Abstract
The association of mitral valve prolapse (MVP) with ventricular arrhythmias has long been known and has generally been considered a benign condition. In recent years, however, a small but not negligible risk of malignant ventricular arrhythmias and sudden cardiac death has been documented in the large population of subjects with MVP. The main predictors of major arrhythmic risk identified so far include history of syncope, ventricular repolarization abnormalities in the inferior-lateral electrocardiogram leads, right bundle branch block morphology of ventricular ectopic beats, finding of areas of myocardial fibrosis on cardiac magnetic resonance, and mitral annular disjunction (MAD) on echocardiogram, as well as a possible pro-arrhythmic genetic substrate. The stratification of arrhythmic risk, with the active search for red flags and in particular of MAD, is important to identify patients with the malignant arrhythmic variant of MVP in whom to implement closer surveillance and possible therapeutic interventions.
Keywords: Mitral valve prolapse, Ventricular arrhythmias, Mitral annular disjunction, Sudden death
Introduction
Mitral valve prolapse (MVP) is an alteration of the mitral valve defined by echocardiography as a dislocation of one or both valve leaflets >2 mm with respect to the plane passing through the mitral valve annulus, evaluated in parasternal long-axis projection. This morphological alteration was first described by Barlow and Bosman1 and is characterized histologically by an abnormal accumulation of proteoglycans which causes redundancy and thickening of the valve leaflets as well as chorda elongation, all elements responsible for the morphological alterations of the valve.
Mitral valve prolapse has a prevalence in the general population of 2–3%2,3 and, unless the coexistence of significant valvular dysfunction, has generally been considered in the past to be a benign condition, only associated with a higher incidence of palpitations due to benign ventricular ectopic beats. However, MVP can be the subject of potentially severe complications, such as severe mitral insufficiency, infective endocarditis, stroke, and finally sudden cardiac death (SCD) and in recent years it has been the subject of studies that have increasingly suggested the existence of an association between MVP, in particular, the forms with myxomatous bileaflet redundancy, and a clinically relevant risk of arrhythmic events, with an annual MVP incidence of 0.2–0.4%, so much so as to suggest the existence of a ‘malignant arrhythmic variant’ of this valvulopathy.4–7
In a recent systematic review of 79 articles relating to the description of 161 cases of MVP with SCD or cardiac arrest with a mean age of 36 ± 16 years, 69% of cases women, the estimated incidence of SCD was 0.21%.8
Evidence in favour of the existence of a malignant arrhythmic variant of mitral valve prolapse
In a large series of the Mayo Clinic, it has been observed that in patients with MVP the presence of significant valvular insufficiency, associated with severe symptoms and left ventricular dysfunction, carries a significant risk of SCD, which is reduced by surgical correction of the valvulopathy. Within this series, however, a non-negligible risk of SCD was also observed in oligo-symptomatic patients with normal left ventricular function.9
To evaluate the correlation between the presence of MVP and the occurrence of life-threatening arrhythmic events, Siram et al.5 starting from a large retrospective study conducted from 2000 to 2009 involving 1200 patients who survived cardiac arrest due to shockable rhythms, evaluated the electrocardiographic and echocardiographic characteristics of 24 patients (2% of the total, 16 of them women, mean age 33 years) in whom organic heart disease, including coronary artery disease and coronary anomalies, or electrical abnormalities, were excluded. An unusually high prevalence of bileaflet MVP was found in this cohort of subjects (10 patients, equal to 42% of the total, in 90% women compared to that of the general population, and generally not associated with significant mitral insufficiency). Abnormalities in ventricular repolarization with biphasic or negative T waves were seen more frequently on baseline electrocardiogram (ECG) of patients with bileaflet MVP, generally in the inferior leads (78% in patients with bileaflet MVP vs. 29% in other patients), while ECG-Holter revealed a significantly higher incidence of ventricular ectopic beats (VEBs) (P = 0.002), bigeminy (P < 0.0001), and sustained or non-sustained ventricular tachycardia (78% vs. 10%), often with different morphologies alternating fascicular origin, origin from papillary muscles and from the left ventricular outflow tract. The authors conclude by suggesting that the presence of a bileaflet MVP, especially in young female subjects, in the presence of ventricular repolarization abnormalities in the inferior leads, and of the ECG-Holter evidence of complicated VEB s, may represent a condition with potential arrhythmic risk, which clinical relevance, however, requires confirmation by further studies.
