Abstract
Acute infections may be complicated by thrombosis occurring in the venous and arterial circulation. This may be observed in patients with community-acquired pneumonia (CAP) and also in patients with coronavirus 2019 (COVID-19), that is a pandemic characterized by severe acute respiratory syndrome (SARS-CoV-2) needing mechanical ventilation and intensive care unit treatment. However, the type and rate of thrombosis can vary according to the cause of pneumonia as is more frequently complicated by arterial thrombosis in CAP, while an equal incidence of venous and arterial thrombosis occurs in SARS-CoV-2. The mechanisms of disease are overall platelet-related in CAP while activation of both platelets and clotting system is implicated in the pathogenesis of thrombosis in SARS-CoV-2; this finding could imply a different therapeutic approach of the two settings. Thrombosis may also occur in subjects vaccinated against SARS-CoV-2 even if its incidence is not so high (1/100 000); this rare effect occurs more prevalently in young women, is independent from known risk factors of thrombosis, is caused by antibodies against platelet PF4 and is counteracted by treatment with immunoglobulin and glucocorticoids.
Keywords: COVID-19, Community-acquired pneumonia, Thrombosis, Vaccine-induced thrombotic thrombocytopenia, Platelets
Introduction
Coronavirus 2019 (COVID-19) is a pandemic characterized by severe acute respiratory syndrome (SARS-CoV-2) needing mechanical ventilation and intensive care unit (ICU) treatment. SARS-CoV-2 is characterized by high mortality rate, which is related not solely to lung disease but also to disease localized in other organs, such as heart, kidney, and cerebellum.1 Thrombosis is among the most important and frequent complication of SARS-CoV-2 and is a strong predictor of survival. Accordingly, clinical studies in COVID-19 demonstrated an early occurrence of venous and arterial thromboembolism coincidentally with a hyper-coagulation state, as depicted by elevated plasma levels of D-dimer.2 This finding is consistent with previous reports in patients with community-acquired pneumonia (CAP), which is prevalently caused by viruses, and is among the commonest cause of hospitalization for pneumonia. Interestingly, CAP share similar complications compared to SARS-CoV-2, as shown by the fact that CAP may experience thrombosis in the acute phase of the disease, suggesting that pneumonia per se may be a trigger for hypercoagulation.3 The thrombotic features of the two settings are, however, different as CAP is prevalently complicated by arterial thrombosis while SARS-CoV-2 is complicated by thrombosis in both venous and arterial circulation. Furthermore, the incidence rate of thrombosis is much higher in SARS-CoV-2 compared to CAP suggesting that a different mechanism of disease occurs in the two settings. The higher predisposition to thrombosis by SARS-CoV-2 is also highlighted by the thrombotic complication, which may rarely occur in the general population undergoing vaccination against SARS-CoV-2.3 These findings raise important issues as to whether the pathophysiology of thrombosis is similar in the pre- and post-vaccination phase of SARS-CoV-2 or a different mechanism is responsible for the thrombosis feature. Thus, the aim of this review is to compare the thrombotic features of CAP and SARS-CoV-2, analyse potential differences in the mechanism of the disease of the two settings and assess a potential relationship between thrombotic complications occurring in the pre- and post-vaccination phase of COVID-19.
Thrombosis features of CAP and SARS-CoV-2
Corrales-Medina et al.4 have been the first to show a relationship between CAP and thrombosis; using CK-MB as biomarker of myocardial necrosis, they reported, in fact, that myocardial (MI) infarction may occur in roughly 4% of patients during the intra-hospital stay. Using serum Troponin as biomarker of myocardial necrosis, our group supported and extended this finding by reporting that MI may occur in about 10% of CAP patients, underscored the peculiarity of MI feature in CAP as.3
As platelets play a key role in the pathogenesis of MI and stroke, previous studies evaluated if the marker of platelet activation can be detected in CAP. Globally considered, the study showed elevated markers of in vivo platelet activation such as plasma P-selection and CD40 Ligand (CD40L) and suggested over-biosynthesis of platelet Thromboxane (Tx)B2, as a potential mechanism. In a recent meta-analysis of 10 cohort studies including roughly 700 000 patients with sepsis, revealing that aspirin may reduce admission to ICU or mortality. Taken together, these findings indicate that CAP is associated with platelet activation but the extent to which antiplatelet treatment can lower MI and stroke remain to be ascertained.
