Graphical abstract
Keywords: Rabbit meat, High intensity ultrasound, Freezing, Meat quality, Yield
Highlights
-
•
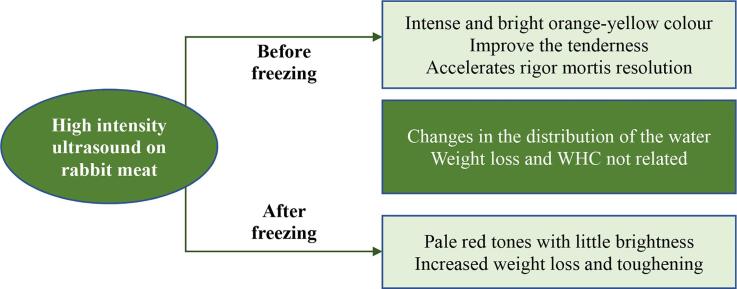
High-intensity ultrasound (HIU) before freezing produced intense and bright yellow-orange tones.
-
•
The post-freezing application of HIU produced pale red tones with little brightness.
-
•
HIU before freezing accelerates rigor mortis resolution.
-
•
HIU before freezing improve the tenderness of rabbit meat.
-
•
HIU after freezing decreased the WHC and yield (weigh loss) of the meat.
Abstract
High intensity ultrasound (HIU) is a technique with the potential to improve meat quality, however, more research is needed on its application within the chain of cold storage and freezing. This study evaluates the effect of HIU (40 kHz, 9.6 W/cm2, 20 and 40 min) and post-mortem development on the yield and physicochemical quality of rabbit meat in samples treated with HIU pre- and post-storage in a freezer (120 h at −20 °C). Twenty rabbit carcasses were vacuum packed 12 h post-mortem, placed in a fridge at 4 °C for 24 h, and divided in two groups (HIU application before or after freezing), before assigning the treatments. The results show that HIU before freezing produced intense and bright orange-yellow colours, whereas its application after freezing resulted in pale red tones. HIU application accelerates rigor mortis resolution when it is applied before freezing and causes a significant decrease in pH immediately following the HIU treatment. Post-freezing application of HIU is not recommended because it considerably increased weight loss and toughening of the meat when long exposure times were used (40 min). In contrast, a short treatment duration with HIU mitigated the effects of freezing and produced significant increases in water-holding capacity (WHC) after cold storage. The yield (weight loss) of the rabbit meat was not affected when HIU was applied pre-freezing. The application of HIU pre-freezing constitutes a promising technology because it increased the tenderness and the WHC of rabbit meat. However, more research is needed to improve the appearance before scaling up to industrial levels.
1. Introduction
The quality of meat is an integral concept that not only considers safety in terms of elimination and/or reduction of physical, chemical, and microbiological risks, but also the sensorial and technological properties during and after cooking [1], [2]. Rabbit meat is characterized by being healthier in comparison to other types of meat because it contains a high content of protein and polyunsaturated fatty acids and a low level of cholesterol [2], [3]. Rabbit meat is lean because it has a low fat content relative to its protein density, for which this meat is perceived as tougher compared to meat from other species [4]. Furthermore, the interest of the meat industry has focused on the improvement of the carcass yield [2]. The quality of meat is also directly influenced by the rapid cooling of the carcass within hours after being slaughtered (post-mortem) [5], [6], and subsequent cold stored. As part of the commercial chain, frozen storage allows for longer preservation. However, water-holding capacity tends to decrease due to tissue lesions during the process of freezing and thawing, whereas lipid oxidation increases and tenderness is not affected [2], [7]. Although frozen meat has a less attractive colour, consumers equally prefer fresh and frozen meat when cooked [7]. The most relevant quality aspect of the meat is its tenderness, which is determined by several factors such as the myofibrillar structure or protein degradation [8]. The use of ultrasound as a supporting technology has demonstrated to be an effective technology to improve the post-mortem tenderness of meat [4], [9], [10], [11], including the improvement of colour parameters such as lightness and colour saturation [12], [13], [14], for which ultrasound is a promising technology for the treatment of rabbit carcasses that are exposed to a process of freezing as part of the commercial supply chain. A study carried out by Gómez-Salazar et al. [14] on rabbit meat reported that ultrasound treatment (2.25 W/cm2, 20 min) reduced the immersion time during marinating and increased lightness; however, weight loss, water-holding capacity, and toughness increased. In contrast, Reyes-Villagrana et al. [15] showed that treatment with ultrasound (12 W/cm2, 24 kHz, 15 min) reduced toughness and increased lightness and yellowness. The discrepancy in these studies can be attributed to differences in factors and levels in the experimental design (durations, intensities, frequencies, temperatures, type of packaging, etc.) and the sample characteristics (species, type of muscle, muscle composition, etc.), for which more research is needed to standardize the parameters that allow for scaling up this technology to industrial levels. Therefore, this research studied the effects of high-intensity ultrasound (HIU, >5 W/cm2 or 10–1000 W/cm2, 20–100 kHz) application and post-mortem duration on the yield (weight loss) and physicochemical quality (color L*a*b*, pH, shear force and water-holding capacity) of rabbit meat in samples treated with HIU before (36 h post-mortem) and after (156 h post-mortem) storage in a freezer for 120 h at −20 °C.
2. Materials and methods
2.1. Description of the samples and treatments
Initially, forty rabbit carcasses (New Zealand × Rex) were obtained 12 h post-mortem from the rabbit production facility at the Faculty of Animal Science and Ecology (Autonomous University of Chihuahua, Mexico). After carcass separation by sex (male/female) and breed (New Zealand × Rex, Azteca × Chinchilla, and Chinchilla) only 27 males were selected to carry out the study, to ensure uniformity of the experimental unit. After slaughter, the carcasses were stored during the night (12 h) at room temperature (12–18 °C). The carcasses were received at 12 °C, cut longitudinally in two symmetric halves, and vacuum packed in thin polyethylene bags (50 μm, Koch Supplies Inc., Kansas, United States of America), and assigned to treatments. The packed samples were divided in two groups: samples treated with ultrasound before freezing (36 h post-mortem) and samples treated with ultrasound after freezing (156 h post-mortem). The samples sonicated prior freezing were vacuum-packaged and placed 24 h at 4 °C, then, they were treated with ultrasound for 20 or 40 min (left in the bath off until completing 1 h at 4 °C, 37 h post-mortem) and frozen for 120 h at −20 °C and finally stored for 48 h at 4 °C (205 h post-mortem). The other group of samples (ultrasound after freezing) was frozen and stored for 120 h at −20 °C immediately after vacuum packing (12 h post-mortem). After that time, they were thawed (156 h post-mortem) at 4 °C for 24 h and then treated with ultrasound for 20 or 40 min (left in the bath off until completing 1 h at 4 °C, 157 h post-mortem), and finally they were stored at 4 °C for 48 h (205 h post-mortem). After completing the ultrasound treatment time (20 or 40 min), the samples remained in the ultrasonic bath (off) at 4 °C until completing 1 h, with the aim of stabilizing the sample before subsequent treatment. For both groups of samples, the control consisted of time 0 of ultrasound treatment, for which the samples were placed in a water bath at 4 °C for 1 h. Nine treatments were assigned to each group of samples with three repetitions each. Both HIU time and post-mortem time had three levels, resulting in nine treatments for each group of samples. Therefore, 27 half carcasses were used for each group of samples (ultrasonication before or after freezing), giving a total of 54 half carcasses. The samples were frozen for 120 h at −20 °C (Fisher Scientific R134A, Massachusetts, United States of America), while cold storage took place for 48 h at 4 °C.
2.2. Ultrasound treatment
The vacuum packed samples were directly placed in the ultrasonic bath (Elmasonic S60H, Singen, Germany) and treated with ultrasound for 20 or 40 min (10, or 20 min per side) at a frequency of 40 kHz and an intensity of 9.6 W/cm2 (determined by calorimetry following Margulis and Margulis [16]). The ultrasonic bath was operating at Normal mode where samples received a homogeneous ultrasound power. Distilled water was used as diffusion medium and the temperature was kept at 4 °C during HIU treatment. The bath temperature during sonication was kept at 4 °C using a refrigeration probe (Julabo® FT200). The control samples (0 min) were kept at 4 °C in distilled water for 1 h. In this way, all treatments were kept at 4 °C, regardless of the ultrasound time (20 or 40 min). The response variables (pH, colour L* a* b*, water-holding capacity, shear force, and weight loss) were measured in the loin (m. Longissimus dorsi) at three times: before the application of the ultrasonic treatment, after the ultrasonic treatment and at the end of the storage period (freezing and / or refrigeration).
2.3. Measurement of physicochemical parameters
Sample pH was obtained with a digital pH meter (Sentron model 1001, Netherlands) coupled to an insertion electrode. The readings were performed at a depth of 2 cm. The probe was previously calibrated at 4 °C using calibration solutions with a pH of 4.0 and 7.0. The average of three measurements at three different points in the muscle was obtained, avoiding contact with fat and remnants of connective tissue.
The colour of the samples was determined with a Minolta colorimeter (CR-400, Konica Minolta Sensing, Inc., Osaka, Japan; Illuminant C, 2° observer angle of measurement), following the methodology from the American Meat Science Association manual [17]. The trichromatic coordinates L* (lightness/darkness), a* (redness/greenness), and b* (yellowness/blueness) were used to calculate C* (chroma or saturation) and Hue (hue angle) using the equations (a*2 + b*2)½ and arctan (b*/a*), respectively. The measurements were performed on parts of the sample that were free of visible connective and adipose tissue, allowing the oxygenation of the myoglobin for 30 min at 4 °C. Three measurements per sample were performed and the averages were recorded.
