Abstract
Forty-eight pneumococci were genotyped by on-line laser fluorescence amplified-fragment length polymorphism (AFLP) and pulsed-field gel electrophoresis (PFGE) analysis of chromosomal restriction fragments. Overall, the data generated by the two methods corresponded well. However, with AFLP, clusters were delineated at a higher similarity level, and isolate differentiation was more pronounced. AFLP and PFGE were equally efficient for assessing intraserotype diversity. We conclude that AFLP is a useful alternative to PFGE.
Various molecular typing methods for pneumococci have been described previously (3, 9, 12, 13, 21, 30, 31). Among these, pulsed-field gel electrophoresis (PFGE) analysis of chromosomal restriction fragments has been shown to be highly informative in various epidemiological studies of Streptococcus pneumoniae (4, 11, 27, 28, 33). Consensus guidelines for interpreting restriction patterns produced by PFGE in an outbreak have been put forward (29), and the use of PFGE as a method for typing pneumococci has been evaluated in a comparative study by Hermans et al. (13). However, PFGE is both labor-intensive and time-consuming and is difficult to automate. Consequently, alternative methods are needed that would provide the same reproducibility and discriminative power, allow large numbers of isolates to be processed in a relatively short period of time, preferably in an automated fashion, and facilitate the storage and comparison of large amounts of data. Amplified-fragment length polymorphism (AFLP) produces stable and highly complex banding patterns and has been evaluated previously for genotypic characterization of bacteria (18). The technique has also been shown to be useful in epidemiological and taxonomic studies (1, 8, 14, 16, 17, 20, 25). Here we compare AFLP to PFGE as a method for typing pneumococci in epidemiological studies.
We examined 48 S. pneumoniae isolates from invasive infections, collected in 1994 in France and Belgium. Isolates were grown overnight on 5% horse blood Mueller-Hinton agar at 37°C under conditions of 5% CO2. Susceptibility testing was performed by the disk diffusion method according to National Committee for Clinical Laboratory Standards guidelines (22). Penicillin MICs were determined with the Epsilon test (15). Pneumococci were classified as penicillin sensitive (Peni-S), penicillin resistant (Peni-R), or intermediately resistant (Peni-I). Serotyping was performed by capsular swelling (Quellung reaction) (22a) with factor-specific antiserum (Statens Seruminstitute, Copenhagen, Denmark).
Fresh cultures for DNA preparation for PFGE were obtained by the inoculation of 2 ml of brain heart infusion (BHI) broth and further growth at 37°C for 4.5 h. Cells were directly embedded in low-melting-temperature agarose (Bio-Rad Labs, Richmond, Va.) as described previously (19). Agarose-embedded DNA was digested with 10 U of SmaI at 30°C for 4 h. PFGE of the restriction fragments was carried out with a contour-clamped homogeneous electric field (CHEF) by using a CHEF-DR III system (Bio-Rad Labs), with alternating pulses at 120°. Electrophoresis was done in 1% (wt/vol) agarose and 0.5× TBE (Tris-borate–EDTA) buffer at 14°C and 200 V. A linearly ramped switching time from 2 to 10 s was applied for 10 h, followed by a linearly ramped switching time from 10 to 20 s for the next 10 h. SmaI-restricted DNA from Staphylococcus aureus NCTC 8325 was used as the standard marker for intergel comparison. Gels were stained with ethidium bromide (0.4 μg/ml) and photographed under UV transillumination (Image Store 5000; Ultra Violet Products, Upland, Calif.). Images were stored in Tagged Image File Format (TIFF) with a horizontal (Xres) and vertical (Yres) resolution of 760 and 500 data points and processed by using GelCompar version 4.1 software (Applied Maths, Kortrijk, Belgium). Conversion to GelCompar format was done with a track resolution of 400. For normalization, the Yres of the TIFF files was further reduced to 350. After normalization and background subtraction, bands were assigned automatically by using band search filter settings at a minimal profiling of 5% and a minimal area of 0.5%. Small variations in band migration occasionally required manual adjustment, with the original photograph used as a reference. Comparative pairwise analysis of PFGE patterns was performed with the Dice band-based similarity coefficient (SD), by using a band position tolerance of 0.8%. All dendrograms were calculated by the unweighted pair-group method by using average linkages (UPGMA) (26).