As for the CAP, SARS-CoV-2 is also complicated by thrombosis, but the incidence rate is quite higher. Several observational studies demonstrated that thrombosis occurs during the hospitalization stay in roughly 20% of patients and may occur with an equivalent incidence in both arterial and venous circulation. MI, stroke, and peripheral embolism are the prevalent clinical feature of arterial thrombosis, while deep venous thrombosis, superficial venous thrombosis, and pulmonary embolism are the most frequent feature of venous thrombosis.3
Thrombotic complications are inconsistently associated with the changes of laboratory clotting time, such as aPTT and PT or low platelet count, while the elevation of D-dimer, a marker of hyperfibrinolysis, is a typical laboratory feature of SARS-CoV-2. The elevation of D-dimer has been considered a marker of hyper-coagulation status and, thereby, a mirror of the thrombotic events occurring in SARS-CoV-2.2 Changes of D-dimer were more marked in patients with severe disease such as those needing ICU and significantly associated with poor survival, it is still to be clarified, however, the extent to which elevation of D-dimer may be predictive of thrombosis and, thereby, potentially usable for thrombosis prevention. In addition to clotting activation, patients with SARS-CoV-2 show changes of platelet activation with over-biosynthesis of TxB2, which has been reported to be significantly associated with thrombosis. For this reason, SARS-CoV-2 seems to be different from CAP as both clotting system and platelet function are over-activated and potentially contribute to thrombosis.3
Mechanism of the disease
As CAP is prevalently due to influenza A RNA virus, a direct platelet interaction between influenza virus and platelets could be a putative mechanism. Thus, influenza A virus particles are detectable within platelets and can mediate platelet aggregation and C3 release-dependent neutrophil-DNA release via Toll-like receptor 7 (TLR7). Furthermore, in animal model of mice infected with the H1N1 virus antiplatelet drugs such as the COX1 inhibitor aspirin or the antagonist of the P2Y12 platelet receptor clopidogrel were able to inhibit virus-induced platelet activation.
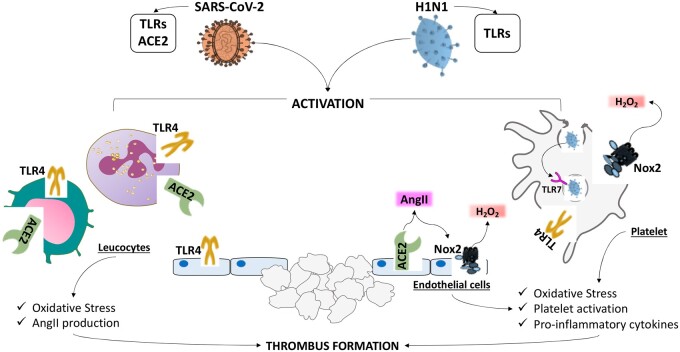
The role of Toll-like receptors (TLRs) in the pathogenesis of thrombosis by rRNA viruses have been demonstrated by Zhao et al. and Choudhury et al. showing that SARS-CoV-2 uses TLR4 to activate cells implicated in the thrombotic process, such as monocytes and leucocytes. The deleterious effects of SARS-CoV-2 binding to TLRs are likely related to up-regulation of Nox2, the most important cellular producer of reactive oxidant species (ROS), resulting in endosomal hydrogen peroxide generation. Nox2 is a key enzyme of the innate immune system, which, intriguingly, shifts endothelial cells and platelets to a prothrombotic profile. In patients affected by chronic granulomatous disease, a rare disease characterized by hereditary deficiency of Nox2, arterial vasoconstriction is lowered and platelets are less prone to promote thrombus growth. Analysis of circulating levels of soluble Nox2, which is a marker of Nox2 activation by blood cells including platelets and leucocytes, revealed that Nox2 activation occurs not only in CAP but also in COVID-19. In the case of COVID-19, the virus entry into the cells may occur by its binding to angiotensin-converting enzyme 2 (ACE2) upon its Spike protein cleavage by a serine protease, i.e. TMPRSS2. COVID-19 RNA has been detected in platelets and endothelial cells from patients with severe and non-severe COVID-19 but it is still unclear if this occurs via the Spike protein-ACE2 axis (Figure 1) as not all agree that platelets and endothelial cells express ACE2. Assuming, however, that spike protein binding and entry into human cells occurs via ACE2, this would result in surface ACE2 expression down-regulation and loss of function with ensuing angiotensin II (AngII) up-regulation as ACE2 degrades Ang II to Ang 1–7. The increase of Ang II could have potentially deleterious effects on both platelets and endothelial cells as Ang II is a pro-oxidant molecule via NOx2 up-regulation Of note, Nox2 is more activated in patients with COVID-19 vs. controls, in severe vs. non severe disease and in patients experiencing thrombotic events3 (Figure 1).
