity and its impact on lung diseases. J Innate Immun. 2020;12(1):31–46. doi: 10.1159/000504344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang S. Significant association between asthma risk and the GSTM1 and GSTT1 deletion polymorphisms: an updated meta-analysis of case-control studies. Respirology. 2013;18(5):774–783. doi: 10.1111/resp.12097. [DOI] [PubMed] [Google Scholar]
- 40.Wu W. Role of GSTM1 in resistance to lung inflammation. Free Radic. Biol. Med. 2012;53(4):721–729. doi: 10.1016/j.freeradbiomed.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroer K.T. Downregulation of glutathione S-transferase pi in asthma contributes to enhanced oxidative stress. J. Allergy Clin. Immunol. 2011;128(3):539–548. doi: 10.1016/j.jaci.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J. Glutathione transferase P1: an endogenous inhibitor of allergic responses in a mouse model of asthma. Am. J. Respir. Crit. Care Med. 2008;178(12):1202–1210. doi: 10.1164/rccm.200801-178OC. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell A.R. Redox regulation of pyruvate kinase M2 by cysteine oxidation and S-nitrosation. Biochem. J. 2018;475(20):3275–3291. doi: 10.1042/BCJ20180556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez-Rodriguez J.C. Human glutathione-S-transferase pi potentiates the cysteine-protease activity of the Der p 1 allergen from house dust mite through a cysteine redox mechanism. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klaus A. Glutathione S-transferases interact with AMP-activated protein kinase: evidence for S-glutathionylation and activation in vitro. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y. GSTpi regulates VE-cadherin stabilization through promoting S-glutathionylation of Src. Redox Biol. 2020;30 doi: 10.1016/j.redox.2019.101416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reynaert N.L. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc. Natl. Acad. Sci. U.S.A. 2006;103(35):13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X. Positive regulation of interleukin-1beta bioactivity by physiological ROS-mediated cysteine S-glutathionylation. Cell Rep. 2017;20(1):224–235. doi: 10.1016/j.celrep.2017.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nath P. Potential role of c-Jun NH2-terminal kinase in allergic airway inflammation and remodelling: effects of SP600125. Eur. J. Pharmacol. 2005;506(3):273–283. doi: 10.1016/j.ejphar.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 50.Wang T. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem. 2001;276(24):20999–21003. doi: 10.1074/jbc.M101355200. [DOI] [PubMed] [Google Scholar]
- 51.Raza A. A phase 2 randomized multicenter study of 2 extended dosing schedules of oral ezatiostat in low to intermediate-1 risk myelodysplastic syndrome. Cancer. 2012;118(8):2138–2147. doi: 10.1002/cncr.26469. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J. Development of telintra as an inhibitor of glutathione S-transferase P. Handb. Exp. Pharmacol. 2021;264:71–91. doi: 10.1007/164_2020_392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.