Water-holding capacity (WHC) was measured using the compression technique from Tsai and Ockerman [18]. 0.3 ± 0.1 g of meat (Ohaus Corporation model AV213, New Jersey, United States of America) was placed between two filter papers (Whatman no. 1) and this pack located between two methacrylate plates with a constant weight pressure of 10 kg for 5 min. Three measurements were obtained from each sample. WHC was then calculated using the following equations:
WHC (%) = 100 – free water
The method of AMSA [19] was used to determine shear force. Samples were cooked on electric grills (George Foreman® model GH0201R-T, Ohio, United States of America) until reaching an internal temperature of 71 °C ± 2 °C in the geometric centre of the sample. Samples were left to cool at ambient temperature and then immediately refrigerated at 4 °C for 12 h. Using a manual punch, three cylinders with a diameter of 10 mm were obtained per sample in a longitudinal direction parallel to the muscle fibres. The cylinders were cut using a “V” shaped Warner-Bratzler knife (triangular opening of 60°) at a speed of 100 mm/min and a height of 30 mm. The maximum force (in Newtons) was determined using a TA-TX-plus texture analyser (Stable Micro Systems Ltd., Surrey, United Kingdom).
2.4. Yield (weight loss)
The percentage of fresh weight loss of the carcass halves was determined using the technique described by Cruz-Garibaldi et al. [20]. A scale (Torrey model LS-Q, Monterrey, Mexico) with a precision of 1 g was used. Sample weight before HIU (36 h and/or 156 h post-mortem) was considered as initial fresh weight, whereas the final fresh weight was obtained after HIU (37 h and/or 205 h post-mortem, and 157 and/or 205 h post-mortem depending on whether the samples were treated with HIU before or after freezing, respectively). The percentage of fresh weight loss was calculated using the following equation:
2.5. Statistical analysis
The results were analysed using statistical package SAS System 9.0. The reported values are means ± standard deviation. The analyses of variance ware performed following a completely randomized 3*3 factorial experimental design (factor “HIU time” with three levels and factor “post-mortem time” with three levels, resulting in a total of 9 treatments). The difference between sample means was determined using Tukey tests (significance levels P < 0.05). According to the limitation of resources (experimental units) and sources of variability, response measures of repetition and not of replications were used. Of 40 initial experimental units, only 27 were used, ensuring homogeneity of sex and race for the allocation of treatments. The limitations and contributions of the study in terms of the number of repetitions and not of replications should be considered as part of the research results obtained.
3. Results and discussion
3.1. Colour space CIE L* a* b*
The results show that the lightness of the meat decreased significantly in samples treated with ultrasound before freezing (P = 0.014, Table 1), independently of the exposure time (20 min or 40 min). In contrast, the meat treated with HIU for 40 min after freezing presented a significant increase in lightness (P = 0.0103). This increase in luminosity seems to be related to the higher content of surface water caused by the exudate releases upon thawing. Although the increase in lightness constitutes a positive attribute of the meat because it improves its appearance and makes it visually more attractive (higher capacity to reflect light), the colour saturation and Hue form colour properties that together with lightness define the consumer’s acceptance and intention to buy. The changes observed in the luminosity of the ultrasound-treated muscle during long exposure times (40 min) are associated with the increase in the temperature of the muscle, which produced lighter tones due to the denaturation of the colour pigments (myoglobin and haemoglobin). This increase in temperature may not be detected in the coupling medium (water) but it could have impacted the sample, due to the effect of the energy lost as heat and the effect of cavitation. When cavitation bubbles implode high local temperatures and pressures are generated resulting in an increase in temperature in some regions of the sonicated meat even if the temperature of the ultrasound bath was kept at 4 °C. The increase in luminosity in meat as the ultrasound time increases (40 kHz, 11 W/cm2) has been documented by Caraveo et al. [21], who observed an increase in the luminosity of 1.27 cm thick bovine Semitendinosus sonicated for 60 or 90 min. In contrast, Chang et al. [22] reported no changes in the luminosity of 2.54 cm thick bovine Semitendinosus treated with ultrasound (40 kHz, 1,500 W) for 10–60 min. This discrepancy in the results appears to be associated with the power level and the thickness of the sample. Regarding the post-mortem time, the samples treated with HIU pre-freezing increased lightness immediately after HIU treatment (37 h); however, after refrigerated and frozen storage the lightness of meat decreased significantly (P = 0.0008) until values similar to 36 h post-mortem. The samples treated with HIU post-freezing significantly increased lightness at 157 h post-mortem (immediately after HIU treatment; P < 0.0001), and after refrigeration (205 h post-mortem). These results suggest that HIU treatment of frozen rabbit meat is a promising technique with positive effects on lightness of the muscle in comparison to HIU application before freezing the meat.
Table 1.
Effects of ultrasound (HIU) time, post-mortem time and HIU time*post-mortem time applied before or after freezing on the colour space CIE L* a* b* of rabbit meat. HIU was applied at 36 h post-mortem (before freezing) or 156 h post-mortem (after freezing).
| Treatment | Colour | ||||
|---|---|---|---|---|---|
| HIU pre-freezing | |||||
| HIU (min) | L* | a* | b* | Hue | Chroma |
| 0 | 58.1 ± 0.6a | 4.3 ± 0.2c | 2.6 ± 0.0c | 22.8 ± 1.2b | 5.0 ± 0.1c |
| 20 | 53.9 ± 2.1b | 8.4 ± 0.4a | 4.2 ± 0.4a | 25.5 ± 4.3b | 9.4 ± 0.2a |
| 40 | 56.2 ± 0.7b | 4.9 ± 0.0b | 3.4 ± 0.1b | 35.9 ± 0.7a | 6.9 ± 0.1b |
| Post-mortem (h) | L* | a* | b* | Hue | Chroma |
| 36 h1 | 53.4 ± 1.9b | 4.3 ± 0.3b | 1.5 ± 0.1c | 18.1 ± 3.1b | 4.6 ± 0.0c |
| 37 h2 | 59.2 ± 1.4a | 4.3 ± 0.2b | 2.9 ± 0.3b | 32.2 ± 2.7a | 5.6 ± 0.2b |
| 205 h3 | 55.5 ± 1.5b | 10.0 ± 0.4a | 5.9 ± 0.1a | 33.9 ± 3.9a | 11.1 ± 0.3a |
| HIU (min)*Post-mortem (h) | L* | a* | b* | Hue | Chroma |
| 0*36 h 0*37 h 0*205 h 20*36 h 20*37 h 20*120 h 40*36 h 40*37 h 40*120 h |
53.0 ± 1.4c 62.3 ± 1.3a 58.9 ± 0.9c 51.9 ± 2.4c 55.0 ± 3.5c 54.8 ± 2.1c 55.4 ± 3.6c 60.4 ± 3.9b 52.8 ± 3.1c |
3.1 ± 0.4f 3.3 ± 0.1f 6.4 ± 0.3d 5.7 ± 0.5d 6.7 ± 0.5c 8.0 ± 0.2b 4.0 ± 0.4e 2.8 ± 0.4f 8.0 ± 0.0b |
0.68 ± 0.0d 0.62 ± 0.0d 6.45 ± 1.0a 2.11 ± 0.4c 3.65 ± 0.1b 6.45 ± 0.3a 1.59 ± 0.4d 4.27 ± 0.2b 4.42 ± 0.1b |
12.4 ± 2.0e 10.8 ± 0.2e 45.2 ± 5.2b 20.2 ± 2.2d 28.6 ± 1.2c 27.6 ± 0.6d 21.6 ± 4.4d 57.3 ± 2.5a 28.9 ± 0.9c |
2.5 ± 0.3e 2.8 ± 0.5b 9.7 ± 0.1d 6.1 ± 0.6d 7.8 ± 0.4c 14.5 ± 0.3a 5.3 ± 0.5d 6.3 ± 0.0d 9.2 ± 0.0b |
| HIU post-freezing | |||||
| HIU (min) | L* | a* | b* | Hue | Chroma |
| 0 | 54.4 ± 0.6b | 5.8 ± 0.2b | 1.7 ± 0.0a | 16.0 ± 1.2a | 6.1 ± 0.1b |
| 20 | 55.0 ± 2.1b | 6.9 ± 0.4a | 0.8 ± 0.4c | 7.0 ± 4.3b | 7.4 ± 0.2a |
| 40 | 56.2 ± 0.7a | 4.4 ± 0.0c | 1.2 ± 0.1b | 16.0 ± 0.7a | 5.0 ± 0.1c |
| Post-mortem (h) | L* | a* | b* | Hue | Chroma |
| 156 h4 | 52.8 ± 1.9c | 5.1 ± 0.3c | 1.5 ± 0.1a | 18.9 ± 3.1a | 5.6 ± 0.0b |
| 157 h5 | 58.2 ± 1.4a | 5.7 ± 0.2b | 1.2 ± 0.3b | 11.3 ± 2.7b | 5.7 ± 0.2b |
| 205 h6 | 54.6 ± 1.5b | 6.3 ± 0.4a | 1.0 ± 0.1c | 8.8 ± 3.9b | 7.2 ± 0.3a |
| HIU (min)*Post-mortem (h | L* | a* | b* | Hue | Chroma |
| 0*36 h 0*37 h 0*205 h 20*36 h 20*37 h 20*120 h 40*36 h 40*37 h 40*120 h |
51.4 ± 0.7e 67.6 ± 0.5b 54.2 ± 0.5e 55.0 ± 1.4c 53.4 ± 0.1c 60.7 ± 2.3c 55.5 ± 1.5c 55.7 ± 1.0b 50.2 ± 0.1c |
4.3 ± 0.5e 6.7 ± 0.5b 6.3 ± 0.4b 7.6 ± 0.0a 5.7 ± 0.5c 7.5 ± 0.4a 3.5 ± 0.1e 4.6 ± 0.3d 5.1 ± 0.2d |
1.1 ± 0.2a 2.4 ± 0.4a 1.5 ± 0.1b 0.9 ± 0.0d 0.8 ± 0.2d 0.8 ± 0.1d 2.4 ± 0.2a 0.5 ± 0.1d 0.5 ± 0.2d |
14.7 ± 1.9e 19.9 ± 4.0b 13.5 ± 1.2d 6.8 ± 0.3e 8.1 ± 2.5e 6.1 ± 0.8e 35.2 ± 2a 5.9 ± 1.5e 6.8 ± 2.3e |
4.7 ± 0.3f 7.1 ± 0.2c 6.7 ± 0.2c 8.2 ± 0.0b 4.1 ± 0.0g 9.8 ± 0.0a 3.8 ± 0.4g 5.9 ± 0.5d 5.3 ± 0.1e |
36 h post-mortem: 12 h at 12 °C + 24 h at 4 °C.
