Abstract
Selective cardiac myosin activators constitute a new class of drugs capable of increasing cardiac contractility independently of intracellular calcium concentrations. In the GALACTIC-HF study, the first of this class of molecules, omecamtiv mercabil, was compared with the standard of care according to current guidelines, showing a significant reduction in the composite endpoint of first episode of heart failure or mortality due to cardiovascular causes in patients exposed to treatment compared with placebo. In particular, the effect was more pronounced for decreasing ejection fraction values, suggesting a potential further benefit of selective cardiac myosin activators in this category of patients.
Keywords: Heart failure, Selective cardiac myosin activators, Cardiac contractility
Introduction and mechanism of action
Cardiac myosin activators constitute a new class of inotropes that improve myocardial contractility by directly stimulating the function of cardiac sarcomeres.1 Omecamtiv mercabil (OM), the first of this class of active compounds, increases cardiac contractility by selective interaction with cardiac myosin, increasing the number of molecules available for binding with actin, and producing a greater amount of energy at the start of systole, all without causing an increase in the consumption of calcium and oxygen. OM does not modify the contractile mechanism or alter the structure of myosin, but binds to an allosteric site that stabilizes the molecule by maximizing the number of actin–myosin interactions and thus increasing the amount of energy generated with each ventricular systole.2
These observations, together with the results of preclinical studies in which intravenous administration of OM was associated with a significant improvement in cardiac performance,3 have increased the interest for this class of molecules in the context of heart failure therapy. In patients with reduced ejection fraction (HFrEF) heart failure enrolled in the COSMIC-HF study, OM administration for 20 weeks was associated with increased systolic output, improved myocardial strain, decreased end-systolic and end-diastolic volumes of the left ventricle, improvement in left ventricular ejection fraction (LVEF) together with significant reduction in natriuretic peptides and heart rate.4
The GALACTIC-HF study: methodological aspects
Based on these observations, the GALACTIC-HF (Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in Heart Failure) trial was designed with the aim of evaluating the impact of OM therapy on the pharmacological and instrumental standard of care of HFrEF therapy on heart failure and cardiovascular death events.
Patients enrolled in GALACTIC-HF were randomized 1:1 to OM vs. placebo and assigned to a dose of 25 mg, 37.5 mg, or 50 mg b.i.d. based on plasma levels of the active substance. The administration of the product was suspended in case of clinical evidences of ischaemia or acute myocardial infarction. The primary endpoint was a composite of first event of HF (need for urgent clinic visit, emergency room access, worsening of compensation requiring higher doses of diuretic or hospitalization for HF) and first event of cardiovascular death. Secondary outcomes included cardiovascular death, changes in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score from baseline to Week 24, first hospitalization for HF, and death from all causes.
Patients studied were New York Heart Association (NYHA) II (53%), III (44%), IV (3.0%), had an LVEF ≤ 35% (mean value 26.5 + 6.3%), and plasma concentrations in NT-proBNP moderately high. Exclusion criteria included haemodynamic instability requiring mechanical circulatory support or intravenous inotropes, systolic blood pressure (SBP) < 85 mmHg, an estimated glomerular filtration rate <20 mL/min, an acute coronary syndrome, or a recently planned myocardial revascularization procedure.