For AFLP, DNA was prepared according to the method of Pitcher et al. (23) with slight modifications. Briefly, 2 ml of cells freshly grown in BHI broth was centrifuged, washed in 500 μl of resuspension buffer (150 mM NaCl, 10 mM EDTA [pH 8.0]), and incubated in 100 μl of mutanolysine solution (20 μg/ml) for 1 h at 37°C. Five hundred microliters of GES (5 M guanidium thiocyanate, 100 mM EDTA, 0.5% [vol/vol] sarcosyl) was added, and cells were kept on ice for 10 min and incubated overnight at 37°C with 130 μg of proteinase K. Cell debris was removed by phenol-chloroform extraction, and DNA was precipitated with 1.5 M ammonium acetate (pH 7.5) and 0.54 volume of isopropanol. The DNA was washed three times with 75% (vol/vol) ethanol, dried, and redissolved in 100 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 7.6]) with 25 μl DNase-free RNase (0.25 mg/ml) for 1.5 h at 37°C. Preparation of AFLP templates was performed as described previously (18). Briefly, 1 μg of DNA was digested with HindIII and TaqI. Subsequently, double-stranded restriction halfsite-specific adapters were ligated to the restriction fragments (32). Adapters were partially complementary oligonucleotides 5′-CTCGTAGACTGCGTACC-3′ and 5′-AGCTGGTACGCAGTC-3′ (for HindIII) and 5′-GACGATGAGTCCTGAC-3′ and 5′-CGGTCAGGACTCAT-3′ (for TaqI). Template DNA was precipitated with ethanol, dissolved in 100 μl of T0.1E buffer, and stored at −20°C. HindIII-TaqI restriction fragments tagged with specific adapters were selectively amplified with primers H01 (5′-GACTGCGTACCAGCTTa-3′) and T01 (5′-GATGAGTCCTGACCGAa-3′) (selective bases are shown in lowercase letters). Primer H01 was obtained from Pharmacia Biotech (Roosendaal, The Netherlands) as a 5′-Cy5-labeled oligonucleotide. All other primers and adapters were from EuroGentec (Seraing, Belgium). Each amplification reaction mixture (10 μl) contained 20 ng of template DNA, 30 ng of primer T01, 6.25 ng of Cy5-labeled primer H01, 100 μM (each) dATP, dCTP, dGTP, and dTTP, 10 mM Tris (pH 8.4), 1.5 mM MgCl2, 50 mM KCl, and 0.7 U of AmpliTaq (Perkin-Elmer, Foster City, Calif.). PCR was performed on a DNA thermal cycler model 480 (Perkin-Elmer). The reaction profile was as follows: 1 cycle of 60 s at 94°C, 30 s at 65°C, and 60 s at 72°C; 8 cycles of 30 s at 94°C, 30 s at an annealing temperature 1°C lower than that of the previous cycle (starting at 64°C), and 60 s at 72°C; and 27 cycles of 30 s at 94°C, 30 s at 56°C, and 60 s at 72°C. Reaction mixtures were overlaid with Ampliwax (Perkin-Elmer) to prevent evaporation. After completion of the PCR, 5 μl of each reaction mixture was mixed with 3 μl of loading dye (Pharmacia), denatured at 95°C for 3 min, and applied to the gel.
Selectively amplified fragments were separated through a 6% (wt/vol) polyacrylamide-7 M urea denaturing slab gel (0.3 mm) (40% Bio-Rad acrylamide-bis solution [29:1]) in 0.6× TBE buffer on an ALFexpress DNA sequencer (Pharmacia). Separation was done at 1,500 V, 60 mA, and 25 W for 700 min at 55°C. Fluorescence signals were collected every 2 s, digitized, and sent to the computer for processing and storage. A fluorescein-labeled molecular size marker (Cy5 Sizer 50-500; Pharmacia) comprising 10 fragments in the size range of 50 to 500 bp was used as an external size marker.