To better investigate the predictive characteristics of significant arrhythmic risk in patients with MVP in the absence of significant valvular dysfunction, Basso et al.7 conducted two studies, comparing their results:
The first is a retrospective autopsy study involving all SCDs occurred in Veneto in subjects aged <40 years between 1982 and 2013 (650 subjects) from whom 46 patients were selected (7%, which rises to 13% considering only the female sex) in whom MVP was the only detectable anomaly, excluding subjects with more than mild associated mitral insufficiency. The pathological characteristics of the heart of these patients were compared with those of 15 subjects of the same age and sex who died of non-cardiac causes, showing a clear prevalence of fibrotic scars in subjects with MVP (100% at the level of the papillary muscles and 88% at level of the inferior-basal wall) absent in the control group. The evaluation of previous ECG tracings (available in 28% of subjects) showed a high prevalence of negative or iso-diphasic T waves in the inferior leads (83%) and ventricular arrhythmias with right bundle branch morphology in all patients.
The second is a prospective study that evaluated patients with MVP undergoing 12-lead ECG-Holter-24 h for arrhythmia evaluation in the period 2010–13, excluding subjects with valvular dysfunction or other associated cardiac pathologies, and subdividing them on the basis of this test in two groups: 30 patients with evidence of complex ventricular arrhythmias (ventricular tachycardia ≥ 3 beats or ventricular fibrillation) compared with 14 patients without such arrhythmias. All subjects with complex ventricular arrhythmias presented ventricular tachycardia originating from the left ventricle (right bundle branch morphology) with a superior (87%) or inferior (43%) axis, and the majority had bileaflet MVP (70% vs. 36% in the patients without complex arrhythmias). All patients underwent cardiac magnetic resonance (CMR) imaging which showed a significantly higher prevalence of late gadolinium enhancement (LGE) in patients with complex arrhythmias (93% vs. 14%; P < 0.001), mainly localized to the papillary muscles (83%, at the base of the papillary muscle or at the level of the mid-apical portion) or of the inferior basal wall (73%). This fibrosis appears histologically different from that with ischaemic genesis, as it is not compact but interposed between hypertrophic cardio-myocytes.
The comparison between the pathological findings of patients with MVP who died suddenly and the appearance detected on CMR in subjects with MVP and complex arrhythmias showed a remarkable agreement in the presence and distribution of fibrosis between these two categories of patients. The authors conclude by hypothesizing that the identification of an arrhythmic substrate by means of CMR could have a significant role in the stratification of the risk of arrhythmic events in patients with MVP. Given the impossibility of extending this investigation indiscriminately, they suggest limiting it to patients with MVP associated with additional risk factors, such as abnormalities in inferior ventricular repolarization, complex ventricular arrhythmias with right bundle branch morphology, or history of relevant clinical events (syncope, pre-syncope, or cardiac arrest).
Stratification of arrhythmic risk
From the analysis of the studies available in patients with MVP and major arrhythmic events, clinical-instrumental features useful for recognizing potentially ‘malignant’ MVP subjects at risk of threatening ventricular arrhythmias were identified (Table 1).
Table1.
Predictive factors of arrhythmic risk in patients with mitral valve prolapse
|
LGE, late gadolinium enhancement; MAD, mitral annular disjunction; RBBB, right bundle branch block; VEB, ventricular ectopic beats.
Demographic characteristics
Female sex and young age appear to be correlated with a greater risk of the occurrence of arrhythmic events in patients with MVP.4,5 In young people aged <40 years, in particular, MVP is considered responsible for 7% of SCDs.
Physical examination
The mid-systolic click is an auscultation finding described in some patients with MVP that follows a sudden tension of the mitral leaflet. In patients with MVP and CMR documentation by LGE Perrazzolo Marra et al.10 found a higher prevalence of mid-systolic click on cardiac auscultation compared to patients with no such finding (72% vs. 38%—P = 0.018). This association is explained by a greater tension overload on some components of the valve attached to the inferior-basal wall, of which the click represents the sound expression, as a result of which areas of myocardial fibrosis can develop more frequently.