Results and analysis of the study
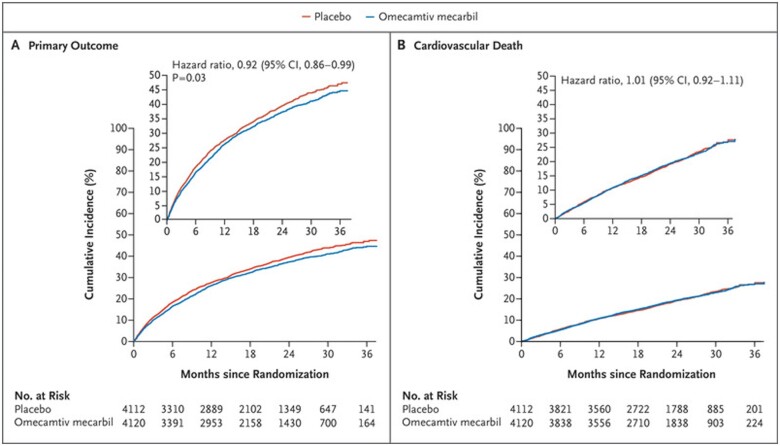
A total of 8256 patients were enrolled in the GALACTIC-HF. Over a median of 21.8 months, the primary outcome of a first hospitalization for heart failure or death from cardiovascular causes occurred in 1523 of 4120 (37%) patients treated with OM and in 1607 of 4112 (39%) patients receiving placebo, showing a statistically significant 8% relative risk reduction (P = 0.025) (Figure 1). Regarding secondary outcomes, no statistically significant differences were observed in terms of cardiovascular death (19.6% in the OM group vs. 19.4% in the placebo group, P = 0.86), incidence of first hospitalization for HF (27.7% in the OM group vs. 28.7% in the placebo group), and death from all causes (25.9% in the OM group vs. 25.9% in the placebo group). There was a significant variation in the quality of life according to the KCCQ score between baseline and the 24th week of observation in favour of OM (P = 0.028) (Table 1).
Figure 1.
Cumulative incidence of the primary outcome.
Table 1.
Primary and secondary endpoints
| Variables | Omecamtiv mercabil | Placebo | Hazard ratio or difference | P values |
|---|---|---|---|---|
| (n = 4120) | (n = 4112) | (95% CI) | ||
| Primary or composite outcome—n (%) | 1523 (37) | 1607 (39.1) | 0.92 (0.86–0.99) | 0.025 |
| Cardiovascular death as first event | 346 (8.4) | 371 (9.0) | ||
| Hospitalization for heart failure as first event | 1107 (26.9) | 1133 (27.6) | ||
| Urgent visit for heart failure as first event | 70 (1.7) | 103 (2.5) | ||
| Secondary outcome | ||||
| Cardiovascular death—n (%) | 808 (19.6) | 798 (19.4) | 1.01 (0.92–1.11) | 0.86 |
| Variation of KCCQ at 24 weeks | 0.03 | |||
| Inpatient | 23.7 ± 0.7 | 21.2 ± 0.7 | 2.5 (0.5–4.5) | |
| Outpatient | 5.8 ± 0.3 | 6.3 ± 0.3 | –0.5 (–1.4 to 0.5) | |
| First hospitalization for heart failure | 1142 (27.7) | 1179 (28.7) | 0.95 (0.87–1.03) | NA |
| All-causes-mortality—n (%) | 1067 (25.9) | 1065 (25.9) | 1.00 (0.92–1.09) | NA |
Over 70% of patients had an LVEF of < 30%; subjects with lower LVEF were younger, more frequently male and non-Caucasian. Additionally, patients with lower LVEF more frequently had non-ischaemic aetiology, more advanced NYHA functional class (mean III–IV), lower Body Mass Index, lower SBP, higher heart rate, higher NT-proBNP and Troponin I, and less frequently had comorbidities such as coronary heart disease, arterial hypertension, type 2 diabetes mellitus, or atrial fibrillation/flutter. Lower LVEF values were also associated with a worse quality of life as determined by the KCCQ, predominantly in patients enrolled during hospitalization, while no significant differences were observed for outpatients. Furthermore, the lower the LVEF, the higher the incidence of primary and secondary outcomes analysed by the study (Figure 2).
Figure 2.
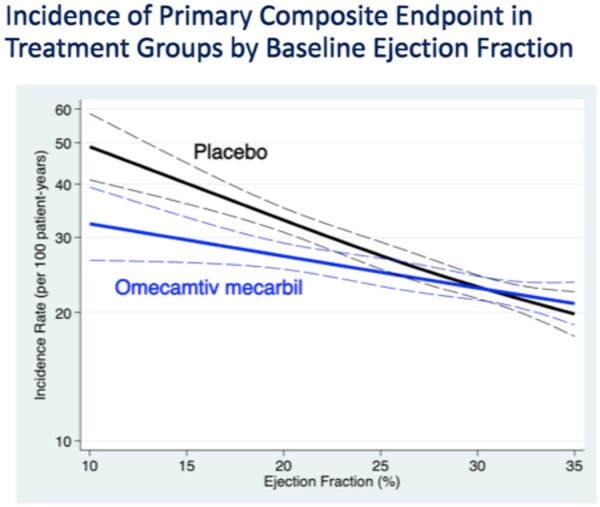
Incidence of primary composite endpoint in treatment groups by baseline ejection fraction.
