Summary
Patients with head and neck squamous cell carcinoma (HNSCC) have an increased risk of developing esophageal second primary tumors (ESPTs). We aimed to determine the incidence, stage, and outcome of synchronous ESPTs in patients with HNSCC in a Western population. We performed a prospective, observational, and cohort study. Patients diagnosed with HNSCC in the oropharynx, hypopharynx, any other sub-location in combination with alcohol abuse, or patients with two synchronous HNSCCs, between February 2019 and February 2020 underwent screening esophagogastroduodenoscopy (EGD). ESPT was defined as presence of esophageal squamous cell carcinoma (ESCC) or high grade dysplasia (HGD). Eighty-five patients were included. A lesion suspected for ESPT was detected in 14 of 85 patients, which was pathologically confirmed in five patients (1 ESCC and 4 HGD). The radiotherapy field was extended to the esophagus in two of five patients, HGD was treated with endoscopic resection in three of five patients. None of the ESPTs were detected on MRI and/or CT-scan prior to EGD. Of the remaining nine patients, three had low grade dysplasia on histology whereas the other six patients had benign lesions. Incidence of synchronous ESPT was 5.9% in our cohort of HNSCC patients. All ESPTs were diagnosed at an early stage and treated with curative intent. We recommend that screening for synchronous ESPTs should be considered in a selected group of patients with HNSCC.
Keywords: esophageal cancer, esophageal second primary tumor, esophageal squamous cell carcinoma, head and neck cancer, head and neck squamous cell carcinoma, lugol chromoendoscopy
INTRODUCTION
Patients with head and neck squamous cell carcinoma (HNSCC) are at increased risk of developing second primary tumors (SPTs).1 The development of SPTs might be explained by the field cancerization theory: premalignant changes of the epithelium around the primary tumor caused by exposure to common carcinogens such as alcohol and tobacco.2 The esophagus in particular is at increased risk of developing SPTs.3
Esophageal cancer is often diagnosed in an advanced stage because these tumors remain asymptomatic for a long period.4 In general, these patients have to be treated with invasive surgery, associated with high morbidity.5 If esophageal cancer is detected in an early stage, patients can be treated with minimal invasive endoscopic resection (ER). Therefore, early diagnosis of esophageal second primary tumor (ESPT) in HNSCC patients is crucial to improve survival with minimum morbidity.6,7 Screening of the esophagus with esophagogastroduodenoscopy (EGD) has the potential to detect ESPTs at an early stage.8 In addition, endoscopic screening is reported to be superior to Positron emission tomography (PET) scan.9
ESPT is often defined as esophageal squamous cell carcinoma (ESCC) or high grade dysplasia (HGD) of squamous epithelium.8 Low grade dysplasia (LGD) is a precursor of ESCC, and requires careful follow-up or ER.10,11 Therefore, LGD is often included in studies on ESPT.6,12
ESPTs are characterized by flat lesions, which are easily overlooked with white light high resolution endoscopy (WLE).13 Narrow-band imaging (NBI) improves the identification of these lesions due to the visibility of intraepithelial papillary capillary loop patterns.14 Still, the gold standard for ESPT detection is Lugol chromoendoscopy (LCE).8,15 Lugol iodine binds to glycogen, which is absent or diminished in dysplastic and neoplastic tissue, and therefore highlights ESPT.16 However, LCE is associated with a high rate of false positive lesions.17 Combining LCE with NBI improves ESPT detection, with a reported accuracy of 91%.18
There are multiple reports on endoscopic screening for ESPTs in HNSCC patients.8 A recent systematic review with meta-analysis by our research group showed a pooled prevalence of 15.2% (95% confidence interval [CI] 11.4–19.0).8 However, 12 of 15 included studies were performed in the Asian population.8 Only very few well-defined screening studies in the Western population exist. The aim of this study was to establish the incidence, stage and outcome of synchronous ESPTs in a selected group of Western patients with HNSCC.
