Abstract
Objective:
To assess reasons for noncompliance with COVID-19 vaccination among healthcare workers (HCWs).
Design:
Cohort observational and surveillance study.
Setting:
Sheba Medical Center, a 1,600-bed tertiary-care medical center in Israel.
Participants:
The study included 10,888 HCWs including all employees, students, and volunteers.
Intervention:
The BNT162b2 mRNA COVID-19 vaccine was offered to all HCWs of the hospital. Noncompliance was assessed, and pre-rollout and post-rollout surveys were conducted. Data regarding uptake of the vaccine as well as demographic data and compliance with prior influenza vaccination were collected, and 2 surveys were distributed. The survey before the rollout pertained to the intention to receive the vaccine, and the survey after the rollout pertained to all unvaccinated HCWs regarding causes of hesitancy.
Results:
In the pre-rollout survey, 1,673 (47%) of 3,563 HCWs declared their intent to receive the vaccine. Overall, 8,108 (79%) HCWs received the COVID-19 vaccine within 40 days of rollout. In a multivariate logistic regression model, the factors that were significant predictors of vaccine uptake were male sex, age 40–59 years, occupation (paramedical professionals and doctors), high socioeconomic level, and compliance with flu vaccine. Among 425 unvaccinated HCWs who answered the second survey, the most common cause for hesitancy was the risk during pregnancy (31%).
Conclusions:
Although vaccine uptake among HCWs was higher than expected, relatively low uptake was observed among young women and those from lower socioeconomic levels and educational backgrounds. Concerns regarding vaccine safety during pregnancy were common and more data about vaccine safety, especially during pregnancy, might improve compliance.
First reported in Wuhan, China, in late 2019, the coronavirus disease 2019 (COVID-19) pandemic has spread worldwide, and severe acute respiratory coronavirus virus 2 (SARS-CoV-2) has been detected among almost 100 million people, causing >2 million deaths.1 The BNT162b2 mRNA COVID-19 vaccine is a lipid nanoparticle-formulated nucleoside modified RNA vaccine that encodes a full-length spike protein.2 A 2-dose regimen of this vaccine confers 95% protection against COVID-19 in clinical trials, and this vaccine was authorized by the US Food and Drug Administration (FDA) in December 2020 for emergency use. Healthcare workers (HCWs) were defined as a priority group in receiving this vaccine. HCWs at the front lines of the pandemic have an increased risk of contracting the virus compared to the general population,3 and they can subsequently suffer from significant morbidity and mortality.4
HCW vaccination has a pivotal role in the prevention of viral transmission in the healthcare setting. This has been well demonstrated in influenza5,6 as well as in measles outbreaks.7 Although the role of vaccination in prevention of disease for both HCWs and patients is well known, vaccine hesitancy among HCWs is a well-described phenomenon.8 Hesitancy is common in annual seasonal influenza vaccines,9 and it can even be more pronounced in novel vaccines.
HCWs, as a priority group, have an important role in the success of COVID-19 vaccination programs. HCWs remain the most trusted advisors and influencers of vaccination decisions,10 and their attitudes toward the vaccine can influence their patients’ decisions regarding vaccination. A preliminary assessment of HCW attitudes toward COVID-19 vaccination revealed that 57%–75% of individuals expressed intent to receive the vaccine and this willingness differed between different hospital roles.11,12
In this study, we assessed factors associated with noncompliance for COVID-19 vaccination among HCWs of a large tertiary-care hospital, and we sought to define the concerns that withheld these participants.
Methods
Sheba Medical Center is a 1,600-bed hospital with 10,888 HCWs, including 8,750 employees and 2,138 students and volunteers. Vaccination status was retrieved for 10,303 of these HCWs: 2,003 doctors, 2,641 nurses, 3,463 administrative and housekeeping staff, 2,175 paramedical professionals, and 21 with unavailable information on health profession. On December 6, 2020, an online pre-rollout survey was distributed via text message to all HCWs in which they were asked about their professional sector and their attitude toward vaccination with a 3 answer options: yes, no, or undecided.