Twelve-lead electrocardiogram
In patients with MVP and arrhythmic episodes, electrocardiographic findings of alterations in ventricular repolarization with biphasic or negative T waves in the inferior-lateral leads or ST-segment elevation in these sites are frequently reported.4,5 This finding appears to be an expression of the presence of a disturbance in the repolarization of these regions, an effect of overload and loco-regional fibrosis generated by the MVP itself.
Electrocardiogram -Holter
The incidence of VEB is extremely common in patients with MVP, equal to 49–85% in 24-h ECG-Holter studies.11 In patients with MVP on evaluation with 12-lead ECG-Holter, a right bundle branch block morphology is observed in most cases with more frequent origin in the papillary muscles, from the postero-basal wall or the outflow tract of the left ventricle, a finding consistent with an irritative genesis produced in these sites by the altered mechanical dynamics induced by MVP.5,12 In a recent work, Essayagh et al.12 analysed the 24-h ECG-Holters of 595 patients with MVP finding a high prevalence VEBs (≥ 5% of total beats in 43% of patients), while severe arrhythmias are were rarer (ventricular tachycardia with frequency> 180 b.p.m. in 9% of patients). The severity of arrhythmic events was directly related to the presence of structural anomalies, such as mitral annular disjunction (MAD), increased redundancy of the leaflets, and the detection of inverted T waves and/or depressed ST segment in the inferior-lateral leads (P < 0.0001), while no association with the degree of mitral insufficiency or left ventricular ejection fraction has been documented. Mortality in the subsequent follow-up of ∼8 years was closely associated with the severity of the arrhythmia, ranging from 10% in the forms without arrhythmias or with mild forms up to 24% in patients with severe arrhythmias (P = 0.02).
Left ventricular fibrosis
Fibrous myocardial replacement is a condition common to many heart diseases and is generally related to a worse prognosis of the underlying disease.13 The histological analysis reported by Basso et al.4 of the fibrotic myocardial areas present almost constantly in the papillary muscles and the basal posterior-lateral wall of the left ventricle in MVP patients who died from SCD has documented a peculiar alternation between areas of fibrosis and viable and hypertrophic cardio-myocytes, a possible cause of electrical inhomogeneity underlying arrhythmic events.
In a recent large study Constant Dit Beaufils et al.,14 400 patients with MVP underwent CMR detecting a higher incidence of cardiovascular events at 4 years in the 110 patients (25%) with LGE generally located in the inferior-posterior region and in the papillary muscles, compared to patients without this finding, with a cardiovascular event-free survival of 49% vs. 74% (P < 0.0001). LGE was also found in 13% of 120 subjects with minimal or mild mitral insufficiency and this finding was related to greater left ventricular dilation, not justified by increased haemodynamic load, so much so that the authors hypothesize the existence of cardiomyopathy associated with MVP. Furthermore, in patients with LGE, a higher incidence of both simple and complex ventricular arrhythmias was observed regardless of the degree of mitral insufficiency, further demonstration of the role of marker of increased arrhythmic risk represented by the evidence of ventricular fibrosis in patients with MVP.