MATERIALS AND METHODS
Study design
We performed a prospective, observational cohort study in a tertiary referral center in the Netherlands. This study was approved by the Medical Ethical Review Committee of the Erasmus MC in Rotterdam, the Netherlands (MEC-2018-1243) and is registered in the Netherlands Trial Register (NL7299). Patients diagnosed with HNSCC between February 2019 and February 2020 were eligible for inclusion. To be included in the study, patients had to have an increased risk of ESPT development: HNSCC located in the oropharynx, hypopharynx, any other head and neck sub-location in combination with alcohol abuse, or the presence of two HNSCCs regardless of location.8 Alcohol abuse was defined according to the classification for ‘risky alcohol use’ of The National Institute on Alcohol Abuse and Alcoholism.19 Patients with history of ESCC, oropharynx carcinoma associated with human papillomavirus infection 20, or incurable HNSCC at time of diagnosis were excluded. In every patient with oropharynx carcinoma, high-risk human papillomavirus testing was performed with immunohistochemistry for a surrogate p16 marker.
EGD was performed within 6 months after HNSCC diagnosis. In general, EGD was performed within 2 weeks after HNSCC diagnosis. All patients underwent routine clinical workup with imaging techniques for HNSCC (i.e. MRI-scan and/or CT-scan). Treatment strategy for HNSCC and ESPT was discussed in a multidisciplinary tumor board meeting consisting of a head and neck surgeon, gastroenterologist, gastrointestinal surgeon, radiotherapist, medical oncologist, and radiologist. If it was deemed impossible to perform EGD during the workup for HNSCC, HNSCC treatment was started and EGD was performed thereafter.
Screening esophagogastroduodenoscopy
EGD was performed with WLE, NBI, and LCE, by an experienced interventional endoscopist (WG; SN; PJ; MS; and AD). All endoscopists participated in dedicated upper gastrointestinal cancer screening programs and had extensive experience with all three screening techniques. EGD was performed as follows: at first, the duodenum, stomach, and esophagus were observed with WLE. Then, the esophagus was observed with NBI for aberrant intraepithelial papillary capillary loop patterns. After observation with NBI, the filter was switched to white light again and LCE was performed. For LCE, the esophagus was stained with 20–30 mL Lugol iodine (1.2%). Incidental findings such as reflux-esophagitis, Barrett’s esophagus or erosive gastritis, not related to this study were treated as per standard clinical practice.
Synchronous ESPT was defined as ESCC (category 5) or HGD of squamous epithelium (category 4) according to the Vienna classification, detected within 6 months after HNSCC diagnosis.21 A lesion was considered a possible ESPT or LGD if it was suspect on at least one of the three endoscopic detection techniques and had a diameter of at least 5 mm. All suspected lesions in the esophagus were systematically assessed for size, location (distance from the incisors), macroscopic appearance according to the Paris Classification, and whether the lesion could be removed by ER.22 ER was preferably performed for proximal lesions in the esophagus rather than being included in the radiotherapy field for HNSCC because (1) ER provides a more precise histopathological staging of early ESCC, (2) curative ER is superior to radiotherapy alone for ESCC, and (3) extending the radiotherapy field is considered a second best because a larger field might lead to more side effects such as stricture development. If ER was deemed possible, a biopsy was preferably avoided to prevent submucosal fibrosis, which might make ER more difficult. All resected specimens and biopsies were reviewed by an expert gastrointestinal pathologist (Supplementary File 1).22,23 All ER specimens were assessed whether they fulfilled the pathological criteria for a curative treatment, according to the ESGE guidelines.24
Study endpoints
The primary endpoint of this study was the incidence of ESPT. Secondary endpoints were: (1) histology and tumor stage of ESPT, (2) the incidence of LGD, (3) treatment and outcome of ESPT and LGD, (4) the number of ESPTs that were not detected with routine imaging techniques for HNSCC workup, and (5) detection rate of ESPT and LGD with WLE, NBI, and LCE.
Statistical analysis
Continuous data were expressed as mean (± standard deviation) for normally distributed data and as median (interquartile range [IQR]) for skewed data. Categorical data were presented with frequencies and percentages. Follow-up data were retrieved until July 2020. Analyses were performed with IBM SPSS Statistics (Version 25).