37 h post-mortem: 12 h at 12 °C + 24 h at 4 °C + HIU (0, 20 or 40 min HIU until completing 1 h at 4 °C).
205 h post-mortem: 12 h at 12 °C + 24 h at 4 °C + HIU + 120 h at −20 °C (freezing) + 48 h at 4 °C (refrigeration).
156 h post-mortem: 12 h at 12 °C + 24 h at 4 °C + 120 h at −20 °C (freezing).
157 h post-mortem: 12 h at 12 °C + 24 h at 4 °C + 120 h at −20 °C + HIU (0, 20 or 40 min HIU until completing 1 h at 4 °C).
205 h post-mortem: 12 h at 12 °C + 24 h at 4 °C + 120 h at −20 °C + HIU + 48 h at 4 °C (refrigeration).
Different letters within the same column indicate significant differences between treatments (p < 0.05).
Results on the chromatic coordinates a* (+a* indicates redness, -a* indicates greeness) and b* (+b indicates yellowness, -b indicates blueness) showed significant differences between HIU times (P < 0.0001) and post-mortem duration (P < 0.0001) for both samples treated pre- and post-freezing with HIU (Table 1). The most relevant coordinate in the meat industry is a* because large positive values indicate a higher tendency towards redness, which is more attractive to the consumer. However, rabbit meat has a low myoglobin concentration (0.02% of the wet muscle weight) in comparison to larger species such as bovines (0.39–0.48 depending on the muscle type) [23]. Myoglobin contributes to the change in red colour of the meat during the ageing depending on the availability of oxygen during cold storage (bright red colour derived from oxymyoglobin and brown colour derived from metamyoglobin) [24]. Samples treated with HIU pre-freezing significantly increased the meat redness, independent of the ultrasound time (20 or 40 min), whereas the samples treated with HIU post-freezing only increased in redness with the short HIU time (20 min). Some degradation of myoglobin takes place when sonication is applied for longer times in frozen meat probably because freezing causes damage to the cells and denaturation of myoglobin, and this pigment could be more susceptible to the effects of ultrasound [25].
The tendency towards redness increased significantly with an increase in post-mortem time. In post-mortem muscle, mitochondria remain active and can influence meat colour by consuming oxygen and reducing metmyoglobin, keeping meat bright cherry and improving colour stability [26]. Thus, the combination of HIU treatment and cold storage in a freezer and fridge (HIU pre-freezing, 20 or 40 min, 120 h post-mortem) favoured the increase in redness. Fresh muscle has a higher tendency to redness than sonicated muscle, probably due to the increase in temperature in some regions of the sonicated meat as mentioned above.
The results (Table 1) showed a significant increase in the hue angle with an increase in HIU time for the samples treated with HIU before freezing (P < 0.0001). This increase was related to a tendency towards an orange colour (≅30°), which constitutes a negative quality of the muscle. Although it was expected that the samples treated pre-freezing would present red tones based on their high a* values, the high b* values (3.4–4.2, Table 1) caused orange-yellow tones. In contrast, the samples treated with HIU post-freezing had lower b* values (0.8–1.2). The meat treated with HIU after freezing for 20 min produced a significant decrease in hue angle (P < 0.0001), for which the meat presented a tendency towards red tones (hue angle < 20 °). In summary, short HIU times (20 min) post-freezing produced an improvement in the red colour and lightness of rabbit meat. This effect seems to be related to the benefits in the use of short ultrasound times after freezing the muscle, since the literature has reported a significant decrease in the red colour in frozen meat. Long ultrasound times (40 min) are negative because they cause denaturation and oxidation of the colour pigments (haemoglobin and myoglobin) [12], [27]. Cavitation produced by ultrasound can induce the formation of free radicals and lead to the oxidation of proteins and lipids [28]. Reactive oxygen species derived from lipid oxidation modify or oxidize intracellular and membrane proteins in muscle [28]. With an increase in the post-mortem time, the tone of the muscle treated with HIU pre-freezing increased significantly (P < 0.0001), producing orange tones, whereas the tone decreased significantly until the end of storage (P < 0.0001) in the samples treated post-freezing, producing red tones. Lan et al. [29] reported that supercooling of rabbit meat (-2.5 and −4 °C) significantly increased its shelf life (from 6 d with traditional refrigeration up to 36 d with supercooling). However, lightness and redness reduced notably with an increase in storage time, especially in samples supercooled at −2.5 °C. The significant increase in the formation of thiobarbituric acid reactive substances (TBARS) suggests that lipid oxidation contributes to an accelerated myoglobin oxidation [29]. A change in colour parameters as a result of freezing was also reported by Chwastowska-Siwiecka et al. [30], who observed a significant increase in yellowness and a decrease in redness in rabbit Longissimus dorsi frozen at −28 °C compared to meat stored at 4 °C. The formation of ice crystals during freezing produces and ultrastructural damage and concentrates the meat solutes, leading to changes in biochemical reactions at a cellular level and meat quality [31].
The results showed that the saturation of the colour increased significantly in the meat treated with HIU pre-freezing (P < 0.0001), independent of the treatment time (20 or 40 min). For the muscle treated with HIU post-freezing, the chroma increased significantly only when the meat was treated with HIU for 20 min (P < 0.0001), whereas long treatment times (40 min) reduced the purity of the colour up to values lower than those of the control samples without HIU. These results suggest that long times of ultrasound treatment in muscle sensitized by frozen storage, cause oxidation and denaturation of colour pigments, causing opaque tones. Changes in Hue and colour saturation in sonicated samples are related to instability of heme pigments due to ultrasound. Regarding the post-mortem time, the results also showed a significant increase in the saturation of the colour with an increase in post-mortem time (after HIU and after freezing and storage) in the meat treated with HIU before freezing. In contrast, the chroma of the meat remained without significant changes after freezing and HIU (157 h post-mortem), and then increased significantly at the end of storage (chroma = 7.2 ± 0.3) but did not match the values obtained when the meat was treated with HIU pre-freezing (11.1 ± 0.3). These high chroma values appear to be associated with the positive effect of ultrasound application prior to freezing. When the muscle is treated with ultrasound, the water migrates out of the cells due to the destruction of the muscular structure, which produces the alteration of the protein conformations and increases the scattering of light in the meat [32]. Thus, the results on the effect of ultrasound on meat colour differ notably. For example, Sikes et al. [33] found that HIU treatment (200 kHz, 48 kPa and 65 kPa of acoustic pressure) in beef loin post-rigor produced a darkening of the steaks; however, after 7 d at 4 °C the colour was similar to control samples without HIU. There was no effect of pre-rigor HIU application on the colour parameters. De Lima Alves et al. [34] observed a significant increase in lightness in samples from bovine Semitendinosus 48 h post-mortem treated with HIU on day 0 only, however, after storage (16 d, 7 °C) there were no differences. In contrast, Gómez-Salazar et al. [14] reported a significant increase in colour parameters of rabbit meat with an increase in the NaCl concentration (70–200 g NaCl/L) in the marinade solution and with the application of ultrasound (40 kHz, 12.25 W/cm2). The studies performed by Stadnik and Dolatowski [12] and Pohlman, Dikeman, and Kropf [13] found that meat treated with ultrasound had lower lightness, lower redness and an orange-yellow tone (higher tone or hue angle) compared to control samples with no ultrasound treatment. These studies were performed on bovine Semitendinosus 24 h post-mortem and bovine pectoralis 24 h post-mortem, respectively. Carrillo-Lopez et al. [35] also observed an increase in hue angle in meat treated with HIU during storage, producing orange tones in the muscle. As mentioned previously, the effect of HIU depends on the equipment and conditions used (intensity, frequency, duration, temperature, pulse or continuum), as well as the sample characteristics (muscle type, thickness, type of packaging, etc.). In the present study, the application of HIU was considered before and after freezing, for which we observed the effect of both techniques on the colour of the meat. The results suggest that post-freezing HIU application on rabbit meat produces red tones, but pale and low in lightness, whereas pre-freezing HIU application resulted in intense and light orange-yellow tones. Therefore, the application of this technology in the meat industry requires further study to determine the optimal conditions that guarantee the combined quality of colour and other quality attributes that are important to the consumer and the industry (water-holding capacity, texture, weight loss, pH, etc.).
3.2. pH
The measurement of the muscle pH is very important because it provides information on the rate of post-mortem glycolysis, in a way that during the sacrifice the pH tends towards neutrality and immediately after it decreases due to acidification Chwastowska-Siniecka et al. [30]. The final pH is an indicator of the quality and shelf life of the meat. Chwastowska-Siniecka et al. [30] obtained an initial pH (45 min post-mortem) of 6.26, which indicates a good quality of the cooled muscle, for which post-mortem acidity should range between 6.1 and 6.9. However, after cold storage the pH of the rabbit meat varied between 5.68 and 5.72.