Figure 1.
Mechanisms of thrombosis in COVID-19 and CAP patients. SARS-CoV-2 and H1N1 by Toll-like receptors (TLRs) activate cells implicated in the thrombotic process such as leucocytes, platelets and endothelial cells. Upon binding to TLRs up-regulation of Nox2, the most important cellular producer of reactive oxidant species (ROS), resulting in hydrogen peroxide generation, may occur. In the case of COVID19 patients, the virus entry into the cells may occur also by its binding to angiotensin-converting enzyme 2 (ACE2) with ensuing loss of function and angiotensin II (AngII)/Nox2 up-regulation.
In alternative to this mechanism, RNA viruses could activate platelets when antibodies opsonize viral particles and interact with platelet FcyRIIA; however, they would be expected to work at later stage of the disease, thereby their potential impact on platelet activation and thrombosis in patients with COVID-19 needs to be elucidated.3
Finally, platelet activation can occur via overproduction of inflammatory pro-aggregating cytokines, which are, in fact, elevated in COVID-19. In accordance with this, incubation of endothelial cells or platelets with plasma from COVID-19 or with specific cytokines such as IL-6, promoted a prothrombotic profile (Table 1).
Table 1.
Case series of patients with vaccine-related thrombosis
| Author/year | Number of patients | Age, years | Sex, % female | Type of vaccine | Site of thrombosis |
|---|---|---|---|---|---|
| Schultz (2021)6 | 5 | 32–54 | 80 | ChAdOx1 nCoV-19 | 4 CVT and 1 PVT, left hepatic vein, splenic vein, azygos vein, hemiazygos vein, and several basivertebral veins |
| See (2021)7 | 12 | 18–59 | 100 | Ad26.COV2.S COVID-19 | CVT |
| Greinacher (2021)11 | 11 | 22–49 | 82 | ChAdOx1 nCov-19 |
9 CVT, 3 had splanchnic-vein thrombosis, 3 PE, and 4 had other thrombosesa |
| Vayne (2021)9 | 11 | 44 | NA | ChAdOx1 nCov-19 | 6 CVT, 5 had splanchnic vein thrombosis |
| Scully (2021)8 | 23 | 21–77 | 52 | ChAdOx1 nCov-19 | 15 CVT, 6 PE/DVT, 3 PVTa |
| Wolf (2021)12 | 3 | 22–46 | 100 | ChAdOx1 nCoV-19 | Intracranial venous sinus thrombosis |
| Pottegard (2021)13 |
148 792 in Denmark 132 472 in Norway |
18–65 |
80 in Denmark 78 in Norway |
ChAdOx1 nCoV-19 |
52 cardiac events 27 cerebrovascular events 59 VTE: 7 CVT, 21 PE, 22 DVT Splanchnic thrombosis <5 |
| Castelli (2021)14 | 1 | 20–50 | 0 | ChAdOx1 nCoV-19 | CVT |
CVT, cerebral venous thrombosis; DVT, deep venous thrombosis; PE, pulmonary embolism; PVT, portal vein thrombosis; VTE, venous thromboembolism.
Patients had one or more thrombosis.
Taken together, these data indicate that in patients with pneumonia by RNA viruses a shift to a prothrombotic profile can be detected in both platelets and endothelial cells. The mechanism of disease may include more than one cellular pathways, which may act at different phase of the disease.3 Thus, the complexity of this phenomenon requires further study for a better comprehension of the disease mechanism and for a more appropriate anti-thrombotic therapy.