RESULTS
Baseline characteristics
Out of 129 eligible patients, 92 patients underwent a screening EGD (Fig. 1). Seven patients with oropharynx carcinoma who underwent EGD were positive for human papillomavirus infection (no ESPT was detected) and were excluded from further analyses. The remaining 85 patients were included in the final analysis. Baseline and HNSCC characteristics are presented in Table 1. Most HNSCCs were located in the hypopharynx (33%) or oropharynx (29%). Six out of 85 patients died within 1 year after HNSCC diagnosis.
Fig. 1.
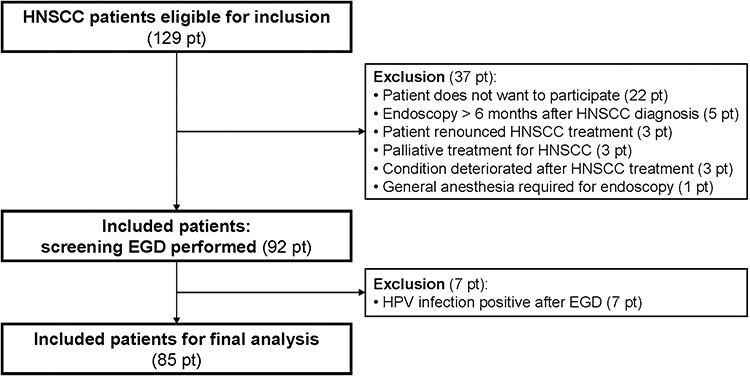
Flow-chart in- and excluded patients. Abbreviations: HPV, human papilloma virus; pt, patient(s).
Table 1.
Baseline characteristics of patients with HNSCC (n = 85)
| Patient characteristics | |
|---|---|
| Male sex, n (%) | 67 (79%) |
| Median age, years (IQR) | 65 (59–70) |
| ASA classification, n (%) I II III |
4 (5%) 69 (81%) 12 (14%) |
| Present alcohol use, n (%) Yes Median units alcohol/week (IQR) No Alcohol use in the past, n Median units alcohol/week (IQR) |
67 (79%) 21 (19–42) 18 (21%) 9 35 (23–77) |
| Current tobacco use, n (%) Yes Median pack years (IQR) No Smoking in the past, n Median pack years (IQR) |
46 (54%) 40 (29–55) 39 (46%) 31 40 (40–50) |
| HNSCC characteristics | |
| Number of HNSCC 1 2 |
79 (93%) 6 (7%) |
| Tumor location, n (%)# Nasopharynx Oral cavity Oropharynx Hypopharynx Larynx |
1 (1%) 15 (16%) 26 (29%) 30 (33%) 19 (21%) |
| Tumor stage, n (%)# Tis T1 T2 T3 T4a/T4b |
7 (8%) 15 (17%) 29 (32%) 24 (26%) 14 (15%)/2 (2%) |
| N stage, n (%)# N0 N1 N2/N2a/N2b/N2c N3b |
49 (55%) 14 (15%) 3 (3%)/2 (2%)/14 (15%)/8 (9%) 1 (1%) |
| M stage, n (%) M0 |
85 (100%) |
| HNSCC treatment, n (%) Chemotherapy Chemoradiotherapy Radiotherapy Surgery Surgery + radiotherapy Surgery + chemoradiotherapy Laser No treatment |
1 (1%) 31 (37%) 25 (30%) 11 (13%) 8 (9%) 1 (1%) 7 (8%) 1 (1%) |
#Calculated for the total number of Head and Neck tumors = 91.
Esophageal second primary tumors
The median time between HNSCC diagnosis and EGD was 9 days (IQR: 6–20). No adverse events occurred during EGD. A total of 15 suspected lesions were detected in 14 patients (16.5%).