COVID-19 vaccines were offered to all HCWs who did not have evidence of prior infection. Beginning December 19, 2020, vaccines were offered in the hospital’s cafeteria 12 hours a day 5 days a week. HCWs were asked to schedule an appointment by phone or online and walk-ins were accepted as well.
An intervention program that included Q&A sessions, a hotline, and various informational pamphlets distributed through organizational e-mail, encouraged hesitant HCWs to be vaccinated. In addition, the vaccination area was pleasant and inviting, and all vaccinated personnel received a “green card” that might grant exemption from social distancing measures in the hospital, entrance to the hospital cafeteria and attending in person meetings and local conferences. Employees who had a history of significant allergic reaction were offered a designated vaccination day when vaccines were administered with closer medical supervision.
On January 28, 2021, 40 days after the initiation of the rollout, all HCWs who still did not receive the first dose of the vaccine received the post-rollout survey with questions regarding their reasons to withhold from vaccination. Options suggested for vaccine hesitancy were concerns regarding short- and long-term side effects, fertility and pregnancy concerns, previous allergic reactions, and mistrust toward vaccines in general and specifically toward the COVID-19 vaccine. They were also asked to suggest steps that could improve their compliance such as better data regarding different aspects of the vaccine, receiving the vaccine in a controlled environment, or a conversation with a physician.
Data regarding HCW age, sex, position, department, city of residence and compliance with prior influenza vaccination were collected from the hospital’s human resources databases. Information about city of residence was used as a surrogate for socioeconomic level through the national statistical offices ranking.13 Information about the cumulative incidence of COVID-19 infections in the cities of residence was obtained from the Israeli Ministry of Health website,14 and cities were divided into regions of endemic level. Departments considered to have high COVID-19 exposure were the emergency department, intensive care units, and all internal medicine and geriatric medicine wards with actively treated COVID-19 patients.
All demographic variables were compared between the vaccinated and unvaccinated groups with a χ2 test. Variables, which were indicated as significant predictors up to the level of P = .20 in the univariate analysis, were tested in a multiple logistic regression model. To identify significant factors influencing the compliance with COVID-19 vaccination, variables were removed if they did not reach a significance level of P < .05
To determine whether the survey responders represent the entire noncompliant group, we compared demographic variables as reported above for the comparison of vaccinated and unvaccinated HCW with a χ2 test, and P < .05 was considered significant.
This study received approval of the institutional ethics committee.
Results
Among all 10,888 HCWs, 3,563 (33%) answered our preliminary pre-rollout survey regarding their attitudes toward the COVID-19 vaccine. Among these 3,563 HCWs, 1,673 (47%) declared their intent to receive the vaccine, 17% declared that they did not intend to receive the vaccine, and 1,261 (35%) of these 3,563 were undecided. The occupational sector that had the highest intention to receive a vaccine comprised doctors: 432 (68%) of 634 declared that they intended to receive the vaccine, and only 54 (8%) declared that they did not intend to receive the vaccine. Nurses had the lowest compliance: only 433 (34%) of 1,261 nurses intended to receive the vaccine, and 362 (29%) of these 1,261 nurses did not intend to receive the vaccine.