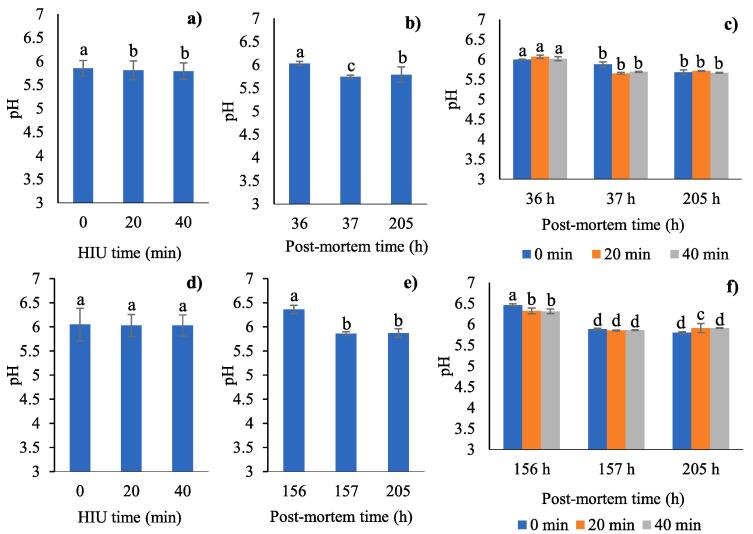
There were significant differences in pH based on HIU time, post-mortem time, and the interaction between both factors (all P < 0.0001) for the samples treated with HIU (before freezing, Fig. 1a, 1b and 1c). The pH decreased with an increase in time of treatment with HIU and post-mortem time. The initial pH of 6.03 (36 h post-mortem) decreased significantly to 5.74 after HIU treatment and then it increased to 5.79 the end of cold storage (205 h post-mortem, Fig. 1b). In living rabbits, the muscular pH is close to neutral and decreases rapidly after sacrifice, from 6.4 to 5.8 between 30 min and 24 h when the carcasses are cooled at 4 °C, so that 20 h after sacrifice the pH is considered as final. Therefore, the ultrasound-treated rabbit meat should have reached the final pH before the application of the ultrasound treatment, that is, at 36 h post-mortem. In fact, in the combination of factors (Fig. 1c) we can see more clearly that the initial pH of the muscle at 36 h post-mortem ranged between 6 and 6.07 in sonicated and non-sonicated samples before treatment with HIU. Our hypothesis is that the absence of pre-cooling after slaughter should have increased the rate of speed of the biochemical mechanisms (conversion of glycogen to lactic acid) for muscle acidification, since the rabbit carcasses remained suspended for 12 h at 12–18 °C before cooling to 4 °C. The pH values obtained are quite close to 6.0, according to Rodríguez-Calleja et al. [36] who reported pH values of 5.98 in chilled rabbit carcasses 24 h post-mortem. While Hulot et al. [6] reported a final pH between 5.45 and 5.8, depending the speed of muscle acidification during the early hours post-mortem. Rodríguez-Calleja et al. [36] attributed the slightly higher pH values to microbial growth in rabbit meat. Bobbitt [37] found Pseudomonas, lactic acid bacteria, B. thermosphacta, clostridia and S. aureus as main microorganisms in rabbit carcasses 24 h post-mortem. In the case of Pseudomonas, they use L-lactate after glucose has been depleted, growing favourably under aerobic conditions. On the other hand, post-mortem proteolysis of myofibrillar and myofibrillar-associated proteins during the resolution of rigor mortis [38] may have slightly increased the pH values in the muscle. The muscle pH tends to decrease significantly due to the effect of ultrasound treatment (from 5.85 in controls to 5.81 with 20 min of HIU), without statistical differences when the HIU time is increased to 40 min (Fig. 1a). The combination of both factors (HIU time*post-mortem time, Fig. 1c) showed final pH values of 5.67–5.71 at the end of refrigerated storage. All the final pH values were within the normal range for rabbit meat. The significant decrease in pH at the end of the refrigerated storage for all the evaluated treatments could be associated with the initial poor microbiological quality due to the incorrect post-mortem acidification, so that the lactic acid bacteria could have contributed to the decrease in the pH due to the anaerobic conditions of vacuum packaging, creating unfavourable conditions for the proliferation of other types of microorganisms such as coliforms [39]. Regarding the effect of ultrasound treatment on muscle pH, the decrease in pH of the samples treated with HIU before freezing (Fig. 1a) is related to the effect of HIU only. In this regard, Alarcon-Rojo et al. [1] reported that a discrepancy exists between results on the effect of ultrasound on the pH of meat due to the conditions under which different studies are performed. For example, Got et al. [40] observed a delay in rigor mortis (increase in pH) when bovine m. Semimembranosus was treated with high intensity and high frequency ultrasound (2.6 MHz, 10 W/cm2, 2x15 s) before rigor (pH 6.2), whereas Stadnik, Dolatowski, and Boranowska [41] found that the pH of bovine m. Semimembranosus decreased at 48 h post-mortem and subsequently increased during storage (72 and 86 h post-mortem) when it was treated with ultrasound (24 kHz, 2 W/cm2, 120 s) 24 h after sacrifice. In line with this, our results in Fig. 1a show that the pH decreased after HIU treatment.
Fig. 1.
Effects of ultrasound (HIU) time, post-mortem time and HIU time*post-mortem time applied before (a,b,c) or after (d,e,f) freezing on the pH of rabbit meat. HIU was applied only at 36 h post-mortem (before freezing) or 156 h post-mortem (after freezing). a,b,c,d Different letters in the columns within the same graph indicate significant differences between treatments (p < 0.05).
HIU time did not have a significant effect on the muscle pH in the meat treated with HIU after freezing (P = 0.5761, Fig. 1d). However, the pH decreased significantly to 5.87 at the end of the cold storage (205 h post-mortem) (P < 0.0001, Fig. 1e). Fig. 1f shows more clearly that the initial pH of the rabbit carcasses before the ultrasonic treatment was between 6.31 and 6.46, higher values than for the group of samples treated with HIU before freezing. This was due to the fact that the samples were frozen at 12 h post mortem, probably before the resolution of rigor mortis. pH values similar to ours were reported by Koziol, Maj & Bieniek [24] in rabbit Longissimus dorsi 3 h post-mortem, who obtained values of 6.25 ± 0.12. When meat is frozen prior to resolution of rigor mortis, glycolysis is not complete and the level of ATP within muscle tissue remains high, so actin and myosin do not bind to form the actin-myosin complex [42]. Muscles frozen prior to rigor mortis are characterized by higher pH values [43]. Similar to the meat treated with HIU before freezing, the meat without HIU (control) presented negligible changes in the pH during the post-mortem stage (from 5.88 to 5.8 at the end of storage), while the samples treated with HIU after freezing showed a sharp drop in pH immediately after HIU treatment (5.85–5.86) with a minimal increase at the end of cold storage (5.91). These changes in pH could be attributed to the equipment uncertainty, rather than a change in the properties of the sample. All the pH values at the end of storage are within the range of what is normal for rabbit meat. Ultrasound treatment may have favoured post-mortem proteolysis during the resolution of rigor mortis, slightly increasing the pH values in the muscle at the end of storage. Changes in pH values in frozen and stored meat have been extensively documented. Lan et al. [29] associated the initial decrease in the pH in supercooled rabbit carcasses (-4 and −2.5 °C) with the accumulation of inorganic phosphoric acid due to the accumulation of adenosine triphosphate (ATP) and the production of lactic acid as a result of the glycogenic decomposition. During the first days of storage (1–10 d) the pH increased slowly, possibly due to the production of ammoniac, amines and basic substances resulting from protein degradation [29]. The decrease in the pH during frozen storage is related to the production of exudates that induce the denaturisation of buffer protein and the freeing of H+ ions, in addition to the increase in the concentration of solutes [31].
3.3. Water-holding capacity (WHC)
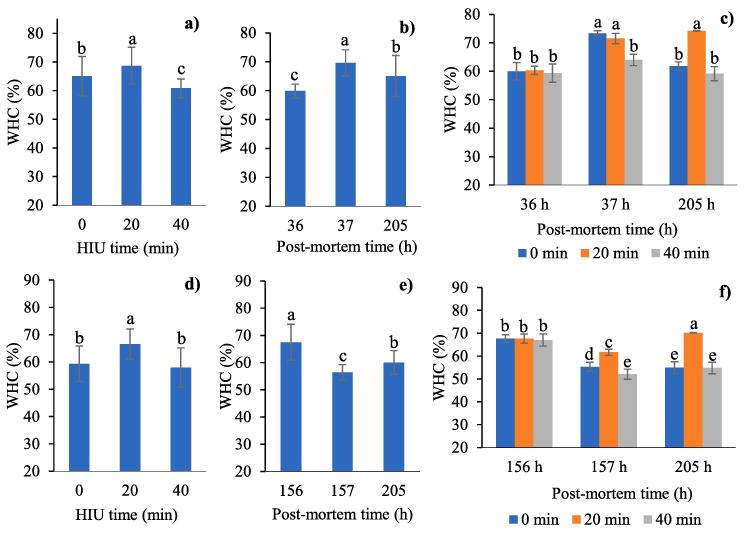
The WHC of the muscle treated with HIU before freezing increased significantly when treatment duration was short (20 min), but decreased with a long ultrasound application (40 min) compared to the control without HIU (P < 0.0001, Fig. 2a). The microstructural modifications that produce high pressures in the muscle produced the release of water as a result of the treatment during long periods of ultrasound (40 min). On the contrary, we consider that the use of short ultrasound times produced an improvement in the water holding capacity because the protein-water interaction was promoted. Several studies have revealed changes in the distribution of water in the muscle as a result of ultrasound treatment, showing that the meat loses water through exudate, pressure, or during cooking. In this regard, Chang et al. [44] reported higher amounts of exudate and water loss in bovine Semitendinosus treated with ultrasound (1500 W, 40 kHz, 20 and 30 min) compared to control, due to the alteration in the cellular structure and high pressure in the medium. Li et al. [45] observed that treatment with ultrasound (40 kHz, 300 W, 10–40 min) increased the proportions of myofibrillar water and produced protein aggregation and cavities in chicken breast meat gels with a low salt content. While using ultrasound (40 kHz, 110 W) as an assisting technology for marinating rabbit meat, Gómez-Salazar et al. [14] also found higher weight loss and a significant decrease in WHC in ultrasonicated samples.
Fig. 2.