Confirmed ESPT
ESPT was histopathologically confirmed in 5 out of 14 patients (Tables 2 and 3; patients 1–5). This was an ESCC in one patient (patient 1) and HGD in four patients (patients 2–5). All ESPTs were ≥20 mm (range 20–80). The radiotherapy field for HNSCC was extended to the esophagus because of the presence HGD (T2 lesion on PET-scan) in one patient (patient 2) and the presence of LVI after ER for T1a ESCC in another patient (patient 1). The remaining three patients with HGD were treated with ER only (patients 3–5).
Table 2.
Patient and tumor characteristics of patients with esophageal second primary tumor or low-grade dysplasia
| Patient & HNSCC characteristics | Screening esophagogastroduodenoscopy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Sex | Age | Alcohol (units/ week) |
Smoking (PY) | HNSCC tumors | HNSCC sub-location | TN stage | Number of lesions, visible with WLE/NBI/LCE | Location esophagus (cm)† | Morphology‡ + diameter lesion (mm) | Pathology | |
| ESPT | 1 | M | 67 | No | Yes (13) | 2 | Oropharynx + Hypopharynx | T2N2c + T2N2c | 1, WLE + NBI* | 20 | 0-IIa (20) | ESD: pT1a ESCC (m3, G1/2, LVI+, R0) |
| 2 | M | 48 | Yes (42) | Yes (31) | 1 | Hypopharynx | T2N2b | 1, WLE + NBI* | 20 | 0-Is +0-II (20) | Biopsy: HGD | |
| 3 | M | 62 | Yes (21) | Yes (20) | 2 | Oropharynx + Hypopharynx | T3N2c + T4aN2c | 1, NBI + LCE | 38 | 0-IIa (20) | Biopsy: LGD EMR: HGD |
|
| 4 | F | 62 | Yes (9) | Yes (30) | 1 | Hypopharynx | T4N2b | 1, WLE + NBI + LCE | 22–31 | 0-IIb + 0-IIa (80) | Biopsy: HGD ESD: HGD |
|
| 5 | M | 67 | No | No | 1 | Oropharynx | T2N0 | 1, WLE + NBI + LCE | 21–24 | 0-IIb (30) | ESD: HGD | |
| LGD | 6 | M | 68 | Yes (28) | No (40) | 1 | Hypopharynx | T3N2b | 1, LCE | 28 | 0-IIb (6) | Biopsy: LGD EMR: ND |
| 7 | F | 67 | Yes (28) | No | 1 | Larynx | T2N0 | 1, LCE | 24 | 0-IIb (5) | EMR: LGD | |
| 8 | M | 77 | No | No | 1 | Hypopharynx | T3N2b | 1, WLE + NBI + LCE | 30 | 0-IIa (20) | Biopsy: LGD | |
* LCE not performed, lesion was visible with WL and NBI.
†From the incisors.
‡According to the Paris classification.20
Abbreviations: EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; F, Female; M, Male; NBI, narrow band imaging; PY, pack years.
Table 3.
Treatment and follow-up information of patients with second primary tumor or low-grade dysplasia
| ID | Treatment | Follow-up | |
|---|---|---|---|
| ESPT | 1 | ESD + radiotherapy field HNSCC extended to the esophagus + chemotherapy | No recurrence |
| 2 | Radiotherapy field HNSCC extended to the esophagus + chemotherapy | Recurrence ESCC after 9 months: laryngeal and pharyngeal extirpation + proximal esophagus resection | |
| 3 | EMR + endoscopic surveillance | No recurrence | |
| 4 | ESD + endoscopic surveillance | No recurrence | |
| 5 | ESD + endoscopic surveillance | No recurrence | |
| LGD | 6 | EMR + endoscopic surveillance | No recurrence |
| 7 | EMR + endoscopic surveillance | No recurrence | |
| 8 | EMR not performed: Patient died | Patient died |
Abbreviations: EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection.
Low grade dysplasia
LGD was found in three patients (Tables 2 and 3; patient 6–8). Two patients underwent ER and one patient died due to HNSCC before ER was performed.