Overall, by January 28, 2021, within 40 days of vaccine rollout, 8,108 HCWs (79%) received the COVID-19 vaccine. Table 1 presents the characteristics of those vaccinated versus the unvaccinated. Male HCWs had a higher compliance rate: 2,673 (83%) of 3,239 male HCWs received the vaccine versus 5,453 (77%) of 7,062 female HCWs (P < .0001). The age group with the highest compliance were those aged >40 year and <60 years, with 87% (3682 of 4226) compliance, and HCWs aged <40 had the lowest compliance 70% (2,972 of 4,219) (P < .0 01). Between the different occupational sectors, doctors had the highest compliance at 82% (1,653 of 2,003) and administrative and housekeeping had the lowest at 76% (2,641 of 3,463) (P < .0001). HCWs working at a department with a high risk of exposure to COVID-19 exhibited compliance similar to those from low-risk departments (79% vs 79%; P = .71). Residence in a highly endemic city had an opposite effect. The higher the prevalence of COVID-19 in a city, the lower the compliance of HCWs residing in it. Cities designated highly endemic had the lowest compliance at 75% (929 of 1,234), and cities with low endemicity had the highest compliance at 82% (2,942 of 3,589; P < .0001). A city’s socioeconomic level was also correlated with the compliance. HCWs living in cities of low socioeconomic level had 73% compliance (603 of 828), and HCWs from cities of high socioeconomic level had 81% compliance (4,244 of 5,244; P < .0001). Among HCWs who did not receive the 2020 annual influenza vaccine, 2,869 did receive the COVID-19 vaccine. For 2020, influenza vaccine compliance was 59%, which is relatively high compared to previous years in which the compliance with the influenza vaccine was 30%–50% among HCWs in our medical center. Yet, the COVID-19 vaccine showed a much higher compliance at 79%. HCWs who had received the influenza vaccine had a higher compliance rate for the COVID-19 vaccine at 87% (5,239 of 6,038) versus those who did not receive the COVID-19 vaccine with compliance at 67% (2,869 of 4,265) (P < .0001). Also, 799 HCWs received the 2020 influenza vaccine but did not receive the COVID 19 vaccine.
Table 1.
Characteristics of Vaccinated Versus Unvaccinated Healthcare Workers (HCWs)
| Variable | Vaccinated, No. (%) | Nonvaccinated, No. (%) | P Value |
|---|---|---|---|
| Total | 8,108 (78.70) | 2,195 (21.30) | |
| Sex, % male | 2,673 of 8,108 (32.97) | 566 of 2,193 (25.81) | <.0001 |
| Age group | |||
| 18–39 y | 2,972 of 8,099 (36.70) | 1,247 of 2,191 (56.91) | <.0001 |
| 40–59 y | 3,682 of 8,099 (45.46) | 544 of 2,191 (24.83) | |
| ≥60 y | 1,445 of 8,099 (17.84) | 400 of 2,191 (18.26) | |
| Woman aged 18–40 ya | 1,923 of 5,430 (35.4) | 971 of 1,623 (59.8) | <.001 |
| Occupational sector | |||
| Doctors | 1,653 of 8,096 (20.42) | 350 of 2,186 (16.01) | <.0001 |
| Nurses | 2,071 of 8,096 (25.58) | 570 of 2,186 (26.08) | |
| Administrative and housekeeping | 2,641 of 8,096 (32.62) | 822 of 2,186 (37.60) | |
| Paramedical | 1,731 of 8,096 (21.38) | 444 of 2,186 (20.31) | |
| High covid exposure department | 831 of 8,108 (10.25) | 219 of 2,195 (9.98) | .71 |
| Endemicity in the city of residence | |||
| Very high | 929 of 7,626 (12.18) | 305 of 2,011 (15.17) | <.0001 |
| High | 3,755 of 7,626 (49.24) | 1,059 of 2,011 (52.66) | |
| Medium | 2,861 of 7,626 (37.52) | 634 of 2,011 (31.53) | |
| Low | 81 of 7,626 (1.06%) | 13 of 2,011 (0.65) | |
| Socioeconomic level | |||
| Low | 603 of 7,553 (7.98) | 225 of 1,997 (11.27) | <.0001 |
| Medium | 2,706 of 7,553 (35.83) | 772 of 1,997 (38.66%) | |
| High | 4,244 of 7,553 (56.19) | 4,244 of 1,997 (56.19) | |
| Influenza vaccine compliance | 5,239 of 8,108 (64.62) | 799 of 2,195 (36.40) | <.0001 |
Childbearing age.
In a multivariate logistic regression model, the significant predictors for noncompliance were female sex, age 18–39 years, nurses, housekeeping and administrative occupations, low socioeconomic level, and noncompliance with the 2020 flu vaccine (Table 2).
Table 2.