Mitral annular disjunction
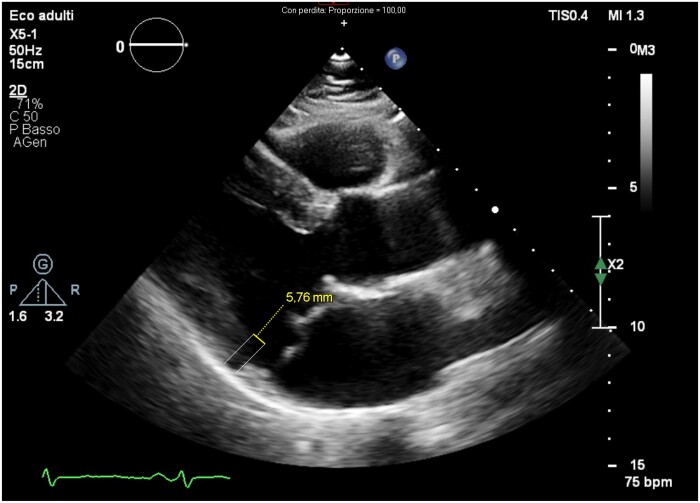
Mitral annular disjunction (MAD), first described by Bharati et al.15 is a frequently encountered anatomical finding in patients with MVP and has been correlated with an increased arrhythmic risk. Mitral annular disjunction is morphologically characterized by an increase in the fibrous separation between the insertion of the posterior mitral leaflet with the atrial wall and the ventricular muscle wall (Figure 1). This measurement is detected in end-systole in the long-axis view at the level of the basal inferior-posterior wall by echocardiogram or CMR. In an initial autopsy study on 900 patients, this anatomic alteration was closely associated with the presence of MVP (92% vs. 5%—P < 0.001), suggesting that the excessive motility of the leaflets generated by this condition could represent the cause of the MVP itself.16 Subsequent studies have scaled down the prevalence of MAD in patients with MVP, reporting a prevalence ranging from 16% to 42% in patients with MVP.6,17,18
Figure 1.
Echocardiogram of a patient with bileaflet mitral valve prolapse The parasternal long-axis view in the end-systolic phase shows a separation of 5.7 mm between the insertion point of the posterior mitral leaflet on the atrial wall and the beginning of the basal muscular portion of the posterior wall of the left ventricle, called annular-mitral disjunction (MAD).
Lee et al.18 analysed the dynamic morphological characteristics of the mitral annulus in 42 patients with MVP and MAD (defined as separation > 5 mm between the mitral–atrial junction and the ventricular wall) by means of a real-time 3D transoesophageal echocardiogram, comparing them to subjects with normal mitral valves. Under normal conditions the mitral annulus passively undergoes the direct effect of the mechanical contraction of the ventricle, showing in systole a reduction in diameters, especially antero-posterior, and an accentuation of the ‘saddle’ morphology due to displacement towards the ventricular apex of its medial and lateral portions. In the presence of MAD, the annulus is no longer affected by the direct effect of ventricular contraction but behaves like the rest of the left atrium wall, undergoing an outward stretch. As a consequence, flattening and paradoxical expansion of the mitral annulus occurs in subjects with MAD. Since the dynamic alterations of the annulus are necessary to correctly balance the haemodynamic stress of systole on all components of the valve, in patients with MAD the different distribution of mechanical stress in the different ventricular portions can cause greater hypertrophy and fibrosis of some ventricular segments, such as the papillary muscles and the inferior-posterior basal wall.
Perrazzolo Marra et al.10 analysed the cardiac anatomical characteristics of 36 consecutive patients with MVP without significant insufficiency but with complex arrhythmic events (ventricular fibrillation or sustained or non-sustained ventricular tachycardia) and evidence of LGE on CMR comparing them with 16 subjects with MVP without evidence of LGE. In patients with LGE, a greater degree of MAD (4.8 vs. 1.8 mm—P < 0.001), dilation of the mitral annulus, ‘systolic curling motion’ (defined as systolic curvature of the posterior mitral annulus) was found on the adjacent myocardium, and thickening >1.5 cm. of the inferior basal wall compared to the inferior wall average, vs. subjects without LGE. The higher prevalence in patients with LGE of meso-systolic click was associated with systolic ‘curling’ of the posterior mitral ring which causes sudden tension in the mitral leaflet with the development of lesions of the inferior-basal wall from tension overload. The authors then analysed the autopsy data of 50 patients who died from SCD in which MVP (64% bileaflet) associated with left ventricular fibrosis was found, comparing them with those of 20 subjects who died of non-cardiac causes and finding in the first group a degree of MAD significantly higher. The authors conclude by stating that the association between MAD and left ventricular fibrosis is a constant finding in patients with ‘arrhythmic’ MVP, proposing it as a morphological marker of increased risk.