Raw data were converted to TIFF format with the alf2tif conversion program (ALFwin version 1.10; Pharmacia) and a resampling value of 10. The resulting dimensions of the gel were 680 by 2,101 data points. The track resolution (Yres) in the GelCompar conversion program was reduced from 2,101 to 1,400 data points, and further to 1,350 data points after normalization. Of these 1,350 data points, only those between the 152nd and 1,334th data points were analyzed, thus excluding the primer front and some of the very large fragments. S. pneumoniae 42984 was included every five lanes in each gel to allow inter- and intragel comparison. After normalization and subtraction of background values, the level of similarity between these reference patterns was at least 94% within the same gel and at least 90% between gels by using the Pearson product-moment correlation coefficient (r). These values should be considered as identity levels (8). Comparative analysis of AFLP patterns obtained with the clinical isolates was also done with the Pearson product-moment correlation coefficient. The Pearson product-moment correlation coefficient was chosen rather than the Dice coefficient of similarity because of the high level of complexity of the AFLP patterns (60 to 70 bands for each pattern). Dendrograms were calculated by the same method (UPGMA) that was used for the PFGE analysis.
Of 48 isolates tested (seven of serotype 6, nine of serotype 9, nine of serotype 14, eight of serotype 19, and 15 of serotype 23F), 29% were Peni-I and 16% were Peni-R. Serotypes 9 and 23F contained 29 and 49% of the Peni-R pneumococci. None of the serotype 19 isolates in this study were Peni-R.
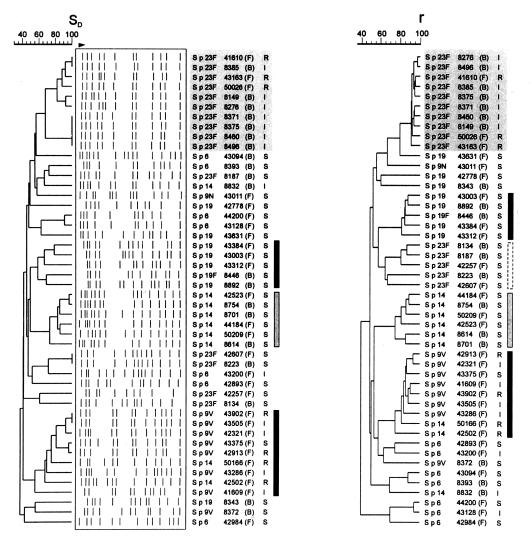
Digestion of S. pneumoniae DNA with SmaI gave 10 to 15 fragments with sizes between 20 and 360 kb. For the 48 isolates, 42 different PFGE restriction patterns were found. The overall genetic similarity, as defined by the Dice coefficient of similarity (SD) of the isolates, was 40%. At the 60% level of similarity, four clusters containing four or more isolates were found (Fig. 1). All 10 Peni-R or Peni-I 23F isolates clearly grouped together, with an SD of 66 to 100%. The remaining five Peni-S 23F isolates were scattered throughout the dendrogram. Five of the eight serotype 19 isolates—two from Belgium and three from France—were placed in a distinct cluster, with a minimal similarity level of 57%. All six Peni-S type 14 isolates (three from Belgium and three from France) grouped tightly together, with an SD of 70% or more, and were also clearly separated from the Peni-R type 14 isolates. Two of the three Peni-R type 14 isolates (no. 50166 and 42502, both from France) clustered with all seven Peni-R type 9V isolates, forming a cluster at a minimal level of similarity of 59%.
FIG. 1.
Dendrograms constructed by using the UPGMA algorithm based on the Dice coefficients (SD) obtained after pairwise comparison of PFGE macrorestriction patterns (left) and on the Pearson product-moment correlations (r) obtained after pairwise comparison of AFLP electropherograms (right). The capsular serotype, strain code number, origin (F, France; B, Belgium), and penicillin susceptibility (R, resistant; I, intermediate; S, susceptible) are also given. Clusters containing isolates at a higher-than-60% level of similarity are indicated by shading (serotype 23F Peni-R strains) or by vertical bars.