Multivariant Regression Model—Odds Ratio of Vaccine Uptake
| Variable | 95% CI | P Value | ||
|---|---|---|---|---|
| OR | Lower | Upper | ||
| Sex, male vs female | 0.652 | 0.579 | 0.733 | <.0001 |
| Age group | <.0001 | |||
| 40–60 vs 18–40 y | 0.336 | 0.299 | 0.379 | <.0001 |
| ≥60 vs 18–40 y | 0.699 | 0.608 | 0.804 | .0057 |
| Occupational sector | <.0001 | |||
| Administrative and housekeeping vs doctor | 1.348 | 1.153 | 1.576 | <.0001 |
| Nurses vs doctor | 1.294 | 1.095 | 1.528 | .0056 |
| Paramedical vs doctor | 0.965 | 0.814 | 1.143 | .0007 |
| Socioeconomic level | .0941 | |||
| 1 to 3 vs 4 to 7 | 1.054 | 0.862 | 1.288 | .089 |
| 8+ vs 4 to 7 | 0.877 | 0.77 | 1 | .6269 |
| Influenza vaccine compliance | 0.318 | 0.287 | 0.352 | <.0001 |
| Endemicity in the city of residence | .0403 | |||
| Medium vs high | 1.771 | 0.952 | 3.295 | .4468 |
| High vs low | 1.777 | 0.95 | 3.324 | .4903 |
| Medium vs low | 1.572 | 0.841 | 2.938 | .5554 |
Note. OR, odds ratio; CI, confidence interval.
The variable endemic region of residence, which was surprisingly negatively associated with compliance (ie, people living in a highly endemic city were less compliant), was excluded from the model because this variable was highly correlated with socioeconomic level, which explains the low compliance.
Of 2,195 unvaccinated HCWs, 425 (20%) answered a survey about the reasons for vaccine hesitancy. The unvaccinated population who answered this survey was not representative of the entire noncompliant group. A comparison of the survey responders to nonresponders revealed that they differed by employment sector. A higher percentage of unvaccinated nurses were survey responders: 40% answered the survey versus 23% who did not (P < .0001). The same was true for unvaccinated paramedical staff: 27% responded versus 19% who did not (P < .0001). These percentages were lower among unvaccinated administrative and housekeeping workers: 21% responded versus 41% who did not (P < .0001). The percentages were also lower among unvaccinated doctors: 13% responded versus 17% who did not (P < .0001). Other groups that were overrepresented were unvaccinated women (87% resonded vs 71% who did not; P < .0001), unvaccinated HCWs aged <40 years (77% responded vs 53% who did not; P < .0001). In addition, those unvaccinated HCWs who answered our questionnaire were more compliant with the flu vaccine (52% vs 33%; P < .0001).
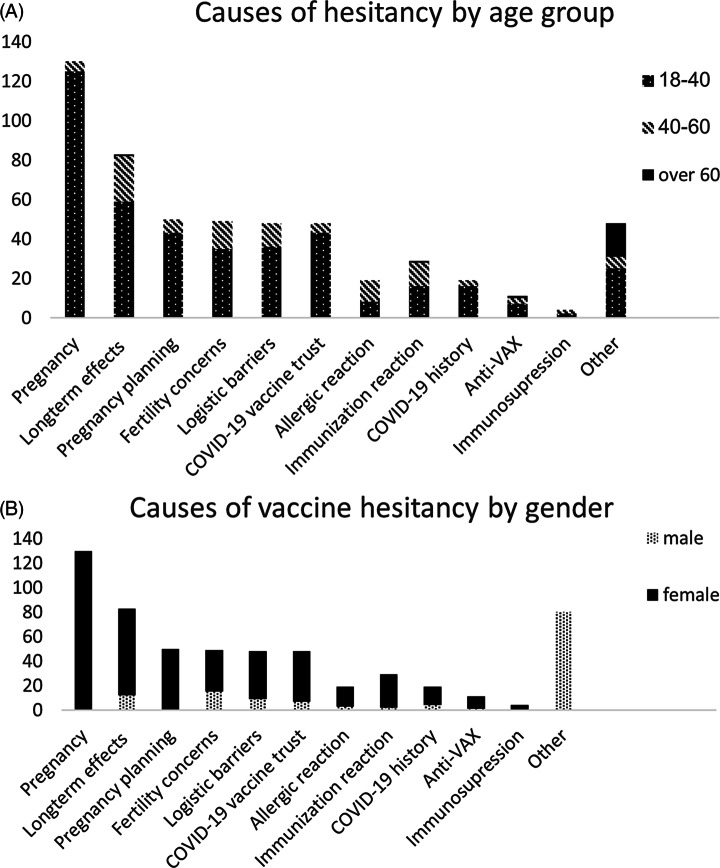