As mentioned, OM has significantly reduced the primary endpoint of time to the first event of HF and death from cardiovascular causes; the incidence of primary outcome in two subgroups of LVEF above and below the median value (<28%) was also assessed in the pre-specified statistical analysis, and it was observed a significant change in the effects of OM treatment by LVEF (interaction P = 0.004). In patients with LVEF <28%, there was a 16% reduction in time to the first event of heart failure or death from cardiovascular causes [hazard ratio (HR): 0.84, 95% confidence interval (CI): 0.77–0.92; P = 0.0003] in favour of OM, while no difference was reported in patients with LVEF >28% (HR: 1.04, 95% CI: 0.94–1.16; P = 0.45). Teerlink et al.5 showed that the effect of OM treatment is more evident when the LVEF decreases: considering four quartiles of LVEF, in the lower quartiles (LVEF <22%), OM produced an absolute reduction of the primary endpoint of 7.4% per 100 patients/year, with a number needed to treat of 11.8 over 3 years to prevent an event. Consistent with the effects on the primary endpoint, the incidence rate of hospitalizations for heart failure increases with decreasing LVEF in both the OM group and in patients exposed to placebo, but was significantly influenced by OM treatment, and showed a progressive reduction, greater than the absolute difference, for decreasing LVEF values.
Other outcomes of interest include the effects of OM on vital signs and laboratory data (Table 2). No differences in SBP changes were observed between baseline, Week 24, or Week 48, between the OM group and placebo; heart rate was slightly lower in the OM group compared with placebo at the two points of observation mentioned above. The reduction in NT-proBNP levels at week 24 from baseline was 10% greater in the OM group compared with placebo.
Table 2.
Labs and vitals
| Variables | Omecamtiv mercabil | Placebo | Relative risk or difference |
|---|---|---|---|
| (n = 4110) | (n = 4101) | (95% CI) | |
| Variation form baseline | |||
| Systolic arterial pressure | |||
| Week 24 | 1.4 ± 15.3 | 1.5 ± 15.6 | −0.1 (−0.9 to 0.6) |
| Week 48 | 2.0 ± 16.1 | 1.9 ± 16.0 | 0.2 (−0.6 to 1.0) |
| Heart rate | |||
| Week 24 | −2.1 ± 12.6 | −0.5 ± 12.8 | −1.6 (−2.2 to − 1.0) |
| Week 48 | −2.0 ± 13.1 | −0.2 ± 13.2 | −1.8 (−2.4 to − 1.1) |
| Potassium | |||
| Week 24 | −0.01 ± 0.57 | −0.01 ± 0.57 | 0.00 (−0.03 to 0.03) |
| Week 48 | −0.03 ± 0.59 | −0.02 ± 0.58 | −0.01 (−0.04 to 0.02) |
| NT-proBNP | |||
| Week 24 | −251 (−1180 to 295) | −180 (−915 to 441) | 0.90 (0.86 to 0.94) |
| Troponin I | |||
| Week 24 | 4 (−2 to 21) | 0 (−9 to 8) | 4 (3 to 5) |
| Week 48 | 2 (−4 to 18) | 0 (−9 to 8) | 2 (1 to 3) |
Regarding safety data, the frequency of cardiac ischaemic events and ventricular arrhythmias did not show significant differences between the two groups (Table 3). Furthermore, no significant differences were observed with regard to serum creatinine and potassium in the two study groups.
Table 3.