With PFGE, serotype 6 isolates did not group together and showed a low-to-moderate similarity to the other pneumococci in this study (SD = 30 to 68%). At an 80% level of similarity, three clusters were visible; the first cluster contained six Peni-I or Peni-R type 23F isolates, the second cluster contained five Peni-S type 14 isolates, and the third cluster contained five Peni-S, Peni-I, or Peni-R type 9V isolates.
For AFLP, the settings for converting the ALF data to the Gelcompar format resulted in approximately 70 peaks (with sizes of 50 to 600 bp) eluting after 105 to 660 min. All fluorograms were of high resolution, with clear separation of all peaks. The overall genetic similarity of the isolates, as defined by the Pearson product-moment correlation coefficient, was 40% and comparable to that obtained by PFGE. At the 60% level of similarity, five clusters of four or more isolates were found (Fig. 1). The 10 Peni-R or Peni-I type 23F isolates grouped together at a similarity level of 90%, whereas the five Peni-S serotype 23F isolates formed a separate group, with an average similarity level of 72%. Five Peni-S serotype 19 isolates, six Peni-S serotype 14 isolates, and seven Peni-R serotype 9 isolates formed three distinct clusters, with minimal similarity levels of 80, 76, and 79%, respectively. Two Peni-R serotype 14 isolates (no. 50166 and 42502) grouped together with the seven Peni-R serotype 9 isolates at similarity levels of 78 and 76%, respectively. Although serotype 6 isolates did not form a distinct group, isolates 42893 and 43200, 43094 and 8393, and 44200 and 43128 were highly similar to each other (Fig. 1).
With AFLP, at an 80% level of similarity, four clusters could be discerned; the first cluster contained all 10 Peni-I or Peni-R type 23F isolates. The second cluster contained four Peni-S 19 isolates and a single Peni-S 19F isolate, the third cluster contained four Peni-S type 14 isolates, and the last cluster contained seven Peni-S, Peni-I, or Peni-R type 9V isolates plus a single Peni-R type 14 isolate.
Assuming an 8-h working day, the turnaround time from starting the broth culture to starting the PFGE gel was 2.5 days. The net total time of all the manipulations and reactions prior to starting the PFGE gel was 31 h. For AFLP, the turnaround time was 2.75 days. The net total time was also 31 h. Run times of the gels were 20 h for PFGE versus 12 h for AFLP. In addition, the AFLP gel can accommodate 33 isolates plus 7 external standards versus only 12 isolates and 3 standards for most PFGE systems.
Typeability for both methods was 100%. The reproducibility of PFGE for a band position tolerance of 0.8% was 100%. For AFLP, 100% reproducibility was obtained at a Pearson correlation coefficient value of 90. At a clone cut-off value for the Dice coefficient of 80%, the discriminatory power for PFGE was 0.95 versus 0.9 for AFLP at the same clone cut-off value for the Pearson coefficient.
The criteria by which different genotyping techniques are usually evaluated are performance (reproducibility, typeability, discriminating power, and interpretability) and convenience (ease of use, hands-on time, rapidity, and cost). PFGE is sensitive, with good reproducibility and well-standardized guidelines for data acquisition and comparison, and it is certainly applicable to the typing of many different species. For certain species, e.g., S. aureus, PFGE typing has even become the “gold standard” (2). For typing of S. pneumoniae, Hermans et al. (13) compared PFGE to four other DNA fingerprinting techniques. These researchers found the highest discriminatory power for BOX PCR, PFGE, and restriction fragment end labeling (RFEL). Because of the ease with which the fingerprint could be computerized and the accuracy of data analysis, Hermans et al. preferred BOX PCR and RFEL over PFGE. PFGE was also found to be time-consuming and laborious, with a limited sample throughput. In addition, the lack of a sufficient number of DNA bands common to all pneumococci, which could serve as internal standards, was considered a limiting factor for computerized analysis of PFGE data.