No dysplasia
In 5 out of 14 patients, ESPT or LGD could not be confirmed on histopathological analysis. These patients are presented in Supplementary file 2; Table S2. The median size of these non-dysplastic lesions was 6 mm (IQR: 5–9). One out of 14 patients had a suspected lesion but histopathology was not obtained because of refusal of further treatment by the patient.
Overall, an ESPT was detected and histopathologically confirmed in 5 out of 85 patients (5.9%, 95% CI 1.9–13.2). LGD was detected in 3 out of 85 patients (3.5%, 95% CI 0.7–10.0). These (pre)malignant lesions were all found in an early stage and could be treated with curative intent. None of the ESPTs and LGD lesions were identified by MRI and/or CT-scan.
Endoscopic detection technique
In two out of five ESPTs, the lesion was detected with WLE and NBI. In both patients, LCE was not performed because this was not considered of additional value and would have only resulted in additional discomfort to the patient. Overall, ESPTs were detected with WLE, NBI, and LCE in four, five, and three patients, respectively. Of the three histopathological confirmed LGD lesions, all lesions were detected with LCE, whereas one was detected with NBI, and another one with WLE. Figure 2 shows two separate ESPTs, one detected by NBI and one by LCE.
Fig. 2.
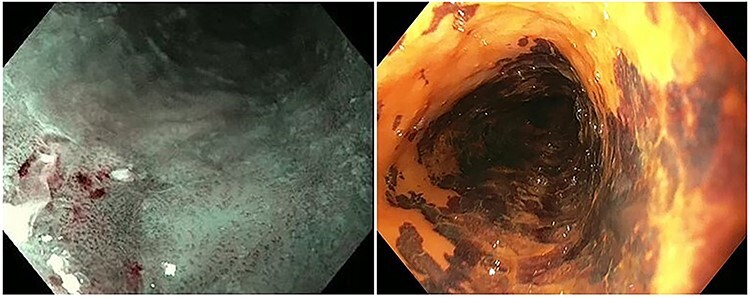
Esophageal second primary tumor visible with NBI and LCE. Left picture: HGD visible with NBI (patient ID: 3), right picture: HGD visible with LCE (patient ID: 4).
Figure 3 shows the number of ESPT, LGD and non-dysplastic lesions detected by WLE, NBI, and LCE. The positive predictive value (PVV) for ESPT detection was highest with NBI (71.4%) and lowest with LCE (27.3%). The false positive detection rate was highest with LCE (45.5%).
Fig. 3.

Detection of esophageal second primary tumors and LGD by EGD. a LCE not performed in two patients; b No pathology obtained in one patient; c calculated for all suspected ESPT of which pathology was obtained. Abbreviation: pt, patients.
DISCUSSION
We performed a prospective endoscopic screening study in patients with HNSCC and found an ESPT incidence of 5.9%. ESPT or LGD was found in approximately one in 10 patients. All esophageal lesions were diagnosed at an early stage and could be treated with curative intent with either ER or radiotherapy. Since none of the ESPTs were identified by other imaging techniques, our findings suggest that screening for ESPT by EGD is of added value for a selected group of HNSCC patients.
Previous screening studies reported prevalences of ESPT between 4.1 and 40.9%.8 A recent meta-analysis by our research group, which included >3000 patients, found a pooled prevalence of 15.2% (95% CI 11.4–19.0).8 This is much higher compared with our current findings, which may be explained by the fact that metachronous ESPTs were also included in the meta-analysis.8 Another possible explanation is that the majority of previously published screening studies were performed in Asia. The prevalence of synchronous ESPTs is higher in the Asian population compared with the Western population.8 This difference might be due to a higher exposure of risk factors (e.g. alcohol and tobacco) in the Asian population and a difference in genetic polymorphisms of alcohol metabolism between these two populations.25 However, the majority of patients in our cohort were exposed to these risk factors. Western gastroenterologists might have a relative lack of experience in screening for early ESCC compared with Asian gastroenterologists, which might contribute to the difference in ESPT prevalence.