Effects of ultrasound (HIU) time, post-mortem time and HIU time*post-mortem time applied before (a,b,c) or after (d,e,f) freezing on the water holding capacity (WHC) of rabbit meat. HIU was applied only at 36 h post-mortem (before freezing) or 156 h post-mortem (after freezing). a,b,c,d,e Different letters in the columns within the same graph indicate significant differences between treatments (p < 0.05).
WHC increased significantly (P < 0.0001) as post-mortem time increased (from 59.88 to 69.61%). However, at the end of storage the WHC decreased to 65.06% (P < 0.0001, Fig. 2b). The interaction between both factors showed a significant increase in WHC (74.17%, Fig. 2c) at the end of storage for meat treated with HIU for 20 min, which resulted to be the best treatment when HIU was applied before freezing (Fig. 2a). In contrast, meat treated with HIU for 40 min showed the lowest WHC at the end of storage, even lower than the control without HIU. Therefore, we think that the effect of ultrasound treatment during long exposure times (40 min) produced ultrastructural modifications that caused water to migrate out from the tissue. Similar as with the pH, the effect of HIU on WHC varies depending on the experimental conditions. Fig. 2b shows that the effect of HIU only increased the WHC immediately after treatment (37 h post-mortem); however, the values are lower than those obtained for the control (Fig. 2c). After freezing and cold storage (205 h post-mortem, Fig. 2c), the WHC increased at the end of storage only in those carcasses treated with 20 min HIU. In this case, HIU appears to reduce the damage produced during freezing and defrosting of the meat. In this regard, Amiri, Sharifian, and Soltanizadeh [46] reported that the increase in the duration and potency of ultrasound (100 y 300 W, 20 kHz, 10–30 min) on myofibrillar proteins of bovine meat improved the WHC due to a better water-protein interaction as a result of the smaller particle size of the myofibrillar proteins, higher pH and specific superficial area. Furthermore, Zhang et al. [47] attributed the increase in WHC in myofibrillar protein gels treated with ultrasound (200–1000 W, 15 min) to the increase in the proportion of bound water and to the cross-linking of water molecules in the pores of the homogenous structure of the protein gel. When comparing studies, it is important to consider the method used to determine WHC, because Zhang et al. [47] indicate that the use of empirical methods such as centrifuging can damage the structure, and therefore recommend the method of low field nuclear magnetic resonance because it can detect changes in the distribution and mobility of water. In agreement with Dolatowski, Stasiak and Latoch [48], there was a higher number of smaller crystals in the meat treated with ultrasound after sacrifice and before freezing compared to the untreated meat, which explains the increase in WHC at the end of storage in the meat treated with HIU for 20 min.
There were significant differences between HIU times, post-mortem time, and the interaction between both factors (all P < 0.0001, Fig. 2d, 2e and 2f) for the samples treated with HIU post-freezing. In contrast to the samples treated before freezing (Fig. 2c), the WHC of the samples treated after freezing decreased significantly after HIU treatement (157 h post-mortem, Fig. 2e). Fig. 2f shows a significant decrease in WHC in the rabbit meat treated for 40 min and the control samples immediately after treatment. Therefore, the effect on the decrease in WHC seems to be related to the damage produced during freezing, which is increased by the rupture of cell membranes that produce water loss when long ultrasound times (40 min) are used. The large decrease in WHC and water loss during cooking of frozen meat has been documented by Daszkiewicz, Kubiak, and Panfil [49] for deer Longissimus thoracis et Lumborum frozen at −26 °C for 6–10 months. According to Ali et al. [50] the loss due to dripping and thawing in chicken breast increases significantly with an increase in the number of freezing-thawing cycles (2–6 cycles). Melting during defrosting and the restructuring of crystals during freezing are detrimental because it causes mechanical damage to the cell membrane which reduces WHC. The WHC of the samples treated for 20 min decreased slightly after ultrasound and then increased significantly up to 70.17% (Fig. 2f). Thus, treating rabbit meat for a short duration (20 min) after freezing appears to attenuate the decrease in WHC caused by storage at −20 °C; in these samples the WHC of the meat was influenced by frozen storage as well as ultrasound. The increase in WHC has been related to the higher protein-water interaction, as a result of the smaller particle size of myofibrillar proteins and a higher specific surface area after short ultrasound treatments. Guo et al. [51] reported that the use of ultrasound (20 kHz, 200–600 W) as a supporting technology during the defrosting of white yak longissimus dorsi significantly decreases defrosting time, lipid oxidation, and loss of water and solutes (vitamines and minerales). Despite sever rupture of the muscle fibre membranes with the application of high potencies (600 W), the barriers between the muscle fibres were clear with the use of low potencies (200 and 400 W) and were blurred in control samples (conventional thawing). Dolatowski, Stasiak, and Latoch [48] found that weight loss decreased and WHC increased in Semimembranosus bovino treated with ultrasound before freezing, thawing, and thermic processing at 50, 60, and 70 °C. Our results were in agreement with this (Fig. 2b). Because of the pH of the control group (Fig. 1c) it is assumed that the meat was in rigor mortis during freezing, and that this caused the increase in water loss compared to the meat treated with HIU for 20 min.
3.4. Weight loss
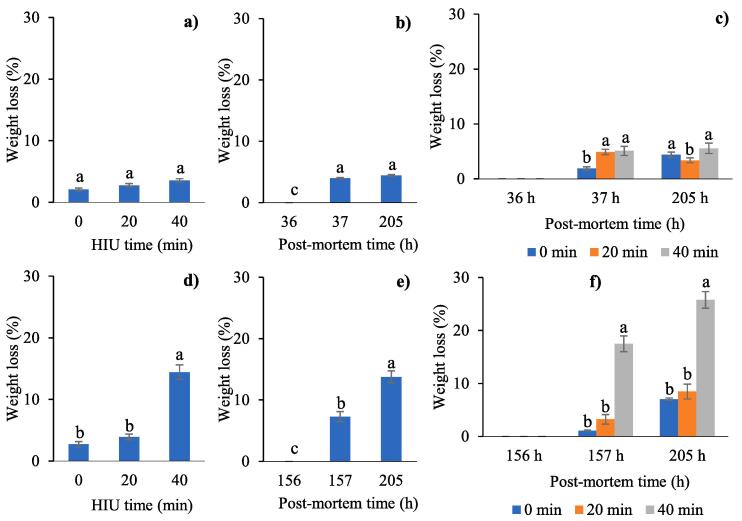
Weight loss after HIU treatment and storage in a freezer or refrigeration allows for a more precise evaluation of the yield related to the initial weight. When meat was treated with HIU before freezing the results did not show significant differences between treatment times (P = 0.1973). However, there were significant differences between post-mortem time and the interaction between both factors (P < 0.0001) for the samples treated with HIU before freezing. The weight loss of the muscle increased significantly immediately after HIU treatment (37 h post-mortem, Fig. 3b). The results shown in Fig. 3c confirm that the weight losses at 37 h post-mortem were significantly higher for the 20 and 40 min treatments. At the end of storage (205 h post-mortem) the weight losses increased only for the controls, while the samples treated with ultrasound for 20 min significantly decreased the weight losses (Fig. 3c). Weight loss in immediately ultrasound samples (37 h post-mortem) is related to the water dynamics due to cavitation, which modifies the structure of myofibrillar proteins, allowing the loss of water as exudate, by pressure or by cooking. However, the decrease in weight loss at the end of storage (156 h post-mortem) in sonicated meat seems to be related to the benefits that the application of short ultrasound times has for the general decrease in water mobility, allowing higher myofibrillar water content in matured meat. The results on weight loss are not related to the results for WHC. Fig. 2c shows a significant increase in the WHC of the muscle inmediately following HIU treatment (37 h post-mortem) for 20 min, whereas Fig. 3c shows a significant increase in weight loss in the same treatment. This is due to loss of free superficial water (extracelular water that causes dripping) during HIU treatment, which could be observed as exudate liquid in the packaging of the meat. Thus, when WHC was determined the amount of free residual water was minimal for these treatments (20 and 40 min of HIU), but the muscle still retained water within its structure, leading to high WHC values when subjected to compression treatment. The compression method to determine WHC is based on the measurement of the water expelled from the meat sample when applying a high pressure using two metacrylate plates [52]. The changes in WHC are related to the inmobile water (water on the surface of proteins, fixed by its loads and inmobilized in the myofibrillar structure), but not to the linked water, which includes constitution water and interfase water close to the proteins. The inmobile water is trapped in the muscle by capilar action located in the thick filaments and between the thick and thin filaments [53]. According to Huff-Lonergan and Lonergan [54] the water in meat is inmobilized and linked to proteins in the interfibrillar spaces, however, in the post-mortem stage the water is freed due to a decrease in pH and the loss of adenosine triphosphate (ATP) and esteric effects produced by myofibrillar contractions (rigor mortis). In this way, the water redistributes in the sacroplasmatic and extracelular spaces. The same was observed by Chwastowska-Siwiecka et al. [30], who reported lower WHC in rabbit Longissimus dorsi cooled at 4 °C compared to the muscle frozen at −28 °C and defrosted with air at 4 °C. Altough the loss due to dripping was lower in the cooled muscle, the loss during processing was higher, resulting in a less juicy product.
Fig. 3.
Effects of ultrasound (HIU) time, post-mortem time and HIU time*post-mortem time applied before (a,b,c) or after (d,e,f) freezing on weight loss of rabbit meat. HIU was applied only at 36 h post-mortem (before freezing) or 156 h post-mortem (after freezing). a,b,c Different letters in the columns within the same graph indicate significant differences between treatments (p < 0.05).