Among the responders, the most frequent cause for hesitancy included risks regarding pregnancy, and 31% responders stated this as a major reason to postpone the vaccine. Also, 20% of responders were concerned about the long-term effects of the vaccine (Fig. 1). Among HCWs aged >40 years, 26% were concerned about the long-term effects, and 16% reported low trust in the COVID-19 vaccine. Furthermore, 24% of male responders were concerned with long-term effects and 29% had trust issues with this vaccine. Other reasons for vaccine hesitancy that responders chose were plans to conceive (12%), concerns regarding fertility effects (11%), logistical issues that caused them to postpone immunization (11%), history of an allergic reaction (7%), history of an adverse reaction to a vaccination (7%), previous COVID-19 infection that was undocumented in the hospital records (5%), general “anti-Vax” agenda (3%), and immunosuppression (1%).
Fig. 1.
Causes of vaccine hesitancy. A-By age group. B-by gender
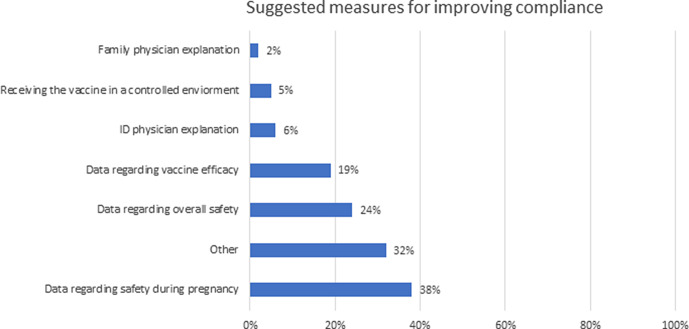
When asked about suggestions to improve hesitancy, 38% of responders suggested receiving more data regarding safety in pregnancy, 24% were interested in more data regarding overall safety, and 19% data regarding vaccine effectiveness (Fig. 2). Receiving a personal explanation by a physician either an infectious disease specialist or a family physician was suggested by only 6% and 2% of responders, respectively. And having the vaccine administered in a more controlled environment was suggested by 4% of responders. Although we provided an option for open-ended answers, the 32% of responders that chose “other” did not elaborate on additional measures that would improve their compliance.
Fig. 2.
Measures suggested by survey responders for improving their compliance with COVID-19 vaccination.
Discussion
The maintenance of an ongoing working health system is crucial for dealing with the epidemic, and protecting those on the frontlines of healthcare is vital. In addition, HCWs are essential for success of the vaccine campaign. A successful vaccination rollout among HCWs can serve as a motivator of others and can have a positive influence on vaccination in the broader population.
Because this vaccine is new, many HCWs had initial hesitation. Di Gennaro and Shekar15,16 reported that only 30%–70% of HCWs were determined to receive the vaccination initially. Receiving information from social media that could be conflicting and confusing affected the attitude toward the vaccine even among those within the health system, and lack of sufficient and reliable data was a major obstacle that caused vaccine hesitancy. An intervention program to address these hesitations was an integral part of our vaccine campaign, we also provided optimal conditions to receive vaccination with high accessibility, short queues, and an inviting vaccination area. These measures increased the vaccination compliance from the expected acceptance of 47%, predicted by a survey on December 6, 2020, 2 weeks before the rollout began, to an actual compliance of 79% by January 28, 2021, within 40 days after the first vaccination.