AFLP combines the advantages of a high degree of discriminatory power with an acceptable turnaround time. The turnaround time reported in this study represents the time elapsed in the normal setting of a routine diagnostic lab and was comparable for both techniques. Two groups have reported rapid (24 h) procedures for PFGE (5, 10) that combine colony selection directly from the plates and reduction of the time for cell lysis, DNA restriction, and running of the gel. A rapid method for DNA extraction for AFLP was recently reported by Clerc et al. (6). Because of the fully computerized data acquisition and analysis inherent in AFLP, there is less risk of observer variability in band identification than by PFGE, facilitating intercenter comparison of data. Other advantages are the use of a nonradioactive label and applicability to many different species. This contrasts, for example, to PCR for the S. pneumoniae-specific BOX segment. An often-reported disadvantage of PCR-based methods, particularly with gram-positive species, is lack of reproducibility. We did not observe this for AFLP, probably due to the stringent PCR conditions and the perfect match of the AFLP primers. Another advantage of AFLP is the high number of bands generated (60 to 70). It has been suggested that the banding pattern generated might be complex enough to provide a quantitative measure of microevolutionary changes (18, 19, 24).
In our study we have used external standards to normalize AFLP patterns. A possible alternative is the use of common pneumococcal fragments for normalization of the lanes. Fragments with identical positions in all isolates were indeed present in all isolates. Another possibility for simplifying the normalization of the lanes and the accurate sizing of the fragments is the use of internal standards marked with a different dye that can be added to every lane without interference with the isolate pattern. Desai et al. (7) recently reported on AFLP with an internal standard in each run for analysis of an outbreak of group A streptococcal invasive disease and found it to be superior to PFGE. Their technique requires the use of a specific sequencing apparatus that has the capacity to detect two different fluorescent dyes. The ALF sequencing apparatus in our study (approximately $57,000) is less expensive than the system used by Desai et al. (7) (approximately $113,500). It is used in many laboratories but detects only one dye. New developments of automated sequencing systems that will further facilitate the use of AFLP as a typing method are automated sample loading and higher throughput of samples, as for example in the CEQ 2000 DNA analysis system (Beckman Coulter Inc., Fullerton, Calif.).
The clusters of related isolates that were found with PFGE were identical to those identified by AFLP. Nevertheless, we found small differences between AFLP and PFGE in the similarity values between isolates within the same cluster and in the genetic similarity values between clusters. By AFLP the genetic dissimilarity between separate clusters was greater than by PFGE. At the same time, however, the genetic similarity between isolates within the same cluster was greater in AFLP (80%) than in PFGE (60%), leading to a lower value for the discriminatory index in AFLP versus PFGE. It should be kept in mind, however, that assessment of the discriminatory power presumes the inclusion of unrelated isolates. This was, for example, not true for the Peni-R and Peni-I 23F isolates that were included in this study.
We conclude that AFLP is a useful alternative to PFGE and other techniques for the molecular typing of S. pneumoniae. It combines the sensitivity and performance of PFGE with more advanced automation, higher throughput, and fully computerized data analysis.
Acknowledgments
This work was financially supported in part by the R. van Furth Chair in Infectious Diseases (W. E. Peetermans) and the Glaxo-Wellcome Chair in Medical Microbiology (J. Van Eldere).
P. Geslin, Centre National De Réference des Pneumocoques, Centre Hospitalier Intercommunal Créteil, France, kindly provided French isolates. Belgian isolates were from the Belgian National Reference Laboratory for Pneumococci (L. Verbist, J. Verhaegen, and J. Van Eldere).