There was a significant decrease in the weight of the samples treated with HIU for 40 min (Fig. 3d, P < 0.0001). Weight losses increased significantly and gradually as the storage time advanced: immediately after the ultrasound treatment (157 h post-mortem) and at the end of the storage period (205 h post-mortem). Water losses increase during storage due to increased proteolytic activity. However, crosslinking between actin and myosin due to myofibrillar contraction during storage led to an increased amount of free water. These losses were significantly higher in samples sonicated for long times (40 min, Fig. 3f). Consequently, the WHC decreased significantly (Fig. 2f). We think that the samples were frozen 12 h post-mortem, it is assumed that this is related to the accelerated rigor mortis of the muscle, which in turn is associated with a decrease in WHC due to a decrease in the filament space [55] and changes in the permeability and osmotic phenomena of the cellular membrane. With the advance of rigor mortis, the myofibrillar space to retain water is reduced and the liquid is forced to displace towards extramyofibrillar spaces, where the water is susceptible to be lost due to dripping [54]. Degradation of cytoskeletal proteins produces an increase in cellular muscle contractions which also results in dripping. Another evidence for the modification of the muscle during the post-mortem stage is related to the pH. As can be seen in Fig. 1f, the pH of the samples treated with ultrasound 156 h post-mortem ranged between 6.31 and 6.46, compared to the samples treated with ultrasound 36 h post-mortem, whose pH values ranged between 6 and 6.07 (Fig. 1c). In the first case, it is assumed that rigor mortis had not yet resolved because the samples were frozen 12 h post-mortem. Huff-Lonergan and Lonergan [54] reported that the variation in WHC at a given pH and storage temperature was due to proteolysis and the resulting contraction of the cellular muscles, as well as the movement from water to the extracellular cavities. In the 20 min treatment and the control without HIU, the low WHC appears to be related to the rupture of cellular tissue and the formation of crystals between and inside fibres, which leads to a redistribution of water and an accumulation of solutes during freezing due to the physical change of the water [25], [31] and the excessive loss during thawing. For these treatments the weight loss was much lower compared to the samples treated with HIU for 40 min. Secci et al. [56] reported an increase in weight loss and a decrease in WHC in rabbit meat balls due to storage in a freezer (20 d, −10 °C). Dalle-Zotte et al. [57] also reported that water loss depends on the muscle type. In Longissimus lumborum they observed more water loss compared to rabbit hind legs due to a partial cleavage of the muscle fibres during the extraction of the carcass, an increase in the lipid and protein oxidation processes, and tissue lesions as a result of freezing and defrosting.
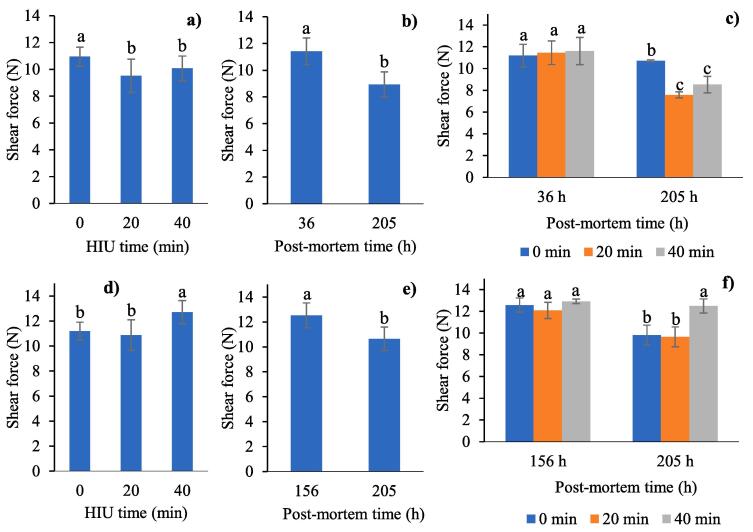
3.5. Shear force
The results showed a significant decrease in shear force in rabbit meat treated with HIU before freezing (20 or 40 min) compared to the control treatment (P = 0.042, Fig. 4a). There also was a post-mortem decrease in the toughness of the muscle (P < 0.0001), such that at the end of storage the meat was more tender for all treatments (Fig. 4b). As is shown in Fig. 4c, the 20 min treatment had a strong effect on the decrease in toughness of the muscle (from 11.45 N before HIU to 7.58 N at the end of storage). We believe there was no acceleration in the post-mortem proteolysis in the control samples without HIU as did happen in the HIU treated muscles, for which the tenderness that is associated with proteolysis of intermediate filaments and enzymatic activity (calpains) did not improve in the control samples [58], [59]. The treatment with ultrasound improves the tenderness of the meat due to fragmentation and denaturation of the collagen macromolecules [60] and the rupture of muscular tissue, which could result in migration of proteins, minerals, and other substances with the resulting acceleration in proteolysis. Many studies have shown an increase in tenderess of meat caused by ultrasound treatment [10], [44], [11]. Jayasooriya et al. [61] also found that the softening of the meat appeared to be associated with proteolysis of myofibrillar proteins by two specific proteases (calpains and cathepsins). Myofibrillar proteins such as desmin, troponin T, and titin degrade and provoke a weakening of the myofibrils and subsequent softening.
Fig. 4.
Effects of ultrasound (HIU) time, post-mortem time and HIU time*post-mortem time applied before (a,b,c) or after (d,e,f) freezing on the shear force in rabbit meat. HIU was applied only at 36 h post-mortem (before freezing) or 156 h post-mortem (after freezing). a,b,c Different letters in the columns within the same graph indicate significant differences between treatments (p < 0.05).
When meat was treated with HIU after freezing there was also a significant decrease in toughness, but only with a short treatment time (20 min). The untreated muscle also had signficantly lower shear force values. The lack of an effect on tenderness in the muscle treated for 40 min appears to be related to the excessive water loss at the end of storage (Fig. 3b). Li et al. [45] observed that longer soniction times reduce WHC and increases water losses preventing the meat to tenderize. More research is needed on the post-mortem distribution and mobility of water in the sacromer and its relation to loss due to dripping to understand why there was no proportionally inverse relation between changes in WHC and weight loss (i.e. more WHC, fewer water loss). The freezing (12 h post-mortem) -ultrasound-storage sequence seems not to be the most appropriate because ultrasound treatment increases the severe myofibrillar damage caused by ice crystals in muscles frozen at −18 °C [26]. On the other hand, the effect produced by ultrasound differs according to the post-mortem age of the muscle. Stadnik, Dolatowski, and Boranowska [41] observed advanced post-mortem changes in bovine m. Semimembranosus (24–96 h post-mortem) treated with ultrasound, suggesting that ultrasound treatment produced and acceleration in rigor mortis followed by a fragmentation of the proteic cell structures which could be concluded based on the changes in the structural elements of the sacromer. Barekat and Soltanizadeh [62] showed that ultrasound treatment (20 kHz, 100 and 300 W, 10–30 min) of bovine Longissimus lumborum causes superficial damage, however, simultaneous application with papain improves penetration of the enzyme in the deeper areas of the meat and increases the proteolytic activity to produce a more uniform and tender texture. According to Stadnik, Dolatowski, and Boranowska [41], meat exposed to ultrasound 24 h post sacrifice increased its WHC immediately after the treatment. However, the transversally applied force on the muscle fibres caused water loss from the extramyofibrillar spaces, whereas the longitudinal pressure on the muscular fibres produces water loss from the extra- and intermyofibrillar structures. Accelerated ageing and destruction of the tissue due to the ultrasound treatment produces extramyofibrillar spaces that can be occupied by water. We believe that a long HIU exposure time (40 min) had a negative effect on the muscle that was susceptible to structural damage because of freezing, added to the post-mortem phase in which the muscle was at 12 h after slaughter. Wang et al. [63] reported that the meat fibre structure collapsed graduately and the porosity and volume of the pores increased with a minimum fluctuation in temperature (-18 °C ± 1 °C) during frozen storage. According to Setyabrata and Kim [64], bovine Longissimus lumborum and Semitendinosus presented larger spaces between fibres, wide open extracelular dripping channels, and more water loss 3 d post-mortem when frozen, thawed and aged, compared to meat that was first aged and then frozen and thawed. The microstructural studies by Lan et al. [29] revealed that supercooling rabbit meat at −4 °C produces gaps and breaks in the myofibrils caused by the formation of ice crystals, with more severe damage in muscles frozen at −18 °C. Therefore, pre-freezing HIU treatment appears to slow down the effect of post-mortem freezing, which is an appropriate sequence (ultrasound-freezing-storage) for the post-mortem treatment of rabbit meat under the experimental conditions of this study. Stadnik, Dolatowski, and Boranowska [41] observed a fast rigor mortis following the fragmentation of the muscle fibre protein structure. Peña-Gonzalez et al. [65] also found and improvement in the textural properties (higher tenderness) and an increase in fragmentation of bovine Longissimus dorsi treated with ultrasound (11 W/cm2, 60 min), with a visible decrease in the size of the fascicles and larger interfibril spaces, as well as an improvement in sensorial attributes (more intensity in the aroma and oily flavour).
4. Conclusions
The application of ultrasound on rabbit meat modified quality variables depending on the post-mortem stage in which this technology was used during storage. The use of HIU before freezing produced intense and bright yellow-orange tones, whereas the post-freezing application of HIU produced pale red tones with little brightness. The high pH values after thawing of frozen muscle 12 h post-mortem suggests that the muscle was in the rigor mortis phase during freezing. Thus, HIU application accelerates the resolution of rigor mortis when applied prior to freezing and causes a significant decrease in pH immediately following the treatment. Weight loss was not directly related to the WHC of the muscle because of changes in the distribution of the water during HIU application and frozen storage. The use of HIU after freezing strongly decreased the WHC of the meat, especially when sonication times were prolonged, whereas short treatment times mitigated the effects of freezing and resulted in significant increases in WHC at the end of cold storage. The yield (weight loss) of the rabbit meat at the end of storage was not affected by applying HIU before freezing. Thus, the application of HIU before freezing is a promising technique to improve the tenderness of rabbit meat. However, more research is needed to improve the appearance (colour parameters) before scaling up to an industrial level.