Prevalence and mechanisms of vaccine-induced thrombosis
With the increasing use of anti-SARS-CoV-2 vaccines to reduce the diffusion of COVID-19 and its related complications, side effects occurring after vaccination have been described. In the phase 1/2 study from the Oxford COVID vaccine Trial Group,5 which included 1077 participants, of whom 543 were randomly assigned to receive ChAdOx1 nCoV-19 (AZD1222), the median age was 35 years and nearly 50% were female. Fatigue (70%) and headache (68%) were the most commonly reported systemic reactions, followed by muscle ache (60%) malaise (61%), chills (56%), and feeling feverish (51%). Transient neutropenia was also reported, but no changes of platelet count or thrombotic events were reported.
Since then, few case reports have been published reporting the onset of thrombosis after ChAdOx1 nCoV-196 and AD26.COV2.S vaccine7 (Table 1).
The majority of these thromboses were at cerebral site, were characterized by a moderate–severe fall in the platelet count in all patients and occurred within 2–3 weeks from the first dose.6 Thrombosis occurred more prevalently in young women aged approximately ≤50 years and were not associated with thrombophilia or other risk factor for thrombosis; the European Medicine Agency (EMA) evaluated in 1 over 125 000 the incidence of thrombosis.1 Thus, the term of vaccine-induced thrombotic thrombocytopenia (VITT) has been proposed to describe this condition.
Given its clinical and biochemical characteristics, the mechanism responsible for VITT has been thought to be similar to that occurring with the heparin-induced thrombocytopenia (HIT). Indeed, HIT is characterized by a platelet count (<150 × 109/L) or a relative decline of 30–50% from baseline values. Thrombocytopenia is usually moderate (50–70 × 109/L) and not causing bleeding complications.1 Thrombosis associated with thrombocytopenia typically occur after 5–15 days after heparin (mostly unfractionated) administration, and primarily affect venous circulation.
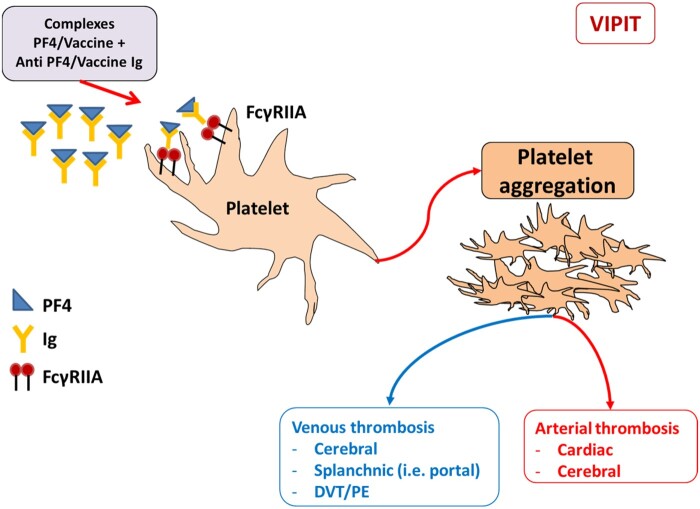
The diagnosis of HIT is confirmed by the presence of anti-Platelet Factor 4 (PF4)/heparin complexes antibodies. In the case of VITT, the production of anti-PF4 antibodies seems to be unrelated from heparin exposure, as it may occur in other clinical conditions. Platelet count, HIT enzyme-linked immunosorbent assay (ELISA), and platelet activation tests in the presence of serum from vaccinated patients have been suggested as laboratory work-up for VITT diagnosis even if there is disagreement regarding the sensitivity of the HIT assay8,9; conversely, the use of PF4-polyvinyl sulphonate provided results consistent with the autoimmune origin of the disease.8,9 It seems, therefore, that for reasons still unknown, vaccines against SAR-CoV-2 promotes the formation of anti-PF4/polyanionic antibodies, which may activate platelets through the Fcγ-receptor IIA (FcγRIIA) (Figure 2). For this reason, high-dose intravenous immunoglobulin (1 g/kg) and glucocorticoids (1 mg/kg) have been suggested as a therapy for VITT.10
Figure 2.