Faced with the evidence of close correlation of MAD with the forms of MVP at greater arrhythmic risk, Basso et al.7 have advanced the hypothesis that MAD and systolic ‘curling’ of the posterior mitral annulus are the initial causes in determining an anomalous traction on the mitral valve, resulting in stretching and excessive overload of the postero-basal wall and of the papillary muscles, with consequent hypertrophy and subsequently loco-regional fibrosis and an increased focal ectopic activity of the Purkinjie fibres; myxomatous degeneration of the mitral valve with progressive valve insufficiency and left ventricular remodelling. The set of these elements, representing the combination of abnormal substrate and mechanical trigger, could explain the higher occurrence of malignant ventricular arrhythmias in patients with MAD and MVP.
More recently Dejgaard et al.19 examined 116 patients with MAD who underwent CMR, 78% of whom with associated MVP, evaluating the distinctive features of 14 patients (12%) with previous severe arrhythmic events (resuscitated cardiac arrest or sustained ventricular tachycardia). In this series, arrhythmic events were more frequently related to the presence of papillary muscle fibrosis (36% vs. 9%) and to the extent of MAD, while no significant correlation with the presence of MVP was found. Based on these results, the authors hypothesized that the MAD may represent a morphological entity in its own right with respect to the MVP, with which it can overlap, with its own arrhythmogenic action and therefore to be actively sought after in the stratification of arrhythmic risk.
A new marker of echocardiographic risk
The abrupt tension towards the apex to which the posterior-basal wall of the left ventricle is subjected in the middle of the systole can be clearly evidenced by the trace of the tissue Doppler (TDI) sampled there in the long axis, with the evidence of a peak in the middle of the systole similar to the tip present on Prussian helmets and therefore called ‘Pickelhaube’s sign’, judged significant when ≥16 cm/s.20 The sudden systolic overload of which this sign is an expression can act as a trigger for the onset of ventricular arrhythmias; however, the role of this marker for arrhythmic risk stratification has not yet been established.
The coexistence of MAD and left ventricular fibrosis constitutes the substrate of the so-called ‘malignant MVP’ which probably represents a more frequent entity in adult women with meso-systolic click on cardiac auscultation, mitral involvement of both leaflets, alterations of ventricular repolarization in the inferior ECG leads, VEB with right bundle branch block or polymorphic morphology and no or mild mitral regurgitation.21
Pro-arrhythmic genetic substrate
Recently, it has been shown that arrhythmogenic bileaflet MVP can recognize an inherited genetic predisposition linked to a mutation of the sarcomeric protein filamin C, associated with different forms of cardiomyopathy: dilated, hypertrophic, and restrictive.22
Practical recommendations
The SCD prevention guidelines published in 2015 by the European Society of Cardiology (ESC) do not even consider MVP as a risk condition worthy of further evaluation.23
The recent ESC guidelines on sport activity in subjects with heart disease24 define the risk of events during sport activity in subjects with MVP in the absence of severe mitral insufficiency as globally low. However, they suggest that these subjects should undergo 12-lead ECG, ergometric test, and ECG-Holter and, in patients with inverted T waves in the inferior leads or evidence of ventricular arrhythmias originating from the left ventricle, also CMR to rule out fibrosis of the inferior-basal wall. The presence of bileaflet MVP, negative T waves in the inferior leads, fibrosis of the inferior-lateral wall, family history of SCD, and evidence of complex arrhythmias are considered sufficient to exclude these subjects from competitive sport allowing only low-intensity aerobics physical activity.
For the purposes of stratifying the arrhythmic risk and with a view to the appropriate use of resources, the algorithm proposed by the Padua group7 seems particularly useful, which recommends stratifying the arrhythmic risk of patients with MVP by looking for ‘red flags’, in particular, the detection of MAD and systolic curling on the echocardiogram in addition to the arrhythmic presentation and ECG alterations, then submitting patients with these markers to CMR for the search for areas of fibrosis and close surveillance for ventricular arrhythmias and the prevention of SCD. The electro-physiology study (EPS) is not routinely recommended since the prognostic significance of any induction of ventricular tachyarrhythmias is not known.
Treatments to reduce the risk of sudden death
There is currently no clear evidence indicating how to manage a patient with MVP judged to be at high arrhythmic risk.
Medical therapy: the empirical use of beta-blocking drugs is often recommended on the basis of the documented elevation of sympathetic tone in patients with MVP. However, this practice is not supported by randomized clinical trials.