CRediT authorship contribution statement
Luis M. Carrillo-Lopez: Conceptualization, Investigation, Formal analysis, Supervision, Visualization, Writing - original draft, Writing - review & editing. Danely Robledo: Investigation, Writing - original draft, Writing - review & editing. Viridiana Martínez: Investigation, Writing - original draft, Writing - review & editing. Mariana Huerta-Jimenez: Investigation, Writing - review & editing. Mieke Titulaer: Investigation, Writing - review & editing. Alma D. Alarcon-Rojo: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. America Chavez-Martinez: Investigation. Lorena Luna-Rodriguez: Resources. Luis R. Garcia-Flores: Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments:
The authors are grateful to Jesús R. Gamez-Piñon and Yair Palma-Rosas for his assistance in the slaughtering and obtention of rabbit carcasses.
Contributor Information
Luis M. Carrillo-Lopez, Email: lmcarrillo@uach.mx.
Danely Robledo, Email: cbs2143053456@titlani.uam.mx.
Viridiana Martínez, Email: 2203700290@alumnos.xoc.uam.mx.
Mariana Huerta-Jimenez, Email: mhuertaj@uach.mx.
Mieke Titulaer, Email: mtitulaer@uach.mx.
Alma D. Alarcon-Rojo, Email: aalarcon@uach.mx.
America Chavez-Martinez, Email: amchavez@uach.mx.
Lorena Luna-Rodriguez, Email: llunaro@xanum.uam.mx.
Luis R. Garcia-Flores, Email: lrgarcia@uach.mx.
References
- 1.Alarcon-Rojo A.D., Carrillo-Lopez L.M., Reyes-Villagrana R., Huerta-Jiménez M., Garcia-Galicia I.A. Ultrasound and meat quality: A review. Ultrason. Sonochem. 2019;55:369–382. doi: 10.1016/j.ultsonch.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Dalle Zotte A. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest. Prod. Sci. 2002;75(1):11–32. doi: 10.1016/S0301-6226(01)00308-6. [DOI] [Google Scholar]
- 3.Polak T., Gašperlin L., Rajar A., Zender B. Influence of genotype Lines, Age at Slaughter and Sexes on the Composition of Rabbit Meat. Food Technol. Biotechnol. 2006;44:65–73. [Google Scholar]
- 4.Warner R.D., McDonnell C.K., Bekhit A.E.D., Claus J., Vaskoska R., Sikes A., Dunshea F.R., Ha M. Systematic review of emerging and innovative technologies for meat tenderization. Meat Sci. 2017;132:72–89. doi: 10.1016/j.meatsci.2017.04.241. [DOI] [PubMed] [Google Scholar]
- 5.Effet du mode de refrigeration sur la biochimie et la contraction des muscles. 1990;11 pages:12–13. [Google Scholar]
- 6.Hulot F., Ouhayoun J. Muscular pH and related traits in rabbits: a review. World Rabbit Sci. 1999;7:15–36. doi: 10.4995/wrs.1999.378. [DOI] [Google Scholar]
- 7.A. Cabanes, J. Ouhayoun, S. Gilbert, Congélation de la viande de lapin. Influence de la durée de conservation sur les propriétés physico-chimiques et sensorielles (3, 6, 9, 12, 18 mois), Viandes Prod. Carnés 17 (1996) 166–171.
- 8.Warner R.D., Greenwood P.L., Pethick D.W., Ferguson D.M. Genetic and environmental effects on meat quality. Meat Sci. 2010;86(1):171–183. doi: 10.1016/j.meatsci.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y., Zhang W., Kang D., Zhou G. Improvement of tenderness and water holding capacity of spiced beef by the application of ultrasound during cooking. Int. J. Food Sci. Technol. 2018;53(3):828–836. doi: 10.1111/ijfs.2018.53.issue-310.1111/ijfs.13659. [DOI] [Google Scholar]
- 10.Peña-González E.M., Alarcón-Rojo A.D., Rentería A., García I., Santellano E., Quintero A., Luna L. Quality and sensory profile of ultrasound-treated beef. Ital. J. Food Sci. 2017;29:463–475. doi: 10.14674/1120-1770/ijfs.v604. [DOI] [Google Scholar]
- 11.Xiong G.-Y., Zhang L.-L., Zhang W., Wu J. Influence of ultrasound and proteolytic enzyme inhibitors on muscle degradation, tenderness, and cooking loss of hens during aging. Czech J. Food Sci. 2012;30(No. 3):195–205. [Google Scholar]
- 12.Stadnik J., Dolatowski Z.J. Influence of sonication on Warner-Bratzler shear force, colour and myoglobin of beef (m. semimembranosus) Eur. Food Res. Technol. 2011;233(4):553–559. doi: 10.1007/s00217-011-1550-5. [DOI] [Google Scholar]
- 13.Pohlman F.W., Dikeman M.E., Kropf D.H. Effects of high intensity ultrasound treatment, storage time and cooking method on shear, sensory, instrumental color and cooking properties of packaged and unpackaged beef pectoralis muscle. Meat Sci. 1997;46(1):89–100. doi: 10.1016/S0309-1740(96)00105-2. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Salazar J.A., Ochoa-Montes D.A., Cerón-García A., Ozuna C., Sosa-Morales M.E. Effect of acid marination assisted by power ultrasound on the quality of rabbit meat. J. Food Qual. 2018;2018:1–6. doi: 10.1155/2018/5754930. [DOI] [Google Scholar]
- 15.Reyes-Villagrana R.A., Huerta-Jimenez M., Salas-Carrazco J.L., Carrillo-Lopez L.M., Alarcon-Rojo A.D., Sanchez-Vega R., Garcia-Galicia I.A. High-intensity ultrasonication of rabbit carcases: a first glance into a small-scale model to improve meat quality traits, Italian. J. Anim. Sci. 2020;19(1):544–550. doi: 10.1080/1828051X.2020.1763212. [DOI] [Google Scholar]
- 16.Margulis M.A., Margulis I.M. Calorimetric method for measurement of acoustic power absorbed in a volume of a liquid. Ultrason. Sonochem. 2003;10(6):343–345. doi: 10.1016/S1350-4177(03)00100-7. [DOI] [PubMed] [Google Scholar]
- 17.AMSA . American Meat Science Association; Champaign, USA: 2012. Meat colour measurement guidelines. [Google Scholar]
- 18.TSAI T.C., OCKERMAN H.W. Water binding measurement of meat. J. Food Sci. 1981;46(3):697–701. doi: 10.1111/jfds.1981.46.issue-310.1111/j.1365-2621.1981.tb15328.x. [DOI] [Google Scholar]
- 19.second, ., editor. AMSA, Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of fresh meat. American Meat Science Association; Champaign, USA: 2016. [Google Scholar]
- 20.Cruz-Garibaldi B.Y., Alarcon-Rojo A.D., Huerta-Jimenez M., Garcia-Galicia I.A., Carrillo-Lopez L.M. Efficacy of Ultrasonic-Assisted Curing Is Dependent on Muscle Size and Ultrasonication System. Processes. 2020;8:1–15. doi: 10.3390/pr8091015. [DOI] [Google Scholar]
- 21.O. Caraveo, A.D. Alarcón-Rojo, A. Rentería, E. Santellano, L. Paniwnyk, Physicochemical and microbiological characteristics of beef treated with high-intensity ultrasound and stored at 4 °C Journal of the Science of Food and Agriculture 95 (2015) 2487-2493. http://dx.doi.org/10.1002/jsfa.6979. [DOI] [PubMed]
- 22.Chang H.-J., Xu X.-L., Zhou G.-H., Li C.-B., Huang M. Effects of Characteristics Changes of Collagen on Meat Physicochemical Properties of Beef Semitendinosus Muscle during Ultrasonic Processing. Food Bioprocess Technol. 2012;5(1):285–297. doi: 10.1007/s11947-009-0269-9. [DOI] [Google Scholar]
- 23.Van Beers R., Kokawa M., Aernouts B., Watté R., De Smet S., Saeys W. Evolution of the bulk optical properties of bovine muscles during wet aging. Meat Science 136. 2018;136:50–58. doi: 10.1016/j.meatsci.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Koziol K., Maj D., Bieniek J. Changes in the colour and pH of rabbit meat in the aging process. Med. Weter. 2015;71:104–108. [Google Scholar]
- 25.Jalang O., Saul J.W., Lawrie R.A. Observations on muscle press juice from bovine, ovine and porcine muscle. Meat Sci. 1987;21:73–76. doi: 10.1016/0309-1740(87)90043-X. [DOI] [PubMed] [Google Scholar]
- 26.Ramanathan R., Mancini R.A. Role of mitochondria in beef color: a review. Meat and Muscle Biology. 2018;2:309–320. doi: 10.22175/mmb2018.05.0013. [DOI] [Google Scholar]
- 27.Cao C., Xiao Z., Tong H., Tao X., Gu D., Wu Y., Xu Z., Ge C. Effect of ultrasound-assisted enzyme treatment on the quality of chicken breast meat. Food and Bioproducts Processing. 2021;125:193–203. doi: 10.1016/j.fbp.2020.11.005. [DOI] [Google Scholar]
- 28.Kang D.-C., Zou Y., Cheng Y., Xing L., Zhou G., Zhang W. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrasonics Sonochemistry. 2016;33:47–53. doi: 10.1016/j.ultsonch.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Lan Y., Shang Y., Song Y., Dong Q. Changes in the quality of superchilled rabbit meat stored at different temperatures. Meat Science. 2016;117:173–181. doi: 10.1016/j.meatsci.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Chwastowska-Siwiecka I., Kondratowicz J., J., A. Gugolek, P. Matusevicius, Changes in the Physicochemical Properties of Deep-Frozen Rabbit Meat as Dependent on Thawing Method. Vet. Zootech-Lith. 2013;62:68–72. [Google Scholar]
- 31.Leygonie C., Britz T.J., Hoffman L.C. Impact of freezing and thawing on the quality of meat: review. Meat Sci. 2012;91(2):93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 32.de Oliveira J., de Souza K.A., Vital A.C.P., Guerrero A., Valero M.V., Kempinski E.M.B.C., Barcelos V.C., Nascimento K.F., I.N. do Prado, Clove and rosemary essential oils and encapsuled active principles (eugenol, thymol and vanillin blend) on meat quality of feedlot-finished heifers. Meat Sci. 2017;130:50–57. doi: 10.1016/j.meatsci.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Sikes A.L., Mawson R., Stark J., Warner R. Quality properties of pre- and post-rigor beef muscle after interventions with high frequency ultrasound. Ultrason. Sonochem. 2014;21(6):2138–2143. doi: 10.1016/j.ultsonch.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 34.L. De Lima Alves, M. Stefanello da Silva, D.R. Martins Flores, D. Rodrigues Athayde, A. Roggia Ruviaro, D. da Silva Brum, V.S. Fagundes Batista, R. de Oliveira Mello, C. Ragagnin de Menezes, P.C. Bastianello Campagnol, R. Wagner, J. Smanioto Barin, A.J. Cichoski, Effect of ultrasound on the physicochemical and microbiological characteristics of Italian salami, Food Res. Int. 106 (2017) 363-373. https://doi.org/10.1016/j.foodres.2017.12.074. [DOI] [PubMed]
- 35.Carrillo-Lopez L.M., Huerta-Jimenez M., Garcia-Galicia I.A., Alarcon-Rojo A.D. Bacterial control and structural and physicochemical modification of bovine Longissimus dorsi by ultrasound. Ultrason. Sonochem. 2019;58:104608. doi: 10.1016/j.ultsonch.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez-Calleja J.M., Santos J.A., Otero A., García-López M.L. Microbiological quality of rabbit meat. J. Food Prot. 2004;67:966–971. doi: 10.4315/0362-028x-67.5.966. [DOI] [PubMed] [Google Scholar]
- 37.J. Bobbitt, Shelf life and microbiological safety of selected new and emerging meats destined for export markets. Rural Industries Research and Development Corporation publ. no. 02-038, 2002. Available at: http://www.rirdc.gov.au/reports/NAP/02–038.pdf. Accesed 22 July 2003.