Safety data
| Variables | Omecamtiv mercabil | Placebo | Relative risk or difference |
|---|---|---|---|
| (N = 4110) | (N = 4101) | (95% CI) | |
| Cardiac arrhythmia | |||
| Ventricular techy-arrhythmia | 290 (7.1) | 304 (7.4) | 0.95 (0.82–1.11) |
| Arrhythmia requiring urgent treatment | 119 (2.9) | 127 (3.1) | 0.93 (0.73–1.20) |
| Ischaemic events | |||
| Myocardial infarction | 122 (3.0) | 118 (2.9) | 1.06 (0.87–1.29) |
| Hospitalization for unstable angina | 115 (2.8) | 117 (2.9) | 1.06 (0.87–1.29) |
Discussion
In the GALACTIC-HF study, an 8% relative risk reduction (absolute difference 2.1%) in the composite primary endpoint of heart failure or death from cardiovascular causes was observed in OM-treated patients compared with placebo. These effects occurred in the absence of a significant increase in the risk of ischaemic events, ventricular arrhythmias, cardiovascular, or all-cause death. This significant, albeit modest, reduction in the incidence of the primary endpoint was observed in both inpatient and outpatient settings; favourable effects were observed in the different subgroups, but a possible heterogeneity of effect was suggested by a potentially greater treatment effect in patients with LVEF below 28%. These observations are actually biologically plausible, since OM specifically impacts cardiac contractility, making the effect more evident as lower is LVEF. Additional analyses will be needed to identify, among patients with an ejection fraction > 28%, those who could derive the greatest benefits. Although OM did not reduce cardiovascular mortality, consistent with the overall GALACTIC-HF results, OM had no adverse effects on blood pressure, heart rate, serum potassium levels, or renal function regardless of LVEF values. The small reduction in heart rate, considered secondary to the lack of the sympathomimetic effect, was consistent within the different LVEF values. As noted in previous studies, a slight increase in Troponin was observed in study patients independent of the ejection fraction values; however, this increase was not clinically significant.6 No differences were observed in the relative risk of adverse events resulting from OM treatment, such as tachyarrhythmias or ischaemic events, compared with placebo. OM, in fact, acting as a selective activator of cardiac myosin, has no effect on intracellular calcium concentrations.7 Therefore, OM therapy could be started at any time in the clinical course without interfering with the initiation or titration of standard of care therapies.
Conclusions
Among patients with HFrEF, those who were treated with OM had a lower incidence of a composite of heart failure events or death from cardiovascular causes, compared with placebo. In GALACTIC-HF, OM therapy resulted in a greater reduction in heart failure events in patients with lower baseline LVEF. Combined with the increased risk of events in this subgroup of patients, the greater relative effect of OM treatment resulted in a progressively greater absolute risk reduction in patients with lower LVEF. These results support the concept that there are subgroups of patients who may derive greater benefit from treatment with direct cardiac myosin activators.
Conflict of interest: none declared.
References
- 1. Psotka MA, Gottlieb SS, Francis GS, Allen LA, Teerlink JR, Adams KF, Rosano GMC, Lancellotti P.. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J Am Coll Cardiol 2019;73:2345–2353. [DOI] [PubMed] [Google Scholar]
- 2. Planelles-Herrero VJ, Hartman JJ, Robert-Paganin J, Malik FI, Houdusse A.. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat Commun 2017;8:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI, Wolff AA.. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet 2011;378:667–675. [DOI] [PubMed] [Google Scholar]
- 4. Teerlink JR, Felker GM, McMurray JJV, Solomon SD, Adams KF, Cleland JGF, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsányi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N;. COSMIC-HF Investigators. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet 2016;388:2895–2903. [DOI] [PubMed] [Google Scholar]
- 5. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, Biering-Sørensen T, Böhm M, Bonderman D, Fang JC, Lanfear DE, Lund M, Momomura S-I, O’Meara E, Ponikowski P, Spinar J, Flores-Arredondo JH, Claggett BL, Heitner SB, Kupfer S, Abbasi SA, Malik FI; GALACTIC-HF Investigators. Effect of ejection fraction on clinical outcomes in patients treated with omecamtiv mecarbil in GALACTIC-HF. J Am Coll Cardiol 2021;S0735-1097:04932-04939. [DOI] [PubMed] [Google Scholar]
- 6. Teerlink JR, Felker GM, McMurray JJV, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JGF, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM.. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: the ATOMIC-AHF study. J Am Coll Cardiol 2016;67:1444–1455. [DOI] [PubMed] [Google Scholar]
- 7. Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen Y-T, Vatner SF, Morgans DJ.. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 2011;331:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]