Some subgroups remained reluctant in their willingness to be vaccinated, such as female workers aged 20–40 years, many of whom were pregnant or were considering a pregnancy. It has been well demonstrated that COVID-19 infection in pregnancy is associated with an increased risk of adverse maternal obstetric complications,17 and pregnancy increases the risk of severe COVID-19 disease.18 On the other hand, pregnant people were excluded from the BNT162b2 mRNA COVID-19 vaccine clinical trials, and data regarding the safety of the vaccine in pregnancy at the time of our initial rollout was sparse.19 Regulatory bodies around the world, such as the FDA and the Israeli Ministry of Health, did not initially recommend the vaccine for pregnant women. This recommendation later shifted so that pregnant people should be offered the vaccine when the benefits outweigh the risks.20 The lack of an unequivocal recommendation might have led many HCWs in this position to delay the vaccine.
In our multivariant analysis, we identified a clear association between the influenza vaccine compliance in 2020 and the COVID-19 vaccine compliance. HCWs who were compliant with the flu vaccine were 3-fold more compliant with the COVID-19 vaccine. However, compliance with the COVID-19 vaccine was even higher than compliance with the influenza vaccine (79% vs 59%). It would have been informative to look at the differences in uptake compared to that for flu vaccines prior to 2020, but these data were not available.
Adverse socioeconomic factors and a low level of education are commonly cited as obstacles to vaccination acceptance.21 Here, we detected a clear association between socioeconomic level, occupational sector (as a marker of educational level), and vaccine acceptance. Our analysis revealed that they resided in a highly endemic city was reversely associated with vaccine acceptance levels, a supposedly unexpected finding. The probable explanation is that the city’s endemicity was highly correlated with its socioeconomic level. Thus, it was not a significant predictor in the multivariant analysis.
Working in a department with high COVID-19 exposure was not associated with higher vaccination rates. Apparently, direct experience in treating COVID-19 patients and the associated increased risk of exposure was not a significant motivator for vaccination, as might have been hypothesized.
Our study has some limitations. Only 34% of HCWs answered our preliminary survey regarding vaccine acceptance and only 20% of unvaccinated HCWs answered our survey regarding their causes to refrain from vaccination. Indeed, our survey likely has a sampling bias, and the respondents likely do not represent the entire non–vaccine-compliant HCW population. Our survey probably did not capture most of the ideological “anti-Vaxxers,” as suggested by the high representation of influenza-noncompliant HCWs among those who did not respond to the surveys. This survey illuminates the reasons for hesitancy among young female HCWs and emphasizes the need for more data and education on pregnancy and fertility fears.
Another limitation was our inability to differentiate administrative versus housekeeping personnel. We observed generally low compliance in the combined group, yet these 2 groups differ in activities and potential risks of exposure. A more accurate characterization of the less compliant group could potentially yield a more targeted intervention. Also, when comparing the compliance with the COVID-19 vaccine to that of the influenza vaccine, we compared only to the 2020 influenza vaccine and not to previous years, when compliance with the vaccine was somewhat lower.
Overall early vaccination rates among HCWs during the first 40 days of vaccine rollout were higher than expected. Some populations were less compliant, such as young women and those with lower socioeconomic level and educational background. The most common causes for vaccine hesitancy were concerns regarding pregnancy and long-term effects of the vaccine. Better data regarding vaccine safety during pregnancy was most common suggestion to improve compliance. Hopefully, better data about the safety and efficacy of the vaccine, especially during pregnancy, which has already began to accumulate since the initial rollout of vaccination,22,23 will help improve compliance.
Acknowledgments
We would like to thank Amit Gotkind, Hofit Amarilyo, Hagit Cohen Aharoni, Etay Menaged and Amir Grinberg for their assistance with the creation and distribution of the pre- and post-rollout surveys. We would also like to thank Vered Roa and Efrat Steinberger for their assistance in this study.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.421.
click here to view supplementary material
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1.WHO coronavirus disease (COVID-19) dashboard. World Health Organization website. https://covid19.who.int/info. Accessed January 25, 2021.