The only two screening studies in Western population reported incidences of 6.9 and 10.0%, which is more in line with our results.12,26 The French study by Dubuc et al., included 393 patients with a history of head and neck (n = 384) or tracheobronchial squamous cell carcinoma (n = 9). ESPT was detected in 27 of 393 patients (6.9%).12 However, the time between HNSCC and ESPT diagnosis was not reported. The proportion of synchronous ESPTs is probably lower than 6.9%. Boller et al. included 40 patients with HNSCC, ESPT was detected in four patients (10.0%).26 The mean time since HNSCC diagnosis was 5.0 years, it is therefore most likely that no synchronous ESPTs are detected in this study.26
All patients with an ESPT in our study had an oropharynx or hypopharynx carcinoma. Several studies have shown that patients with an HNSCC in these sub-locations have a higher risk of developing ESPT.27 An endoscopic screening study by Gong et al., showed that the ESPT prevalence was highest in patients with hypopharynx carcinoma (21%).27 Wang et al. reported an ESPT prevalence of 36% in patients with an oropharynx carcinoma and 29% in patients with a hypopharynx carcinoma, in contrast to an ESPT prevalence of only 9% in patients with laryngeal cancer.28 According to a pooled analysis, the ESPT incidences are 14 and 28% for patients with an HNSCC in the oropharynx and hypopharynx, respectively.8 This suggests that endoscopic screening for ESPT is most effective in these patients.
It is well established that esophageal lesion size is associated with malignancy with 20 mm as the most common cut-off value.26,29 In an endoscopic screening study by Boller et al., none of the Lugol voiding lesions (LVL) < 20 mm showed dysplasia on histopathological assessment, whereas dysplasia was found in 80% of lesions ≥ 20 mm.26 In another endoscopic screening study, 37% of the LVL > 10 mm showed dysplasia or neoplasia compared with only 5% of the LVL between 5 and 10 mm.17 In our study, all ESPTs were ≥20 mm, whereas the six non-dysplastic lesions had a median diameter of only 6 mm. Therefore, we would suggest follow-up with repeat EGD or biopsy instead of ER in esophageal lesions smaller than 20 mm.
Although LCE is considered the gold standard for ESPT detection by many, its application is subject to debate because of its side effects and prolonged procedure time.12 In addition, the specificity of LCE is low, since non-dysplastic lesions can also be unstained.13 An endoscopic screening study by Shao et al., found that 74% of the LVL showed no dysplasia on histopathological assessment.17 Another endoscopic screening study in patients with HNSCC showed that 82% of the LVL were non dysplastic.26 A high false positive detection rate is also reflected by our results: 46% of lesions that were suspicious on LCE were false positive, compared with 14% in NBI.
Although our endoscopists have extensive experience in assessing esophageal lesions, the relatively high number of false positive lesions detected by LCE might indicate that LVL were easily misinterpreted by the endoscopists. However, our study was not designed to calculate the accuracy rates of endoscopic detection techniques. As reported in a systematic review and meta-analysis by Morita et al., NBI was superior to LCE in differentiating ESPTs from other esophageal mucosa alterations, but the sensitivity rates of these techniques to detect ESPTs were comparable.30 LCE is helpful to highlight suspected lesions but endoscopist’ experience is still key in the characterization and detection of suspected ESPT.31
Our study is subject to certain limitations. First, we included relatively few patients. This made it impossible to perform risk factor analysis. Second, a large number of patients were excluded and these patients could potentially have had a synchronous ESPT. This might lead to a chance of bias skewing the incidence of ESPTs. Third, several patients had an incurable HNSCC, which came to light after they had underwent endoscopic screening. Since these patients would not have benefitted from endoscopic screening it would have been better if screening was performed after workup for HNSCC was completed. If endoscopic screening is implemented in daily practice, patients with incurable HNSCC will most likely not be included. Fourth, patient burden was not taken into account. Screening EGD is an invasive examination for patients. Patient burden is an important parameter for the decision whether screening should be performed.
The major strength of our study is its prospective design. All eligible patients were asked to participate, which prevented selection bias. This design also ensured that we had no missing data. Another strength is that screening EGD was performed in a systematic manner with three different endoscopic techniques. This presumably lead to a high detection rate with only minimal missed lesions.