Mechanism of VIPIT. Vaccines against SAR-CoV-2 promote the formation of anti-PF4/polyanionic antibodies, which may activate platelets through the Fcγ-receptor IIA (FcγRIIA).
Conclusions
The data here reported show that thrombosis may occur in SAR-CoV-2 patients and, very rarely, in subjects vaccinated against SAR-CoV-2. The underlying mechanism are apparently different as in the first case the thrombotic process seems to be related to the interplay between the S protein and intracellular pathways promoting platelet and clotting activation; this sequence of events requires the use of anticoagulant which are usually give as prophylactic doses. Conversely, VIPIT is an autoimmune disease associated with thrombocytopenia and platelet-related thrombosis occurring in unusual sites, where antibodies against platelet PF4 play a major role, thereby needing an immunosuppressive therapy. Further study is, however, necessary to better elucidate the mechanism of disease.
Conflict of interest: none declared.
References
- 1. Aleem A, Nadeem AJ. Coronavirus (COVID-19) vaccine-induced immune thrombotic thrombocytopenia (VITT). In: StatPearls (internet). Treasure Island (FL): StatPearls Publishing, 2021. [PubMed]
- 2. Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L.. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost 2020;120:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Violi F, Cammisotto V, Pignatelli P.. Thrombosis in COVID-19 and non-COVID-19 pneumonia: role of platelets. Platelets 2021;doi: 10.1080/09537104.2021.1936478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corrales-Medina VF, Musher DM, Wells GA, Chirinos JA, Chen L, Fine MJ.. Cardiac complications in patients with community-acquired pneumonia: incidence, timing, risk factors, and association with short-term mortality. Circulation 2012;125:773–781. [DOI] [PubMed] [Google Scholar]
- 5. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Aboagye J, Adams K, Ali A, Allen E, Allison JL, Anslow R, Arbe-Barnes EH, Babbage G, Baillie K, Baker M, Baker N, Baker P, Baleanu I, Ballaminut J, Barnes E, Barrett J, Bates L, Batten A, Beadon K, Beckley R, Berrie E, Berry L, Beveridge A, Bewley KR, Bijker EM, Bingham T, Blackwell L, Blundell CL, Bolam E, Boland E, Borthwick N, Bower T, Boyd A, Brenner T, Bright PD, Brown-O'Sullivan C, Brunt E, Burbage J, Burge S, Buttigieg KR, Byard N, Cabera Puig I, Calvert A, Camara S, Cao M, Cappuccini F, Carr M, Carroll MW, Carter V, Cathie K, Challis RJ, Charlton S, Chelysheva I, Cho J-S, Cicconi P, Cifuentes L, Clark H, Clark E, Cole T, Colin-Jones R, Conlon CP, Cook A, Coombes NS, Cooper R, Cosgrove CA, Coy K, Crocker WEM, Cunningham CJ, Damratoski BE, Dando L, Datoo MS, Davies H, De Graaf H, Demissie T, Di Maso C, Dietrich I, Dong T, Donnellan FR, Douglas N, Downing C, Drake J, Drake-Brockman R, Drury RE, Dunachie SJ, Edwards NJ, Edwards FDL, Edwards CJ, Elias SC, Elmore MJ, Emary KRW, English MR, Fagerbrink S, Felle S, Feng S, Field S, Fixmer C, Fletcher C, Ford KJ, Fowler J, Fox P, Francis E, Frater J, Furze J, Fuskova M, Galiza E, Gbesemete D, Gilbride C, Godwin K, Gorini G, Goulston L, Grabau C, Gracie L, Gray Z, Guthrie LB, Hackett M, Halwe S, Hamilton E, Hamlyn J, Hanumunthadu B, Harding I, Harris SA, Harris A, Harrison D, Harrison C, Hart TC, Haskell L, Hawkins S, Head I, Henry JA, Hill J, Hodgson SHC, Hou MM, Howe E, Howell N, Hutlin C, Ikram S, Isitt C, Iveson P, Jackson S, Jackson F, James SW, Jenkins M, Jones E, Jones K, Jones CE, Jones B, Kailath R, Karampatsas K, Keen J, Kelly S, Kelly D, Kerr D, Kerridge S, Khan L, Khan U, Killen