REFERENCES
- 1.Aarts H J, van Lith L A, Keijer J. High-resolution genotyping of Salmonella isolates by AFLP-fingerprinting. Lett Appl Microbiol. 1998;26:131–135. doi: 10.1046/j.1472-765x.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman T L, Hancock G A, Tenover F C, Miller J M. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos J, Fuste M C, Trujillo G, Saez-Nieto J, Vazquez J, Loren J G, Vinas M, Spratt B G. Genetic diversity of penicillin-resistant Neisseria meningitidis. J Infect Dis. 1992;166:173–177. doi: 10.1093/infdis/166.1.173. [DOI] [PubMed] [Google Scholar]
- 4.Carvalho C, Geslin P, Vaz-Pato M V. Pulsed field gel electrophoresis in Streptococcus pneumoniae isolated in France and Portugal. Pathol Biol (Paris) 1996;44:430–434. [PubMed] [Google Scholar]
- 5.Chang N, Chui L. A standardized protocol for the rapid preparation of bacterial DNA for pulsed-field gel electrophoresis. Diagn Microbiol Infect Dis. 1998;31:275–279. doi: 10.1016/s0732-8893(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 6.Clerc A, Manceau C, Nesme X. Comparison of randomly amplified polymorphic DNA with amplified fragment length polymorphism to assess genetic diversity and genetic relatedness within genospecies III of Pseudomonas syringae. Appl Environ Microbiol. 1998;64:1180–1187. doi: 10.1128/aem.64.4.1180-1187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai M, Tanna A, Wall R, Efstratiou A, George R, Stanley J. Fluorescent amplified-fragment length polymorphism analysis of an outbreak of group A streptococcal invasive disease. J Clin Microbiol. 1998;36:3133–3137. doi: 10.1128/jcm.36.11.3133-3137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkshoorn L, Aucken H, Gerner-Smidt P, Janssen P, Kaufmann M E, Garaizar J, Ursing J, Pitt T L. Comparison of outbreak and nonoutbreak Acinetobacter baumannii isolates by genotypic and phenotypic methods. J Clin Microbiol. 1996;34:1519–1525. doi: 10.1128/jcm.34.6.1519-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doit C, Denamur E, Picard B, Geslin P, Elion J, Bingen E. Mechanisms of the spread of penicillin resistance in Streptococcus pneumoniae strains causing meningitis in children in France. J Infect Dis. 1996;174:520–528. doi: 10.1093/infdis/174.3.520. [DOI] [PubMed] [Google Scholar]
- 10.Gautom R K. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J Clin Microbiol. 1997;35:2977–2980. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall L M, Whiley R A, Duke B, George R C, Efstratiou A. Genetic relatedness within and between serotypes of Streptococcus pneumoniae from the United Kingdom: analysis of multilocus enzyme electrophoresis, pulsed-field gel electrophoresis, and antimicrobial resistance patterns. J Clin Microbiol. 1996;34:853–859. doi: 10.1128/jcm.34.4.853-859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harakeh H, Bosley G S, Keihlbauch J A, Fields B S. Heterogeneity of rRNA gene restriction patterns of multiresistant serotype 6B Streptococcus pneumoniae strains. J Clin Microbiol. 1994;32:3046–3048. doi: 10.1128/jcm.32.12.3046-3048.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermans P, Sluijter M, Hoogenboezem T, Heersma H, van Belkum A, de Groot R. Comparative study of five different DNA fingerprint techniques for molecular typing of Streptococcus pneumoniae isolates. J Clin Microbiol. 1995;33:1606–1612. doi: 10.1128/jcm.33.6.1606-1612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huys G, Coopman R, Janssen P, Kersters K. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int J Syst Bacteriol. 1996;46:572–580. doi: 10.1099/00207713-46-2-572. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs M R, Bajaksouzian S, Appelbaum P C, Bolmstrom A. Evaluation of the E-Test for susceptibility testing of pneumococci. Diagn Microbiol Infect Dis. 1992;15:473–478. doi: 10.1016/0732-8893(92)90093-9. [DOI] [PubMed] [Google Scholar]
- 16.Janssen P, Dijkshoorn L. High resolution DNA fingerprinting of Acinetobacter outbreak isolates. FEMS Microbiol Lett. 1996;142:191–194. doi: 10.1111/j.1574-6968.1996.tb08429.x. [DOI] [PubMed] [Google Scholar]