Electrophysiology study and ablation of the arrhythmic substrate:
Evidence in this regard is very scarce. EPS may be indicated in patients with MVP, suspected arrhythmic syncope, and evidence of myocardial fibrosis. Syed et al.25 treated 14 consecutive patients with EFS and catheter ablation, with ‘arrhythmic’ bileaflet MVP and mild mitral insufficiency, comparing the results between the 6 subjects with previous cardiac arrest and with implantable defibrillator (ICD) and the 8 patients with symptomatic complex ventricular arrhythmias. In all patients, it was possible to identify an arrhythmic focus at the fascicular or papillary muscle level. Inducibility of pacing ventricular fibrillation in Purkinje tissue was documented in all patients with previous cardiac arrest and in most of those in the other group. Transcatheter ablation of the arrhythmic substrate resulted in a reduction in symptomatic ventricular arrhythmias in 85% of patients in the follow-up and a reduction in appropriate shocks in ICD patients despite the need to repeat the procedure in the follow-up for the development of new arrhythmic foci. An important consideration regarding this study is that the effectiveness of the procedure in reducing the frequency of VEBs does not automatically translate into a reduction in the risk of potentially fatal arrhythmias.
ICD implant. It is currently indicated only in secondary prevention in patients with MVP in whom the diagnostic work-up has not revealed any reversible causes.
Mitral valve repair or replacement surgery. Mitral surgery has been shown in small studies to reduce malignant ventricular arrhythmias in patients with MVP and severe mitral insufficiency, possibly by reduction of left ventricular overload, while there is no evidence to support surgery aimed solely at reducing the arrhythmic risk in subjects with MVP in the absence of significant valvular dysfunction.
In conclusion, there are currently insufficient data available to draw conclusions regarding the efficacy of surgical or percutaneous procedures in reducing the arrhythmic risk of patients with MVP.
Conclusion
MVP is a widespread and generally benign condition. However, when MVP is associated with the presence of specific markers of arrhythmic risk, such as inferior-lateral electrocardiographic changes, MAD or the presence of inferior-postero basal fibrosis, it can be related to an increased arrhythmic risk and rarely to SCD. Further studies are needed to improve prognostic stratification and evaluate the indication and effectiveness of therapeutic interventions in reducing the incidence of arrhythmic events and SCDs in the large population of patients with MVP.
Conflict of interest: none declared.
References
- 1. Barlow JB, Bosman CK.. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. Am Heart J 1966;71:166–178. [DOI] [PubMed] [Google Scholar]
- 2. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ.. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1–7. [DOI] [PubMed] [Google Scholar]
- 3. Delling FN, Vasan RS.. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation 2014;129:2158–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani A, Frigo AC, Rigato I, Migliore F, Pilichou K, Bertaglia E, Cacciavillani L, Bauce B, Corrado D, Thiene G, Iliceto S.. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015;132:556–566. [DOI] [PubMed] [Google Scholar]
- 5. Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ.. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol 2013;62:222–230. [DOI] [PubMed] [Google Scholar]
- 6. Miller MA, Dukkipati S, Turagam M, Liao SL, Adams DH, Reddy VY.. Arrhythmic mitral valve prolapse. J Am Coll Cardiol 2018;72:2904–2914. [DOI] [PubMed] [Google Scholar]
- 7. Basso C, Iliceto S, Thiene G, Perazzolo Marra M.. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation 2019;140:952–964. [DOI] [PubMed] [Google Scholar]