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in healthcare workers in Denmark: an observational cohort study, 2020. [DOI] [PMC free article] [PubMed]
- 4. Gómez-Ochoa SA, Franco OH, Rojas L, et al. COVID-19 in healthcare workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol 2021;190:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in healthcare settings. Clin Infect Dis 2003;37:1094–1101. [DOI] [PubMed] [Google Scholar]
- 6. Carman WF, Elder AG, Wallace LA, et al. Effects of influenza vaccination of healthcare workers on mortality of elderly people in long-term care: a randomised controlled trial, Lancet 2000;355:93–97. [DOI] [PubMed] [Google Scholar]
- 7. Maltezou HC, Wicker S. Measles in health-care settings. Am J Infect Control 2013;41:661–663. [DOI] [PubMed] [Google Scholar]
- 8. Karafillakis E, Dinca I, Apfel F, et al. Vaccine hesitancy among healthcare workers in Europe: a qualitative study. Vaccine 2016;34:5013–5020. [DOI] [PubMed] [Google Scholar]
- 9. Rebmann T, Wright KS, Anthony J, Knaup RC, Peters EB. Seasonal influenza vaccine compliance among hospital-based and nonhospital-based healthcare workers. Infect Control Hosp Epidemiol 2012;33:243–249. [DOI] [PubMed] [Google Scholar]
- 10. Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine 2016;34:6700–6706. [DOI] [PubMed] [Google Scholar]
- 11. Shaw J, Stewart T, Anderson KB, et al. Assessment of US healthcare personnel (HCP) attitudes toward COVID-19 vaccination in a large university healthcare system. Clin Infect Dis 2021. doi: 10.1093/cid/ciab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gagneux-Brunon A, Detoc M, Bruel S, et al. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: a cross-sectional survey. J Hosp Infect 2021;108:168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Israeli statistics bureau. (2006) list of municipalities by socio-demographic cluster https://www.molsa.gov.il/CommunityInfo/ResearchAndEvaluation/Documents/%D7%A0%D7%A1%D7%A4%D7%97%20-%20%D7%A8%D7%A9%D7%99%D7%9E%D7%AA%20%D7%A8%D7%A9%D7%95%D7%99%D7%95%D7%AA%20%D7%9C%D7%A4%D7%99%20%D7%9E%D7%97%D7%95%D7%96%D7%95%D7%AA%20%D7%95%D7%90%D7%A9%D7%9B%D7%95%D7%9C%20%D7%97%D7%91-%D7%9B%D7%9C%D7%9B%D7%9C%D7%99%20-%20571-580%20-%20%D7%A1%D7%A7%D7%99%D7%A8%D7%94%202010.pdf Accessed March 7, 2021.
- 14. Concentration of corona data in Israel. Israeli government website. https://www.gov.il/he/departments/guides/information-corona?chapterIndex=1. Accessed March 7, 2021.
- 15. Di Gennaro F, Murri R, Segala FV, et al. Attitudes toward anti–SARS-CoV-2 vaccination among healthcare workers: results from a national survey in Italy. Viruses 2021;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shekhar R, Sheikh AB, Upadhyay S, et al. COVID-19 vaccine acceptance among healthcare workers in the United States. Vaccines 2021;9:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res 2020;25:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oakes MC, Kernberg AS, Carter EB, et al. Pregnancy as a risk factor for severe coronavirus 2019 (COVID-19) disease using standardized clinical criteria. Am J Obstet Gynecol 2021;3:100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Male V. Are COVID-19 vaccines safe in pregnancy? Nat Rev Immunol 2021;21:200–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fact sheet for healthcare providers administering vaccine (vaccination providers).
- 21. Guzman-Holst A, DeAntonio R, Prado-Cohrs D, Juliao P. Barriers to vaccination in Latin America: a systematic literature review. Vaccine 2020;38:470–481. [DOI] [PubMed] [Google Scholar]
- 22. Collier ARY, McMahan K, Yu J, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA 2021;325:2370–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Eng J Med 2021;384:2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/ice.2021.421.
click here to view supplementary material