- 17.Janssen P, Maquelin K, Coopman R, Tjernberg I, Bouvet P, Kersters K, Dijkshoorn L. Discrimination of Acinetobacter genomic species by AFLP fingerprinting. Int J Syst Bacteriol. 1997;47:1179–1187. doi: 10.1099/00207713-47-4-1179. [DOI] [PubMed] [Google Scholar]
- 18.Janssen P, Coopman R, Huys G, Swings J, Bleeker M, Vos P, Zabeau M, Kersters K. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology. 1996;142:1881–1893. doi: 10.1099/13500872-142-7-1881. [DOI] [PubMed] [Google Scholar]
- 19.Keim P, Kalif A, Schupp J, Hill K, Travis S E, Richmond K, Adair D M, Hugh-Jones M, Kuske C R, Jackson P. Molecular evolution and diversity in Bacillus anthracis as detected by amplified fragment length polymorphism markers. J Bacteriol. 1997;179:818–824. doi: 10.1128/jb.179.3.818-824.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koeleman J G, Parlevliet G A, Dijkshoorn L, Savelkoul P H, Vandenbroucke-Grauls C M. Nosocomial outbreak of multi-resistant Acinetobacter baumannii on a surgical ward: epidemiology and risk factors for acquisition. J Hosp Infect. 1997;37:113–123. doi: 10.1016/s0195-6701(97)90181-x. [DOI] [PubMed] [Google Scholar]
- 21.Lefevre J C, Faucon G, Sicard A M, Gasc A M. DNA fingerprinting of Streptococcus pneumoniae isolates by pulsed-field gel electrophoresis. J Clin Microbiol. 1993;31:2724–2728. doi: 10.1128/jcm.31.10.2724-2728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: eighth informational supplement. M100-S8. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 22a.Neufeld F. Ueber die Agglutination der Pneumokokken und über die Theorien der Agglutination. Z Hyg Infektionskr. 1902;40:54–72. [Google Scholar]
- 23.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 24.Restrepo S, Duque M, Tohme J, Verdier V. AFLP fingerprinting: an efficient technique for detecting genetic variation of Xanthomonas axonopodis pv. manihotis. Microbiology. 1999;145:107–114. doi: 10.1099/13500872-145-1-107. [DOI] [PubMed] [Google Scholar]
- 25.Sloos J H, Janssen P, van Boven C P A, Dijkshoorn L. AFLPTM typing of Staphylococcus epidermidis in multiple sequential blood cultures. Res Microbiol. 1998;149:221–228. doi: 10.1016/s0923-2508(98)80082-x. [DOI] [PubMed] [Google Scholar]
- 26.Sneath P H A, Sokal R R. Numerical taxonomy: the principles and practice of numerical classification. W. H. San Francisco, Calif: Freeman; 1973. [Google Scholar]
- 27.Soares S, Kristinsson K G, Musser J M, Tomasz A. Evidence for the introduction of a multiresistant clone of serotype 6B Streptococcus pneumoniae from Spain to Iceland in the late 1980s. J Infect Dis. 1993;168:158–163. doi: 10.1093/infdis/168.1.158. [DOI] [PubMed] [Google Scholar]
- 28.Tarasi A, Sterk-Kuzmanovic N, Sieradzki K, Schoenwald S, Austrian R, Tomasz A. Penicillin-resistant and multidrug-resistant Streptococcus pneumoniae in a pediatric hospital in Zagreb, Croatia. Microb Drug Resist. 1995;1:169–176. doi: 10.1089/mdr.1995.1.169. [DOI] [PubMed] [Google Scholar]
- 29.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial isolate typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Belkum A, Sluijter M, De Groot R, Verbrugh H, Hermans P W M. A novel BOX repeat PCR assay for high-resolution typing of Streptococcus pneumoniae isolates. J Clin Microbiol. 1996;34:1176–1179. doi: 10.1128/jcm.34.5.1176-1179.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Versalovic J V, Kapur V, Mason E O, Shah U, Koeuth T, Lupski J R, Musser J M. Penicillin-resistant Streptococcus pneumoniae strains recovered in Houston: identification and molecular characterization of multiple clones. J Infect Dis. 1992;167:850–856. doi: 10.1093/infdis/167.4.850. [DOI] [PubMed] [Google Scholar]
- 32.Vos P, Hogers R, Bleeker M, Reijans M, van der Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida R, Hirakata Y, Kaku M, Takemura H, Tanaka H, Tomono K, Koga H, Kohno S, Kamihira S. Genetic relationship of Peni-R Streptococcus pneumoniae serotype 19B isolates in Japan. Epidemiol Infect. 1997;118:105–110. doi: 10.1017/s0950268896007273. [DOI] [PMC free article] [PubMed] [Google Scholar]