- 8. Han H-C, Ha FJ, Teh AW, Calafiore P, Jones EF, Johns J, Koshy AN, O'Donnell D, Hare DL, Farouque O, Lim HS.. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc 2018;7:e010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grigioni F, Enriquez-Sarano M, Ling LH, Bailey KR, Seward JB, Tajik AJ, Frye RL.. Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol 1999;34:2078–2085. [DOI] [PubMed] [Google Scholar]
- 10. Perazzolo Marra M, Basso C, De Lazzari M, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016;9:e005030. doi:10.1161/CIRCIMAGING.116.005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Savage DD, Levy D, Garrison RJ, Castelli WP, Kligfield P, Devereux RB, Anderson SJ, Kannel WB, Feinleib M.. Mitral valve prolapse in the general population. 3. Dysrhythmias: the Framingham Study. Am Heart J 1983;106:582–586. [DOI] [PubMed] [Google Scholar]
- 12. Essayagh B, Sabbag A, Antoine C, Benfari G, Yang L-T, Maalouf J, Asirvatham S, Michelena H, Enriquez-Sarano M.. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol 2020;76:637–649. [DOI] [PubMed] [Google Scholar]
- 13. Patel AR, Kramer CM.. Role of cardiac magnetic resonance in the diagnosis and prognosis of nonischemic cardiomyopathy. J Am Coll Cardiol Cardiovasc Imaging 2017;10:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Constant Dit Beaufils A-L, Huttin O, Jobbe-Duval A, Senage T, Filippetti L, Piriou N, Cueff C, Venner C, Mandry D, Sellal J-M, Le Scouarnec S, Capoulade R, Marrec M, Thollet A, Beaumont M, Hossu G, Toquet C, Gourraud J-B, Trochu J-N, Warin-Fresse K, Marie P-Y, Schott J-J, Roussel J-C, Serfaty J-M, Selton-Suty C, Le Tourneau T.. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling and arrhythmia. Circulation 2021;143:1763–1774. [DOI] [PubMed] [Google Scholar]
- 15. Bharati S, Granston AS, Liebson PR, Loeb HS, Rosen KM, Lev M.. The conduction system in mitral valve prolapse syndrome with sudden death. Am Heart J 1981;101:667–670. [DOI] [PubMed] [Google Scholar]
- 16. Hutchins GM, Moore GW, Skoog DK.. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med 1986;314:535–540. [DOI] [PubMed] [Google Scholar]
- 17. Mantegazza V, Tamborini G, Muratori M, et al. Mitral annular disjunction in a large cohort of patients with mitral valve prolapse and significant regurgitation. J Am Coll Cardiol Cardiovasc Imaging 2019;12:2266–2284. [DOI] [PubMed] [Google Scholar]
- 18. Lee APW, Jin CN, Fan Y, Wong RHL, Malcolm J, Underwood MJ, Wan S.. Functional implication of mitral annular disjunction in mitral valve prolapse. A quantitative dynamic 3D echocardiographic study. J Am Coll Cardiol Cardiovasc Imaging 2017;10:1424–1433. [DOI] [PubMed] [Google Scholar]
- 19. Dejgaard LA, Skjølsvik ET, Lie ØH, Ribe M, Stokke MK, Hegbom F, Scheirlynck ES, Gjertsen E, Andresen K, Helle-Valle TM, Hopp E, Edvardsen T, Haugaa KH.. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol 2018;72:1600–1609. [DOI] [PubMed] [Google Scholar]
- 20. Muthukumar L, Rahman F, Jan MF, Shaikh A, Kalvin L, Dhala A, Jahangir A, Tajik AJ.. The Pickelhaube sign: novel echocardiographic risk marker for malignant mitral valve prolapse syndrome. J Am Coll Cardiovasc Imaging 2017;10:1078–1080. [DOI] [PubMed] [Google Scholar]
- 21. Lancellotti P, Garbi M; Malignant mitral valve prolapse. Substrates to ventricular remodeling and arrhythmias. Circ Cardiovasc Imaging 2016;9:e005248. [DOI] [PubMed] [Google Scholar]
- 22. Bains S, Tester DJ, Asirvatham SJ, Noseworthy PA, Ackerman MJ, Giudicessi JR.. A novel truncating variant in FLNC-encoded filamin C may serve as a proarrhythmic genetic substrate for arrhythmogenic bileaflet mitral valve prolapse syndrome. Mayo Clin Proc 2019;94:906–913. [DOI] [PubMed] [Google Scholar]
- 23. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck K-H, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ; ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015;36:2793–2867.26320108 [Google Scholar]
- 24. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet J-P, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M; ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
- 25. Syed FF, Ackerman MJ, McLeod CJ.. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol 2016;9:e004005. [DOI] [PubMed] [Google Scholar]