A, Kinch J, King TB, King L, King J, Kingham-Page L, Klenerman P, Knapper F, Knight JC, Knott D, Koleva S, Kupke A, Larkworthy CW, Larwood JPJ, Laskey A, Lawrie AM, Lee A, Ngan Lee KY, Lees EA, Legge H, Lelliott A, Lemm N-M, Lias AM, Linder A, Lipworth S, Liu X, Liu S, Lopez Ramon R, Lwin M, Mabesa F, Madhavan M, Mallett G, Mansatta K, Marcal I, Marinou S, Marlow E, Marshall JL, Martin J, McEwan J, McInroy L, Meddaugh G, Mentzer AJ, Mirtorabi N, Moore M, Moran E, Morey E, Morgan V, Morris SJ, Morrison H, Morshead G, Morter R, Mujadidi YF, Muller J, Munera-Huertas T, Munro C, Munro A, Murphy S, Munster VJ, Mweu P, Noé A, Nugent FL, Nuthall E, O'Brien K, O'Connor D, Oguti B, Oliver JL, Oliveira C, O'Reilly PJ, Osborn M, Osborne P, Owen C, Owens D, Owino N, Pacurar M, Parker K, Parracho H, Patrick-Smith M, Payne V, Pearce J, Peng Y, Peralta Alvarez MP, Perring J, Pfafferott K, Pipini D, Plested E, Pluess-Hall H, Pollock K, Poulton I, Presland L, Provstgaard-Morys S, Pulido D, Radia K, Ramos Lopez F, Rand J, Ratcliffe H, Rawlinson T, Rhead S, Riddell A, Ritchie AJ, Roberts H, Robson J, Roche S, Rohde C, Rollier CS, Romani R, Rudiansyah I, Saich S, Sajjad S, Salvador S, Sanchez Riera L, Sanders H, Sanders K, Sapaun S, Sayce C, Schofield E, Screaton G, Selby B, Semple C, Sharpe HR, Shaik I, Shea A, Shelton H, Silk S, Silva-Reyes L, Skelly DT, Smee H, Smith CC, Smith DJ, Song R, Spencer AJ, Stafford E, Steele A, Stefanova E, Stockdale L, Szigeti A, Tahiri-Alaoui A, Tait M, Talbot H, Tanner R, Taylor IJ, Taylor V, Te Water Naude R, Thakur N, Themistocleous Y, Themistocleous A, Thomas M, Thomas TM, Thompson A, Thomson-Hill S, Tomlins J, Tonks S, Towner J, Tran N, Tree JA, Truby A, Turkentine K, Turner C, Turner N, Turner S, Tuthill T, Ulaszewska M, Varughese R, Van Doremalen N, Veighey K, Verheul MK, Vichos I, Vitale E, Walker L, Watson MEE, Welham B, Wheat J, White C, White R, Worth AT, Wright D, Wright S, Yao XL, Yau Y.. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt A-H, Skattør TH, Tjønnfjord GE, Holme PA.. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021;325:2448--2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, Lester W.. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;384:2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vayne C, Rollin J, Gruel Y, Pouplard C, Galinat H, Huet O, Mémier V, Geeraerts T, Marlu R, Pernod G, Mourey G, Fournel A, Cordonnier C, Susen S.. PF4 immunoassays in vaccine-induced thrombotic thrombocytopenia. N Engl J Med 2021;385:376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karnam A, Lacroix-Desmazes S, Kaveri SV, Bayry J.. Vaccine-induced prothrombotic immune thrombocytopenia (VIPIT): consider IVIG batch in the treatment. J Thromb Haemost 2021;19:1838–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S.. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med 2021;384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf ME, Luz B, Niehaus L, Bhogal P, Bazner H, Henkes H.. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 Vaccine AstraZeneca” exposure. J Clin Med 2021;10:1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, Lidegaard Ø, Tapia G, Gulseth HL, Ruiz PL-D, Watle SV, Mikkelsen AP, Pedersen L, Sørensen HT, Thomsen RW, Hviid A.. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ 2021;373:n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Castelli GP, Pognani C, Sozzi C, Franchini M, Vivona L.. Cerebral venous sinus thrombosis associated with thrombocytopenia post-vaccination for COVID-19. Crit Care 2021;25:137. [DOI] [PMC free article] [PubMed] [Google Scholar]