We believe that screening for synchronous ESPTs in patients with HNSCC is promising. Screening should be first considered in high-risk patients (e.g. HNSCC located in the oropharynx and hypopharynx, patients with alcohol abuse). The combination of WLE and NBI is probably the most sensitive method. Although LCE can be performed, extra awareness is indicated in case of lesions <20 mm because of the high rate of false positive lesions.
However, more research is necessary before screening for ESPT can be implemented. More studies with a larger patient cohort are necessary, preferably in a multicenter setting. This would enable a solid risk factor analysis and identify a specific subgroup of HNSCC patients who would benefit most from screening. Future studies should also take patient burden, survival benefit and cost-effectiveness of screening into account.
Supplementary Material
Contributor Information
Steffi E M van de Ven, Department of Gastroenterology and Hepatology, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Wilmar de Graaf, Department of Gastroenterology and Hepatology, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Oisín Bugter, Department of Otorhinolaryngology and Head and Neck Surgery, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Manon C W Spaander, Department of Gastroenterology and Hepatology, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Suzan Nikkessen, Department of Gastroenterology and Hepatology, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Pieter Jan F de Jonge, Department of Gastroenterology and Hepatology, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Jose A Hardillo, Department of Otorhinolaryngology and Head and Neck Surgery, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Aniel Sewnaik, Department of Otorhinolaryngology and Head and Neck Surgery, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Dominiek A Monserez, Department of Otorhinolaryngology and Head and Neck Surgery, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Hetty Mast, Department of Oral and Maxillofacial surgery, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Stijn Keereweer, Department of Otorhinolaryngology and Head and Neck Surgery, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Marco J Bruno, Department of Gastroenterology and Hepatology, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Robert J Baatenburg de Jong, Department of Otorhinolaryngology and Head and Neck Surgery, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Arjun D Koch, Department of Gastroenterology and Hepatology, Erasmus MC Cancer Institute, University Medical Center, Rotterdam, the Netherlands.
Funding
The Dutch Digestive Foundation (SK18-12).
Conflict of interest
None declared.
References
- 1. Priante A V, Castilho E C, Kowalski L P. Second primary tumors in patients with head and neck cancer. Curr Oncol Rep 2011; 13: 132–7. [DOI] [PubMed] [Google Scholar]
- 2. Slaughter D P, Southwick H W, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953; 6: 963–8. [DOI] [PubMed] [Google Scholar]
- 3. Atienza J A, Dasanu C A. Incidence of second primary malignancies in patients with treated head and neck cancer: a comprehensive review of literature. Curr Med Res Opin 2012; 28: 1899–909. [DOI] [PubMed] [Google Scholar]
- 4. Enzinger P C, Mayer R J. Esophageal cancer. N Engl J Med 2003; 349: 2241–52. [DOI] [PubMed] [Google Scholar]
- 5. Linden P A, Towe C W, Watson T J et al. Mortality after esophagectomy: analysis of individual complications and their association with mortality. J Gastrointest Surg 2019; 24: 1948–54. [DOI] [PubMed] [Google Scholar]
- 6. Chung C S, Lo W C, Chen K C et al. Clinical benefits from endoscopy screening of esophageal second primary tumor for head and neck cancer patients: analysis of a hospital-based registry. Oral Oncol 2019; 96: 27–33. [DOI] [PubMed] [Google Scholar]
- 7. Bradley P J, Bradley P T. Searching for metachronous tumours in patients with head and neck cancer: the ideal protocol! Curr Opin Otolaryngol Head Neck Surg 2010; 18: 124–33. [DOI] [PubMed] [Google Scholar]