- 38.Koohmaraie M., Geesink G.H. Contribution of post-mortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Science. 2006;74(1):34–43. doi: 10.1016/j.meatsci.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Cullere M., Dalle Zotte A., Tasoniero G., Giaccone V., Szendrő Z., Szín M., Odermatt M., Gerencsér Z., Dal Bosco A., Matics Z. Effect of diet and packaging system on the microbial status, pH, color and sensory traits of rabbit meat evaluated during chilled storage. Meat Science. 2018;141:36–43. doi: 10.1016/j.meatsci.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Got F., Culioli J., Berge P., Vignon X., Astruc T., Quideau J.M., Lethiecq M. Effects of high-intensity high-frequency ultrasound on ageing rate, ultrastructure and some physico-chemical properties of beef. Meat Sci. 1999;51(1):35–42. doi: 10.1016/S0309-1740(98)00094-1. [DOI] [PubMed] [Google Scholar]
- 41.Stadnik J., Dolatowski Z.J., Baranowska H.M. Effect of ultrasound treatment on water holding properties and microstructure of beef (m. semimembranosus) during ageing, LWT –. Food Sci. Technol. 2008;41(10):2151–2158. doi: 10.1016/j.lwt.2007.12.003. [DOI] [Google Scholar]
- 42.Feiner G. In: Woodhead Publishing Series in Food Science, Technology and Nutrition, Meat Products Handbook. Feiner G., editor. Woodhead Publishing; 2006. 4. Definitions of terms used in meat science and technology. [DOI] [Google Scholar]
- 43.Sabikun N., Bakhsh A., Ismail I., Hwang Y.-H., Rahman M.S., Joo S.-T. Changes in physicochemical characteristics and oxidative stability of pre- and post-rigor frozen chicken muscles during cold storage. J Food Sci Technol. 2019;56(11):4809–4816. doi: 10.1007/s13197-019-03941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang H.-J., Wang Q., Tang C.-H., Zhou G.-H. Effects of ultrasound treatment on connective tissue collagen and meat quality of beef semitendinosus muscle. J. Food Qual. 2015;38(4):256–267. doi: 10.1111/jfq.12141. [DOI] [Google Scholar]
- 45.Li K., Kang Z.-L., Zou Y.-F., Xu X.-L., Zhou G.-H. Effect of ultrasound treatment on functional properties of reduced-salt chicken breast meat batter. J. Food Sci. Technol. 2015;52(5):2622–2633. doi: 10.1007/s13197-014-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amiri A., Sharifian P., Soltanizadeh N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018;111:139–147. doi: 10.1016/j.ijbiomac.2017.12.167. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z., Regenstein M.J., Zhou P., Yang Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017;34:960–967. doi: 10.1016/j.ultsonch.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Dolatowski Z., Stasiak D.M., Latoch A. Effect of ultrasound processing of meat before freezing on its texture after thawing. Electron J. Pol. Agric. Univ. 2000;3 http://www.ejpau.media.pl [Google Scholar]
- 49.Daszkiewicz T., Kubiak D., Panfil A. The effect of long-term frozen storage on the quality of meat (Longissimus thoracis et Lumborum) from female roe deer (Capreolus capreolus L.) J Food Qual. 2018;2018:1–7. doi: 10.1155/2018/4691542. [DOI] [Google Scholar]
- 50.Ali S., Rajput N., Li C., Zhang W., Zhou G. Effect of Freeze-Thaw Cycles on Lipid Oxidation and Myowater in Broiler Chickens, Brazilian. Journal of Poultry Science. 2016;18(1):35–40. doi: 10.1590/1516-635x1801035-040. [DOI] [Google Scholar]
- 51.Guo Z., Ge X., Yang L., Ma G., Ma J., Yu Q.-li., Han L. Ultrasound-assisted thawing of frozen white yak meat: Effects on thawing rate, meat quality, nutrients, and microstructure. Ultrason. Sonochem. 2021;70:105345. doi: 10.1016/j.ultsonch.2020.105345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barge M.T., Destefanis G., Toscano G.P., Brugiapaglia A. Two reading techniques of the filter paper press method for measuring meat water-holding capacity. Meat Science. 1991;29(2):183–189. doi: 10.1016/0309-1740(91)90065-X. [DOI] [PubMed] [Google Scholar]
- 53.Hamm R. In: Muscle as food. Bechtel P.J., editor. Academic Press; New York: 1986. Functional properties of the myofibrillar system and their measurements; pp. 135–199. [Google Scholar]
- 54.Huff-Lonergan E., Lonergan S.M. Mechanism of water- holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Science. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Honikel K.O., Kim C.J., Hamm R., Roncales P. Sarcomere shortening of prerigor muscles and its influence on drip loss. Meat Sci. 1986;16(4):267–282. doi: 10.1016/0309-1740(86)90038-0. [DOI] [PubMed] [Google Scholar]
- 56.Secci G., Bovera F., Musco N., Husein Y., Parisi G. Use of mirrors into free-range areas: effects on rabbit meat quality and storage stability. Livest. Sci. 2020;239:104094. doi: 10.1016/j.livsci.2020.104094. [DOI] [Google Scholar]
- 57.Dalle Zotte A., Cullere M., Rémignon H., Alberghini L., Paci G. Meat physical quality and muscle fibre properties of rabbit meat as affected by the sire breed, season, parity order and gender in an organic production system, World Rabbit Sci. [S.l.] 2016;24(2):145. doi: 10.4995/wrs.2016.4300. [DOI] [Google Scholar]
- 58.Melody J.L., Lonergan S.M., Rowe L.J., Huiatt T.W., Mayes M.S., Huff-Lonergan E. Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. J. Anim. Sci. 2004;8:1195–1205. doi: 10.2527/2004.8241195x. [DOI] [PubMed] [Google Scholar]
- 59.Huff-Lonergan E., Lonergan S.M. In: Quality attributes of muscle foods. Xiong Y.L., Ho C.-.-T., Shahidi F., editors. Kluwer Academic/Plenum Publishers; New York: 1999. Postmortem mechanisms of meat tenderization: the roles of the structural proteins and the calpain system; pp. 229–251. [Google Scholar]
- 60.LYNG J.G., ALLEN P., McKENNA B.M. The influence of high intensity ultrasound baths on aspects of beef tenderness. J. Muscle Foods. 1997;8(3):237–249. doi: 10.1111/jmf.1997.8.issue-310.1111/j.1745-4573.1997.tb00630.x. [DOI] [Google Scholar]
- 61.Jayasooriya S.D., Bhandari B.R., Torley P., D'Arcy B.R. Effect of high power ultrasound waves on properties of meat: a review. Int. J. Food Prop. 2004;7(2):301–319. doi: 10.1081/JFP-120030039. [DOI] [Google Scholar]
- 62.Barekat S., Soltanizadeh N. Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innov. Food Sci. Emerg. Technol. 2017;39:223–229. doi: 10.1016/j.ifset.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y., Liang H., Xu R.Y., Lu B.Y., Song X.Y., Liu B.L. Effects of temperature fluctuations on the meat quality and muscle microstructure of frozen beef. Int. J. Refrig. 2020;116:1–8. doi: 10.1016/j.ijrefrig.2019.12.025. [DOI] [Google Scholar]
- 64.Setyabrata D., Kim Y.H.B. Impacts of aging/freezing sequence on microstructure, protein degradation and physico-chemical properties of beef muscles. Meat Sci. 2019;151:64–74. doi: 10.1016/j.meatsci.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 65.Peña-Gonzalez E., Alarcon-Rojo A.D., Garcia-Galicia I., Carrillo-Lopez L., Huerta-Jimenez M. Ultrasound as a potential process to tenderize beef: sensory and technological parameters. Ultrason. Sonochem. 2019;53:134–141. doi: 10.1016/j.ultsonch.2018.12.045. [DOI] [PubMed] [Google Scholar]