- 8. Bugter O, van de Ven S E M, Hardillo J A et al. Early detection of esophageal second primary tumors using Lugol chromoendoscopy in patients with head and neck cancer: a systematic review and meta-analysis. Head Neck 2019; 41: 1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su H A, Hsiao S W, Hsu Y C et al. Superiority of NBI endoscopy to PET/CT scan in detecting esophageal cancer among head and neck cancer patients: a retrospective cohort analysis. BMC Cancer 2020; 20: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mandard A M, Hainaut P, Hollstein M. Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res 2000; 462: 335–42. [DOI] [PubMed] [Google Scholar]
- 11. Wang G Q, Abnet C C, Shen Q et al. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut 2005; 54: 187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubuc J, Legoux J, Winnock M et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: a prospective study conducted in 62 French endoscopy centers. Endoscopy 2006; 38: 690–5. [DOI] [PubMed] [Google Scholar]
- 13. Goda K, Dobashi A, Yoshimura N et al. Narrow-band imaging magnifying endoscopy versus lugol chromoendoscopy with pink-color sign assessment in the diagnosis of superficial esophageal squamous neoplasms: a randomised noninferiority trial. Gastroenterol Res Pract 2015; 2015: 639462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshida T, Inoue H, Usui S et al. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc 2004; 59: 288–95. [DOI] [PubMed] [Google Scholar]
- 15. Inoue H, Rey J F, Lightdale C. Lugol chromoendoscopy for esophageal squamous cell cancer. Endoscopy 2001; 33: 75–9. [PubMed] [Google Scholar]
- 16. Mori M, Adachi Y, Matsushima T et al. Lugol staining pattern and histology of esophageal lesions. Am J Gastroenterol 1993; 88: 701–5. [PubMed] [Google Scholar]
- 17. Shao Y, Yu Z L, Ji M et al. Lugol chromoendoscopic screening for esophageal dysplasia/early squamous cell carcinoma in patients with esophageal symptoms in low-risk region in China. Oncol Lett 2015; 10: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C H, Lee Y C, Wang C P et al. Use of transnasal endoscopy for screening of esophageal squamous cell carcinoma in high-risk patients: yield rate, completion rate, and safety. Dig Endosc 2014; 26: 24–31. [DOI] [PubMed] [Google Scholar]
- 19. [Anonymous]. National Institute on Alcohol Abuse and Alcoholism . Helping Patients Who Drink Too Much: A Clinician's Guide. Bethesda: NIH Publication no 05-3769, 2005; DOI:. [Google Scholar]
- 20. Morris L G, Sikora A G, Patel S G et al. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol 2011; 29: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schlemper R J, Riddell R H, Kato Y et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anonymous . The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003; 58: S3–43. [DOI] [PubMed] [Google Scholar]
- 23. Bosman F T C F, Hruban R H et al. WHO Classification of Tumours of the Digestive System, vol. 3, 4th edn. Lyon: International Agency for Research on Cancer, 2010. [Google Scholar]
- 24. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2015; 47: 829–54. [DOI] [PubMed] [Google Scholar]
- 25. Li H, Borinskaya S, Yoshimura K et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet 2009; 73: 335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boller D, Spieler P, Schoenegg R et al. Lugol chromoendoscopy combined with brush cytology in patients at risk for esophageal squamous cell carcinoma. Surg Endosc 2009; 23: 2748–54. [DOI] [PubMed] [Google Scholar]
- 27. Gong E J, Kim D H, Ahn J Y et al. Routine endoscopic screening for synchronous esophageal neoplasm in patients with head and neck squamous cell carcinoma: a prospective study. Dis Esophagus 2016; 29: 752–9. [DOI] [PubMed] [Google Scholar]
- 28. Wang W L, Lee C T, Lee Y C et al. Risk factors for developing synchronous esophageal neoplasia in patients with head and neck cancer. Head Neck 2011; 33: 77–81. [DOI] [PubMed] [Google Scholar]
- 29. Fukuzawa K, Noguchi Y, Yoshikawa T et al. High incidence of synchronous cancer of the oral cavity and the upper gastrointestinal tract. Cancer Lett 1999; 144: 145–51. [DOI] [PubMed] [Google Scholar]
- 30. Morita F H, Bernardo W M, Ide E et al. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer 2017; 17: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van de Ven S E M, Koch A D. When is Lugol still necessary in 2020? Endosc Int Open 2020; 8: E1478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


